 Found 368 hits with Last Name = 'kim' and Initial = 'is'
Found 368 hits with Last Name = 'kim' and Initial = 'is' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Protein kinase C alpha type
(Homo sapiens (Human)) | BDBM50048890
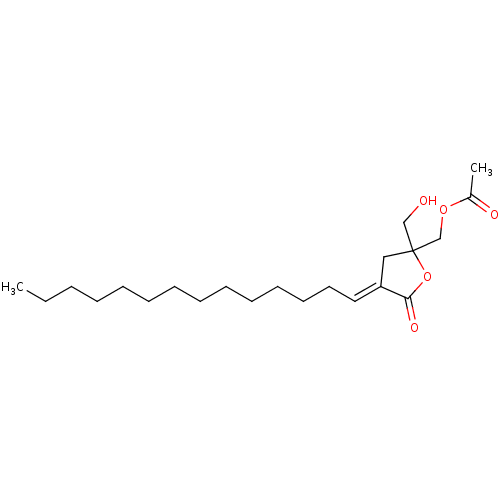
(Acetic acid 2-hydroxymethyl-5-oxo-4-tetradec-(E)-y...)Show InChI InChI=1S/C22H38O5/c1-3-4-5-6-7-8-9-10-11-12-13-14-15-20-16-22(17-23,27-21(20)25)18-26-19(2)24/h15,23H,3-14,16-18H2,1-2H3/b20-15+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 78 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Cancer Institute
Curated by ChEMBL
| Assay Description
Displacement of [20-3H]phorbol12,13-dibutyrate from a recombinant PK-C alpha |
Bioorg Med Chem Lett 8: 1757-62 (1999)
BindingDB Entry DOI: 10.7270/Q2TH8KT2 |
More data for this
Ligand-Target Pair | |
Protein kinase C alpha type
(Homo sapiens (Human)) | BDBM50070858
(CHEMBL49945 | Tetradecane-1-sulfonic acid 2-hydrox...)Show InChI InChI=1S/C20H38O6S/c1-2-3-4-5-6-7-8-9-10-11-12-13-16-27(23,24)25-18-20(17-21)15-14-19(22)26-20/h21H,2-18H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| 91 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Cancer Institute
Curated by ChEMBL
| Assay Description
Displacement of [20-3H]phorbol12,13-dibutyrate from a recombinant PK-C alpha |
Bioorg Med Chem Lett 8: 1757-62 (1999)
BindingDB Entry DOI: 10.7270/Q2TH8KT2 |
More data for this
Ligand-Target Pair | |
Protein kinase C alpha type
(Homo sapiens (Human)) | BDBM50048889

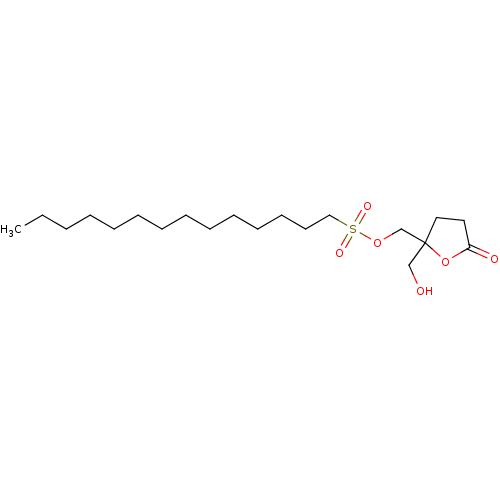
(CHEMBL299225 | Tetradecanoic acid 2-hydroxymethyl-...)Show InChI InChI=1S/C20H36O5/c1-2-3-4-5-6-7-8-9-10-11-12-13-18(22)24-17-20(16-21)15-14-19(23)25-20/h21H,2-17H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 138 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Cancer Institute
Curated by ChEMBL
| Assay Description
Displacement of [20-3H]phorbol12,13-dibutyrate from a recombinant PK-C alpha |
Bioorg Med Chem Lett 8: 1757-62 (1999)
BindingDB Entry DOI: 10.7270/Q2TH8KT2 |
More data for this
Ligand-Target Pair | |
Protein kinase C alpha type
(Homo sapiens (Human)) | BDBM50070859
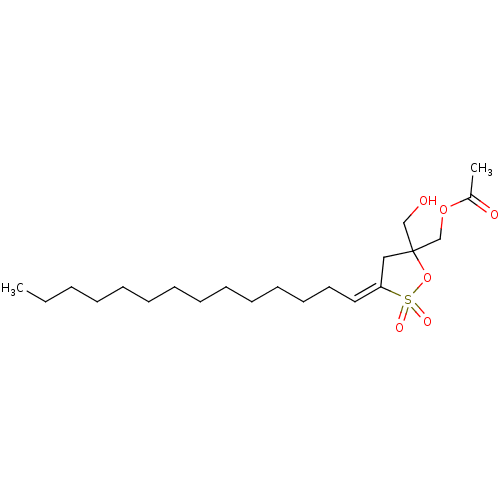
(Acetic acid 5-hydroxymethyl-2,2-dioxo-3-tetradec-(...)Show SMILES CCCCCCCCCCCCC\C=C1/CC(CO)(COC(C)=O)OS1(=O)=O Show InChI InChI=1S/C21H38O6S/c1-3-4-5-6-7-8-9-10-11-12-13-14-15-20-16-21(17-22,18-26-19(2)23)27-28(20,24)25/h15,22H,3-14,16-18H2,1-2H3/b20-15+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| 320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Cancer Institute
Curated by ChEMBL
| Assay Description
Displacement of [20-3H]phorbol12,13-dibutyrate from a recombinant PK-C alpha |
Bioorg Med Chem Lett 8: 1757-62 (1999)
BindingDB Entry DOI: 10.7270/Q2TH8KT2 |
More data for this
Ligand-Target Pair | |
Protein kinase C alpha type
(Homo sapiens (Human)) | BDBM50070861
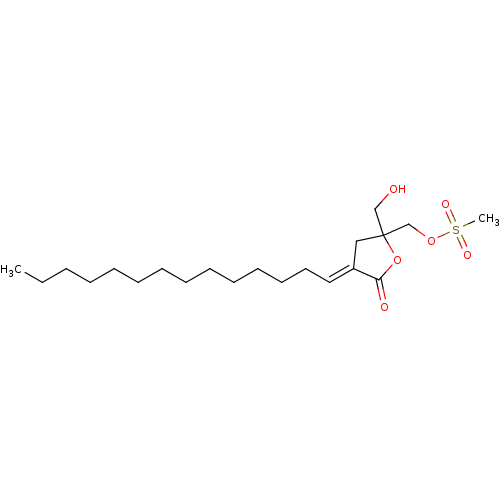
(CHEMBL48577 | Methanesulfonic acid 2-hydroxymethyl...)Show SMILES CCCCCCCCCCCCC\C=C1/CC(CO)(COS(C)(=O)=O)OC1=O Show InChI InChI=1S/C21H38O6S/c1-3-4-5-6-7-8-9-10-11-12-13-14-15-19-16-21(17-22,27-20(19)23)18-26-28(2,24)25/h15,22H,3-14,16-18H2,1-2H3/b19-15+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| 823 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Cancer Institute
Curated by ChEMBL
| Assay Description
Displacement of [20-3H]phorbol12,13-dibutyrate from a recombinant PK-C alpha |
Bioorg Med Chem Lett 8: 1757-62 (1999)
BindingDB Entry DOI: 10.7270/Q2TH8KT2 |
More data for this
Ligand-Target Pair | |
Protein kinase C alpha type
(Homo sapiens (Human)) | BDBM50070860
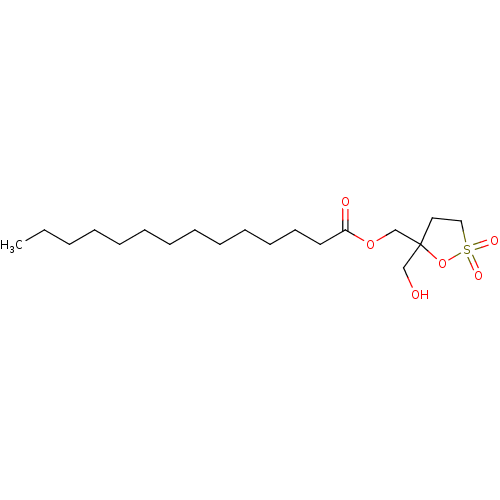
(CHEMBL51992 | Tetradecanoic acid 5-hydroxymethyl-2...)Show InChI InChI=1S/C19H36O6S/c1-2-3-4-5-6-7-8-9-10-11-12-13-18(21)24-17-19(16-20)14-15-26(22,23)25-19/h20H,2-17H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| 1.04E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Cancer Institute
Curated by ChEMBL
| Assay Description
Displacement of [20-3H]phorbol12,13-dibutyrate from a recombinant PK-C alpha |
Bioorg Med Chem Lett 8: 1757-62 (1999)
BindingDB Entry DOI: 10.7270/Q2TH8KT2 |
More data for this
Ligand-Target Pair | |
Hepatocyte growth factor receptor
(Homo sapiens (Human)) | BDBM50306682

((R)-3-(1-(2,6-dichloro-3-fluorophenyl)ethoxy)-5-(1...)Show SMILES C[C@@H](Oc1cc(cnc1N)-c1cnn(c1)C1CCNCC1)c1c(Cl)ccc(F)c1Cl |r| Show InChI InChI=1S/C21H22Cl2FN5O/c1-12(19-16(22)2-3-17(24)20(19)23)30-18-8-13(9-27-21(18)25)14-10-28-29(11-14)15-4-6-26-7-5-15/h2-3,8-12,15,26H,4-7H2,1H3,(H2,25,27)/t12-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Sungkyunkwan University
Curated by ChEMBL
| Assay Description
Inhibition of c-Met (unknown origin) by ADP-Glo kinase assay |
Bioorg Med Chem Lett 30: (2020)
Article DOI: 10.1016/j.bmcl.2020.127189
BindingDB Entry DOI: 10.7270/Q23F4T7M |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Prolyl endopeptidase
(Homo sapiens (Human)) | BDBM50557413
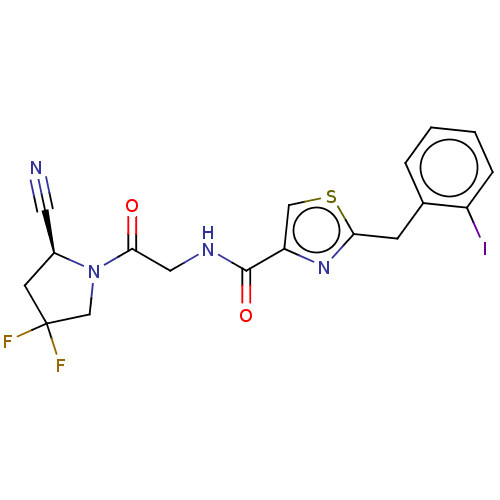
(CHEMBL4758377)Show SMILES FC1(F)C[C@@H](C#N)N(C1)C(=O)CNC(=O)c1csc(Cc2ccccc2I)n1 |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of PREP (unknown origin) using AMC substrate by fluorometric assay |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.127846
BindingDB Entry DOI: 10.7270/Q2PN999J |
More data for this
Ligand-Target Pair | |
Prolyl endopeptidase
(Homo sapiens (Human)) | BDBM50557433
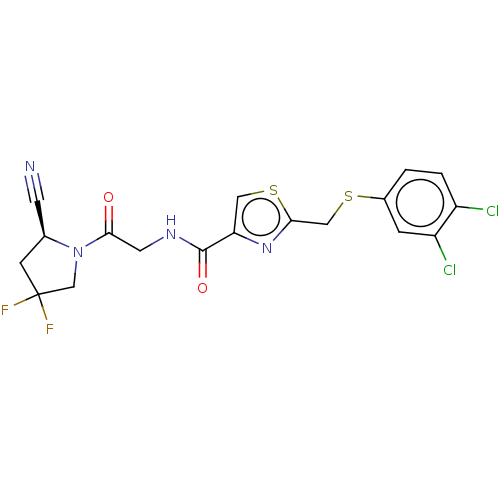
(CHEMBL4746891)Show SMILES FC1(F)C[C@@H](C#N)N(C1)C(=O)CNC(=O)c1csc(CSc2ccc(Cl)c(Cl)c2)n1 |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of PREP (unknown origin) using AMC substrate by fluorometric assay |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.127846
BindingDB Entry DOI: 10.7270/Q2PN999J |
More data for this
Ligand-Target Pair | |
Prolyl endopeptidase
(Homo sapiens (Human)) | BDBM50557417

(CHEMBL4762591)Show SMILES FC1(F)C[C@@H](C#N)N(C1)C(=O)CNC(=O)c1csc(Cc2ccc(Cl)cc2Cl)n1 |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of PREP (unknown origin) using AMC substrate by fluorometric assay |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.127846
BindingDB Entry DOI: 10.7270/Q2PN999J |
More data for this
Ligand-Target Pair | |
Acyl-protein thioesterase 1
(Rattus norvegicus) | BDBM50557417

(CHEMBL4762591)Show SMILES FC1(F)C[C@@H](C#N)N(C1)C(=O)CNC(=O)c1csc(Cc2ccc(Cl)cc2Cl)n1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of FAP (unknown origin) using AMC substrate by fluorometric assay |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.127846
BindingDB Entry DOI: 10.7270/Q2PN999J |
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50005711

(CHEBI:46024 | GNF-Pf-1011 | TRICHOSTATIN | Trichos...)Show SMILES C[C@H](\C=C(/C)\C=C\C(=O)NO)C(=O)c1ccc(cc1)N(C)C |r| Show InChI InChI=1S/C17H22N2O3/c1-12(5-10-16(20)18-22)11-13(2)17(21)14-6-8-15(9-7-14)19(3)4/h5-11,13,22H,1-4H3,(H,18,20)/b10-5+,12-11+/t13-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Sungkyunkwan University
Curated by ChEMBL
| Assay Description
Inhibition of full length human recombinant N-terminal GST-tagged HDAC6 (1 to 1215 residues) expressed in sf9 cells using RHK-K(Ac)-AMC as substrate ... |
Eur J Med Chem 116: 126-135 (2016)
Article DOI: 10.1016/j.ejmech.2016.03.046
BindingDB Entry DOI: 10.7270/Q2057HTZ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50557396
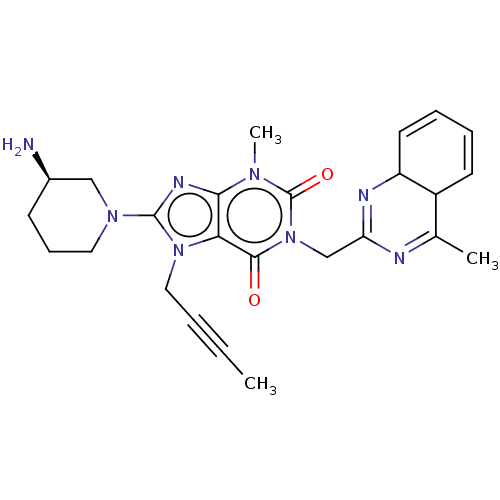
(CHEMBL4747683)Show SMILES CC#CCn1c(nc2n(C)c(=O)n(CC3=NC4C=CC=CC4C(C)=N3)c(=O)c12)N1CCC[C@@H](N)C1 |r,c:17,19,24,t:14| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of DPP4 (unknown origin) using AMC substrate by fluorometric assay |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.127846
BindingDB Entry DOI: 10.7270/Q2PN999J |
More data for this
Ligand-Target Pair | |
Acyl-protein thioesterase 1
(Rattus norvegicus) | BDBM50557416

(CHEMBL4786982)Show SMILES FC1(F)C[C@@H](C#N)N(C1)C(=O)CNC(=O)c1csc(Cc2ccc(Cl)c(Cl)c2)n1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of FAP (unknown origin) using AMC substrate by fluorometric assay |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.127846
BindingDB Entry DOI: 10.7270/Q2PN999J |
More data for this
Ligand-Target Pair | |
Hepatocyte growth factor receptor
(Homo sapiens (Human)) | BDBM50543787
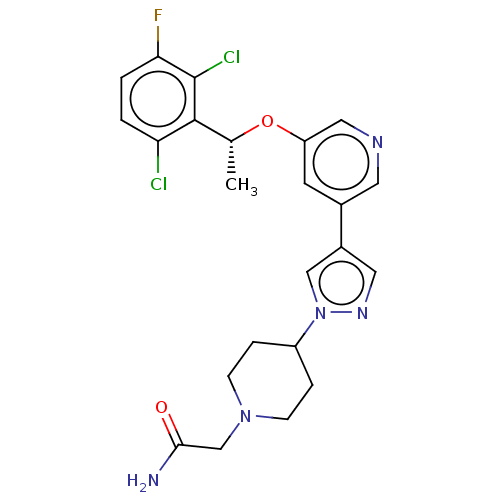
(CHEMBL4645582)Show SMILES C[C@@H](Oc1cncc(c1)-c1cnn(c1)C1CCN(CC(N)=O)CC1)c1c(Cl)ccc(F)c1Cl |r| Show InChI InChI=1S/C23H24Cl2FN5O2/c1-14(22-19(24)2-3-20(26)23(22)25)33-18-8-15(9-28-11-18)16-10-29-31(12-16)17-4-6-30(7-5-17)13-21(27)32/h2-3,8-12,14,17H,4-7,13H2,1H3,(H2,27,32)/t14-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Sungkyunkwan University
Curated by ChEMBL
| Assay Description
Inhibition of c-Met (unknown origin) by ADP-Glo kinase assay |
Bioorg Med Chem Lett 30: (2020)
Article DOI: 10.1016/j.bmcl.2020.127189
BindingDB Entry DOI: 10.7270/Q23F4T7M |
More data for this
Ligand-Target Pair | |
Acyl-protein thioesterase 1
(Rattus norvegicus) | BDBM50009366

(CHEMBL3233842 | US11504364, Compound Ref.-Comp. | ...)Show SMILES FC1(F)C[C@@H](C#N)N(C1)C(=O)CNC(=O)c1ccnc2ccccc12 |r| Show InChI InChI=1S/C17H14F2N4O2/c18-17(19)7-11(8-20)23(10-17)15(24)9-22-16(25)13-5-6-21-14-4-2-1-3-12(13)14/h1-6,11H,7,9-10H2,(H,22,25)/t11-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of FAP (unknown origin) using AMC substrate by fluorometric assay |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.127846
BindingDB Entry DOI: 10.7270/Q2PN999J |
More data for this
Ligand-Target Pair | |
Acyl-protein thioesterase 1
(Rattus norvegicus) | BDBM50557415
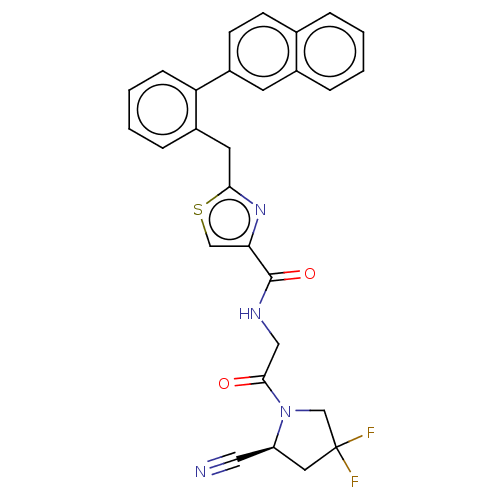
(CHEMBL4750058)Show SMILES FC1(F)C[C@@H](C#N)N(C1)C(=O)CNC(=O)c1csc(Cc2ccccc2-c2ccc3ccccc3c2)n1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of FAP (unknown origin) using AMC substrate by fluorometric assay |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.127846
BindingDB Entry DOI: 10.7270/Q2PN999J |
More data for this
Ligand-Target Pair | |
Hepatocyte growth factor receptor
(Homo sapiens (Human)) | BDBM50543784
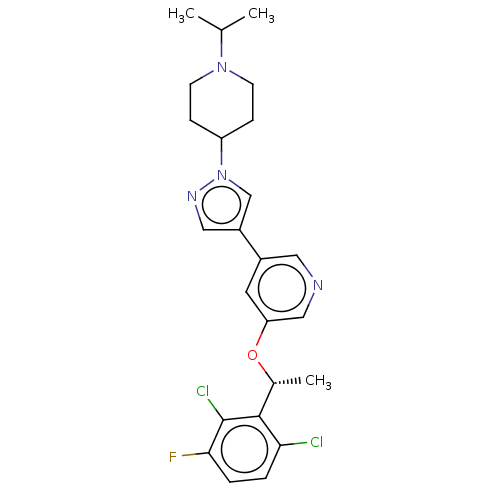
(CHEMBL4649193)Show SMILES CC(C)N1CCC(CC1)n1cc(cn1)-c1cncc(O[C@H](C)c2c(Cl)ccc(F)c2Cl)c1 |r| Show InChI InChI=1S/C24H27Cl2FN4O/c1-15(2)30-8-6-19(7-9-30)31-14-18(12-29-31)17-10-20(13-28-11-17)32-16(3)23-21(25)4-5-22(27)24(23)26/h4-5,10-16,19H,6-9H2,1-3H3/t16-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Sungkyunkwan University
Curated by ChEMBL
| Assay Description
Inhibition of c-Met (unknown origin) by ADP-Glo kinase assay |
Bioorg Med Chem Lett 30: (2020)
Article DOI: 10.1016/j.bmcl.2020.127189
BindingDB Entry DOI: 10.7270/Q23F4T7M |
More data for this
Ligand-Target Pair | |
Acyl-protein thioesterase 1
(Rattus norvegicus) | BDBM50557439

(CHEMBL4758299)Show SMILES FC(F)(F)c1ccc(cc1)S(=O)(=O)Cc1nc(cs1)C(=O)NCC(=O)N1CC(F)(F)C[C@H]1C#N |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of FAP (unknown origin) using AMC substrate by fluorometric assay |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.127846
BindingDB Entry DOI: 10.7270/Q2PN999J |
More data for this
Ligand-Target Pair | |
Prolyl endopeptidase
(Homo sapiens (Human)) | BDBM50557414
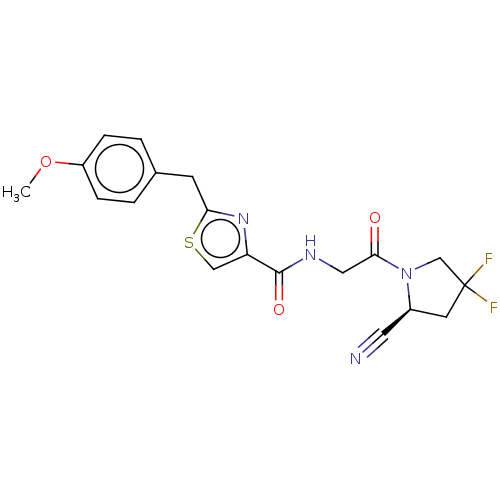
(CHEMBL4748328)Show SMILES COc1ccc(Cc2nc(cs2)C(=O)NCC(=O)N2CC(F)(F)C[C@H]2C#N)cc1 |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of PREP (unknown origin) using AMC substrate by fluorometric assay |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.127846
BindingDB Entry DOI: 10.7270/Q2PN999J |
More data for this
Ligand-Target Pair | |
Acyl-protein thioesterase 1
(Rattus norvegicus) | BDBM50557434
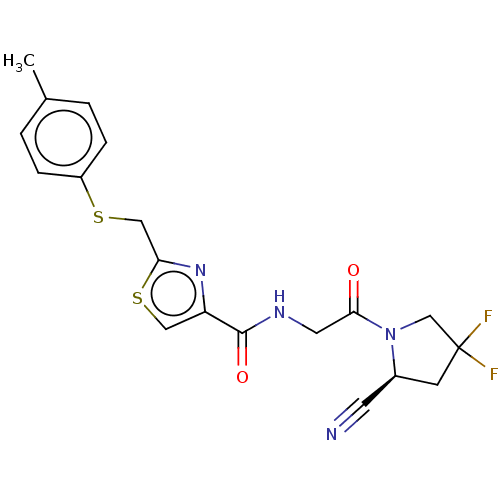
(CHEMBL4780357)Show SMILES Cc1ccc(SCc2nc(cs2)C(=O)NCC(=O)N2CC(F)(F)C[C@H]2C#N)cc1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of FAP (unknown origin) using AMC substrate by fluorometric assay |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.127846
BindingDB Entry DOI: 10.7270/Q2PN999J |
More data for this
Ligand-Target Pair | |
Hepatocyte growth factor receptor
(Homo sapiens (Human)) | BDBM50543785
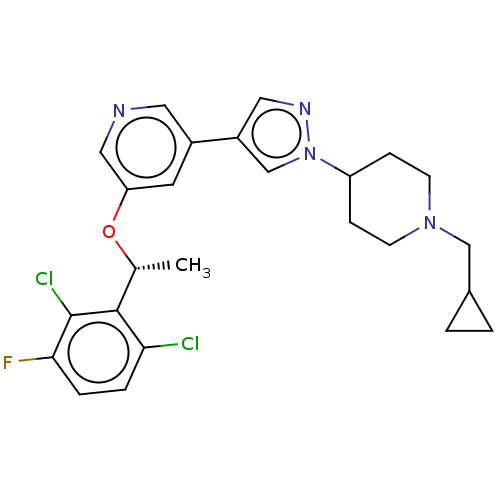
(CHEMBL4646769)Show SMILES C[C@@H](Oc1cncc(c1)-c1cnn(c1)C1CCN(CC2CC2)CC1)c1c(Cl)ccc(F)c1Cl |r| Show InChI InChI=1S/C25H27Cl2FN4O/c1-16(24-22(26)4-5-23(28)25(24)27)33-21-10-18(11-29-13-21)19-12-30-32(15-19)20-6-8-31(9-7-20)14-17-2-3-17/h4-5,10-13,15-17,20H,2-3,6-9,14H2,1H3/t16-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Sungkyunkwan University
Curated by ChEMBL
| Assay Description
Inhibition of c-Met (unknown origin) by ADP-Glo kinase assay |
Bioorg Med Chem Lett 30: (2020)
Article DOI: 10.1016/j.bmcl.2020.127189
BindingDB Entry DOI: 10.7270/Q23F4T7M |
More data for this
Ligand-Target Pair | |
Hepatocyte growth factor receptor
(Homo sapiens (Human)) | BDBM50543788
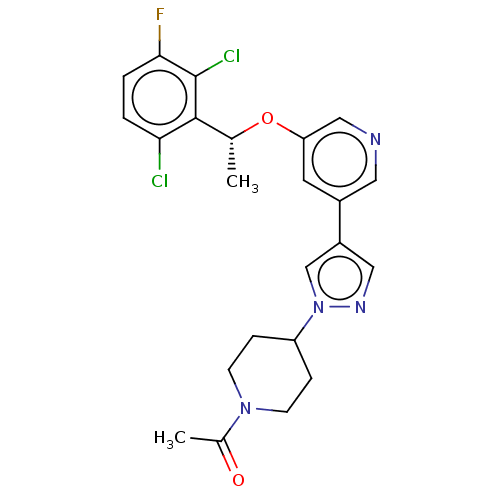
(CHEMBL4638327)Show SMILES C[C@@H](Oc1cncc(c1)-c1cnn(c1)C1CCN(CC1)C(C)=O)c1c(Cl)ccc(F)c1Cl |r| Show InChI InChI=1S/C23H23Cl2FN4O2/c1-14(22-20(24)3-4-21(26)23(22)25)32-19-9-16(10-27-12-19)17-11-28-30(13-17)18-5-7-29(8-6-18)15(2)31/h3-4,9-14,18H,5-8H2,1-2H3/t14-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Sungkyunkwan University
Curated by ChEMBL
| Assay Description
Inhibition of c-Met (unknown origin) by ADP-Glo kinase assay |
Bioorg Med Chem Lett 30: (2020)
Article DOI: 10.1016/j.bmcl.2020.127189
BindingDB Entry DOI: 10.7270/Q23F4T7M |
More data for this
Ligand-Target Pair | |
Hepatocyte growth factor receptor
(Homo sapiens (Human)) | BDBM50469052
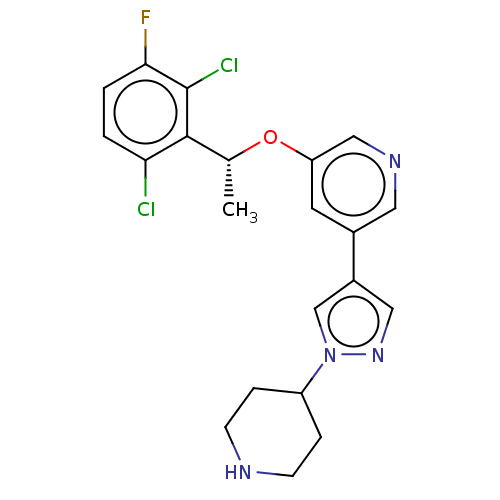
(CHEMBL4286383)Show SMILES C[C@@H](Oc1cncc(c1)-c1cnn(c1)C1CCNCC1)c1c(Cl)ccc(F)c1Cl |r| Show InChI InChI=1S/C21H21Cl2FN4O/c1-13(20-18(22)2-3-19(24)21(20)23)29-17-8-14(9-26-11-17)15-10-27-28(12-15)16-4-6-25-7-5-16/h2-3,8-13,16,25H,4-7H2,1H3/t13-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Sungkyunkwan University
Curated by ChEMBL
| Assay Description
Inhibition of c-Met (unknown origin) by ADP-Glo kinase assay |
Bioorg Med Chem Lett 30: (2020)
Article DOI: 10.1016/j.bmcl.2020.127189
BindingDB Entry DOI: 10.7270/Q23F4T7M |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50005711

(CHEBI:46024 | GNF-Pf-1011 | TRICHOSTATIN | Trichos...)Show SMILES C[C@H](\C=C(/C)\C=C\C(=O)NO)C(=O)c1ccc(cc1)N(C)C |r| Show InChI InChI=1S/C17H22N2O3/c1-12(5-10-16(20)18-22)11-13(2)17(21)14-6-8-15(9-7-14)19(3)4/h5-11,13,22H,1-4H3,(H,18,20)/b10-5+,12-11+/t13-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 6.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Sungkyunkwan University
Curated by ChEMBL
| Assay Description
Inhibition of full length human recombinant C-terminal FLAG-His-tagged HDAC1 (1 to 482 residues) expressed in Sf21 cells using RHK-K(Ac)-AMC as subst... |
Eur J Med Chem 116: 126-135 (2016)
Article DOI: 10.1016/j.ejmech.2016.03.046
BindingDB Entry DOI: 10.7270/Q2057HTZ |
More data for this
Ligand-Target Pair | |
Acyl-protein thioesterase 1
(Rattus norvegicus) | BDBM50557442
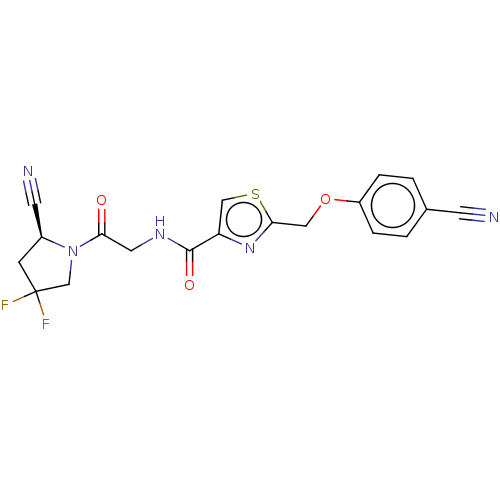
(CHEMBL4747575)Show SMILES FC1(F)C[C@@H](C#N)N(C1)C(=O)CNC(=O)c1csc(COc2ccc(cc2)C#N)n1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of FAP (unknown origin) using AMC substrate by fluorometric assay |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.127846
BindingDB Entry DOI: 10.7270/Q2PN999J |
More data for this
Ligand-Target Pair | |
Prolyl endopeptidase
(Homo sapiens (Human)) | BDBM50557410
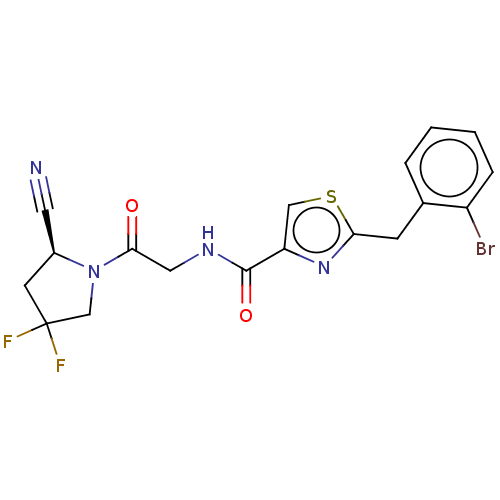
(CHEMBL4751873)Show SMILES FC1(F)C[C@@H](C#N)N(C1)C(=O)CNC(=O)c1csc(Cc2ccccc2Br)n1 |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of PREP (unknown origin) using AMC substrate by fluorometric assay |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.127846
BindingDB Entry DOI: 10.7270/Q2PN999J |
More data for this
Ligand-Target Pair | |
Acyl-protein thioesterase 1
(Rattus norvegicus) | BDBM50557438

(CHEMBL4789965)Show SMILES FC1(F)C[C@@H](C#N)N(C1)C(=O)CNC(=O)c1csc(CS(=O)(=O)c2ccc(cc2)C#N)n1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of FAP (unknown origin) using AMC substrate by fluorometric assay |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.127846
BindingDB Entry DOI: 10.7270/Q2PN999J |
More data for this
Ligand-Target Pair | |
Histone deacetylase 2
(Homo sapiens (Human)) | BDBM50005711

(CHEBI:46024 | GNF-Pf-1011 | TRICHOSTATIN | Trichos...)Show SMILES C[C@H](\C=C(/C)\C=C\C(=O)NO)C(=O)c1ccc(cc1)N(C)C |r| Show InChI InChI=1S/C17H22N2O3/c1-12(5-10-16(20)18-22)11-13(2)17(21)14-6-8-15(9-7-14)19(3)4/h5-11,13,22H,1-4H3,(H,18,20)/b10-5+,12-11+/t13-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 10.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Sungkyunkwan University
Curated by ChEMBL
| Assay Description
Inhibition of full length human recombinant C-terminal GST-tagged HDAC2 (1 to 488 residues) expressed in insect cells using RHK-K(Ac)-AMC as substrat... |
Eur J Med Chem 116: 126-135 (2016)
Article DOI: 10.1016/j.ejmech.2016.03.046
BindingDB Entry DOI: 10.7270/Q2057HTZ |
More data for this
Ligand-Target Pair | |
Acyl-protein thioesterase 1
(Rattus norvegicus) | BDBM50557436

(CHEMBL4776967)Show SMILES FC(F)(F)c1ccc(SCc2nc(cs2)C(=O)NCC(=O)N2CC(F)(F)C[C@H]2C#N)cc1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of FAP (unknown origin) using AMC substrate by fluorometric assay |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.127846
BindingDB Entry DOI: 10.7270/Q2PN999J |
More data for this
Ligand-Target Pair | |
Histone deacetylase 3/Nuclear receptor corepressor 2 (HDAC3/NCoR2)
(Homo sapiens (Human)) | BDBM50005711

(CHEBI:46024 | GNF-Pf-1011 | TRICHOSTATIN | Trichos...)Show SMILES C[C@H](\C=C(/C)\C=C\C(=O)NO)C(=O)c1ccc(cc1)N(C)C |r| Show InChI InChI=1S/C17H22N2O3/c1-12(5-10-16(20)18-22)11-13(2)17(21)14-6-8-15(9-7-14)19(3)4/h5-11,13,22H,1-4H3,(H,18,20)/b10-5+,12-11+/t13-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 11.1 | n/a | n/a | n/a | n/a | n/a | n/a |
Sungkyunkwan University
Curated by ChEMBL
| Assay Description
Inhibition of full length human recombinant C-terminal His-tagged HDAC3 (1 to 428 residues)/human recombinant N-terminal GST-tagged NCOR2 expressed i... |
Eur J Med Chem 116: 126-135 (2016)
Article DOI: 10.1016/j.ejmech.2016.03.046
BindingDB Entry DOI: 10.7270/Q2057HTZ |
More data for this
Ligand-Target Pair | |
Histone deacetylase 11
(Homo sapiens (Human)) | BDBM50005711

(CHEBI:46024 | GNF-Pf-1011 | TRICHOSTATIN | Trichos...)Show SMILES C[C@H](\C=C(/C)\C=C\C(=O)NO)C(=O)c1ccc(cc1)N(C)C |r| Show InChI InChI=1S/C17H22N2O3/c1-12(5-10-16(20)18-22)11-13(2)17(21)14-6-8-15(9-7-14)19(3)4/h5-11,13,22H,1-4H3,(H,18,20)/b10-5+,12-11+/t13-/m1/s1 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 11.4 | n/a | n/a | n/a | n/a | n/a | n/a |
Sungkyunkwan University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant N-terminal His-tagged HDAC11 (1 to 347 residues) expressed in insect cells/baculovirus expression system using RHK-K(... |
Eur J Med Chem 116: 126-135 (2016)
Article DOI: 10.1016/j.ejmech.2016.03.046
BindingDB Entry DOI: 10.7270/Q2057HTZ |
More data for this
Ligand-Target Pair | |
Hepatocyte growth factor receptor
(Homo sapiens (Human)) | BDBM50543791
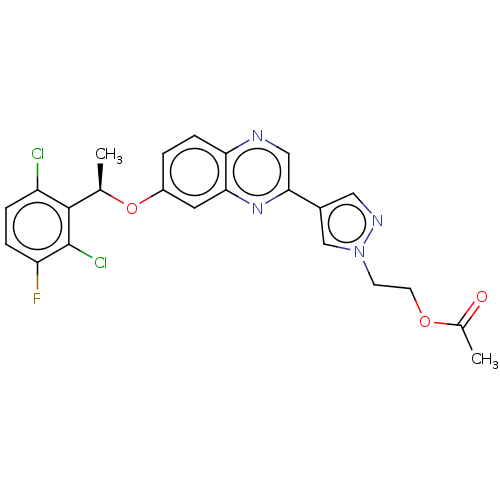
(CHEMBL4648693)Show SMILES C[C@@H](Oc1ccc2ncc(nc2c1)-c1cnn(CCOC(C)=O)c1)c1c(Cl)ccc(F)c1Cl |r| Show InChI InChI=1S/C23H19Cl2FN4O3/c1-13(22-17(24)4-5-18(26)23(22)25)33-16-3-6-19-20(9-16)29-21(11-27-19)15-10-28-30(12-15)7-8-32-14(2)31/h3-6,9-13H,7-8H2,1-2H3/t13-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Sungkyunkwan University
Curated by ChEMBL
| Assay Description
Inhibition of c-Met (unknown origin) by ADP-Glo kinase assay |
Bioorg Med Chem Lett 30: (2020)
Article DOI: 10.1016/j.bmcl.2020.127189
BindingDB Entry DOI: 10.7270/Q23F4T7M |
More data for this
Ligand-Target Pair | |
Hepatocyte growth factor receptor
(Homo sapiens (Human)) | BDBM50543793
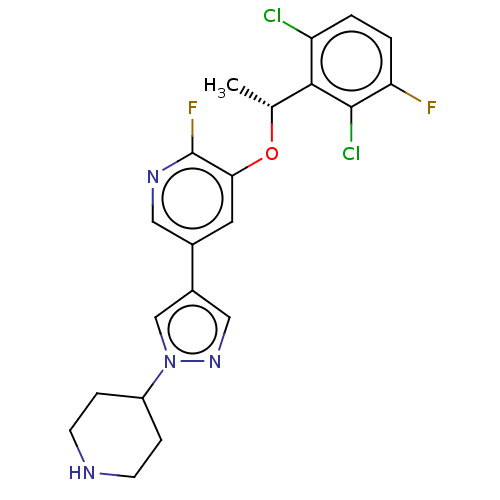
(CHEMBL4638210)Show SMILES Cl.C[C@@H](Oc1cc(cnc1F)-c1cnn(c1)C1CCNCC1)c1c(Cl)ccc(F)c1Cl |r| Show InChI InChI=1S/C21H20Cl2F2N4O.ClH/c1-12(19-16(22)2-3-17(24)20(19)23)30-18-8-13(9-27-21(18)25)14-10-28-29(11-14)15-4-6-26-7-5-15;/h2-3,8-12,15,26H,4-7H2,1H3;1H/t12-;/m1./s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Sungkyunkwan University
Curated by ChEMBL
| Assay Description
Inhibition of c-Met (unknown origin) by ADP-Glo kinase assay |
Bioorg Med Chem Lett 30: (2020)
Article DOI: 10.1016/j.bmcl.2020.127189
BindingDB Entry DOI: 10.7270/Q23F4T7M |
More data for this
Ligand-Target Pair | |
Acyl-protein thioesterase 1
(Rattus norvegicus) | BDBM50557424
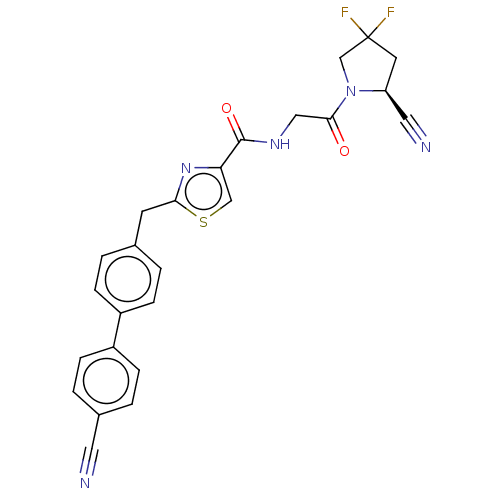
(CHEMBL4753316)Show SMILES FC1(F)C[C@@H](C#N)N(C1)C(=O)CNC(=O)c1csc(Cc2ccc(cc2)-c2ccc(cc2)C#N)n1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of FAP (unknown origin) using AMC substrate by fluorometric assay |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.127846
BindingDB Entry DOI: 10.7270/Q2PN999J |
More data for this
Ligand-Target Pair | |
Hepatocyte growth factor receptor
(Homo sapiens (Human)) | BDBM50543790
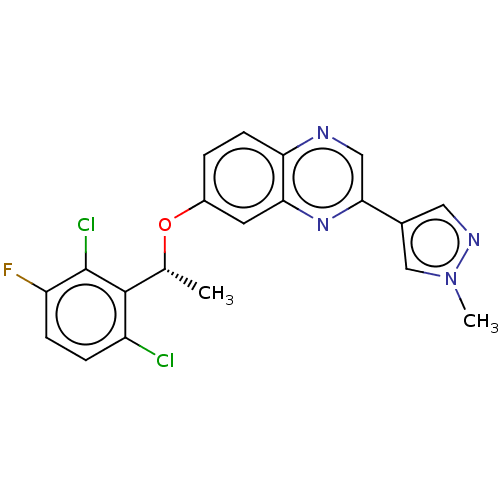
(CHEMBL4642284)Show SMILES C[C@@H](Oc1ccc2ncc(nc2c1)-c1cnn(C)c1)c1c(Cl)ccc(F)c1Cl |r| Show InChI InChI=1S/C20H15Cl2FN4O/c1-11(19-14(21)4-5-15(23)20(19)22)28-13-3-6-16-17(7-13)26-18(9-24-16)12-8-25-27(2)10-12/h3-11H,1-2H3/t11-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Sungkyunkwan University
Curated by ChEMBL
| Assay Description
Inhibition of c-Met (unknown origin) by ADP-Glo kinase assay |
Bioorg Med Chem Lett 30: (2020)
Article DOI: 10.1016/j.bmcl.2020.127189
BindingDB Entry DOI: 10.7270/Q23F4T7M |
More data for this
Ligand-Target Pair | |
Acyl-protein thioesterase 1
(Rattus norvegicus) | BDBM50557423
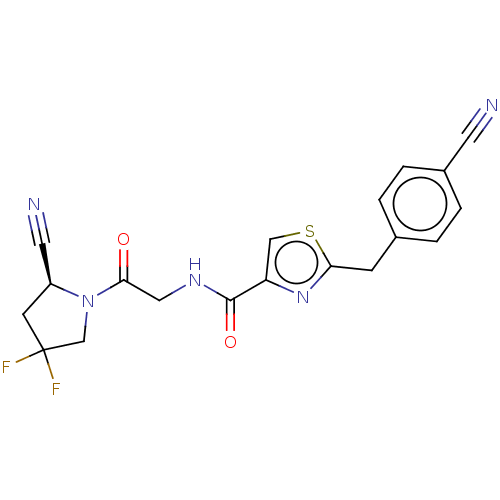
(CHEMBL4799823)Show SMILES FC1(F)C[C@@H](C#N)N(C1)C(=O)CNC(=O)c1csc(Cc2ccc(cc2)C#N)n1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of FAP (unknown origin) using AMC substrate by fluorometric assay |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.127846
BindingDB Entry DOI: 10.7270/Q2PN999J |
More data for this
Ligand-Target Pair | |
Hepatocyte growth factor receptor
(Homo sapiens (Human)) | BDBM50543792
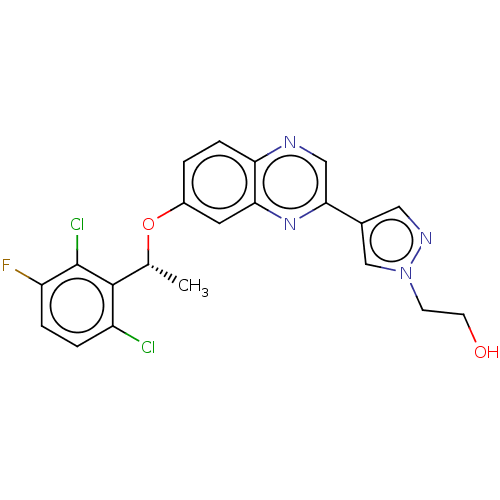
(CHEMBL4636429)Show SMILES C[C@@H](Oc1ccc2ncc(nc2c1)-c1cnn(CCO)c1)c1c(Cl)ccc(F)c1Cl |r| Show InChI InChI=1S/C21H17Cl2FN4O2/c1-12(20-15(22)3-4-16(24)21(20)23)30-14-2-5-17-18(8-14)27-19(10-25-17)13-9-26-28(11-13)6-7-29/h2-5,8-12,29H,6-7H2,1H3/t12-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Sungkyunkwan University
Curated by ChEMBL
| Assay Description
Inhibition of c-Met (unknown origin) by ADP-Glo kinase assay |
Bioorg Med Chem Lett 30: (2020)
Article DOI: 10.1016/j.bmcl.2020.127189
BindingDB Entry DOI: 10.7270/Q23F4T7M |
More data for this
Ligand-Target Pair | |
Prolyl endopeptidase
(Homo sapiens (Human)) | BDBM50557418
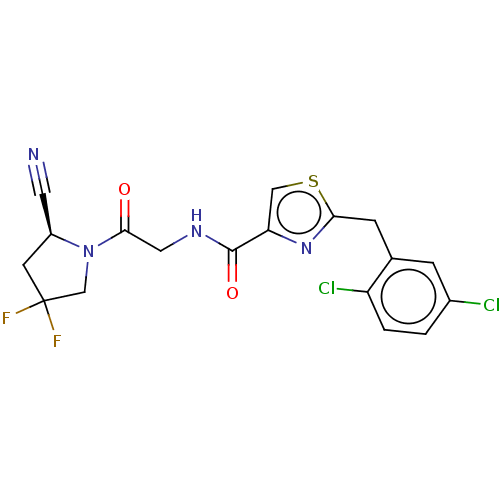
(CHEMBL4792518)Show SMILES FC1(F)C[C@@H](C#N)N(C1)C(=O)CNC(=O)c1csc(Cc2cc(Cl)ccc2Cl)n1 |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of PREP (unknown origin) using AMC substrate by fluorometric assay |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.127846
BindingDB Entry DOI: 10.7270/Q2PN999J |
More data for this
Ligand-Target Pair | |
Acyl-protein thioesterase 1
(Rattus norvegicus) | BDBM50557412
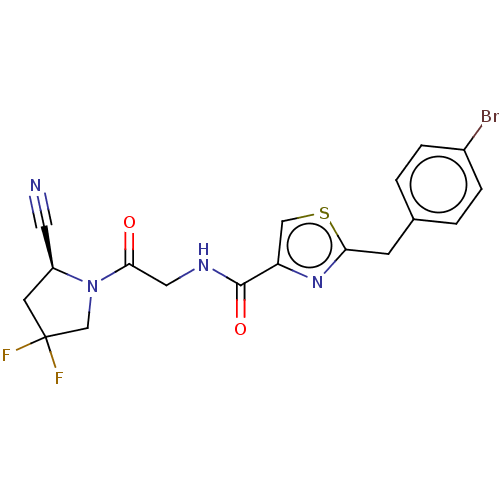
(CHEMBL4746868)Show SMILES FC1(F)C[C@@H](C#N)N(C1)C(=O)CNC(=O)c1csc(Cc2ccc(Br)cc2)n1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of FAP (unknown origin) using AMC substrate by fluorometric assay |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.127846
BindingDB Entry DOI: 10.7270/Q2PN999J |
More data for this
Ligand-Target Pair | |
Hepatocyte growth factor receptor
(Homo sapiens (Human)) | BDBM50543789
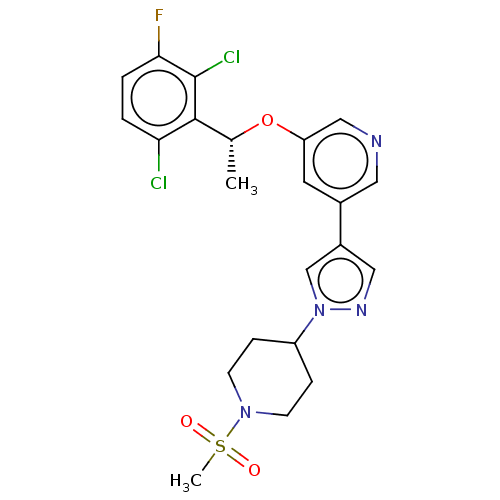
(CHEMBL4648551)Show SMILES C[C@@H](Oc1cncc(c1)-c1cnn(c1)C1CCN(CC1)S(C)(=O)=O)c1c(Cl)ccc(F)c1Cl |r| Show InChI InChI=1S/C22H23Cl2FN4O3S/c1-14(21-19(23)3-4-20(25)22(21)24)32-18-9-15(10-26-12-18)16-11-27-29(13-16)17-5-7-28(8-6-17)33(2,30)31/h3-4,9-14,17H,5-8H2,1-2H3/t14-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Sungkyunkwan University
Curated by ChEMBL
| Assay Description
Inhibition of c-Met (unknown origin) by ADP-Glo kinase assay |
Bioorg Med Chem Lett 30: (2020)
Article DOI: 10.1016/j.bmcl.2020.127189
BindingDB Entry DOI: 10.7270/Q23F4T7M |
More data for this
Ligand-Target Pair | |
Hepatocyte growth factor receptor
(Homo sapiens (Human)) | BDBM50543783
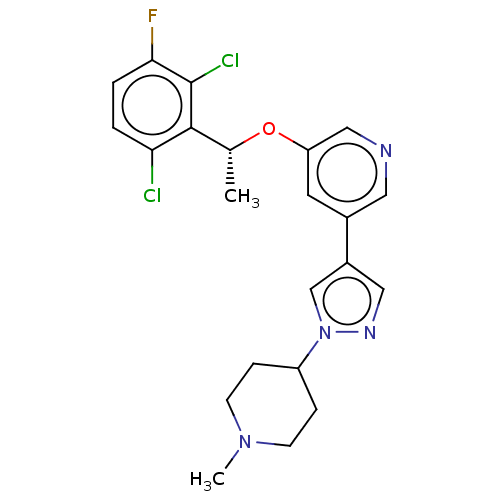
(CHEMBL4635560)Show SMILES C[C@@H](Oc1cncc(c1)-c1cnn(c1)C1CCN(C)CC1)c1c(Cl)ccc(F)c1Cl |r| Show InChI InChI=1S/C22H23Cl2FN4O/c1-14(21-19(23)3-4-20(25)22(21)24)30-18-9-15(10-26-12-18)16-11-27-29(13-16)17-5-7-28(2)8-6-17/h3-4,9-14,17H,5-8H2,1-2H3/t14-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Sungkyunkwan University
Curated by ChEMBL
| Assay Description
Inhibition of c-Met (unknown origin) by ADP-Glo kinase assay |
Bioorg Med Chem Lett 30: (2020)
Article DOI: 10.1016/j.bmcl.2020.127189
BindingDB Entry DOI: 10.7270/Q23F4T7M |
More data for this
Ligand-Target Pair | |
Acyl-protein thioesterase 1
(Rattus norvegicus) | BDBM50557437
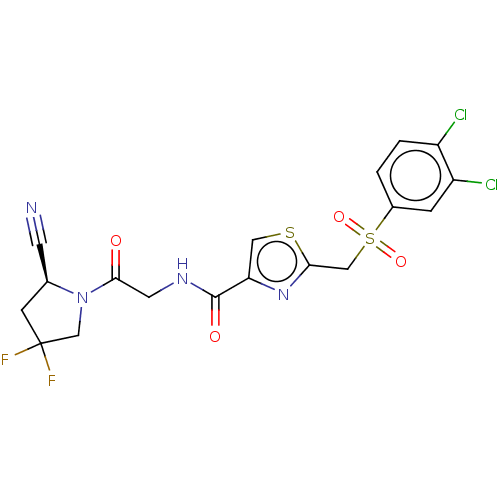
(CHEMBL4791505)Show SMILES FC1(F)C[C@@H](C#N)N(C1)C(=O)CNC(=O)c1csc(CS(=O)(=O)c2ccc(Cl)c(Cl)c2)n1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of FAP (unknown origin) using AMC substrate by fluorometric assay |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.127846
BindingDB Entry DOI: 10.7270/Q2PN999J |
More data for this
Ligand-Target Pair | |
Acyl-protein thioesterase 1
(Rattus norvegicus) | BDBM50557440
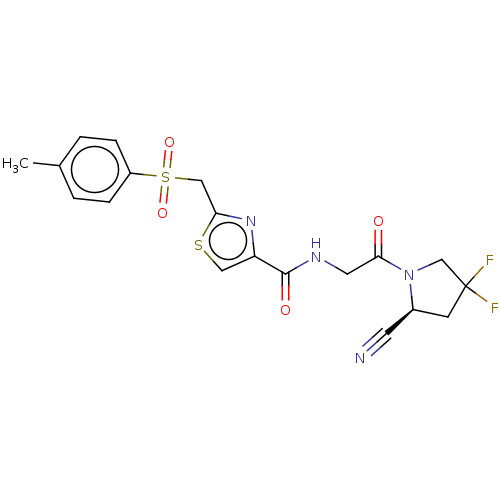
(CHEMBL4759201)Show SMILES Cc1ccc(cc1)S(=O)(=O)Cc1nc(cs1)C(=O)NCC(=O)N1CC(F)(F)C[C@H]1C#N |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of FAP (unknown origin) using AMC substrate by fluorometric assay |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.127846
BindingDB Entry DOI: 10.7270/Q2PN999J |
More data for this
Ligand-Target Pair | |
Acyl-protein thioesterase 1
(Rattus norvegicus) | BDBM50557428
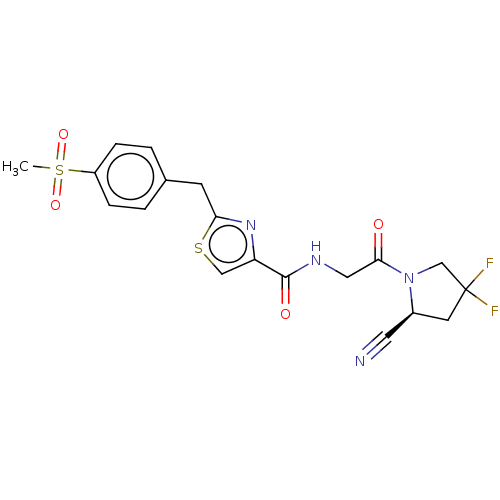
(CHEMBL4797479)Show SMILES CS(=O)(=O)c1ccc(Cc2nc(cs2)C(=O)NCC(=O)N2CC(F)(F)C[C@H]2C#N)cc1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of FAP (unknown origin) using AMC substrate by fluorometric assay |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.127846
BindingDB Entry DOI: 10.7270/Q2PN999J |
More data for this
Ligand-Target Pair | |
Acyl-protein thioesterase 1
(Rattus norvegicus) | BDBM50557409
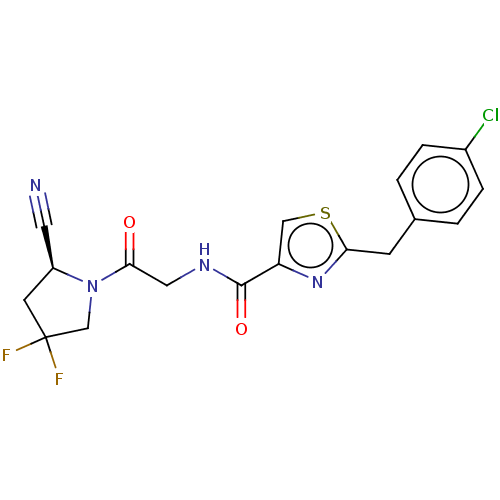
(CHEMBL4789754)Show SMILES FC1(F)C[C@@H](C#N)N(C1)C(=O)CNC(=O)c1csc(Cc2ccc(Cl)cc2)n1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of FAP (unknown origin) using AMC substrate by fluorometric assay |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.127846
BindingDB Entry DOI: 10.7270/Q2PN999J |
More data for this
Ligand-Target Pair | |
Acyl-protein thioesterase 1
(Rattus norvegicus) | BDBM50557432
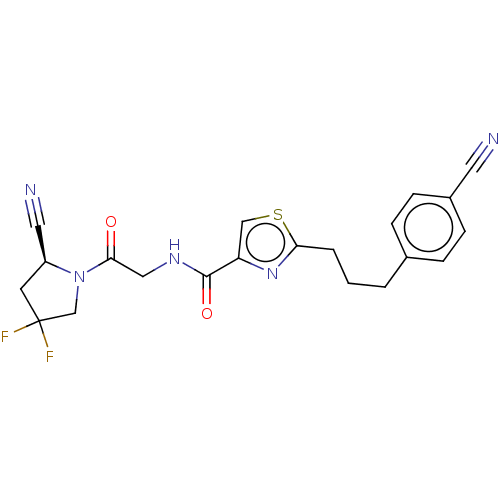
(CHEMBL4781018)Show SMILES FC1(F)C[C@@H](C#N)N(C1)C(=O)CNC(=O)c1csc(CCCc2ccc(cc2)C#N)n1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of FAP (unknown origin) using AMC substrate by fluorometric assay |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.127846
BindingDB Entry DOI: 10.7270/Q2PN999J |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50050513

((R)-1-((S)-2-amino-3-methylbutanoyl)pyrrolidin-2-y...)Show InChI InChI=1S/C9H19BN2O3/c1-6(2)8(11)9(13)12-5-3-4-7(12)10(14)15/h6-8,14-15H,3-5,11H2,1-2H3/t7-,8-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of DPP4 (unknown origin) using AMC substrate by fluorometric assay |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.127846
BindingDB Entry DOI: 10.7270/Q2PN999J |
More data for this
Ligand-Target Pair | |
Acyl-protein thioesterase 1
(Rattus norvegicus) | BDBM50557422
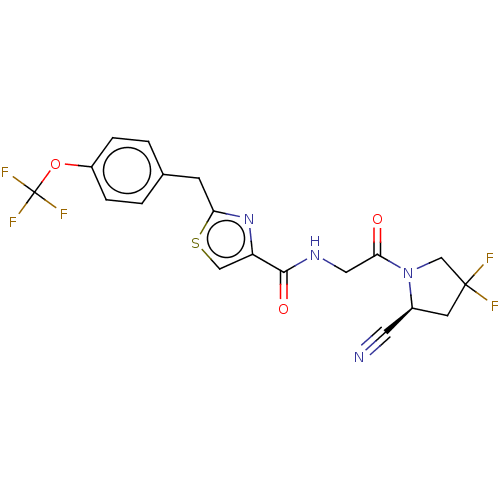
(CHEMBL4744633)Show SMILES FC(F)(F)Oc1ccc(Cc2nc(cs2)C(=O)NCC(=O)N2CC(F)(F)C[C@H]2C#N)cc1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of FAP (unknown origin) using AMC substrate by fluorometric assay |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.127846
BindingDB Entry DOI: 10.7270/Q2PN999J |
More data for this
Ligand-Target Pair | |
Acyl-protein thioesterase 1
(Rattus norvegicus) | BDBM50557429
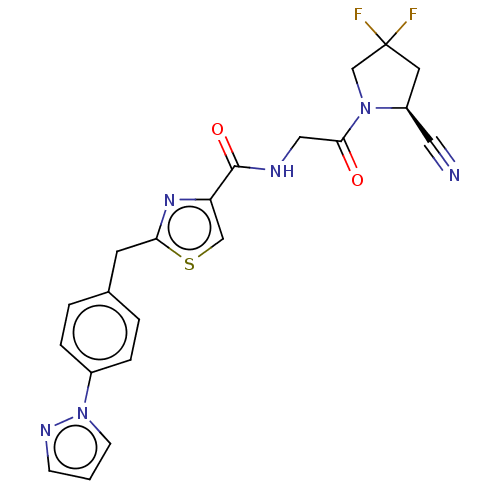
(CHEMBL4742308)Show SMILES FC1(F)C[C@@H](C#N)N(C1)C(=O)CNC(=O)c1csc(Cc2ccc(cc2)-n2cccn2)n1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of FAP (unknown origin) using AMC substrate by fluorometric assay |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.127846
BindingDB Entry DOI: 10.7270/Q2PN999J |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data