

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM21281 ((11R)-2-methyl-11-(morpholin-4-ylmethyl)-3-(naphth...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PubMed | 23 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Mississippi Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 from human recombinant CB1 receptor expressed in HEK293 cells | J Nat Prod 64: 1322-5 (2001) BindingDB Entry DOI: 10.7270/Q2C24W57 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasmepsin II (Plasmodium falciparum) | BDBM50377588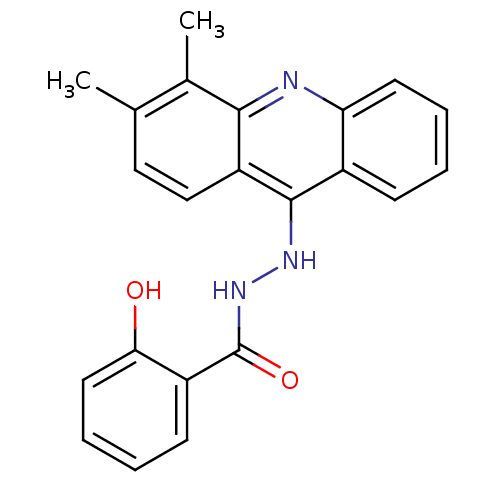 (CHEMBL257594) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.130 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Karachi Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum plasmepsin-2 by FRET assay | Bioorg Med Chem Lett 18: 3011-5 (2008) Article DOI: 10.1016/j.bmcl.2008.02.060 BindingDB Entry DOI: 10.7270/Q2TT4RV5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin D (Homo sapiens (Human)) | BDBM50377588 (CHEMBL257594) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Karachi Curated by ChEMBL | Assay Description Inhibition of human cathepsin D by FRET assay | Bioorg Med Chem Lett 18: 3011-5 (2008) Article DOI: 10.1016/j.bmcl.2008.02.060 BindingDB Entry DOI: 10.7270/Q2TT4RV5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin D (Homo sapiens (Human)) | BDBM912 ((3S,4S)-3-hydroxy-4-[(2S)-2-[(3S,4S)-3-hydroxy-6-m...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB Article PubMed | n/a | n/a | 0.550 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Karachi Curated by ChEMBL | Assay Description Inhibition of human cathepsin D by FRET assay | Bioorg Med Chem Lett 18: 3011-5 (2008) Article DOI: 10.1016/j.bmcl.2008.02.060 BindingDB Entry DOI: 10.7270/Q2TT4RV5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasmepsin II (Plasmodium falciparum) | BDBM912 ((3S,4S)-3-hydroxy-4-[(2S)-2-[(3S,4S)-3-hydroxy-6-m...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | n/a | n/a | 0.680 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Karachi Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum plasmepsin-2 by FRET assay | Bioorg Med Chem Lett 18: 3011-5 (2008) Article DOI: 10.1016/j.bmcl.2008.02.060 BindingDB Entry DOI: 10.7270/Q2TT4RV5 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Plasmepsin II (Plasmodium falciparum) | BDBM50377587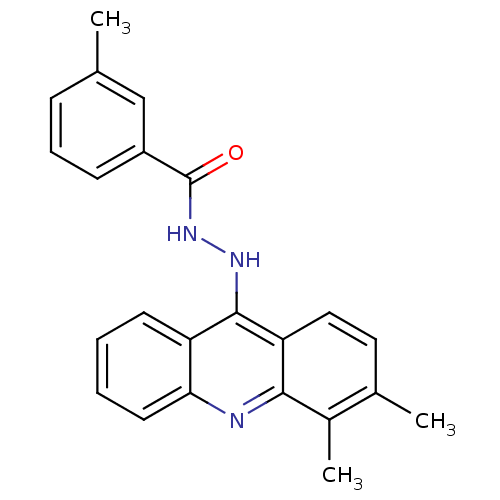 (CHEMBL404394) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Karachi Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum plasmepsin-2 by FRET assay | Bioorg Med Chem Lett 18: 3011-5 (2008) Article DOI: 10.1016/j.bmcl.2008.02.060 BindingDB Entry DOI: 10.7270/Q2TT4RV5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin D (Homo sapiens (Human)) | BDBM50377587 (CHEMBL404394) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Karachi Curated by ChEMBL | Assay Description Inhibition of human cathepsin D by FRET assay | Bioorg Med Chem Lett 18: 3011-5 (2008) Article DOI: 10.1016/j.bmcl.2008.02.060 BindingDB Entry DOI: 10.7270/Q2TT4RV5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional protein GlmU (Escherichia coli) | BDBM50075297 (CHEMBL2207606) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIR-Indian Institute of Integrative Medicine Curated by ChEMBL | Assay Description Inhibition of Escherichia coli GlmU acetyltransferase activity assessed as coenzyme A production using acetyl CoA substrate | Eur J Med Chem 92: 78-90 (2015) Article DOI: 10.1016/j.ejmech.2014.12.030 BindingDB Entry DOI: 10.7270/Q2RJ4M55 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional protein GlmU (Escherichia coli) | BDBM50075297 (CHEMBL2207606) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIR-Indian Institute of Integrative Medicine Curated by ChEMBL | Assay Description Inhibition of Escherichia coli GlmU acetyltransferase activity assessed as coenzyme A production using acetyl CoA substrate | Eur J Med Chem 92: 78-90 (2015) Article DOI: 10.1016/j.ejmech.2014.12.030 BindingDB Entry DOI: 10.7270/Q2RJ4M55 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional protein GlmU (Escherichia coli) | BDBM92474 (Sulfonamide, 5) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIR-Indian Institute of Integrative Medicine Curated by ChEMBL | Assay Description Inhibition of Escherichia coli GlmU acetyltransferase activity assessed as coenzyme A production using acetyl CoA substrate | Eur J Med Chem 92: 78-90 (2015) Article DOI: 10.1016/j.ejmech.2014.12.030 BindingDB Entry DOI: 10.7270/Q2RJ4M55 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Bifunctional protein GlmU (Escherichia coli) | BDBM92474 (Sulfonamide, 5) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIR-Indian Institute of Integrative Medicine Curated by ChEMBL | Assay Description Inhibition of Escherichia coli GlmU acetyltransferase activity assessed as coenzyme A production using acetyl CoA substrate | Eur J Med Chem 92: 78-90 (2015) Article DOI: 10.1016/j.ejmech.2014.12.030 BindingDB Entry DOI: 10.7270/Q2RJ4M55 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Bifunctional protein GlmU (Escherichia coli) | BDBM92472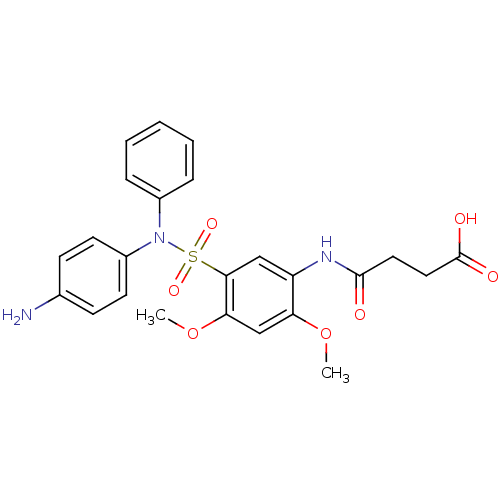 (Sulfonamide, 3) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIR-Indian Institute of Integrative Medicine Curated by ChEMBL | Assay Description Inhibition of Escherichia coli GlmU acetyltransferase activity assessed as coenzyme A production using acetyl CoA substrate | Eur J Med Chem 92: 78-90 (2015) Article DOI: 10.1016/j.ejmech.2014.12.030 BindingDB Entry DOI: 10.7270/Q2RJ4M55 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Bifunctional protein GlmU (Escherichia coli) | BDBM92472 (Sulfonamide, 3) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIR-Indian Institute of Integrative Medicine Curated by ChEMBL | Assay Description Inhibition of Escherichia coli GlmU acetyltransferase activity assessed as coenzyme A production using acetyl CoA substrate | Eur J Med Chem 92: 78-90 (2015) Article DOI: 10.1016/j.ejmech.2014.12.030 BindingDB Entry DOI: 10.7270/Q2RJ4M55 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Bifunctional protein GlmU (Escherichia coli) | BDBM50075298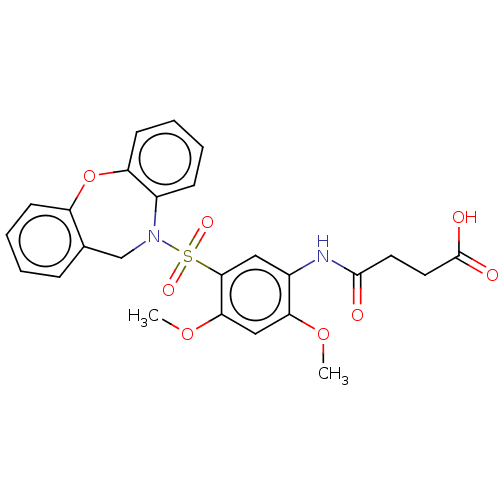 (CHEMBL2207605) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIR-Indian Institute of Integrative Medicine Curated by ChEMBL | Assay Description Inhibition of Escherichia coli GlmU acetyltransferase activity assessed as coenzyme A production using acetyl CoA substrate | Eur J Med Chem 92: 78-90 (2015) Article DOI: 10.1016/j.ejmech.2014.12.030 BindingDB Entry DOI: 10.7270/Q2RJ4M55 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional protein GlmU (Escherichia coli) | BDBM50075387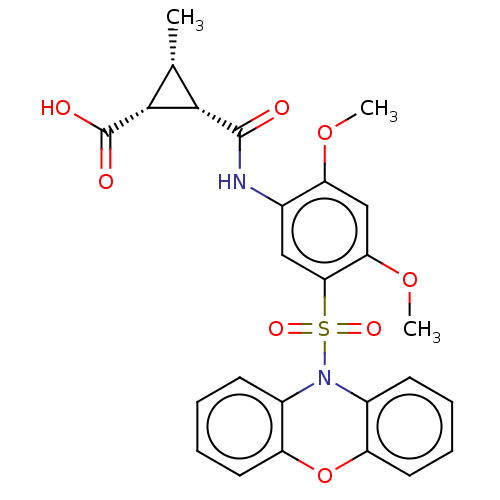 (CHEMBL3415068) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIR-Indian Institute of Integrative Medicine Curated by ChEMBL | Assay Description Inhibition of Escherichia coli GlmU acetyltransferase activity assessed as coenzyme A production using acetyl CoA substrate | Eur J Med Chem 92: 78-90 (2015) Article DOI: 10.1016/j.ejmech.2014.12.030 BindingDB Entry DOI: 10.7270/Q2RJ4M55 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional protein GlmU (Escherichia coli) | BDBM50075387 (CHEMBL3415068) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIR-Indian Institute of Integrative Medicine Curated by ChEMBL | Assay Description Inhibition of Escherichia coli GlmU acetyltransferase activity assessed as coenzyme A production using acetyl CoA substrate | Eur J Med Chem 92: 78-90 (2015) Article DOI: 10.1016/j.ejmech.2014.12.030 BindingDB Entry DOI: 10.7270/Q2RJ4M55 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional protein GlmU (Escherichia coli) | BDBM50075298 (CHEMBL2207605) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIR-Indian Institute of Integrative Medicine Curated by ChEMBL | Assay Description Inhibition of Escherichia coli GlmU acetyltransferase activity assessed as coenzyme A production using acetyl CoA substrate | Eur J Med Chem 92: 78-90 (2015) Article DOI: 10.1016/j.ejmech.2014.12.030 BindingDB Entry DOI: 10.7270/Q2RJ4M55 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional protein GlmU (Escherichia coli) | BDBM50075300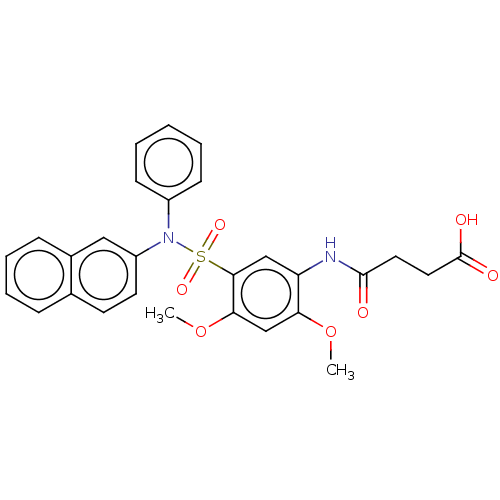 (CHEMBL2207602) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIR-Indian Institute of Integrative Medicine Curated by ChEMBL | Assay Description Inhibition of Escherichia coli GlmU acetyltransferase activity assessed as coenzyme A production using acetyl CoA substrate | Eur J Med Chem 92: 78-90 (2015) Article DOI: 10.1016/j.ejmech.2014.12.030 BindingDB Entry DOI: 10.7270/Q2RJ4M55 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional protein GlmU (Escherichia coli) | BDBM50075301 (CHEMBL2207600) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIR-Indian Institute of Integrative Medicine Curated by ChEMBL | Assay Description Inhibition of Escherichia coli GlmU acetyltransferase activity assessed as coenzyme A production using acetyl CoA substrate | Eur J Med Chem 92: 78-90 (2015) Article DOI: 10.1016/j.ejmech.2014.12.030 BindingDB Entry DOI: 10.7270/Q2RJ4M55 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional protein GlmU (Escherichia coli) | BDBM50075301 (CHEMBL2207600) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIR-Indian Institute of Integrative Medicine Curated by ChEMBL | Assay Description Inhibition of Escherichia coli GlmU acetyltransferase activity assessed as coenzyme A production using acetyl CoA substrate | Eur J Med Chem 92: 78-90 (2015) Article DOI: 10.1016/j.ejmech.2014.12.030 BindingDB Entry DOI: 10.7270/Q2RJ4M55 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional protein GlmU (Escherichia coli) | BDBM50075325 (CHEMBL2207599) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIR-Indian Institute of Integrative Medicine Curated by ChEMBL | Assay Description Inhibition of Escherichia coli GlmU acetyltransferase activity assessed as coenzyme A production using acetyl CoA substrate | Eur J Med Chem 92: 78-90 (2015) Article DOI: 10.1016/j.ejmech.2014.12.030 BindingDB Entry DOI: 10.7270/Q2RJ4M55 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional protein GlmU (Escherichia coli) | BDBM50075300 (CHEMBL2207602) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIR-Indian Institute of Integrative Medicine Curated by ChEMBL | Assay Description Inhibition of Escherichia coli GlmU acetyltransferase activity assessed as coenzyme A production using acetyl CoA substrate | Eur J Med Chem 92: 78-90 (2015) Article DOI: 10.1016/j.ejmech.2014.12.030 BindingDB Entry DOI: 10.7270/Q2RJ4M55 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional protein GlmU (Escherichia coli) | BDBM50075325 (CHEMBL2207599) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIR-Indian Institute of Integrative Medicine Curated by ChEMBL | Assay Description Inhibition of Escherichia coli GlmU acetyltransferase activity assessed as coenzyme A production using acetyl CoA substrate | Eur J Med Chem 92: 78-90 (2015) Article DOI: 10.1016/j.ejmech.2014.12.030 BindingDB Entry DOI: 10.7270/Q2RJ4M55 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional protein GlmU (Escherichia coli) | BDBM50075381 (CHEMBL3415072) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIR-Indian Institute of Integrative Medicine Curated by ChEMBL | Assay Description Inhibition of Escherichia coli GlmU acetyltransferase activity assessed as coenzyme A production using acetyl CoA substrate | Eur J Med Chem 92: 78-90 (2015) Article DOI: 10.1016/j.ejmech.2014.12.030 BindingDB Entry DOI: 10.7270/Q2RJ4M55 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional protein GlmU (Escherichia coli) | BDBM50075381 (CHEMBL3415072) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIR-Indian Institute of Integrative Medicine Curated by ChEMBL | Assay Description Inhibition of Escherichia coli GlmU acetyltransferase activity assessed as coenzyme A production using acetyl CoA substrate | Eur J Med Chem 92: 78-90 (2015) Article DOI: 10.1016/j.ejmech.2014.12.030 BindingDB Entry DOI: 10.7270/Q2RJ4M55 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional protein GlmU (Escherichia coli) | BDBM50075385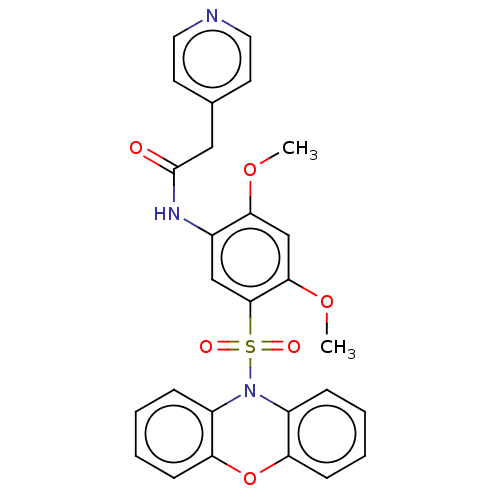 (CHEMBL2207224) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIR-Indian Institute of Integrative Medicine Curated by ChEMBL | Assay Description Inhibition of Escherichia coli GlmU acetyltransferase activity assessed as coenzyme A production using acetyl CoA substrate | Eur J Med Chem 92: 78-90 (2015) Article DOI: 10.1016/j.ejmech.2014.12.030 BindingDB Entry DOI: 10.7270/Q2RJ4M55 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional protein GlmU (Escherichia coli) | BDBM50075385 (CHEMBL2207224) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIR-Indian Institute of Integrative Medicine Curated by ChEMBL | Assay Description Inhibition of Escherichia coli GlmU acetyltransferase activity assessed as coenzyme A production using acetyl CoA substrate | Eur J Med Chem 92: 78-90 (2015) Article DOI: 10.1016/j.ejmech.2014.12.030 BindingDB Entry DOI: 10.7270/Q2RJ4M55 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional protein GlmU (Escherichia coli) | BDBM50075379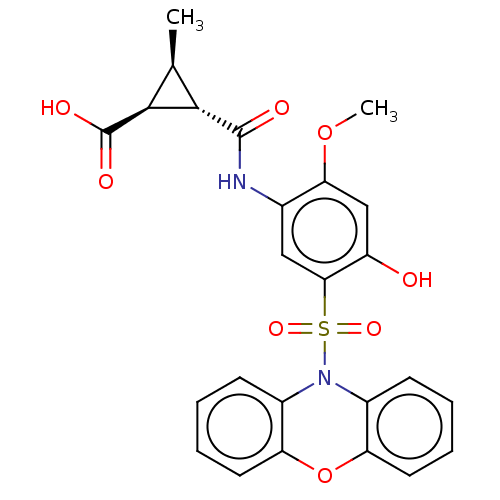 (CHEMBL3415115) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIR-Indian Institute of Integrative Medicine Curated by ChEMBL | Assay Description Inhibition of Escherichia coli GlmU acetyltransferase activity assessed as coenzyme A production using acetyl CoA substrate | Eur J Med Chem 92: 78-90 (2015) Article DOI: 10.1016/j.ejmech.2014.12.030 BindingDB Entry DOI: 10.7270/Q2RJ4M55 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional protein GlmU (Escherichia coli) | BDBM50075379 (CHEMBL3415115) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIR-Indian Institute of Integrative Medicine Curated by ChEMBL | Assay Description Inhibition of Escherichia coli GlmU acetyltransferase activity assessed as coenzyme A production using acetyl CoA substrate | Eur J Med Chem 92: 78-90 (2015) Article DOI: 10.1016/j.ejmech.2014.12.030 BindingDB Entry DOI: 10.7270/Q2RJ4M55 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Enoyl-acyl-carrier protein reductase (Plasmodium falciparum) | BDBM8726 (5-chloro-2-(2,4-dichlorophenoxy)phenol | CHEMBL849...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Karachi Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum enoyl-ACP reductase assessed as oxidation of NADH to NAD+ after 10 mins by spectrophotometric analysis | Bioorg Med Chem Lett 22: 610-2 (2011) Article DOI: 10.1016/j.bmcl.2011.10.072 BindingDB Entry DOI: 10.7270/Q2K35V3H | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Bifunctional protein GlmU (Escherichia coli) | BDBM50075383 (CHEMBL2207226) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 90 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIR-Indian Institute of Integrative Medicine Curated by ChEMBL | Assay Description Inhibition of Escherichia coli GlmU acetyltransferase activity assessed as coenzyme A production using acetyl CoA substrate | Eur J Med Chem 92: 78-90 (2015) Article DOI: 10.1016/j.ejmech.2014.12.030 BindingDB Entry DOI: 10.7270/Q2RJ4M55 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional protein GlmU (Escherichia coli) | BDBM50075383 (CHEMBL2207226) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 90 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIR-Indian Institute of Integrative Medicine Curated by ChEMBL | Assay Description Inhibition of Escherichia coli GlmU acetyltransferase activity assessed as coenzyme A production using acetyl CoA substrate | Eur J Med Chem 92: 78-90 (2015) Article DOI: 10.1016/j.ejmech.2014.12.030 BindingDB Entry DOI: 10.7270/Q2RJ4M55 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional protein GlmU (Escherichia coli) | BDBM50075299 (CHEMBL2207604) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 90 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIR-Indian Institute of Integrative Medicine Curated by ChEMBL | Assay Description Inhibition of Escherichia coli GlmU acetyltransferase activity assessed as coenzyme A production using acetyl CoA substrate | Eur J Med Chem 92: 78-90 (2015) Article DOI: 10.1016/j.ejmech.2014.12.030 BindingDB Entry DOI: 10.7270/Q2RJ4M55 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional protein GlmU (Escherichia coli) | BDBM50075370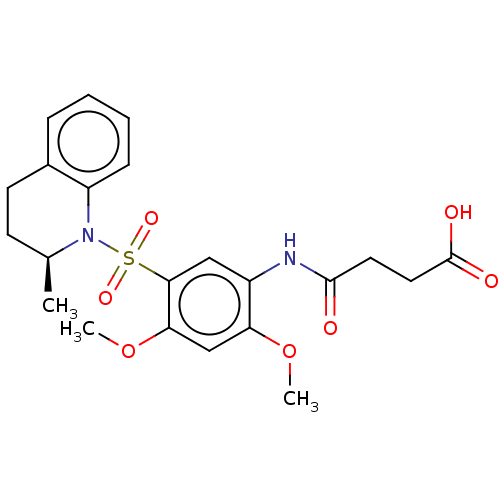 (CHEMBL3414903) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 90 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIR-Indian Institute of Integrative Medicine Curated by ChEMBL | Assay Description Inhibition of Escherichia coli GlmU acetyltransferase activity assessed as coenzyme A production using acetyl CoA substrate | Eur J Med Chem 92: 78-90 (2015) Article DOI: 10.1016/j.ejmech.2014.12.030 BindingDB Entry DOI: 10.7270/Q2RJ4M55 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional protein GlmU (Escherichia coli) | BDBM50075299 (CHEMBL2207604) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 90 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIR-Indian Institute of Integrative Medicine Curated by ChEMBL | Assay Description Inhibition of Escherichia coli GlmU acetyltransferase activity assessed as coenzyme A production using acetyl CoA substrate | Eur J Med Chem 92: 78-90 (2015) Article DOI: 10.1016/j.ejmech.2014.12.030 BindingDB Entry DOI: 10.7270/Q2RJ4M55 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional protein GlmU (Escherichia coli) | BDBM50075370 (CHEMBL3414903) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 90 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIR-Indian Institute of Integrative Medicine Curated by ChEMBL | Assay Description Inhibition of Escherichia coli GlmU acetyltransferase activity assessed as coenzyme A production using acetyl CoA substrate | Eur J Med Chem 92: 78-90 (2015) Article DOI: 10.1016/j.ejmech.2014.12.030 BindingDB Entry DOI: 10.7270/Q2RJ4M55 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional protein GlmU (Escherichia coli) | BDBM50075369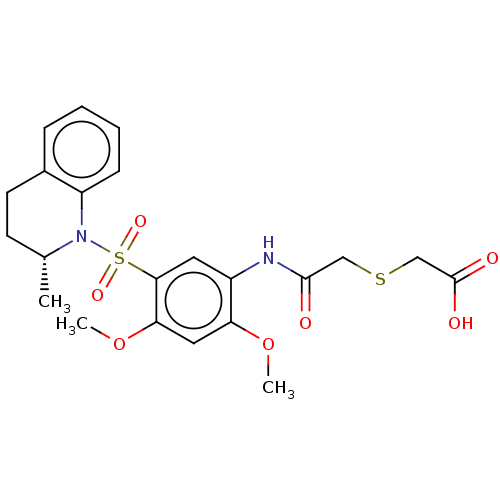 (CHEMBL3414904) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIR-Indian Institute of Integrative Medicine Curated by ChEMBL | Assay Description Inhibition of Escherichia coli GlmU acetyltransferase activity assessed as coenzyme A production using acetyl CoA substrate | Eur J Med Chem 92: 78-90 (2015) Article DOI: 10.1016/j.ejmech.2014.12.030 BindingDB Entry DOI: 10.7270/Q2RJ4M55 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional protein GlmU (Escherichia coli) | BDBM50075369 (CHEMBL3414904) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIR-Indian Institute of Integrative Medicine Curated by ChEMBL | Assay Description Inhibition of Escherichia coli GlmU acetyltransferase activity assessed as coenzyme A production using acetyl CoA substrate | Eur J Med Chem 92: 78-90 (2015) Article DOI: 10.1016/j.ejmech.2014.12.030 BindingDB Entry DOI: 10.7270/Q2RJ4M55 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase (Toxoplasma gondii) | BDBM13336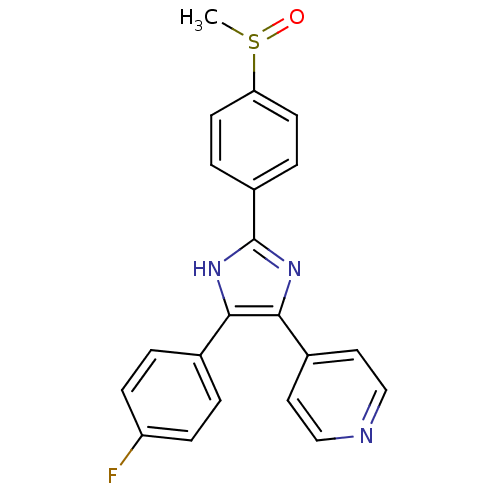 (4-[4-(4-fluorophenyl)-2-(4-methanesulfinylphenyl)-...) | UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Tulane University School of Medicine Curated by ChEMBL | Assay Description Inhibition of Toxoplasma gondii recombinant MAPK1 expressed in Escherichia coli | Antimicrob Agents Chemother 51: 4324-8 (2007) Article DOI: 10.1128/AAC.00680-07 BindingDB Entry DOI: 10.7270/Q2Q52QJG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholesterol side-chain cleavage enzyme, mitochondrial (Rattus norvegicus) | BDBM50498223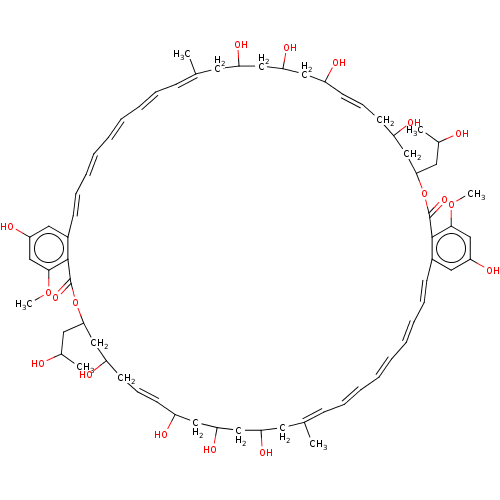 (CHEMBL3577359) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 167 | n/a | n/a | n/a | n/a | n/a | n/a |
Lucigen Corporation Curated by ChEMBL | Assay Description Antibacterial activity against Enterococcus faecalis ATCC 51299 assessed as growth inhibition using fresh sample in DMSO by CLSI method | J Nat Prod 78: 924-8 (2015) Article DOI: 10.1021/np500911k BindingDB Entry DOI: 10.7270/Q2GT5R6X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase (Toxoplasma gondii) | BDBM50295553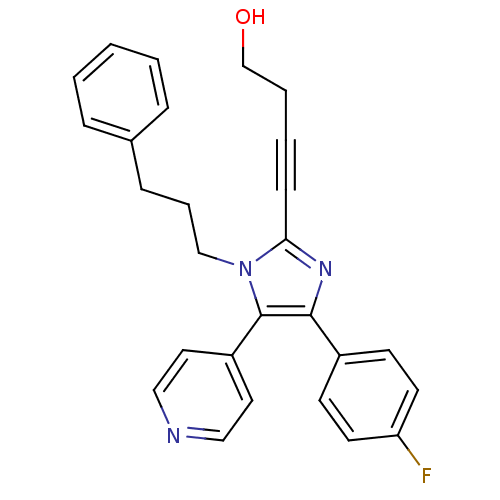 (4-(4-(4-fluorophenyl)-1-(3-phenylpropyl)-5-(pyridi...) | UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Tulane University School of Medicine Curated by ChEMBL | Assay Description Inhibition of Toxoplasma gondii recombinant MAPK1 expressed in Escherichia coli | Antimicrob Agents Chemother 51: 4324-8 (2007) Article DOI: 10.1128/AAC.00680-07 BindingDB Entry DOI: 10.7270/Q2Q52QJG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional protein GlmU (Escherichia coli) | BDBM50075372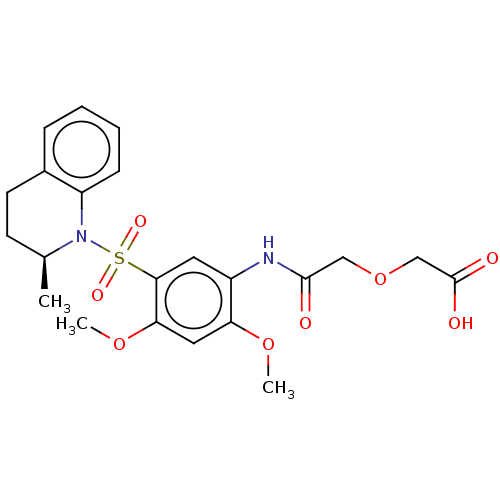 (CHEMBL3414901) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIR-Indian Institute of Integrative Medicine Curated by ChEMBL | Assay Description Inhibition of Escherichia coli GlmU acetyltransferase activity assessed as coenzyme A production using acetyl CoA substrate | Eur J Med Chem 92: 78-90 (2015) Article DOI: 10.1016/j.ejmech.2014.12.030 BindingDB Entry DOI: 10.7270/Q2RJ4M55 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional protein GlmU (Escherichia coli) | BDBM50075372 (CHEMBL3414901) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIR-Indian Institute of Integrative Medicine Curated by ChEMBL | Assay Description Inhibition of Escherichia coli GlmU acetyltransferase activity assessed as coenzyme A production using acetyl CoA substrate | Eur J Med Chem 92: 78-90 (2015) Article DOI: 10.1016/j.ejmech.2014.12.030 BindingDB Entry DOI: 10.7270/Q2RJ4M55 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional protein GlmU (Escherichia coli) | BDBM50365095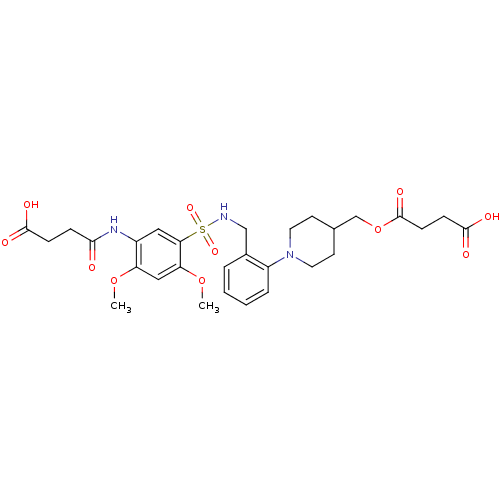 (CHEMBL1951184) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 250 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIR-Indian Institute of Integrative Medicine Curated by ChEMBL | Assay Description Inhibition of Escherichia coli GlmU acetyltransferase activity assessed as coenzyme A production using acetyl CoA substrate | Eur J Med Chem 92: 78-90 (2015) Article DOI: 10.1016/j.ejmech.2014.12.030 BindingDB Entry DOI: 10.7270/Q2RJ4M55 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional protein GlmU (Escherichia coli) | BDBM50365095 (CHEMBL1951184) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 250 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIR-Indian Institute of Integrative Medicine Curated by ChEMBL | Assay Description Inhibition of Escherichia coli GlmU acetyltransferase activity assessed as coenzyme A production using acetyl CoA substrate | Eur J Med Chem 92: 78-90 (2015) Article DOI: 10.1016/j.ejmech.2014.12.030 BindingDB Entry DOI: 10.7270/Q2RJ4M55 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM13531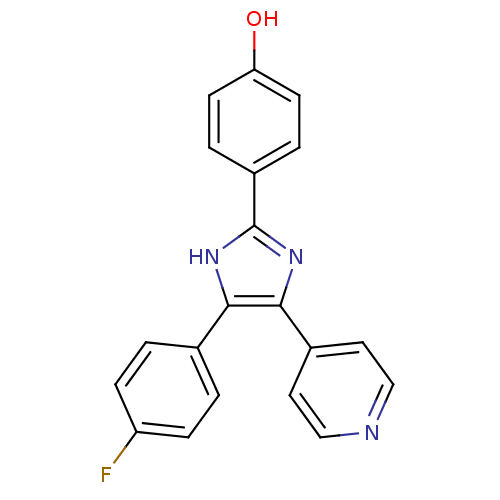 (4-(4-Fluorophenyl)-2-(4-hydroxyphenyl)-5-(4-pyridy...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
Tulane University School of Medicine Curated by ChEMBL | Assay Description Inhibition of human recombinant p38alpha assessed as [gamma33P]ATP utilization by microplate scintillation counting | Antimicrob Agents Chemother 51: 4324-8 (2007) Article DOI: 10.1128/AAC.00680-07 BindingDB Entry DOI: 10.7270/Q2Q52QJG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional protein GlmU (Escherichia coli) | BDBM50365098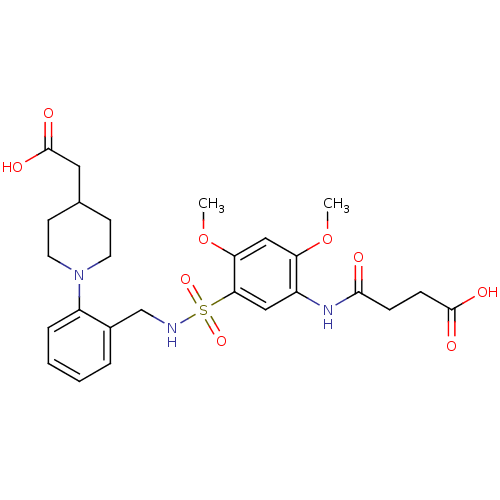 (CHEMBL1951187) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIR-Indian Institute of Integrative Medicine Curated by ChEMBL | Assay Description Inhibition of Escherichia coli GlmU acetyltransferase activity assessed as coenzyme A production using acetyl CoA substrate | Eur J Med Chem 92: 78-90 (2015) Article DOI: 10.1016/j.ejmech.2014.12.030 BindingDB Entry DOI: 10.7270/Q2RJ4M55 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional protein GlmU (Escherichia coli) | BDBM50075386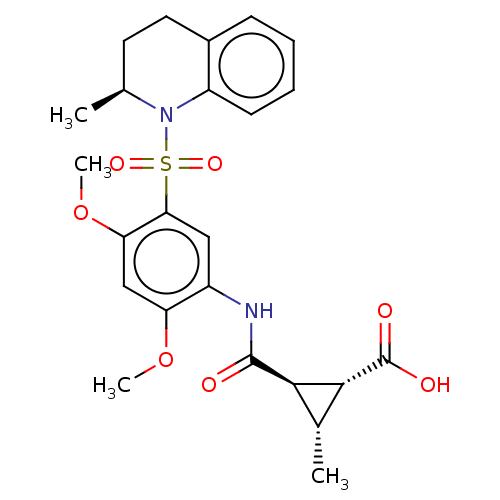 (CHEMBL3415069) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIR-Indian Institute of Integrative Medicine Curated by ChEMBL | Assay Description Inhibition of Escherichia coli GlmU acetyltransferase activity assessed as coenzyme A production using acetyl CoA substrate | Eur J Med Chem 92: 78-90 (2015) Article DOI: 10.1016/j.ejmech.2014.12.030 BindingDB Entry DOI: 10.7270/Q2RJ4M55 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional protein GlmU (Escherichia coli) | BDBM50075386 (CHEMBL3415069) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIR-Indian Institute of Integrative Medicine Curated by ChEMBL | Assay Description Inhibition of Escherichia coli GlmU acetyltransferase activity assessed as coenzyme A production using acetyl CoA substrate | Eur J Med Chem 92: 78-90 (2015) Article DOI: 10.1016/j.ejmech.2014.12.030 BindingDB Entry DOI: 10.7270/Q2RJ4M55 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional protein GlmU (Escherichia coli) | BDBM50365098 (CHEMBL1951187) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIR-Indian Institute of Integrative Medicine Curated by ChEMBL | Assay Description Inhibition of Escherichia coli GlmU acetyltransferase activity assessed as coenzyme A production using acetyl CoA substrate | Eur J Med Chem 92: 78-90 (2015) Article DOI: 10.1016/j.ejmech.2014.12.030 BindingDB Entry DOI: 10.7270/Q2RJ4M55 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 225 total ) | Next | Last >> |