 Found 274 hits with Last Name = 'keith' and Initial = 'd'
Found 274 hits with Last Name = 'keith' and Initial = 'd' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Bifunctional glutamate/proline--tRNA ligase
(Homo sapiens (Human)) | BDBM50097096

(2-(4-Bromo-phenyl)-6-chloro-8-methyl-quinoline-4-c...)Show InChI InChI=1S/C17H11BrClNO2/c1-9-6-12(19)7-13-14(17(21)22)8-15(20-16(9)13)10-2-4-11(18)5-3-10/h2-8H,1H3,(H,21,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 5.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cubist Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Binding affinity towards 5-hydroxytryptamine 3 receptor |
Bioorg Med Chem Lett 11: 541-4 (2001)
BindingDB Entry DOI: 10.7270/Q2Q23ZH8 |
More data for this
Ligand-Target Pair | |
Bifunctional glutamate/proline--tRNA ligase
(Homo sapiens (Human)) | BDBM50097096

(2-(4-Bromo-phenyl)-6-chloro-8-methyl-quinoline-4-c...)Show InChI InChI=1S/C17H11BrClNO2/c1-9-6-12(19)7-13-14(17(21)22)8-15(20-16(9)13)10-2-4-11(18)5-3-10/h2-8H,1H3,(H,21,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cubist Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Binding affinity towards 5-hydroxytryptamine 3 receptor |
Bioorg Med Chem Lett 11: 541-4 (2001)
BindingDB Entry DOI: 10.7270/Q2Q23ZH8 |
More data for this
Ligand-Target Pair | |
Glutamate carboxypeptidase 2
(Homo sapiens (Human)) | BDBM50265453
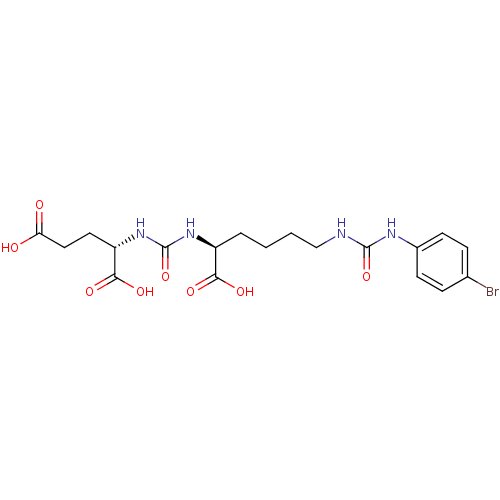
((S)-2-(3-((S)-1-Carboxy-5-(3-(4-bromophenyl)ureido...)Show SMILES OC(=O)CC[C@H](NC(=O)N[C@@H](CCCCNC(=O)Nc1ccc(Br)cc1)C(O)=O)C(O)=O |r| Show InChI InChI=1S/C19H25BrN4O8/c20-11-4-6-12(7-5-11)22-18(31)21-10-2-1-3-13(16(27)28)23-19(32)24-14(17(29)30)8-9-15(25)26/h4-7,13-14H,1-3,8-10H2,(H,25,26)(H,27,28)(H,29,30)(H2,21,22,31)(H2,23,24,32)/t13-,14-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Molecular Insight Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of [131I]DCIT from PSMA in human LNCAP cells |
J Med Chem 52: 347-57 (2009)
Article DOI: 10.1021/jm800994j
BindingDB Entry DOI: 10.7270/Q2V987ZZ |
More data for this
Ligand-Target Pair | |
Glutamate carboxypeptidase 2
(Homo sapiens (Human)) | BDBM50265381
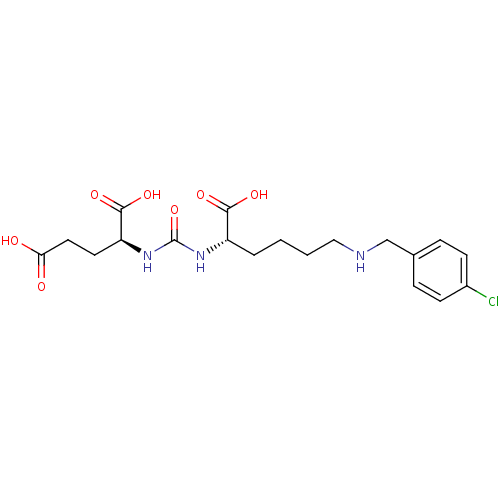
((S)-2-(3-((S)-1-Carboxy-5-(4-chlorobenzylamino)pen...)Show SMILES OC(=O)CC[C@H](NC(=O)N[C@@H](CCCCNCc1ccc(Cl)cc1)C(O)=O)C(O)=O |r| Show InChI InChI=1S/C19H26ClN3O7/c20-13-6-4-12(5-7-13)11-21-10-2-1-3-14(17(26)27)22-19(30)23-15(18(28)29)8-9-16(24)25/h4-7,14-15,21H,1-3,8-11H2,(H,24,25)(H,26,27)(H,28,29)(H2,22,23,30)/t14-,15-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Molecular Insight Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of [131I]DCIT from PSMA in human LNCAP cells |
J Med Chem 52: 347-57 (2009)
Article DOI: 10.1021/jm800994j
BindingDB Entry DOI: 10.7270/Q2V987ZZ |
More data for this
Ligand-Target Pair | |
Phenylalanine--tRNA ligase alpha subunit
(Streptococcus pyogenes serotype M18) | BDBM50140706

(5-(3,4-dichlorophenyl)-3-phenyl-(3R,3aR,6aS)-spiro...)Show SMILES Oc1c2C(OC3(C(=O)c4ccccc4C3=O)c2c(O)n1-c1ccc(Cl)c(Cl)c1)c1ccccc1 Show InChI InChI=1S/C26H15Cl2NO5/c27-17-11-10-14(12-18(17)28)29-24(32)19-20(25(29)33)26(34-21(19)13-6-2-1-3-7-13)22(30)15-8-4-5-9-16(15)23(26)31/h1-12,21,32-33H | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Cubist Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against human phenylalanyl-tRNA synthetase was determined |
Bioorg Med Chem Lett 14: 1339-42 (2004)
Article DOI: 10.1016/j.bmcl.2003.11.081
BindingDB Entry DOI: 10.7270/Q2PR7VDS |
More data for this
Ligand-Target Pair | |
Glutamate carboxypeptidase 2
(Homo sapiens (Human)) | BDBM50265471
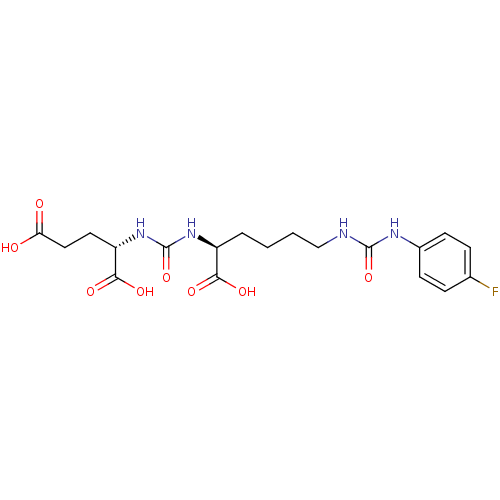
((S)-2-(3-((S)-1-Carboxy-5-(3-(4-fluorophenyl)ureid...)Show SMILES OC(=O)CC[C@H](NC(=O)N[C@@H](CCCCNC(=O)Nc1ccc(F)cc1)C(O)=O)C(O)=O |r| Show InChI InChI=1S/C19H25FN4O8/c20-11-4-6-12(7-5-11)22-18(31)21-10-2-1-3-13(16(27)28)23-19(32)24-14(17(29)30)8-9-15(25)26/h4-7,13-14H,1-3,8-10H2,(H,25,26)(H,27,28)(H,29,30)(H2,21,22,31)(H2,23,24,32)/t13-,14-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Molecular Insight Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of [131I]DCIT from PSMA in human LNCAP cells |
J Med Chem 52: 347-57 (2009)
Article DOI: 10.1021/jm800994j
BindingDB Entry DOI: 10.7270/Q2V987ZZ |
More data for this
Ligand-Target Pair | |
Glutamate carboxypeptidase 2
(Homo sapiens (Human)) | BDBM50265454
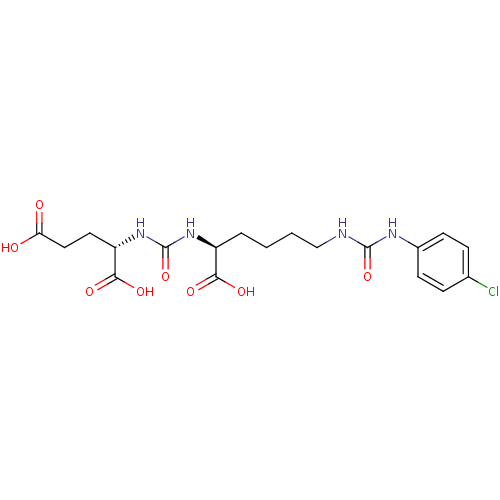
((S)-2-(3-((S)-1-Carboxy-5-(3-(4-chlorophenyl)ureid...)Show SMILES OC(=O)CC[C@H](NC(=O)N[C@@H](CCCCNC(=O)Nc1ccc(Cl)cc1)C(O)=O)C(O)=O |r| Show InChI InChI=1S/C19H25ClN4O8/c20-11-4-6-12(7-5-11)22-18(31)21-10-2-1-3-13(16(27)28)23-19(32)24-14(17(29)30)8-9-15(25)26/h4-7,13-14H,1-3,8-10H2,(H,25,26)(H,27,28)(H,29,30)(H2,21,22,31)(H2,23,24,32)/t13-,14-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Molecular Insight Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of [131I]DCIT from PSMA in human LNCAP cells |
J Med Chem 52: 347-57 (2009)
Article DOI: 10.1021/jm800994j
BindingDB Entry DOI: 10.7270/Q2V987ZZ |
More data for this
Ligand-Target Pair | |
Phenylalanine--tRNA ligase alpha subunit
(Streptococcus pyogenes serotype M18) | BDBM50140706

(5-(3,4-dichlorophenyl)-3-phenyl-(3R,3aR,6aS)-spiro...)Show SMILES Oc1c2C(OC3(C(=O)c4ccccc4C3=O)c2c(O)n1-c1ccc(Cl)c(Cl)c1)c1ccccc1 Show InChI InChI=1S/C26H15Cl2NO5/c27-17-11-10-14(12-18(17)28)29-24(32)19-20(25(29)33)26(34-21(19)13-6-2-1-3-7-13)22(30)15-8-4-5-9-16(15)23(26)31/h1-12,21,32-33H | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Cubist Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against phenylalanyl-tRNA synthetase from Enterococcus faecalis |
Bioorg Med Chem Lett 14: 1339-42 (2004)
Article DOI: 10.1016/j.bmcl.2003.11.081
BindingDB Entry DOI: 10.7270/Q2PR7VDS |
More data for this
Ligand-Target Pair | |
Proline--tRNA ligase, cytoplasmic
(Candida albicans) | BDBM50097096

(2-(4-Bromo-phenyl)-6-chloro-8-methyl-quinoline-4-c...)Show InChI InChI=1S/C17H11BrClNO2/c1-9-6-12(19)7-13-14(17(21)22)8-15(20-16(9)13)10-2-4-11(18)5-3-10/h2-8H,1H3,(H,21,22) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Cubist Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Aminoacylation activity against Candida albicans prolyl-tRNA synthetase |
Bioorg Med Chem Lett 11: 541-4 (2001)
BindingDB Entry DOI: 10.7270/Q2Q23ZH8 |
More data for this
Ligand-Target Pair | |
Glutamate carboxypeptidase 2
(Homo sapiens (Human)) | BDBM50265474

((S)-2-(3-((S)-1-Carboxy-5-(4-iodophenylsulfonamido...)Show SMILES OC(=O)CC[C@H](NC(=O)N[C@@H](CCCCNS(=O)(=O)c1ccc(I)cc1)C(O)=O)C(O)=O |r| Show InChI InChI=1S/C18H24IN3O9S/c19-11-4-6-12(7-5-11)32(30,31)20-10-2-1-3-13(16(25)26)21-18(29)22-14(17(27)28)8-9-15(23)24/h4-7,13-14,20H,1-3,8-10H2,(H,23,24)(H,25,26)(H,27,28)(H2,21,22,29)/t13-,14-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Molecular Insight Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of [131I]DCIT from PSMA in human LNCAP cells |
J Med Chem 52: 347-57 (2009)
Article DOI: 10.1021/jm800994j
BindingDB Entry DOI: 10.7270/Q2V987ZZ |
More data for this
Ligand-Target Pair | |
Glutamate carboxypeptidase 2
(Homo sapiens (Human)) | BDBM50265783

((S)-2-(3-((S)-1-Carboxy-5-(3-(4-iodophenyl)ureido)...)Show SMILES OC(=O)CC[C@H](NC(=O)N[C@@H](CCCCNC(=O)Nc1ccc(I)cc1)C(O)=O)C(O)=O |r| Show InChI InChI=1S/C19H25IN4O8/c20-11-4-6-12(7-5-11)22-18(31)21-10-2-1-3-13(16(27)28)23-19(32)24-14(17(29)30)8-9-15(25)26/h4-7,13-14H,1-3,8-10H2,(H,25,26)(H,27,28)(H,29,30)(H2,21,22,31)(H2,23,24,32)/t13-,14-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Molecular Insight Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of [131I]DCIT from PSMA in human LNCAP cells |
J Med Chem 52: 347-57 (2009)
Article DOI: 10.1021/jm800994j
BindingDB Entry DOI: 10.7270/Q2V987ZZ |
More data for this
Ligand-Target Pair | |
Glutamate carboxypeptidase 2
(Homo sapiens (Human)) | BDBM50265472

((S)-2-(3-((S)-1-Carboxy-5-(3-phenylureido)pentyl)u...)Show SMILES OC(=O)CC[C@H](NC(=O)N[C@@H](CCCCNC(=O)Nc1ccccc1)C(O)=O)C(O)=O |r| Show InChI InChI=1S/C19H26N4O8/c24-15(25)10-9-14(17(28)29)23-19(31)22-13(16(26)27)8-4-5-11-20-18(30)21-12-6-2-1-3-7-12/h1-3,6-7,13-14H,4-5,8-11H2,(H,24,25)(H,26,27)(H,28,29)(H2,20,21,30)(H2,22,23,31)/t13-,14-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Molecular Insight Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of [131I]DCIT from PSMA in human LNCAP cells |
J Med Chem 52: 347-57 (2009)
Article DOI: 10.1021/jm800994j
BindingDB Entry DOI: 10.7270/Q2V987ZZ |
More data for this
Ligand-Target Pair | |
Methionine--tRNA ligase, cytoplasmic
(Homo sapiens (Human)) | BDBM50143330
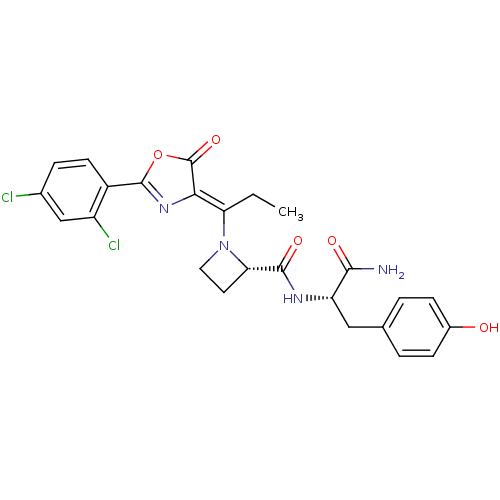
((S)-1-{1-[2-(2,4-Dichloro-phenyl)-5-oxo-oxazol-(4Z...)Show SMILES CC\C(N1CC[C@H]1C(=O)N[C@@H](Cc1ccc(O)cc1)C(N)=O)=C1\N=C(OC1=O)c1ccc(Cl)cc1Cl |c:25| Show InChI InChI=1S/C25H24Cl2N4O5/c1-2-19(21-25(35)36-24(30-21)16-8-5-14(26)12-17(16)27)31-10-9-20(31)23(34)29-18(22(28)33)11-13-3-6-15(32)7-4-13/h3-8,12,18,20,32H,2,9-11H2,1H3,(H2,28,33)(H,29,34)/b21-19-/t18-,20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
ArQule Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Staphylococcus aureus methionyl-tRNA synthetase (SaMetRS) |
Bioorg Med Chem Lett 14: 1909-11 (2004)
Article DOI: 10.1016/j.bmcl.2004.01.094
BindingDB Entry DOI: 10.7270/Q2NG4Q2F |
More data for this
Ligand-Target Pair | |
Glutamate carboxypeptidase 2
(Homo sapiens (Human)) | BDBM50265473
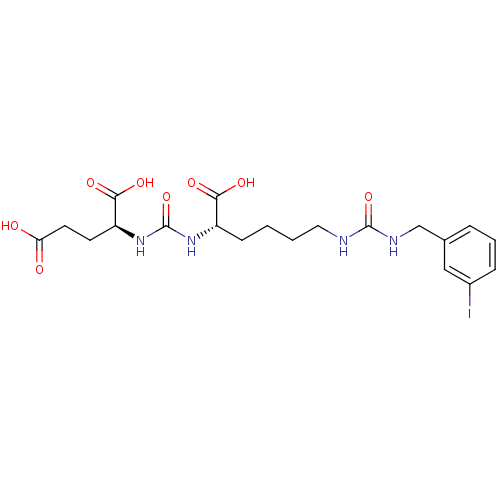
((9S,13S)-1-(3-iodophenyl)-3,11-dioxo-2,4,10,12-tet...)Show SMILES OC(=O)CC[C@H](NC(=O)N[C@@H](CCCCNC(=O)NCc1cccc(I)c1)C(O)=O)C(O)=O |r| Show InChI InChI=1S/C20H27IN4O8/c21-13-5-3-4-12(10-13)11-23-19(32)22-9-2-1-6-14(17(28)29)24-20(33)25-15(18(30)31)7-8-16(26)27/h3-5,10,14-15H,1-2,6-9,11H2,(H,26,27)(H,28,29)(H,30,31)(H2,22,23,32)(H2,24,25,33)/t14-,15-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Molecular Insight Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of [131I]DCIT from PSMA in human LNCAP cells |
J Med Chem 52: 347-57 (2009)
Article DOI: 10.1021/jm800994j
BindingDB Entry DOI: 10.7270/Q2V987ZZ |
More data for this
Ligand-Target Pair | |
Proline--tRNA ligase, cytoplasmic
(Candida albicans) | BDBM50097082

(2-(4-Bromo-phenyl)-6-iodo-quinoline-4-carboxylic a...)Show InChI InChI=1S/C16H9BrINO2/c17-10-3-1-9(2-4-10)15-8-13(16(20)21)12-7-11(18)5-6-14(12)19-15/h1-8H,(H,20,21) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Cubist Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Aminoacylation activity against Candida albicans prolyl-tRNA synthetase |
Bioorg Med Chem Lett 11: 541-4 (2001)
BindingDB Entry DOI: 10.7270/Q2Q23ZH8 |
More data for this
Ligand-Target Pair | |
Proline--tRNA ligase, cytoplasmic
(Candida albicans) | BDBM50097095

(2-(4-Bromo-phenyl)-6-chloro-8-methyl-quinoline-4-c...)Show SMILES Cc1cc(Cl)cc2c(cc(nc12)-c1ccc(Br)cc1)C(=O)OCc1ccc(Cl)c(Cl)c1 Show InChI InChI=1S/C24H15BrCl3NO2/c1-13-8-17(26)10-18-19(24(30)31-12-14-2-7-20(27)21(28)9-14)11-22(29-23(13)18)15-3-5-16(25)6-4-15/h2-11H,12H2,1H3 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Cubist Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Aminoacylation activity against Candida albicans prolyl-tRNA synthetase |
Bioorg Med Chem Lett 11: 541-4 (2001)
BindingDB Entry DOI: 10.7270/Q2Q23ZH8 |
More data for this
Ligand-Target Pair | |
Glutamate carboxypeptidase 2
(Homo sapiens (Human)) | BDBM50265377

((S)-2-(3-((S)-1-Carboxy-5-(4-iodobenzylamino)penty...)Show SMILES OC(=O)CC[C@H](NC(=O)N[C@@H](CCCCNCc1ccc(I)cc1)C(O)=O)C(O)=O |r| Show InChI InChI=1S/C19H26IN3O7/c20-13-6-4-12(5-7-13)11-21-10-2-1-3-14(17(26)27)22-19(30)23-15(18(28)29)8-9-16(24)25/h4-7,14-15,21H,1-3,8-11H2,(H,24,25)(H,26,27)(H,28,29)(H2,22,23,30)/t14-,15-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Molecular Insight Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of [131I]DCIT from PSMA in human LNCAP cells |
J Med Chem 52: 347-57 (2009)
Article DOI: 10.1021/jm800994j
BindingDB Entry DOI: 10.7270/Q2V987ZZ |
More data for this
Ligand-Target Pair | |
Proline--tRNA ligase, cytoplasmic
(Candida albicans) | BDBM50097105
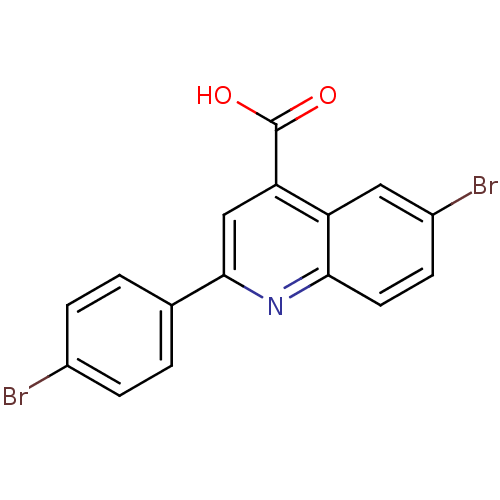
(6-Bromo-2-(4-bromo-phenyl)-quinoline-4-carboxylic ...)Show InChI InChI=1S/C16H9Br2NO2/c17-10-3-1-9(2-4-10)15-8-13(16(20)21)12-7-11(18)5-6-14(12)19-15/h1-8H,(H,20,21) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Cubist Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Aminoacylation activity against Candida albicans prolyl-tRNA synthetase |
Bioorg Med Chem Lett 11: 541-4 (2001)
BindingDB Entry DOI: 10.7270/Q2Q23ZH8 |
More data for this
Ligand-Target Pair | |
Proline--tRNA ligase, cytoplasmic
(Candida albicans) | BDBM50097106

(2-(4-Bromo-phenyl)-8-chloro-6-methyl-quinoline-4-c...)Show InChI InChI=1S/C17H11BrClNO2/c1-9-6-12-13(17(21)22)8-15(20-16(12)14(19)7-9)10-2-4-11(18)5-3-10/h2-8H,1H3,(H,21,22) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Cubist Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Aminoacylation activity against Candida albicans prolyl-tRNA synthetase |
Bioorg Med Chem Lett 11: 541-4 (2001)
BindingDB Entry DOI: 10.7270/Q2Q23ZH8 |
More data for this
Ligand-Target Pair | |
Proline--tRNA ligase, cytoplasmic
(Candida albicans) | BDBM50097090
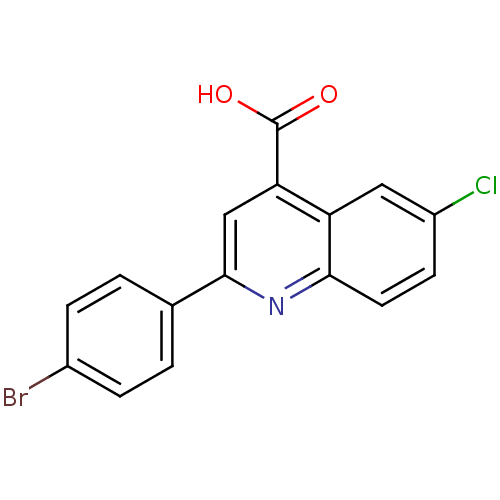
(2-(4-Bromo-phenyl)-6-chloro-quinoline-4-carboxylic...)Show InChI InChI=1S/C16H9BrClNO2/c17-10-3-1-9(2-4-10)15-8-13(16(20)21)12-7-11(18)5-6-14(12)19-15/h1-8H,(H,20,21) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
Cubist Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Aminoacylation activity against Candida albicans prolyl-tRNA synthetase |
Bioorg Med Chem Lett 11: 541-4 (2001)
BindingDB Entry DOI: 10.7270/Q2Q23ZH8 |
More data for this
Ligand-Target Pair | |
Proline--tRNA ligase, cytoplasmic
(Candida albicans) | BDBM50097086
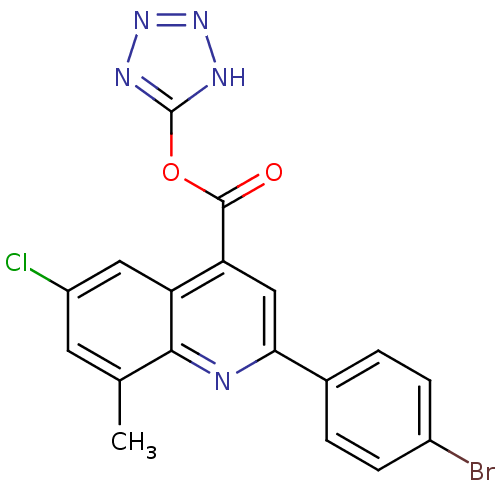
(2-(4-Bromo-phenyl)-6-chloro-8-methyl-quinoline-4-c...)Show SMILES Cc1cc(Cl)cc2c(cc(nc12)-c1ccc(Br)cc1)C(=O)Oc1nnn[nH]1 Show InChI InChI=1S/C18H11BrClN5O2/c1-9-6-12(20)7-13-14(17(26)27-18-22-24-25-23-18)8-15(21-16(9)13)10-2-4-11(19)5-3-10/h2-8H,1H3,(H,22,23,24,25) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Cubist Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Aminoacylation activity against Candida albicans prolyl-tRNA synthetase |
Bioorg Med Chem Lett 11: 541-4 (2001)
BindingDB Entry DOI: 10.7270/Q2Q23ZH8 |
More data for this
Ligand-Target Pair | |
Glutamate carboxypeptidase 2
(Homo sapiens (Human)) | BDBM50265378
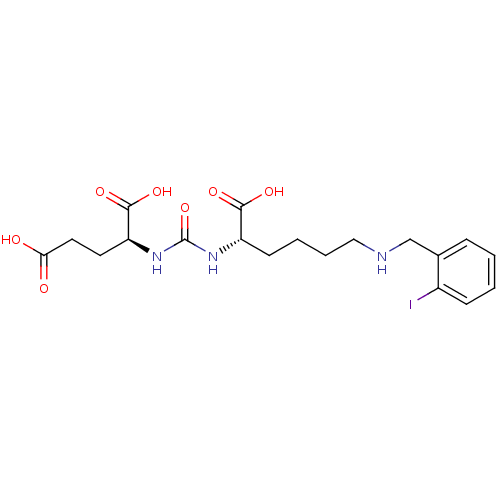
((S)-2-(3-((S)-1-Carboxy-5-(2-iodobenzylamino)penty...)Show SMILES OC(=O)CC[C@H](NC(=O)N[C@@H](CCCCNCc1ccccc1I)C(O)=O)C(O)=O |r| Show InChI InChI=1S/C19H26IN3O7/c20-13-6-2-1-5-12(13)11-21-10-4-3-7-14(17(26)27)22-19(30)23-15(18(28)29)8-9-16(24)25/h1-2,5-6,14-15,21H,3-4,7-11H2,(H,24,25)(H,26,27)(H,28,29)(H2,22,23,30)/t14-,15-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 37 | n/a | n/a | n/a | n/a | n/a | n/a |
Molecular Insight Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of [131I]DCIT from PSMA in human LNCAP cells |
J Med Chem 52: 347-57 (2009)
Article DOI: 10.1021/jm800994j
BindingDB Entry DOI: 10.7270/Q2V987ZZ |
More data for this
Ligand-Target Pair | |
Methionine--tRNA ligase, cytoplasmic
(Homo sapiens (Human)) | BDBM50143333
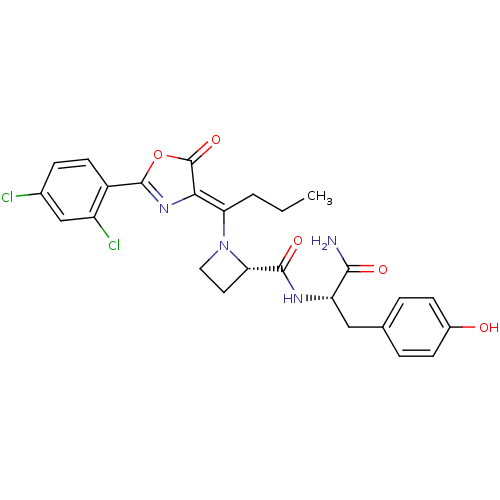
((S)-1-{1-[2-(2,4-Dichloro-phenyl)-5-oxo-oxazol-(4Z...)Show SMILES CCC\C(N1CC[C@H]1C(=O)N[C@@H](Cc1ccc(O)cc1)C(N)=O)=C1\N=C(OC1=O)c1ccc(Cl)cc1Cl |c:26| Show InChI InChI=1S/C26H26Cl2N4O5/c1-2-3-20(22-26(36)37-25(31-22)17-9-6-15(27)13-18(17)28)32-11-10-21(32)24(35)30-19(23(29)34)12-14-4-7-16(33)8-5-14/h4-9,13,19,21,33H,2-3,10-12H2,1H3,(H2,29,34)(H,30,35)/b22-20-/t19-,21-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
ArQule Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Staphylococcus aureus methionyl-tRNA synthetase (SaMetRS) |
Bioorg Med Chem Lett 14: 1909-11 (2004)
Article DOI: 10.1016/j.bmcl.2004.01.094
BindingDB Entry DOI: 10.7270/Q2NG4Q2F |
More data for this
Ligand-Target Pair | |
Phenylalanine--tRNA ligase alpha subunit
(Streptococcus pyogenes serotype M18) | BDBM50140709
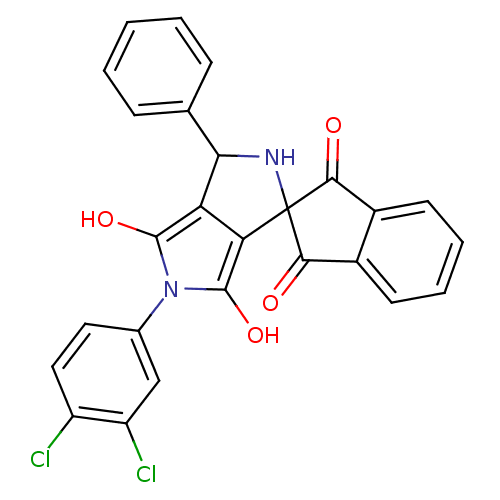
(5'-(3,4-dichlorophenyl)-3'-phenyl-(3'R,3a'S,6a'R)-...)Show SMILES Oc1c2C(NC3(C(=O)c4ccccc4C3=O)c2c(O)n1-c1ccc(Cl)c(Cl)c1)c1ccccc1 Show InChI InChI=1S/C26H16Cl2N2O4/c27-17-11-10-14(12-18(17)28)30-24(33)19-20(25(30)34)26(29-21(19)13-6-2-1-3-7-13)22(31)15-8-4-5-9-16(15)23(26)32/h1-12,21,29,33-34H | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Cubist Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against phenylalanyl-tRNA synthetase from Enterococcus faecalis |
Bioorg Med Chem Lett 14: 1339-42 (2004)
Article DOI: 10.1016/j.bmcl.2003.11.081
BindingDB Entry DOI: 10.7270/Q2PR7VDS |
More data for this
Ligand-Target Pair | |
Phenylalanine--tRNA ligase alpha subunit
(Streptococcus pyogenes serotype M18) | BDBM50140709

(5'-(3,4-dichlorophenyl)-3'-phenyl-(3'R,3a'S,6a'R)-...)Show SMILES Oc1c2C(NC3(C(=O)c4ccccc4C3=O)c2c(O)n1-c1ccc(Cl)c(Cl)c1)c1ccccc1 Show InChI InChI=1S/C26H16Cl2N2O4/c27-17-11-10-14(12-18(17)28)30-24(33)19-20(25(30)34)26(29-21(19)13-6-2-1-3-7-13)22(31)15-8-4-5-9-16(15)23(26)32/h1-12,21,29,33-34H | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Cubist Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against phenylalanyl-tRNA synthetase from Staphylococcus aureus |
Bioorg Med Chem Lett 14: 1339-42 (2004)
Article DOI: 10.1016/j.bmcl.2003.11.081
BindingDB Entry DOI: 10.7270/Q2PR7VDS |
More data for this
Ligand-Target Pair | |
Glutamate carboxypeptidase 2
(Homo sapiens (Human)) | BDBM50265380
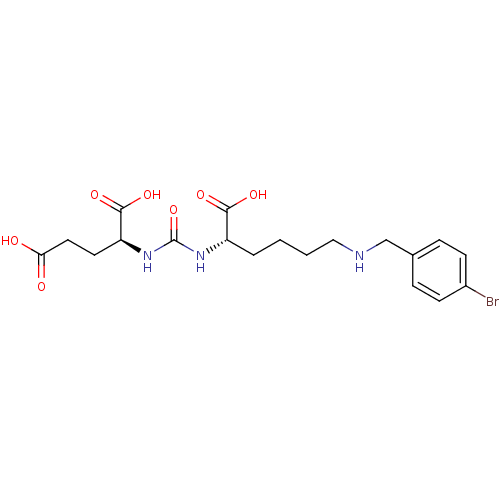
((S)-2-(3-((S)-1-Carboxy-5-(4-bromobenzylamino)pent...)Show SMILES OC(=O)CC[C@H](NC(=O)N[C@@H](CCCCNCc1ccc(Br)cc1)C(O)=O)C(O)=O |r| Show InChI InChI=1S/C19H26BrN3O7/c20-13-6-4-12(5-7-13)11-21-10-2-1-3-14(17(26)27)22-19(30)23-15(18(28)29)8-9-16(24)25/h4-7,14-15,21H,1-3,8-11H2,(H,24,25)(H,26,27)(H,28,29)(H2,22,23,30)/t14-,15-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 43 | n/a | n/a | n/a | n/a | n/a | n/a |
Molecular Insight Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of [131I]DCIT from PSMA in human LNCAP cells |
J Med Chem 52: 347-57 (2009)
Article DOI: 10.1021/jm800994j
BindingDB Entry DOI: 10.7270/Q2V987ZZ |
More data for this
Ligand-Target Pair | |
Proline--tRNA ligase, cytoplasmic
(Candida albicans) | BDBM50097113
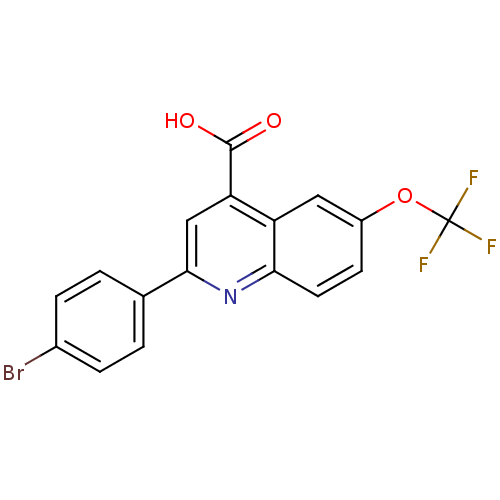
(2-(4-Bromo-phenyl)-6-trifluoromethoxy-quinoline-4-...)Show SMILES OC(=O)c1cc(nc2ccc(OC(F)(F)F)cc12)-c1ccc(Br)cc1 Show InChI InChI=1S/C17H9BrF3NO3/c18-10-3-1-9(2-4-10)15-8-13(16(23)24)12-7-11(25-17(19,20)21)5-6-14(12)22-15/h1-8H,(H,23,24) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 53 | n/a | n/a | n/a | n/a | n/a | n/a |
Cubist Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Aminoacylation activity against Candida albicans prolyl-tRNA synthetase |
Bioorg Med Chem Lett 11: 541-4 (2001)
BindingDB Entry DOI: 10.7270/Q2Q23ZH8 |
More data for this
Ligand-Target Pair | |
Methionine--tRNA ligase, cytoplasmic
(Homo sapiens (Human)) | BDBM50143329
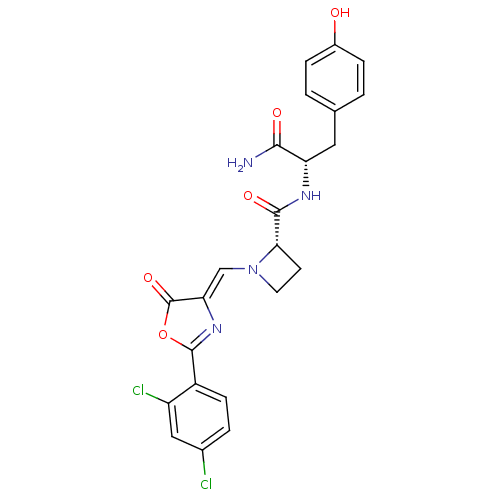
((S)-1-[2-(2,4-Dichloro-phenyl)-5-oxo-oxazol-(4Z)-y...)Show SMILES NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@@H]1CCN1\C=C1/N=C(OC1=O)c1ccc(Cl)cc1Cl |r,c:23| Show InChI InChI=1S/C23H20Cl2N4O5/c24-13-3-6-15(16(25)10-13)22-28-18(23(33)34-22)11-29-8-7-19(29)21(32)27-17(20(26)31)9-12-1-4-14(30)5-2-12/h1-6,10-11,17,19,30H,7-9H2,(H2,26,31)(H,27,32)/b18-11-/t17-,19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
ArQule Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human methionyl-tRNA synthetase (fMetRS) |
Bioorg Med Chem Lett 14: 1909-11 (2004)
Article DOI: 10.1016/j.bmcl.2004.01.094
BindingDB Entry DOI: 10.7270/Q2NG4Q2F |
More data for this
Ligand-Target Pair | |
Phenylalanine--tRNA ligase alpha subunit
(Streptococcus pyogenes serotype M18) | BDBM50140707
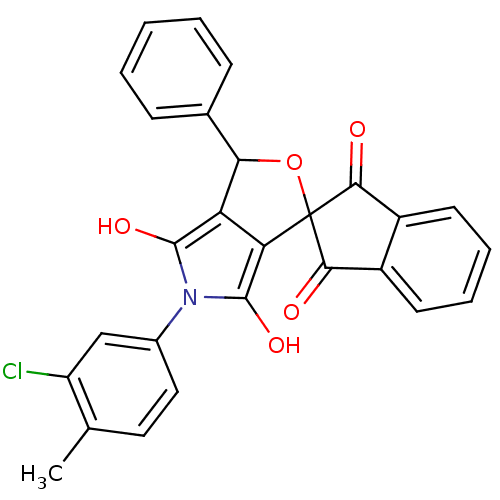
(5-(3-chloro-4-methylphenyl)-3-phenyl-(3R,3aS,6aR)-...)Show SMILES Cc1ccc(cc1Cl)-n1c(O)c2C(OC3(C(=O)c4ccccc4C3=O)c2c1O)c1ccccc1 Show InChI InChI=1S/C27H18ClNO5/c1-14-11-12-16(13-19(14)28)29-25(32)20-21(26(29)33)27(34-22(20)15-7-3-2-4-8-15)23(30)17-9-5-6-10-18(17)24(27)31/h2-13,22,32-33H,1H3 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
Cubist Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against phenylalanyl-tRNA synthetase from Staphylococcus aureus |
Bioorg Med Chem Lett 14: 1339-42 (2004)
Article DOI: 10.1016/j.bmcl.2003.11.081
BindingDB Entry DOI: 10.7270/Q2PR7VDS |
More data for this
Ligand-Target Pair | |
Proline--tRNA ligase, cytoplasmic
(Candida albicans) | BDBM50097102

(2-(4-Bromo-phenyl)-6-chloro-8-methyl-quinoline-4-c...)Show SMILES Cc1cc(Cl)cc2c(cc(nc12)-c1ccc(Br)cc1)C(=O)OCc1ccco1 Show InChI InChI=1S/C22H15BrClNO3/c1-13-9-16(24)10-18-19(22(26)28-12-17-3-2-8-27-17)11-20(25-21(13)18)14-4-6-15(23)7-5-14/h2-11H,12H2,1H3 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
Cubist Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Aminoacylation activity against Candida albicans prolyl-tRNA synthetase |
Bioorg Med Chem Lett 11: 541-4 (2001)
BindingDB Entry DOI: 10.7270/Q2Q23ZH8 |
More data for this
Ligand-Target Pair | |
Methionine--tRNA ligase, cytoplasmic
(Homo sapiens (Human)) | BDBM50143334
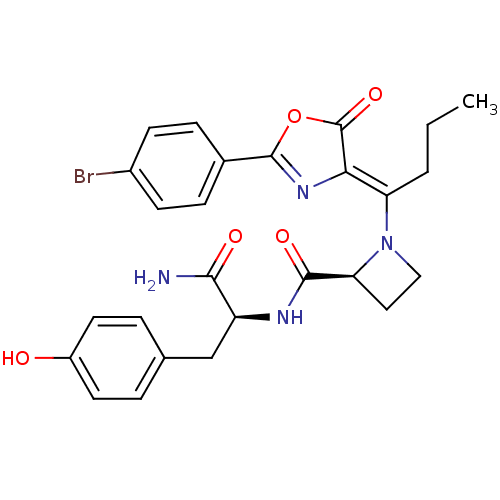
((S)-1-{1-[2-(4-Bromo-phenyl)-5-oxo-oxazol-(4Z)-yli...)Show SMILES CCC\C(N1CC[C@H]1C(=O)N[C@@H](Cc1ccc(O)cc1)C(N)=O)=C1\N=C(OC1=O)c1ccc(Br)cc1 |c:26| Show InChI InChI=1S/C26H27BrN4O5/c1-2-3-20(22-26(35)36-25(30-22)16-6-8-17(27)9-7-16)31-13-12-21(31)24(34)29-19(23(28)33)14-15-4-10-18(32)11-5-15/h4-11,19,21,32H,2-3,12-14H2,1H3,(H2,28,33)(H,29,34)/b22-20-/t19-,21-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 87 | n/a | n/a | n/a | n/a | n/a | n/a |
ArQule Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Staphylococcus aureus methionyl-tRNA synthetase (SaMetRS) |
Bioorg Med Chem Lett 14: 1909-11 (2004)
Article DOI: 10.1016/j.bmcl.2004.01.094
BindingDB Entry DOI: 10.7270/Q2NG4Q2F |
More data for this
Ligand-Target Pair | |
Proline--tRNA ligase, cytoplasmic
(Candida albicans) | BDBM50097107

(2-(4-Bromo-phenyl)-7-fluoro-8-methyl-quinoline-4-c...)Show InChI InChI=1S/C17H11BrFNO2/c1-9-14(19)7-6-12-13(17(21)22)8-15(20-16(9)12)10-2-4-11(18)5-3-10/h2-8H,1H3,(H,21,22) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
Cubist Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Aminoacylation activity against Candida albicans prolyl-tRNA synthetase |
Bioorg Med Chem Lett 11: 541-4 (2001)
BindingDB Entry DOI: 10.7270/Q2Q23ZH8 |
More data for this
Ligand-Target Pair | |
Methionine--tRNA ligase, cytoplasmic
(Homo sapiens (Human)) | BDBM50129541
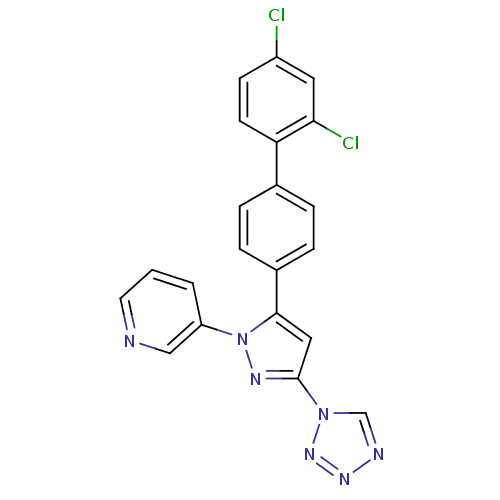
(3-[5-(2',4'-Dichloro-biphenyl-4-yl)-3-tetrazol-1-y...)Show SMILES Clc1ccc(c(Cl)c1)-c1ccc(cc1)-c1cc(nn1-c1cccnc1)-n1cnnn1 Show InChI InChI=1S/C21H13Cl2N7/c22-16-7-8-18(19(23)10-16)14-3-5-15(6-4-14)20-11-21(29-13-25-27-28-29)26-30(20)17-2-1-9-24-12-17/h1-13H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
Cubist Pharmaceutical Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against Staphylococcus aureus methionyl-tRNA synthetase (SaMetRS) |
Bioorg Med Chem Lett 13: 2231-4 (2003)
BindingDB Entry DOI: 10.7270/Q24Q7TCM |
More data for this
Ligand-Target Pair | |
Methionine--tRNA ligase, cytoplasmic
(Homo sapiens (Human)) | BDBM50129527
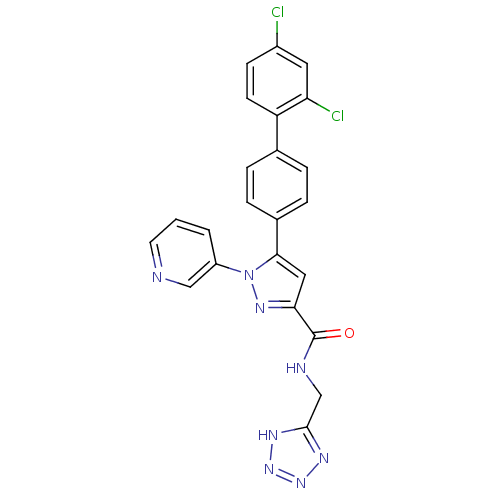
(5-(2',4'-Dichloro-biphenyl-4-yl)-1-pyridin-3-yl-1H...)Show SMILES Clc1ccc(c(Cl)c1)-c1ccc(cc1)-c1cc(nn1-c1cccnc1)C(=O)NCc1nnn[nH]1 Show InChI InChI=1S/C23H16Cl2N8O/c24-16-7-8-18(19(25)10-16)14-3-5-15(6-4-14)21-11-20(23(34)27-13-22-28-31-32-29-22)30-33(21)17-2-1-9-26-12-17/h1-12H,13H2,(H,27,34)(H,28,29,31,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
Cubist Pharmaceutical Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against human methionyl-tRNA synthetase (hMetRS). |
Bioorg Med Chem Lett 13: 2231-4 (2003)
BindingDB Entry DOI: 10.7270/Q24Q7TCM |
More data for this
Ligand-Target Pair | |
Proline--tRNA ligase, cytoplasmic
(Candida albicans) | BDBM50097091
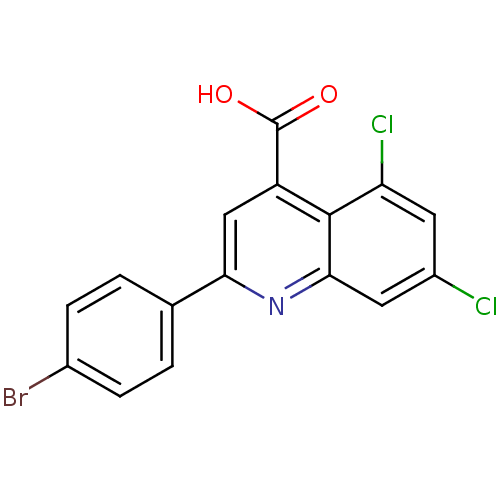
(2-(4-Bromo-phenyl)-5,7-dichloro-quinoline-4-carbox...)Show SMILES OC(=O)c1cc(nc2cc(Cl)cc(Cl)c12)-c1ccc(Br)cc1 Show InChI InChI=1S/C16H8BrCl2NO2/c17-9-3-1-8(2-4-9)13-7-11(16(21)22)15-12(19)5-10(18)6-14(15)20-13/h1-7H,(H,21,22) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
Cubist Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Aminoacylation activity against Candida albicans prolyl-tRNA synthetase |
Bioorg Med Chem Lett 11: 541-4 (2001)
BindingDB Entry DOI: 10.7270/Q2Q23ZH8 |
More data for this
Ligand-Target Pair | |
Proline--tRNA ligase, cytoplasmic
(Candida albicans) | BDBM50097093

(7-Bromo-2-(4-bromo-phenyl)-8-methyl-quinoline-4-ca...)Show InChI InChI=1S/C17H11Br2NO2/c1-9-14(19)7-6-12-13(17(21)22)8-15(20-16(9)12)10-2-4-11(18)5-3-10/h2-8H,1H3,(H,21,22) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
Cubist Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Aminoacylation activity against Candida albicans prolyl-tRNA synthetase |
Bioorg Med Chem Lett 11: 541-4 (2001)
BindingDB Entry DOI: 10.7270/Q2Q23ZH8 |
More data for this
Ligand-Target Pair | |
Methionine--tRNA ligase, cytoplasmic
(Homo sapiens (Human)) | BDBM50129528

(2-[5-(2',4'-Dichloro-biphenyl-4-yl)-3-tetrazol-1-y...)Show SMILES Clc1ccc(c(Cl)c1)-c1ccc(cc1)-c1cc(nn1-c1ccccn1)-n1cnnn1 Show InChI InChI=1S/C21H13Cl2N7/c22-16-8-9-17(18(23)11-16)14-4-6-15(7-5-14)19-12-21(29-13-25-27-28-29)26-30(19)20-3-1-2-10-24-20/h1-13H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
Cubist Pharmaceutical Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against Staphylococcus aureus methionyl-tRNA synthetase (SaMetRS) |
Bioorg Med Chem Lett 13: 2231-4 (2003)
BindingDB Entry DOI: 10.7270/Q24Q7TCM |
More data for this
Ligand-Target Pair | |
Glutamate carboxypeptidase 2
(Homo sapiens (Human)) | BDBM50265489
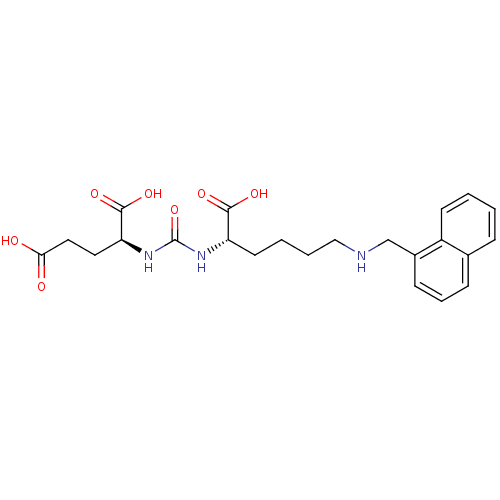
((S)-2-(3-((S)-1-Carboxy-5-(naphthalen-1-ylmethylam...)Show SMILES OC(=O)CC[C@H](NC(=O)N[C@@H](CCCCNCc1cccc2ccccc12)C(O)=O)C(O)=O |r| Show InChI InChI=1S/C23H29N3O7/c27-20(28)12-11-19(22(31)32)26-23(33)25-18(21(29)30)10-3-4-13-24-14-16-8-5-7-15-6-1-2-9-17(15)16/h1-2,5-9,18-19,24H,3-4,10-14H2,(H,27,28)(H,29,30)(H,31,32)(H2,25,26,33)/t18-,19-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 154 | n/a | n/a | n/a | n/a | n/a | n/a |
Molecular Insight Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of [131I]DCIT from PSMA in human LNCAP cells |
J Med Chem 52: 347-57 (2009)
Article DOI: 10.1021/jm800994j
BindingDB Entry DOI: 10.7270/Q2V987ZZ |
More data for this
Ligand-Target Pair | |
Methionine--tRNA ligase, cytoplasmic
(Homo sapiens (Human)) | BDBM50143331
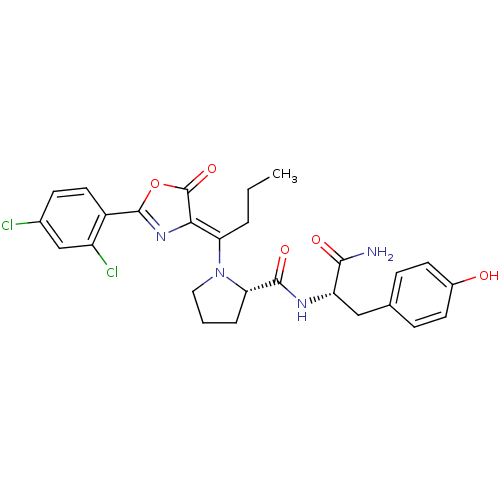
((S)-1-{1-[2-(2,4-Dichloro-phenyl)-5-oxo-oxazol-(4Z...)Show SMILES CCC\C(N1CCC[C@H]1C(=O)N[C@@H](Cc1ccc(O)cc1)C(N)=O)=C1\N=C(OC1=O)c1ccc(Cl)cc1Cl |c:27| Show InChI InChI=1S/C27H28Cl2N4O5/c1-2-4-21(23-27(37)38-26(32-23)18-11-8-16(28)14-19(18)29)33-12-3-5-22(33)25(36)31-20(24(30)35)13-15-6-9-17(34)10-7-15/h6-11,14,20,22,34H,2-5,12-13H2,1H3,(H2,30,35)(H,31,36)/b23-21-/t20-,22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 170 | n/a | n/a | n/a | n/a | n/a | n/a |
ArQule Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Staphylococcus aureus methionyl-tRNA synthetase (SaMetRS) |
Bioorg Med Chem Lett 14: 1909-11 (2004)
Article DOI: 10.1016/j.bmcl.2004.01.094
BindingDB Entry DOI: 10.7270/Q2NG4Q2F |
More data for this
Ligand-Target Pair | |
Phenylalanine--tRNA ligase, mitochondrial
(Homo sapiens (Human)) | BDBM50140728
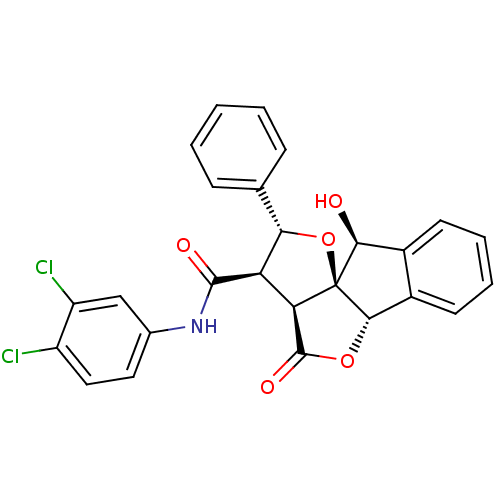
(13N-(3,4-dichlorophenyl)-2-hydroxy-11-oxo-14-pheny...)Show SMILES O[C@H]1c2ccccc2[C@@H]2OC(=O)[C@H]3[C@H]([C@@H](O[C@@]123)c1ccccc1)C(=O)Nc1ccc(Cl)c(Cl)c1 Show InChI InChI=1S/C26H19Cl2NO5/c27-17-11-10-14(12-18(17)28)29-24(31)19-20-25(32)33-23-16-9-5-4-8-15(16)22(30)26(20,23)34-21(19)13-6-2-1-3-7-13/h1-12,19-23,30H,(H,29,31)/t19-,20-,21+,22+,23+,26+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 170 | n/a | n/a | n/a | n/a | n/a | n/a |
Cubist Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Compound was evaluated for the inhibitory activity against Escherichia coli phenylalanyl-tRNA synthetase |
Bioorg Med Chem Lett 14: 1343-6 (2004)
Article DOI: 10.1016/j.bmcl.2003.11.082
BindingDB Entry DOI: 10.7270/Q2K073Q6 |
More data for this
Ligand-Target Pair | |
Methionine--tRNA ligase, cytoplasmic
(Homo sapiens (Human)) | BDBM50143337
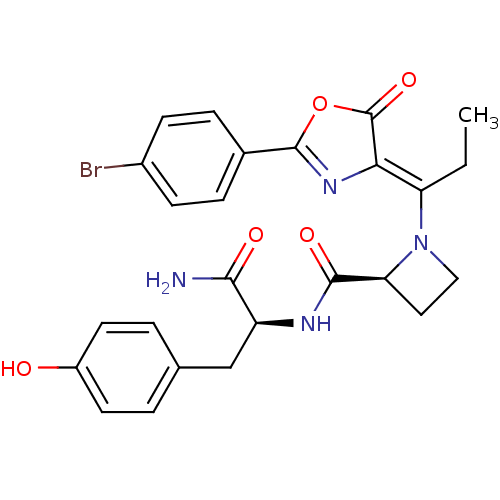
((S)-1-{1-[2-(4-Bromo-phenyl)-5-oxo-oxazol-(4Z)-yli...)Show SMILES CC\C(N1CC[C@H]1C(=O)N[C@@H](Cc1ccc(O)cc1)C(N)=O)=C1\N=C(OC1=O)c1ccc(Br)cc1 |c:25| Show InChI InChI=1S/C25H25BrN4O5/c1-2-19(21-25(34)35-24(29-21)15-5-7-16(26)8-6-15)30-12-11-20(30)23(33)28-18(22(27)32)13-14-3-9-17(31)10-4-14/h3-10,18,20,31H,2,11-13H2,1H3,(H2,27,32)(H,28,33)/b21-19-/t18-,20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 170 | n/a | n/a | n/a | n/a | n/a | n/a |
ArQule Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human methionyl-tRNA synthetase (fMetRS) |
Bioorg Med Chem Lett 14: 1909-11 (2004)
Article DOI: 10.1016/j.bmcl.2004.01.094
BindingDB Entry DOI: 10.7270/Q2NG4Q2F |
More data for this
Ligand-Target Pair | |
Methionine--tRNA ligase, cytoplasmic
(Homo sapiens (Human)) | BDBM50143332
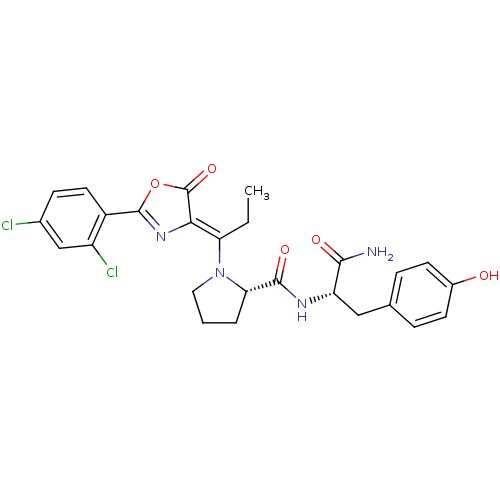
((S)-1-{1-[2-(2,4-Dichloro-phenyl)-5-oxo-oxazol-(4Z...)Show SMILES CC\C(N1CCC[C@H]1C(=O)N[C@@H](Cc1ccc(O)cc1)C(N)=O)=C1\N=C(OC1=O)c1ccc(Cl)cc1Cl |c:26| Show InChI InChI=1S/C26H26Cl2N4O5/c1-2-20(22-26(36)37-25(31-22)17-10-7-15(27)13-18(17)28)32-11-3-4-21(32)24(35)30-19(23(29)34)12-14-5-8-16(33)9-6-14/h5-10,13,19,21,33H,2-4,11-12H2,1H3,(H2,29,34)(H,30,35)/b22-20-/t19-,21-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 180 | n/a | n/a | n/a | n/a | n/a | n/a |
ArQule Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Staphylococcus aureus methionyl-tRNA synthetase (SaMetRS) |
Bioorg Med Chem Lett 14: 1909-11 (2004)
Article DOI: 10.1016/j.bmcl.2004.01.094
BindingDB Entry DOI: 10.7270/Q2NG4Q2F |
More data for this
Ligand-Target Pair | |
Proline--tRNA ligase, cytoplasmic
(Candida albicans) | BDBM50097104
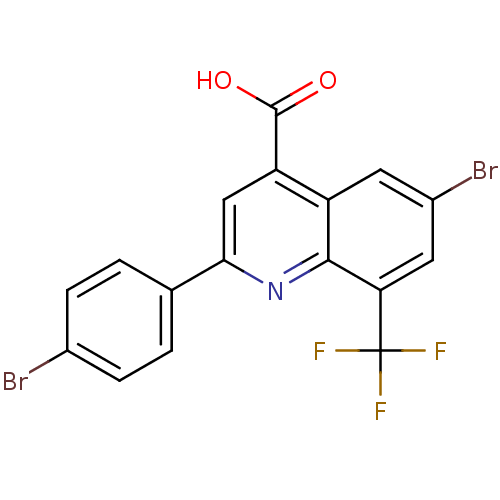
(6-Bromo-2-(4-bromo-phenyl)-8-trifluoromethyl-quino...)Show SMILES OC(=O)c1cc(nc2c(cc(Br)cc12)C(F)(F)F)-c1ccc(Br)cc1 Show InChI InChI=1S/C17H8Br2F3NO2/c18-9-3-1-8(2-4-9)14-7-12(16(24)25)11-5-10(19)6-13(15(11)23-14)17(20,21)22/h1-7H,(H,24,25) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 220 | n/a | n/a | n/a | n/a | n/a | n/a |
Cubist Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Aminoacylation activity against Candida albicans prolyl-tRNA synthetase |
Bioorg Med Chem Lett 11: 541-4 (2001)
BindingDB Entry DOI: 10.7270/Q2Q23ZH8 |
More data for this
Ligand-Target Pair | |
Phenylalanine--tRNA ligase alpha subunit
(Streptococcus pyogenes serotype M18) | BDBM50140707

(5-(3-chloro-4-methylphenyl)-3-phenyl-(3R,3aS,6aR)-...)Show SMILES Cc1ccc(cc1Cl)-n1c(O)c2C(OC3(C(=O)c4ccccc4C3=O)c2c1O)c1ccccc1 Show InChI InChI=1S/C27H18ClNO5/c1-14-11-12-16(13-19(14)28)29-25(32)20-21(26(29)33)27(34-22(20)15-7-3-2-4-8-15)23(30)17-9-5-6-10-18(17)24(27)31/h2-13,22,32-33H,1H3 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 220 | n/a | n/a | n/a | n/a | n/a | n/a |
Cubist Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against phenylalanyl-tRNA synthetase from Enterococcus faecalis |
Bioorg Med Chem Lett 14: 1339-42 (2004)
Article DOI: 10.1016/j.bmcl.2003.11.081
BindingDB Entry DOI: 10.7270/Q2PR7VDS |
More data for this
Ligand-Target Pair | |
Glutamate carboxypeptidase 2
(Homo sapiens (Human)) | BDBM50265391
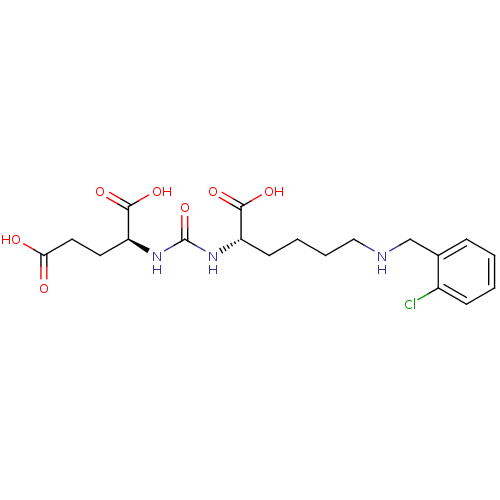
((S)-2-(3-((S)-1-Carboxy-5-(2-chlorobenzylamino)pen...)Show SMILES OC(=O)CC[C@H](NC(=O)N[C@@H](CCCCNCc1ccccc1Cl)C(O)=O)C(O)=O |r| Show InChI InChI=1S/C19H26ClN3O7/c20-13-6-2-1-5-12(13)11-21-10-4-3-7-14(17(26)27)22-19(30)23-15(18(28)29)8-9-16(24)25/h1-2,5-6,14-15,21H,3-4,7-11H2,(H,24,25)(H,26,27)(H,28,29)(H2,22,23,30)/t14-,15-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 245 | n/a | n/a | n/a | n/a | n/a | n/a |
Molecular Insight Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of [131I]DCIT from PSMA in human LNCAP cells |
J Med Chem 52: 347-57 (2009)
Article DOI: 10.1021/jm800994j
BindingDB Entry DOI: 10.7270/Q2V987ZZ |
More data for this
Ligand-Target Pair | |
Phenylalanine--tRNA ligase, mitochondrial
(Homo sapiens (Human)) | BDBM50140728

(13N-(3,4-dichlorophenyl)-2-hydroxy-11-oxo-14-pheny...)Show SMILES O[C@H]1c2ccccc2[C@@H]2OC(=O)[C@H]3[C@H]([C@@H](O[C@@]123)c1ccccc1)C(=O)Nc1ccc(Cl)c(Cl)c1 Show InChI InChI=1S/C26H19Cl2NO5/c27-17-11-10-14(12-18(17)28)29-24(31)19-20-25(32)33-23-16-9-5-4-8-15(16)22(30)26(20,23)34-21(19)13-6-2-1-3-7-13/h1-12,19-23,30H,(H,29,31)/t19-,20-,21+,22+,23+,26+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 260 | n/a | n/a | n/a | n/a | n/a | n/a |
Cubist Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Compound was evaluated for the inhibitory activity against Staphylococcus aureus phenylalanyl-tRNA synthetase |
Bioorg Med Chem Lett 14: 1343-6 (2004)
Article DOI: 10.1016/j.bmcl.2003.11.082
BindingDB Entry DOI: 10.7270/Q2K073Q6 |
More data for this
Ligand-Target Pair | |
Glutamate carboxypeptidase 2
(Homo sapiens (Human)) | BDBM50265451

((S)-2-(3-((S)-1-Carboxy-5-(3-chlorobenzylamino)pen...)Show SMILES OC(=O)CC[C@H](NC(=O)N[C@@H](CCCCNCc1cccc(Cl)c1)C(O)=O)C(O)=O |r| Show InChI InChI=1S/C19H26ClN3O7/c20-13-5-3-4-12(10-13)11-21-9-2-1-6-14(17(26)27)22-19(30)23-15(18(28)29)7-8-16(24)25/h3-5,10,14-15,21H,1-2,6-9,11H2,(H,24,25)(H,26,27)(H,28,29)(H2,22,23,30)/t14-,15-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 277 | n/a | n/a | n/a | n/a | n/a | n/a |
Molecular Insight Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of [131I]DCIT from PSMA in human LNCAP cells |
J Med Chem 52: 347-57 (2009)
Article DOI: 10.1021/jm800994j
BindingDB Entry DOI: 10.7270/Q2V987ZZ |
More data for this
Ligand-Target Pair | |
Methionine--tRNA ligase, cytoplasmic
(Homo sapiens (Human)) | BDBM50129551
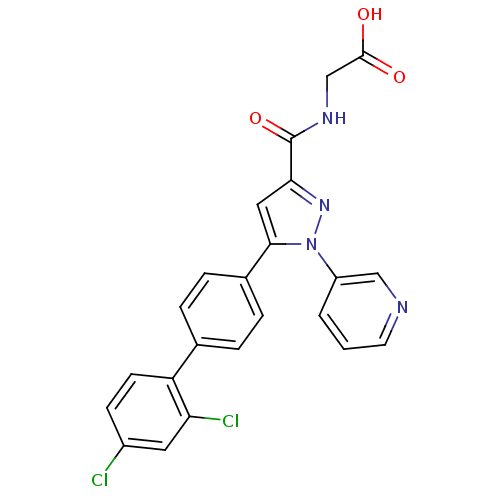
(CHEMBL74224 | {[5-(2',4'-Dichloro-biphenyl-4-yl)-1...)Show SMILES OC(=O)CNC(=O)c1cc(-c2ccc(cc2)-c2ccc(Cl)cc2Cl)n(n1)-c1cccnc1 Show InChI InChI=1S/C23H16Cl2N4O3/c24-16-7-8-18(19(25)10-16)14-3-5-15(6-4-14)21-11-20(23(32)27-13-22(30)31)28-29(21)17-2-1-9-26-12-17/h1-12H,13H2,(H,27,32)(H,30,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
Cubist Pharmaceutical Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against Staphylococcus aureus methionyl-tRNA synthetase (SaMetRS) |
Bioorg Med Chem Lett 13: 2231-4 (2003)
BindingDB Entry DOI: 10.7270/Q24Q7TCM |
More data for this
Ligand-Target Pair | |
Methionine--tRNA ligase, cytoplasmic
(Homo sapiens (Human)) | BDBM50129534

(5-(2',4'-Dichloro-biphenyl-4-yl)-1-pyridin-3-yl-1H...)Show SMILES OC(=O)c1cc(-c2ccc(cc2)-c2ccc(Cl)cc2Cl)n(n1)-c1cccnc1 Show InChI InChI=1S/C21H13Cl2N3O2/c22-15-7-8-17(18(23)10-15)13-3-5-14(6-4-13)20-11-19(21(27)28)25-26(20)16-2-1-9-24-12-16/h1-12H,(H,27,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
Cubist Pharmaceutical Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against human methionyl-tRNA synthetase (hMetRS). |
Bioorg Med Chem Lett 13: 2231-4 (2003)
BindingDB Entry DOI: 10.7270/Q24Q7TCM |
More data for this
Ligand-Target Pair | |
Methionine--tRNA ligase, cytoplasmic
(Homo sapiens (Human)) | BDBM50143343
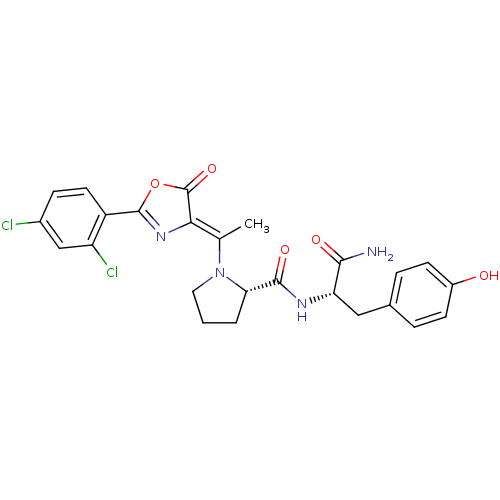
((S)-1-{1-[2-(2,4-Dichloro-phenyl)-5-oxo-oxazol-(4Z...)Show SMILES C\C(N1CCC[C@H]1C(=O)N[C@@H](Cc1ccc(O)cc1)C(N)=O)=C1\N=C(OC1=O)c1ccc(Cl)cc1Cl |c:25| Show InChI InChI=1S/C25H24Cl2N4O5/c1-13(21-25(35)36-24(30-21)17-9-6-15(26)12-18(17)27)31-10-2-3-20(31)23(34)29-19(22(28)33)11-14-4-7-16(32)8-5-14/h4-9,12,19-20,32H,2-3,10-11H2,1H3,(H2,28,33)(H,29,34)/b21-13-/t19-,20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 340 | n/a | n/a | n/a | n/a | n/a | n/a |
ArQule Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human methionyl-tRNA synthetase (fMetRS) |
Bioorg Med Chem Lett 14: 1909-11 (2004)
Article DOI: 10.1016/j.bmcl.2004.01.094
BindingDB Entry DOI: 10.7270/Q2NG4Q2F |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data