 Found 288 hits with Last Name = 'kar' and Initial = 'nf'
Found 288 hits with Last Name = 'kar' and Initial = 'nf' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50146155
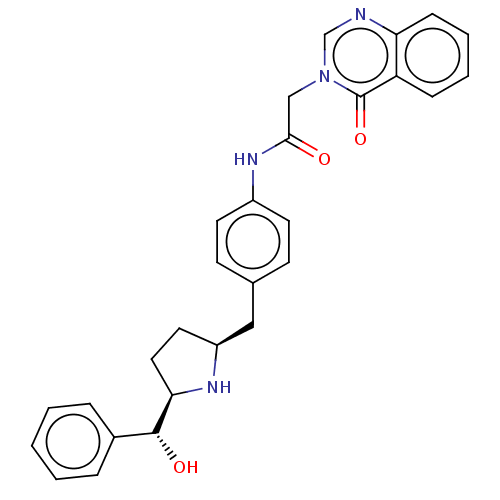
(CHEMBL3764774)Show SMILES [H][C@@]1(CC[C@@H](Cc2ccc(NC(=O)Cn3cnc4ccccc4c3=O)cc2)N1)[C@H](O)c1ccccc1 |r| Show InChI InChI=1S/C28H28N4O3/c33-26(17-32-18-29-24-9-5-4-8-23(24)28(32)35)31-21-12-10-19(11-13-21)16-22-14-15-25(30-22)27(34)20-6-2-1-3-7-20/h1-13,18,22,25,27,30,34H,14-17H2,(H,31,33)/t22-,25+,27+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human SERT expressed in HEK293 cells preincubated for 30 mins followed by fluorescent substrate addition measured after 30 mins by plat... |
J Med Chem 59: 609-23 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01372
BindingDB Entry DOI: 10.7270/Q2M047B3 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50092645
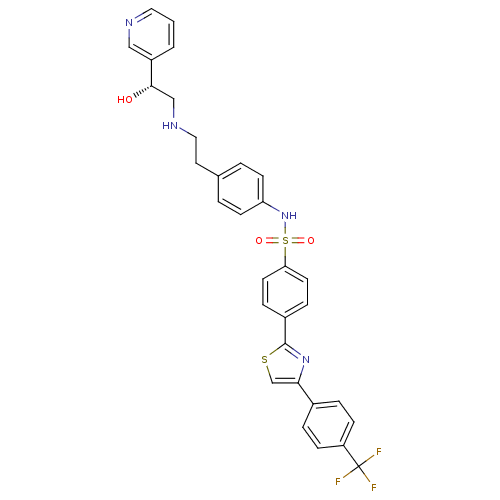
((R)-N-(4-(2-(2-hydroxy-2-(pyridin-3-yl)ethylamino)...)Show SMILES O[C@@H](CNCCc1ccc(NS(=O)(=O)c2ccc(cc2)-c2nc(cs2)-c2ccc(cc2)C(F)(F)F)cc1)c1cccnc1 Show InChI InChI=1S/C31H27F3N4O3S2/c32-31(33,34)25-9-5-22(6-10-25)28-20-42-30(37-28)23-7-13-27(14-8-23)43(40,41)38-26-11-3-21(4-12-26)15-17-36-19-29(39)24-2-1-16-35-18-24/h1-14,16,18,20,29,36,38-39H,15,17,19H2/t29-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 220 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity to human ERG channel |
J Med Chem 59: 609-23 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01372
BindingDB Entry DOI: 10.7270/Q2M047B3 |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50448787

(CHEMBL3128178)Show SMILES O[C@@H]([C@H]1CC[C@@H](Cc2ccc(NC(=O)[C@@H]3CCc4scnc34)cc2)N1)c1cccnc1 |r| Show InChI InChI=1S/C24H26N4O2S/c29-23(16-2-1-11-25-13-16)20-9-7-18(27-20)12-15-3-5-17(6-4-15)28-24(30)19-8-10-21-22(19)26-14-31-21/h1-6,11,13-14,18-20,23,27,29H,7-10,12H2,(H,28,30)/t18-,19+,20+,23+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 420 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human SERT expressed in HEK293 cells preincubated for 30 mins followed by fluorescent substrate addition measured after 30 mins by plat... |
J Med Chem 59: 609-23 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01372
BindingDB Entry DOI: 10.7270/Q2M047B3 |
More data for this
Ligand-Target Pair | |
Beta-2 adrenergic receptor
(Homo sapiens (Human)) | BDBM50092645

((R)-N-(4-(2-(2-hydroxy-2-(pyridin-3-yl)ethylamino)...)Show SMILES O[C@@H](CNCCc1ccc(NS(=O)(=O)c2ccc(cc2)-c2nc(cs2)-c2ccc(cc2)C(F)(F)F)cc1)c1cccnc1 Show InChI InChI=1S/C31H27F3N4O3S2/c32-31(33,34)25-9-5-22(6-10-25)28-20-42-30(37-28)23-7-13-27(14-8-23)43(40,41)38-26-11-3-21(4-12-26)15-17-36-19-29(39)24-2-1-16-35-18-24/h1-14,16,18,20,29,36,38-39H,15,17,19H2/t29-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 670 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [125]I-cyanopindolol from human recombinant beta2 adrenergic receptor after 1 hr by scintillation counting method |
J Med Chem 59: 609-23 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01372
BindingDB Entry DOI: 10.7270/Q2M047B3 |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50146157
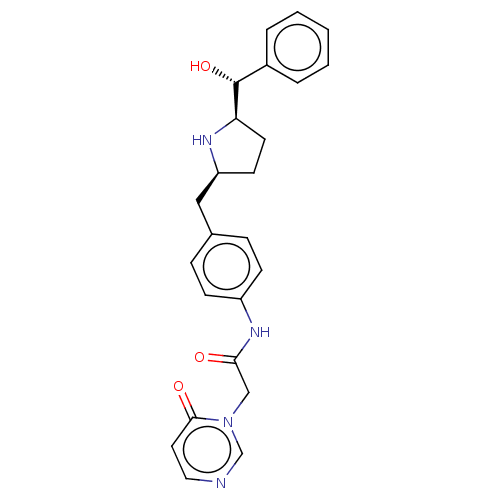
(CHEMBL3764950)Show SMILES [H][C@@]1(CC[C@@H](Cc2ccc(NC(=O)Cn3cnccc3=O)cc2)N1)[C@H](O)c1ccccc1 |r| Show InChI InChI=1S/C24H26N4O3/c29-22(15-28-16-25-13-12-23(28)30)27-19-8-6-17(7-9-19)14-20-10-11-21(26-20)24(31)18-4-2-1-3-5-18/h1-9,12-13,16,20-21,24,26,31H,10-11,14-15H2,(H,27,29)/t20-,21+,24+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 700 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human SERT expressed in HEK293 cells preincubated for 30 mins followed by fluorescent substrate addition measured after 30 mins by plat... |
J Med Chem 59: 609-23 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01372
BindingDB Entry DOI: 10.7270/Q2M047B3 |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50146161
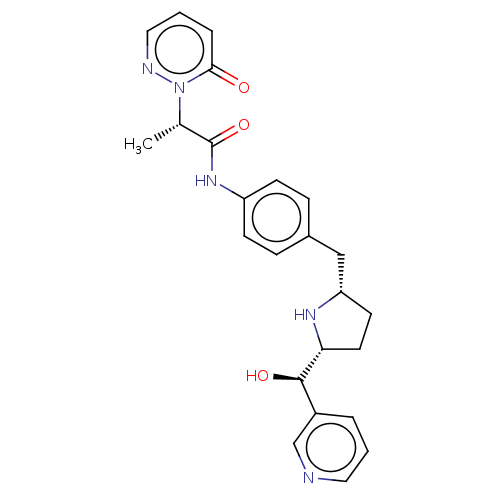
(CHEMBL3763998)Show SMILES [H][C@@]1(CC[C@@H](Cc2ccc(NC(=O)[C@H](C)n3ncccc3=O)cc2)N1)[C@H](O)c1cccnc1 |r| Show InChI InChI=1S/C24H27N5O3/c1-16(29-22(30)5-3-13-26-29)24(32)28-19-8-6-17(7-9-19)14-20-10-11-21(27-20)23(31)18-4-2-12-25-15-18/h2-9,12-13,15-16,20-21,23,27,31H,10-11,14H2,1H3,(H,28,32)/t16-,20-,21+,23+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 800 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human SERT expressed in HEK293 cells preincubated for 30 mins followed by fluorescent substrate addition measured after 30 mins by plat... |
J Med Chem 59: 609-23 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01372
BindingDB Entry DOI: 10.7270/Q2M047B3 |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50448789

(CHEMBL3128188)Show SMILES O[C@@H]([C@H]1CC[C@@H](Cc2ccc(NC(=O)[C@@H]3CCc4ccnn34)cc2)N1)c1cccnc1 |r| Show InChI InChI=1S/C24H27N5O2/c30-23(17-2-1-12-25-15-17)21-9-7-19(27-21)14-16-3-5-18(6-4-16)28-24(31)22-10-8-20-11-13-26-29(20)22/h1-6,11-13,15,19,21-23,27,30H,7-10,14H2,(H,28,31)/t19-,21+,22-,23+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 900 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human SERT expressed in HEK293 cells preincubated for 30 mins followed by fluorescent substrate addition measured after 30 mins by plat... |
J Med Chem 59: 609-23 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01372
BindingDB Entry DOI: 10.7270/Q2M047B3 |
More data for this
Ligand-Target Pair | |
Beta-1 adrenergic receptor
(Homo sapiens (Human)) | BDBM50092645

((R)-N-(4-(2-(2-hydroxy-2-(pyridin-3-yl)ethylamino)...)Show SMILES O[C@@H](CNCCc1ccc(NS(=O)(=O)c2ccc(cc2)-c2nc(cs2)-c2ccc(cc2)C(F)(F)F)cc1)c1cccnc1 Show InChI InChI=1S/C31H27F3N4O3S2/c32-31(33,34)25-9-5-22(6-10-25)28-20-42-30(37-28)23-7-13-27(14-8-23)43(40,41)38-26-11-3-21(4-12-26)15-17-36-19-29(39)24-2-1-16-35-18-24/h1-14,16,18,20,29,36,38-39H,15,17,19H2/t29-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [125]I-cyanopindolol from recombinant human beta1 adrenergic receptor after 1 hr by scintillation counting method |
J Med Chem 59: 609-23 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01372
BindingDB Entry DOI: 10.7270/Q2M047B3 |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50146159
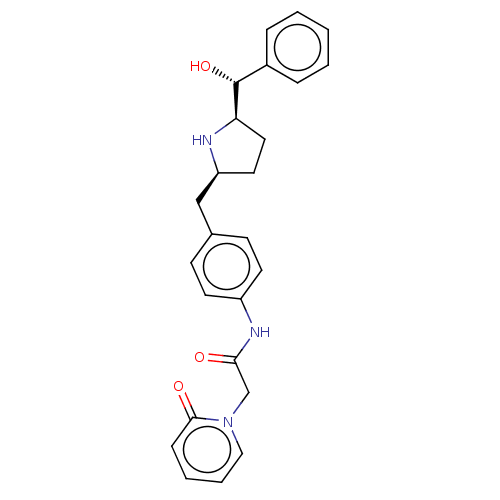
(CHEMBL3764592)Show SMILES [H][C@@]1(CC[C@@H](Cc2ccc(NC(=O)Cn3ccccc3=O)cc2)N1)[C@H](O)c1ccccc1 |r| Show InChI InChI=1S/C25H27N3O3/c29-23(17-28-15-5-4-8-24(28)30)27-20-11-9-18(10-12-20)16-21-13-14-22(26-21)25(31)19-6-2-1-3-7-19/h1-12,15,21-22,25-26,31H,13-14,16-17H2,(H,27,29)/t21-,22+,25+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human SERT expressed in HEK293 cells preincubated for 30 mins followed by fluorescent substrate addition measured after 30 mins by plat... |
J Med Chem 59: 609-23 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01372
BindingDB Entry DOI: 10.7270/Q2M047B3 |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50338821
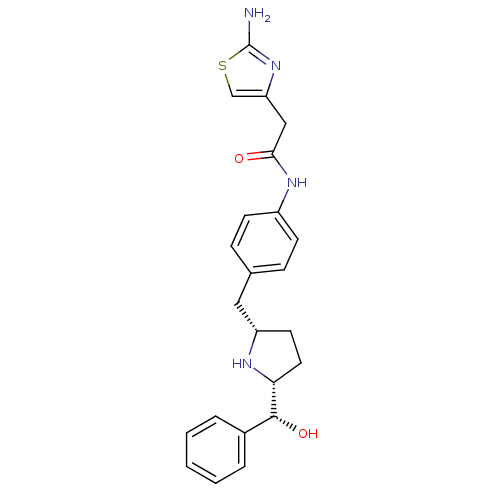
(2-(2-aminothiazol-4-yl)-N-(4-(((2S,5R)-5-((R)-hydr...)Show SMILES Nc1nc(CC(=O)Nc2ccc(C[C@@H]3CC[C@@H](N3)[C@H](O)c3ccccc3)cc2)cs1 |r| Show InChI InChI=1S/C23H26N4O2S/c24-23-27-19(14-30-23)13-21(28)26-17-8-6-15(7-9-17)12-18-10-11-20(25-18)22(29)16-4-2-1-3-5-16/h1-9,14,18,20,22,25,29H,10-13H2,(H2,24,27)(H,26,28)/t18-,20+,22+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human SERT expressed in HEK293 cells preincubated for 30 mins followed by fluorescent substrate addition measured after 30 mins by plat... |
J Med Chem 59: 609-23 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01372
BindingDB Entry DOI: 10.7270/Q2M047B3 |
More data for this
Ligand-Target Pair | |
Voltage-dependent L-type calcium channel subunit alpha-1C
(Homo sapiens (Human)) | BDBM50092645

((R)-N-(4-(2-(2-hydroxy-2-(pyridin-3-yl)ethylamino)...)Show SMILES O[C@@H](CNCCc1ccc(NS(=O)(=O)c2ccc(cc2)-c2nc(cs2)-c2ccc(cc2)C(F)(F)F)cc1)c1cccnc1 Show InChI InChI=1S/C31H27F3N4O3S2/c32-31(33,34)25-9-5-22(6-10-25)28-20-42-30(37-28)23-7-13-27(14-8-23)43(40,41)38-26-11-3-21(4-12-26)15-17-36-19-29(39)24-2-1-16-35-18-24/h1-14,16,18,20,29,36,38-39H,15,17,19H2/t29-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]-diltiazem from human Cav1.2 channel |
J Med Chem 59: 609-23 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01372
BindingDB Entry DOI: 10.7270/Q2M047B3 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50146155

(CHEMBL3764774)Show SMILES [H][C@@]1(CC[C@@H](Cc2ccc(NC(=O)Cn3cnc4ccccc4c3=O)cc2)N1)[C@H](O)c1ccccc1 |r| Show InChI InChI=1S/C28H28N4O3/c33-26(17-32-18-29-24-9-5-4-8-23(24)28(32)35)31-21-12-10-19(11-13-21)16-22-14-15-25(30-22)27(34)20-6-2-1-3-7-20/h1-13,18,22,25,27,30,34H,14-17H2,(H,31,33)/t22-,25+,27+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity to human ERG channel |
J Med Chem 59: 609-23 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01372
BindingDB Entry DOI: 10.7270/Q2M047B3 |
More data for this
Ligand-Target Pair | |
Beta-2 adrenergic receptor
(Homo sapiens (Human)) | BDBM50146155

(CHEMBL3764774)Show SMILES [H][C@@]1(CC[C@@H](Cc2ccc(NC(=O)Cn3cnc4ccccc4c3=O)cc2)N1)[C@H](O)c1ccccc1 |r| Show InChI InChI=1S/C28H28N4O3/c33-26(17-32-18-29-24-9-5-4-8-23(24)28(32)35)31-21-12-10-19(11-13-21)16-22-14-15-25(30-22)27(34)20-6-2-1-3-7-20/h1-13,18,22,25,27,30,34H,14-17H2,(H,31,33)/t22-,25+,27+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [125]I-cyanopindolol from human recombinant beta2 adrenergic receptor after 1 hr by scintillation counting method |
J Med Chem 59: 609-23 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01372
BindingDB Entry DOI: 10.7270/Q2M047B3 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50338821

(2-(2-aminothiazol-4-yl)-N-(4-(((2S,5R)-5-((R)-hydr...)Show SMILES Nc1nc(CC(=O)Nc2ccc(C[C@@H]3CC[C@@H](N3)[C@H](O)c3ccccc3)cc2)cs1 |r| Show InChI InChI=1S/C23H26N4O2S/c24-23-27-19(14-30-23)13-21(28)26-17-8-6-15(7-9-17)12-18-10-11-20(25-18)22(29)16-4-2-1-3-5-16/h1-9,14,18,20,22,25,29H,10-13H2,(H2,24,27)(H,26,28)/t18-,20+,22+/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 2.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of CYP2D6 in human liver microsomes assessed as dextraomethorphan O-demethylation |
J Med Chem 59: 609-23 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01372
BindingDB Entry DOI: 10.7270/Q2M047B3 |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50146158
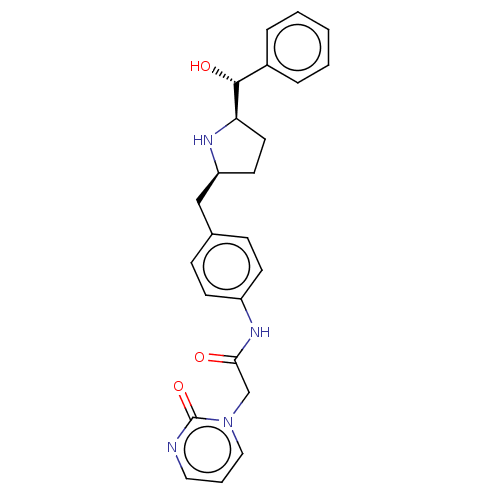
(CHEMBL3763594)Show SMILES [H][C@@]1(CC[C@@H](Cc2ccc(NC(=O)Cn3cccnc3=O)cc2)N1)[C@H](O)c1ccccc1 |r| Show InChI InChI=1S/C24H26N4O3/c29-22(16-28-14-4-13-25-24(28)31)27-19-9-7-17(8-10-19)15-20-11-12-21(26-20)23(30)18-5-2-1-3-6-18/h1-10,13-14,20-21,23,26,30H,11-12,15-16H2,(H,27,29)/t20-,21+,23+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human SERT expressed in HEK293 cells preincubated for 30 mins followed by fluorescent substrate addition measured after 30 mins by plat... |
J Med Chem 59: 609-23 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01372
BindingDB Entry DOI: 10.7270/Q2M047B3 |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50146156
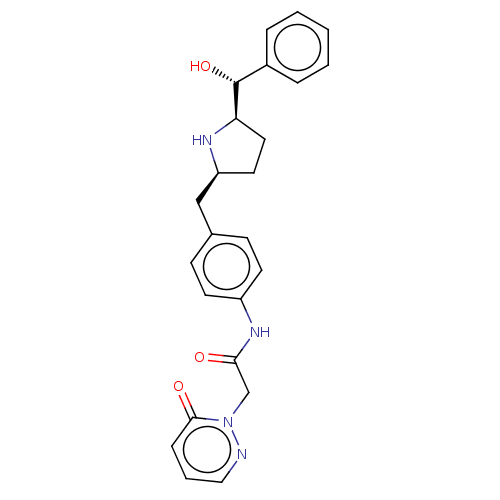
(CHEMBL3764088)Show SMILES [H][C@@]1(CC[C@@H](Cc2ccc(NC(=O)Cn3ncccc3=O)cc2)N1)[C@H](O)c1ccccc1 |r| Show InChI InChI=1S/C24H26N4O3/c29-22(16-28-23(30)7-4-14-25-28)27-19-10-8-17(9-11-19)15-20-12-13-21(26-20)24(31)18-5-2-1-3-6-18/h1-11,14,20-21,24,26,31H,12-13,15-16H2,(H,27,29)/t20-,21+,24+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human SERT expressed in HEK293 cells preincubated for 30 mins followed by fluorescent substrate addition measured after 30 mins by plat... |
J Med Chem 59: 609-23 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01372
BindingDB Entry DOI: 10.7270/Q2M047B3 |
More data for this
Ligand-Target Pair | |
Sodium-dependent dopamine transporter
(Homo sapiens (Human)) | BDBM50146162

(CHEMBL3763934)Show SMILES [H][C@@]1(CC[C@@H](Cc2ccc(NC(=O)[C@@H]3CCc4cccc(=O)n34)cc2)N1)[C@H](O)c1ccccc1 |r| Show InChI InChI=1S/C27H29N3O3/c31-25-8-4-7-22-14-16-24(30(22)25)27(33)29-20-11-9-18(10-12-20)17-21-13-15-23(28-21)26(32)19-5-2-1-3-6-19/h1-12,21,23-24,26,28,32H,13-17H2,(H,29,33)/t21-,23+,24-,26+/m0/s1 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human dopamine transporter |
J Med Chem 59: 609-23 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01372
BindingDB Entry DOI: 10.7270/Q2M047B3 |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50092645

((R)-N-(4-(2-(2-hydroxy-2-(pyridin-3-yl)ethylamino)...)Show SMILES O[C@@H](CNCCc1ccc(NS(=O)(=O)c2ccc(cc2)-c2nc(cs2)-c2ccc(cc2)C(F)(F)F)cc1)c1cccnc1 Show InChI InChI=1S/C31H27F3N4O3S2/c32-31(33,34)25-9-5-22(6-10-25)28-20-42-30(37-28)23-7-13-27(14-8-23)43(40,41)38-26-11-3-21(4-12-26)15-17-36-19-29(39)24-2-1-16-35-18-24/h1-14,16,18,20,29,36,38-39H,15,17,19H2/t29-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human SERT expressed in HEK293 cells preincubated for 30 mins followed by fluorescent substrate addition measured after 30 mins by plat... |
J Med Chem 59: 609-23 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01372
BindingDB Entry DOI: 10.7270/Q2M047B3 |
More data for this
Ligand-Target Pair | |
Beta-2 adrenergic receptor
(Homo sapiens (Human)) | BDBM50500472

(CHEMBL3746068)Show SMILES [H][C@@]1(CC[C@@H](Cc2ccc(cc2)C(=O)NCc2csc(N)n2)N1)[C@H](O)c1ccccc1 |r| Show InChI InChI=1S/C23H26N4O2S/c24-23-27-19(14-30-23)13-25-22(29)17-8-6-15(7-9-17)12-18-10-11-20(26-18)21(28)16-4-2-1-3-5-16/h1-9,14,18,20-21,26,28H,10-13H2,(H2,24,27)(H,25,29)/t18-,20+,21+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck and Co.
Curated by ChEMBL
| Assay Description
Displacement of [125I]CYP from human beta-2 adrenergic receptor expressed in CHO cell membrane after 1 hr by scintillation counting method |
Bioorg Med Chem Lett 26: 55-9 (2016)
Article DOI: 10.1016/j.bmcl.2015.11.030
BindingDB Entry DOI: 10.7270/Q29C71DN |
More data for this
Ligand-Target Pair | |
Beta-2 adrenergic receptor
(Homo sapiens (Human)) | BDBM50500475
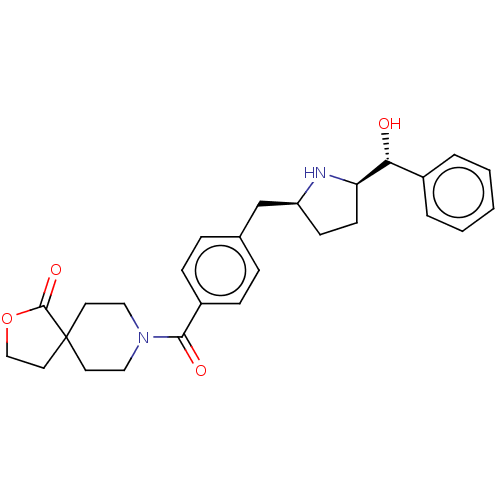
(CHEMBL3747244)Show SMILES [H][C@@]1(CC[C@@H](Cc2ccc(cc2)C(=O)N2CCC3(CCOC3=O)CC2)N1)[C@H](O)c1ccccc1 |r| Show InChI InChI=1S/C27H32N2O4/c30-24(20-4-2-1-3-5-20)23-11-10-22(28-23)18-19-6-8-21(9-7-19)25(31)29-15-12-27(13-16-29)14-17-33-26(27)32/h1-9,22-24,28,30H,10-18H2/t22-,23+,24+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck and Co.
Curated by ChEMBL
| Assay Description
Displacement of [125I]CYP from human beta-2 adrenergic receptor expressed in CHO cell membrane after 1 hr by scintillation counting method |
Bioorg Med Chem Lett 26: 55-9 (2016)
Article DOI: 10.1016/j.bmcl.2015.11.030
BindingDB Entry DOI: 10.7270/Q29C71DN |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50448787

(CHEMBL3128178)Show SMILES O[C@@H]([C@H]1CC[C@@H](Cc2ccc(NC(=O)[C@@H]3CCc4scnc34)cc2)N1)c1cccnc1 |r| Show InChI InChI=1S/C24H26N4O2S/c29-23(16-2-1-11-25-13-16)20-9-7-18(27-20)12-15-3-5-17(6-4-15)28-24(30)19-8-10-21-22(19)26-14-31-21/h1-6,11,13-14,18-20,23,27,29H,7-10,12H2,(H,28,30)/t18-,19+,20+,23+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity to human ERG channel |
J Med Chem 59: 609-23 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01372
BindingDB Entry DOI: 10.7270/Q2M047B3 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50500480

(CHEMBL3747546)Show SMILES [H][C@@]12CN(C[C@]1([H])[C@H]2C(=O)OCC)C(=O)c1ccc(C[C@@H]2CC[C@@]([H])(N2)[C@H](O)c2ccccc2)cc1 |r| Show InChI InChI=1S/C27H32N2O4/c1-2-33-27(32)24-21-15-29(16-22(21)24)26(31)19-10-8-17(9-11-19)14-20-12-13-23(28-20)25(30)18-6-4-3-5-7-18/h3-11,20-25,28,30H,2,12-16H2,1H3/t20-,21-,22+,23+,24+,25+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck and Co.
Curated by ChEMBL
| Assay Description
Inhibition of human ERG by IKr binding assay |
Bioorg Med Chem Lett 26: 55-9 (2016)
Article DOI: 10.1016/j.bmcl.2015.11.030
BindingDB Entry DOI: 10.7270/Q29C71DN |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50146162

(CHEMBL3763934)Show SMILES [H][C@@]1(CC[C@@H](Cc2ccc(NC(=O)[C@@H]3CCc4cccc(=O)n34)cc2)N1)[C@H](O)c1ccccc1 |r| Show InChI InChI=1S/C27H29N3O3/c31-25-8-4-7-22-14-16-24(30(22)25)27(33)29-20-11-9-18(10-12-20)17-21-13-15-23(28-21)26(32)19-5-2-1-3-6-19/h1-12,21,23-24,26,28,32H,13-17H2,(H,29,33)/t21-,23+,24-,26+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human SERT expressed in HEK293 cells preincubated for 30 mins followed by fluorescent substrate addition measured after 30 mins by plat... |
J Med Chem 59: 609-23 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01372
BindingDB Entry DOI: 10.7270/Q2M047B3 |
More data for this
Ligand-Target Pair | |
Voltage-dependent L-type calcium channel subunit alpha-1C
(Homo sapiens (Human)) | BDBM50338821

(2-(2-aminothiazol-4-yl)-N-(4-(((2S,5R)-5-((R)-hydr...)Show SMILES Nc1nc(CC(=O)Nc2ccc(C[C@@H]3CC[C@@H](N3)[C@H](O)c3ccccc3)cc2)cs1 |r| Show InChI InChI=1S/C23H26N4O2S/c24-23-27-19(14-30-23)13-21(28)26-17-8-6-15(7-9-17)12-18-10-11-20(25-18)22(29)16-4-2-1-3-5-16/h1-9,14,18,20,22,25,29H,10-13H2,(H2,24,27)(H,26,28)/t18-,20+,22+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 8.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]-diltiazem from human Cav1.2 channel |
J Med Chem 59: 609-23 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01372
BindingDB Entry DOI: 10.7270/Q2M047B3 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50500474
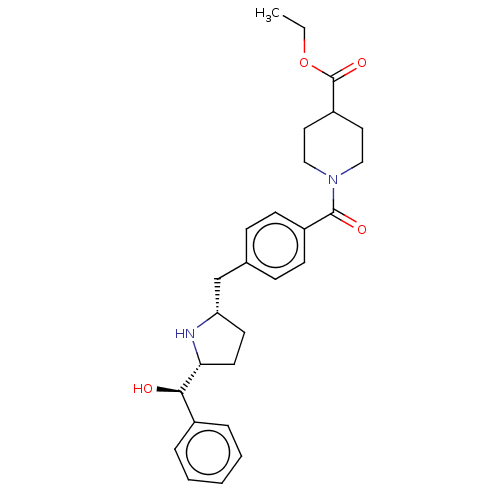
(CHEMBL3747487)Show SMILES [H][C@@]1(CC[C@@H](Cc2ccc(cc2)C(=O)N2CCC(CC2)C(=O)OCC)N1)[C@H](O)c1ccccc1 |r| Show InChI InChI=1S/C27H34N2O4/c1-2-33-27(32)22-14-16-29(17-15-22)26(31)21-10-8-19(9-11-21)18-23-12-13-24(28-23)25(30)20-6-4-3-5-7-20/h3-11,22-25,28,30H,2,12-18H2,1H3/t23-,24+,25+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 8.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck and Co.
Curated by ChEMBL
| Assay Description
Inhibition of human ERG by IKr binding assay |
Bioorg Med Chem Lett 26: 55-9 (2016)
Article DOI: 10.1016/j.bmcl.2015.11.030
BindingDB Entry DOI: 10.7270/Q29C71DN |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50235077

(CHEMBL4074920)Show SMILES [H][C@@]1(CC[C@@H](Cc2ccc(cc2)C(=O)N2CCN(Cc3cccc(n3)-c3nc(C)no3)CC2)N1)[C@H](O)c1ccccc1 |r| Show InChI InChI=1S/C32H36N6O3/c1-22-33-31(41-36-22)29-9-5-8-27(35-29)21-37-16-18-38(19-17-37)32(40)25-12-10-23(11-13-25)20-26-14-15-28(34-26)30(39)24-6-3-2-4-7-24/h2-13,26,28,30,34,39H,14-21H2,1H3/t26-,28+,30+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.79E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck& Co. Inc.
Curated by ChEMBL
| Assay Description
Inhibition of SERT (unknown origin) |
Bioorg Med Chem Lett 27: 1094-1098 (2017)
Article DOI: 10.1016/j.bmcl.2016.12.033
BindingDB Entry DOI: 10.7270/Q2QC05RV |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50092645

((R)-N-(4-(2-(2-hydroxy-2-(pyridin-3-yl)ethylamino)...)Show SMILES O[C@@H](CNCCc1ccc(NS(=O)(=O)c2ccc(cc2)-c2nc(cs2)-c2ccc(cc2)C(F)(F)F)cc1)c1cccnc1 Show InChI InChI=1S/C31H27F3N4O3S2/c32-31(33,34)25-9-5-22(6-10-25)28-20-42-30(37-28)23-7-13-27(14-8-23)43(40,41)38-26-11-3-21(4-12-26)15-17-36-19-29(39)24-2-1-16-35-18-24/h1-14,16,18,20,29,36,38-39H,15,17,19H2/t29-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 in human liver microsomes assessed as testosterone 6beta-hydroxylation |
J Med Chem 59: 609-23 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01372
BindingDB Entry DOI: 10.7270/Q2M047B3 |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50235078

(CHEMBL4092912)Show SMILES [H][C@@]1(CC[C@@H](Cc2ccc(cc2)C(=O)N2CCN(Cc3nnn(CC4CC4)n3)CC2)N1)[C@H](O)c1ccccc1 |r| Show InChI InChI=1S/C29H37N7O2/c37-28(23-4-2-1-3-5-23)26-13-12-25(30-26)18-21-8-10-24(11-9-21)29(38)35-16-14-34(15-17-35)20-27-31-33-36(32-27)19-22-6-7-22/h1-5,8-11,22,25-26,28,30,37H,6-7,12-20H2/t25-,26+,28+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck& Co. Inc.
Curated by ChEMBL
| Assay Description
Inhibition of SERT (unknown origin) |
Bioorg Med Chem Lett 27: 1094-1098 (2017)
Article DOI: 10.1016/j.bmcl.2016.12.033
BindingDB Entry DOI: 10.7270/Q2QC05RV |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50235076

(CHEMBL4073137)Show SMILES [H][C@@]1(CC[C@@H](Cc2ccc(cc2)C(=O)N2CCN(Cc3ccccn3)CC2)N1)[C@H](O)c1ccccc1 |r| Show InChI InChI=1S/C29H34N4O2/c34-28(23-6-2-1-3-7-23)27-14-13-25(31-27)20-22-9-11-24(12-10-22)29(35)33-18-16-32(17-19-33)21-26-8-4-5-15-30-26/h1-12,15,25,27-28,31,34H,13-14,16-21H2/t25-,27+,28+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck& Co. Inc.
Curated by ChEMBL
| Assay Description
Inhibition of SERT (unknown origin) |
Bioorg Med Chem Lett 27: 1094-1098 (2017)
Article DOI: 10.1016/j.bmcl.2016.12.033
BindingDB Entry DOI: 10.7270/Q2QC05RV |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50146160
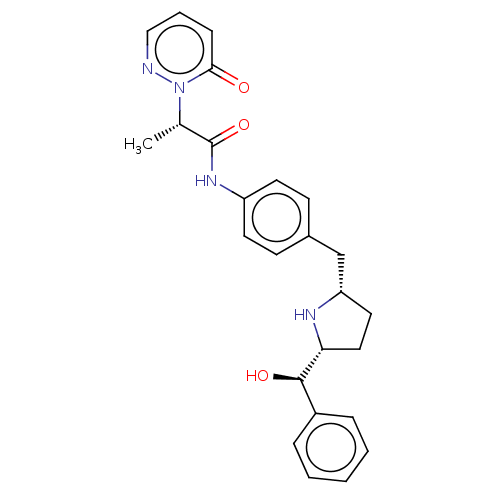
(CHEMBL3765335)Show SMILES [H][C@@]1(CC[C@@H](Cc2ccc(NC(=O)[C@H](C)n3ncccc3=O)cc2)N1)[C@H](O)c1ccccc1 |r| Show InChI InChI=1S/C25H28N4O3/c1-17(29-23(30)8-5-15-26-29)25(32)28-20-11-9-18(10-12-20)16-21-13-14-22(27-21)24(31)19-6-3-2-4-7-19/h2-12,15,17,21-22,24,27,31H,13-14,16H2,1H3,(H,28,32)/t17-,21-,22+,24+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human SERT expressed in HEK293 cells preincubated for 30 mins followed by fluorescent substrate addition measured after 30 mins by plat... |
J Med Chem 59: 609-23 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01372
BindingDB Entry DOI: 10.7270/Q2M047B3 |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50235122
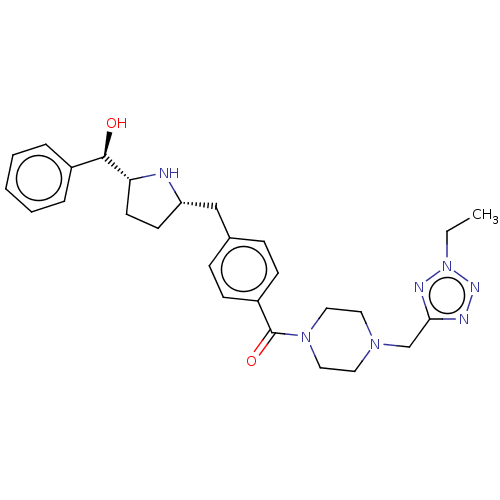
(CHEMBL4065251)Show SMILES [H][C@@]1(CC[C@@H](Cc2ccc(cc2)C(=O)N2CCN(Cc3nnn(CC)n3)CC2)N1)[C@H](O)c1ccccc1 |r| Show InChI InChI=1S/C27H35N7O2/c1-2-34-30-25(29-31-34)19-32-14-16-33(17-15-32)27(36)22-10-8-20(9-11-22)18-23-12-13-24(28-23)26(35)21-6-4-3-5-7-21/h3-11,23-24,26,28,35H,2,12-19H2,1H3/t23-,24+,26+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck& Co. Inc.
Curated by ChEMBL
| Assay Description
Inhibition of SERT (unknown origin) |
Bioorg Med Chem Lett 27: 1094-1098 (2017)
Article DOI: 10.1016/j.bmcl.2016.12.033
BindingDB Entry DOI: 10.7270/Q2QC05RV |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50235120

(CHEMBL4087670)Show SMILES [H][C@@]1(CC[C@@H](Cc2ccc(cc2)C(=O)N2CCN(Cc3ncn(CC(F)F)n3)CC2)N1)[C@H](O)c1ccccc1 |r| Show InChI InChI=1S/C28H34F2N6O2/c29-25(30)17-36-19-31-26(33-36)18-34-12-14-35(15-13-34)28(38)22-8-6-20(7-9-22)16-23-10-11-24(32-23)27(37)21-4-2-1-3-5-21/h1-9,19,23-25,27,32,37H,10-18H2/t23-,24+,27+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck& Co. Inc.
Curated by ChEMBL
| Assay Description
Inhibition of SERT (unknown origin) |
Bioorg Med Chem Lett 27: 1094-1098 (2017)
Article DOI: 10.1016/j.bmcl.2016.12.033
BindingDB Entry DOI: 10.7270/Q2QC05RV |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50235075

(CHEMBL4095361)Show SMILES [H][C@@]1(CC[C@@H](Cc2ccc(cc2)C(=O)N2CCN(Cc3cccc(n3)-c3nnc(C)o3)CC2)N1)[C@H](O)c1ccccc1 |r| Show InChI InChI=1S/C32H36N6O3/c1-22-35-36-31(41-22)29-9-5-8-27(34-29)21-37-16-18-38(19-17-37)32(40)25-12-10-23(11-13-25)20-26-14-15-28(33-26)30(39)24-6-3-2-4-7-24/h2-13,26,28,30,33,39H,14-21H2,1H3/t26-,28+,30+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck& Co. Inc.
Curated by ChEMBL
| Assay Description
Inhibition of SERT (unknown origin) |
Bioorg Med Chem Lett 27: 1094-1098 (2017)
Article DOI: 10.1016/j.bmcl.2016.12.033
BindingDB Entry DOI: 10.7270/Q2QC05RV |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50146154
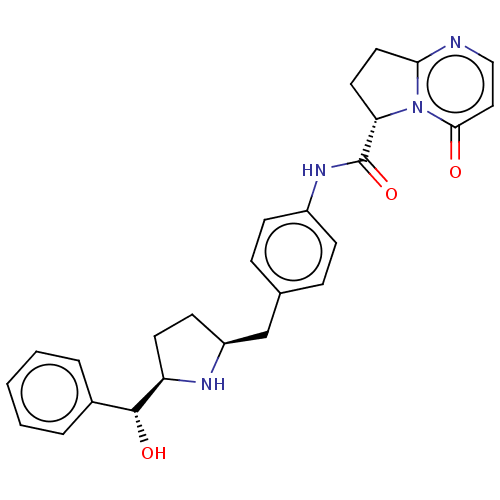
(KRP-114V | MK-4618 | Vibegron)Show SMILES [H][C@@](O)(c1ccccc1)[C@@]1([H])CC[C@@]([H])(Cc2ccc(NC(=O)[C@]3([H])CCc4nccc(=O)n34)cc2)N1 |r| Show InChI InChI=1S/C26H28N4O3/c31-24-14-15-27-23-13-12-22(30(23)24)26(33)29-19-8-6-17(7-9-19)16-20-10-11-21(28-20)25(32)18-4-2-1-3-5-18/h1-9,14-15,20-22,25,28,32H,10-13,16H2,(H,29,33)/t20-,21+,22-,25+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human SERT expressed in HEK293 cells preincubated for 30 mins followed by fluorescent substrate addition measured after 30 mins by plat... |
J Med Chem 59: 609-23 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01372
BindingDB Entry DOI: 10.7270/Q2M047B3 |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50235121

(CHEMBL4066314)Show SMILES [H][C@@]1(CC[C@@H](Cc2ccc(cc2)C(=O)N2CCN(Cc3ncn(CC(F)(F)F)n3)CC2)N1)[C@H](O)c1ccccc1 |r| Show InChI InChI=1S/C28H33F3N6O2/c29-28(30,31)18-37-19-32-25(34-37)17-35-12-14-36(15-13-35)27(39)22-8-6-20(7-9-22)16-23-10-11-24(33-23)26(38)21-4-2-1-3-5-21/h1-9,19,23-24,26,33,38H,10-18H2/t23-,24+,26+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck& Co. Inc.
Curated by ChEMBL
| Assay Description
Inhibition of SERT (unknown origin) |
Bioorg Med Chem Lett 27: 1094-1098 (2017)
Article DOI: 10.1016/j.bmcl.2016.12.033
BindingDB Entry DOI: 10.7270/Q2QC05RV |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50235119

(CHEMBL4085173)Show SMILES [H][C@@]1(CC[C@@H](Cc2ccc(cc2)C(=O)N2CCN(Cc3nnn(CC(F)(F)F)n3)CC2)N1)[C@H](O)c1ccccc1 |r| Show InChI InChI=1S/C27H32F3N7O2/c28-27(29,30)18-37-33-24(32-34-37)17-35-12-14-36(15-13-35)26(39)21-8-6-19(7-9-21)16-22-10-11-23(31-22)25(38)20-4-2-1-3-5-20/h1-9,22-23,25,31,38H,10-18H2/t22-,23+,25+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck& Co. Inc.
Curated by ChEMBL
| Assay Description
Inhibition of SERT (unknown origin) |
Bioorg Med Chem Lett 27: 1094-1098 (2017)
Article DOI: 10.1016/j.bmcl.2016.12.033
BindingDB Entry DOI: 10.7270/Q2QC05RV |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50235079

(CHEMBL4103112)Show SMILES [H][C@@]1(CC[C@@H](Cc2ccc(cc2)C(=O)N2CCN(Cc3nnn(CC(F)F)n3)CC2)N1)[C@H](O)c1ccccc1 |r| Show InChI InChI=1S/C27H33F2N7O2/c28-24(29)17-36-32-25(31-33-36)18-34-12-14-35(15-13-34)27(38)21-8-6-19(7-9-21)16-22-10-11-23(30-22)26(37)20-4-2-1-3-5-20/h1-9,22-24,26,30,37H,10-18H2/t22-,23+,26+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck& Co. Inc.
Curated by ChEMBL
| Assay Description
Inhibition of SERT (unknown origin) |
Bioorg Med Chem Lett 27: 1094-1098 (2017)
Article DOI: 10.1016/j.bmcl.2016.12.033
BindingDB Entry DOI: 10.7270/Q2QC05RV |
More data for this
Ligand-Target Pair | |
Beta-2 adrenergic receptor
(Homo sapiens (Human)) | BDBM50500474

(CHEMBL3747487)Show SMILES [H][C@@]1(CC[C@@H](Cc2ccc(cc2)C(=O)N2CCC(CC2)C(=O)OCC)N1)[C@H](O)c1ccccc1 |r| Show InChI InChI=1S/C27H34N2O4/c1-2-33-27(32)22-14-16-29(17-15-22)26(31)21-10-8-19(9-11-21)18-23-12-13-24(28-23)25(30)20-6-4-3-5-7-20/h3-11,22-25,28,30H,2,12-18H2,1H3/t23-,24+,25+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.09E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck and Co.
Curated by ChEMBL
| Assay Description
Displacement of [125I]CYP from human beta-2 adrenergic receptor expressed in CHO cell membrane after 1 hr by scintillation counting method |
Bioorg Med Chem Lett 26: 55-9 (2016)
Article DOI: 10.1016/j.bmcl.2015.11.030
BindingDB Entry DOI: 10.7270/Q29C71DN |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50092645

((R)-N-(4-(2-(2-hydroxy-2-(pyridin-3-yl)ethylamino)...)Show SMILES O[C@@H](CNCCc1ccc(NS(=O)(=O)c2ccc(cc2)-c2nc(cs2)-c2ccc(cc2)C(F)(F)F)cc1)c1cccnc1 Show InChI InChI=1S/C31H27F3N4O3S2/c32-31(33,34)25-9-5-22(6-10-25)28-20-42-30(37-28)23-7-13-27(14-8-23)43(40,41)38-26-11-3-21(4-12-26)15-17-36-19-29(39)24-2-1-16-35-18-24/h1-14,16,18,20,29,36,38-39H,15,17,19H2/t29-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of CYP2D6 in human liver microsomes assessed as dextraomethorphan O-demethylation |
J Med Chem 59: 609-23 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01372
BindingDB Entry DOI: 10.7270/Q2M047B3 |
More data for this
Ligand-Target Pair | |
Beta-2 adrenergic receptor
(Homo sapiens (Human)) | BDBM50500481
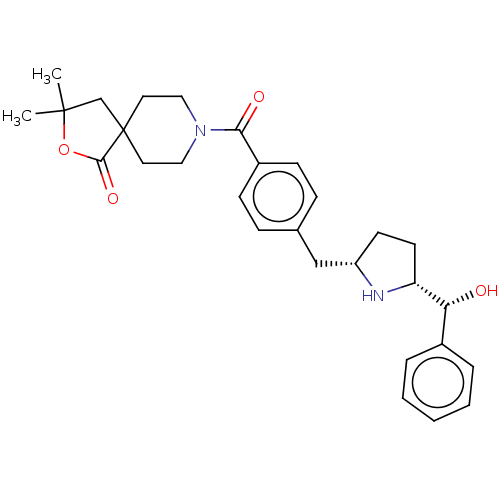
(CHEMBL3746280)Show SMILES [H][C@@]1(CC[C@@H](Cc2ccc(cc2)C(=O)N2CCC3(CC(C)(C)OC3=O)CC2)N1)[C@H](O)c1ccccc1 |r| Show InChI InChI=1S/C29H36N2O4/c1-28(2)19-29(27(34)35-28)14-16-31(17-15-29)26(33)22-10-8-20(9-11-22)18-23-12-13-24(30-23)25(32)21-6-4-3-5-7-21/h3-11,23-25,30,32H,12-19H2,1-2H3/t23-,24+,25+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck and Co.
Curated by ChEMBL
| Assay Description
Displacement of [125I]CYP from human beta-2 adrenergic receptor expressed in CHO cell membrane after 1 hr by scintillation counting method |
Bioorg Med Chem Lett 26: 55-9 (2016)
Article DOI: 10.1016/j.bmcl.2015.11.030
BindingDB Entry DOI: 10.7270/Q29C71DN |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50500471

(CHEMBL3746885)Show SMILES [H][C@@]12CN(C[C@]1([H])[C@@H]2C(=O)OCC)C(=O)c1ccc(C[C@@H]2CC[C@@]([H])(N2)[C@H](O)c2ccccc2)cc1 |r| Show InChI InChI=1S/C27H32N2O4/c1-2-33-27(32)24-21-15-29(16-22(21)24)26(31)19-10-8-17(9-11-19)14-20-12-13-23(28-20)25(30)18-6-4-3-5-7-18/h3-11,20-25,28,30H,2,12-16H2,1H3/t20-,21-,22+,23+,24-,25+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck and Co.
Curated by ChEMBL
| Assay Description
Inhibition of human ERG by IKr binding assay |
Bioorg Med Chem Lett 26: 55-9 (2016)
Article DOI: 10.1016/j.bmcl.2015.11.030
BindingDB Entry DOI: 10.7270/Q29C71DN |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50122844
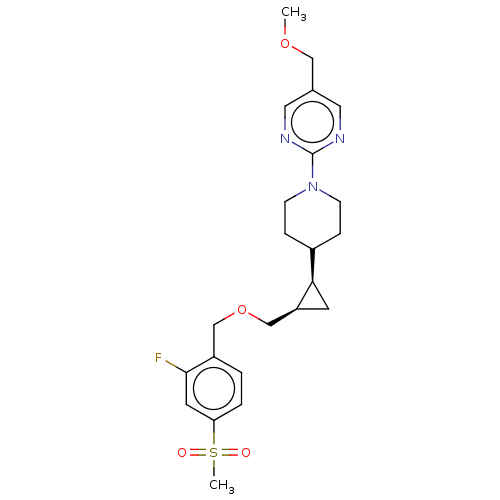
(CHEMBL3622175)Show SMILES COCc1cnc(nc1)N1CCC(CC1)[C@H]1C[C@H]1COCc1ccc(cc1F)S(C)(=O)=O |r| Show InChI InChI=1S/C23H30FN3O4S/c1-30-13-16-11-25-23(26-12-16)27-7-5-17(6-8-27)21-9-19(21)15-31-14-18-3-4-20(10-22(18)24)32(2,28)29/h3-4,10-12,17,19,21H,5-9,13-15H2,1-2H3/t19-,21+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human ERG by by patchXpress method |
ACS Med Chem Lett 6: 936-41 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00207
BindingDB Entry DOI: 10.7270/Q2BK1F5K |
More data for this
Ligand-Target Pair | |
Voltage-dependent L-type calcium channel subunit alpha-1C
(Homo sapiens (Human)) | BDBM50146155

(CHEMBL3764774)Show SMILES [H][C@@]1(CC[C@@H](Cc2ccc(NC(=O)Cn3cnc4ccccc4c3=O)cc2)N1)[C@H](O)c1ccccc1 |r| Show InChI InChI=1S/C28H28N4O3/c33-26(17-32-18-29-24-9-5-4-8-23(24)28(32)35)31-21-12-10-19(11-13-21)16-22-14-15-25(30-22)27(34)20-6-2-1-3-7-20/h1-13,18,22,25,27,30,34H,14-17H2,(H,31,33)/t22-,25+,27+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]-diltiazem from human Cav1.2 channel |
J Med Chem 59: 609-23 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01372
BindingDB Entry DOI: 10.7270/Q2M047B3 |
More data for this
Ligand-Target Pair | |
Beta-2 adrenergic receptor
(Homo sapiens (Human)) | BDBM50500480

(CHEMBL3747546)Show SMILES [H][C@@]12CN(C[C@]1([H])[C@H]2C(=O)OCC)C(=O)c1ccc(C[C@@H]2CC[C@@]([H])(N2)[C@H](O)c2ccccc2)cc1 |r| Show InChI InChI=1S/C27H32N2O4/c1-2-33-27(32)24-21-15-29(16-22(21)24)26(31)19-10-8-17(9-11-19)14-20-12-13-23(28-20)25(30)18-6-4-3-5-7-18/h3-11,20-25,28,30H,2,12-16H2,1H3/t20-,21-,22+,23+,24+,25+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.34E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck and Co.
Curated by ChEMBL
| Assay Description
Displacement of [125I]CYP from human beta-2 adrenergic receptor expressed in CHO cell membrane after 1 hr by scintillation counting method |
Bioorg Med Chem Lett 26: 55-9 (2016)
Article DOI: 10.1016/j.bmcl.2015.11.030
BindingDB Entry DOI: 10.7270/Q29C71DN |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50500478
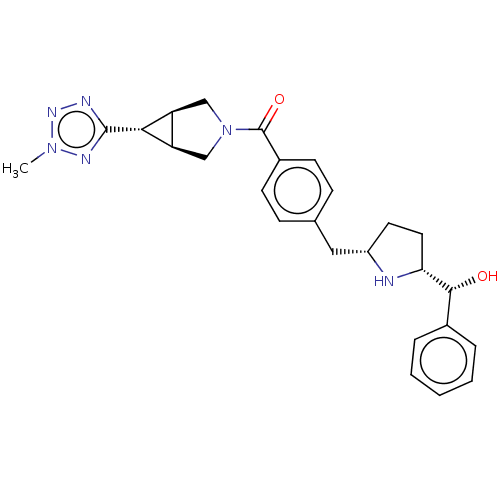
(CHEMBL3745890)Show SMILES [H][C@@]12CN(C[C@]1([H])[C@H]2c1nnn(C)n1)C(=O)c1ccc(C[C@@H]2CC[C@@]([H])(N2)[C@H](O)c2ccccc2)cc1 |r| Show InChI InChI=1S/C26H30N6O2/c1-31-29-25(28-30-31)23-20-14-32(15-21(20)23)26(34)18-9-7-16(8-10-18)13-19-11-12-22(27-19)24(33)17-5-3-2-4-6-17/h2-10,19-24,27,33H,11-15H2,1H3/t19-,20-,21+,22+,23+,24+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck and Co.
Curated by ChEMBL
| Assay Description
Inhibition of human ERG by IKr binding assay |
Bioorg Med Chem Lett 26: 55-9 (2016)
Article DOI: 10.1016/j.bmcl.2015.11.030
BindingDB Entry DOI: 10.7270/Q29C71DN |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50338821

(2-(2-aminothiazol-4-yl)-N-(4-(((2S,5R)-5-((R)-hydr...)Show SMILES Nc1nc(CC(=O)Nc2ccc(C[C@@H]3CC[C@@H](N3)[C@H](O)c3ccccc3)cc2)cs1 |r| Show InChI InChI=1S/C23H26N4O2S/c24-23-27-19(14-30-23)13-21(28)26-17-8-6-15(7-9-17)12-18-10-11-20(25-18)22(29)16-4-2-1-3-5-16/h1-9,14,18,20,22,25,29H,10-13H2,(H2,24,27)(H,26,28)/t18-,20+,22+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 1.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity to human ERG channel |
J Med Chem 59: 609-23 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01372
BindingDB Entry DOI: 10.7270/Q2M047B3 |
More data for this
Ligand-Target Pair | |
Beta-2 adrenergic receptor
(Homo sapiens (Human)) | BDBM50500479

(CHEMBL3747356)Show SMILES [H][C@@]12CN(C[C@]1([H])[C@H]2c1nnco1)C(=O)c1ccc(C[C@@H]2CC[C@@]([H])(N2)[C@H](O)c2ccccc2)cc1 |r| Show InChI InChI=1S/C26H28N4O3/c31-24(17-4-2-1-3-5-17)22-11-10-19(28-22)12-16-6-8-18(9-7-16)26(32)30-13-20-21(14-30)23(20)25-29-27-15-33-25/h1-9,15,19-24,28,31H,10-14H2/t19-,20-,21+,22+,23+,24+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck and Co.
Curated by ChEMBL
| Assay Description
Displacement of [125I]CYP from human beta-2 adrenergic receptor expressed in CHO cell membrane after 1 hr by scintillation counting method |
Bioorg Med Chem Lett 26: 55-9 (2016)
Article DOI: 10.1016/j.bmcl.2015.11.030
BindingDB Entry DOI: 10.7270/Q29C71DN |
More data for this
Ligand-Target Pair | |
Beta-2 adrenergic receptor
(Homo sapiens (Human)) | BDBM50500478

(CHEMBL3745890)Show SMILES [H][C@@]12CN(C[C@]1([H])[C@H]2c1nnn(C)n1)C(=O)c1ccc(C[C@@H]2CC[C@@]([H])(N2)[C@H](O)c2ccccc2)cc1 |r| Show InChI InChI=1S/C26H30N6O2/c1-31-29-25(28-30-31)23-20-14-32(15-21(20)23)26(34)18-9-7-16(8-10-18)13-19-11-12-22(27-19)24(33)17-5-3-2-4-6-17/h2-10,19-24,27,33H,11-15H2,1H3/t19-,20-,21+,22+,23+,24+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck and Co.
Curated by ChEMBL
| Assay Description
Displacement of [125I]CYP from human beta-2 adrenergic receptor expressed in CHO cell membrane after 1 hr by scintillation counting method |
Bioorg Med Chem Lett 26: 55-9 (2016)
Article DOI: 10.1016/j.bmcl.2015.11.030
BindingDB Entry DOI: 10.7270/Q29C71DN |
More data for this
Ligand-Target Pair | |
Beta-1 adrenergic receptor
(Homo sapiens (Human)) | BDBM50500479

(CHEMBL3747356)Show SMILES [H][C@@]12CN(C[C@]1([H])[C@H]2c1nnco1)C(=O)c1ccc(C[C@@H]2CC[C@@]([H])(N2)[C@H](O)c2ccccc2)cc1 |r| Show InChI InChI=1S/C26H28N4O3/c31-24(17-4-2-1-3-5-17)22-11-10-19(28-22)12-16-6-8-18(9-7-16)26(32)30-13-20-21(14-30)23(20)25-29-27-15-33-25/h1-9,15,19-24,28,31H,10-14H2/t19-,20-,21+,22+,23+,24+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck and Co.
Curated by ChEMBL
| Assay Description
Displacement of [125I]CYP from human beta-1 adrenergic receptor expressed in CHO cell membrane after 1 hr by scintillation counting method |
Bioorg Med Chem Lett 26: 55-9 (2016)
Article DOI: 10.1016/j.bmcl.2015.11.030
BindingDB Entry DOI: 10.7270/Q29C71DN |
More data for this
Ligand-Target Pair | |
Beta-1 adrenergic receptor
(Homo sapiens (Human)) | BDBM50500481

(CHEMBL3746280)Show SMILES [H][C@@]1(CC[C@@H](Cc2ccc(cc2)C(=O)N2CCC3(CC(C)(C)OC3=O)CC2)N1)[C@H](O)c1ccccc1 |r| Show InChI InChI=1S/C29H36N2O4/c1-28(2)19-29(27(34)35-28)14-16-31(17-15-29)26(33)22-10-8-20(9-11-22)18-23-12-13-24(30-23)25(32)21-6-4-3-5-7-21/h3-11,23-25,30,32H,12-19H2,1-2H3/t23-,24+,25+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck and Co.
Curated by ChEMBL
| Assay Description
Displacement of [125I]CYP from human beta-1 adrenergic receptor expressed in CHO cell membrane after 1 hr by scintillation counting method |
Bioorg Med Chem Lett 26: 55-9 (2016)
Article DOI: 10.1016/j.bmcl.2015.11.030
BindingDB Entry DOI: 10.7270/Q29C71DN |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data