 Found 359 hits with Last Name = 'jeppesen' and Initial = 'cb'
Found 359 hits with Last Name = 'jeppesen' and Initial = 'cb' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50193204
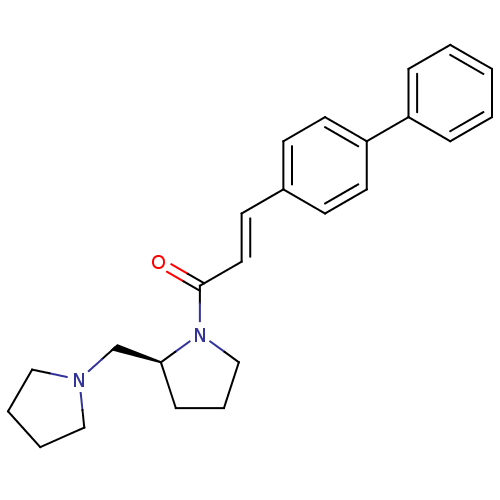
(3-biphenyl-4-yl-1-((S)-2-pyrrolidin-1-ylmethyl-pyr...)Show SMILES O=C(\C=C\c1ccc(cc1)-c1ccccc1)N1CCC[C@H]1CN1CCCC1 Show InChI InChI=1S/C24H28N2O/c27-24(26-18-6-9-23(26)19-25-16-4-5-17-25)15-12-20-10-13-22(14-11-20)21-7-2-1-3-8-21/h1-3,7-8,10-15,23H,4-6,9,16-19H2/b15-12+/t23-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 3.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
Binding affinity at histamine H3 receptor by hH3-[35S]GTPgamma[S] binding assay |
Bioorg Med Chem Lett 16: 5303-8 (2006)
Article DOI: 10.1016/j.bmcl.2006.07.093
BindingDB Entry DOI: 10.7270/Q2RV0NBD |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50193199
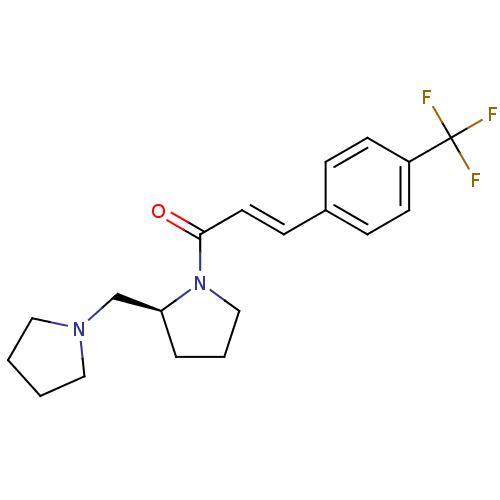
((S)-1-(2-(pyrrolidin-1-ylmethyl)pyrrolidin-1-yl)-3...)Show SMILES FC(F)(F)c1ccc(\C=C\C(=O)N2CCC[C@H]2CN2CCCC2)cc1 |r| Show InChI InChI=1S/C19H23F3N2O/c20-19(21,22)16-8-5-15(6-9-16)7-10-18(25)24-13-3-4-17(24)14-23-11-1-2-12-23/h5-10,17H,1-4,11-14H2/b10-7+/t17-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 4.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
Binding affinity at histamine H3 receptor by hH3-[35S]GTPgamma[S] binding assay |
Bioorg Med Chem Lett 16: 5303-8 (2006)
Article DOI: 10.1016/j.bmcl.2006.07.093
BindingDB Entry DOI: 10.7270/Q2RV0NBD |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50193175
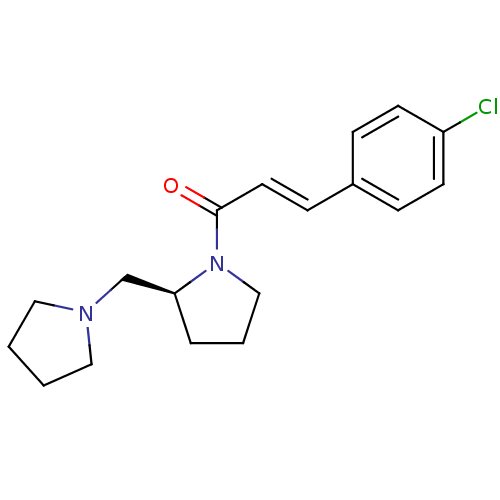
((S)-3-(4-chlorophenyl)-1-(2-(pyrrolidin-1-ylmethyl...)Show InChI InChI=1S/C18H23ClN2O/c19-16-8-5-15(6-9-16)7-10-18(22)21-13-3-4-17(21)14-20-11-1-2-12-20/h5-10,17H,1-4,11-14H2/b10-7+/t17-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 11.2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
Binding affinity at histamine H3 receptor by hH3-[35S]GTPgamma[S] binding assay |
Bioorg Med Chem Lett 16: 5303-8 (2006)
Article DOI: 10.1016/j.bmcl.2006.07.093
BindingDB Entry DOI: 10.7270/Q2RV0NBD |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50193185

((S)-1-(2-(pyrrolidin-1-ylmethyl)pyrrolidin-1-yl)-3...)Show SMILES FC(F)(F)Oc1ccc(\C=C\C(=O)N2CCC[C@H]2CN2CCCC2)cc1 Show InChI InChI=1S/C19H23F3N2O2/c20-19(21,22)26-17-8-5-15(6-9-17)7-10-18(25)24-13-3-4-16(24)14-23-11-1-2-12-23/h5-10,16H,1-4,11-14H2/b10-7+/t16-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 13.4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
Binding affinity at histamine H3 receptor by hH3-[35S]GTPgamma[S] binding assay |
Bioorg Med Chem Lett 16: 5303-8 (2006)
Article DOI: 10.1016/j.bmcl.2006.07.093
BindingDB Entry DOI: 10.7270/Q2RV0NBD |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50193209
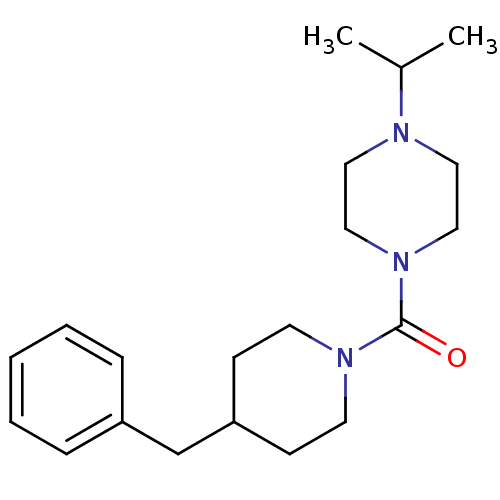
((4-benzylpiperidin-1-yl)(4-isopropylpiperazin-1-yl...)Show InChI InChI=1S/C20H31N3O/c1-17(2)21-12-14-23(15-13-21)20(24)22-10-8-19(9-11-22)16-18-6-4-3-5-7-18/h3-7,17,19H,8-16H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
Binding affinity at histamine H3 receptor by hH3-[35S]GTPgamma[S] binding assay |
Bioorg Med Chem Lett 16: 5303-8 (2006)
Article DOI: 10.1016/j.bmcl.2006.07.093
BindingDB Entry DOI: 10.7270/Q2RV0NBD |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50193189

(CHEMBL218834 | [4-(3-Aza-bicyclo[3.2.2]nonane-3-ca...)Show SMILES CC(C)N1CCN(CC1)C(=O)N1CCC(CC1)C(=O)N1CC2CCC(CC2)C1 |(-10.27,-12.49,;-8.93,-13.25,;-8.92,-14.79,;-7.6,-12.47,;-7.61,-10.93,;-6.29,-10.15,;-4.94,-10.9,;-4.93,-12.44,;-6.26,-13.23,;-3.62,-10.12,;-3.64,-8.58,;-2.28,-10.87,;-2.27,-12.41,;-.93,-13.16,;.4,-12.38,;.38,-10.84,;-.96,-10.08,;1.74,-13.14,;1.75,-14.68,;3.07,-12.37,;4.64,-12.83,;5.99,-11.86,;7.33,-12.56,;6.6,-11.37,;4.98,-10.51,;5.01,-9.04,;6.04,-10.28,;4.29,-11.83,)| Show InChI InChI=1S/C22H38N4O2/c1-17(2)23-11-13-25(14-12-23)22(28)24-9-7-20(8-10-24)21(27)26-15-18-3-4-19(16-26)6-5-18/h17-20H,3-16H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
Binding affinity at histamine H3 receptor by hH3-[35S]GTPgamma[S] binding assay |
Bioorg Med Chem Lett 16: 5303-8 (2006)
Article DOI: 10.1016/j.bmcl.2006.07.093
BindingDB Entry DOI: 10.7270/Q2RV0NBD |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50193171
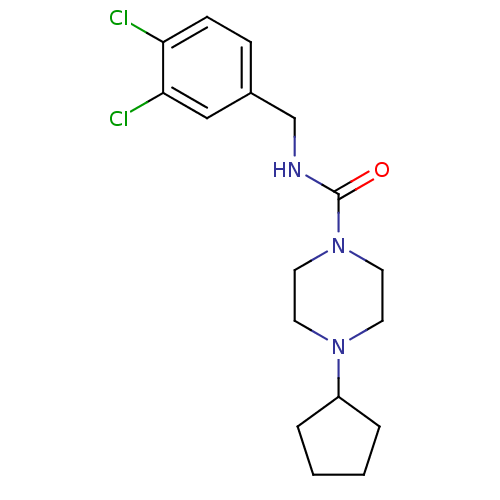
(CHEMBL218984 | N-(3,4-dichlorobenzyl)-4-cyclopenty...)Show InChI InChI=1S/C17H23Cl2N3O/c18-15-6-5-13(11-16(15)19)12-20-17(23)22-9-7-21(8-10-22)14-3-1-2-4-14/h5-6,11,14H,1-4,7-10,12H2,(H,20,23) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
Binding affinity at histamine H3 receptor by hH3-[35S]GTPgamma[S] binding assay |
Bioorg Med Chem Lett 16: 5303-8 (2006)
Article DOI: 10.1016/j.bmcl.2006.07.093
BindingDB Entry DOI: 10.7270/Q2RV0NBD |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50193208
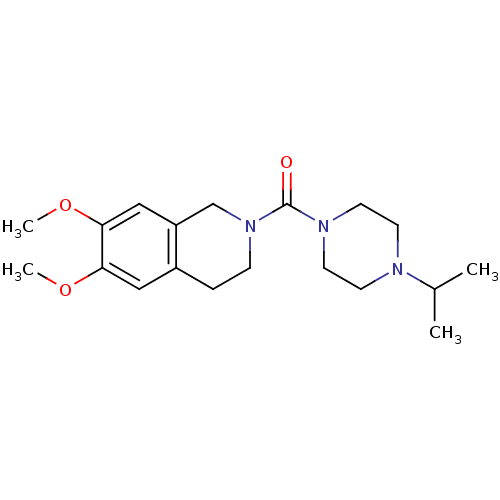
((6,7-dimethoxy-3,4-dihydroisoquinolin-2(1H)-yl)(4-...)Show InChI InChI=1S/C19H29N3O3/c1-14(2)20-7-9-21(10-8-20)19(23)22-6-5-15-11-17(24-3)18(25-4)12-16(15)13-22/h11-12,14H,5-10,13H2,1-4H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
Binding affinity at histamine H3 receptor by hH3-[35S]GTPgamma[S] binding assay |
Bioorg Med Chem Lett 16: 5303-8 (2006)
Article DOI: 10.1016/j.bmcl.2006.07.093
BindingDB Entry DOI: 10.7270/Q2RV0NBD |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50193180
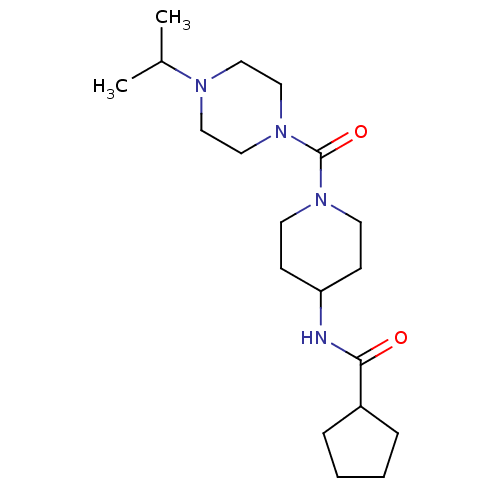
(CHEMBL220407 | N-(1-(1-isopropylpiperazine-4-carbo...)Show SMILES CC(C)N1CCN(CC1)C(=O)N1CCC(CC1)NC(=O)C1CCCC1 Show InChI InChI=1S/C19H34N4O2/c1-15(2)21-11-13-23(14-12-21)19(25)22-9-7-17(8-10-22)20-18(24)16-5-3-4-6-16/h15-17H,3-14H2,1-2H3,(H,20,24) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
Binding affinity at histamine H3 receptor by hH3-[35S]GTPgamma[S] binding assay |
Bioorg Med Chem Lett 16: 5303-8 (2006)
Article DOI: 10.1016/j.bmcl.2006.07.093
BindingDB Entry DOI: 10.7270/Q2RV0NBD |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50193203
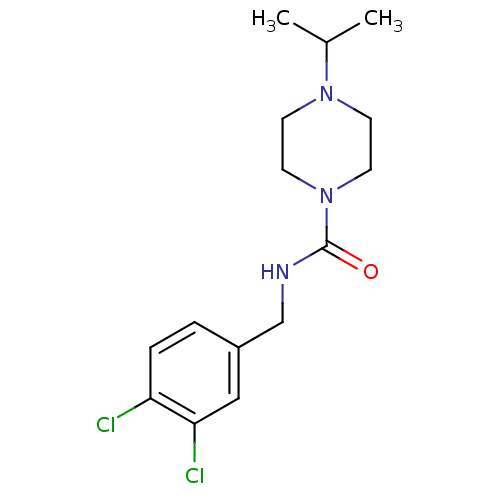
(CHEMBL374023 | N-(3,4-dichlorobenzyl)-4-isopropylp...)Show InChI InChI=1S/C15H21Cl2N3O/c1-11(2)19-5-7-20(8-6-19)15(21)18-10-12-3-4-13(16)14(17)9-12/h3-4,9,11H,5-8,10H2,1-2H3,(H,18,21) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
Binding affinity at histamine H3 receptor by hH3-[35S]GTPgamma[S] binding assay |
Bioorg Med Chem Lett 16: 5303-8 (2006)
Article DOI: 10.1016/j.bmcl.2006.07.093
BindingDB Entry DOI: 10.7270/Q2RV0NBD |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50193210
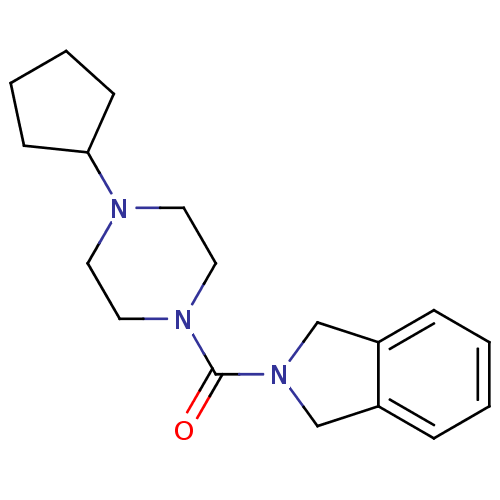
((4-cyclopentylpiperazin-1-yl)(isoindolin-2-yl)meth...)Show InChI InChI=1S/C18H25N3O/c22-18(21-13-15-5-1-2-6-16(15)14-21)20-11-9-19(10-12-20)17-7-3-4-8-17/h1-2,5-6,17H,3-4,7-14H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 23 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
Binding affinity at histamine H3 receptor by hH3-[35S]GTPgamma[S] binding assay |
Bioorg Med Chem Lett 16: 5303-8 (2006)
Article DOI: 10.1016/j.bmcl.2006.07.093
BindingDB Entry DOI: 10.7270/Q2RV0NBD |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50193179
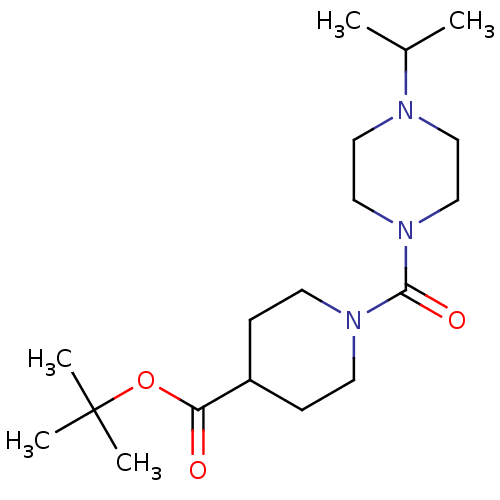
(CHEMBL218410 | tert-butyl 1-(1-isopropylpiperazine...)Show SMILES CC(C)N1CCN(CC1)C(=O)N1CCC(CC1)C(=O)OC(C)(C)C Show InChI InChI=1S/C18H33N3O3/c1-14(2)19-10-12-21(13-11-19)17(23)20-8-6-15(7-9-20)16(22)24-18(3,4)5/h14-15H,6-13H2,1-5H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
Binding affinity at histamine H3 receptor by hH3-[35S]GTPgamma[S] binding assay |
Bioorg Med Chem Lett 16: 5303-8 (2006)
Article DOI: 10.1016/j.bmcl.2006.07.093
BindingDB Entry DOI: 10.7270/Q2RV0NBD |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50193197
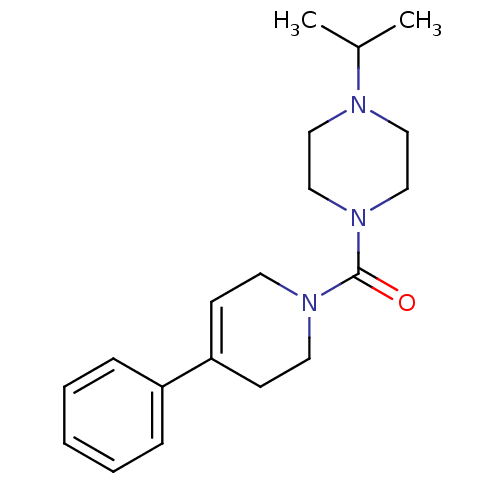
((4-isopropylpiperazin-1-yl)(4-phenyl-5,6-dihydropy...)Show SMILES CC(C)N1CCN(CC1)C(=O)N1CCC(=CC1)c1ccccc1 |c:15| Show InChI InChI=1S/C19H27N3O/c1-16(2)20-12-14-22(15-13-20)19(23)21-10-8-18(9-11-21)17-6-4-3-5-7-17/h3-8,16H,9-15H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 27 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
Binding affinity at histamine H3 receptor by hH3-[35S]GTPgamma[S] binding assay |
Bioorg Med Chem Lett 16: 5303-8 (2006)
Article DOI: 10.1016/j.bmcl.2006.07.093
BindingDB Entry DOI: 10.7270/Q2RV0NBD |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50193201
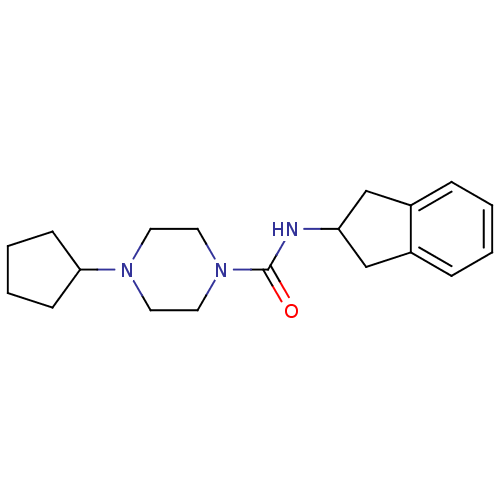
(4-cyclopentyl-N-(2,3-dihydro-1H-inden-2-yl)piperaz...)Show InChI InChI=1S/C19H27N3O/c23-19(20-17-13-15-5-1-2-6-16(15)14-17)22-11-9-21(10-12-22)18-7-3-4-8-18/h1-2,5-6,17-18H,3-4,7-14H2,(H,20,23) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 29 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
Binding affinity at histamine H3 receptor by hH3-[35S]GTPgamma[S] binding assay |
Bioorg Med Chem Lett 16: 5303-8 (2006)
Article DOI: 10.1016/j.bmcl.2006.07.093
BindingDB Entry DOI: 10.7270/Q2RV0NBD |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50193181

(CHEMBL219368 | N-(2,3-dihydro-1H-inden-2-yl)-4-iso...)Show InChI InChI=1S/C17H25N3O/c1-13(2)19-7-9-20(10-8-19)17(21)18-16-11-14-5-3-4-6-15(14)12-16/h3-6,13,16H,7-12H2,1-2H3,(H,18,21) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
Binding affinity at histamine H3 receptor by hH3-[35S]GTPgamma[S] binding assay |
Bioorg Med Chem Lett 16: 5303-8 (2006)
Article DOI: 10.1016/j.bmcl.2006.07.093
BindingDB Entry DOI: 10.7270/Q2RV0NBD |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50193178
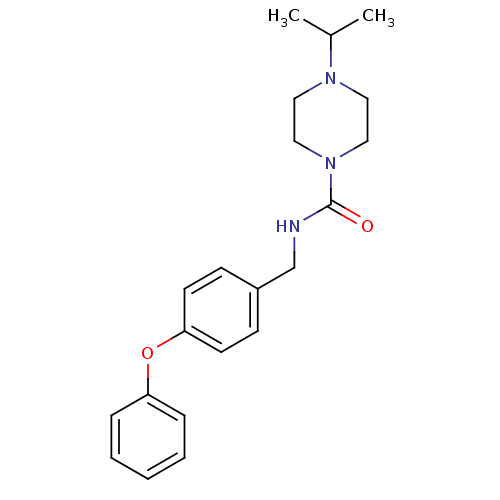
(CHEMBL441708 | N-(4-phenoxybenzyl)-4-isopropylpipe...)Show InChI InChI=1S/C21H27N3O2/c1-17(2)23-12-14-24(15-13-23)21(25)22-16-18-8-10-20(11-9-18)26-19-6-4-3-5-7-19/h3-11,17H,12-16H2,1-2H3,(H,22,25) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 34 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
Binding affinity at histamine H3 receptor by hH3-[35S]GTPgamma[S] binding assay |
Bioorg Med Chem Lett 16: 5303-8 (2006)
Article DOI: 10.1016/j.bmcl.2006.07.093
BindingDB Entry DOI: 10.7270/Q2RV0NBD |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50193196
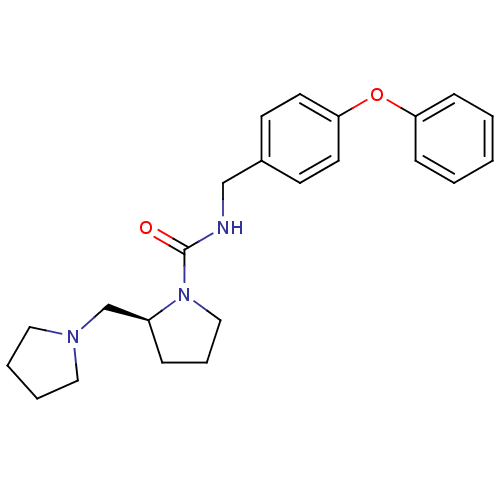
((S)-N-(4-phenoxybenzyl)-2-(pyrrolidin-1-ylmethyl)p...)Show SMILES O=C(NCc1ccc(Oc2ccccc2)cc1)N1CCC[C@H]1CN1CCCC1 Show InChI InChI=1S/C23H29N3O2/c27-23(26-16-6-7-20(26)18-25-14-4-5-15-25)24-17-19-10-12-22(13-11-19)28-21-8-2-1-3-9-21/h1-3,8-13,20H,4-7,14-18H2,(H,24,27)/t20-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 34 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
Binding affinity at histamine H3 receptor by hH3-[35S]GTPgamma[S] binding assay |
Bioorg Med Chem Lett 16: 5303-8 (2006)
Article DOI: 10.1016/j.bmcl.2006.07.093
BindingDB Entry DOI: 10.7270/Q2RV0NBD |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50193211
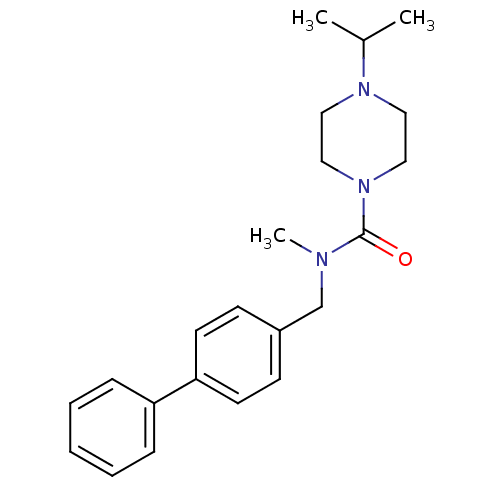
(4-isopropyl-piperazine-1-carboxylic acid biphenyl-...)Show SMILES CC(C)N1CCN(CC1)C(=O)N(C)Cc1ccc(cc1)-c1ccccc1 Show InChI InChI=1S/C22H29N3O/c1-18(2)24-13-15-25(16-14-24)22(26)23(3)17-19-9-11-21(12-10-19)20-7-5-4-6-8-20/h4-12,18H,13-17H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 37 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
Binding affinity at histamine H3 receptor by hH3-[35S]GTPgamma[S] binding assay |
Bioorg Med Chem Lett 16: 5303-8 (2006)
Article DOI: 10.1016/j.bmcl.2006.07.093
BindingDB Entry DOI: 10.7270/Q2RV0NBD |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50193187

(CHEMBL218860 | N-(4-phenoxybenzyl)-4-cyclopentylpi...)Show SMILES O=C(NCc1ccc(Oc2ccccc2)cc1)N1CCN(CC1)C1CCCC1 Show InChI InChI=1S/C23H29N3O2/c27-23(26-16-14-25(15-17-26)20-6-4-5-7-20)24-18-19-10-12-22(13-11-19)28-21-8-2-1-3-9-21/h1-3,8-13,20H,4-7,14-18H2,(H,24,27) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 38 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
Binding affinity at histamine H3 receptor by hH3-[35S]GTPgamma[S] binding assay |
Bioorg Med Chem Lett 16: 5303-8 (2006)
Article DOI: 10.1016/j.bmcl.2006.07.093
BindingDB Entry DOI: 10.7270/Q2RV0NBD |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50193193
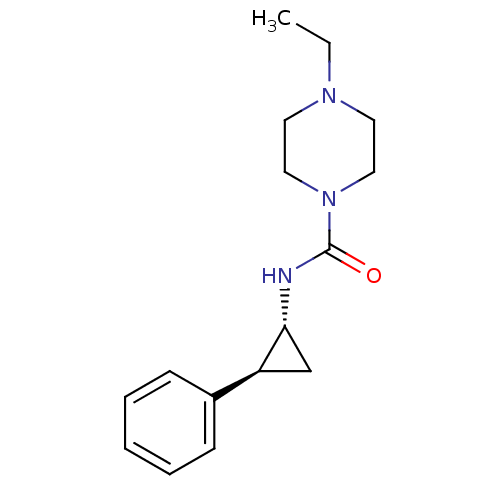
(4-ethyl-N-((1R,2S)-2-phenylcyclopropyl)piperazine-...)Show InChI InChI=1S/C16H23N3O/c1-2-18-8-10-19(11-9-18)16(20)17-15-12-14(15)13-6-4-3-5-7-13/h3-7,14-15H,2,8-12H2,1H3,(H,17,20)/t14-,15+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
Binding affinity at histamine H3 receptor by hH3-[35S]GTPgamma[S] binding assay |
Bioorg Med Chem Lett 16: 5303-8 (2006)
Article DOI: 10.1016/j.bmcl.2006.07.093
BindingDB Entry DOI: 10.7270/Q2RV0NBD |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50019293
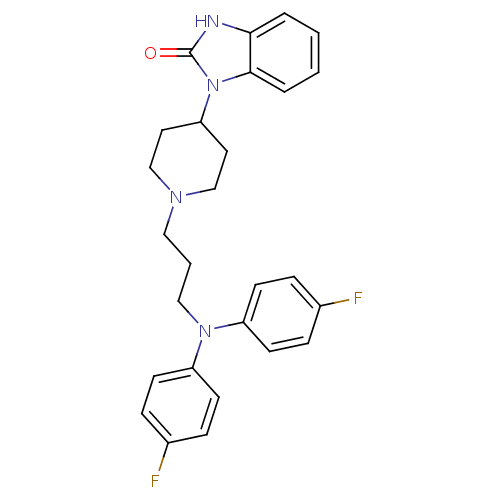
(1-(1-{3-[Bis-(4-fluoro-phenyl)-amino]-propyl}-pipe...)Show SMILES Fc1ccc(cc1)N(CCCN1CCC(CC1)n1c2ccccc2[nH]c1=O)c1ccc(F)cc1 Show InChI InChI=1S/C27H28F2N4O/c28-20-6-10-22(11-7-20)32(23-12-8-21(29)9-13-23)17-3-16-31-18-14-24(15-19-31)33-26-5-2-1-4-25(26)30-27(33)34/h1-2,4-13,24H,3,14-19H2,(H,30,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 42 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
Displacement of [3H]Astemizole from hERG expressed in HEK293 cells at 10 uM |
Bioorg Med Chem Lett 16: 5303-8 (2006)
Article DOI: 10.1016/j.bmcl.2006.07.093
BindingDB Entry DOI: 10.7270/Q2RV0NBD |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50193176
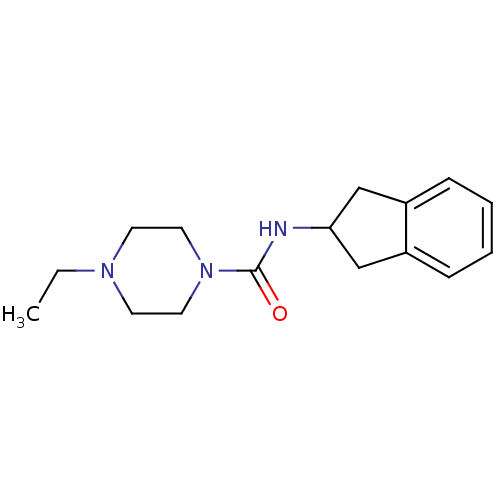
(CHEMBL373540 | N-(2,3-dihydro-1H-inden-2-yl)-4-eth...)Show InChI InChI=1S/C16H23N3O/c1-2-18-7-9-19(10-8-18)16(20)17-15-11-13-5-3-4-6-14(13)12-15/h3-6,15H,2,7-12H2,1H3,(H,17,20) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 44 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
Binding affinity at histamine H3 receptor by hH3-[35S]GTPgamma[S] binding assay |
Bioorg Med Chem Lett 16: 5303-8 (2006)
Article DOI: 10.1016/j.bmcl.2006.07.093
BindingDB Entry DOI: 10.7270/Q2RV0NBD |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50193198
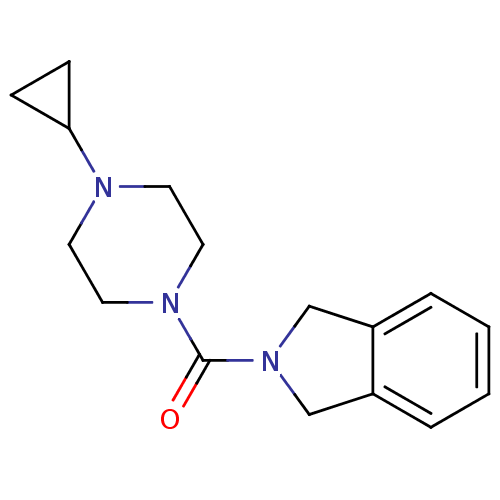
((4-cyclopropylpiperazin-1-yl)(isoindolin-2-yl)meth...)Show InChI InChI=1S/C16H21N3O/c20-16(18-9-7-17(8-10-18)15-5-6-15)19-11-13-3-1-2-4-14(13)12-19/h1-4,15H,5-12H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 45 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
Binding affinity at histamine H3 receptor by hH3-[35S]GTPgamma[S] binding assay |
Bioorg Med Chem Lett 16: 5303-8 (2006)
Article DOI: 10.1016/j.bmcl.2006.07.093
BindingDB Entry DOI: 10.7270/Q2RV0NBD |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50193205
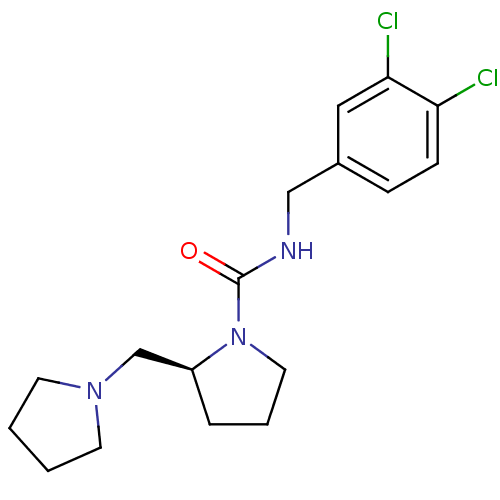
((S)-N-(3,4-dichlorobenzyl)-2-(pyrrolidin-1-ylmethy...)Show InChI InChI=1S/C17H23Cl2N3O/c18-15-6-5-13(10-16(15)19)11-20-17(23)22-9-3-4-14(22)12-21-7-1-2-8-21/h5-6,10,14H,1-4,7-9,11-12H2,(H,20,23)/t14-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 47 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
Binding affinity at histamine H3 receptor by hH3-[35S]GTPgamma[S] binding assay |
Bioorg Med Chem Lett 16: 5303-8 (2006)
Article DOI: 10.1016/j.bmcl.2006.07.093
BindingDB Entry DOI: 10.7270/Q2RV0NBD |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50193172
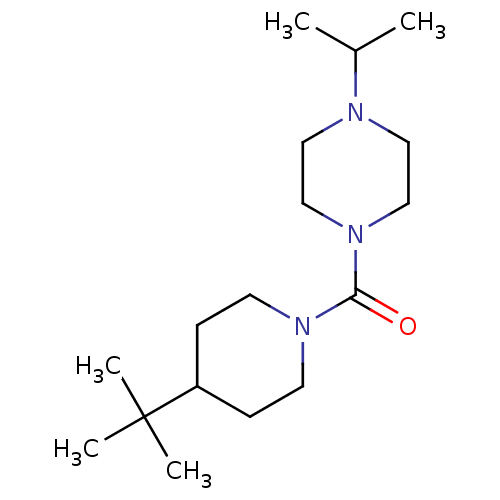
((4-tert-butylpiperidin-1-yl)(4-isopropylpiperazin-...)Show InChI InChI=1S/C17H33N3O/c1-14(2)18-10-12-20(13-11-18)16(21)19-8-6-15(7-9-19)17(3,4)5/h14-15H,6-13H2,1-5H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 47 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
Binding affinity at histamine H3 receptor by hH3-[35S]GTPgamma[S] binding assay |
Bioorg Med Chem Lett 16: 5303-8 (2006)
Article DOI: 10.1016/j.bmcl.2006.07.093
BindingDB Entry DOI: 10.7270/Q2RV0NBD |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50193213
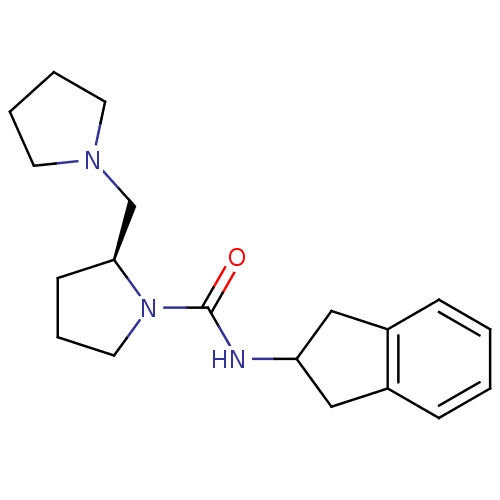
((S)-N-(2,3-dihydro-1H-inden-2-yl)-2-(pyrrolidin-1-...)Show InChI InChI=1S/C19H27N3O/c23-19(20-17-12-15-6-1-2-7-16(15)13-17)22-11-5-8-18(22)14-21-9-3-4-10-21/h1-2,6-7,17-18H,3-5,8-14H2,(H,20,23)/t18-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 52 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
Binding affinity at histamine H3 receptor by hH3-[35S]GTPgamma[S] binding assay |
Bioorg Med Chem Lett 16: 5303-8 (2006)
Article DOI: 10.1016/j.bmcl.2006.07.093
BindingDB Entry DOI: 10.7270/Q2RV0NBD |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50193184
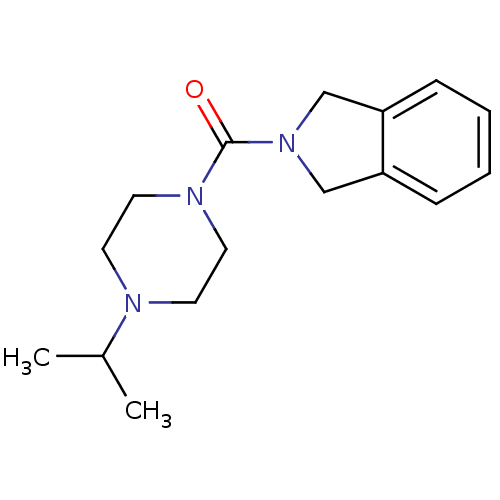
(CHEMBL219592 | isoindolin-2-yl(4-isopropylpiperazi...)Show InChI InChI=1S/C16H23N3O/c1-13(2)17-7-9-18(10-8-17)16(20)19-11-14-5-3-4-6-15(14)12-19/h3-6,13H,7-12H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 59 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
Binding affinity at histamine H3 receptor by hH3-[35S]GTPgamma[S] binding assay |
Bioorg Med Chem Lett 16: 5303-8 (2006)
Article DOI: 10.1016/j.bmcl.2006.07.093
BindingDB Entry DOI: 10.7270/Q2RV0NBD |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50193207
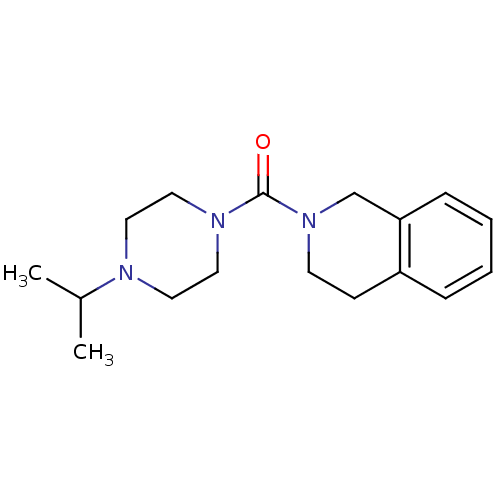
((3,4-dihydroisoquinolin-2(1H)-yl)(4-isopropylpiper...)Show InChI InChI=1S/C17H25N3O/c1-14(2)18-9-11-19(12-10-18)17(21)20-8-7-15-5-3-4-6-16(15)13-20/h3-6,14H,7-13H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 73 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
Binding affinity at histamine H3 receptor by hH3-[35S]GTPgamma[S] binding assay |
Bioorg Med Chem Lett 16: 5303-8 (2006)
Article DOI: 10.1016/j.bmcl.2006.07.093
BindingDB Entry DOI: 10.7270/Q2RV0NBD |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50193212
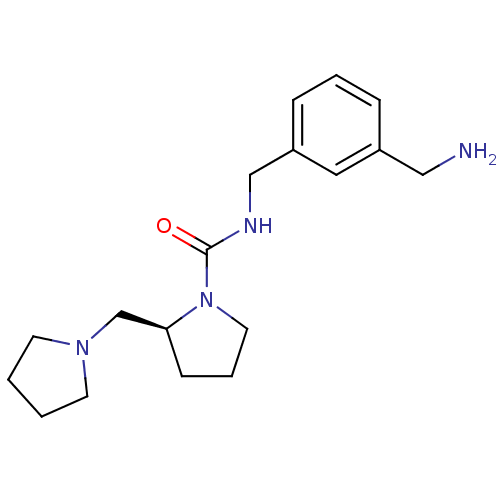
((S)-N-(3-(aminomethyl)benzyl)-2-(pyrrolidin-1-ylme...)Show InChI InChI=1S/C18H28N4O/c19-12-15-5-3-6-16(11-15)13-20-18(23)22-10-4-7-17(22)14-21-8-1-2-9-21/h3,5-6,11,17H,1-2,4,7-10,12-14,19H2,(H,20,23)/t17-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 78 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
Binding affinity at histamine H3 receptor by hH3-[35S]GTPgamma[S] binding assay |
Bioorg Med Chem Lett 16: 5303-8 (2006)
Article DOI: 10.1016/j.bmcl.2006.07.093
BindingDB Entry DOI: 10.7270/Q2RV0NBD |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50193195

((4-isopropylpiperazin-1-yl)(4-phenylpiperidin-1-yl...)Show InChI InChI=1S/C19H29N3O/c1-16(2)20-12-14-22(15-13-20)19(23)21-10-8-18(9-11-21)17-6-4-3-5-7-17/h3-7,16,18H,8-15H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 86 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
Binding affinity at histamine H3 receptor by hH3-[35S]GTPgamma[S] binding assay |
Bioorg Med Chem Lett 16: 5303-8 (2006)
Article DOI: 10.1016/j.bmcl.2006.07.093
BindingDB Entry DOI: 10.7270/Q2RV0NBD |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50193190
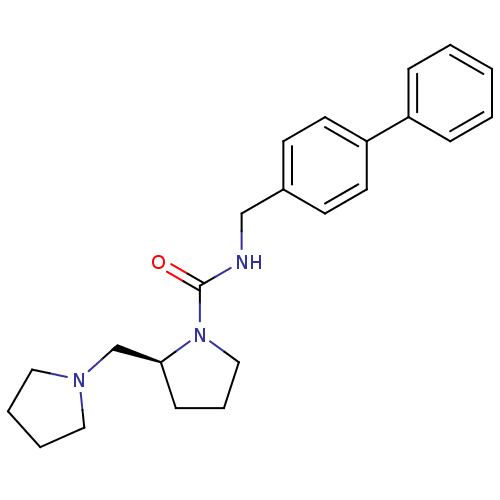
((S)-2-pyrrolidin-1-ylmethyl-pyrrolidine-1-carboxyl...)Show SMILES O=C(NCc1ccc(cc1)-c1ccccc1)N1CCC[C@H]1CN1CCCC1 Show InChI InChI=1S/C23H29N3O/c27-23(26-16-6-9-22(26)18-25-14-4-5-15-25)24-17-19-10-12-21(13-11-19)20-7-2-1-3-8-20/h1-3,7-8,10-13,22H,4-6,9,14-18H2,(H,24,27)/t22-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 87 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
Binding affinity at histamine H3 receptor by hH3-[35S]GTPgamma[S] binding assay |
Bioorg Med Chem Lett 16: 5303-8 (2006)
Article DOI: 10.1016/j.bmcl.2006.07.093
BindingDB Entry DOI: 10.7270/Q2RV0NBD |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50193214
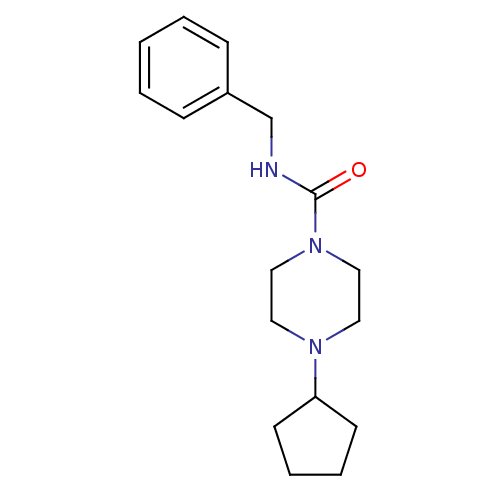
(CHEMBL219757 | N-benzyl-4-cyclopentylpiperazine-1-...)Show InChI InChI=1S/C17H25N3O/c21-17(18-14-15-6-2-1-3-7-15)20-12-10-19(11-13-20)16-8-4-5-9-16/h1-3,6-7,16H,4-5,8-14H2,(H,18,21) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 94 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
Binding affinity at histamine H3 receptor by hH3-[35S]GTPgamma[S] binding assay |
Bioorg Med Chem Lett 16: 5303-8 (2006)
Article DOI: 10.1016/j.bmcl.2006.07.093
BindingDB Entry DOI: 10.7270/Q2RV0NBD |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50193200
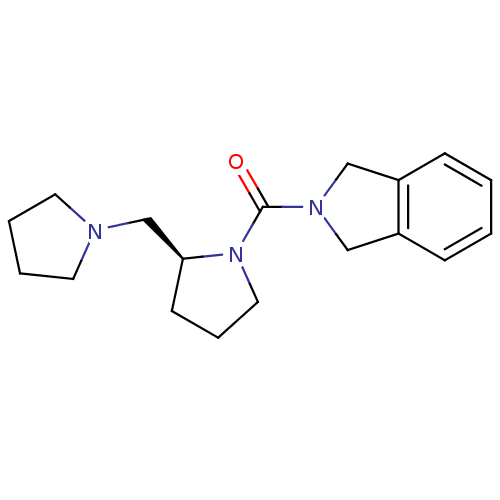
((S)-isoindolin-2-yl(2-(pyrrolidin-1-ylmethyl)pyrro...)Show InChI InChI=1S/C18H25N3O/c22-18(20-12-15-6-1-2-7-16(15)13-20)21-11-5-8-17(21)14-19-9-3-4-10-19/h1-2,6-7,17H,3-5,8-14H2/t17-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 94 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
Binding affinity at histamine H3 receptor by hH3-[35S]GTPgamma[S] binding assay |
Bioorg Med Chem Lett 16: 5303-8 (2006)
Article DOI: 10.1016/j.bmcl.2006.07.093
BindingDB Entry DOI: 10.7270/Q2RV0NBD |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50193194
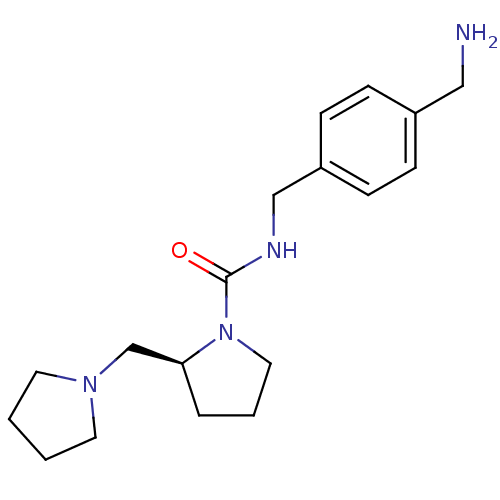
((S)-N-(4-(aminomethyl)benzyl)-2-(pyrrolidin-1-ylme...)Show InChI InChI=1S/C18H28N4O/c19-12-15-5-7-16(8-6-15)13-20-18(23)22-11-3-4-17(22)14-21-9-1-2-10-21/h5-8,17H,1-4,9-14,19H2,(H,20,23)/t17-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 109 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
Binding affinity at histamine H3 receptor by hH3-[35S]GTPgamma[S] binding assay |
Bioorg Med Chem Lett 16: 5303-8 (2006)
Article DOI: 10.1016/j.bmcl.2006.07.093
BindingDB Entry DOI: 10.7270/Q2RV0NBD |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50193202
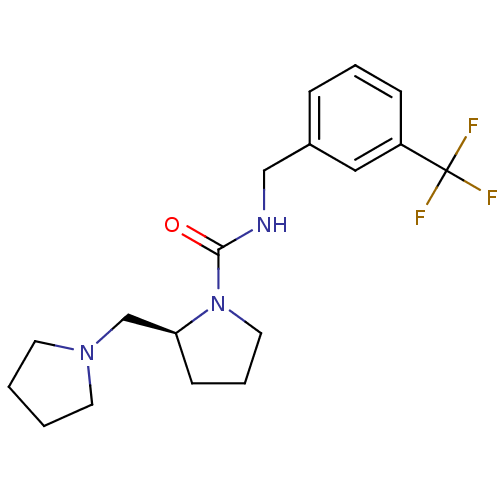
((S)-N-(3-(trifluoromethyl)benzyl)-2-(pyrrolidin-1-...)Show SMILES FC(F)(F)c1cccc(CNC(=O)N2CCC[C@H]2CN2CCCC2)c1 Show InChI InChI=1S/C18H24F3N3O/c19-18(20,21)15-6-3-5-14(11-15)12-22-17(25)24-10-4-7-16(24)13-23-8-1-2-9-23/h3,5-6,11,16H,1-2,4,7-10,12-13H2,(H,22,25)/t16-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 117 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
Binding affinity at histamine H3 receptor by hH3-[35S]GTPgamma[S] binding assay |
Bioorg Med Chem Lett 16: 5303-8 (2006)
Article DOI: 10.1016/j.bmcl.2006.07.093
BindingDB Entry DOI: 10.7270/Q2RV0NBD |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50193174
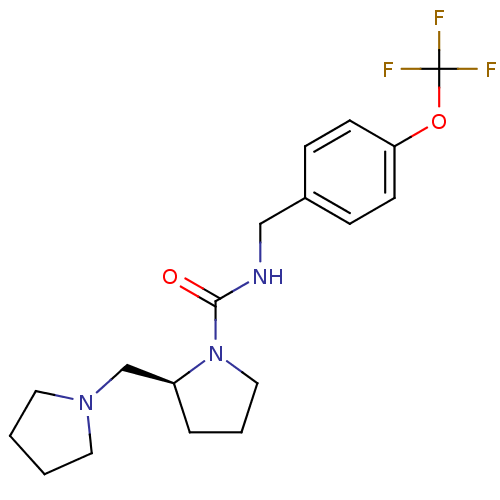
((S)-N-(4-(trifluoromethoxy)benzyl)-2-(pyrrolidin-1...)Show SMILES FC(F)(F)Oc1ccc(CNC(=O)N2CCC[C@H]2CN2CCCC2)cc1 Show InChI InChI=1S/C18H24F3N3O2/c19-18(20,21)26-16-7-5-14(6-8-16)12-22-17(25)24-11-3-4-15(24)13-23-9-1-2-10-23/h5-8,15H,1-4,9-13H2,(H,22,25)/t15-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 121 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
Binding affinity at histamine H3 receptor by hH3-[35S]GTPgamma[S] binding assay |
Bioorg Med Chem Lett 16: 5303-8 (2006)
Article DOI: 10.1016/j.bmcl.2006.07.093
BindingDB Entry DOI: 10.7270/Q2RV0NBD |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50193188
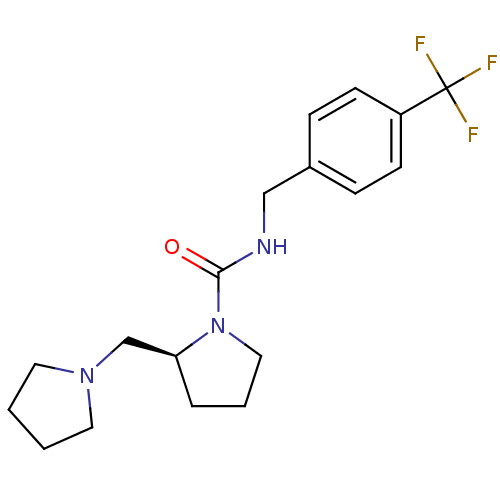
((S)-N-(4-(trifluoromethyl)benzyl)-2-(pyrrolidin-1-...)Show SMILES FC(F)(F)c1ccc(CNC(=O)N2CCC[C@H]2CN2CCCC2)cc1 Show InChI InChI=1S/C18H24F3N3O/c19-18(20,21)15-7-5-14(6-8-15)12-22-17(25)24-11-3-4-16(24)13-23-9-1-2-10-23/h5-8,16H,1-4,9-13H2,(H,22,25)/t16-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 162 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
Binding affinity at histamine H3 receptor by hH3-[35S]GTPgamma[S] binding assay |
Bioorg Med Chem Lett 16: 5303-8 (2006)
Article DOI: 10.1016/j.bmcl.2006.07.093
BindingDB Entry DOI: 10.7270/Q2RV0NBD |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50193191
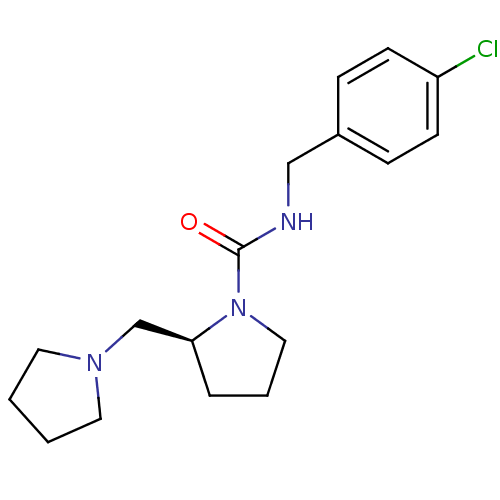
((S)-N-(4-chlorobenzyl)-2-(pyrrolidin-1-ylmethyl)py...)Show InChI InChI=1S/C17H24ClN3O/c18-15-7-5-14(6-8-15)12-19-17(22)21-11-3-4-16(21)13-20-9-1-2-10-20/h5-8,16H,1-4,9-13H2,(H,19,22)/t16-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 165 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
Binding affinity at histamine H3 receptor by hH3-[35S]GTPgamma[S] binding assay |
Bioorg Med Chem Lett 16: 5303-8 (2006)
Article DOI: 10.1016/j.bmcl.2006.07.093
BindingDB Entry DOI: 10.7270/Q2RV0NBD |
More data for this
Ligand-Target Pair | |
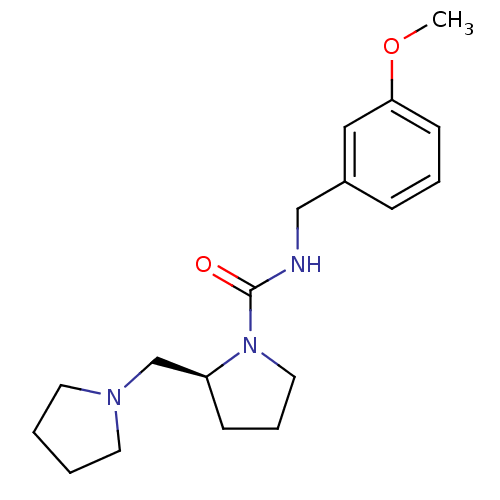
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50193177
((S)-N-(3-methoxybenzyl)-2-(pyrrolidin-1-ylmethyl)p...)Show InChI InChI=1S/C18H27N3O2/c1-23-17-8-4-6-15(12-17)13-19-18(22)21-11-5-7-16(21)14-20-9-2-3-10-20/h4,6,8,12,16H,2-3,5,7,9-11,13-14H2,1H3,(H,19,22)/t16-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 188 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
Binding affinity at histamine H3 receptor by hH3-[35S]GTPgamma[S] binding assay |
Bioorg Med Chem Lett 16: 5303-8 (2006)
Article DOI: 10.1016/j.bmcl.2006.07.093
BindingDB Entry DOI: 10.7270/Q2RV0NBD |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50193192

((S)-N-benzyl-2-(pyrrolidin-1-ylmethyl)pyrrolidine-...)Show InChI InChI=1S/C17H25N3O/c21-17(18-13-15-7-2-1-3-8-15)20-12-6-9-16(20)14-19-10-4-5-11-19/h1-3,7-8,16H,4-6,9-14H2,(H,18,21)/t16-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 196 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
Binding affinity at histamine H3 receptor by hH3-[35S]GTPgamma[S] binding assay |
Bioorg Med Chem Lett 16: 5303-8 (2006)
Article DOI: 10.1016/j.bmcl.2006.07.093
BindingDB Entry DOI: 10.7270/Q2RV0NBD |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
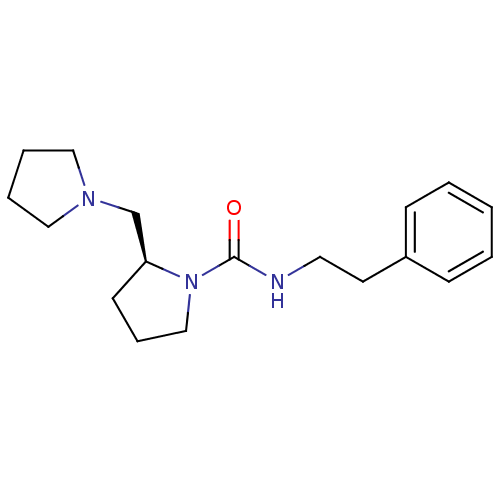
(Homo sapiens (Human)) | BDBM50193183
((S)-N-phenethyl-2-(pyrrolidin-1-ylmethyl)pyrrolidi...)Show InChI InChI=1S/C18H27N3O/c22-18(19-11-10-16-7-2-1-3-8-16)21-14-6-9-17(21)15-20-12-4-5-13-20/h1-3,7-8,17H,4-6,9-15H2,(H,19,22)/t17-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 226 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
Binding affinity at histamine H3 receptor by hH3-[35S]GTPgamma[S] binding assay |
Bioorg Med Chem Lett 16: 5303-8 (2006)
Article DOI: 10.1016/j.bmcl.2006.07.093
BindingDB Entry DOI: 10.7270/Q2RV0NBD |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
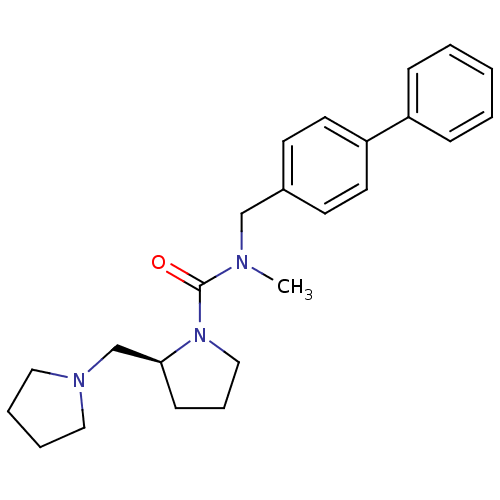
(Homo sapiens (Human)) | BDBM50193182
((S)-2-pyrrolidin-1-ylmethyl-pyrrolidine-1-carboxyl...)Show SMILES CN(Cc1ccc(cc1)-c1ccccc1)C(=O)N1CCC[C@H]1CN1CCCC1 Show InChI InChI=1S/C24H31N3O/c1-25(18-20-11-13-22(14-12-20)21-8-3-2-4-9-21)24(28)27-17-7-10-23(27)19-26-15-5-6-16-26/h2-4,8-9,11-14,23H,5-7,10,15-19H2,1H3/t23-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 281 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
Binding affinity at histamine H3 receptor by hH3-[35S]GTPgamma[S] binding assay |
Bioorg Med Chem Lett 16: 5303-8 (2006)
Article DOI: 10.1016/j.bmcl.2006.07.093
BindingDB Entry DOI: 10.7270/Q2RV0NBD |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50193173
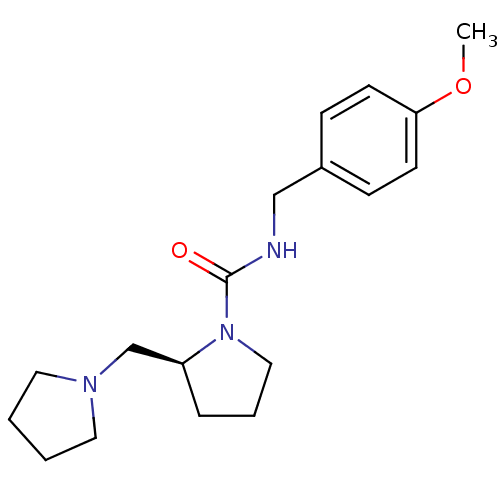
((S)-N-(4-methoxybenzyl)-2-(pyrrolidin-1-ylmethyl)p...)Show InChI InChI=1S/C18H27N3O2/c1-23-17-8-6-15(7-9-17)13-19-18(22)21-12-4-5-16(21)14-20-10-2-3-11-20/h6-9,16H,2-5,10-14H2,1H3,(H,19,22)/t16-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 295 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
Binding affinity at histamine H3 receptor by hH3-[35S]GTPgamma[S] binding assay |
Bioorg Med Chem Lett 16: 5303-8 (2006)
Article DOI: 10.1016/j.bmcl.2006.07.093
BindingDB Entry DOI: 10.7270/Q2RV0NBD |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM78577
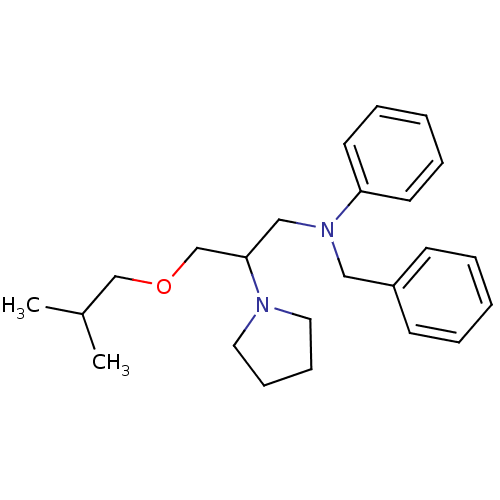
(BEPRIDIL HYDROCHLORIDE | Bepridil | MLS000028456 |...)Show InChI InChI=1S/C24H34N2O/c1-21(2)19-27-20-24(25-15-9-10-16-25)18-26(23-13-7-4-8-14-23)17-22-11-5-3-6-12-22/h3-8,11-14,21,24H,9-10,15-20H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 444 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
Displacement of [3H]Astemizole from hERG expressed in HEK293 cells at 10 uM |
Bioorg Med Chem Lett 16: 5303-8 (2006)
Article DOI: 10.1016/j.bmcl.2006.07.093
BindingDB Entry DOI: 10.7270/Q2RV0NBD |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50193206
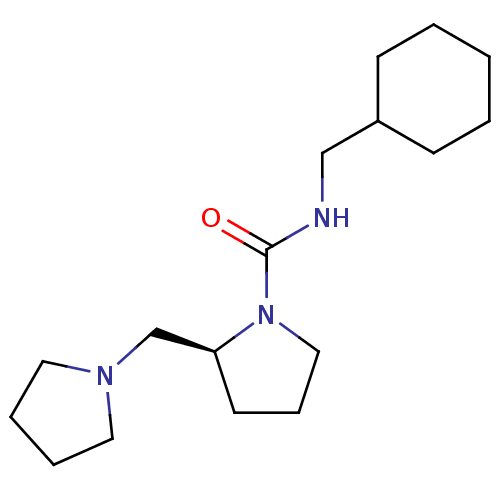
((S)-N-(cyclohexylmethyl)-2-(pyrrolidin-1-ylmethyl)...)Show InChI InChI=1S/C17H31N3O/c21-17(18-13-15-7-2-1-3-8-15)20-12-6-9-16(20)14-19-10-4-5-11-19/h15-16H,1-14H2,(H,18,21)/t16-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 458 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
Binding affinity at histamine H3 receptor by hH3-[35S]GTPgamma[S] binding assay |
Bioorg Med Chem Lett 16: 5303-8 (2006)
Article DOI: 10.1016/j.bmcl.2006.07.093
BindingDB Entry DOI: 10.7270/Q2RV0NBD |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM21398

(4-[4-(4-Chloro-phenyl)-4-hydroxy-piperidin-1-yl]-1...)Show SMILES OC1(CCN(CCCC(=O)c2ccc(F)cc2)CC1)c1ccc(Cl)cc1 Show InChI InChI=1S/C21H23ClFNO2/c22-18-7-5-17(6-8-18)21(26)11-14-24(15-12-21)13-1-2-20(25)16-3-9-19(23)10-4-16/h3-10,26H,1-2,11-15H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 512 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
Displacement of [3H]Astemizole from hERG expressed in HEK293 cells at 10 uM |
Bioorg Med Chem Lett 16: 5303-8 (2006)
Article DOI: 10.1016/j.bmcl.2006.07.093
BindingDB Entry DOI: 10.7270/Q2RV0NBD |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50002338
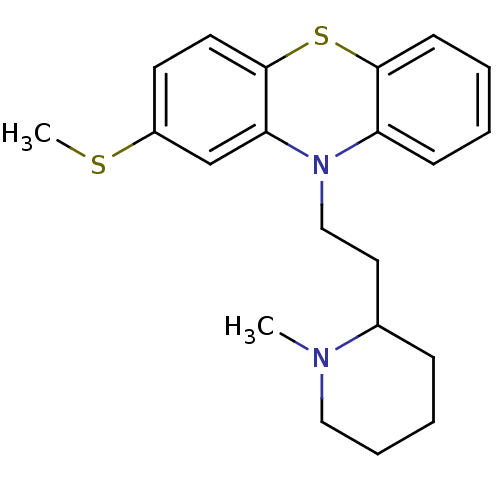
((Thioridazine)10-[2-(1-Methyl-piperidin-2-yl)-ethy...)Show InChI InChI=1S/C21H26N2S2/c1-22-13-6-5-7-16(22)12-14-23-18-8-3-4-9-20(18)25-21-11-10-17(24-2)15-19(21)23/h3-4,8-11,15-16H,5-7,12-14H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 869 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
Displacement of [3H]Astemizole from hERG expressed in HEK293 cells at 10 uM |
Bioorg Med Chem Lett 16: 5303-8 (2006)
Article DOI: 10.1016/j.bmcl.2006.07.093
BindingDB Entry DOI: 10.7270/Q2RV0NBD |
More data for this
Ligand-Target Pair | |
Melanocyte-stimulating hormone receptor
(Homo sapiens (Human)) | BDBM50134946
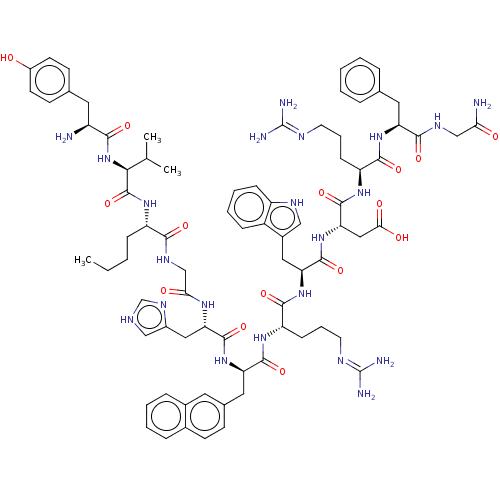
(CHEMBL2370964 | H-Tyr-Val-Nle-Gly-His-D-Nal(2')-Ar...)Show SMILES CCCC[C@H](NC(=O)[C@@H](NC(=O)[C@@H](N)Cc1ccc(O)cc1)C(C)C)C(=O)NCC(=O)N[C@@H](Cc1c[nH]cn1)C(=O)N[C@H](Cc1ccc2ccccc2c1)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](Cc1ccccc1)C(=O)NCC(N)=O |wU:68.72,8.22,90.96,wD:82.88,32.33,42.44,101.107,12.13,57.61,4.3,(2.47,-9.76,;1.17,-9.01,;1.19,-7.5,;-.16,-6.75,;-.14,-5.24,;-1.49,-4.47,;-2.84,-5.23,;-2.85,-6.73,;-4.17,-4.45,;-5.53,-5.2,;-6.86,-4.44,;-6.86,-2.92,;-8.22,-5.18,;-9.55,-4.42,;-8.24,-6.7,;-6.9,-7.44,;-5.56,-6.71,;-4.2,-7.47,;-4.23,-8.99,;-2.87,-9.75,;-5.57,-9.73,;-6.91,-8.97,;-4.17,-2.95,;-5.5,-2.19,;-2.82,-2.2,;1.2,-4.49,;1.22,-2.98,;2.54,-5.26,;3.9,-4.5,;5.23,-5.27,;5.21,-6.79,;6.58,-4.52,;7.92,-5.29,;7.92,-6.79,;9.25,-7.55,;10.69,-6.96,;11.71,-8.09,;10.92,-9.39,;9.41,-9.05,;9.28,-4.55,;9.3,-3.03,;10.61,-5.31,;11.96,-4.56,;11.96,-3.02,;11.17,-1.68,;9.62,-1.68,;8.85,-.34,;9.62,1.02,;8.85,2.36,;9.61,3.72,;11.17,3.72,;11.95,2.36,;11.17,1.02,;11.95,-.32,;13.31,-5.32,;13.29,-6.82,;14.65,-4.56,;15.99,-5.32,;15.98,-6.85,;17.32,-7.61,;17.3,-9.11,;18.65,-9.89,;18.63,-11.4,;19.98,-12.15,;17.29,-12.13,;17.35,-4.59,;17.36,-3.08,;18.69,-5.35,;20.05,-4.61,;20.05,-3.09,;21.4,-2.35,;22.81,-2.98,;23.86,-1.85,;23.1,-.55,;23.58,.89,;22.56,2.03,;21.03,1.71,;20.53,.28,;21.57,-.85,;21.37,-5.37,;21.37,-6.88,;22.73,-4.62,;24.06,-5.4,;24.06,-6.9,;25.41,-7.67,;26.74,-6.9,;25.39,-9.17,;25.41,-4.64,;25.43,-3.14,;26.76,-5.4,;28.12,-4.66,;28.13,-3.15,;29.48,-2.4,;29.48,-.89,;30.83,-.15,;30.84,1.36,;32.2,2.11,;29.51,2.12,;29.46,-5.43,;29.45,-6.93,;30.8,-4.67,;32.14,-5.44,;32.13,-6.95,;33.47,-7.71,;34.83,-6.96,;36.17,-7.72,;36.15,-9.24,;34.8,-9.99,;33.47,-9.22,;33.5,-4.69,;33.5,-3.19,;34.83,-5.46,;36.18,-4.72,;37.53,-5.48,;38.89,-4.73,;37.51,-6.99,)| Show InChI InChI=1S/C79H104N22O15/c1-4-5-20-56(96-77(116)67(44(2)3)101-68(107)54(80)33-46-25-28-52(102)29-26-46)69(108)91-42-65(104)93-62(37-51-40-86-43-92-51)75(114)98-60(35-47-24-27-48-17-9-10-18-49(48)32-47)73(112)94-58(23-14-31-88-79(84)85)72(111)99-61(36-50-39-89-55-21-12-11-19-53(50)55)74(113)100-63(38-66(105)106)76(115)95-57(22-13-30-87-78(82)83)71(110)97-59(70(109)90-41-64(81)103)34-45-15-7-6-8-16-45/h6-12,15-19,21,24-29,32,39-40,43-44,54,56-63,67,89,102H,4-5,13-14,20,22-23,30-31,33-38,41-42,80H2,1-3H3,(H2,81,103)(H,86,92)(H,90,109)(H,91,108)(H,93,104)(H,94,112)(H,95,115)(H,96,116)(H,97,110)(H,98,114)(H,99,111)(H,100,113)(H,101,107)(H,105,106)(H4,82,83,87)(H4,84,85,88)/t54-,56-,57-,58-,59-,60?,61-,62-,63-,67-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0740 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01295
BindingDB Entry DOI: 10.7270/Q2GX4GPJ |
More data for this
Ligand-Target Pair | |
Melanocyte-stimulating hormone receptor
(Homo sapiens (Human)) | BDBM50134946

(CHEMBL2370964 | H-Tyr-Val-Nle-Gly-His-D-Nal(2')-Ar...)Show SMILES CCCC[C@H](NC(=O)[C@@H](NC(=O)[C@@H](N)Cc1ccc(O)cc1)C(C)C)C(=O)NCC(=O)N[C@@H](Cc1c[nH]cn1)C(=O)N[C@H](Cc1ccc2ccccc2c1)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](Cc1ccccc1)C(=O)NCC(N)=O |wU:68.72,8.22,90.96,wD:82.88,32.33,42.44,101.107,12.13,57.61,4.3,(2.47,-9.76,;1.17,-9.01,;1.19,-7.5,;-.16,-6.75,;-.14,-5.24,;-1.49,-4.47,;-2.84,-5.23,;-2.85,-6.73,;-4.17,-4.45,;-5.53,-5.2,;-6.86,-4.44,;-6.86,-2.92,;-8.22,-5.18,;-9.55,-4.42,;-8.24,-6.7,;-6.9,-7.44,;-5.56,-6.71,;-4.2,-7.47,;-4.23,-8.99,;-2.87,-9.75,;-5.57,-9.73,;-6.91,-8.97,;-4.17,-2.95,;-5.5,-2.19,;-2.82,-2.2,;1.2,-4.49,;1.22,-2.98,;2.54,-5.26,;3.9,-4.5,;5.23,-5.27,;5.21,-6.79,;6.58,-4.52,;7.92,-5.29,;7.92,-6.79,;9.25,-7.55,;10.69,-6.96,;11.71,-8.09,;10.92,-9.39,;9.41,-9.05,;9.28,-4.55,;9.3,-3.03,;10.61,-5.31,;11.96,-4.56,;11.96,-3.02,;11.17,-1.68,;9.62,-1.68,;8.85,-.34,;9.62,1.02,;8.85,2.36,;9.61,3.72,;11.17,3.72,;11.95,2.36,;11.17,1.02,;11.95,-.32,;13.31,-5.32,;13.29,-6.82,;14.65,-4.56,;15.99,-5.32,;15.98,-6.85,;17.32,-7.61,;17.3,-9.11,;18.65,-9.89,;18.63,-11.4,;19.98,-12.15,;17.29,-12.13,;17.35,-4.59,;17.36,-3.08,;18.69,-5.35,;20.05,-4.61,;20.05,-3.09,;21.4,-2.35,;22.81,-2.98,;23.86,-1.85,;23.1,-.55,;23.58,.89,;22.56,2.03,;21.03,1.71,;20.53,.28,;21.57,-.85,;21.37,-5.37,;21.37,-6.88,;22.73,-4.62,;24.06,-5.4,;24.06,-6.9,;25.41,-7.67,;26.74,-6.9,;25.39,-9.17,;25.41,-4.64,;25.43,-3.14,;26.76,-5.4,;28.12,-4.66,;28.13,-3.15,;29.48,-2.4,;29.48,-.89,;30.83,-.15,;30.84,1.36,;32.2,2.11,;29.51,2.12,;29.46,-5.43,;29.45,-6.93,;30.8,-4.67,;32.14,-5.44,;32.13,-6.95,;33.47,-7.71,;34.83,-6.96,;36.17,-7.72,;36.15,-9.24,;34.8,-9.99,;33.47,-9.22,;33.5,-4.69,;33.5,-3.19,;34.83,-5.46,;36.18,-4.72,;37.53,-5.48,;38.89,-4.73,;37.51,-6.99,)| Show InChI InChI=1S/C79H104N22O15/c1-4-5-20-56(96-77(116)67(44(2)3)101-68(107)54(80)33-46-25-28-52(102)29-26-46)69(108)91-42-65(104)93-62(37-51-40-86-43-92-51)75(114)98-60(35-47-24-27-48-17-9-10-18-49(48)32-47)73(112)94-58(23-14-31-88-79(84)85)72(111)99-61(36-50-39-89-55-21-12-11-19-53(50)55)74(113)100-63(38-66(105)106)76(115)95-57(22-13-30-87-78(82)83)71(110)97-59(70(109)90-41-64(81)103)34-45-15-7-6-8-16-45/h6-12,15-19,21,24-29,32,39-40,43-44,54,56-63,67,89,102H,4-5,13-14,20,22-23,30-31,33-38,41-42,80H2,1-3H3,(H2,81,103)(H,86,92)(H,90,109)(H,91,108)(H,93,104)(H,94,112)(H,95,115)(H,96,116)(H,97,110)(H,98,114)(H,99,111)(H,100,113)(H,101,107)(H,105,106)(H4,82,83,87)(H4,84,85,88)/t54-,56-,57-,58-,59-,60?,61-,62-,63-,67-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0741 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01295
BindingDB Entry DOI: 10.7270/Q2GX4GPJ |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 4
(Homo sapiens (Human)) | BDBM50134946

(CHEMBL2370964 | H-Tyr-Val-Nle-Gly-His-D-Nal(2')-Ar...)Show SMILES CCCC[C@H](NC(=O)[C@@H](NC(=O)[C@@H](N)Cc1ccc(O)cc1)C(C)C)C(=O)NCC(=O)N[C@@H](Cc1c[nH]cn1)C(=O)N[C@H](Cc1ccc2ccccc2c1)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](Cc1ccccc1)C(=O)NCC(N)=O |wU:68.72,8.22,90.96,wD:82.88,32.33,42.44,101.107,12.13,57.61,4.3,(2.47,-9.76,;1.17,-9.01,;1.19,-7.5,;-.16,-6.75,;-.14,-5.24,;-1.49,-4.47,;-2.84,-5.23,;-2.85,-6.73,;-4.17,-4.45,;-5.53,-5.2,;-6.86,-4.44,;-6.86,-2.92,;-8.22,-5.18,;-9.55,-4.42,;-8.24,-6.7,;-6.9,-7.44,;-5.56,-6.71,;-4.2,-7.47,;-4.23,-8.99,;-2.87,-9.75,;-5.57,-9.73,;-6.91,-8.97,;-4.17,-2.95,;-5.5,-2.19,;-2.82,-2.2,;1.2,-4.49,;1.22,-2.98,;2.54,-5.26,;3.9,-4.5,;5.23,-5.27,;5.21,-6.79,;6.58,-4.52,;7.92,-5.29,;7.92,-6.79,;9.25,-7.55,;10.69,-6.96,;11.71,-8.09,;10.92,-9.39,;9.41,-9.05,;9.28,-4.55,;9.3,-3.03,;10.61,-5.31,;11.96,-4.56,;11.96,-3.02,;11.17,-1.68,;9.62,-1.68,;8.85,-.34,;9.62,1.02,;8.85,2.36,;9.61,3.72,;11.17,3.72,;11.95,2.36,;11.17,1.02,;11.95,-.32,;13.31,-5.32,;13.29,-6.82,;14.65,-4.56,;15.99,-5.32,;15.98,-6.85,;17.32,-7.61,;17.3,-9.11,;18.65,-9.89,;18.63,-11.4,;19.98,-12.15,;17.29,-12.13,;17.35,-4.59,;17.36,-3.08,;18.69,-5.35,;20.05,-4.61,;20.05,-3.09,;21.4,-2.35,;22.81,-2.98,;23.86,-1.85,;23.1,-.55,;23.58,.89,;22.56,2.03,;21.03,1.71,;20.53,.28,;21.57,-.85,;21.37,-5.37,;21.37,-6.88,;22.73,-4.62,;24.06,-5.4,;24.06,-6.9,;25.41,-7.67,;26.74,-6.9,;25.39,-9.17,;25.41,-4.64,;25.43,-3.14,;26.76,-5.4,;28.12,-4.66,;28.13,-3.15,;29.48,-2.4,;29.48,-.89,;30.83,-.15,;30.84,1.36,;32.2,2.11,;29.51,2.12,;29.46,-5.43,;29.45,-6.93,;30.8,-4.67,;32.14,-5.44,;32.13,-6.95,;33.47,-7.71,;34.83,-6.96,;36.17,-7.72,;36.15,-9.24,;34.8,-9.99,;33.47,-9.22,;33.5,-4.69,;33.5,-3.19,;34.83,-5.46,;36.18,-4.72,;37.53,-5.48,;38.89,-4.73,;37.51,-6.99,)| Show InChI InChI=1S/C79H104N22O15/c1-4-5-20-56(96-77(116)67(44(2)3)101-68(107)54(80)33-46-25-28-52(102)29-26-46)69(108)91-42-65(104)93-62(37-51-40-86-43-92-51)75(114)98-60(35-47-24-27-48-17-9-10-18-49(48)32-47)73(112)94-58(23-14-31-88-79(84)85)72(111)99-61(36-50-39-89-55-21-12-11-19-53(50)55)74(113)100-63(38-66(105)106)76(115)95-57(22-13-30-87-78(82)83)71(110)97-59(70(109)90-41-64(81)103)34-45-15-7-6-8-16-45/h6-12,15-19,21,24-29,32,39-40,43-44,54,56-63,67,89,102H,4-5,13-14,20,22-23,30-31,33-38,41-42,80H2,1-3H3,(H2,81,103)(H,86,92)(H,90,109)(H,91,108)(H,93,104)(H,94,112)(H,95,115)(H,96,116)(H,97,110)(H,98,114)(H,99,111)(H,100,113)(H,101,107)(H,105,106)(H4,82,83,87)(H4,84,85,88)/t54-,56-,57-,58-,59-,60?,61-,62-,63-,67-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01295
BindingDB Entry DOI: 10.7270/Q2GX4GPJ |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data