

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Amyloid-beta precursor protein (Homo sapiens (Human)) | BDBM50483880 (CHEMBL1774642) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University Bundang Hospital Curated by ChEMBL | Assay Description Displacement of [3H]PIB from amyloid beta (1 to 42) aggregates in Alzheimer's disease-human brain homogenate after 3 hrs by gamma counting | Bioorg Med Chem 19: 2980-90 (2011) Article DOI: 10.1016/j.bmc.2011.03.029 BindingDB Entry DOI: 10.7270/Q21839CG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amyloid-beta precursor protein (Homo sapiens (Human)) | BDBM50483881 (CHEMBL1774643) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University Bundang Hospital Curated by ChEMBL | Assay Description Displacement of [3H]PIB from amyloid beta (1 to 42) aggregates in Alzheimer's disease-human brain homogenate after 3 hrs by gamma counting | Bioorg Med Chem 19: 2980-90 (2011) Article DOI: 10.1016/j.bmc.2011.03.029 BindingDB Entry DOI: 10.7270/Q21839CG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-4/beta-2 (Homo sapiens (Human)) | BDBM50135703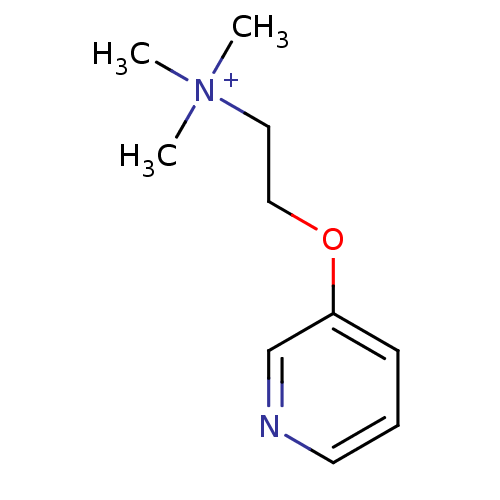 (CHEMBL345732 | CHEMBL99367 | Trimethyl-[2-(pyridin...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Binding affinity at alpha4beta2 | Bioorg Med Chem Lett 16: 4283-6 (2006) Article DOI: 10.1016/j.bmcl.2006.05.080 BindingDB Entry DOI: 10.7270/Q2CJ8F8J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amyloid-beta precursor protein (Homo sapiens (Human)) | BDBM50129793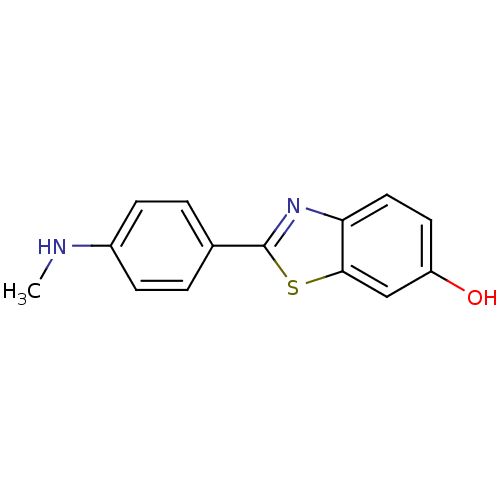 (2-(4''-methylaminophenyl)-6-hydroxybenzothiazole |...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University Bundang Hospital Curated by ChEMBL | Assay Description Displacement of [3H]PIB from amyloid beta (1 to 42) aggregates in Alzheimer's disease-human brain homogenate after 3 hrs by gamma counting | Bioorg Med Chem 19: 2980-90 (2011) Article DOI: 10.1016/j.bmc.2011.03.029 BindingDB Entry DOI: 10.7270/Q21839CG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amyloid-beta precursor protein (Homo sapiens (Human)) | BDBM50483880 (CHEMBL1774642) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 5.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University Bundang Hospital Curated by ChEMBL | Assay Description Displacement of [125I]TZDM from amyloid beta (1 to 42) aggregates in Alzheimer's disease-human brain homogenate after 3 hrs by gamma counting | Bioorg Med Chem 19: 2980-90 (2011) Article DOI: 10.1016/j.bmc.2011.03.029 BindingDB Entry DOI: 10.7270/Q21839CG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amyloid-beta precursor protein (Homo sapiens (Human)) | BDBM50129793 (2-(4''-methylaminophenyl)-6-hydroxybenzothiazole |...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 5.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University Bundang Hospital Curated by ChEMBL | Assay Description Displacement of [125I]TZDM from amyloid beta (1 to 42) aggregates in Alzheimer's disease-human brain homogenate after 3 hrs by gamma counting | Bioorg Med Chem 19: 2980-90 (2011) Article DOI: 10.1016/j.bmc.2011.03.029 BindingDB Entry DOI: 10.7270/Q21839CG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amyloid-beta precursor protein (Homo sapiens (Human)) | BDBM50483881 (CHEMBL1774643) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 5.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University Bundang Hospital Curated by ChEMBL | Assay Description Displacement of [125I]TZDM from amyloid beta (1 to 42) aggregates in Alzheimer's disease-human brain homogenate after 3 hrs by gamma counting | Bioorg Med Chem 19: 2980-90 (2011) Article DOI: 10.1016/j.bmc.2011.03.029 BindingDB Entry DOI: 10.7270/Q21839CG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amyloid-beta precursor protein (Homo sapiens (Human)) | BDBM50483882 (CHEMBL284575) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 26 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University Bundang Hospital Curated by ChEMBL | Assay Description Displacement of [125I]TZDM from amyloid beta (1 to 42) aggregates in Alzheimer's disease-human brain homogenate after 3 hrs by gamma counting | Bioorg Med Chem 19: 2980-90 (2011) Article DOI: 10.1016/j.bmc.2011.03.029 BindingDB Entry DOI: 10.7270/Q21839CG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-7 (Rattus norvegicus (Rat)) | BDBM50135703 (CHEMBL345732 | CHEMBL99367 | Trimethyl-[2-(pyridin...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Displacement of [125I]iodo-MLA from alpha-7 nAChR in rat cerebral cortex | Bioorg Med Chem Lett 16: 4283-6 (2006) Article DOI: 10.1016/j.bmcl.2006.05.080 BindingDB Entry DOI: 10.7270/Q2CJ8F8J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-7 (Rattus norvegicus (Rat)) | BDBM82070 (CAS_29790-52-1 | NICOTINE-L (BASE) | Nicotine-D sa...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase KEGG PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 750 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Displacement of [125I]iodo-MLA from alpha-7 nAChR in rat cerebral cortex | Bioorg Med Chem Lett 16: 4283-6 (2006) Article DOI: 10.1016/j.bmcl.2006.05.080 BindingDB Entry DOI: 10.7270/Q2CJ8F8J | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Neuronal acetylcholine receptor subunit alpha-7 (Rattus norvegicus (Rat)) | BDBM50188956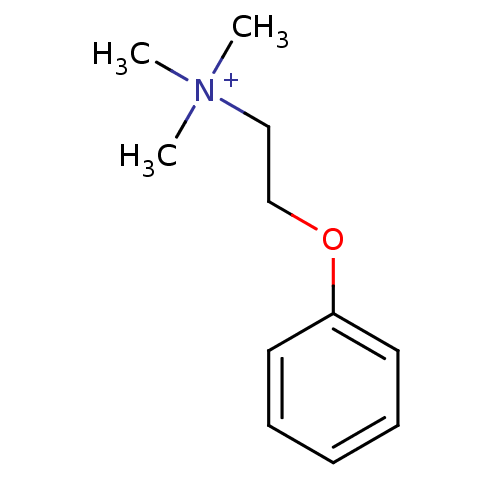 (CHEMBL209891 | N,N,N-trimethyl-2-phenoxyethanamini...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Displacement of [125I]iodo-MLA from alpha-7 nAChR in rat cerebral cortex | Bioorg Med Chem Lett 16: 4283-6 (2006) Article DOI: 10.1016/j.bmcl.2006.05.080 BindingDB Entry DOI: 10.7270/Q2CJ8F8J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-7 (Rattus norvegicus (Rat)) | BDBM50188954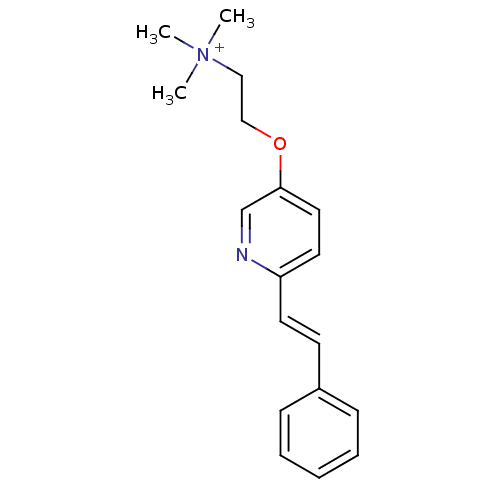 (CHEMBL212819 | N,N,N-trimethyl-2-(6-styrylpyridin-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.25E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Displacement of [125I]iodo-MLA from alpha-7 nAChR in rat cerebral cortex | Bioorg Med Chem Lett 16: 4283-6 (2006) Article DOI: 10.1016/j.bmcl.2006.05.080 BindingDB Entry DOI: 10.7270/Q2CJ8F8J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-7 (Rattus norvegicus (Rat)) | BDBM50188952 (CHEMBL379830 | N,N,N-trimethyl-2-(4-styrylphenoxy)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.17E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Displacement of [125I]iodo-MLA from alpha-7 nAChR in rat cerebral cortex | Bioorg Med Chem Lett 16: 4283-6 (2006) Article DOI: 10.1016/j.bmcl.2006.05.080 BindingDB Entry DOI: 10.7270/Q2CJ8F8J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-7 (Rattus norvegicus (Rat)) | BDBM50188948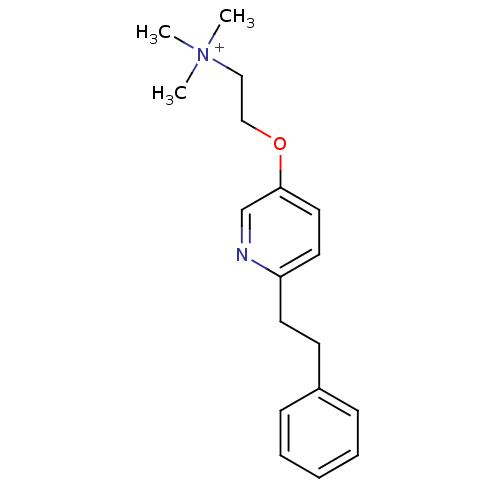 (CHEMBL209636 | N,N,N-trimethyl-2-(6-phenethylpyrid...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.32E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Displacement of [125I]iodo-MLA from alpha-7 nAChR in rat cerebral cortex | Bioorg Med Chem Lett 16: 4283-6 (2006) Article DOI: 10.1016/j.bmcl.2006.05.080 BindingDB Entry DOI: 10.7270/Q2CJ8F8J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-7 (Rattus norvegicus (Rat)) | BDBM50188957 (CHEMBL209788 | N,N,N-trimethyl-2-(4-phenethylpheno...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Displacement of [125I]iodo-MLA from alpha-7 nAChR in rat cerebral cortex | Bioorg Med Chem Lett 16: 4283-6 (2006) Article DOI: 10.1016/j.bmcl.2006.05.080 BindingDB Entry DOI: 10.7270/Q2CJ8F8J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-7 (Rattus norvegicus (Rat)) | BDBM50188964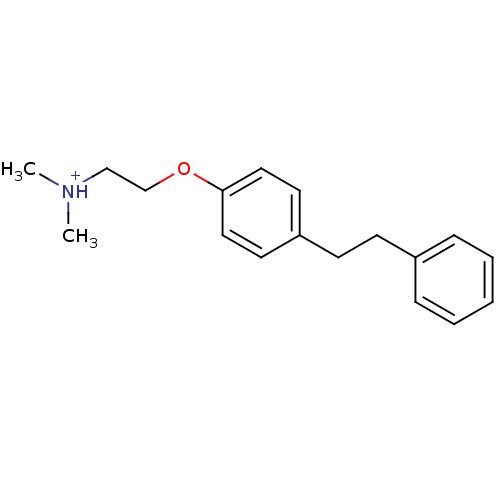 (N,N-dimethyl-2-(4-phenethylphenoxy)ethanaminium) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Displacement of [125I]iodo-MLA from alpha-7 nAChR in rat cerebral cortex | Bioorg Med Chem Lett 16: 4283-6 (2006) Article DOI: 10.1016/j.bmcl.2006.05.080 BindingDB Entry DOI: 10.7270/Q2CJ8F8J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-7 (Rattus norvegicus (Rat)) | BDBM50119573 (CHEMBL142418 | Dimethyl-(3-pyridin-3-yl-propyl)-am...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Displacement of [125I]iodo-MLA from alpha-7 nAChR in rat cerebral cortex | Bioorg Med Chem Lett 16: 4283-6 (2006) Article DOI: 10.1016/j.bmcl.2006.05.080 BindingDB Entry DOI: 10.7270/Q2CJ8F8J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-7 (Rattus norvegicus (Rat)) | BDBM50188963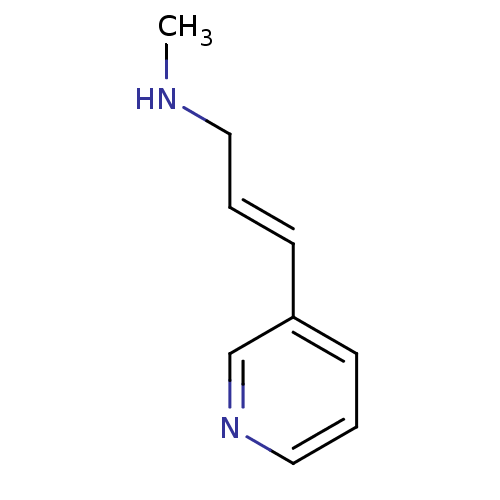 (CHEMBL150421 | Methyl-((E)-3-pyridin-3-yl-allyl)-a...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Displacement of [125I]iodo-MLA from alpha-7 nAChR in rat cerebral cortex | Bioorg Med Chem Lett 16: 4283-6 (2006) Article DOI: 10.1016/j.bmcl.2006.05.080 BindingDB Entry DOI: 10.7270/Q2CJ8F8J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-7 (Rattus norvegicus (Rat)) | BDBM50188962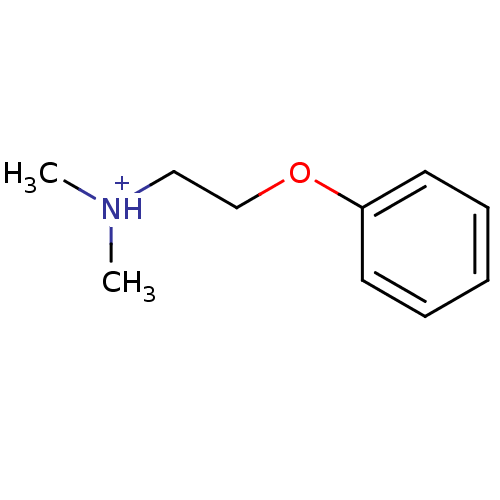 (N,N-dimethyl-2-phenoxyethanaminium) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Displacement of [125I]iodo-MLA from alpha-7 nAChR in rat cerebral cortex | Bioorg Med Chem Lett 16: 4283-6 (2006) Article DOI: 10.1016/j.bmcl.2006.05.080 BindingDB Entry DOI: 10.7270/Q2CJ8F8J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-7 (Rattus norvegicus (Rat)) | BDBM50119558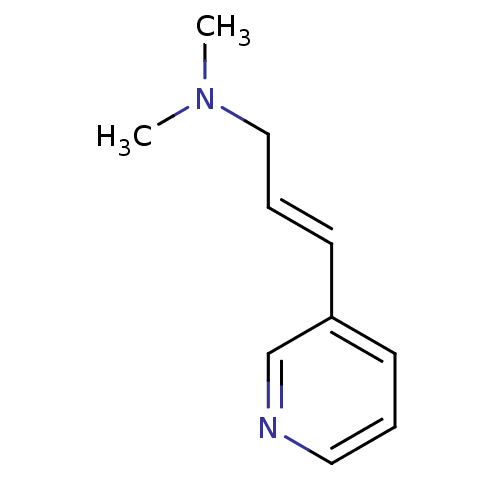 (CHEMBL142000 | N,N-dimethyl-3-(pyridin-3-yl)prop-2...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Displacement of [125I]iodo-MLA from alpha-7 nAChR in rat cerebral cortex | Bioorg Med Chem Lett 16: 4283-6 (2006) Article DOI: 10.1016/j.bmcl.2006.05.080 BindingDB Entry DOI: 10.7270/Q2CJ8F8J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-7 (Rattus norvegicus (Rat)) | BDBM50188960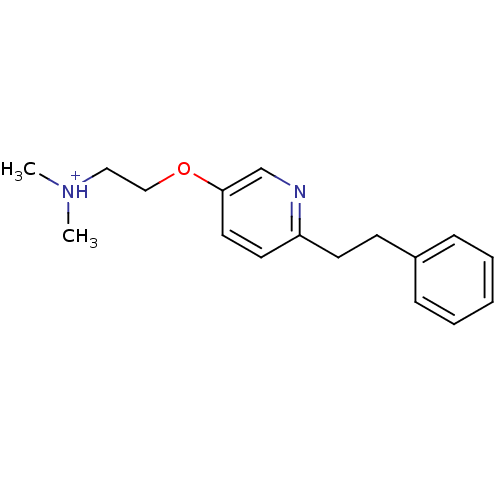 (N,N-dimethyl-2-(6-phenethylpyridin-3-yloxy)ethanam...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Displacement of [125I]iodo-MLA from alpha-7 nAChR in rat cerebral cortex | Bioorg Med Chem Lett 16: 4283-6 (2006) Article DOI: 10.1016/j.bmcl.2006.05.080 BindingDB Entry DOI: 10.7270/Q2CJ8F8J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-7 (Rattus norvegicus (Rat)) | BDBM50115811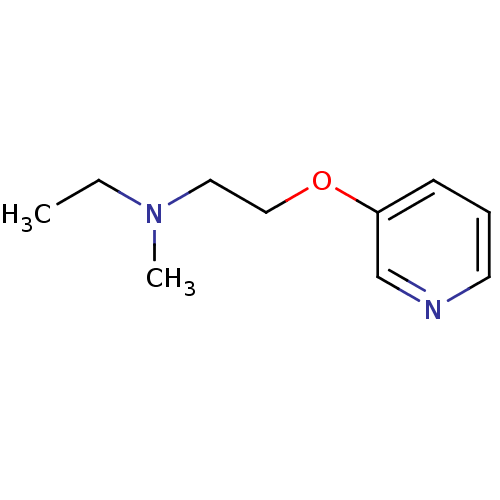 (CHEMBL57912 | Ethyl-methyl-[2-(pyridin-3-yloxy)-et...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Displacement of [125I]iodo-MLA from alpha-7 nAChR in rat cerebral cortex | Bioorg Med Chem Lett 16: 4283-6 (2006) Article DOI: 10.1016/j.bmcl.2006.05.080 BindingDB Entry DOI: 10.7270/Q2CJ8F8J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-7 (Rattus norvegicus (Rat)) | BDBM50120586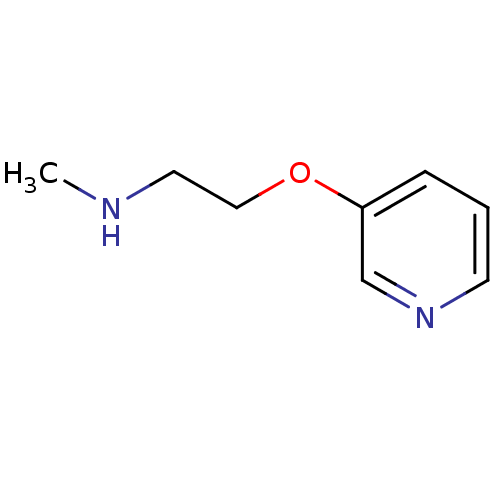 (CHEMBL112221 | Methyl-[2-(pyridin-3-yloxy)-ethyl]-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Displacement of [125I]iodo-MLA from alpha-7 nAChR in rat cerebral cortex | Bioorg Med Chem Lett 16: 4283-6 (2006) Article DOI: 10.1016/j.bmcl.2006.05.080 BindingDB Entry DOI: 10.7270/Q2CJ8F8J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-7 (Rattus norvegicus (Rat)) | BDBM50188958 (N,N-dimethyl-2-(6-styrylpyridin-3-yloxy)ethanamini...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Displacement of [125I]iodo-MLA from alpha-7 nAChR in rat cerebral cortex | Bioorg Med Chem Lett 16: 4283-6 (2006) Article DOI: 10.1016/j.bmcl.2006.05.080 BindingDB Entry DOI: 10.7270/Q2CJ8F8J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-7 (Rattus norvegicus (Rat)) | BDBM50168255 ((+/-)6-(2-Phenylethyl)nicotine | 5-(1-Methyl-pyrro...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Displacement of [125I]iodo-MLA from alpha-7 nAChR in rat cerebral cortex | Bioorg Med Chem Lett 16: 4283-6 (2006) Article DOI: 10.1016/j.bmcl.2006.05.080 BindingDB Entry DOI: 10.7270/Q2CJ8F8J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-7 (Rattus norvegicus (Rat)) | BDBM50188955 (N,N-dimethyl-2-(4-styrylphenoxy)ethanaminium) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Displacement of [125I]iodo-MLA from alpha-7 nAChR in rat cerebral cortex | Bioorg Med Chem Lett 16: 4283-6 (2006) Article DOI: 10.1016/j.bmcl.2006.05.080 BindingDB Entry DOI: 10.7270/Q2CJ8F8J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-7 (Rattus norvegicus (Rat)) | BDBM50188953 (CHEMBL215721 | N,N,N-trimethyl-3-(pyridin-3-yloxy)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Displacement of [125I]iodo-MLA from alpha-7 nAChR in rat cerebral cortex | Bioorg Med Chem Lett 16: 4283-6 (2006) Article DOI: 10.1016/j.bmcl.2006.05.080 BindingDB Entry DOI: 10.7270/Q2CJ8F8J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-7 (Rattus norvegicus (Rat)) | BDBM50188951 (CHEMBL438638 | N-methyl-3-(pyridin-3-yl)prop-2-en-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Displacement of [125I]iodo-MLA from alpha-7 nAChR in rat cerebral cortex | Bioorg Med Chem Lett 16: 4283-6 (2006) Article DOI: 10.1016/j.bmcl.2006.05.080 BindingDB Entry DOI: 10.7270/Q2CJ8F8J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-7 (Rattus norvegicus (Rat)) | BDBM50188950 (CHEMBL212134 | N-methyl-3-(pyridin-3-yl)propan-1-a...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Displacement of [125I]iodo-MLA from alpha-7 nAChR in rat cerebral cortex | Bioorg Med Chem Lett 16: 4283-6 (2006) Article DOI: 10.1016/j.bmcl.2006.05.080 BindingDB Entry DOI: 10.7270/Q2CJ8F8J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-7 (Rattus norvegicus (Rat)) | BDBM50115808 (CHEMBL61616 | Dimethyl-[2-(pyridin-3-yloxy)-ethyl]...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Displacement of [125I]iodo-MLA from alpha-7 nAChR in rat cerebral cortex | Bioorg Med Chem Lett 16: 4283-6 (2006) Article DOI: 10.1016/j.bmcl.2006.05.080 BindingDB Entry DOI: 10.7270/Q2CJ8F8J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Homo sapiens (Human)) | BDBM50291820 (CHEMBL4159155) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.290 | n/a | n/a | n/a | n/a | n/a | n/a |
TiumBio Company Ltd. Curated by ChEMBL | Assay Description Displacement of [125I]D-Trp6-LHRH from human GnRH receptor expressed in CHO-K1 cells after 1 hr by microbeta scintillation counting method | Eur J Med Chem 145: 413-424 (2018) Article DOI: 10.1016/j.ejmech.2017.12.095 BindingDB Entry DOI: 10.7270/Q2XK8J2D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Homo sapiens (Human)) | BDBM50291735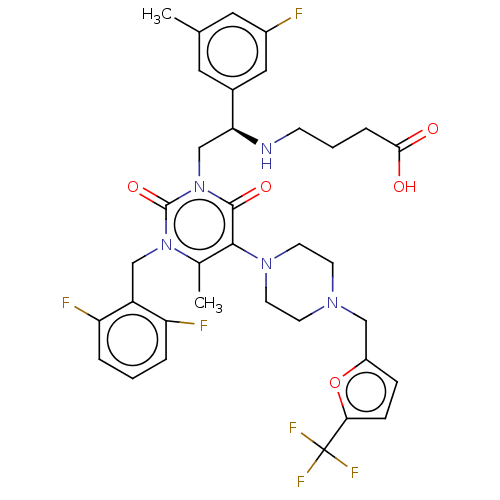 (CHEMBL4169891) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.330 | n/a | n/a | n/a | n/a | n/a | n/a |
TiumBio Company Ltd. Curated by ChEMBL | Assay Description Binding affinity of compound to human recombinant Ribonuclease L was evaluated | Eur J Med Chem 145: 413-424 (2018) Article DOI: 10.1016/j.ejmech.2017.12.095 BindingDB Entry DOI: 10.7270/Q2XK8J2D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Homo sapiens (Human)) | BDBM50291816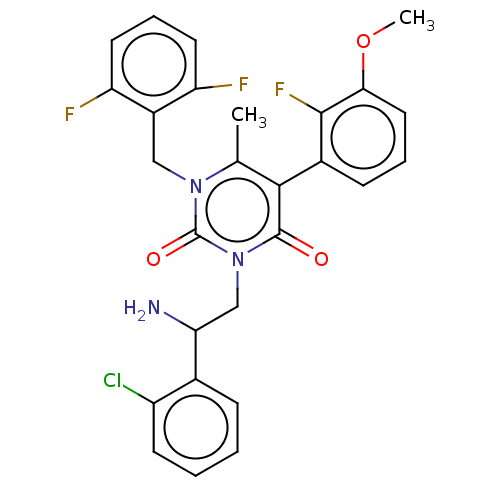 (CHEMBL4165983) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.380 | n/a | n/a | n/a | n/a | n/a | n/a |
TiumBio Company Ltd. Curated by ChEMBL | Assay Description Displacement of [125I]D-Trp6-LHRH from human GnRH receptor expressed in CHO-K1 cells after 1 hr by microbeta scintillation counting method | Eur J Med Chem 145: 413-424 (2018) Article DOI: 10.1016/j.ejmech.2017.12.095 BindingDB Entry DOI: 10.7270/Q2XK8J2D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein kinase FLT3 (Homo sapiens (Human)) | BDBM476709 (2-((5-chloro-4-(6-methyl-1H-indole-3-yl)pyrimidine...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
HANMI PHARM. CO.. LTD. US Patent | Assay Description The inhibitory activity of some of the compounds described above against FLT3-ITD, FLT3 wild type (WT), VEGFR2 (KDR), and SYK kinase was measured.The... | US Patent US10870639 (2020) BindingDB Entry DOI: 10.7270/Q20G3P70 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein kinase FLT3 (Homo sapiens (Human)) | BDBM476709 (2-((5-chloro-4-(6-methyl-1H-indole-3-yl)pyrimidine...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The inhibitory activity of some of the compounds described above against FLT3-ITD, FLT3 wild type (WT), VEGFR2 (KDR), and SYK kinase was measured. Th... | Citation and Details BindingDB Entry DOI: 10.7270/Q2VX0KQ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein kinase FLT3 (Homo sapiens (Human)) | BDBM546731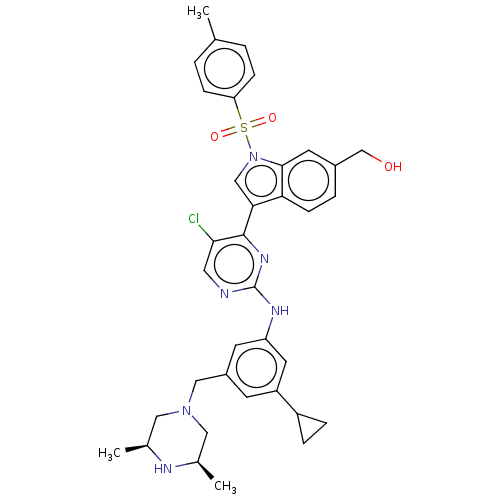 ((3-(5-chloro-2-((3-cyclopropyl-5-(((3R, 5S)-3,5-di...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The inhibitory activity of some of the compounds described above against FLT3-ITD, FLT3 wild type (WT), VEGFR2 (KDR), and SYK kinase was measured. Th... | Citation and Details BindingDB Entry DOI: 10.7270/Q2VX0KQ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein kinase FLT3 (Homo sapiens (Human)) | BDBM476704 ( (3-(5-chloro-2-((3-cyclopropyl-5-(((3R,5S)-3,5-di...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
HANMI PHARM. CO.. LTD. US Patent | Assay Description The inhibitory activity of some of the compounds described above against FLT3-ITD, FLT3 wild type (WT), VEGFR2 (KDR), and SYK kinase was measured.The... | US Patent US10870639 (2020) BindingDB Entry DOI: 10.7270/Q20G3P70 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Homo sapiens (Human)) | BDBM50291733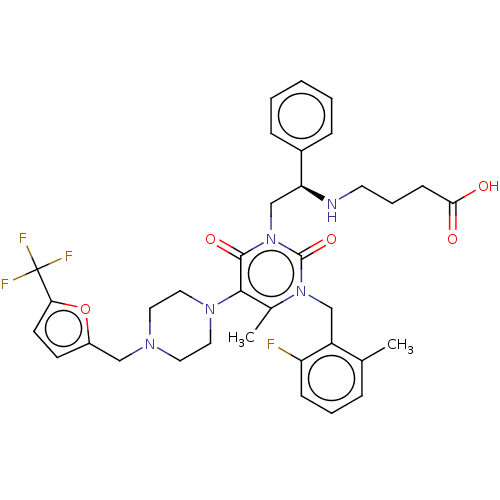 (CHEMBL4173065) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.460 | n/a | n/a | n/a | n/a | n/a | n/a |
TiumBio Company Ltd. Curated by ChEMBL | Assay Description Displacement of [125I]D-Trp6-LHRH from human GnRH receptor expressed in CHO-K1 cells after 1 hr by microbeta scintillation counting method | Eur J Med Chem 145: 413-424 (2018) Article DOI: 10.1016/j.ejmech.2017.12.095 BindingDB Entry DOI: 10.7270/Q2XK8J2D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Receptor-type tyrosine-protein kinase FLT3 (Homo sapiens (Human)) | BDBM476704 ( (3-(5-chloro-2-((3-cyclopropyl-5-(((3R,5S)-3,5-di...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
HANMI PHARM. CO.. LTD. US Patent | Assay Description The inhibitory activity of some of the compounds described above against FLT3-ITD, FLT3 wild type (WT), VEGFR2 (KDR), and SYK kinase was measured.The... | US Patent US10870639 (2020) BindingDB Entry DOI: 10.7270/Q20G3P70 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Receptor-type tyrosine-protein kinase FLT3 (Homo sapiens (Human)) | BDBM546731 ((3-(5-chloro-2-((3-cyclopropyl-5-(((3R, 5S)-3,5-di...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The inhibitory activity of some of the compounds described above against FLT3-ITD, FLT3 wild type (WT), VEGFR2 (KDR), and SYK kinase was measured. Th... | Citation and Details BindingDB Entry DOI: 10.7270/Q2VX0KQ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase receptor UFO (Homo sapiens (Human)) | BDBM50506637 (CHEMBL4476226) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | <0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea University Curated by ChEMBL | Assay Description Inhibition of human AXL using EAIYAAPFAKKK as substrate by [gamma-33P]-ATP assay | Bioorg Med Chem Lett 28: 3761-3765 (2018) Article DOI: 10.1016/j.bmcl.2018.10.013 BindingDB Entry DOI: 10.7270/Q23T9MHD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase receptor UFO (Homo sapiens (Human)) | BDBM50506637 (CHEMBL4476226) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | <0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea University Curated by ChEMBL | Assay Description Inhibition of N-terminal His-tagged recombinant human AXL (473 to end amino acids) expressed by baculovirus in Sf9 cells using axltide substrate and ... | Bioorg Med Chem Lett 28: 3761-3765 (2018) Article DOI: 10.1016/j.bmcl.2018.10.013 BindingDB Entry DOI: 10.7270/Q23T9MHD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Homo sapiens (Human)) | BDBM50291734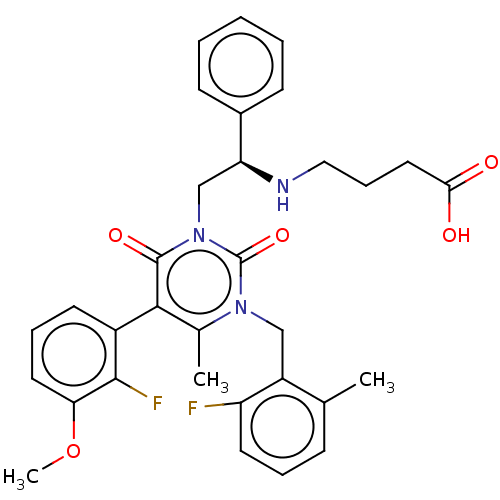 (CHEMBL4159247) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.580 | n/a | n/a | n/a | n/a | n/a | n/a |
TiumBio Company Ltd. Curated by ChEMBL | Assay Description Displacement of [125I]D-Trp6-LHRH from human GnRH receptor expressed in CHO-K1 cells after 1 hr by microbeta scintillation counting method | Eur J Med Chem 145: 413-424 (2018) Article DOI: 10.1016/j.ejmech.2017.12.095 BindingDB Entry DOI: 10.7270/Q2XK8J2D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Homo sapiens (Human)) | BDBM50162007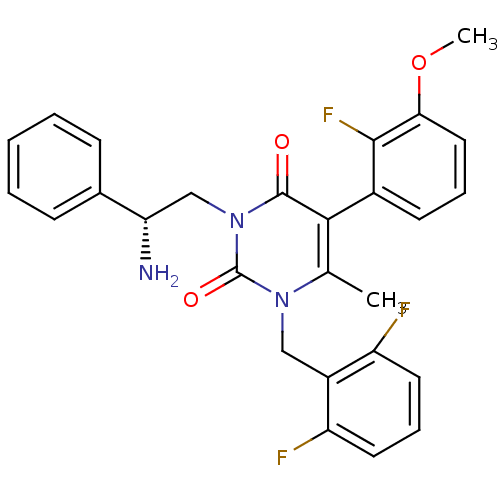 ((R)-1-(2,6-difluorobenzyl)-3-(2-amino-2-phenylethy...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.590 | n/a | n/a | n/a | n/a | n/a | n/a |
TiumBio Company Ltd. Curated by ChEMBL | Assay Description Displacement of [125I]D-Trp6-LHRH from human GnRH receptor expressed in CHO-K1 cells after 1 hr by microbeta scintillation counting method | Eur J Med Chem 145: 413-424 (2018) Article DOI: 10.1016/j.ejmech.2017.12.095 BindingDB Entry DOI: 10.7270/Q2XK8J2D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Homo sapiens (Human)) | BDBM50291819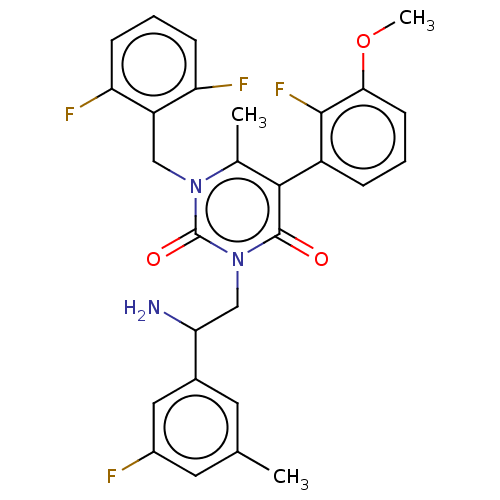 (CHEMBL4170478) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.740 | n/a | n/a | n/a | n/a | n/a | n/a |
TiumBio Company Ltd. Curated by ChEMBL | Assay Description Displacement of [125I]D-Trp6-LHRH from human GnRH receptor expressed in CHO-K1 cells after 1 hr by microbeta scintillation counting method | Eur J Med Chem 145: 413-424 (2018) Article DOI: 10.1016/j.ejmech.2017.12.095 BindingDB Entry DOI: 10.7270/Q2XK8J2D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM50066870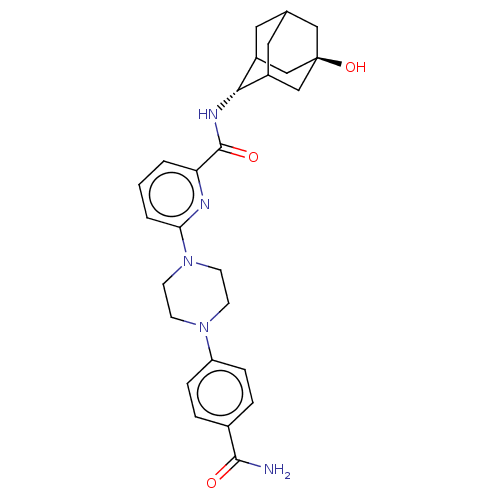 (CHEMBL3401672) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description Inhibition of human 11beta-HSD1 expressed in HEK293 cells using NADPH assessed as conversion of cortisone to cortisol by cell-based assay | Bioorg Med Chem Lett 25: 1679-83 (2015) Article DOI: 10.1016/j.bmcl.2015.03.003 BindingDB Entry DOI: 10.7270/Q2X3504Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase receptor UFO (Homo sapiens (Human)) | BDBM50506648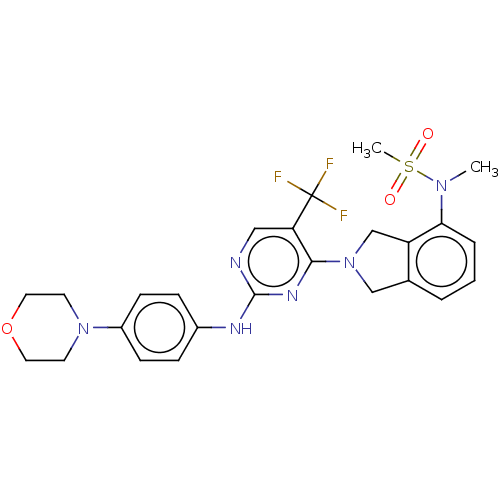 (CHEMBL4445940) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.940 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea University Curated by ChEMBL | Assay Description Inhibition of N-terminal His-tagged recombinant human AXL (473 to end amino acids) expressed by baculovirus in Sf9 cells using axltide substrate and ... | Bioorg Med Chem Lett 28: 3761-3765 (2018) Article DOI: 10.1016/j.bmcl.2018.10.013 BindingDB Entry DOI: 10.7270/Q23T9MHD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein kinase FLT3 (Homo sapiens (Human)) | BDBM476700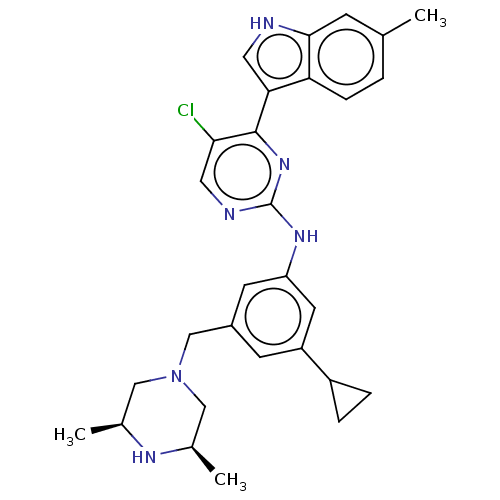 (5-chloro-N-(3-cyclopropyl-5-(((3R,5S)-3,5-dimethyl...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | US Patent | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The inhibitory activity of some of the compounds described above against FLT3-ITD, FLT3 wild type (WT), VEGFR2 (KDR), and SYK kinase was measured. Th... | Citation and Details BindingDB Entry DOI: 10.7270/Q2VX0KQ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein kinase FLT3 (Homo sapiens (Human)) | BDBM476700 (5-chloro-N-(3-cyclopropyl-5-(((3R,5S)-3,5-dimethyl...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | US Patent | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
HANMI PHARM. CO.. LTD. US Patent | Assay Description The inhibitory activity of some of the compounds described above against FLT3-ITD, FLT3 wild type (WT), VEGFR2 (KDR), and SYK kinase was measured.The... | US Patent US10870639 (2020) BindingDB Entry DOI: 10.7270/Q20G3P70 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteinase-activated receptor 1 (Homo sapiens (Human)) | BDBM50261110 (CHEMBL493982 | Ethyl [(3aR,4aR,8aR,9aS)-9(S)-[(E)-...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Technology Curated by ChEMBL | Assay Description Displacement of [3H]haTRAP from PAR1 in human platelet membranes after 60 mins by scintillation counting analysis | ACS Med Chem Lett 4: 1054-8 (2013) Article DOI: 10.1021/ml400235c BindingDB Entry DOI: 10.7270/Q2KH0PSN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 843 total ) | Next | Last >> |