

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM19149 (CHEMBL98 | N-hydroxy-N'-phenyloctanediamide | SAHA...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Medicinal Chemistry, Key Laboratory of Chemical Biology (Ministry of Education), School of Pharmaceutical Sciences, Shandong University, Jinan 250012, China. Curated by ChEMBL | Assay Description Inhibition of HDAC1 (unknown origin) using Ac-Leu-GlyLys(Ac)-AMC as substrate preincubated for 10 mins followed by substrate addition measured after ... | Bioorg Med Chem 25: 4123-4132 (2017) Article DOI: 10.1016/j.bmc.2017.05.062 BindingDB Entry DOI: 10.7270/Q27H1N23 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Gamma-aminobutyric acid receptor subunit alpha-1/beta-2/gamma-2 (Homo sapiens (Human)) | BDBM50537125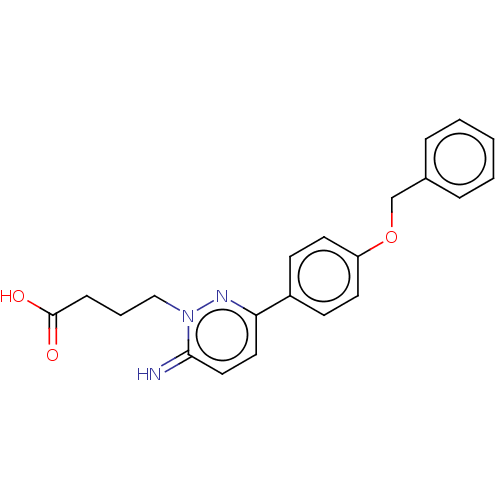 (CHEMBL1851667) | PDB UniProtKB/SwissProt antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Copenhagen Curated by ChEMBL | Assay Description Antagonist activity at human alpha1beta2gamma2S GABA(A) receptor expressed in human tsA201 cells assessed as inhibition of GABA-induced membrane pote... | J Med Chem 62: 2798-2813 (2019) Article DOI: 10.1021/acs.jmedchem.9b00131 BindingDB Entry DOI: 10.7270/Q2D2225V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 2 (Homo sapiens (Human)) | BDBM19149 (CHEMBL98 | N-hydroxy-N'-phenyloctanediamide | SAHA...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Medicinal Chemistry, Key Laboratory of Chemical Biology (Ministry of Education), School of Pharmaceutical Sciences, Shandong University, Jinan 250012, China. Curated by ChEMBL | Assay Description Inhibition of HDAC2 (unknown origin) using Ac-Leu-GlyLys(Ac)-AMC as substrate preincubated for 10 mins followed by substrate addition measured after ... | Bioorg Med Chem 25: 4123-4132 (2017) Article DOI: 10.1016/j.bmc.2017.05.062 BindingDB Entry DOI: 10.7270/Q27H1N23 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Gamma-aminobutyric acid receptor subunit alpha-1/beta-2/gamma-2 (Homo sapiens (Human)) | BDBM50378769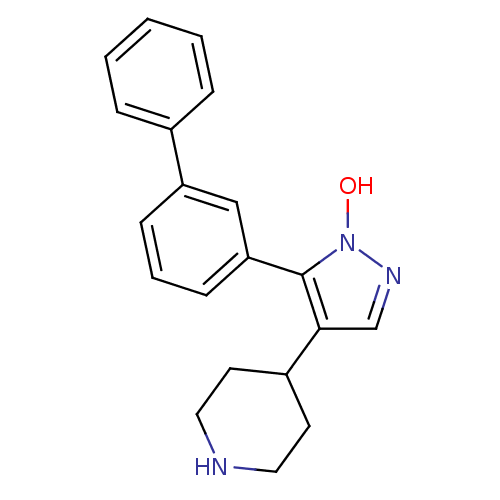 (CHEMBL1204074) | PDB UniProtKB/SwissProt antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Copenhagen Curated by ChEMBL | Assay Description Antagonist activity at human alpha1beta2gamma2S GABA(A) receptor expressed in human tsA201 cells assessed as inhibition of GABA-induced membrane pote... | J Med Chem 62: 2798-2813 (2019) Article DOI: 10.1021/acs.jmedchem.9b00131 BindingDB Entry DOI: 10.7270/Q2D2225V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM50268330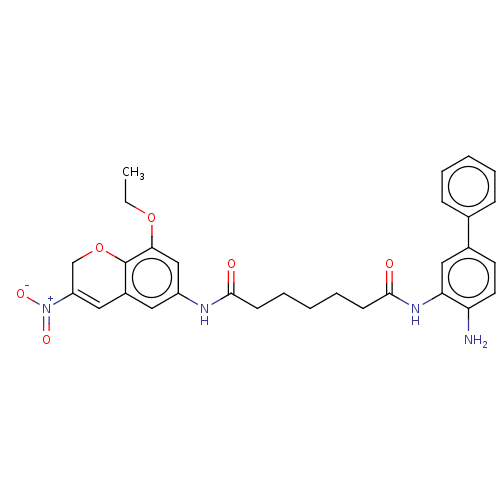 (CHEMBL4060953) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 128 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Medicinal Chemistry, Key Laboratory of Chemical Biology (Ministry of Education), School of Pharmaceutical Sciences, Shandong University, Jinan 250012, China. Curated by ChEMBL | Assay Description Inhibition of HDAC1 (unknown origin) using Ac-Leu-GlyLys(Ac)-AMC as substrate preincubated for 10 mins followed by substrate addition measured after ... | Bioorg Med Chem 25: 4123-4132 (2017) Article DOI: 10.1016/j.bmc.2017.05.062 BindingDB Entry DOI: 10.7270/Q27H1N23 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM50268329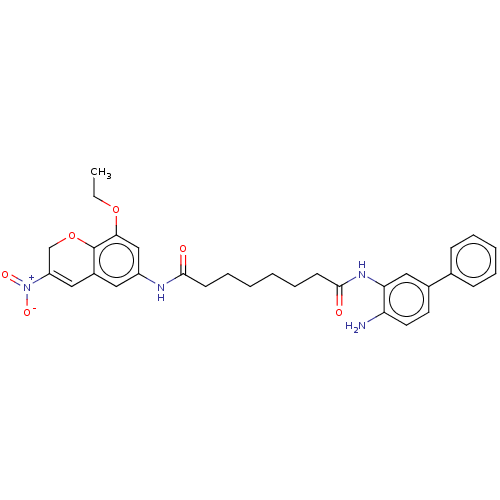 (CHEMBL4095143) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 179 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Medicinal Chemistry, Key Laboratory of Chemical Biology (Ministry of Education), School of Pharmaceutical Sciences, Shandong University, Jinan 250012, China. Curated by ChEMBL | Assay Description Inhibition of HDAC1 (unknown origin) using Ac-Leu-GlyLys(Ac)-AMC as substrate preincubated for 10 mins followed by substrate addition measured after ... | Bioorg Med Chem 25: 4123-4132 (2017) Article DOI: 10.1016/j.bmc.2017.05.062 BindingDB Entry DOI: 10.7270/Q27H1N23 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gamma-aminobutyric acid receptor subunit alpha-1/beta-2/gamma-2 (Homo sapiens (Human)) | BDBM50378763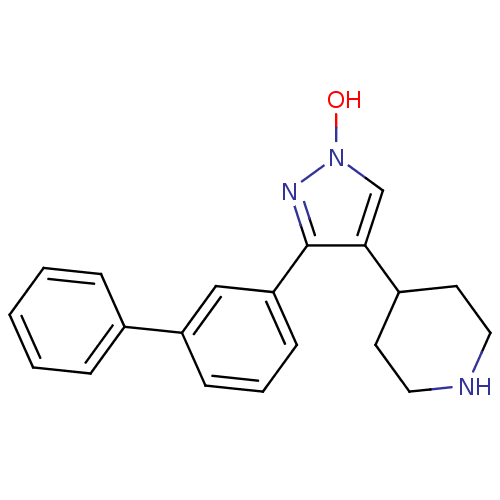 (CHEMBL1204077) | PDB UniProtKB/SwissProt antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 210 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Copenhagen Curated by ChEMBL | Assay Description Antagonist activity at human alpha1beta2gamma2S GABA(A) receptor expressed in human tsA201 cells assessed as inhibition of GABA-induced membrane pote... | J Med Chem 62: 2798-2813 (2019) Article DOI: 10.1021/acs.jmedchem.9b00131 BindingDB Entry DOI: 10.7270/Q2D2225V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM50268331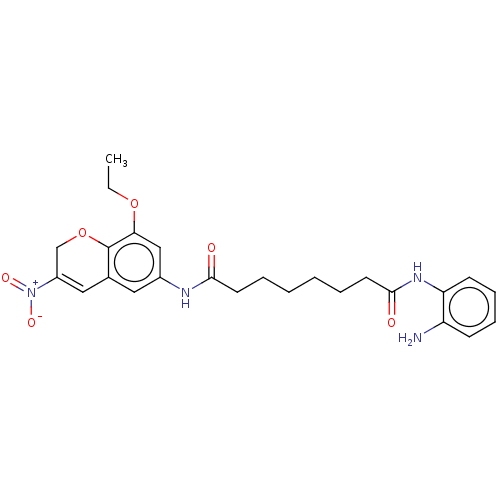 (CHEMBL4069623) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 232 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Medicinal Chemistry, Key Laboratory of Chemical Biology (Ministry of Education), School of Pharmaceutical Sciences, Shandong University, Jinan 250012, China. Curated by ChEMBL | Assay Description Inhibition of HDAC1 (unknown origin) using Ac-Leu-GlyLys(Ac)-AMC as substrate preincubated for 10 mins followed by substrate addition measured after ... | Bioorg Med Chem 25: 4123-4132 (2017) Article DOI: 10.1016/j.bmc.2017.05.062 BindingDB Entry DOI: 10.7270/Q27H1N23 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gamma-aminobutyric acid receptor subunit alpha-1/beta-2/gamma-2 (Homo sapiens (Human)) | BDBM50001572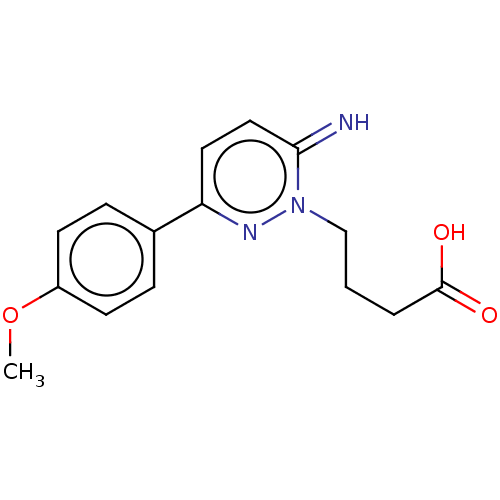 ((gabazine)4-[6-Imino-3-(4-methoxy-phenyl)-6H-pyrid...) | PDB UniProtKB/SwissProt antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 240 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Copenhagen Curated by ChEMBL | Assay Description Antagonist activity at human alpha1beta2gamma2S GABA(A) receptor expressed in human tsA201 cells assessed as inhibition of GABA-induced membrane pote... | J Med Chem 62: 2798-2813 (2019) Article DOI: 10.1021/acs.jmedchem.9b00131 BindingDB Entry DOI: 10.7270/Q2D2225V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM19410 (CHEMBL27759 | MS-275 | US11377423, MS-275 | US1167...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 271 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Medicinal Chemistry, Key Laboratory of Chemical Biology (Ministry of Education), School of Pharmaceutical Sciences, Shandong University, Jinan 250012, China. Curated by ChEMBL | Assay Description Inhibition of HDAC1 (unknown origin) using Ac-Leu-GlyLys(Ac)-AMC as substrate preincubated for 10 mins followed by substrate addition measured after ... | Bioorg Med Chem 25: 4123-4132 (2017) Article DOI: 10.1016/j.bmc.2017.05.062 BindingDB Entry DOI: 10.7270/Q27H1N23 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM50268332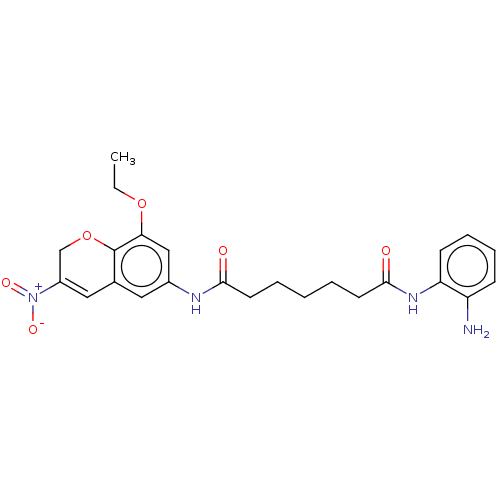 (CHEMBL4103874) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 288 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Medicinal Chemistry, Key Laboratory of Chemical Biology (Ministry of Education), School of Pharmaceutical Sciences, Shandong University, Jinan 250012, China. Curated by ChEMBL | Assay Description Inhibition of HDAC1 (unknown origin) using Ac-Leu-GlyLys(Ac)-AMC as substrate preincubated for 10 mins followed by substrate addition measured after ... | Bioorg Med Chem 25: 4123-4132 (2017) Article DOI: 10.1016/j.bmc.2017.05.062 BindingDB Entry DOI: 10.7270/Q27H1N23 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM50268328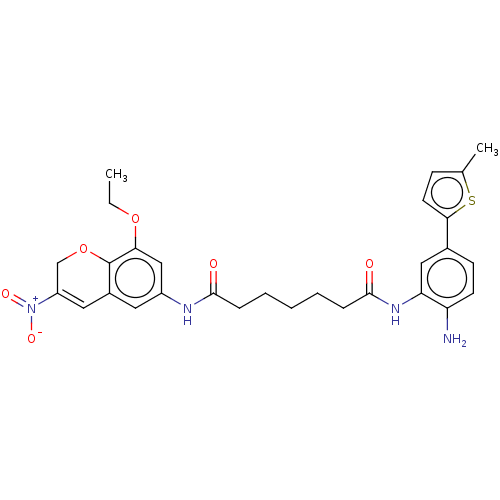 (CHEMBL4072341) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 366 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Medicinal Chemistry, Key Laboratory of Chemical Biology (Ministry of Education), School of Pharmaceutical Sciences, Shandong University, Jinan 250012, China. Curated by ChEMBL | Assay Description Inhibition of HDAC1 (unknown origin) using Ac-Leu-GlyLys(Ac)-AMC as substrate preincubated for 10 mins followed by substrate addition measured after ... | Bioorg Med Chem 25: 4123-4132 (2017) Article DOI: 10.1016/j.bmc.2017.05.062 BindingDB Entry DOI: 10.7270/Q27H1N23 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 2 (Homo sapiens (Human)) | BDBM50268331 (CHEMBL4069623) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 439 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Medicinal Chemistry, Key Laboratory of Chemical Biology (Ministry of Education), School of Pharmaceutical Sciences, Shandong University, Jinan 250012, China. Curated by ChEMBL | Assay Description Inhibition of HDAC2 (unknown origin) using Ac-Leu-GlyLys(Ac)-AMC as substrate preincubated for 10 mins followed by substrate addition measured after ... | Bioorg Med Chem 25: 4123-4132 (2017) Article DOI: 10.1016/j.bmc.2017.05.062 BindingDB Entry DOI: 10.7270/Q27H1N23 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM50268327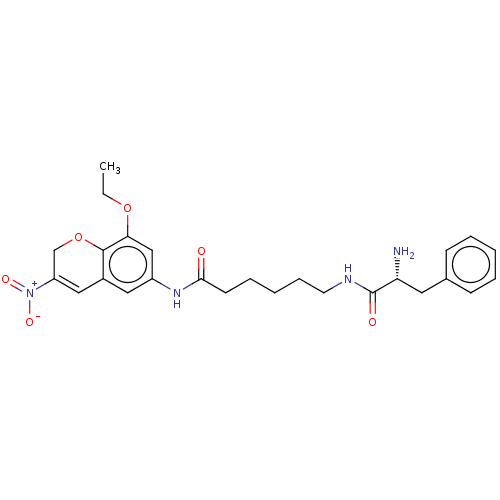 (CHEMBL4075124) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 448 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Medicinal Chemistry, Key Laboratory of Chemical Biology (Ministry of Education), School of Pharmaceutical Sciences, Shandong University, Jinan 250012, China. Curated by ChEMBL | Assay Description Inhibition of HDAC1 (unknown origin) using Ac-Leu-GlyLys(Ac)-AMC as substrate preincubated for 10 mins followed by substrate addition measured after ... | Bioorg Med Chem 25: 4123-4132 (2017) Article DOI: 10.1016/j.bmc.2017.05.062 BindingDB Entry DOI: 10.7270/Q27H1N23 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 2 (Homo sapiens (Human)) | BDBM19410 (CHEMBL27759 | MS-275 | US11377423, MS-275 | US1167...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 505 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Medicinal Chemistry, Key Laboratory of Chemical Biology (Ministry of Education), School of Pharmaceutical Sciences, Shandong University, Jinan 250012, China. Curated by ChEMBL | Assay Description Inhibition of HDAC2 (unknown origin) using Ac-Leu-GlyLys(Ac)-AMC as substrate preincubated for 10 mins followed by substrate addition measured after ... | Bioorg Med Chem 25: 4123-4132 (2017) Article DOI: 10.1016/j.bmc.2017.05.062 BindingDB Entry DOI: 10.7270/Q27H1N23 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 2 (Homo sapiens (Human)) | BDBM50268330 (CHEMBL4060953) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 659 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Medicinal Chemistry, Key Laboratory of Chemical Biology (Ministry of Education), School of Pharmaceutical Sciences, Shandong University, Jinan 250012, China. Curated by ChEMBL | Assay Description Inhibition of HDAC2 (unknown origin) using Ac-Leu-GlyLys(Ac)-AMC as substrate preincubated for 10 mins followed by substrate addition measured after ... | Bioorg Med Chem 25: 4123-4132 (2017) Article DOI: 10.1016/j.bmc.2017.05.062 BindingDB Entry DOI: 10.7270/Q27H1N23 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM50268319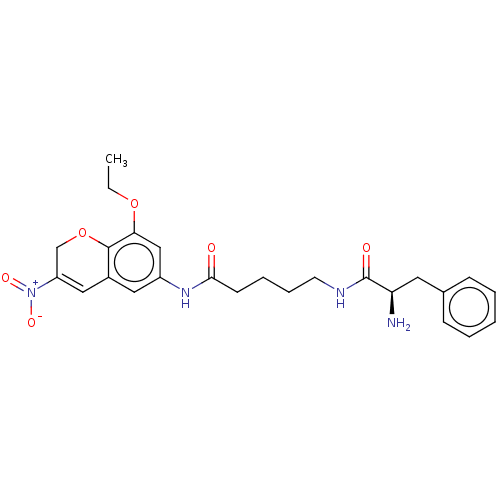 (CHEMBL4103290) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 742 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Medicinal Chemistry, Key Laboratory of Chemical Biology (Ministry of Education), School of Pharmaceutical Sciences, Shandong University, Jinan 250012, China. Curated by ChEMBL | Assay Description Inhibition of HDAC1 (unknown origin) using Ac-Leu-GlyLys(Ac)-AMC as substrate preincubated for 10 mins followed by substrate addition measured after ... | Bioorg Med Chem 25: 4123-4132 (2017) Article DOI: 10.1016/j.bmc.2017.05.062 BindingDB Entry DOI: 10.7270/Q27H1N23 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 2 (Homo sapiens (Human)) | BDBM50268332 (CHEMBL4103874) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 759 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Medicinal Chemistry, Key Laboratory of Chemical Biology (Ministry of Education), School of Pharmaceutical Sciences, Shandong University, Jinan 250012, China. Curated by ChEMBL | Assay Description Inhibition of HDAC2 (unknown origin) using Ac-Leu-GlyLys(Ac)-AMC as substrate preincubated for 10 mins followed by substrate addition measured after ... | Bioorg Med Chem 25: 4123-4132 (2017) Article DOI: 10.1016/j.bmc.2017.05.062 BindingDB Entry DOI: 10.7270/Q27H1N23 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 2 (Homo sapiens (Human)) | BDBM50268329 (CHEMBL4095143) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 827 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Medicinal Chemistry, Key Laboratory of Chemical Biology (Ministry of Education), School of Pharmaceutical Sciences, Shandong University, Jinan 250012, China. Curated by ChEMBL | Assay Description Inhibition of HDAC2 (unknown origin) using Ac-Leu-GlyLys(Ac)-AMC as substrate preincubated for 10 mins followed by substrate addition measured after ... | Bioorg Med Chem 25: 4123-4132 (2017) Article DOI: 10.1016/j.bmc.2017.05.062 BindingDB Entry DOI: 10.7270/Q27H1N23 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50333649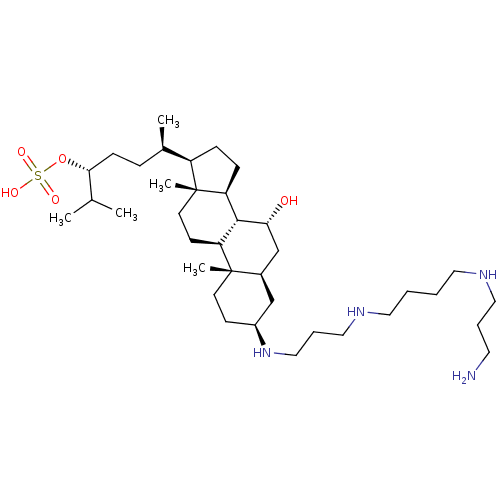 ((3R,6R)-6-((3S,5R,7R,8R,9S,10S,13R,14S,17R)-3-(3-(...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human recombinant PTP1B (1 to 322 residues) expressed in Escherichia coli using pNPP as substrate preincubated with substrate for 10 mi... | Citation and Details Article DOI: 10.1021/acs.jnatprod.1c00454 BindingDB Entry DOI: 10.7270/Q2BC43C6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 2 (Homo sapiens (Human)) | BDBM50268327 (CHEMBL4075124) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.29E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Medicinal Chemistry, Key Laboratory of Chemical Biology (Ministry of Education), School of Pharmaceutical Sciences, Shandong University, Jinan 250012, China. Curated by ChEMBL | Assay Description Inhibition of HDAC2 (unknown origin) using Ac-Leu-GlyLys(Ac)-AMC as substrate preincubated for 10 mins followed by substrate addition measured after ... | Bioorg Med Chem 25: 4123-4132 (2017) Article DOI: 10.1016/j.bmc.2017.05.062 BindingDB Entry DOI: 10.7270/Q27H1N23 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50535803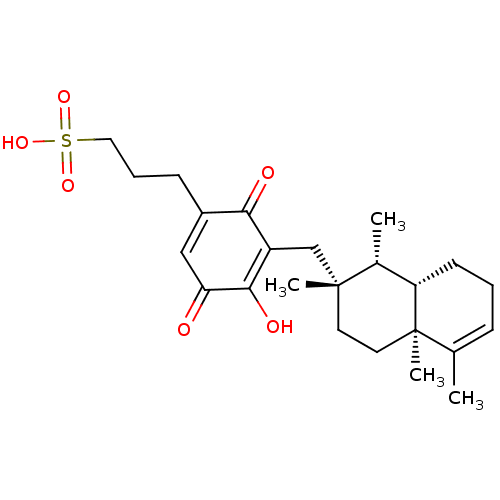 (CHEMBL4554678) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Copenhagen Curated by ChEMBL | Assay Description Inhibition of GST-tagged human PTP1B expressed in Escherichia coli using pNPP substrate assessed as reduction in p-nitrophenol release | J Nat Prod 79: 1063-72 (2016) Article DOI: 10.1021/acs.jnatprod.5b01128 BindingDB Entry DOI: 10.7270/Q2Z89GXC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50209683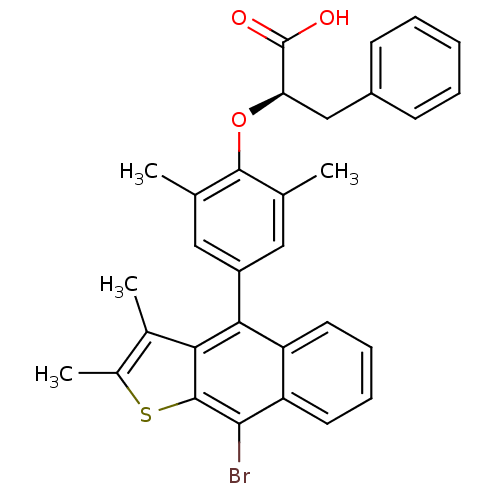 ((R)-2-(4-(9-bromo-2,3-dimethylnaphtho[2,3-b]thioph...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human recombinant PTP1B (1 to 322 residues) expressed in Escherichia coli using pNPP as substrate preincubated with substrate for 10 mi... | Citation and Details Article DOI: 10.1021/acs.jnatprod.1c00454 BindingDB Entry DOI: 10.7270/Q2BC43C6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 2 (Homo sapiens (Human)) | BDBM50268328 (CHEMBL4072341) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.79E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Medicinal Chemistry, Key Laboratory of Chemical Biology (Ministry of Education), School of Pharmaceutical Sciences, Shandong University, Jinan 250012, China. Curated by ChEMBL | Assay Description Inhibition of HDAC2 (unknown origin) using Ac-Leu-GlyLys(Ac)-AMC as substrate preincubated for 10 mins followed by substrate addition measured after ... | Bioorg Med Chem 25: 4123-4132 (2017) Article DOI: 10.1016/j.bmc.2017.05.062 BindingDB Entry DOI: 10.7270/Q27H1N23 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium- and chloride-dependent GABA transporter 3 (Homo sapiens (Human)) | BDBM50525189 (CHEMBL4458674) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Copenhagen Curated by ChEMBL | Assay Description Inhibition of human GAT3 expressed in Flp-In CHO cells assessed as reduction in [3H]GABA uptake incubated for 3 mins by liquid scintillation counting... | J Med Chem 62: 5797-5809 (2019) Article DOI: 10.1021/acs.jmedchem.9b00026 BindingDB Entry DOI: 10.7270/Q2D2222H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium- and chloride-dependent GABA transporter 3 (Homo sapiens (Human)) | BDBM50525189 (CHEMBL4458674) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Copenhagen Curated by ChEMBL | Assay Description Inhibition of human GAT3 expressed in Flp-In CHO cells assessed as reduction in [3H]GABA uptake incubated for 3 mins by liquid scintillation counting... | J Med Chem 62: 5797-5809 (2019) Article DOI: 10.1021/acs.jmedchem.9b00026 BindingDB Entry DOI: 10.7270/Q2D2222H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium- and chloride-dependent GABA transporter 3 (Homo sapiens (Human)) | BDBM50367949 (CHEMBL1788265) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Copenhagen Curated by ChEMBL | Assay Description Inhibition of human GAT3 expressed in Flp-In CHO cells assessed as reduction in [3H]GABA uptake incubated for 3 mins by liquid scintillation counting... | J Med Chem 62: 5797-5809 (2019) Article DOI: 10.1021/acs.jmedchem.9b00026 BindingDB Entry DOI: 10.7270/Q2D2222H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50575001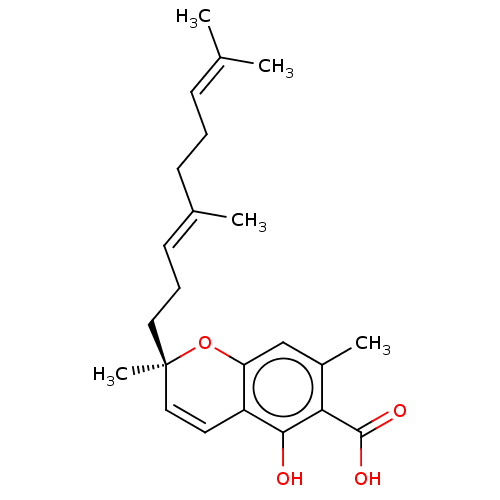 (CHEMBL2310311) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human recombinant PTP1B (1 to 322 residues) expressed in Escherichia coli using pNPP as substrate preincubated with substrate for 10 mi... | Citation and Details Article DOI: 10.1021/acs.jnatprod.1c00454 BindingDB Entry DOI: 10.7270/Q2BC43C6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium- and chloride-dependent GABA transporter 3 (Homo sapiens (Human)) | BDBM50367949 (CHEMBL1788265) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.51E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Copenhagen Curated by ChEMBL | Assay Description Inhibition of human GAT3 expressed in Flp-In CHO cells assessed as reduction in [3H]GABA uptake incubated for 3 mins by liquid scintillation counting... | J Med Chem 62: 5797-5809 (2019) Article DOI: 10.1021/acs.jmedchem.9b00026 BindingDB Entry DOI: 10.7270/Q2D2222H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium- and chloride-dependent GABA transporter 3 (Homo sapiens (Human)) | BDBM50013223 (CHEMBL349005 | Pyrrolidin-3-yl-acetic acid ((S)-(+...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.51E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Copenhagen Curated by ChEMBL | Assay Description Inhibition of human GAT3 expressed in Flp-In CHO cells assessed as reduction in [3H]GABA uptake incubated for 3 mins by liquid scintillation counting... | J Med Chem 62: 5797-5809 (2019) Article DOI: 10.1021/acs.jmedchem.9b00026 BindingDB Entry DOI: 10.7270/Q2D2222H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium- and chloride-dependent GABA transporter 3 (Homo sapiens (Human)) | BDBM50013223 (CHEMBL349005 | Pyrrolidin-3-yl-acetic acid ((S)-(+...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Copenhagen Curated by ChEMBL | Assay Description Inhibition of human GAT3 expressed in Flp-In CHO cells assessed as reduction in [3H]GABA uptake incubated for 3 mins by liquid scintillation counting... | J Med Chem 62: 5797-5809 (2019) Article DOI: 10.1021/acs.jmedchem.9b00026 BindingDB Entry DOI: 10.7270/Q2D2222H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium- and chloride-dependent GABA transporter 2 (Homo sapiens (Human)) | BDBM50525189 (CHEMBL4458674) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Copenhagen Curated by ChEMBL | Assay Description Inhibition of human GAT2 expressed in Flp-In CHO cells assessed as reduction in [3H]GABA uptake incubated for 3 mins by liquid scintillation counting... | J Med Chem 62: 5797-5809 (2019) Article DOI: 10.1021/acs.jmedchem.9b00026 BindingDB Entry DOI: 10.7270/Q2D2222H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 2 (Homo sapiens (Human)) | BDBM50268319 (CHEMBL4103290) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.92E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Medicinal Chemistry, Key Laboratory of Chemical Biology (Ministry of Education), School of Pharmaceutical Sciences, Shandong University, Jinan 250012, China. Curated by ChEMBL | Assay Description Inhibition of HDAC2 (unknown origin) using Ac-Leu-GlyLys(Ac)-AMC as substrate preincubated for 10 mins followed by substrate addition measured after ... | Bioorg Med Chem 25: 4123-4132 (2017) Article DOI: 10.1016/j.bmc.2017.05.062 BindingDB Entry DOI: 10.7270/Q27H1N23 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium- and chloride-dependent betaine transporter (Homo sapiens (Human)) | BDBM50525188 (CHEMBL4526098) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Copenhagen Curated by ChEMBL | Assay Description Inhibition of human BGT1 expressed in Flp-In CHO cells assessed as reduction in [3H]GABA uptake incubated for 3 mins by liquid scintillation counting... | J Med Chem 62: 5797-5809 (2019) Article DOI: 10.1021/acs.jmedchem.9b00026 BindingDB Entry DOI: 10.7270/Q2D2222H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium- and chloride-dependent GABA transporter 2 (Homo sapiens (Human)) | BDBM50525189 (CHEMBL4458674) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.16E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Copenhagen Curated by ChEMBL | Assay Description Inhibition of human GAT2 expressed in Flp-In CHO cells assessed as reduction in [3H]GABA uptake incubated for 3 mins by liquid scintillation counting... | J Med Chem 62: 5797-5809 (2019) Article DOI: 10.1021/acs.jmedchem.9b00026 BindingDB Entry DOI: 10.7270/Q2D2222H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium- and chloride-dependent betaine transporter (Homo sapiens (Human)) | BDBM50525188 (CHEMBL4526098) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.16E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Copenhagen Curated by ChEMBL | Assay Description Inhibition of human BGT1 expressed in Flp-In CHO cells assessed as reduction in [3H]GABA uptake incubated for 3 mins by liquid scintillation counting... | J Med Chem 62: 5797-5809 (2019) Article DOI: 10.1021/acs.jmedchem.9b00026 BindingDB Entry DOI: 10.7270/Q2D2222H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50535802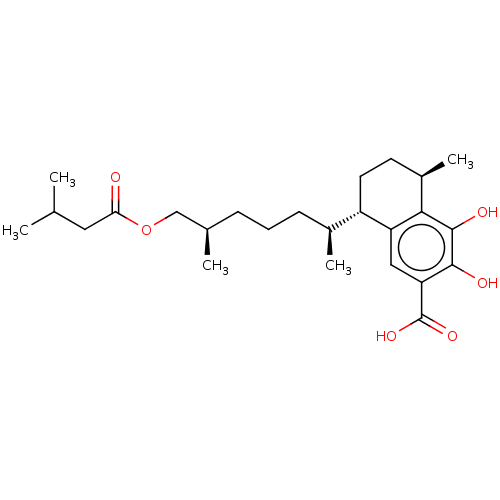 (CHEMBL4567748) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.44E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Copenhagen Curated by ChEMBL | Assay Description Inhibition of recombinant human PTP1B preincubated for 10 mins followed by protein addition using pNPP substrate | J Nat Prod 79: 1063-72 (2016) Article DOI: 10.1021/acs.jnatprod.5b01128 BindingDB Entry DOI: 10.7270/Q2Z89GXC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50374277 (CHEMBL403094) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Copenhagen Curated by ChEMBL | Assay Description Inhibition of GST-tagged human PTP1B expressed in Escherichia coli using pNPP substrate assessed as reduction in p-nitrophenol release | J Nat Prod 79: 1063-72 (2016) Article DOI: 10.1021/acs.jnatprod.5b01128 BindingDB Entry DOI: 10.7270/Q2Z89GXC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50374280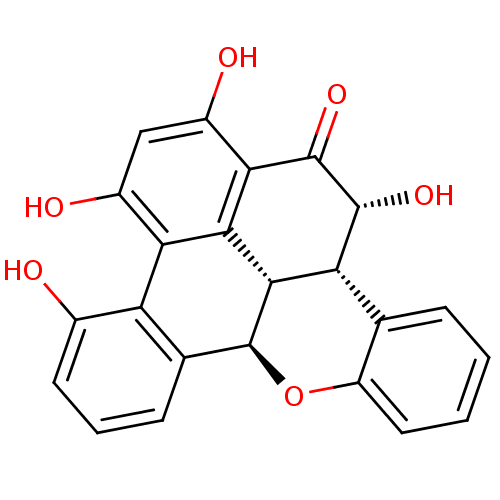 (CHEMBL403411) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Copenhagen Curated by ChEMBL | Assay Description Inhibition of GST-tagged human PTP1B expressed in Escherichia coli using pNPP substrate assessed as reduction in p-nitrophenol release | J Nat Prod 79: 1063-72 (2016) Article DOI: 10.1021/acs.jnatprod.5b01128 BindingDB Entry DOI: 10.7270/Q2Z89GXC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50535808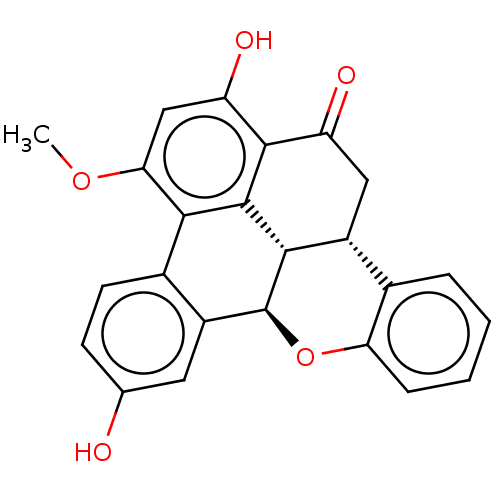 (CHEMBL4524583) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Copenhagen Curated by ChEMBL | Assay Description Inhibition of GST-tagged human PTP1B expressed in Escherichia coli using pNPP substrate assessed as reduction in p-nitrophenol release | J Nat Prod 79: 1063-72 (2016) Article DOI: 10.1021/acs.jnatprod.5b01128 BindingDB Entry DOI: 10.7270/Q2Z89GXC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50374279 (OHIOENSIN A) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Copenhagen Curated by ChEMBL | Assay Description Inhibition of GST-tagged human PTP1B expressed in Escherichia coli using pNPP substrate assessed as reduction in p-nitrophenol release | J Nat Prod 79: 1063-72 (2016) Article DOI: 10.1021/acs.jnatprod.5b01128 BindingDB Entry DOI: 10.7270/Q2Z89GXC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50537951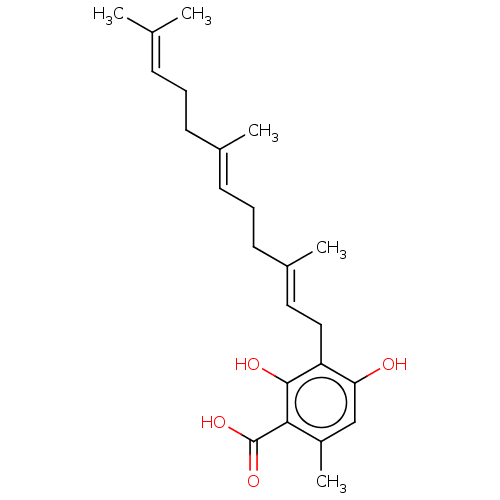 (Grifolic Acid) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human recombinant PTP1B (1 to 322 residues) expressed in Escherichia coli using pNPP as substrate preincubated with substrate for 10 mi... | Citation and Details Article DOI: 10.1021/acs.jnatprod.1c00454 BindingDB Entry DOI: 10.7270/Q2BC43C6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium- and chloride-dependent GABA transporter 1 (Homo sapiens (Human)) | BDBM50367949 (CHEMBL1788265) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Copenhagen Curated by ChEMBL | Assay Description Inhibition of human GAT1 expressed in Flp-In CHO cells assessed as reduction in [3H]GABA uptake incubated for 3 mins by liquid scintillation counting... | J Med Chem 62: 5797-5809 (2019) Article DOI: 10.1021/acs.jmedchem.9b00026 BindingDB Entry DOI: 10.7270/Q2D2222H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium- and chloride-dependent GABA transporter 1 (Homo sapiens (Human)) | BDBM50367949 (CHEMBL1788265) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Copenhagen Curated by ChEMBL | Assay Description Inhibition of human GAT1 expressed in Flp-In CHO cells assessed as reduction in [3H]GABA uptake incubated for 3 mins by liquid scintillation counting... | J Med Chem 62: 5797-5809 (2019) Article DOI: 10.1021/acs.jmedchem.9b00026 BindingDB Entry DOI: 10.7270/Q2D2222H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium- and chloride-dependent GABA transporter 1 (Homo sapiens (Human)) | BDBM50013223 (CHEMBL349005 | Pyrrolidin-3-yl-acetic acid ((S)-(+...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Copenhagen Curated by ChEMBL | Assay Description Inhibition of human GAT1 expressed in Flp-In CHO cells assessed as reduction in [3H]GABA uptake incubated for 3 mins by liquid scintillation counting... | J Med Chem 62: 5797-5809 (2019) Article DOI: 10.1021/acs.jmedchem.9b00026 BindingDB Entry DOI: 10.7270/Q2D2222H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gamma-aminobutyric acid receptor subunit alpha-1/beta-2/gamma-2 (Homo sapiens (Human)) | BDBM50537127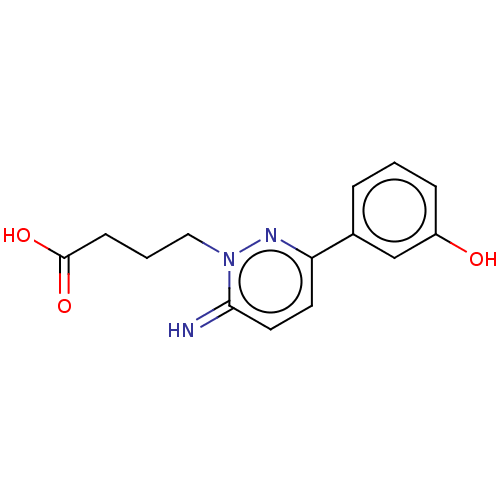 (CHEMBL4589563) | PDB UniProtKB/SwissProt antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Copenhagen Curated by ChEMBL | Assay Description Antagonist activity at human alpha1beta2gamma2S GABA(A) receptor expressed in human tsA201 cells assessed as inhibition of GABA-induced membrane pote... | J Med Chem 62: 2798-2813 (2019) Article DOI: 10.1021/acs.jmedchem.9b00131 BindingDB Entry DOI: 10.7270/Q2D2225V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium- and chloride-dependent GABA transporter 1 (Homo sapiens (Human)) | BDBM50013223 (CHEMBL349005 | Pyrrolidin-3-yl-acetic acid ((S)-(+...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Copenhagen Curated by ChEMBL | Assay Description Inhibition of human GAT1 expressed in Flp-In CHO cells assessed as reduction in [3H]GABA uptake incubated for 3 mins by liquid scintillation counting... | J Med Chem 62: 5797-5809 (2019) Article DOI: 10.1021/acs.jmedchem.9b00026 BindingDB Entry DOI: 10.7270/Q2D2222H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gamma-aminobutyric acid receptor subunit alpha-1/beta-2/gamma-2 (Homo sapiens (Human)) | BDBM50537127 (CHEMBL4589563) | PDB UniProtKB/SwissProt antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.98E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Copenhagen Curated by ChEMBL | Assay Description Antagonist activity at human alpha1beta2gamma2S GABA(A) receptor expressed in human tsA201 cells assessed as inhibition of GABA-induced membrane pote... | J Med Chem 62: 2798-2813 (2019) Article DOI: 10.1021/acs.jmedchem.9b00131 BindingDB Entry DOI: 10.7270/Q2D2225V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gamma-aminobutyric acid receptor subunit alpha-1/beta-2/gamma-2 (Homo sapiens (Human)) | BDBM50537128 (CHEMBL4586737) | PDB UniProtKB/SwissProt antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.37E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Copenhagen Curated by ChEMBL | Assay Description Antagonist activity at human alpha1beta2gamma2S GABA(A) receptor expressed in human tsA201 cells assessed as inhibition of GABA-induced membrane pote... | J Med Chem 62: 2798-2813 (2019) Article DOI: 10.1021/acs.jmedchem.9b00131 BindingDB Entry DOI: 10.7270/Q2D2225V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50093526 (CHEMBL426373 | RK-682) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Copenhagen Curated by ChEMBL | Assay Description Inhibition of recombinant human PTP1B preincubated for 10 mins followed by protein addition using pNPP substrate | J Nat Prod 79: 1063-72 (2016) Article DOI: 10.1021/acs.jnatprod.5b01128 BindingDB Entry DOI: 10.7270/Q2Z89GXC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 225 total ) | Next | Last >> |