

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| L-lactate dehydrogenase A chain (Homo sapiens (Human)) | BDBM50546980 (CHEMBL4792513) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human LDHA using sodium pyruvate as substrate preincubated for 5 mins followed by diaphorase/resazurin addition and measure... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00916 BindingDB Entry DOI: 10.7270/Q2057KJ6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| L-lactate dehydrogenase A chain (Homo sapiens (Human)) | BDBM50546969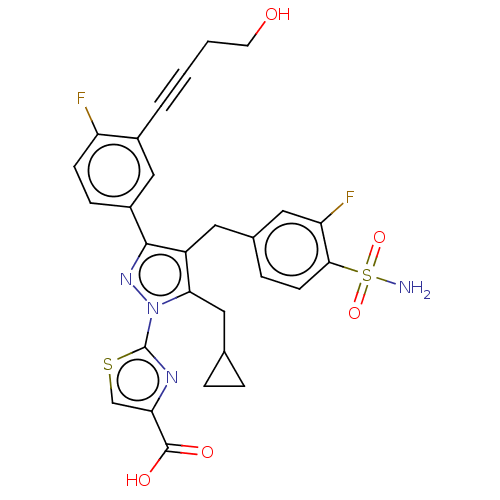 (CHEMBL4786682 | US11247971, Cmpd ID 409) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human LDHA using sodium pyruvate as substrate preincubated for 5 mins followed by diaphorase/resazurin addition and measure... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00916 BindingDB Entry DOI: 10.7270/Q2057KJ6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| L-lactate dehydrogenase A chain (Homo sapiens (Human)) | BDBM537077 (US11247971, Cmpd ID 400) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human LDHA using sodium pyruvate as substrate preincubated for 5 mins followed by diaphorase/resazurin addition and measure... | Citation and Details Article DOI: 10.1016/j.bmcl.2021.127974 BindingDB Entry DOI: 10.7270/Q24J0JV6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| L-lactate dehydrogenase A chain (Homo sapiens (Human)) | BDBM489090 (2-(5- (cyclopropylmethyl)- 3-(4-fluoro-3- ((tetrah...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human LDHA using sodium pyruvate as substrate preincubated for 5 mins followed by diaphorase/resazurin addition and measure... | Citation and Details Article DOI: 10.1016/j.bmcl.2021.127974 BindingDB Entry DOI: 10.7270/Q24J0JV6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| L-lactate dehydrogenase A chain (Homo sapiens (Human)) | BDBM489091 (2-(5- (cyclopropylmethyl)- 3-(4-fluoro-3- ((tetrah...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human LDHA using sodium pyruvate as substrate preincubated for 5 mins followed by diaphorase/resazurin addition and measure... | Citation and Details Article DOI: 10.1016/j.bmcl.2021.127974 BindingDB Entry DOI: 10.7270/Q24J0JV6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| L-lactate dehydrogenase A chain (Homo sapiens (Human)) | BDBM50546981 (CHEMBL4797357) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human LDHA using sodium pyruvate as substrate preincubated for 5 mins followed by diaphorase/resazurin addition and measure... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00916 BindingDB Entry DOI: 10.7270/Q2057KJ6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 3-kinase regulatory subunit beta (Homo sapiens (Human)) | BDBM338879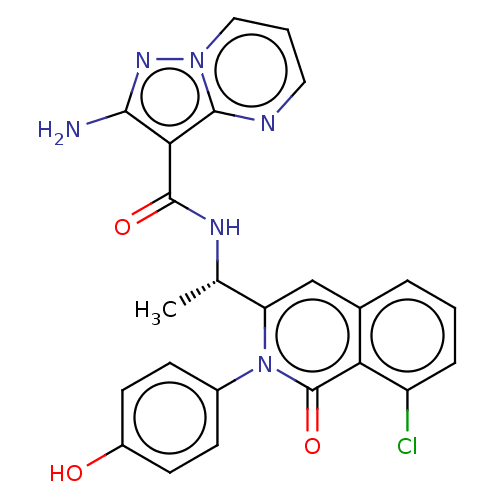 (US9751888, Compound 70) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 5.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Infinity Pharmaceuticals, Inc. US Patent | Assay Description Class I PI3-Ks can be either purchased (p110α/p85α, p110β/p85α, p110δ/p85α from Upstate, and p110γ from Sigma) or ... | US Patent US9751888 (2017) BindingDB Entry DOI: 10.7270/Q2TB190M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| L-lactate dehydrogenase A chain (Homo sapiens (Human)) | BDBM489160 (2-(3-(3-(tert- butylcarbamoyl)-4- fluorophenyl)-5-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human LDHA using sodium pyruvate as substrate preincubated for 5 mins followed by diaphorase/resazurin addition and measure... | Citation and Details Article DOI: 10.1016/j.bmcl.2021.127974 BindingDB Entry DOI: 10.7270/Q24J0JV6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| L-lactate dehydrogenase A chain (Homo sapiens (Human)) | BDBM50546978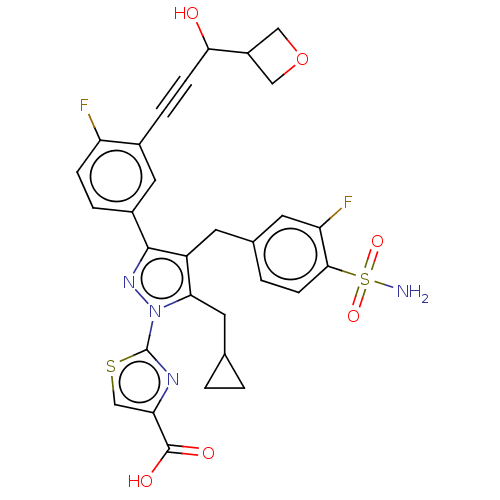 (CHEMBL4752940) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human LDHA using sodium pyruvate as substrate preincubated for 5 mins followed by diaphorase/resazurin addition and measure... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00916 BindingDB Entry DOI: 10.7270/Q2057KJ6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| L-lactate dehydrogenase A chain (Homo sapiens (Human)) | BDBM50546975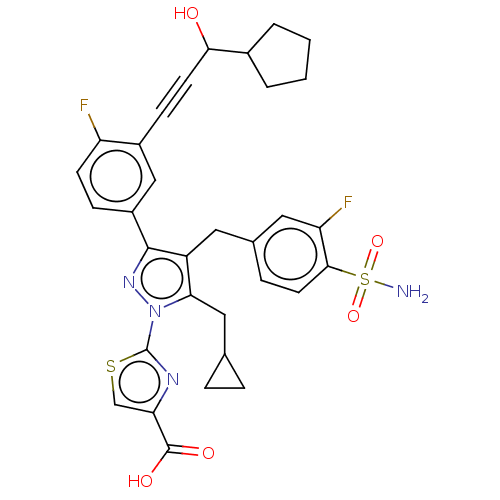 (CHEMBL4749903) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human LDHA using sodium pyruvate as substrate preincubated for 5 mins followed by diaphorase/resazurin addition and measure... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00916 BindingDB Entry DOI: 10.7270/Q2057KJ6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| L-lactate dehydrogenase A chain (Homo sapiens (Human)) | BDBM50569440 (CHEMBL4877988 | US11752138, Compound 152) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human LDHA using sodium pyruvate as substrate preincubated for 5 mins followed by diaphorase/resazurin addition and measure... | Citation and Details Article DOI: 10.1016/j.bmcl.2021.127974 BindingDB Entry DOI: 10.7270/Q24J0JV6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| L-lactate dehydrogenase A chain (Homo sapiens (Human)) | BDBM50546979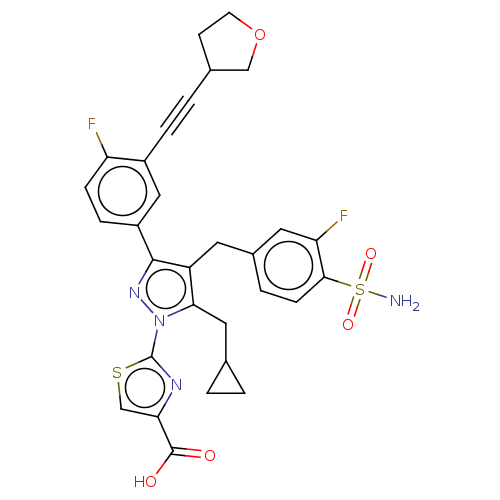 (CHEMBL4747300 | US11247971, Cmpd ID 423) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human LDHA using sodium pyruvate as substrate preincubated for 5 mins followed by diaphorase/resazurin addition and measure... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00916 BindingDB Entry DOI: 10.7270/Q2057KJ6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| L-lactate dehydrogenase A chain (Homo sapiens (Human)) | BDBM50546977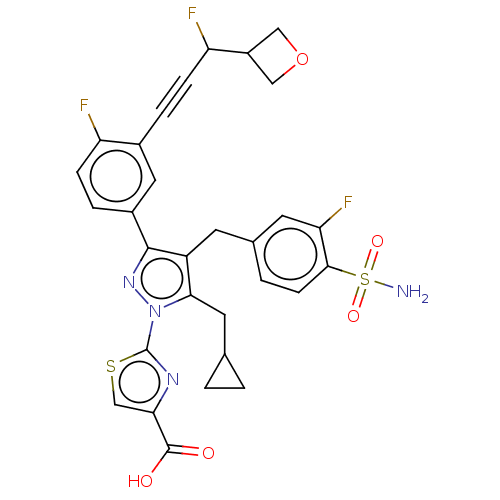 (CHEMBL4759378) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human LDHA using sodium pyruvate as substrate preincubated for 5 mins followed by diaphorase/resazurin addition and measure... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00916 BindingDB Entry DOI: 10.7270/Q2057KJ6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| L-lactate dehydrogenase A chain (Homo sapiens (Human)) | BDBM50546989 (CHEMBL4759499 | US11247971, Cmpd ID 417) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human LDHA using sodium pyruvate as substrate preincubated for 5 mins followed by diaphorase/resazurin addition and measure... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00916 BindingDB Entry DOI: 10.7270/Q2057KJ6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| L-lactate dehydrogenase A chain (Homo sapiens (Human)) | BDBM489161 (2-(3-(3- (benzylcarbamoyl)-4- fluorophenyl)-5- (cy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human LDHA using sodium pyruvate as substrate preincubated for 5 mins followed by diaphorase/resazurin addition and measure... | Citation and Details Article DOI: 10.1016/j.bmcl.2021.127974 BindingDB Entry DOI: 10.7270/Q24J0JV6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| L-lactate dehydrogenase A chain (Homo sapiens (Human)) | BDBM489092 (2-(3-(3- cydopropoxy-4- fluorophenyl)-5- (cyclopro...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human LDHA using sodium pyruvate as substrate preincubated for 5 mins followed by diaphorase/resazurin addition and measure... | Citation and Details Article DOI: 10.1016/j.bmcl.2021.127974 BindingDB Entry DOI: 10.7270/Q24J0JV6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| L-lactate dehydrogenase A chain (Homo sapiens (Human)) | BDBM536955 (US11247971, Cmpd ID 278) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human LDHA using sodium pyruvate as substrate preincubated for 5 mins followed by diaphorase/resazurin addition and measure... | Citation and Details Article DOI: 10.1016/j.bmcl.2021.127974 BindingDB Entry DOI: 10.7270/Q24J0JV6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| L-lactate dehydrogenase A chain (Homo sapiens (Human)) | BDBM50546970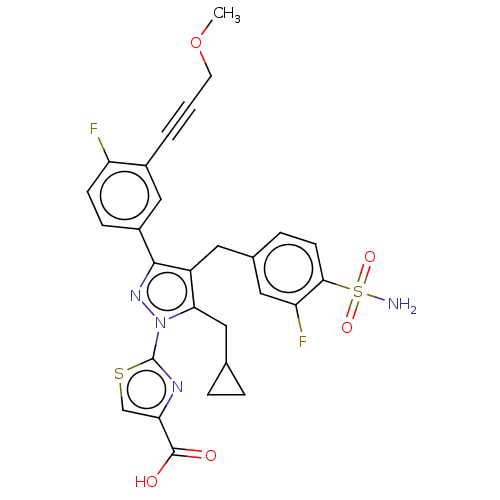 (CHEMBL4783945 | US11247971, Cmpd ID 404) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human LDHA using sodium pyruvate as substrate preincubated for 5 mins followed by diaphorase/resazurin addition and measure... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00916 BindingDB Entry DOI: 10.7270/Q2057KJ6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| L-lactate dehydrogenase A chain (Homo sapiens (Human)) | BDBM50546998 (CHEMBL4790159 | US11247971, Cmpd ID 405) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human LDHA using sodium pyruvate as substrate preincubated for 5 mins followed by diaphorase/resazurin addition and measure... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00916 BindingDB Entry DOI: 10.7270/Q2057KJ6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| L-lactate dehydrogenase A chain (Homo sapiens (Human)) | BDBM50547000 (CHEMBL4783252 | US11247971, Cmpd ID 270) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human LDHA using sodium pyruvate as substrate preincubated for 5 mins followed by diaphorase/resazurin addition and measure... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00916 BindingDB Entry DOI: 10.7270/Q2057KJ6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| L-lactate dehydrogenase A chain (Homo sapiens (Human)) | BDBM50546999 (CHEMBL4786717) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human LDHA using sodium pyruvate as substrate preincubated for 5 mins followed by diaphorase/resazurin addition and measure... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00916 BindingDB Entry DOI: 10.7270/Q2057KJ6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| L-lactate dehydrogenase A chain (Homo sapiens (Human)) | BDBM50546990 (CHEMBL4794789) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human LDHA using sodium pyruvate as substrate preincubated for 5 mins followed by diaphorase/resazurin addition and measure... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00916 BindingDB Entry DOI: 10.7270/Q2057KJ6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| L-lactate dehydrogenase A chain (Homo sapiens (Human)) | BDBM50546963 (CHEMBL4760911 | US11247971, Cmpd ID 410) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human LDHA using sodium pyruvate as substrate preincubated for 5 mins followed by diaphorase/resazurin addition and measure... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00916 BindingDB Entry DOI: 10.7270/Q2057KJ6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| L-lactate dehydrogenase A chain (Homo sapiens (Human)) | BDBM50546971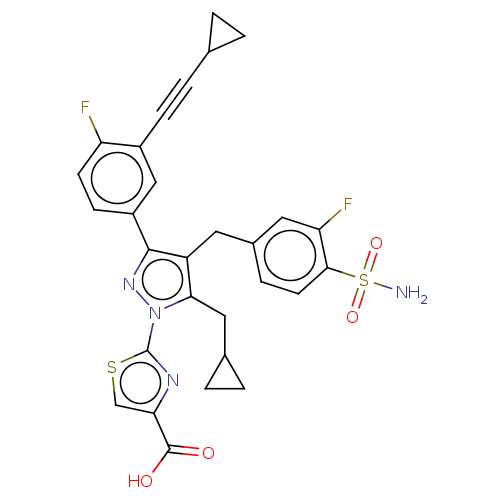 (CHEMBL4777867 | US11247971, Cmpd ID 262) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human LDHA using sodium pyruvate as substrate preincubated for 5 mins followed by diaphorase/resazurin addition and measure... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00916 BindingDB Entry DOI: 10.7270/Q2057KJ6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| L-lactate dehydrogenase A chain (Homo sapiens (Human)) | BDBM50546976 (CHEMBL4751495) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human LDHA using sodium pyruvate as substrate preincubated for 5 mins followed by diaphorase/resazurin addition and measure... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00916 BindingDB Entry DOI: 10.7270/Q2057KJ6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Casein kinase I isoform delta (Homo sapiens (Human)) | BDBM302809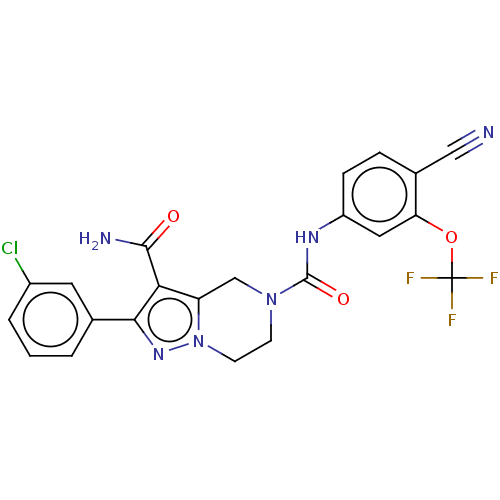 (2-(3-Chlorophenyl)-N5- (4-cyano-3- (trifluorometho...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description The kinase assay was performed in V-bottom 384-well plates. The final assay volume was 30 μl prepared from 15 μl additions of enzyme, subst... | US Patent US9598423 (2017) BindingDB Entry DOI: 10.7270/Q2FJ2JT9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Casein kinase I isoform epsilon (Homo sapiens (Human)) | BDBM302810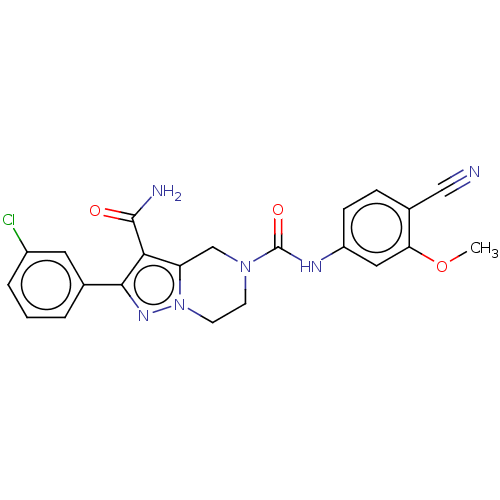 (2-(3-Chlorophenyl)-N5- (4-cyano-3- methoxyphenyl)-...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description The kinase assay was performed in V-bottom 384-well plates. The final assay volume was 30 μl prepared from 15 μl additions of enzyme, subst... | US Patent US9598423 (2017) BindingDB Entry DOI: 10.7270/Q2FJ2JT9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Casein kinase I isoform delta (Homo sapiens (Human)) | BDBM302436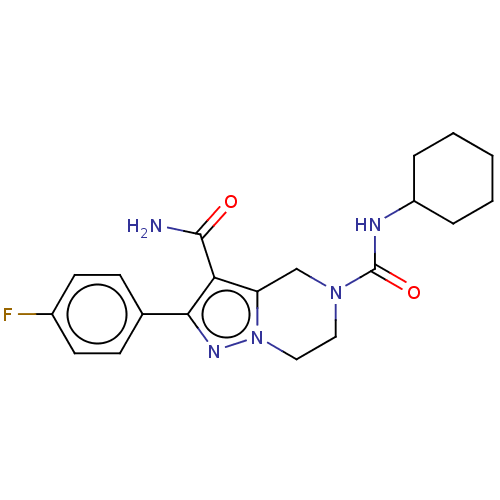 (N5-Cyclohexyl-2-(4-fluorophenyl)-6,7- dihydropyraz...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description The kinase assay was performed in V-bottom 384-well plates. The final assay volume was 30 μl prepared from 15 μl additions of enzyme, subst... | US Patent US9598423 (2017) BindingDB Entry DOI: 10.7270/Q2FJ2JT9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Casein kinase I isoform epsilon (Homo sapiens (Human)) | BDBM302440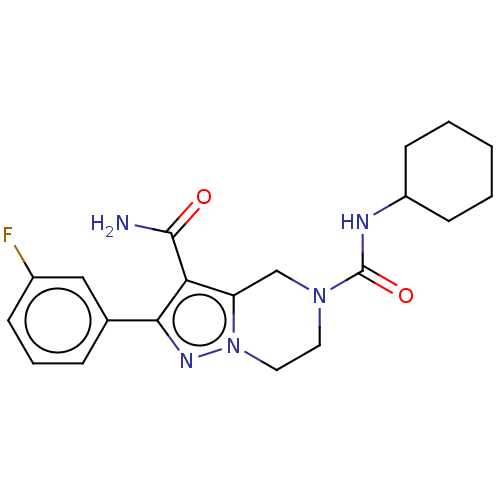 (N5-Cyclohexyl-2-(3- fluorophenyl)-6,7- dihydropyra...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description The kinase assay was performed in V-bottom 384-well plates. The final assay volume was 30 μl prepared from 15 μl additions of enzyme, subst... | US Patent US9598423 (2017) BindingDB Entry DOI: 10.7270/Q2FJ2JT9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Casein kinase I isoform delta (Homo sapiens (Human)) | BDBM302440 (N5-Cyclohexyl-2-(3- fluorophenyl)-6,7- dihydropyra...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description The kinase assay was performed in V-bottom 384-well plates. The final assay volume was 30 μl prepared from 15 μl additions of enzyme, subst... | US Patent US9598423 (2017) BindingDB Entry DOI: 10.7270/Q2FJ2JT9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Casein kinase I isoform epsilon (Homo sapiens (Human)) | BDBM302776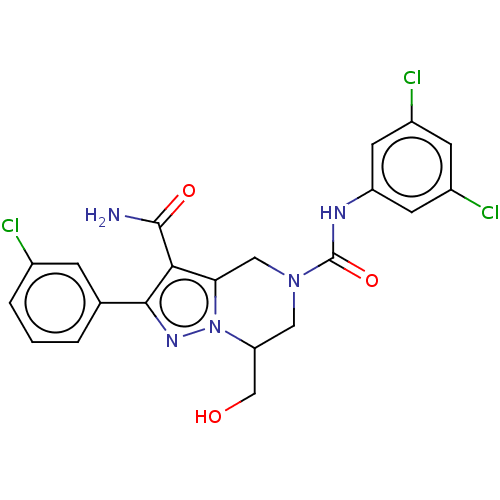 (2-(3-Chlorophenyl)-N5- (3,5-dichlorophenyl)-7- (hy...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description The kinase assay was performed in V-bottom 384-well plates. The final assay volume was 30 μl prepared from 15 μl additions of enzyme, subst... | US Patent US9598423 (2017) BindingDB Entry DOI: 10.7270/Q2FJ2JT9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Casein kinase I isoform delta (Homo sapiens (Human)) | BDBM302839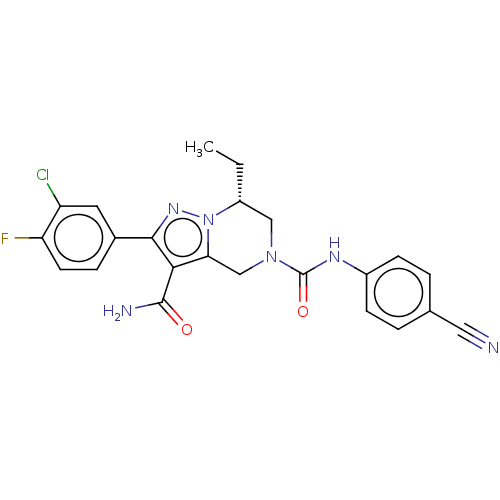 (2-(3-Chloro-4-fluorophenyl)-N5-(4-cyanophenyl)-7-e...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description The kinase assay was performed in V-bottom 384-well plates. The final assay volume was 30 μl prepared from 15 μl additions of enzyme, subst... | US Patent US9598423 (2017) BindingDB Entry DOI: 10.7270/Q2FJ2JT9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Casein kinase I isoform delta (Homo sapiens (Human)) | BDBM302473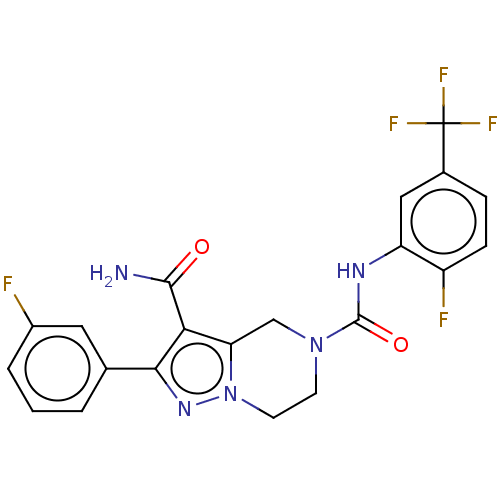 (N5-(2-Fluoro-5- (trifluoromethyl)phenyl)- 2-(3-flu...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description The kinase assay was performed in V-bottom 384-well plates. The final assay volume was 30 μl prepared from 15 μl additions of enzyme, subst... | US Patent US9598423 (2017) BindingDB Entry DOI: 10.7270/Q2FJ2JT9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Casein kinase I isoform epsilon (Homo sapiens (Human)) | BDBM302527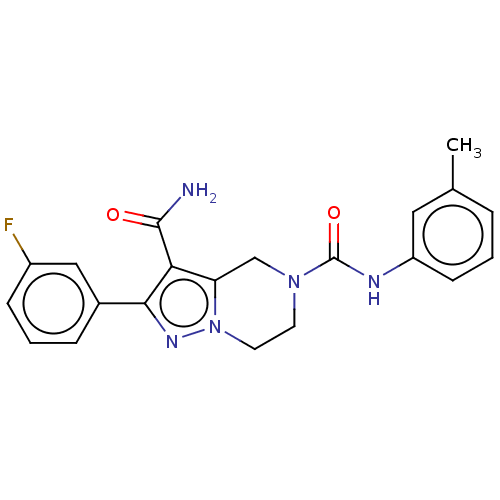 (2-(3-Fluorophenyl)-N5-(3- (methylsulfonyl)phenyl)-...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description The kinase assay was performed in V-bottom 384-well plates. The final assay volume was 30 μl prepared from 15 μl additions of enzyme, subst... | US Patent US9598423 (2017) BindingDB Entry DOI: 10.7270/Q2FJ2JT9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Casein kinase I isoform epsilon (Homo sapiens (Human)) | BDBM302538 (US9598423, Example 104) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description The kinase assay was performed in V-bottom 384-well plates. The final assay volume was 30 μl prepared from 15 μl additions of enzyme, subst... | US Patent US9598423 (2017) BindingDB Entry DOI: 10.7270/Q2FJ2JT9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Casein kinase I isoform delta (Homo sapiens (Human)) | BDBM302597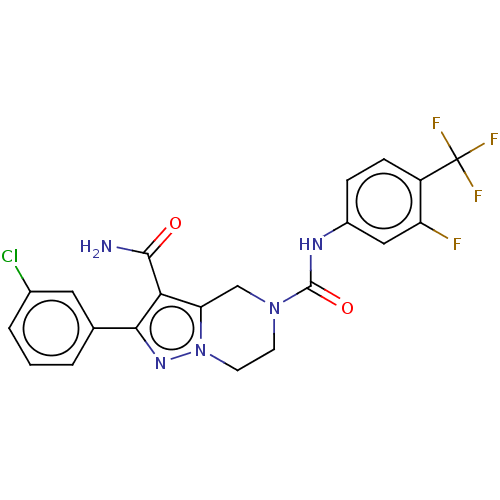 (2-(3-Chlorophenyl)-N5- (3-fluoro-4-(trifluoromethy...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description The kinase assay was performed in V-bottom 384-well plates. The final assay volume was 30 μl prepared from 15 μl additions of enzyme, subst... | US Patent US9598423 (2017) BindingDB Entry DOI: 10.7270/Q2FJ2JT9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Casein kinase I isoform delta (Homo sapiens (Human)) | BDBM302643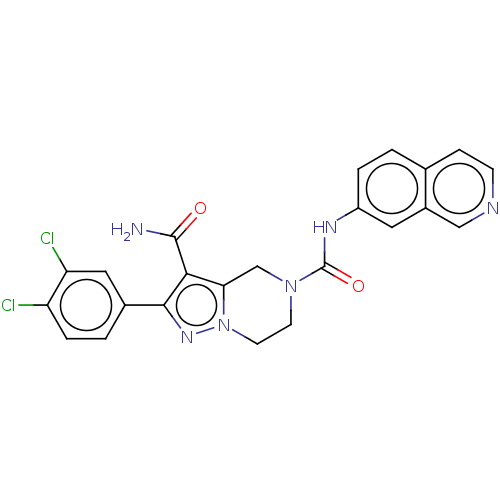 (2-(3,4-Dichlorophenyl)-N5- (isoquinolin-7-yl)-6,7-...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description The kinase assay was performed in V-bottom 384-well plates. The final assay volume was 30 μl prepared from 15 μl additions of enzyme, subst... | US Patent US9598423 (2017) BindingDB Entry DOI: 10.7270/Q2FJ2JT9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Casein kinase I isoform delta (Homo sapiens (Human)) | BDBM302659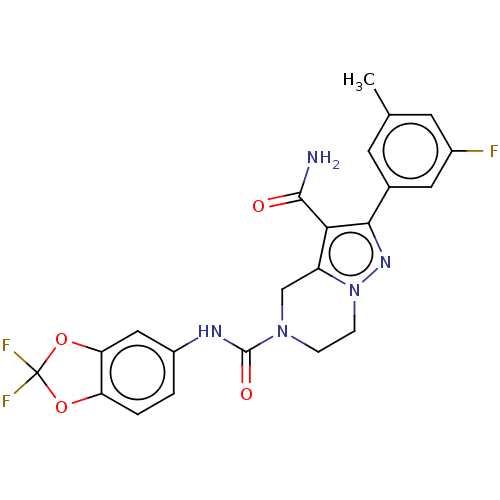 (N5-(2,2-Difluorobenzo[d] [1,3]dioxol-5-yl)-2-(3- f...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description The kinase assay was performed in V-bottom 384-well plates. The final assay volume was 30 μl prepared from 15 μl additions of enzyme, subst... | US Patent US9598423 (2017) BindingDB Entry DOI: 10.7270/Q2FJ2JT9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Casein kinase I isoform epsilon (Homo sapiens (Human)) | BDBM302712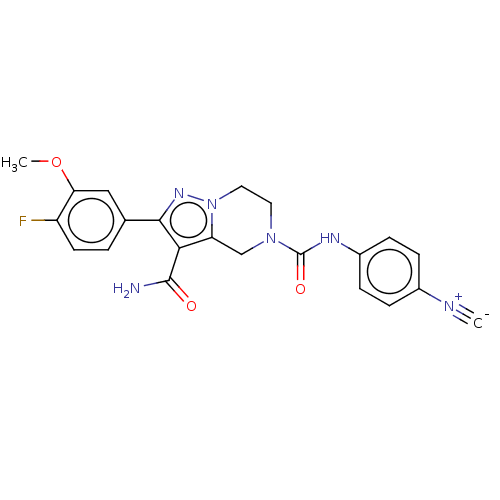 (N5-(4-Cyanophenyl)-2-(4- fluoro-3-methoxyphenyl)-6...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description The kinase assay was performed in V-bottom 384-well plates. The final assay volume was 30 μl prepared from 15 μl additions of enzyme, subst... | US Patent US9598423 (2017) BindingDB Entry DOI: 10.7270/Q2FJ2JT9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Casein kinase I isoform epsilon (Homo sapiens (Human)) | BDBM302726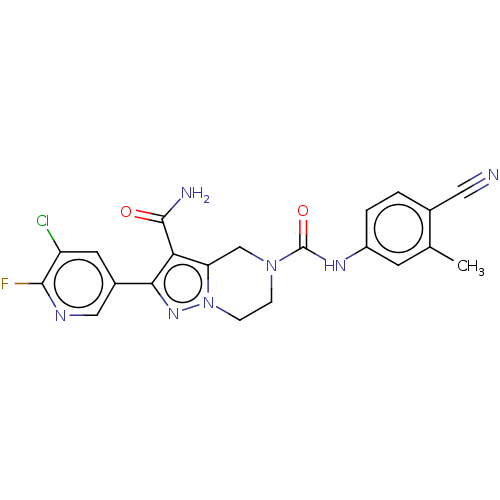 (2-(5-Chloro-6-fluoropyridin- 3-yl)-N5-(4-cyano-3- ...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description The kinase assay was performed in V-bottom 384-well plates. The final assay volume was 30 μl prepared from 15 μl additions of enzyme, subst... | US Patent US9598423 (2017) BindingDB Entry DOI: 10.7270/Q2FJ2JT9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Casein kinase I isoform delta (Homo sapiens (Human)) | BDBM302774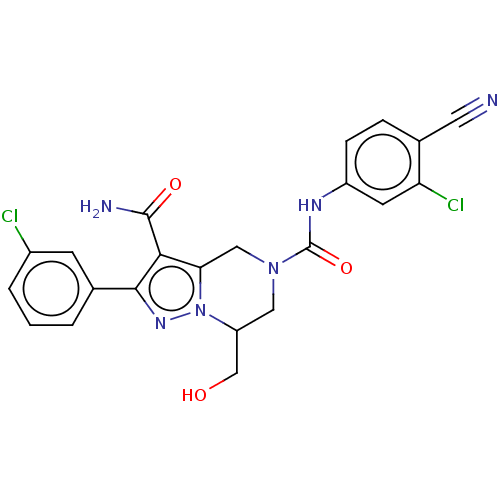 (N5-(3- Chloro-4-cyanophenyl)- 2-(3-chlorophenyl)-7...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description The kinase assay was performed in V-bottom 384-well plates. The final assay volume was 30 μl prepared from 15 μl additions of enzyme, subst... | US Patent US9598423 (2017) BindingDB Entry DOI: 10.7270/Q2FJ2JT9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Casein kinase I isoform epsilon (Homo sapiens (Human)) | BDBM302775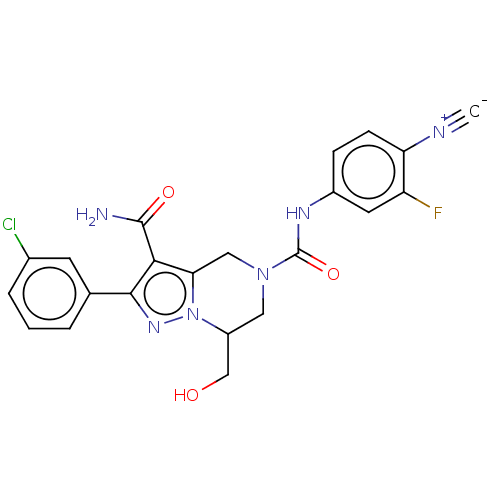 (2-(3-Chlorophenyl)-N5-(4- cyano-3-fluorophenyl)-7-...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description The kinase assay was performed in V-bottom 384-well plates. The final assay volume was 30 μl prepared from 15 μl additions of enzyme, subst... | US Patent US9598423 (2017) BindingDB Entry DOI: 10.7270/Q2FJ2JT9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Casein kinase I isoform delta (Homo sapiens (Human)) | BDBM302775 (2-(3-Chlorophenyl)-N5-(4- cyano-3-fluorophenyl)-7-...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description The kinase assay was performed in V-bottom 384-well plates. The final assay volume was 30 μl prepared from 15 μl additions of enzyme, subst... | US Patent US9598423 (2017) BindingDB Entry DOI: 10.7270/Q2FJ2JT9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Casein kinase I isoform delta (Homo sapiens (Human)) | BDBM302776 (2-(3-Chlorophenyl)-N5- (3,5-dichlorophenyl)-7- (hy...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description The kinase assay was performed in V-bottom 384-well plates. The final assay volume was 30 μl prepared from 15 μl additions of enzyme, subst... | US Patent US9598423 (2017) BindingDB Entry DOI: 10.7270/Q2FJ2JT9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Casein kinase I isoform epsilon (Homo sapiens (Human)) | BDBM302777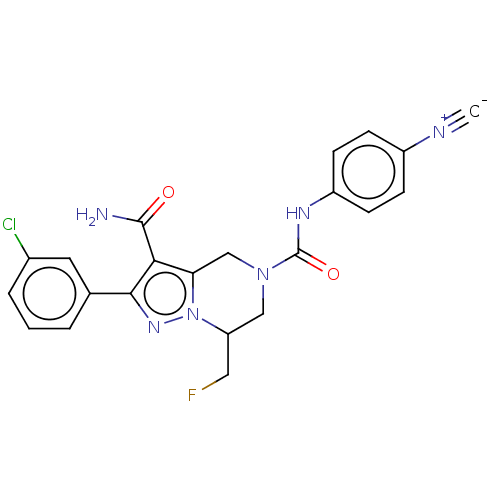 (US9598423, Example A5) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description The kinase assay was performed in V-bottom 384-well plates. The final assay volume was 30 μl prepared from 15 μl additions of enzyme, subst... | US Patent US9598423 (2017) BindingDB Entry DOI: 10.7270/Q2FJ2JT9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Casein kinase I isoform delta (Homo sapiens (Human)) | BDBM302777 (US9598423, Example A5) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description The kinase assay was performed in V-bottom 384-well plates. The final assay volume was 30 μl prepared from 15 μl additions of enzyme, subst... | US Patent US9598423 (2017) BindingDB Entry DOI: 10.7270/Q2FJ2JT9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Casein kinase I isoform delta (Homo sapiens (Human)) | BDBM302778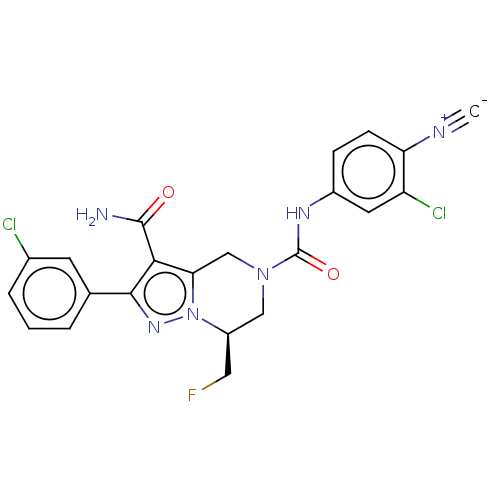 ((S)-N5-(3-Chloro- 4-cyanophenyl)- 2-(3-chloropheny...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description The kinase assay was performed in V-bottom 384-well plates. The final assay volume was 30 μl prepared from 15 μl additions of enzyme, subst... | US Patent US9598423 (2017) BindingDB Entry DOI: 10.7270/Q2FJ2JT9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Casein kinase I isoform delta (Homo sapiens (Human)) | BDBM302779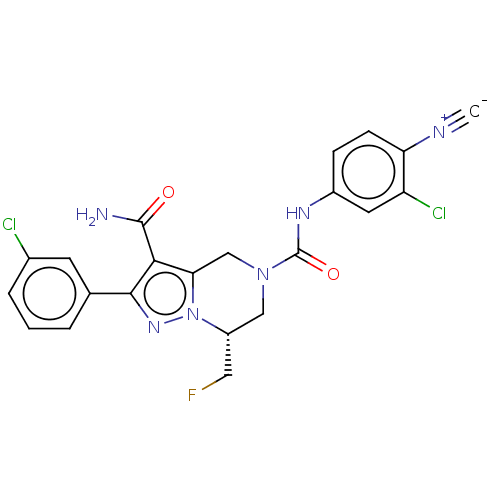 ((R)-N5-(3-Chloro- 4-cyanophenyl)- 2-(3-chloropheny...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description The kinase assay was performed in V-bottom 384-well plates. The final assay volume was 30 μl prepared from 15 μl additions of enzyme, subst... | US Patent US9598423 (2017) BindingDB Entry DOI: 10.7270/Q2FJ2JT9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Casein kinase I isoform epsilon (Homo sapiens (Human)) | BDBM302780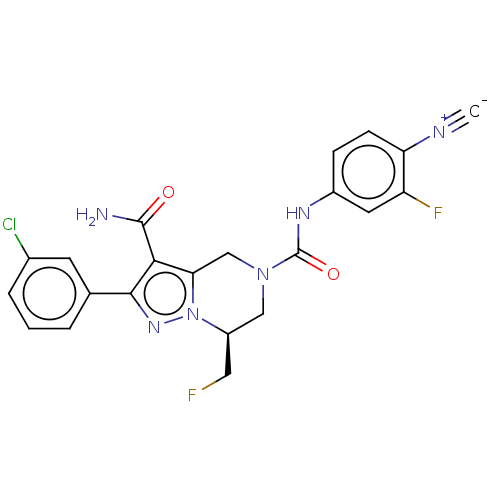 ((S)-2-(3- Chlorophenyl)-N5- (4-cyano-3-fluoropheny...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description The kinase assay was performed in V-bottom 384-well plates. The final assay volume was 30 μl prepared from 15 μl additions of enzyme, subst... | US Patent US9598423 (2017) BindingDB Entry DOI: 10.7270/Q2FJ2JT9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Casein kinase I isoform delta (Homo sapiens (Human)) | BDBM302780 ((S)-2-(3- Chlorophenyl)-N5- (4-cyano-3-fluoropheny...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description The kinase assay was performed in V-bottom 384-well plates. The final assay volume was 30 μl prepared from 15 μl additions of enzyme, subst... | US Patent US9598423 (2017) BindingDB Entry DOI: 10.7270/Q2FJ2JT9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 3523 total ) | Next | Last >> |