

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Carbonic anhydrase 1 (Homo sapiens (Human)) | BDBM50252992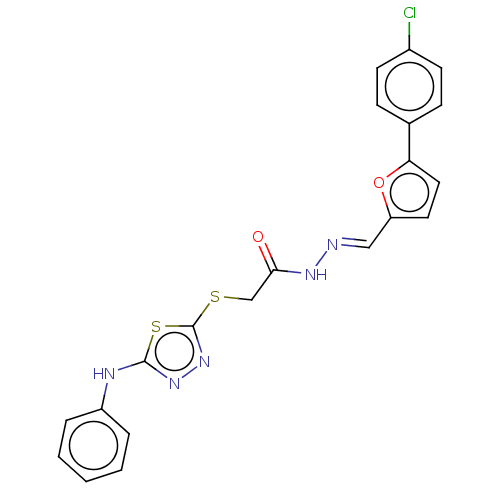 (CHEMBL4100496) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Pharmaceutical Chemistry, Faculty of Pharmacy, Anadolu University, 26470 Eski?ehir, Turkey. Electronic address: mdaltintop@anadolu.edu.tr. Curated by ChEMBL | Assay Description Competitive inhibition of carbonic anhydrase-1 in human erythrocyte membranes assessed as reduction in H+ release using CO2 as substrate by Lineweave... | Bioorg Med Chem 25: 3547-3554 (2017) Article DOI: 10.1016/j.bmc.2017.05.005 BindingDB Entry DOI: 10.7270/Q28G8P41 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 1 (Homo sapiens (Human)) | BDBM50252993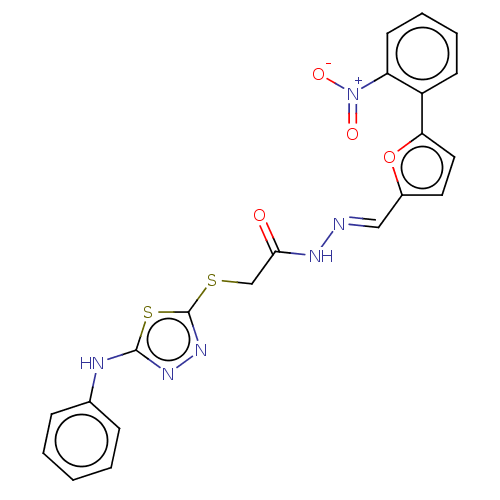 (CHEMBL4073678) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Pharmaceutical Chemistry, Faculty of Pharmacy, Anadolu University, 26470 Eski?ehir, Turkey. Electronic address: mdaltintop@anadolu.edu.tr. Curated by ChEMBL | Assay Description Non-competitive inhibition of carbonic anhydrase-1 in human erythrocyte membranes assessed as reduction in H+ release using CO2 as substrate by Linew... | Bioorg Med Chem 25: 3547-3554 (2017) Article DOI: 10.1016/j.bmc.2017.05.005 BindingDB Entry DOI: 10.7270/Q28G8P41 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50252992 (CHEMBL4100496) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Pharmaceutical Chemistry, Faculty of Pharmacy, Anadolu University, 26470 Eski?ehir, Turkey. Electronic address: mdaltintop@anadolu.edu.tr. Curated by ChEMBL | Assay Description Competitive inhibition of carbonic anhydrase-2 in human erythrocyte membranes assessed as reduction in H+ release using CO2 as substrate by Lineweave... | Bioorg Med Chem 25: 3547-3554 (2017) Article DOI: 10.1016/j.bmc.2017.05.005 BindingDB Entry DOI: 10.7270/Q28G8P41 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 1 (Homo sapiens (Human)) | BDBM50252996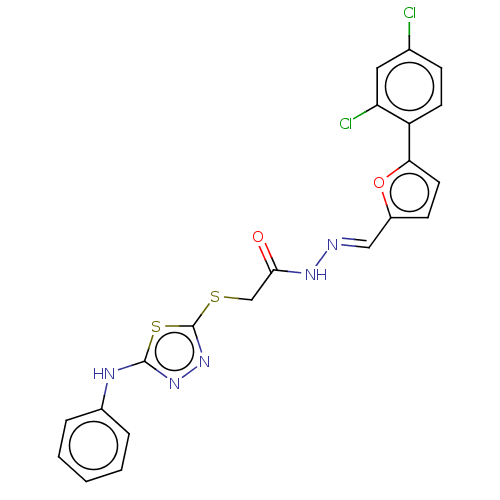 (CHEMBL4097972) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.370 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Pharmaceutical Chemistry, Faculty of Pharmacy, Anadolu University, 26470 Eski?ehir, Turkey. Electronic address: mdaltintop@anadolu.edu.tr. Curated by ChEMBL | Assay Description Competitive inhibition of carbonic anhydrase-1 in human erythrocyte membranes assessed as reduction in H+ release using CO2 as substrate by Lineweave... | Bioorg Med Chem 25: 3547-3554 (2017) Article DOI: 10.1016/j.bmc.2017.05.005 BindingDB Entry DOI: 10.7270/Q28G8P41 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50252991 (CHEMBL4064130) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 0.610 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Pharmaceutical Chemistry, Faculty of Pharmacy, Anadolu University, 26470 Eski?ehir, Turkey. Electronic address: mdaltintop@anadolu.edu.tr. Curated by ChEMBL | Assay Description Competitive inhibition of carbonic anhydrase-2 in human erythrocyte membranes assessed as reduction in H+ release using CO2 as substrate by Lineweave... | Bioorg Med Chem 25: 3547-3554 (2017) Article DOI: 10.1016/j.bmc.2017.05.005 BindingDB Entry DOI: 10.7270/Q28G8P41 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 1 (Homo sapiens (Human)) | BDBM50252994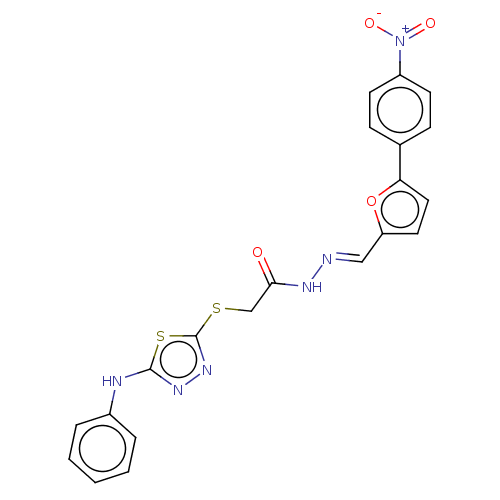 (CHEMBL4082551) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.630 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Pharmaceutical Chemistry, Faculty of Pharmacy, Anadolu University, 26470 Eski?ehir, Turkey. Electronic address: mdaltintop@anadolu.edu.tr. Curated by ChEMBL | Assay Description Non-competitive inhibition of carbonic anhydrase-1 in human erythrocyte membranes assessed as reduction in H+ release using CO2 as substrate by Linew... | Bioorg Med Chem 25: 3547-3554 (2017) Article DOI: 10.1016/j.bmc.2017.05.005 BindingDB Entry DOI: 10.7270/Q28G8P41 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50252998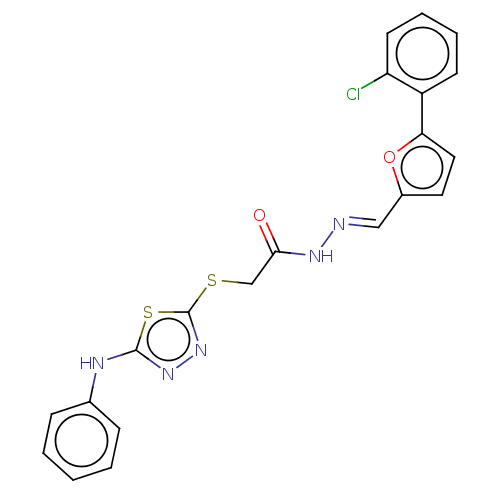 (CHEMBL4105008) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.640 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Pharmaceutical Chemistry, Faculty of Pharmacy, Anadolu University, 26470 Eski?ehir, Turkey. Electronic address: mdaltintop@anadolu.edu.tr. Curated by ChEMBL | Assay Description Competitive inhibition of carbonic anhydrase-2 in human erythrocyte membranes assessed as reduction in H+ release using CO2 as substrate by Lineweave... | Bioorg Med Chem 25: 3547-3554 (2017) Article DOI: 10.1016/j.bmc.2017.05.005 BindingDB Entry DOI: 10.7270/Q28G8P41 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50252996 (CHEMBL4097972) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.660 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Pharmaceutical Chemistry, Faculty of Pharmacy, Anadolu University, 26470 Eski?ehir, Turkey. Electronic address: mdaltintop@anadolu.edu.tr. Curated by ChEMBL | Assay Description Competitive inhibition of carbonic anhydrase-2 in human erythrocyte membranes assessed as reduction in H+ release using CO2 as substrate by Lineweave... | Bioorg Med Chem 25: 3547-3554 (2017) Article DOI: 10.1016/j.bmc.2017.05.005 BindingDB Entry DOI: 10.7270/Q28G8P41 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50253004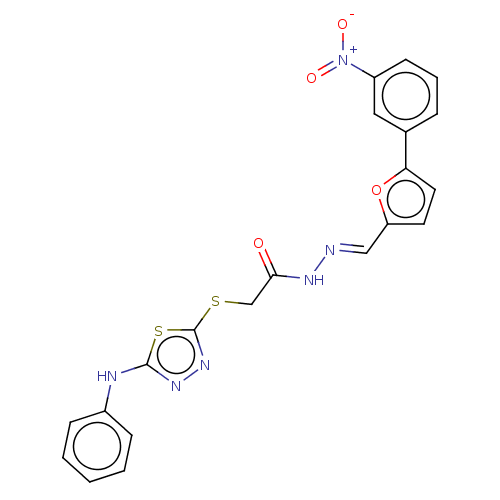 (CHEMBL4072122) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.660 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Pharmaceutical Chemistry, Faculty of Pharmacy, Anadolu University, 26470 Eski?ehir, Turkey. Electronic address: mdaltintop@anadolu.edu.tr. Curated by ChEMBL | Assay Description Competitive inhibition of carbonic anhydrase-2 in human erythrocyte membranes assessed as reduction in H+ release using CO2 as substrate by Lineweave... | Bioorg Med Chem 25: 3547-3554 (2017) Article DOI: 10.1016/j.bmc.2017.05.005 BindingDB Entry DOI: 10.7270/Q28G8P41 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 1 (Homo sapiens (Human)) | BDBM50253005 (CHEMBL4076887) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.690 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Pharmaceutical Chemistry, Faculty of Pharmacy, Anadolu University, 26470 Eski?ehir, Turkey. Electronic address: mdaltintop@anadolu.edu.tr. Curated by ChEMBL | Assay Description Competitive inhibition of carbonic anhydrase-1 in human erythrocyte membranes assessed as reduction in H+ release using CO2 as substrate by Lineweave... | Bioorg Med Chem 25: 3547-3554 (2017) Article DOI: 10.1016/j.bmc.2017.05.005 BindingDB Entry DOI: 10.7270/Q28G8P41 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 1 (Homo sapiens (Human)) | BDBM50252991 (CHEMBL4064130) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 0.770 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Pharmaceutical Chemistry, Faculty of Pharmacy, Anadolu University, 26470 Eski?ehir, Turkey. Electronic address: mdaltintop@anadolu.edu.tr. Curated by ChEMBL | Assay Description Competitive inhibition of carbonic anhydrase-1 in human erythrocyte membranes assessed as reduction in H+ release using CO2 as substrate by Lineweave... | Bioorg Med Chem 25: 3547-3554 (2017) Article DOI: 10.1016/j.bmc.2017.05.005 BindingDB Entry DOI: 10.7270/Q28G8P41 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50252993 (CHEMBL4073678) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.880 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Pharmaceutical Chemistry, Faculty of Pharmacy, Anadolu University, 26470 Eski?ehir, Turkey. Electronic address: mdaltintop@anadolu.edu.tr. Curated by ChEMBL | Assay Description Competitive inhibition of carbonic anhydrase-2 in human erythrocyte membranes assessed as reduction in H+ release using CO2 as substrate by Lineweave... | Bioorg Med Chem 25: 3547-3554 (2017) Article DOI: 10.1016/j.bmc.2017.05.005 BindingDB Entry DOI: 10.7270/Q28G8P41 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 1 (Homo sapiens (Human)) | BDBM50253004 (CHEMBL4072122) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Pharmaceutical Chemistry, Faculty of Pharmacy, Anadolu University, 26470 Eski?ehir, Turkey. Electronic address: mdaltintop@anadolu.edu.tr. Curated by ChEMBL | Assay Description Non-competitive inhibition of carbonic anhydrase-1 in human erythrocyte membranes assessed as reduction in H+ release using CO2 as substrate by Linew... | Bioorg Med Chem 25: 3547-3554 (2017) Article DOI: 10.1016/j.bmc.2017.05.005 BindingDB Entry DOI: 10.7270/Q28G8P41 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 1 (Homo sapiens (Human)) | BDBM50252997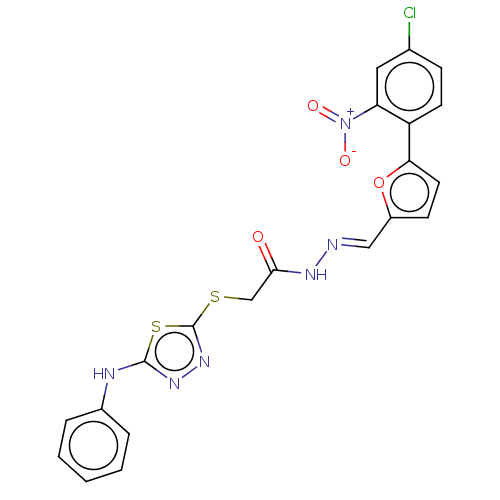 (CHEMBL4098116) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Pharmaceutical Chemistry, Faculty of Pharmacy, Anadolu University, 26470 Eski?ehir, Turkey. Electronic address: mdaltintop@anadolu.edu.tr. Curated by ChEMBL | Assay Description Competitive inhibition of carbonic anhydrase-1 in human erythrocyte membranes assessed as reduction in H+ release using CO2 as substrate by Lineweave... | Bioorg Med Chem 25: 3547-3554 (2017) Article DOI: 10.1016/j.bmc.2017.05.005 BindingDB Entry DOI: 10.7270/Q28G8P41 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 1 (Homo sapiens (Human)) | BDBM50252998 (CHEMBL4105008) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Pharmaceutical Chemistry, Faculty of Pharmacy, Anadolu University, 26470 Eski?ehir, Turkey. Electronic address: mdaltintop@anadolu.edu.tr. Curated by ChEMBL | Assay Description Competitive inhibition of carbonic anhydrase-1 in human erythrocyte membranes assessed as reduction in H+ release using CO2 as substrate by Lineweave... | Bioorg Med Chem 25: 3547-3554 (2017) Article DOI: 10.1016/j.bmc.2017.05.005 BindingDB Entry DOI: 10.7270/Q28G8P41 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 5A (Homo sapiens (Human)) | BDBM50585913 (CHEMBL5093295) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-5-CT from human 5-HT5A receptor at 32 uM incubated for 2 hr by radioligand binding assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c02031 BindingDB Entry DOI: 10.7270/Q2G73JM6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 5A (Homo sapiens (Human)) | BDBM50585914 (CHEMBL5079273) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-5-CT from human 5-HT5A receptor at 1 uM incubated for 2 hr by radioligand binding assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c02031 BindingDB Entry DOI: 10.7270/Q2G73JM6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 5A (Homo sapiens (Human)) | BDBM50585912 (CHEMBL5075486) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-5-CT from human 5-HT5A receptor at 32 uM incubated for 2 hr by radioligand binding assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c02031 BindingDB Entry DOI: 10.7270/Q2G73JM6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 5A (Homo sapiens (Human)) | BDBM50585909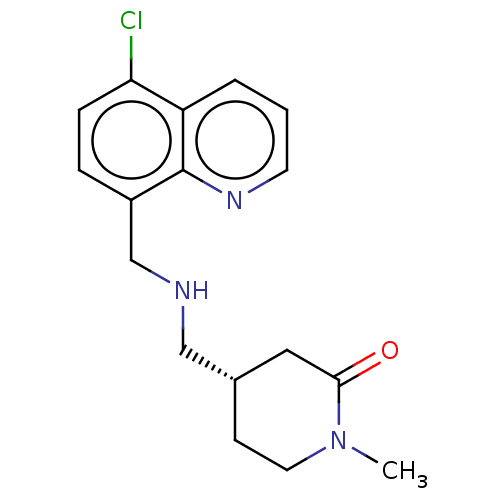 (CHEMBL5089996) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-5-CT from human 5-HT5A receptor at 32 uM incubated for 2 hr by radioligand binding assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c02031 BindingDB Entry DOI: 10.7270/Q2G73JM6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A (Homo sapiens (Human)) | BDBM50289513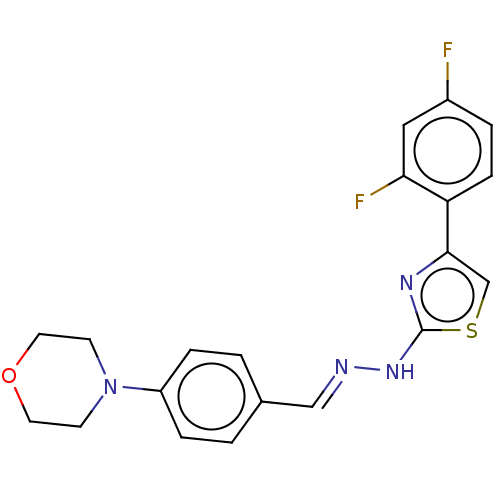 (CHEMBL4169527) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 4.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Anadolu University Curated by ChEMBL | Assay Description Reversible-competitive inhibition of human MAO-A using varying levels of tyramine as substrate after 30 mins by Lineweaver-Burk plot | Eur J Med Chem 144: 68-81 (2018) Article DOI: 10.1016/j.ejmech.2017.12.013 BindingDB Entry DOI: 10.7270/Q2833VK7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50252995 (CHEMBL4075535) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 5.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Pharmaceutical Chemistry, Faculty of Pharmacy, Anadolu University, 26470 Eski?ehir, Turkey. Electronic address: mdaltintop@anadolu.edu.tr. Curated by ChEMBL | Assay Description Competitive inhibition of carbonic anhydrase-2 in human erythrocyte membranes assessed as reduction in H+ release using CO2 as substrate by Lineweave... | Bioorg Med Chem 25: 3547-3554 (2017) Article DOI: 10.1016/j.bmc.2017.05.005 BindingDB Entry DOI: 10.7270/Q28G8P41 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 1 (Homo sapiens (Human)) | BDBM50252995 (CHEMBL4075535) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 6.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Pharmaceutical Chemistry, Faculty of Pharmacy, Anadolu University, 26470 Eski?ehir, Turkey. Electronic address: mdaltintop@anadolu.edu.tr. Curated by ChEMBL | Assay Description Competitive inhibition of carbonic anhydrase-1 in human erythrocyte membranes assessed as reduction in H+ release using CO2 as substrate by Lineweave... | Bioorg Med Chem 25: 3547-3554 (2017) Article DOI: 10.1016/j.bmc.2017.05.005 BindingDB Entry DOI: 10.7270/Q28G8P41 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A (Homo sapiens (Human)) | BDBM50289515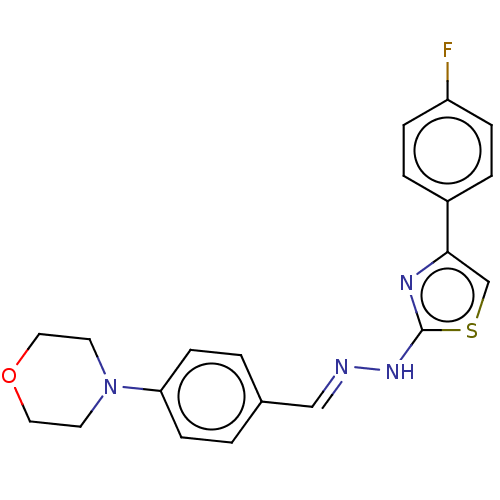 (CHEMBL4172923) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 6.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Anadolu University Curated by ChEMBL | Assay Description Reversible-competitive inhibition of human MAO-A using varying levels of tyramine as substrate after 30 mins by Lineweaver-Burk plot | Eur J Med Chem 144: 68-81 (2018) Article DOI: 10.1016/j.ejmech.2017.12.013 BindingDB Entry DOI: 10.7270/Q2833VK7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 5A (Homo sapiens (Human)) | BDBM50585911 (CHEMBL5093969) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 6.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-5-CT from human 5-HT5A receptor at 32 uM incubated for 2 hr by radioligand binding assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c02031 BindingDB Entry DOI: 10.7270/Q2G73JM6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 5A (Homo sapiens (Human)) | BDBM50585910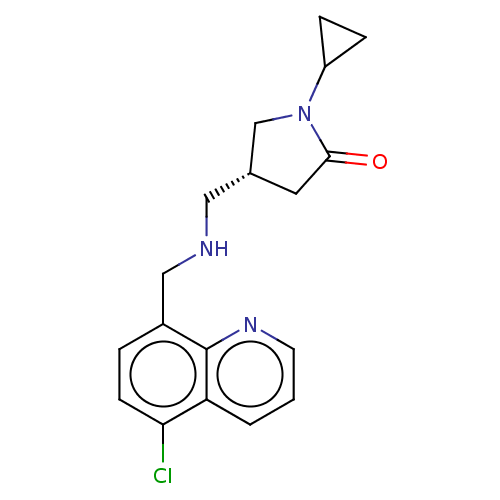 (CHEMBL5094012) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 6.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-5-CT from human 5-HT5A receptor at 32 uM incubated for 2 hr by radioligand binding assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c02031 BindingDB Entry DOI: 10.7270/Q2G73JM6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 1 (Homo sapiens (Human)) | BDBM10880 (AZA | AZA2 | AZM acetazolamide | Acerazolamide, AA...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | 8.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Pharmaceutical Chemistry, Faculty of Pharmacy, Anadolu University, 26470 Eski?ehir, Turkey. Electronic address: mdaltintop@anadolu.edu.tr. Curated by ChEMBL | Assay Description Inhibition of carbonic anhydrase-1 in human erythrocyte membranes assessed as reduction in H+ release using CO2 as substrate by bromine thymol blue i... | Bioorg Med Chem 25: 3547-3554 (2017) Article DOI: 10.1016/j.bmc.2017.05.005 BindingDB Entry DOI: 10.7270/Q28G8P41 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM10880 (AZA | AZA2 | AZM acetazolamide | Acerazolamide, AA...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank MMDB PDB Article PubMed | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Pharmaceutical Chemistry, Faculty of Pharmacy, Anadolu University, 26470 Eski?ehir, Turkey. Electronic address: mdaltintop@anadolu.edu.tr. Curated by ChEMBL | Assay Description Inhibition of carbonic anhydrase-2 in human erythrocyte membranes assessed as reduction in H+ release using CO2 as substrate by bromine thymol blue i... | Bioorg Med Chem 25: 3547-3554 (2017) Article DOI: 10.1016/j.bmc.2017.05.005 BindingDB Entry DOI: 10.7270/Q28G8P41 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50552011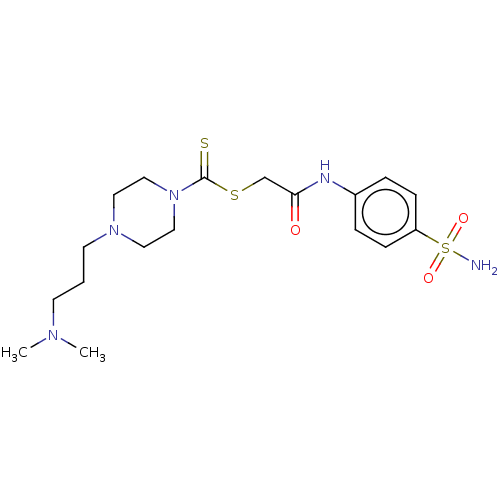 (CHEMBL4792992) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human erythrocyte CA2 using 4-nitrophenyl acetate as substrate by Lineweaver-Burk plot analysis | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112392 BindingDB Entry DOI: 10.7270/Q2C2512X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50552018 (CHEMBL4764100) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 21 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human erythrocyte CA2 using 4-nitrophenyl acetate as substrate by Lineweaver-Burk plot analysis | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112392 BindingDB Entry DOI: 10.7270/Q2C2512X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50552012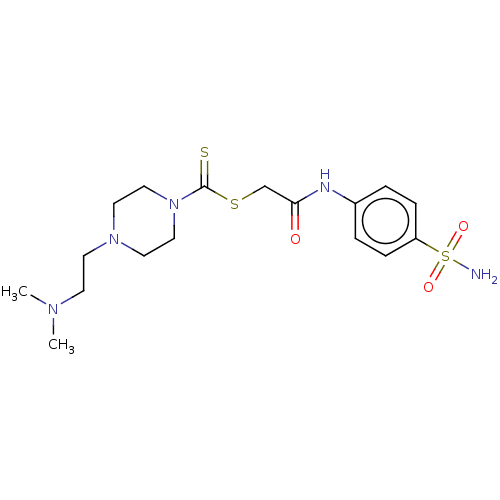 (CHEMBL4786423) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 26 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human erythrocyte CA2 using 4-nitrophenyl acetate as substrate by Lineweaver-Burk plot analysis | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112392 BindingDB Entry DOI: 10.7270/Q2C2512X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50552009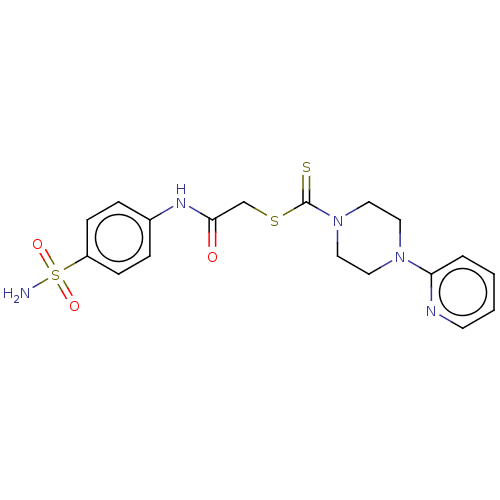 (CHEMBL4747356) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 28 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human erythrocyte CA2 using 4-nitrophenyl acetate as substrate by Lineweaver-Burk plot analysis | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112392 BindingDB Entry DOI: 10.7270/Q2C2512X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 1 (Homo sapiens (Human)) | BDBM50552011 (CHEMBL4792992) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 32 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human erythrocyte CA1 b using 4-nitrophenyl acetate as substrate by Lineweaver-Burk plot analysis | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112392 BindingDB Entry DOI: 10.7270/Q2C2512X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A (Homo sapiens (Human)) | BDBM50553240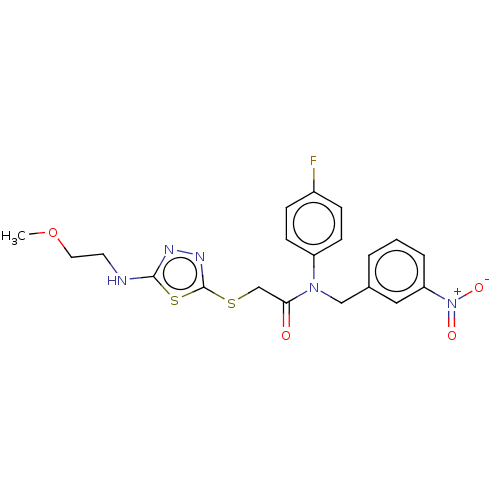 (CHEMBL4798970) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 33 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Competitive inhibition of human recombinant MAO-A using tyramine as substrate preincubated with enzyme for 30 mins followed by substrate addition and... | Citation and Details Article DOI: 10.1039/d0md00150c BindingDB Entry DOI: 10.7270/Q2183B56 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 1 (Homo sapiens (Human)) | BDBM50552008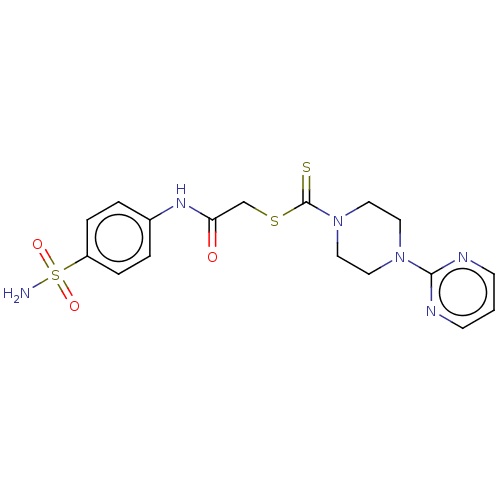 (CHEMBL4750069) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 41 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human erythrocyte CA1 b using 4-nitrophenyl acetate as substrate by Lineweaver-Burk plot analysis | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112392 BindingDB Entry DOI: 10.7270/Q2C2512X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 1 (Homo sapiens (Human)) | BDBM50552012 (CHEMBL4786423) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 49 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human erythrocyte CA1 b using 4-nitrophenyl acetate as substrate by Lineweaver-Burk plot analysis | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112392 BindingDB Entry DOI: 10.7270/Q2C2512X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50552014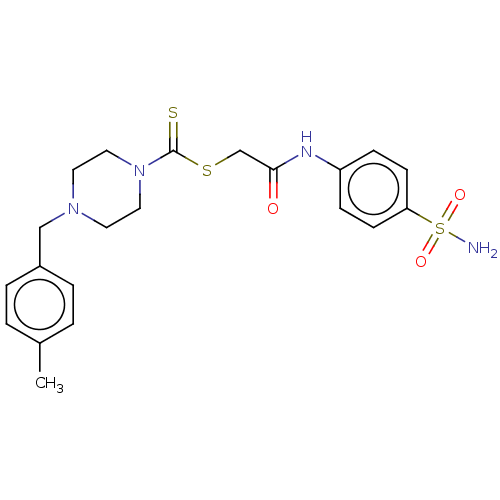 (CHEMBL4763412) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 87 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human erythrocyte CA2 using 4-nitrophenyl acetate as substrate by Lineweaver-Burk plot analysis | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112392 BindingDB Entry DOI: 10.7270/Q2C2512X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 1 (Homo sapiens (Human)) | BDBM50552018 (CHEMBL4764100) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 98 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human erythrocyte CA1 b using 4-nitrophenyl acetate as substrate by Lineweaver-Burk plot analysis | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112392 BindingDB Entry DOI: 10.7270/Q2C2512X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50143951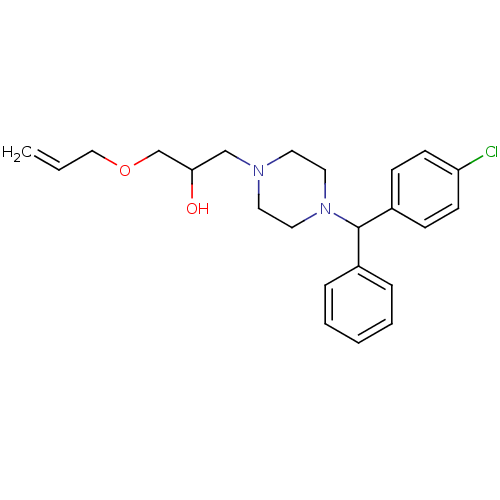 (1-Allyloxy-3-{4-[(4-chloro-phenyl)-phenyl-methyl]-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ArQule Inc Curated by ChEMBL | Assay Description Predictive competitive inhibition of cytochrome P450 2D6 was determined in silico | Bioorg Med Chem Lett 14: 2025-30 (2004) Article DOI: 10.1016/j.bmcl.2004.02.078 BindingDB Entry DOI: 10.7270/Q22J6B8R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50552008 (CHEMBL4750069) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 107 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human erythrocyte CA2 using 4-nitrophenyl acetate as substrate by Lineweaver-Burk plot analysis | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112392 BindingDB Entry DOI: 10.7270/Q2C2512X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 5A (Homo sapiens (Human)) | BDBM50585918 (CHEMBL5078567) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 107 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-5-CT from human 5-HT5A receptor incubated for 2 hr by radioligand binding assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c02031 BindingDB Entry DOI: 10.7270/Q2G73JM6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM10880 (AZA | AZA2 | AZM acetazolamide | Acerazolamide, AA...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank MMDB PDB Article PubMed | 135 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human erythrocyte CA2 using 4-nitrophenyl acetate as substrate by Lineweaver-Burk plot analysis | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112392 BindingDB Entry DOI: 10.7270/Q2C2512X | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Amine oxidase [flavin-containing] B (Homo sapiens (Human)) | BDBM50256449 (CHEMBL4068302) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Pharmacology, Faculty of Pharmacy, Anadolu University, 26470 Eski?ehir, Turkey. Curated by ChEMBL | Assay Description Non-competitive inhibition of recombinant human MAO-B expressed in baculovirus infected BTI insect cells using varying levels of tyramine as substrat... | Eur J Med Chem 131: 92-106 (2017) Article DOI: 10.1016/j.ejmech.2017.03.009 BindingDB Entry DOI: 10.7270/Q2MG7RZB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 1 (Homo sapiens (Human)) | BDBM50552009 (CHEMBL4747356) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 164 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human erythrocyte CA1 b using 4-nitrophenyl acetate as substrate by Lineweaver-Burk plot analysis | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112392 BindingDB Entry DOI: 10.7270/Q2C2512X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50552015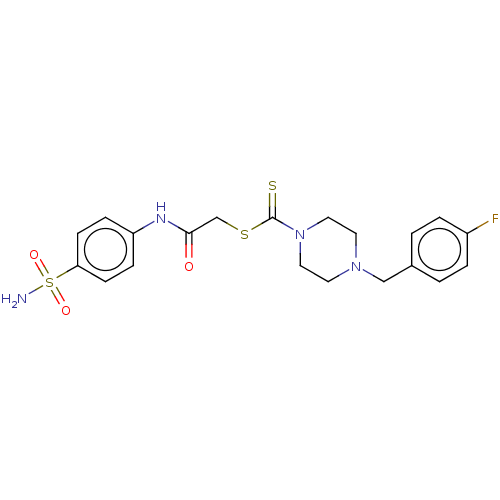 (CHEMBL4783836) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human erythrocyte CA2 using 4-nitrophenyl acetate as substrate by Lineweaver-Burk plot analysis | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112392 BindingDB Entry DOI: 10.7270/Q2C2512X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 5A (Homo sapiens (Human)) | BDBM50585915 (CHEMBL5082151) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 208 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-5-CT from human 5-HT5A receptor at 1 uM incubated for 2 hr by radioligand binding assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c02031 BindingDB Entry DOI: 10.7270/Q2G73JM6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 5A (Homo sapiens (Human)) | BDBM50585919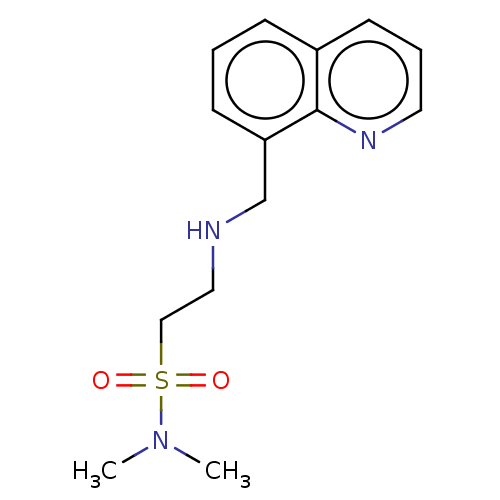 (CHEMBL5090584) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 229 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-5-CT from human 5-HT5A receptor incubated for 2 hr by radioligand binding assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c02031 BindingDB Entry DOI: 10.7270/Q2G73JM6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 5A (Homo sapiens (Human)) | BDBM50585920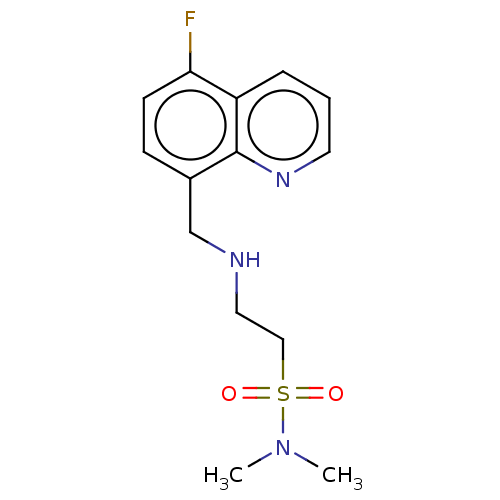 (CHEMBL5092524) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 271 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-5-CT from human 5-HT5A receptor incubated for 2 hr by radioligand binding assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c02031 BindingDB Entry DOI: 10.7270/Q2G73JM6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 1 (Homo sapiens (Human)) | BDBM50552014 (CHEMBL4763412) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human erythrocyte CA1 b using 4-nitrophenyl acetate as substrate by Lineweaver-Burk plot analysis | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112392 BindingDB Entry DOI: 10.7270/Q2C2512X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 1 (Homo sapiens (Human)) | BDBM10880 (AZA | AZA2 | AZM acetazolamide | Acerazolamide, AA...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | 289 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human erythrocyte CA1 b using 4-nitrophenyl acetate as substrate by Lineweaver-Burk plot analysis | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112392 BindingDB Entry DOI: 10.7270/Q2C2512X | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Carbonic anhydrase 1 (Homo sapiens (Human)) | BDBM50552015 (CHEMBL4783836) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 299 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human erythrocyte CA1 b using 4-nitrophenyl acetate as substrate by Lineweaver-Burk plot analysis | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112392 BindingDB Entry DOI: 10.7270/Q2C2512X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 6172 total ) | Next | Last >> |