

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Serine/threonine-protein kinase 17B (Homo sapiens (Human)) | BDBM50166121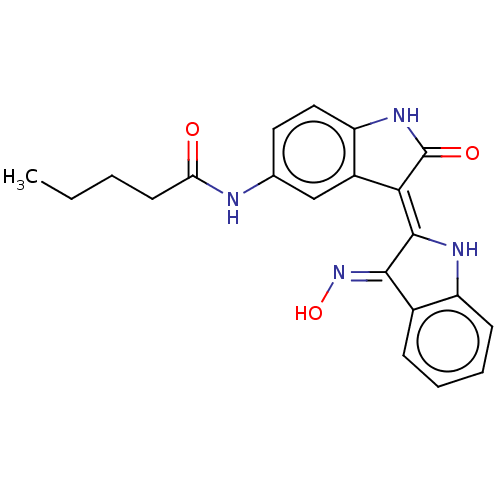 (CHEMBL3797480) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology Curated by ChEMBL | Assay Description Competitive inhibition of DRAK2 (unknown origin) using MRLC3 peptide as substrate incubated for 2 hrs by Lineweaver-Burk plot analysis in presence of... | Bioorg Med Chem Lett 26: 2719-23 (2016) Article DOI: 10.1016/j.bmcl.2016.03.111 BindingDB Entry DOI: 10.7270/Q2N29ZTZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM50568146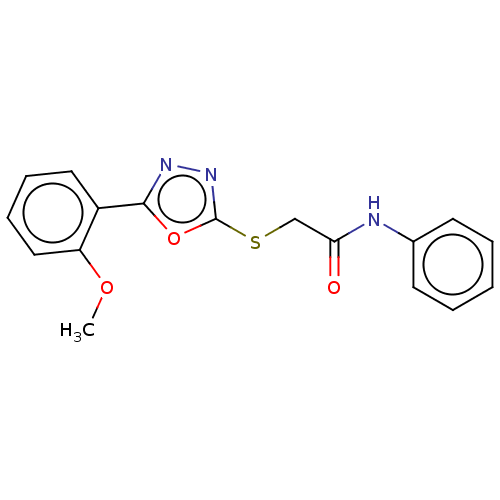 (CHEMBL1481541) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Noncompetitive inhibition of mushroom tyrosinase assessed as dissociation constant using L-DOPA as substrate by Lineweaver-Burk plot analysis | Citation and Details Article DOI: 10.1016/j.bmc.2021.116222 BindingDB Entry DOI: 10.7270/Q24171T1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A [1-429] (Clostridium botulinum (strain Hall / ATCC 3502 / N...) | BDBM506811 (US11046652, Example 1-5) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description In a 96 well, clear bottom black plate, the following was added to each well: 5 nM BoNT/A LC1-429, 28 μM substrate in 30 mM HEPES pH 7.3, 0.05 m... | Citation and Details BindingDB Entry DOI: 10.7270/Q2VQ35TK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A [1-429] (Clostridium botulinum (strain Hall / ATCC 3502 / N...) | BDBM506825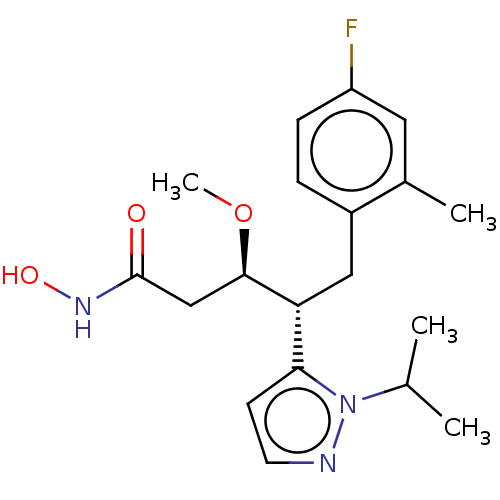 (US11046652, Example 2-4) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 4.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description In a 96 well, clear bottom black plate, the following was added to each well: 5 nM BoNT/A LC1-429, 28 μM substrate in 30 mM HEPES pH 7.3, 0.05 m... | Citation and Details BindingDB Entry DOI: 10.7270/Q2VQ35TK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A [1-429] (Clostridium botulinum (strain Hall / ATCC 3502 / N...) | BDBM506834 (US11046652, Example 3-7) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 5.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description In a 96 well, clear bottom black plate, the following was added to each well: 5 nM BoNT/A LC1-429, 28 μM substrate in 30 mM HEPES pH 7.3, 0.05 m... | Citation and Details BindingDB Entry DOI: 10.7270/Q2VQ35TK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A [1-429] (Clostridium botulinum (strain Hall / ATCC 3502 / N...) | BDBM506829 (US11046652, Example 3-2) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 5.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description In a 96 well, clear bottom black plate, the following was added to each well: 5 nM BoNT/A LC1-429, 28 μM substrate in 30 mM HEPES pH 7.3, 0.05 m... | Citation and Details BindingDB Entry DOI: 10.7270/Q2VQ35TK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A [1-429] (Clostridium botulinum (strain Hall / ATCC 3502 / N...) | BDBM506835 (US11046652, Example 3-8) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 5.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description In a 96 well, clear bottom black plate, the following was added to each well: 5 nM BoNT/A LC1-429, 28 μM substrate in 30 mM HEPES pH 7.3, 0.05 m... | Citation and Details BindingDB Entry DOI: 10.7270/Q2VQ35TK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A [1-429] (Clostridium botulinum (strain Hall / ATCC 3502 / N...) | BDBM506807 (US11046652, Example 1-1 | US11046652, Example 2-1) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 5.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description In a 96 well, clear bottom black plate, the following was added to each well: 5 nM BoNT/A LC1-429, 28 μM substrate in 30 mM HEPES pH 7.3, 0.05 m... | Citation and Details BindingDB Entry DOI: 10.7270/Q2VQ35TK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A [1-429] (Clostridium botulinum (strain Hall / ATCC 3502 / N...) | BDBM506807 (US11046652, Example 1-1 | US11046652, Example 2-1) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 6.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description In a 96 well, clear bottom black plate, the following was added to each well: 5 nM BoNT/A LC1-429, 28 μM substrate in 30 mM HEPES pH 7.3, 0.05 m... | Citation and Details BindingDB Entry DOI: 10.7270/Q2VQ35TK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A [1-429] (Clostridium botulinum (strain Hall / ATCC 3502 / N...) | BDBM506813 (US11046652, Example 1-7) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 6.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description In a 96 well, clear bottom black plate, the following was added to each well: 5 nM BoNT/A LC1-429, 28 μM substrate in 30 mM HEPES pH 7.3, 0.05 m... | Citation and Details BindingDB Entry DOI: 10.7270/Q2VQ35TK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A [1-429] (Clostridium botulinum (strain Hall / ATCC 3502 / N...) | BDBM506832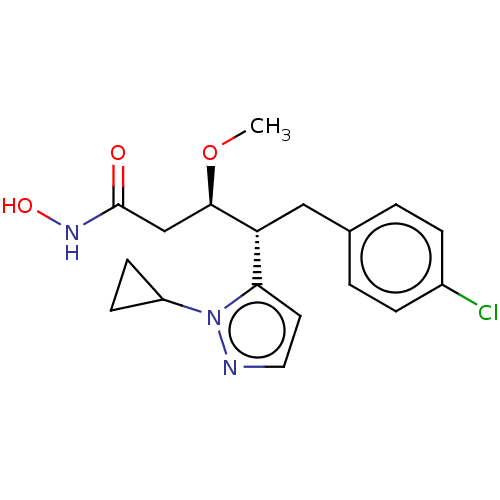 (US11046652, Example 3-5) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 6.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description In a 96 well, clear bottom black plate, the following was added to each well: 5 nM BoNT/A LC1-429, 28 μM substrate in 30 mM HEPES pH 7.3, 0.05 m... | Citation and Details BindingDB Entry DOI: 10.7270/Q2VQ35TK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A [1-429] (Clostridium botulinum (strain Hall / ATCC 3502 / N...) | BDBM506808 (US11046652, Example 1-2 | US11046652, Example 1-3) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 6.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description In a 96 well, clear bottom black plate, the following was added to each well: 5 nM BoNT/A LC1-429, 28 μM substrate in 30 mM HEPES pH 7.3, 0.05 m... | Citation and Details BindingDB Entry DOI: 10.7270/Q2VQ35TK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A [1-429] (Clostridium botulinum (strain Hall / ATCC 3502 / N...) | BDBM506841 (US11046652, Example 5-1) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 7.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description In a 96 well, clear bottom black plate, the following was added to each well: 5 nM BoNT/A LC1-429, 28 μM substrate in 30 mM HEPES pH 7.3, 0.05 m... | Citation and Details BindingDB Entry DOI: 10.7270/Q2VQ35TK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A [1-429] (Clostridium botulinum (strain Hall / ATCC 3502 / N...) | BDBM506815 (US11046652, Example 1-9) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 8.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description In a 96 well, clear bottom black plate, the following was added to each well: 5 nM BoNT/A LC1-429, 28 μM substrate in 30 mM HEPES pH 7.3, 0.05 m... | Citation and Details BindingDB Entry DOI: 10.7270/Q2VQ35TK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A [1-429] (Clostridium botulinum (strain Hall / ATCC 3502 / N...) | BDBM506833 (US11046652, Example 3-6) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 9.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description In a 96 well, clear bottom black plate, the following was added to each well: 5 nM BoNT/A LC1-429, 28 μM substrate in 30 mM HEPES pH 7.3, 0.05 m... | Citation and Details BindingDB Entry DOI: 10.7270/Q2VQ35TK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A [1-429] (Clostridium botulinum (strain Hall / ATCC 3502 / N...) | BDBM506828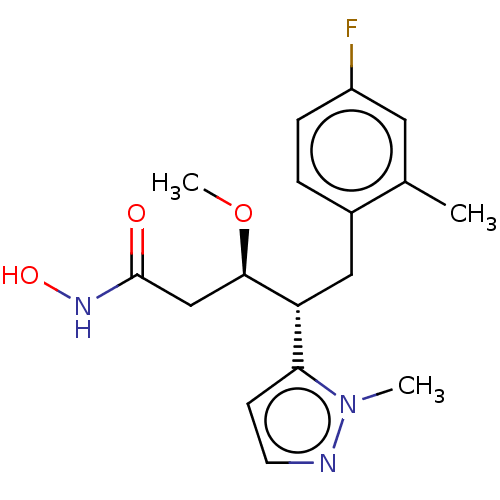 (US11046652, Example 3-1) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 10.2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description In a 96 well, clear bottom black plate, the following was added to each well: 5 nM BoNT/A LC1-429, 28 μM substrate in 30 mM HEPES pH 7.3, 0.05 m... | Citation and Details BindingDB Entry DOI: 10.7270/Q2VQ35TK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A [1-429] (Clostridium botulinum (strain Hall / ATCC 3502 / N...) | BDBM506810 (US11046652, Example 1-4) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 13.8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description In a 96 well, clear bottom black plate, the following was added to each well: 5 nM BoNT/A LC1-429, 28 μM substrate in 30 mM HEPES pH 7.3, 0.05 m... | Citation and Details BindingDB Entry DOI: 10.7270/Q2VQ35TK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A [1-429] (Clostridium botulinum (strain Hall / ATCC 3502 / N...) | BDBM506814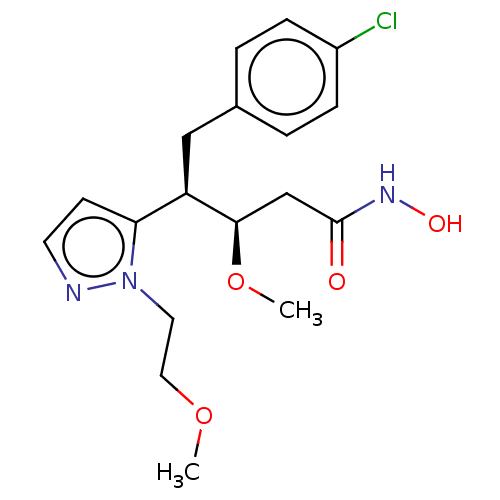 (US11046652, Example 1-8) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 14.7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description In a 96 well, clear bottom black plate, the following was added to each well: 5 nM BoNT/A LC1-429, 28 μM substrate in 30 mM HEPES pH 7.3, 0.05 m... | Citation and Details BindingDB Entry DOI: 10.7270/Q2VQ35TK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A [1-429] (Clostridium botulinum (strain Hall / ATCC 3502 / N...) | BDBM506821 (US11046652, Example 1-15) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 17.8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description In a 96 well, clear bottom black plate, the following was added to each well: 5 nM BoNT/A LC1-429, 28 μM substrate in 30 mM HEPES pH 7.3, 0.05 m... | Citation and Details BindingDB Entry DOI: 10.7270/Q2VQ35TK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A [1-429] (Clostridium botulinum (strain Hall / ATCC 3502 / N...) | BDBM506812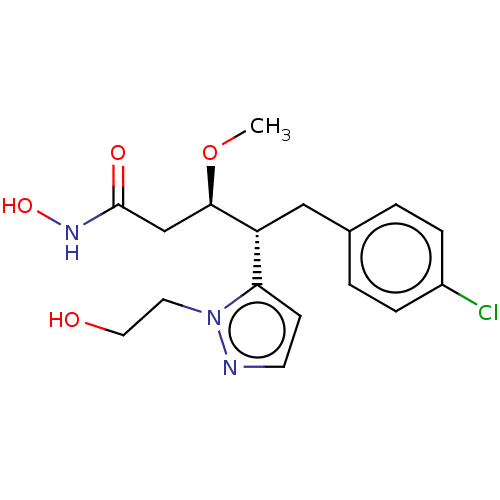 (US11046652, Example 1-6) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description In a 96 well, clear bottom black plate, the following was added to each well: 5 nM BoNT/A LC1-429, 28 μM substrate in 30 mM HEPES pH 7.3, 0.05 m... | Citation and Details BindingDB Entry DOI: 10.7270/Q2VQ35TK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A [1-429] (Clostridium botulinum (strain Hall / ATCC 3502 / N...) | BDBM506819 (US11046652, Example 1-13) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description In a 96 well, clear bottom black plate, the following was added to each well: 5 nM BoNT/A LC1-429, 28 μM substrate in 30 mM HEPES pH 7.3, 0.05 m... | Citation and Details BindingDB Entry DOI: 10.7270/Q2VQ35TK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A [1-429] (Clostridium botulinum (strain Hall / ATCC 3502 / N...) | BDBM506820 (US11046652, Example 1-14) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 20.4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description In a 96 well, clear bottom black plate, the following was added to each well: 5 nM BoNT/A LC1-429, 28 μM substrate in 30 mM HEPES pH 7.3, 0.05 m... | Citation and Details BindingDB Entry DOI: 10.7270/Q2VQ35TK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A [1-429] (Clostridium botulinum (strain Hall / ATCC 3502 / N...) | BDBM506808 (US11046652, Example 1-2 | US11046652, Example 1-3) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 21.3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description In a 96 well, clear bottom black plate, the following was added to each well: 5 nM BoNT/A LC1-429, 28 μM substrate in 30 mM HEPES pH 7.3, 0.05 m... | Citation and Details BindingDB Entry DOI: 10.7270/Q2VQ35TK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A [1-429] (Clostridium botulinum (strain Hall / ATCC 3502 / N...) | BDBM506823 (US11046652, Example 2-2) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 24.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description In a 96 well, clear bottom black plate, the following was added to each well: 5 nM BoNT/A LC1-429, 28 μM substrate in 30 mM HEPES pH 7.3, 0.05 m... | Citation and Details BindingDB Entry DOI: 10.7270/Q2VQ35TK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A [1-429] (Clostridium botulinum (strain Hall / ATCC 3502 / N...) | BDBM506824 (US11046652, Example 2-3) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 26 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description In a 96 well, clear bottom black plate, the following was added to each well: 5 nM BoNT/A LC1-429, 28 μM substrate in 30 mM HEPES pH 7.3, 0.05 m... | Citation and Details BindingDB Entry DOI: 10.7270/Q2VQ35TK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A [1-429] (Clostridium botulinum (strain Hall / ATCC 3502 / N...) | BDBM506818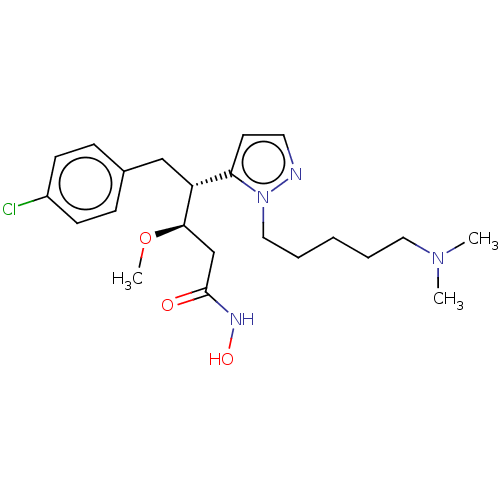 (US11046652, Example 1-12) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 28 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description In a 96 well, clear bottom black plate, the following was added to each well: 5 nM BoNT/A LC1-429, 28 μM substrate in 30 mM HEPES pH 7.3, 0.05 m... | Citation and Details BindingDB Entry DOI: 10.7270/Q2VQ35TK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A [1-429] (Clostridium botulinum (strain Hall / ATCC 3502 / N...) | BDBM506837 (US11046652, Example 3-10 | US11046652, Example 3-1...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 30.6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description In a 96 well, clear bottom black plate, the following was added to each well: 5 nM BoNT/A LC1-429, 28 μM substrate in 30 mM HEPES pH 7.3, 0.05 m... | Citation and Details BindingDB Entry DOI: 10.7270/Q2VQ35TK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A [1-429] (Clostridium botulinum (strain Hall / ATCC 3502 / N...) | BDBM506836 (US11046652, Example 3-9) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 31.7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description In a 96 well, clear bottom black plate, the following was added to each well: 5 nM BoNT/A LC1-429, 28 μM substrate in 30 mM HEPES pH 7.3, 0.05 m... | Citation and Details BindingDB Entry DOI: 10.7270/Q2VQ35TK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A [1-429] (Clostridium botulinum (strain Hall / ATCC 3502 / N...) | BDBM506840 (US11046652, Example 4-2) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 33.3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description In a 96 well, clear bottom black plate, the following was added to each well: 5 nM BoNT/A LC1-429, 28 μM substrate in 30 mM HEPES pH 7.3, 0.05 m... | Citation and Details BindingDB Entry DOI: 10.7270/Q2VQ35TK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A [1-429] (Clostridium botulinum (strain Hall / ATCC 3502 / N...) | BDBM506816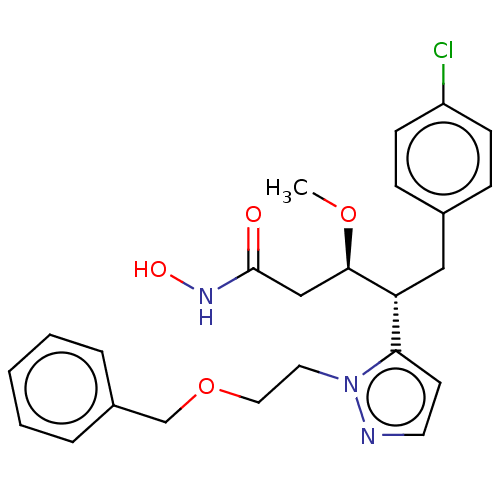 (US11046652, Example 1-10) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 39.8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description In a 96 well, clear bottom black plate, the following was added to each well: 5 nM BoNT/A LC1-429, 28 μM substrate in 30 mM HEPES pH 7.3, 0.05 m... | Citation and Details BindingDB Entry DOI: 10.7270/Q2VQ35TK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A [1-429] (Clostridium botulinum (strain Hall / ATCC 3502 / N...) | BDBM506817 (US11046652, Example 1-11) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 44 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description In a 96 well, clear bottom black plate, the following was added to each well: 5 nM BoNT/A LC1-429, 28 μM substrate in 30 mM HEPES pH 7.3, 0.05 m... | Citation and Details BindingDB Entry DOI: 10.7270/Q2VQ35TK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A [1-429] (Clostridium botulinum (strain Hall / ATCC 3502 / N...) | BDBM506837 (US11046652, Example 3-10 | US11046652, Example 3-1...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 53 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description In a 96 well, clear bottom black plate, the following was added to each well: 5 nM BoNT/A LC1-429, 28 μM substrate in 30 mM HEPES pH 7.3, 0.05 m... | Citation and Details BindingDB Entry DOI: 10.7270/Q2VQ35TK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A [1-429] (Clostridium botulinum (strain Hall / ATCC 3502 / N...) | BDBM506827 (US11046652, Example 2-6) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 118 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description In a 96 well, clear bottom black plate, the following was added to each well: 5 nM BoNT/A LC1-429, 28 μM substrate in 30 mM HEPES pH 7.3, 0.05 m... | Citation and Details BindingDB Entry DOI: 10.7270/Q2VQ35TK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A [1-429] (Clostridium botulinum (strain Hall / ATCC 3502 / N...) | BDBM506839 (US11046652, Example 4-1) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 182 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description In a 96 well, clear bottom black plate, the following was added to each well: 5 nM BoNT/A LC1-429, 28 μM substrate in 30 mM HEPES pH 7.3, 0.05 m... | Citation and Details BindingDB Entry DOI: 10.7270/Q2VQ35TK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A [1-429] (Clostridium botulinum (strain Hall / ATCC 3502 / N...) | BDBM506831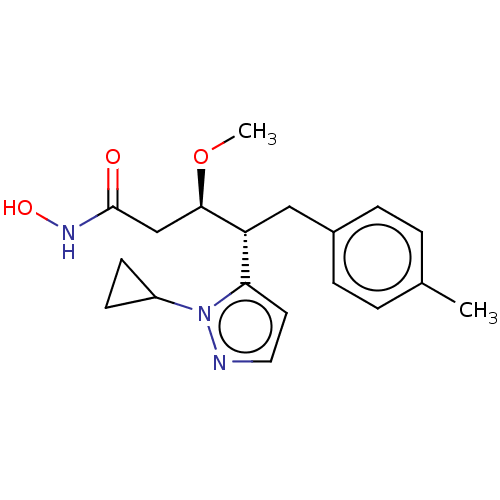 (US11046652, Example 3-4) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 207 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description In a 96 well, clear bottom black plate, the following was added to each well: 5 nM BoNT/A LC1-429, 28 μM substrate in 30 mM HEPES pH 7.3, 0.05 m... | Citation and Details BindingDB Entry DOI: 10.7270/Q2VQ35TK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A [1-429] (Clostridium botulinum (strain Hall / ATCC 3502 / N...) | BDBM506826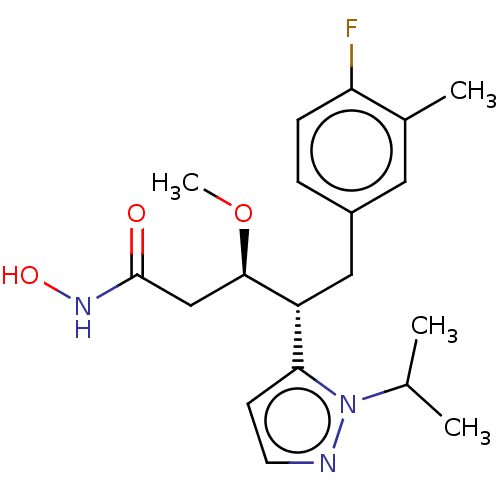 (US11046652, Example 2-5) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 214 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description In a 96 well, clear bottom black plate, the following was added to each well: 5 nM BoNT/A LC1-429, 28 μM substrate in 30 mM HEPES pH 7.3, 0.05 m... | Citation and Details BindingDB Entry DOI: 10.7270/Q2VQ35TK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A [1-429] (Clostridium botulinum (strain Hall / ATCC 3502 / N...) | BDBM506842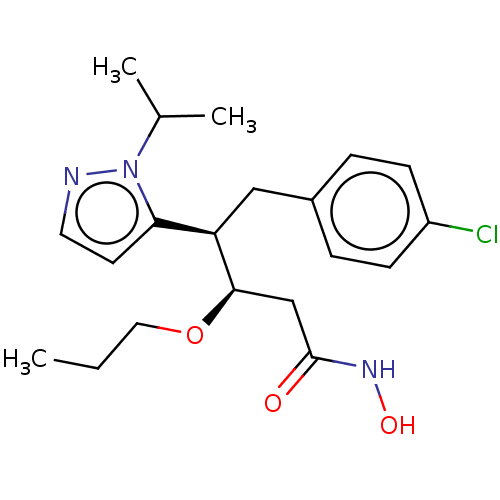 (US11046652, Example 5-2) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 246 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description In a 96 well, clear bottom black plate, the following was added to each well: 5 nM BoNT/A LC1-429, 28 μM substrate in 30 mM HEPES pH 7.3, 0.05 m... | Citation and Details BindingDB Entry DOI: 10.7270/Q2VQ35TK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A [1-429] (Clostridium botulinum (strain Hall / ATCC 3502 / N...) | BDBM506843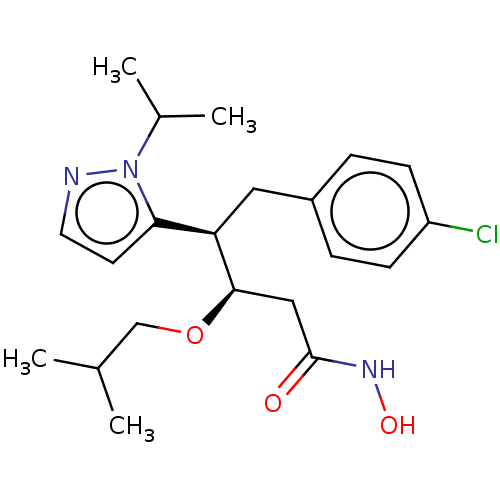 (US11046652, Example 5-3) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 299 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description In a 96 well, clear bottom black plate, the following was added to each well: 5 nM BoNT/A LC1-429, 28 μM substrate in 30 mM HEPES pH 7.3, 0.05 m... | Citation and Details BindingDB Entry DOI: 10.7270/Q2VQ35TK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A [1-429] (Clostridium botulinum (strain Hall / ATCC 3502 / N...) | BDBM506830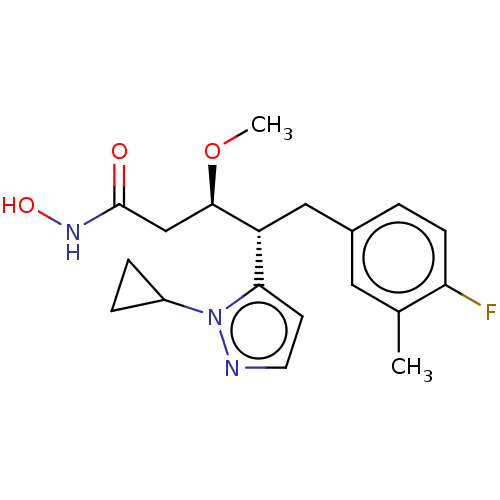 (US11046652, Example 3-3) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 623 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description In a 96 well, clear bottom black plate, the following was added to each well: 5 nM BoNT/A LC1-429, 28 μM substrate in 30 mM HEPES pH 7.3, 0.05 m... | Citation and Details BindingDB Entry DOI: 10.7270/Q2VQ35TK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A [1-429] (Clostridium botulinum (strain Hall / ATCC 3502 / N...) | BDBM506844 (US11046652, Example 5-4) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 629 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description In a 96 well, clear bottom black plate, the following was added to each well: 5 nM BoNT/A LC1-429, 28 μM substrate in 30 mM HEPES pH 7.3, 0.05 m... | Citation and Details BindingDB Entry DOI: 10.7270/Q2VQ35TK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxypeptidase A1 (Bos taurus (bovine)) | BDBM50121272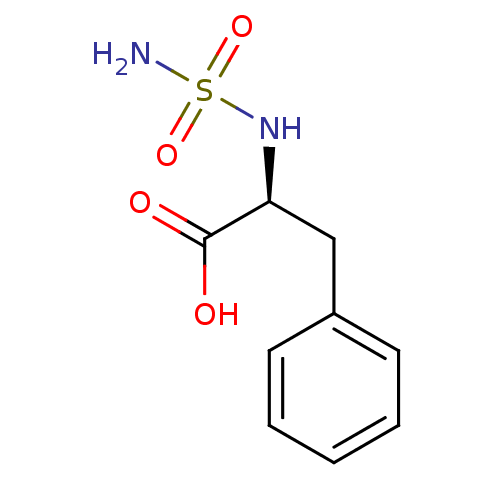 ((2S)-2-[(aminosulfonyl)amino]-3-phenylpropanoic ac...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Similars | DrugBank MMDB PDB PubMed | 650 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pohang University of Science and Technology Curated by ChEMBL | Assay Description Inhibitory activity of the compound against Carboxypeptidase A | J Med Chem 45: 5295-302 (2002) BindingDB Entry DOI: 10.7270/Q2BR8RJM | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Botulinum neurotoxin type A [1-429] (Clostridium botulinum (strain Hall / ATCC 3502 / N...) | BDBM506845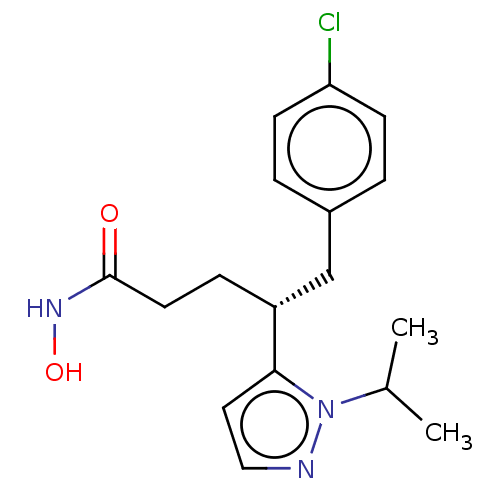 (US11046652, Example 5-5) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 7.45E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description In a 96 well, clear bottom black plate, the following was added to each well: 5 nM BoNT/A LC1-429, 28 μM substrate in 30 mM HEPES pH 7.3, 0.05 m... | Citation and Details BindingDB Entry DOI: 10.7270/Q2VQ35TK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| M-phase inducer phosphatase 1 (Homo sapiens (Human)) | BDBM50253594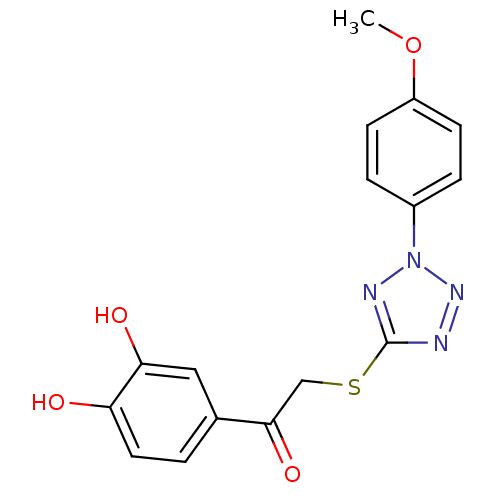 (1-(3,4-dihydroxyphenyl)-2-[2-(4-methoxyphenyl)-2H-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 2.04E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sejong University Curated by ChEMBL | Assay Description Binding affinity to Cdc25A (336-523) (unknown origin) expressed in Escherichia coli by steady-state kinetic assay | J Med Chem 51: 5533-41 (2008) Article DOI: 10.1021/jm701157g BindingDB Entry DOI: 10.7270/Q20V8CMW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| M-phase inducer phosphatase 1 (Homo sapiens (Human)) | BDBM50253593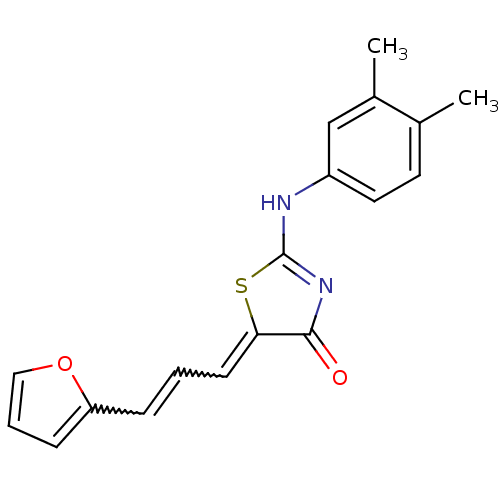 (2-(3,4-Dimethylphenylamino)-5-(3-furan-2-ylallylid...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.14E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sejong University Curated by ChEMBL | Assay Description Binding affinity to Cdc25A (336-523) (unknown origin) expressed in Escherichia coli by steady-state kinetic assay | J Med Chem 51: 5533-41 (2008) Article DOI: 10.1021/jm701157g BindingDB Entry DOI: 10.7270/Q20V8CMW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| M-phase inducer phosphatase 1 (Homo sapiens (Human)) | BDBM50237948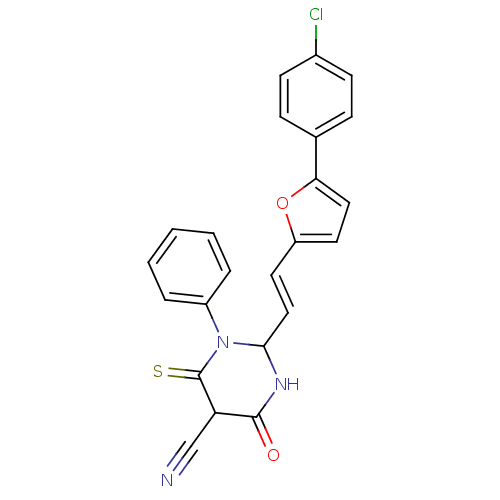 ((E)-2-(2-(5-(4-chlorophenyl)furan-2-yl)vinyl)-6-me...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 2.92E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sejong University Curated by ChEMBL | Assay Description Binding affinity to Cdc25A (336-523) (unknown origin) expressed in Escherichia coli by steady-state kinetic assay | J Med Chem 51: 5533-41 (2008) Article DOI: 10.1021/jm701157g BindingDB Entry DOI: 10.7270/Q20V8CMW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxypeptidase A1 (Bos taurus (bovine)) | BDBM50121270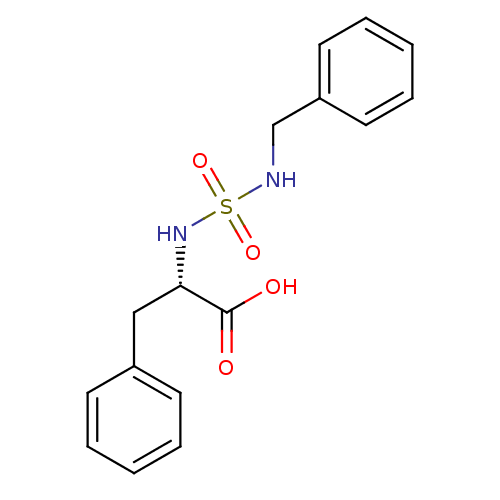 ((2S)-2-({[(phenylmethyl)amino]sulfonyl}amino)-3-ph...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 3.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pohang University of Science and Technology Curated by ChEMBL | Assay Description Inhibitory activity of the compound against Carboxypeptidase A | J Med Chem 45: 5295-302 (2002) BindingDB Entry DOI: 10.7270/Q2BR8RJM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| M-phase inducer phosphatase 1 (Homo sapiens (Human)) | BDBM50253622 (2-[4-(4-chlorophenyl)-5-p-tolyl-4H-[1,2,4]triazol-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.75E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sejong University Curated by ChEMBL | Assay Description Binding affinity to Cdc25A (336-523) (unknown origin) expressed in Escherichia coli by steady-state kinetic assay | J Med Chem 51: 5533-41 (2008) Article DOI: 10.1021/jm701157g BindingDB Entry DOI: 10.7270/Q20V8CMW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxypeptidase A1 (Bos taurus (bovine)) | BDBM50121275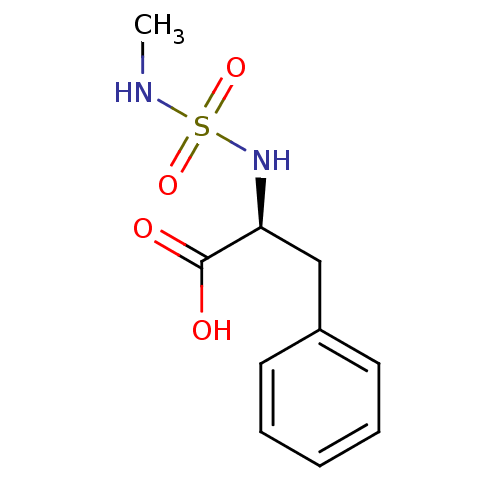 ((2S)-2-{[(methylamino)sulfonyl]amino}-3-phenylprop...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.80E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pohang University of Science and Technology Curated by ChEMBL | Assay Description Inhibitory activity of the compound against Carboxypeptidase A | J Med Chem 45: 5295-302 (2002) BindingDB Entry DOI: 10.7270/Q2BR8RJM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxypeptidase A1 (Bos taurus (bovine)) | BDBM50121276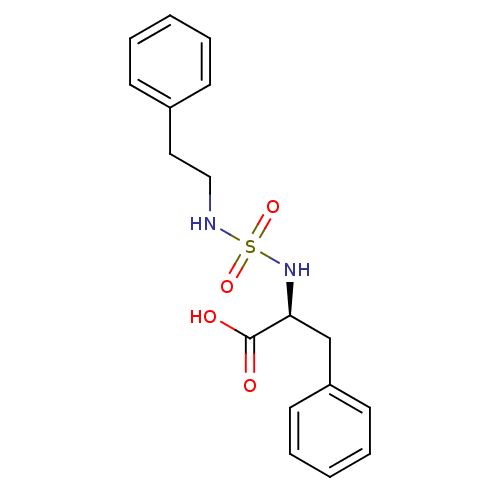 ((2S)-2-({[(2-phenylethyl)amino]sulfonyl}amino)-3-p...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.80E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pohang University of Science and Technology Curated by ChEMBL | Assay Description Inhibitory activity of the compound against Carboxypeptidase A | J Med Chem 45: 5295-302 (2002) BindingDB Entry DOI: 10.7270/Q2BR8RJM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxypeptidase A1 (Bos taurus (bovine)) | BDBM50121269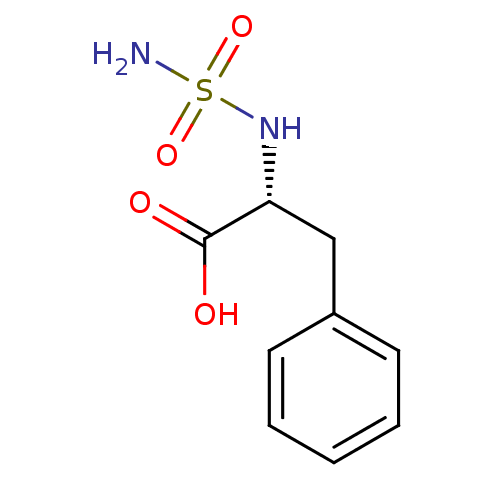 ((2R)-2-[(aminosulfonyl)amino]-3-phenylpropanoic ac...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 4.70E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pohang University of Science and Technology Curated by ChEMBL | Assay Description Inhibitory activity of the compound against Carboxypeptidase A | J Med Chem 45: 5295-302 (2002) BindingDB Entry DOI: 10.7270/Q2BR8RJM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 767 total ) | Next | Last >> |