 Found 98 hits with Last Name = 'kitagawa' and Initial = 'h'
Found 98 hits with Last Name = 'kitagawa' and Initial = 'h' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Lysine-specific histone demethylase 1A
(Homo sapiens (Human)) | BDBM50586369

(CHEMBL5094265)Show SMILES CC(C)C[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](CO)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H]1CCCN1)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCCNC(N)=N)C(O)=O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 5.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human LSD1 using K4me2 peptide as substrate measured after 10 mins by peroxidase-coupled reaction assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01371
BindingDB Entry DOI: 10.7270/Q2KS6WFP |
More data for this
Ligand-Target Pair | |
Lysine-specific histone demethylase 1A
(Homo sapiens (Human)) | BDBM50586368

(CHEMBL5073848)Show SMILES CC(C)C[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](CO)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H]1CCCN1)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCCCN)C(O)=O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 7.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human LSD1 using K4me2 peptide as substrate measured after 10 mins by peroxidase-coupled reaction assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01371
BindingDB Entry DOI: 10.7270/Q2KS6WFP |
More data for this
Ligand-Target Pair | |
REST corepressor 1
(Homo sapiens (Human)) | BDBM50586370
(CHEMBL5084197)Show SMILES CC(C)C[C@@H]1NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](CO)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H]2CCCN2C(=O)OCCSSCCn2cc(COc3cc(OCc4cn(CCSSCCOC(=O)NCCCC[C@H](NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H](NC1=O)C(C)C)C(N)=O)nn4)cc(c3)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCCNC(N)=N)C(N)=O)nn2 |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human LSD1/CoREST |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01371
BindingDB Entry DOI: 10.7270/Q2KS6WFP |
More data for this
Ligand-Target Pair | |
REST corepressor 1
(Homo sapiens (Human)) | BDBM50586371
(CHEMBL5089876)Show SMILES CC(C)C[C@@H]1NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](CO)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H]2CCCN2C(=O)OCCOCCn2cc(COc3cc(OCc4cn(CCOCCOC(=O)NCCCC[C@H](NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H](NC1=O)C(C)C)C(N)=O)nn4)cc(c3)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCCNC(N)=N)C(N)=O)nn2 |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human LSD1/CoREST |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01371
BindingDB Entry DOI: 10.7270/Q2KS6WFP |
More data for this
Ligand-Target Pair | |
Lysine-specific histone demethylase 1A
(Homo sapiens (Human)) | BDBM50586366

(CHEMBL5093950)Show SMILES CC(C)C[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](CO)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H]1CCCN1)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCCNC(N)=N)C(O)=O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human LSD1 using K4me2 peptide as substrate measured after 10 mins by peroxidase-coupled reaction assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01371
BindingDB Entry DOI: 10.7270/Q2KS6WFP |
More data for this
Ligand-Target Pair | |
Lysine-specific histone demethylase 1A
(Homo sapiens (Human)) | BDBM50586367

(CHEMBL5089144)Show SMILES CC(C)C[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](CO)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H]1CCCN1)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CCCNC(N)=N)C(O)=O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human LSD1 using K4me2 peptide as substrate measured after 10 mins by peroxidase-coupled reaction assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01371
BindingDB Entry DOI: 10.7270/Q2KS6WFP |
More data for this
Ligand-Target Pair | |
REST corepressor 1
(Homo sapiens (Human)) | BDBM50586373

(CHEMBL5075544)Show SMILES NC(=N)NCCC[C@H](NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CCCNC(N)=N)NC(=O)c1cc(OCc2cn(CCO)nn2)cc(OCc2cn(CCO)nn2)c1)C(N)=O |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human LSD1/CoREST |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01371
BindingDB Entry DOI: 10.7270/Q2KS6WFP |
More data for this
Ligand-Target Pair | |
Lysine-specific histone demethylase 1A
(Homo sapiens (Human)) | BDBM50586365

(CHEMBL5078239)Show SMILES CC(C)C[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](CO)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H]1CCCN1)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N1CCC[C@H]1C(O)=O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human LSD1 using K4me2 peptide as substrate measured after 10 mins by peroxidase-coupled reaction assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01371
BindingDB Entry DOI: 10.7270/Q2KS6WFP |
More data for this
Ligand-Target Pair | |
REST corepressor 1
(Homo sapiens (Human)) | BDBM50586372
(CHEMBL5085737)Show SMILES CC(C)C[C@@H]1NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](CO)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H]2CCCN2C(=O)OCCSSCCn2cc(COc3cccc(OCc4cn(CCSSCCOC(=O)NCCCC[C@H](NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H](NC1=O)C(C)C)C(N)=O)nn4)c3)nn2 |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 640 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human LSD1/CoREST |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01371
BindingDB Entry DOI: 10.7270/Q2KS6WFP |
More data for this
Ligand-Target Pair | |
Lysine-specific histone demethylase 1A
(Homo sapiens (Human)) | BDBM50586364
(CHEMBL5084292)Show SMILES CC(C)C[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](CO)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H]1CCCN1)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCCCN)C(O)=O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 950 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human LSD1 using K4me2 peptide as substrate measured after 10 mins by peroxidase-coupled reaction assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01371
BindingDB Entry DOI: 10.7270/Q2KS6WFP |
More data for this
Ligand-Target Pair | |
Lysine-specific histone demethylase 1A
(Homo sapiens (Human)) | BDBM50586363
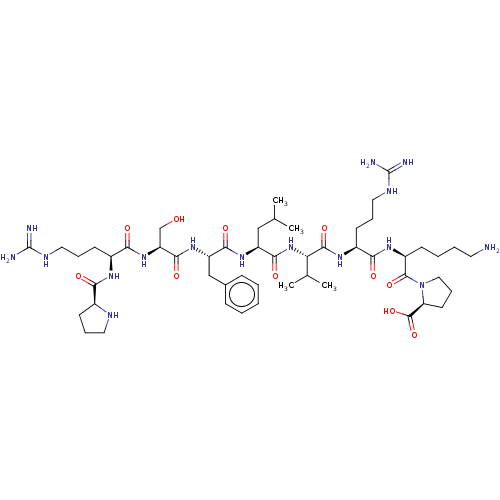
(CHEMBL5079374)Show SMILES CC(C)C[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](CO)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H]1CCCN1)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCCCN)C(=O)N1CCC[C@H]1C(O)=O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human LSD1 using K4me2 peptide as substrate measured after 10 mins by peroxidase-coupled reaction assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01371
BindingDB Entry DOI: 10.7270/Q2KS6WFP |
More data for this
Ligand-Target Pair | |
Trans-2-enoyl-ACP reductase II
(Streptococcus pneumoniae) | BDBM50219763
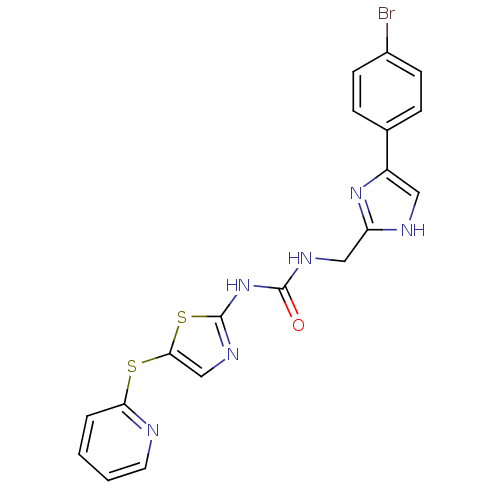
(1-((4-(4-bromophenyl)-1H-imidazol-2-yl)methyl)-3-(...)Show SMILES Brc1ccc(cc1)-c1c[nH]c(CNC(=O)Nc2ncc(Sc3ccccn3)s2)n1 Show InChI InChI=1S/C19H15BrN6OS2/c20-13-6-4-12(5-7-13)14-9-22-15(25-14)10-23-18(27)26-19-24-11-17(29-19)28-16-3-1-2-8-21-16/h1-9,11H,10H2,(H,22,25)(H2,23,24,26,27) | PDB
MMDB
KEGG
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Meiji Seika Kaisha, Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of Streptococcus pneumoniae FabK |
J Med Chem 50: 4710-20 (2007)
Article DOI: 10.1021/jm0705354
BindingDB Entry DOI: 10.7270/Q2NZ88GS |
More data for this
Ligand-Target Pair | |
Trans-2-enoyl-ACP reductase II
(Streptococcus pneumoniae) | BDBM50219763

(1-((4-(4-bromophenyl)-1H-imidazol-2-yl)methyl)-3-(...)Show SMILES Brc1ccc(cc1)-c1c[nH]c(CNC(=O)Nc2ncc(Sc3ccccn3)s2)n1 Show InChI InChI=1S/C19H15BrN6OS2/c20-13-6-4-12(5-7-13)14-9-22-15(25-14)10-23-18(27)26-19-24-11-17(29-19)28-16-3-1-2-8-21-16/h1-9,11H,10H2,(H,22,25)(H2,23,24,26,27) | PDB
MMDB
KEGG
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Meiji Seika Kaisha, Ltd
Curated by ChEMBL
| Assay Description
Inhibition of Streptococcus pneumoniae enoyl-ACP reductase FabK |
Bioorg Med Chem 15: 7325-36 (2007)
Article DOI: 10.1016/j.bmc.2007.08.050
BindingDB Entry DOI: 10.7270/Q25T3MBH |
More data for this
Ligand-Target Pair | |
Trans-2-enoyl-ACP reductase II
(Streptococcus pneumoniae) | BDBM50223360
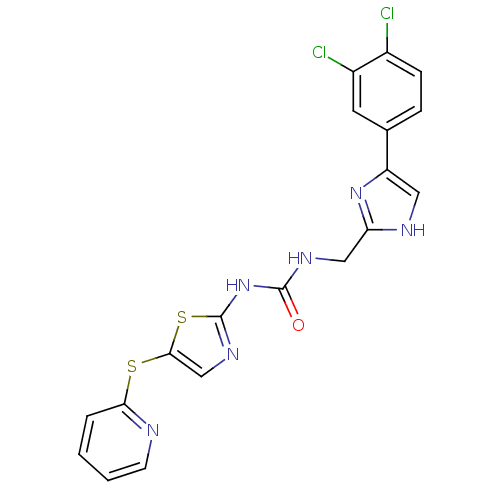
(1-((5-(3,4-dichlorophenyl)-1H-imidazol-2-yl)methyl...)Show SMILES Clc1ccc(cc1Cl)-c1c[nH]c(CNC(=O)Nc2ncc(Sc3ccccn3)s2)n1 Show InChI InChI=1S/C19H14Cl2N6OS2/c20-12-5-4-11(7-13(12)21)14-8-23-15(26-14)9-24-18(28)27-19-25-10-17(30-19)29-16-3-1-2-6-22-16/h1-8,10H,9H2,(H,23,26)(H2,24,25,27,28) | PDB
MMDB
KEGG
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Meiji Seika Kaisha, Ltd
Curated by ChEMBL
| Assay Description
Inhibition of Streptococcus pneumoniae enoyl-ACP reductase FabK |
Bioorg Med Chem 15: 7325-36 (2007)
Article DOI: 10.1016/j.bmc.2007.08.050
BindingDB Entry DOI: 10.7270/Q25T3MBH |
More data for this
Ligand-Target Pair | |
Trans-2-enoyl-ACP reductase II
(Streptococcus pneumoniae) | BDBM50371156

(CHEMBL390211)Show SMILES Cc1c(Cc2c(Cl)cccc2Cl)c(=O)ccn1CCc1ccc(cc1)-c1c[nH]c(CNC(=O)Nc2nc3ccc(cc3s2)S(C)(=O)=O)n1 Show InChI InChI=1S/C34H30Cl2N6O4S2/c1-20-24(17-25-26(35)4-3-5-27(25)36)30(43)13-15-42(20)14-12-21-6-8-22(9-7-21)29-18-37-32(39-29)19-38-33(44)41-34-40-28-11-10-23(48(2,45)46)16-31(28)47-34/h3-11,13,15-16,18H,12,14,17,19H2,1-2H3,(H,37,39)(H2,38,40,41,44) | PDB
MMDB
KEGG
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Meiji Seika Kaisha, Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of Streptococcus pneumoniae FabK |
J Med Chem 50: 4710-20 (2007)
Article DOI: 10.1021/jm0705354
BindingDB Entry DOI: 10.7270/Q2NZ88GS |
More data for this
Ligand-Target Pair | |
Trans-2-enoyl-ACP reductase II
(Streptococcus pneumoniae) | BDBM50219766
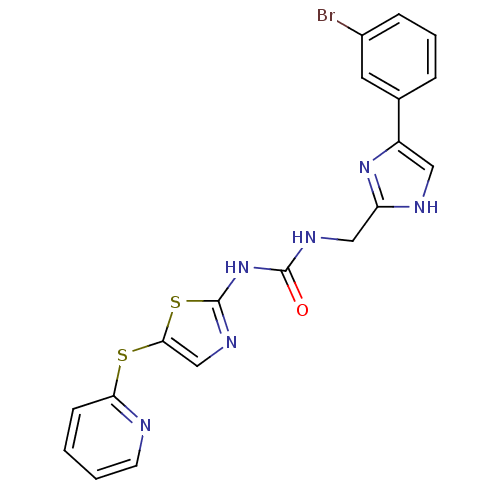
(1-((4-(3-bromophenyl)-1H-imidazol-2-yl)methyl)-3-(...)Show SMILES Brc1cccc(c1)-c1c[nH]c(CNC(=O)Nc2ncc(Sc3ccccn3)s2)n1 Show InChI InChI=1S/C19H15BrN6OS2/c20-13-5-3-4-12(8-13)14-9-22-15(25-14)10-23-18(27)26-19-24-11-17(29-19)28-16-6-1-2-7-21-16/h1-9,11H,10H2,(H,22,25)(H2,23,24,26,27) | PDB
MMDB
KEGG
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Meiji Seika Kaisha, Ltd
Curated by ChEMBL
| Assay Description
Inhibition of Streptococcus pneumoniae enoyl-ACP reductase FabK |
Bioorg Med Chem 15: 7325-36 (2007)
Article DOI: 10.1016/j.bmc.2007.08.050
BindingDB Entry DOI: 10.7270/Q25T3MBH |
More data for this
Ligand-Target Pair | |
Trans-2-enoyl-ACP reductase II
(Streptococcus pneumoniae) | BDBM50219766

(1-((4-(3-bromophenyl)-1H-imidazol-2-yl)methyl)-3-(...)Show SMILES Brc1cccc(c1)-c1c[nH]c(CNC(=O)Nc2ncc(Sc3ccccn3)s2)n1 Show InChI InChI=1S/C19H15BrN6OS2/c20-13-5-3-4-12(8-13)14-9-22-15(25-14)10-23-18(27)26-19-24-11-17(29-19)28-16-6-1-2-7-21-16/h1-9,11H,10H2,(H,22,25)(H2,23,24,26,27) | PDB
MMDB
KEGG
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Meiji Seika Kaisha, Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of Streptococcus pneumoniae FabK |
J Med Chem 50: 4710-20 (2007)
Article DOI: 10.1021/jm0705354
BindingDB Entry DOI: 10.7270/Q2NZ88GS |
More data for this
Ligand-Target Pair | |
Trans-2-enoyl-ACP reductase II
(Streptococcus pneumoniae) | BDBM50371158
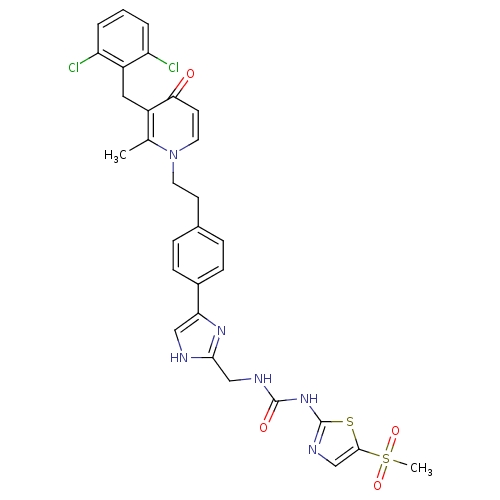
(CHEMBL175157)Show SMILES Cc1c(Cc2c(Cl)cccc2Cl)c(=O)ccn1CCc1ccc(cc1)-c1c[nH]c(CNC(=O)Nc2ncc(s2)S(C)(=O)=O)n1 Show InChI InChI=1S/C30H28Cl2N6O4S2/c1-18-21(14-22-23(31)4-3-5-24(22)32)26(39)11-13-38(18)12-10-19-6-8-20(9-7-19)25-15-33-27(36-25)16-34-29(40)37-30-35-17-28(43-30)44(2,41)42/h3-9,11,13,15,17H,10,12,14,16H2,1-2H3,(H,33,36)(H2,34,35,37,40) | PDB
MMDB
KEGG
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Meiji Seika Kaisha, Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of Streptococcus pneumoniae FabK |
J Med Chem 50: 4710-20 (2007)
Article DOI: 10.1021/jm0705354
BindingDB Entry DOI: 10.7270/Q2NZ88GS |
More data for this
Ligand-Target Pair | |
Trans-2-enoyl-ACP reductase II
(Streptococcus pneumoniae) | BDBM50371150

(CHEMBL244962)Show SMILES Cc1c(Cc2c(Cl)cccc2Cl)c(=O)ccn1CCc1ccc(cc1)-c1c[nH]c(CNC(=O)Nc2ncc(Sc3ccccn3)s2)n1 Show InChI InChI=1S/C34H29Cl2N7O2S2/c1-21-24(17-25-26(35)5-4-6-27(25)36)29(44)13-16-43(21)15-12-22-8-10-23(11-9-22)28-18-38-30(41-28)19-39-33(45)42-34-40-20-32(47-34)46-31-7-2-3-14-37-31/h2-11,13-14,16,18,20H,12,15,17,19H2,1H3,(H,38,41)(H2,39,40,42,45) | PDB
MMDB
KEGG
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Meiji Seika Kaisha, Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of Streptococcus pneumoniae FabK |
J Med Chem 50: 4710-20 (2007)
Article DOI: 10.1021/jm0705354
BindingDB Entry DOI: 10.7270/Q2NZ88GS |
More data for this
Ligand-Target Pair | |
Trans-2-enoyl-ACP reductase II
(Streptococcus pneumoniae) | BDBM50371149
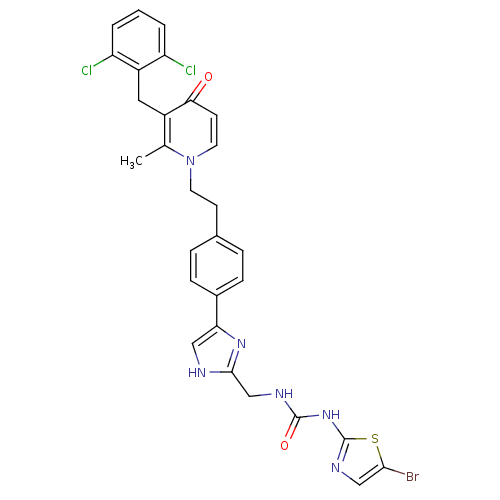
(CHEMBL390895)Show SMILES Cc1c(Cc2c(Cl)cccc2Cl)c(=O)ccn1CCc1ccc(cc1)-c1c[nH]c(CNC(=O)Nc2ncc(Br)s2)n1 Show InChI InChI=1S/C29H25BrCl2N6O2S/c1-17-20(13-21-22(31)3-2-4-23(21)32)25(39)10-12-38(17)11-9-18-5-7-19(8-6-18)24-14-33-27(36-24)16-34-28(40)37-29-35-15-26(30)41-29/h2-8,10,12,14-15H,9,11,13,16H2,1H3,(H,33,36)(H2,34,35,37,40) | PDB
MMDB
KEGG
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
Meiji Seika Kaisha, Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of Streptococcus pneumoniae FabK |
J Med Chem 50: 4710-20 (2007)
Article DOI: 10.1021/jm0705354
BindingDB Entry DOI: 10.7270/Q2NZ88GS |
More data for this
Ligand-Target Pair | |
Trans-2-enoyl-ACP reductase II
(Streptococcus pneumoniae) | BDBM50371154

(CHEMBL242241)Show SMILES [O-]C(=O)COc1ccc(cc1)-c1c[nH]c(CNC(=O)Nc2ncc(Sc3ccccn3)s2)n1 Show InChI InChI=1S/C21H18N6O4S2/c28-18(29)12-31-14-6-4-13(5-7-14)15-9-23-16(26-15)10-24-20(30)27-21-25-11-19(33-21)32-17-3-1-2-8-22-17/h1-9,11H,10,12H2,(H,23,26)(H,28,29)(H2,24,25,27,30)/p-1 | PDB
MMDB
KEGG
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 34 | n/a | n/a | n/a | n/a | n/a | n/a |
Meiji Seika Kaisha, Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of Streptococcus pneumoniae FabK |
J Med Chem 50: 4710-20 (2007)
Article DOI: 10.1021/jm0705354
BindingDB Entry DOI: 10.7270/Q2NZ88GS |
More data for this
Ligand-Target Pair | |
Trans-2-enoyl-ACP reductase II
(Streptococcus pneumoniae) | BDBM50223356

(4-(2-(3-((4-phenyl-1H-imidazol-2-yl)methyl)ureido)...)Show SMILES NC(=O)c1ccc(Sc2cnc(NC(=O)NCc3nc(c[nH]3)-c3ccccc3)s2)cc1 Show InChI InChI=1S/C21H18N6O2S2/c22-19(28)14-6-8-15(9-7-14)30-18-12-25-21(31-18)27-20(29)24-11-17-23-10-16(26-17)13-4-2-1-3-5-13/h1-10,12H,11H2,(H2,22,28)(H,23,26)(H2,24,25,27,29) | PDB
MMDB
KEGG
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 37 | n/a | n/a | n/a | n/a | n/a | n/a |
Meiji Seika Kaisha, Ltd
Curated by ChEMBL
| Assay Description
Inhibition of Streptococcus pneumoniae enoyl-ACP reductase FabK |
Bioorg Med Chem 15: 7325-36 (2007)
Article DOI: 10.1016/j.bmc.2007.08.050
BindingDB Entry DOI: 10.7270/Q25T3MBH |
More data for this
Ligand-Target Pair | |
Trans-2-enoyl-ACP reductase II
(Streptococcus pneumoniae) | BDBM50223364
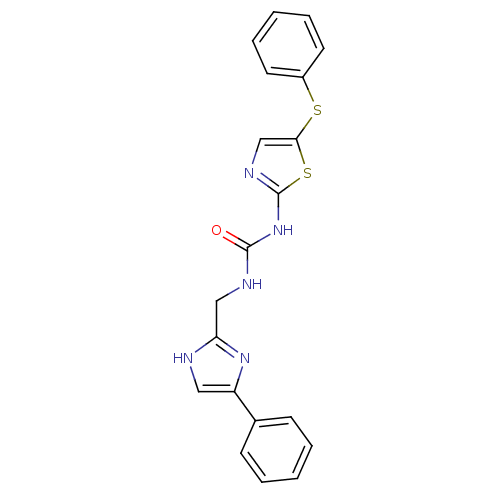
(1-((4-phenyl-1H-imidazol-2-yl)methyl)-3-(5-(phenyl...)Show SMILES O=C(NCc1nc(c[nH]1)-c1ccccc1)Nc1ncc(Sc2ccccc2)s1 Show InChI InChI=1S/C20H17N5OS2/c26-19(22-12-17-21-11-16(24-17)14-7-3-1-4-8-14)25-20-23-13-18(28-20)27-15-9-5-2-6-10-15/h1-11,13H,12H2,(H,21,24)(H2,22,23,25,26) | PDB
MMDB
KEGG
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 37 | n/a | n/a | n/a | n/a | n/a | n/a |
Meiji Seika Kaisha, Ltd
Curated by ChEMBL
| Assay Description
Inhibition of Streptococcus pneumoniae enoyl-ACP reductase FabK |
Bioorg Med Chem 15: 7325-36 (2007)
Article DOI: 10.1016/j.bmc.2007.08.050
BindingDB Entry DOI: 10.7270/Q25T3MBH |
More data for this
Ligand-Target Pair | |
Trans-2-enoyl-ACP reductase II
(Streptococcus pneumoniae) | BDBM50219767
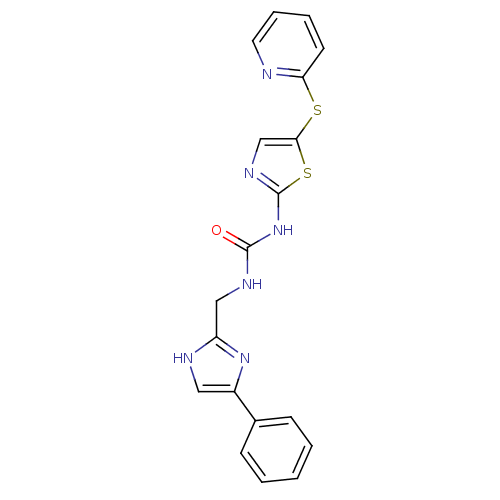
(1-((4-phenyl-1H-imidazol-2-yl)methyl)-3-(5-(pyridi...)Show SMILES O=C(NCc1nc(c[nH]1)-c1ccccc1)Nc1ncc(Sc2ccccn2)s1 Show InChI InChI=1S/C19H16N6OS2/c26-18(22-11-15-21-10-14(24-15)13-6-2-1-3-7-13)25-19-23-12-17(28-19)27-16-8-4-5-9-20-16/h1-10,12H,11H2,(H,21,24)(H2,22,23,25,26) | PDB
MMDB
KEGG
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 42 | n/a | n/a | n/a | n/a | n/a | n/a |
Meiji Seika Kaisha, Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of Streptococcus pneumoniae FabK |
J Med Chem 50: 4710-20 (2007)
Article DOI: 10.1021/jm0705354
BindingDB Entry DOI: 10.7270/Q2NZ88GS |
More data for this
Ligand-Target Pair | |
Trans-2-enoyl-ACP reductase II
(Streptococcus pneumoniae) | BDBM50219767

(1-((4-phenyl-1H-imidazol-2-yl)methyl)-3-(5-(pyridi...)Show SMILES O=C(NCc1nc(c[nH]1)-c1ccccc1)Nc1ncc(Sc2ccccn2)s1 Show InChI InChI=1S/C19H16N6OS2/c26-18(22-11-15-21-10-14(24-15)13-6-2-1-3-7-13)25-19-23-12-17(28-19)27-16-8-4-5-9-20-16/h1-10,12H,11H2,(H,21,24)(H2,22,23,25,26) | PDB
MMDB
KEGG
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 42 | n/a | n/a | n/a | n/a | n/a | n/a |
Meiji Seika Kaisha, Ltd
Curated by ChEMBL
| Assay Description
Inhibition of Streptococcus pneumoniae enoyl-ACP reductase FabK |
Bioorg Med Chem 15: 7325-36 (2007)
Article DOI: 10.1016/j.bmc.2007.08.050
BindingDB Entry DOI: 10.7270/Q25T3MBH |
More data for this
Ligand-Target Pair | |
Trans-2-enoyl-ACP reductase II
(Streptococcus pneumoniae) | BDBM50216891
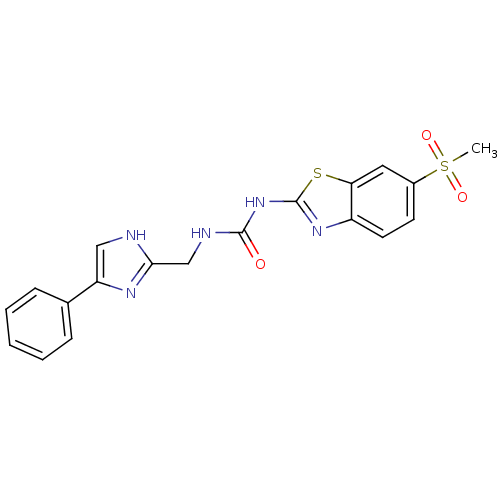
(1-((4-phenyl-1H-imidazol-2-yl)methyl)-3-(6-(methyl...)Show SMILES CS(=O)(=O)c1ccc2nc(NC(=O)NCc3nc(c[nH]3)-c3ccccc3)sc2c1 Show InChI InChI=1S/C19H17N5O3S2/c1-29(26,27)13-7-8-14-16(9-13)28-19(23-14)24-18(25)21-11-17-20-10-15(22-17)12-5-3-2-4-6-12/h2-10H,11H2,1H3,(H,20,22)(H2,21,23,24,25) | PDB
MMDB
KEGG
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 88 | n/a | n/a | n/a | n/a | n/a | n/a |
Meiji Seika Kaisha, Ltd
Curated by ChEMBL
| Assay Description
Inhibition of Streptococcus pneumoniae enoyl-ACP reductase FabK |
Bioorg Med Chem 15: 7325-36 (2007)
Article DOI: 10.1016/j.bmc.2007.08.050
BindingDB Entry DOI: 10.7270/Q25T3MBH |
More data for this
Ligand-Target Pair | |
Trans-2-enoyl-ACP reductase II
(Streptococcus pneumoniae) | BDBM50223363
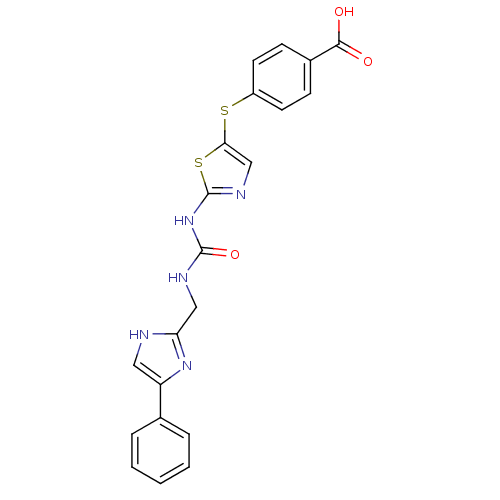
(4-(2-(3-((4-phenyl-1H-imidazol-2-yl)methyl)ureido)...)Show SMILES OC(=O)c1ccc(Sc2cnc(NC(=O)NCc3nc(c[nH]3)-c3ccccc3)s2)cc1 Show InChI InChI=1S/C21H17N5O3S2/c27-19(28)14-6-8-15(9-7-14)30-18-12-24-21(31-18)26-20(29)23-11-17-22-10-16(25-17)13-4-2-1-3-5-13/h1-10,12H,11H2,(H,22,25)(H,27,28)(H2,23,24,26,29) | PDB
MMDB
KEGG
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 98 | n/a | n/a | n/a | n/a | n/a | n/a |
Meiji Seika Kaisha, Ltd
Curated by ChEMBL
| Assay Description
Inhibition of Streptococcus pneumoniae enoyl-ACP reductase FabK |
Bioorg Med Chem 15: 7325-36 (2007)
Article DOI: 10.1016/j.bmc.2007.08.050
BindingDB Entry DOI: 10.7270/Q25T3MBH |
More data for this
Ligand-Target Pair | |
Trans-2-enoyl-ACP reductase II
(Streptococcus pneumoniae) | BDBM50216891

(1-((4-phenyl-1H-imidazol-2-yl)methyl)-3-(6-(methyl...)Show SMILES CS(=O)(=O)c1ccc2nc(NC(=O)NCc3nc(c[nH]3)-c3ccccc3)sc2c1 Show InChI InChI=1S/C19H17N5O3S2/c1-29(26,27)13-7-8-14-16(9-13)28-19(23-14)24-18(25)21-11-17-20-10-15(22-17)12-5-3-2-4-6-12/h2-10H,11H2,1H3,(H,20,22)(H2,21,23,24,25) | PDB
MMDB
KEGG
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
Meiji Seika Kaisha Ltd
Curated by ChEMBL
| Assay Description
Inhibition of Streptococcus pneumoniae KU197 FabK |
Bioorg Med Chem Lett 17: 4982-6 (2007)
Article DOI: 10.1016/j.bmcl.2007.06.040
BindingDB Entry DOI: 10.7270/Q2C53MP9 |
More data for this
Ligand-Target Pair | |
Trans-2-enoyl-ACP reductase II
(Streptococcus pneumoniae) | BDBM50223357

(1-((4-phenyl-1H-imidazol-2-yl)methyl)-3-(5-phenylt...)Show SMILES O=C(NCc1nc(c[nH]1)-c1ccccc1)Nc1ncc(s1)-c1ccccc1 Show InChI InChI=1S/C20H17N5OS/c26-19(25-20-23-12-17(27-20)15-9-5-2-6-10-15)22-13-18-21-11-16(24-18)14-7-3-1-4-8-14/h1-12H,13H2,(H,21,24)(H2,22,23,25,26) | PDB
MMDB
KEGG
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 380 | n/a | n/a | n/a | n/a | n/a | n/a |
Meiji Seika Kaisha, Ltd
Curated by ChEMBL
| Assay Description
Inhibition of Streptococcus pneumoniae enoyl-ACP reductase FabK |
Bioorg Med Chem 15: 7325-36 (2007)
Article DOI: 10.1016/j.bmc.2007.08.050
BindingDB Entry DOI: 10.7270/Q25T3MBH |
More data for this
Ligand-Target Pair | |
Trans-2-enoyl-ACP reductase II
(Streptococcus pneumoniae) | BDBM50216895

(1-((1H-benzo[d]imidazol-2-yl)methyl)-3-(6-(methyls...)Show SMILES CS(=O)(=O)c1ccc2nc(NC(=O)NCc3nc4ccccc4[nH]3)sc2c1 Show InChI InChI=1S/C17H15N5O3S2/c1-27(24,25)10-6-7-13-14(8-10)26-17(21-13)22-16(23)18-9-15-19-11-4-2-3-5-12(11)20-15/h2-8H,9H2,1H3,(H,19,20)(H2,18,21,22,23) | PDB
MMDB
KEGG
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 740 | n/a | n/a | n/a | n/a | n/a | n/a |
Meiji Seika Kaisha, Ltd
Curated by ChEMBL
| Assay Description
Inhibition of Streptococcus pneumoniae enoyl-ACP reductase FabK |
Bioorg Med Chem 15: 7325-36 (2007)
Article DOI: 10.1016/j.bmc.2007.08.050
BindingDB Entry DOI: 10.7270/Q25T3MBH |
More data for this
Ligand-Target Pair | |
Trans-2-enoyl-ACP reductase II
(Streptococcus pneumoniae) | BDBM50216895

(1-((1H-benzo[d]imidazol-2-yl)methyl)-3-(6-(methyls...)Show SMILES CS(=O)(=O)c1ccc2nc(NC(=O)NCc3nc4ccccc4[nH]3)sc2c1 Show InChI InChI=1S/C17H15N5O3S2/c1-27(24,25)10-6-7-13-14(8-10)26-17(21-13)22-16(23)18-9-15-19-11-4-2-3-5-12(11)20-15/h2-8H,9H2,1H3,(H,19,20)(H2,18,21,22,23) | PDB
MMDB
KEGG
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 760 | n/a | n/a | n/a | n/a | n/a | n/a |
Meiji Seika Kaisha Ltd
Curated by ChEMBL
| Assay Description
Inhibition of Streptococcus pneumoniae KU197 FabK |
Bioorg Med Chem Lett 17: 4982-6 (2007)
Article DOI: 10.1016/j.bmcl.2007.06.040
BindingDB Entry DOI: 10.7270/Q2C53MP9 |
More data for this
Ligand-Target Pair | |
Enoyl-[acyl-carrier-protein] reductase [NADH] FabI
(Escherichia coli) | BDBM8726

(5-chloro-2-(2,4-dichlorophenoxy)phenol | CHEMBL849...)Show InChI InChI=1S/C12H7Cl3O2/c13-7-1-3-11(9(15)5-7)17-12-4-2-8(14)6-10(12)16/h1-6,16H | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 900 | n/a | n/a | n/a | n/a | n/a | n/a |
Meiji Seika Kaisha, Ltd
Curated by ChEMBL
| Assay Description
Inhibition of Escherichia coli enoyl-ACP reductase FabI |
Bioorg Med Chem 15: 7325-36 (2007)
Article DOI: 10.1016/j.bmc.2007.08.050
BindingDB Entry DOI: 10.7270/Q25T3MBH |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Trans-2-enoyl-ACP reductase II
(Streptococcus pneumoniae) | BDBM50223362
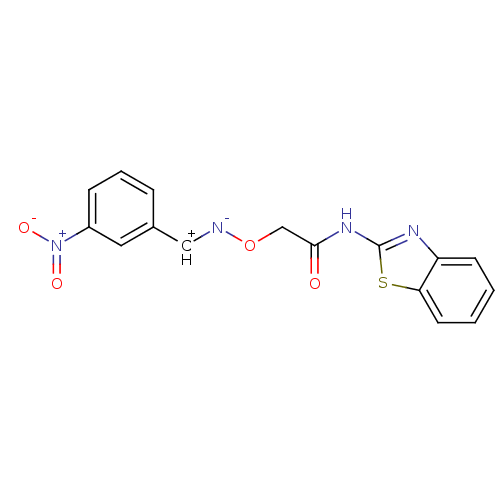
(CHEMBL237447 | N-benzothiazol-2-yl-2-[1-(3-nitro-p...)Show SMILES [O-][N+](=O)c1cccc([CH+][N-]OCC(=O)Nc2nc3ccccc3s2)c1 Show InChI InChI=1S/C16H12N4O4S/c21-15(19-16-18-13-6-1-2-7-14(13)25-16)10-24-17-9-11-4-3-5-12(8-11)20(22)23/h1-9H,10H2,(H,18,19,21) | PDB
MMDB
KEGG
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Meiji Seika Kaisha, Ltd
Curated by ChEMBL
| Assay Description
Inhibition of Streptococcus pneumoniae enoyl-ACP reductase FabK |
Bioorg Med Chem 15: 7325-36 (2007)
Article DOI: 10.1016/j.bmc.2007.08.050
BindingDB Entry DOI: 10.7270/Q25T3MBH |
More data for this
Ligand-Target Pair | |
Trans-2-enoyl-ACP reductase II
(Streptococcus pneumoniae) | BDBM50216894

(1-((1H-benzo[d]imidazol-2-yl)methyl)-3-(6-methoxyb...)Show InChI InChI=1S/C17H15N5O2S/c1-24-10-6-7-13-14(8-10)25-17(21-13)22-16(23)18-9-15-19-11-4-2-3-5-12(11)20-15/h2-8H,9H2,1H3,(H,19,20)(H2,18,21,22,23) | PDB
MMDB
KEGG
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Meiji Seika Kaisha, Ltd
Curated by ChEMBL
| Assay Description
Inhibition of Streptococcus pneumoniae enoyl-ACP reductase FabK |
Bioorg Med Chem 15: 7325-36 (2007)
Article DOI: 10.1016/j.bmc.2007.08.050
BindingDB Entry DOI: 10.7270/Q25T3MBH |
More data for this
Ligand-Target Pair | |
Trans-2-enoyl-ACP reductase II
(Streptococcus pneumoniae) | BDBM50223365
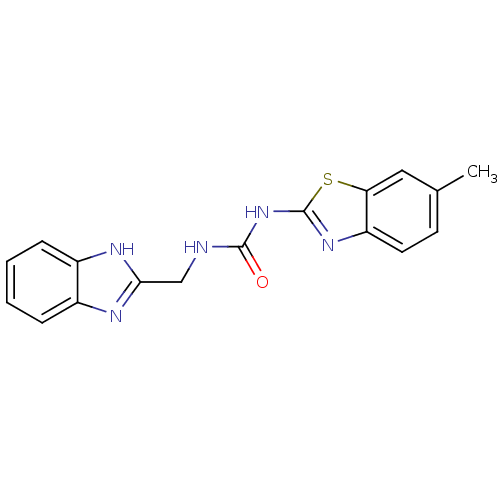
(1-((1H-benzo[d]imidazol-2-yl)methyl)-3-(6-methylbe...)Show InChI InChI=1S/C17H15N5OS/c1-10-6-7-13-14(8-10)24-17(21-13)22-16(23)18-9-15-19-11-4-2-3-5-12(11)20-15/h2-8H,9H2,1H3,(H,19,20)(H2,18,21,22,23) | PDB
MMDB
KEGG
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Meiji Seika Kaisha, Ltd
Curated by ChEMBL
| Assay Description
Inhibition of Streptococcus pneumoniae enoyl-ACP reductase FabK |
Bioorg Med Chem 15: 7325-36 (2007)
Article DOI: 10.1016/j.bmc.2007.08.050
BindingDB Entry DOI: 10.7270/Q25T3MBH |
More data for this
Ligand-Target Pair | |
Trans-2-enoyl-ACP reductase II
(Streptococcus pneumoniae) | BDBM50223358

(1-(6-(methylsulfonyl)benzo[d]thiazol-2-yl)-3-((5-(...)Show SMILES CS(=O)(=O)c1ccc2nc(NC(=O)NCc3ccc(s3)-c3ccccn3)sc2c1 Show InChI InChI=1S/C19H16N4O3S3/c1-29(25,26)13-6-7-15-17(10-13)28-19(22-15)23-18(24)21-11-12-5-8-16(27-12)14-4-2-3-9-20-14/h2-10H,11H2,1H3,(H2,21,22,23,24) | PDB
MMDB
KEGG
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Meiji Seika Kaisha, Ltd
Curated by ChEMBL
| Assay Description
Inhibition of Streptococcus pneumoniae enoyl-ACP reductase FabK |
Bioorg Med Chem 15: 7325-36 (2007)
Article DOI: 10.1016/j.bmc.2007.08.050
BindingDB Entry DOI: 10.7270/Q25T3MBH |
More data for this
Ligand-Target Pair | |
Trans-2-enoyl-ACP reductase II
(Streptococcus pneumoniae) | BDBM50223355
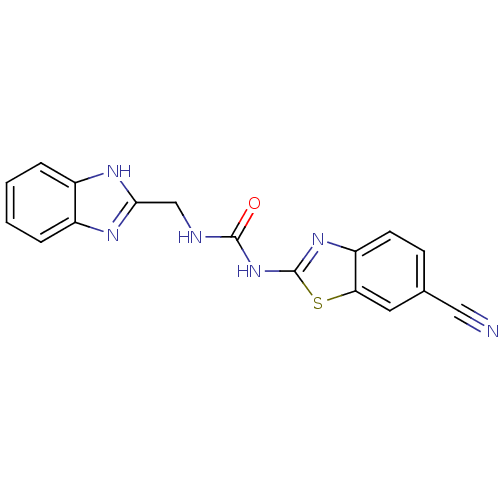
(1-((1H-benzo[d]imidazol-2-yl)methyl)-3-(6-cyanoben...)Show InChI InChI=1S/C17H12N6OS/c18-8-10-5-6-13-14(7-10)25-17(22-13)23-16(24)19-9-15-20-11-3-1-2-4-12(11)21-15/h1-7H,9H2,(H,20,21)(H2,19,22,23,24) | PDB
MMDB
KEGG
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Meiji Seika Kaisha, Ltd
Curated by ChEMBL
| Assay Description
Inhibition of Streptococcus pneumoniae enoyl-ACP reductase FabK |
Bioorg Med Chem 15: 7325-36 (2007)
Article DOI: 10.1016/j.bmc.2007.08.050
BindingDB Entry DOI: 10.7270/Q25T3MBH |
More data for this
Ligand-Target Pair | |
Trans-2-enoyl-ACP reductase II
(Streptococcus pneumoniae) | BDBM50371223

(CHEMBL246581)Show InChI InChI=1S/C17H12N6OS/c18-8-10-5-6-14-13(7-10)22-17(25-14)23-16(24)19-9-15-20-11-3-1-2-4-12(11)21-15/h1-7H,9H2,(H,20,21)(H2,19,22,23,24) | PDB
MMDB
KEGG
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Meiji Seika Kaisha Ltd
Curated by ChEMBL
| Assay Description
Inhibition of Streptococcus pneumoniae KU197 FabK |
Bioorg Med Chem Lett 17: 4982-6 (2007)
Article DOI: 10.1016/j.bmcl.2007.06.040
BindingDB Entry DOI: 10.7270/Q2C53MP9 |
More data for this
Ligand-Target Pair | |
Trans-2-enoyl-ACP reductase II
(Streptococcus pneumoniae) | BDBM50223361
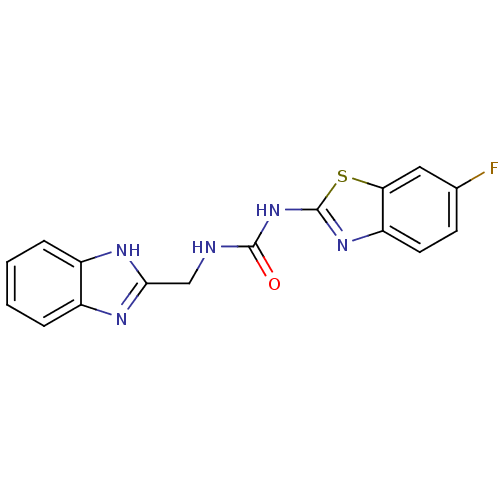
(1-((1H-benzo[d]imidazol-2-yl)methyl)-3-(6-fluorobe...)Show InChI InChI=1S/C16H12FN5OS/c17-9-5-6-12-13(7-9)24-16(21-12)22-15(23)18-8-14-19-10-3-1-2-4-11(10)20-14/h1-7H,8H2,(H,19,20)(H2,18,21,22,23) | PDB
MMDB
KEGG
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Meiji Seika Kaisha, Ltd
Curated by ChEMBL
| Assay Description
Inhibition of Streptococcus pneumoniae enoyl-ACP reductase FabK |
Bioorg Med Chem 15: 7325-36 (2007)
Article DOI: 10.1016/j.bmc.2007.08.050
BindingDB Entry DOI: 10.7270/Q25T3MBH |
More data for this
Ligand-Target Pair | |
Trans-2-enoyl-ACP reductase II
(Streptococcus pneumoniae) | BDBM50371157

(CHEMBL390218)Show SMILES Cc1c(Cc2c(Cl)cccc2Cl)c(=O)ccn1CCCc1ccc(cc1)-c1c[nH]c(CNC(=O)Nc2ncc(Br)s2)n1 Show InChI InChI=1S/C30H27BrCl2N6O2S/c1-18-21(14-22-23(32)5-2-6-24(22)33)26(40)11-13-39(18)12-3-4-19-7-9-20(10-8-19)25-15-34-28(37-25)17-35-29(41)38-30-36-16-27(31)42-30/h2,5-11,13,15-16H,3-4,12,14,17H2,1H3,(H,34,37)(H2,35,36,38,41) | PDB
MMDB
KEGG
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Meiji Seika Kaisha, Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of Streptococcus pneumoniae FabK |
J Med Chem 50: 4710-20 (2007)
Article DOI: 10.1021/jm0705354
BindingDB Entry DOI: 10.7270/Q2NZ88GS |
More data for this
Ligand-Target Pair | |
Trans-2-enoyl-ACP reductase II
(Streptococcus pneumoniae) | BDBM50216886
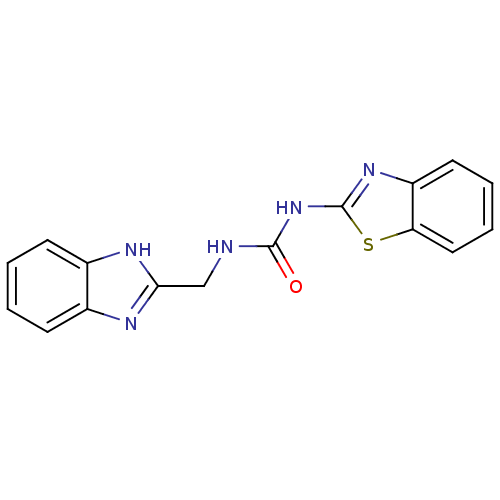
(1-((1H-benzo[d]imidazol-2-yl)methyl)-3-(benzo[d]th...)Show InChI InChI=1S/C16H13N5OS/c22-15(21-16-20-12-7-3-4-8-13(12)23-16)17-9-14-18-10-5-1-2-6-11(10)19-14/h1-8H,9H2,(H,18,19)(H2,17,20,21,22) | PDB
MMDB
KEGG
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Meiji Seika Kaisha, Ltd
Curated by ChEMBL
| Assay Description
Inhibition of Streptococcus pneumoniae enoyl-ACP reductase FabK |
Bioorg Med Chem 15: 7325-36 (2007)
Article DOI: 10.1016/j.bmc.2007.08.050
BindingDB Entry DOI: 10.7270/Q25T3MBH |
More data for this
Ligand-Target Pair | |
Trans-2-enoyl-ACP reductase II
(Streptococcus pneumoniae) | BDBM50216894

(1-((1H-benzo[d]imidazol-2-yl)methyl)-3-(6-methoxyb...)Show InChI InChI=1S/C17H15N5O2S/c1-24-10-6-7-13-14(8-10)25-17(21-13)22-16(23)18-9-15-19-11-4-2-3-5-12(11)20-15/h2-8H,9H2,1H3,(H,19,20)(H2,18,21,22,23) | PDB
MMDB
KEGG
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Meiji Seika Kaisha Ltd
Curated by ChEMBL
| Assay Description
Inhibition of Streptococcus pneumoniae KU197 FabK |
Bioorg Med Chem Lett 17: 4982-6 (2007)
Article DOI: 10.1016/j.bmcl.2007.06.040
BindingDB Entry DOI: 10.7270/Q2C53MP9 |
More data for this
Ligand-Target Pair | |
Trans-2-enoyl-ACP reductase II
(Streptococcus pneumoniae) | BDBM50371152
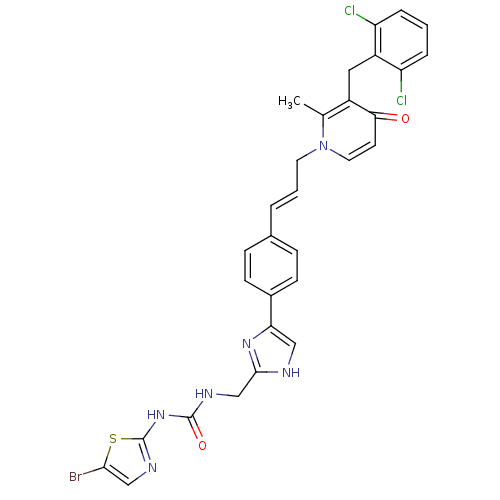
(CHEMBL242277)Show SMILES Cc1c(Cc2c(Cl)cccc2Cl)c(=O)ccn1C\C=C\c1ccc(cc1)-c1c[nH]c(CNC(=O)Nc2ncc(Br)s2)n1 Show InChI InChI=1S/C30H25BrCl2N6O2S/c1-18-21(14-22-23(32)5-2-6-24(22)33)26(40)11-13-39(18)12-3-4-19-7-9-20(10-8-19)25-15-34-28(37-25)17-35-29(41)38-30-36-16-27(31)42-30/h2-11,13,15-16H,12,14,17H2,1H3,(H,34,37)(H2,35,36,38,41)/b4-3+ | PDB
MMDB
KEGG
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Meiji Seika Kaisha, Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of Streptococcus pneumoniae FabK |
J Med Chem 50: 4710-20 (2007)
Article DOI: 10.1021/jm0705354
BindingDB Entry DOI: 10.7270/Q2NZ88GS |
More data for this
Ligand-Target Pair | |
Trans-2-enoyl-ACP reductase II
(Streptococcus pneumoniae) | BDBM50371153
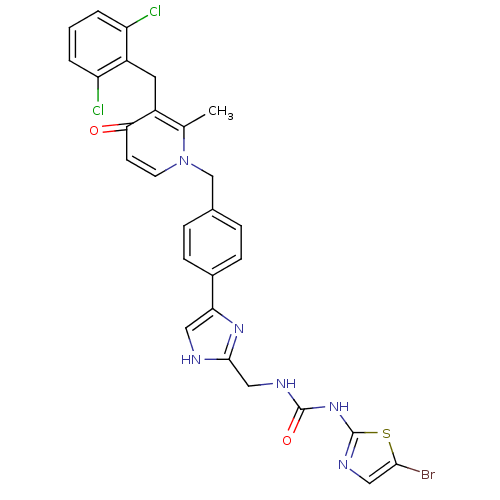
(CHEMBL242242)Show SMILES Cc1c(Cc2c(Cl)cccc2Cl)c(=O)ccn1Cc1ccc(cc1)-c1c[nH]c(CNC(=O)Nc2ncc(Br)s2)n1 Show InChI InChI=1S/C28H23BrCl2N6O2S/c1-16-19(11-20-21(30)3-2-4-22(20)31)24(38)9-10-37(16)15-17-5-7-18(8-6-17)23-12-32-26(35-23)14-33-27(39)36-28-34-13-25(29)40-28/h2-10,12-13H,11,14-15H2,1H3,(H,32,35)(H2,33,34,36,39) | PDB
MMDB
KEGG
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Meiji Seika Kaisha, Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of Streptococcus pneumoniae FabK |
J Med Chem 50: 4710-20 (2007)
Article DOI: 10.1021/jm0705354
BindingDB Entry DOI: 10.7270/Q2NZ88GS |
More data for this
Ligand-Target Pair | |
Trans-2-enoyl-ACP reductase II
(Streptococcus pneumoniae) | BDBM50216888
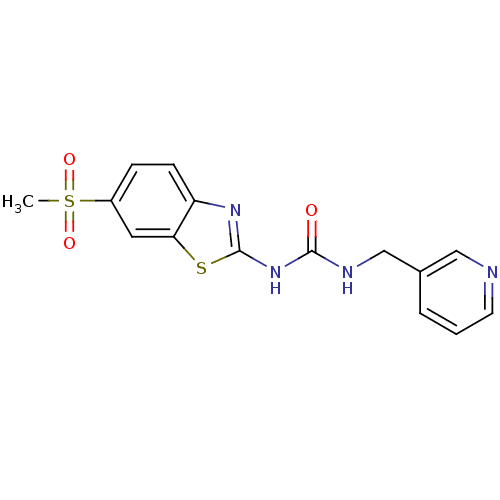
(1-(6-(methylsulfonyl)benzo[d]thiazol-2-yl)-3-(pyri...)Show InChI InChI=1S/C15H14N4O3S2/c1-24(21,22)11-4-5-12-13(7-11)23-15(18-12)19-14(20)17-9-10-3-2-6-16-8-10/h2-8H,9H2,1H3,(H2,17,18,19,20) | PDB
MMDB
KEGG
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Meiji Seika Kaisha, Ltd
Curated by ChEMBL
| Assay Description
Inhibition of Streptococcus pneumoniae enoyl-ACP reductase FabK |
Bioorg Med Chem 15: 7325-36 (2007)
Article DOI: 10.1016/j.bmc.2007.08.050
BindingDB Entry DOI: 10.7270/Q25T3MBH |
More data for this
Ligand-Target Pair | |
Trans-2-enoyl-ACP reductase II
(Streptococcus pneumoniae) | BDBM50216899
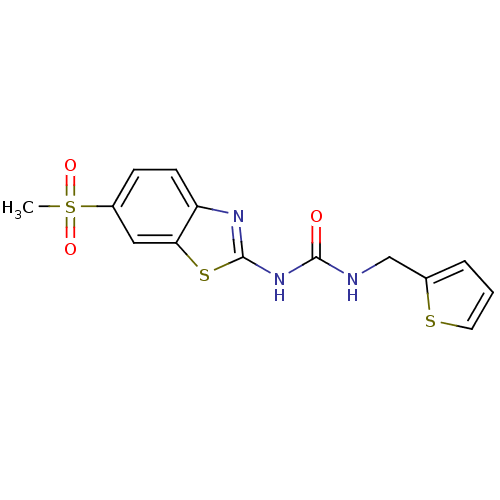
(1-(6-(methylsulfonyl)benzo[d]thiazol-2-yl)-3-(thio...)Show InChI InChI=1S/C14H13N3O3S3/c1-23(19,20)10-4-5-11-12(7-10)22-14(16-11)17-13(18)15-8-9-3-2-6-21-9/h2-7H,8H2,1H3,(H2,15,16,17,18) | PDB
MMDB
KEGG
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Meiji Seika Kaisha Ltd
Curated by ChEMBL
| Assay Description
Inhibition of Streptococcus pneumoniae KU197 FabK |
Bioorg Med Chem Lett 17: 4982-6 (2007)
Article DOI: 10.1016/j.bmcl.2007.06.040
BindingDB Entry DOI: 10.7270/Q2C53MP9 |
More data for this
Ligand-Target Pair | |
Trans-2-enoyl-ACP reductase II
(Streptococcus pneumoniae) | BDBM50216888

(1-(6-(methylsulfonyl)benzo[d]thiazol-2-yl)-3-(pyri...)Show InChI InChI=1S/C15H14N4O3S2/c1-24(21,22)11-4-5-12-13(7-11)23-15(18-12)19-14(20)17-9-10-3-2-6-16-8-10/h2-8H,9H2,1H3,(H2,17,18,19,20) | PDB
MMDB
KEGG
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Meiji Seika Kaisha Ltd
Curated by ChEMBL
| Assay Description
Inhibition of Streptococcus pneumoniae KU197 FabK |
Bioorg Med Chem Lett 17: 4982-6 (2007)
Article DOI: 10.1016/j.bmcl.2007.06.040
BindingDB Entry DOI: 10.7270/Q2C53MP9 |
More data for this
Ligand-Target Pair | |
Trans-2-enoyl-ACP reductase II
(Streptococcus pneumoniae) | BDBM50216886

(1-((1H-benzo[d]imidazol-2-yl)methyl)-3-(benzo[d]th...)Show InChI InChI=1S/C16H13N5OS/c22-15(21-16-20-12-7-3-4-8-13(12)23-16)17-9-14-18-10-5-1-2-6-11(10)19-14/h1-8H,9H2,(H,18,19)(H2,17,20,21,22) | PDB
MMDB
KEGG
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Meiji Seika Kaisha Ltd
Curated by ChEMBL
| Assay Description
Inhibition of Streptococcus pneumoniae KU197 FabK |
Bioorg Med Chem Lett 17: 4982-6 (2007)
Article DOI: 10.1016/j.bmcl.2007.06.040
BindingDB Entry DOI: 10.7270/Q2C53MP9 |
More data for this
Ligand-Target Pair | |
Trans-2-enoyl-ACP reductase II
(Streptococcus pneumoniae) | BDBM50371148
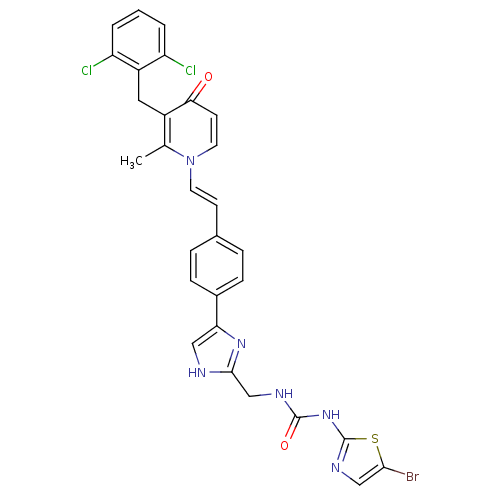
(CHEMBL428739)Show SMILES Cc1c(Cc2c(Cl)cccc2Cl)c(=O)ccn1\C=C\c1ccc(cc1)-c1c[nH]c(CNC(=O)Nc2ncc(Br)s2)n1 Show InChI InChI=1S/C29H23BrCl2N6O2S/c1-17-20(13-21-22(31)3-2-4-23(21)32)25(39)10-12-38(17)11-9-18-5-7-19(8-6-18)24-14-33-27(36-24)16-34-28(40)37-29-35-15-26(30)41-29/h2-12,14-15H,13,16H2,1H3,(H,33,36)(H2,34,35,37,40)/b11-9+ | PDB
MMDB
KEGG
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Meiji Seika Kaisha, Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of Streptococcus pneumoniae FabK |
J Med Chem 50: 4710-20 (2007)
Article DOI: 10.1021/jm0705354
BindingDB Entry DOI: 10.7270/Q2NZ88GS |
More data for this
Ligand-Target Pair | |
Trans-2-enoyl-ACP reductase II
(Streptococcus pneumoniae) | BDBM50216899

(1-(6-(methylsulfonyl)benzo[d]thiazol-2-yl)-3-(thio...)Show InChI InChI=1S/C14H13N3O3S3/c1-23(19,20)10-4-5-11-12(7-10)22-14(16-11)17-13(18)15-8-9-3-2-6-21-9/h2-7H,8H2,1H3,(H2,15,16,17,18) | PDB
MMDB
KEGG
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Meiji Seika Kaisha, Ltd
Curated by ChEMBL
| Assay Description
Inhibition of Streptococcus pneumoniae enoyl-ACP reductase FabK |
Bioorg Med Chem 15: 7325-36 (2007)
Article DOI: 10.1016/j.bmc.2007.08.050
BindingDB Entry DOI: 10.7270/Q25T3MBH |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data