

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bile salt export pump (Rattus norvegicus) | BDBM50375575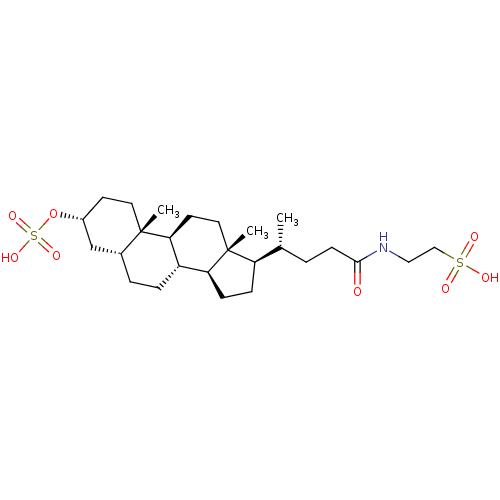 (CHEMBL270493) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Tokyo Curated by ChEMBL | Assay Description TP_TRANSPORTER: inhibition of Taurocholate uptake (Taurocholate: 1 uM) in canalicular membrane vesicles from SD rat | Biochim Biophys Acta 1511: 7-16 (2001) BindingDB Entry DOI: 10.7270/Q2KW5H4R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bile salt export pump (Rattus norvegicus) | BDBM50391001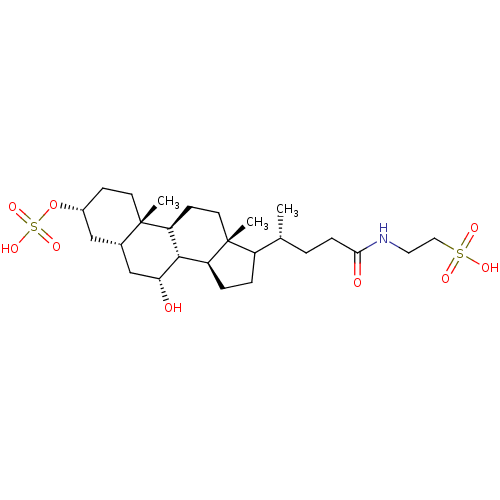 (CHEMBL2074736) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Tokyo Curated by ChEMBL | Assay Description TP_TRANSPORTER: inhibition of Taurocholate uptake (Taurocholate: 1 uM) in canalicular membrane vesicles from SD rat | Biochim Biophys Acta 1511: 7-16 (2001) BindingDB Entry DOI: 10.7270/Q2KW5H4R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bile salt export pump (Rattus norvegicus) | BDBM50375575 (CHEMBL270493) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 6.19E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Tokyo Curated by ChEMBL | Assay Description TP_TRANSPORTER: inhibition of Taurocholate uptake (Taurocholate: 1 uM) in membrane vesicles isolated from Bsep-expressing Sf9 cells | Biochim Biophys Acta 1511: 7-16 (2001) BindingDB Entry DOI: 10.7270/Q2KW5H4R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bile salt export pump (Rattus norvegicus) | BDBM50391001 (CHEMBL2074736) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 6.79E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Tokyo Curated by ChEMBL | Assay Description TP_TRANSPORTER: inhibition of Taurocholate uptake (Taurocholate: 1 uM) in membrane vesicles isolated from Bsep-expressing Sf9 cells | Biochim Biophys Acta 1511: 7-16 (2001) BindingDB Entry DOI: 10.7270/Q2KW5H4R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor alpha (Homo sapiens (Human)) | BDBM50075315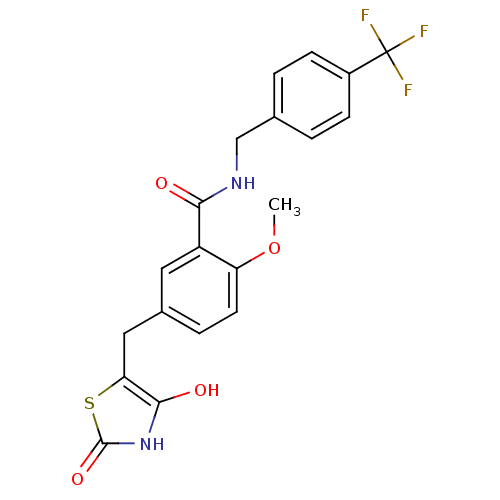 (5-(2,4-Dioxo-thiazolidin-5-ylmethyl)-2-methoxy-N-(...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | n/a | n/a | n/a | 228 | n/a | n/a | n/a | n/a | n/a |
Kyorin Pharmaceutical Co., Ltd. Curated by ChEMBL | Assay Description Binding affinity for Peroxisome proliferator activated receptor alpha (PPAR alpha) | Bioorg Med Chem Lett 9: 533-8 (1999) BindingDB Entry DOI: 10.7270/Q25X283W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50075315 (5-(2,4-Dioxo-thiazolidin-5-ylmethyl)-2-methoxy-N-(...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | n/a | n/a | n/a | 326 | n/a | n/a | n/a | n/a | n/a |
Kyorin Pharmaceutical Co., Ltd. Curated by ChEMBL | Assay Description Binding affinity for Peroxisome proliferator activated receptor gamma (PPAR gamma) | Bioorg Med Chem Lett 9: 533-8 (1999) BindingDB Entry DOI: 10.7270/Q25X283W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||