

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50004000 ((3aS,8aR)-1,3a,8-trimethyl-1,2,3,3a,8,8a-hexahydro...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | DrugBank Article PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
University of the Punjab Curated by ChEMBL | Assay Description Inhibition of AChE (unknown origin) using acetylthiocholine iodide as substrate preincubated for 30 mins before substrate addition measured after 30 ... | Bioorg Med Chem 23: 6014-24 (2015) Article DOI: 10.1016/j.bmc.2015.06.051 BindingDB Entry DOI: 10.7270/Q2TT4SQS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50004000 ((3aS,8aR)-1,3a,8-trimethyl-1,2,3,3a,8,8a-hexahydro...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 850 | n/a | n/a | n/a | n/a | n/a | n/a |
University of the Punjab Curated by ChEMBL | Assay Description Inhibition of BChE (unknown origin) using butyrylthiocholine chloride as substrate preincubated for 30 mins before substrate addition measured after ... | Bioorg Med Chem 23: 6014-24 (2015) Article DOI: 10.1016/j.bmc.2015.06.051 BindingDB Entry DOI: 10.7270/Q2TT4SQS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50110324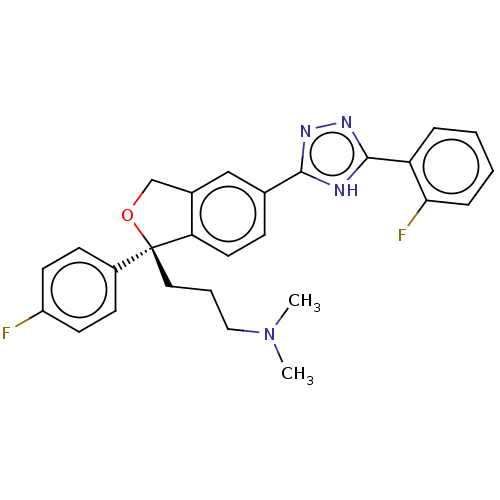 (CHEMBL3605424) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.52E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of the Punjab Curated by ChEMBL | Assay Description Inhibition of BChE (unknown origin) using butyrylthiocholine chloride as substrate preincubated for 30 mins before substrate addition measured after ... | Bioorg Med Chem 23: 6014-24 (2015) Article DOI: 10.1016/j.bmc.2015.06.051 BindingDB Entry DOI: 10.7270/Q2TT4SQS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50110322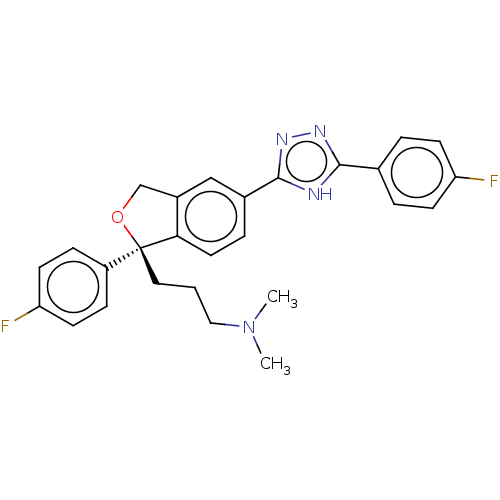 (CHEMBL3605426) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.31E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of the Punjab Curated by ChEMBL | Assay Description Inhibition of BChE (unknown origin) using butyrylthiocholine chloride as substrate preincubated for 30 mins before substrate addition measured after ... | Bioorg Med Chem 23: 6014-24 (2015) Article DOI: 10.1016/j.bmc.2015.06.051 BindingDB Entry DOI: 10.7270/Q2TT4SQS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50110325 (CHEMBL3605423) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6.71E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of the Punjab Curated by ChEMBL | Assay Description Inhibition of AChE (unknown origin) using acetylthiocholine iodide as substrate preincubated for 30 mins before substrate addition measured after 30 ... | Bioorg Med Chem 23: 6014-24 (2015) Article DOI: 10.1016/j.bmc.2015.06.051 BindingDB Entry DOI: 10.7270/Q2TT4SQS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50110253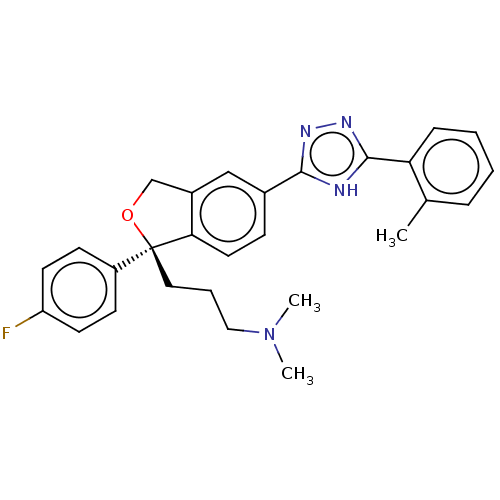 (CHEMBL3605418) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.52E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of the Punjab Curated by ChEMBL | Assay Description Inhibition of AChE (unknown origin) using acetylthiocholine iodide as substrate preincubated for 30 mins before substrate addition measured after 30 ... | Bioorg Med Chem 23: 6014-24 (2015) Article DOI: 10.1016/j.bmc.2015.06.051 BindingDB Entry DOI: 10.7270/Q2TT4SQS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oligo-1,6-glucosidase IMA1 (Saccharomyces cerevisiae S288c (Baker's yeast)) | BDBM50445973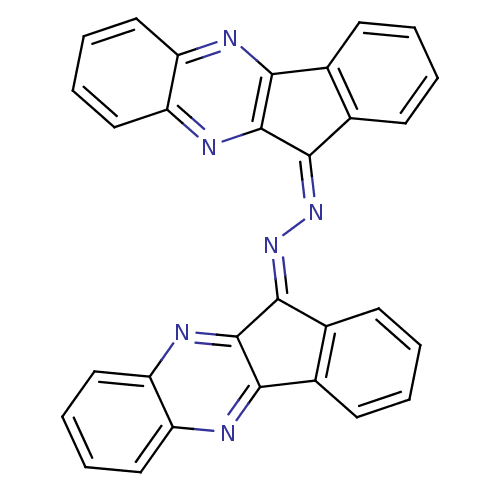 (CHEMBL3103095) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.82E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of the Punjab Curated by ChEMBL | Assay Description Inhibition of Saccharomyces cerevisiae alpha-glucosidase using p-nitrophenyl-alpha-D-glucopyranoside as substrate preincubated for 10 mins measured a... | Bioorg Med Chem 22: 1195-200 (2014) Article DOI: 10.1016/j.bmc.2013.12.024 BindingDB Entry DOI: 10.7270/Q2F1916H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oligo-1,6-glucosidase IMA1 (Saccharomyces cerevisiae S288c (Baker's yeast)) | BDBM50445984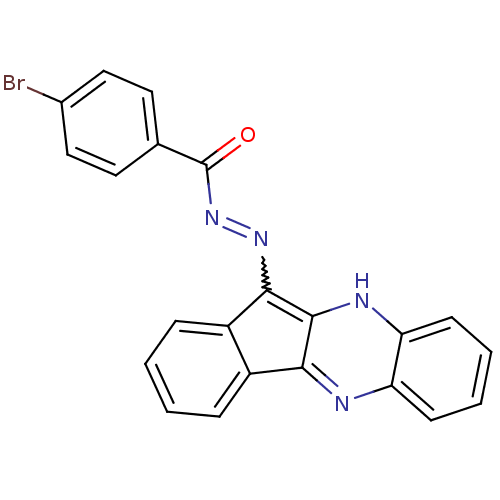 (CHEMBL3103158) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.27E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of the Punjab Curated by ChEMBL | Assay Description Inhibition of Saccharomyces cerevisiae alpha-glucosidase using p-nitrophenyl-alpha-D-glucopyranoside as substrate preincubated for 10 mins measured a... | Bioorg Med Chem 22: 1195-200 (2014) Article DOI: 10.1016/j.bmc.2013.12.024 BindingDB Entry DOI: 10.7270/Q2F1916H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oligo-1,6-glucosidase IMA1 (Saccharomyces cerevisiae S288c (Baker's yeast)) | BDBM50445986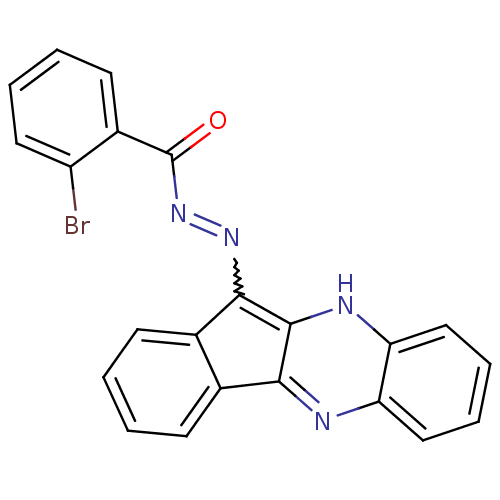 (CHEMBL3103156) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.31E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of the Punjab Curated by ChEMBL | Assay Description Inhibition of Saccharomyces cerevisiae alpha-glucosidase using p-nitrophenyl-alpha-D-glucopyranoside as substrate preincubated for 10 mins measured a... | Bioorg Med Chem 22: 1195-200 (2014) Article DOI: 10.1016/j.bmc.2013.12.024 BindingDB Entry DOI: 10.7270/Q2F1916H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oligo-1,6-glucosidase IMA1 (Saccharomyces cerevisiae S288c (Baker's yeast)) | BDBM50445976 (CHEMBL3103091) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.35E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of the Punjab Curated by ChEMBL | Assay Description Inhibition of Saccharomyces cerevisiae alpha-glucosidase using p-nitrophenyl-alpha-D-glucopyranoside as substrate preincubated for 10 mins measured a... | Bioorg Med Chem 22: 1195-200 (2014) Article DOI: 10.1016/j.bmc.2013.12.024 BindingDB Entry DOI: 10.7270/Q2F1916H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50110315 (CHEMBL3605436) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.41E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of the Punjab Curated by ChEMBL | Assay Description Inhibition of BChE (unknown origin) using butyrylthiocholine chloride as substrate preincubated for 30 mins before substrate addition measured after ... | Bioorg Med Chem 23: 6014-24 (2015) Article DOI: 10.1016/j.bmc.2015.06.051 BindingDB Entry DOI: 10.7270/Q2TT4SQS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oligo-1,6-glucosidase IMA1 (Saccharomyces cerevisiae S288c (Baker's yeast)) | BDBM50445983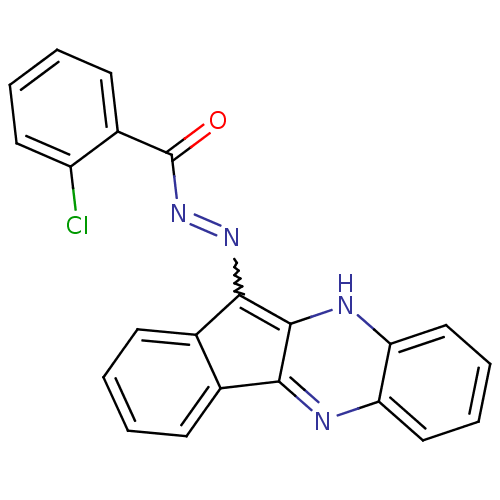 (CHEMBL3103159) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.59E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of the Punjab Curated by ChEMBL | Assay Description Inhibition of Saccharomyces cerevisiae alpha-glucosidase using p-nitrophenyl-alpha-D-glucopyranoside as substrate preincubated for 10 mins measured a... | Bioorg Med Chem 22: 1195-200 (2014) Article DOI: 10.1016/j.bmc.2013.12.024 BindingDB Entry DOI: 10.7270/Q2F1916H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50110320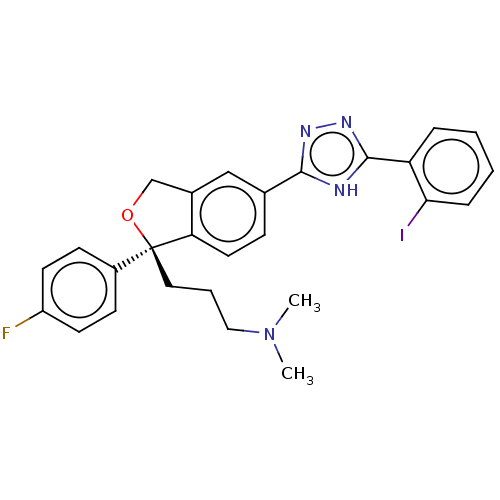 (CHEMBL3605430) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.59E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of the Punjab Curated by ChEMBL | Assay Description Inhibition of BChE (unknown origin) using butyrylthiocholine chloride as substrate preincubated for 30 mins before substrate addition measured after ... | Bioorg Med Chem 23: 6014-24 (2015) Article DOI: 10.1016/j.bmc.2015.06.051 BindingDB Entry DOI: 10.7270/Q2TT4SQS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50110326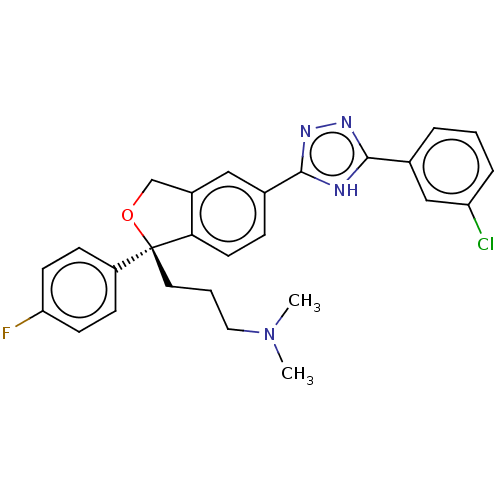 (CHEMBL3605422) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.65E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of the Punjab Curated by ChEMBL | Assay Description Inhibition of BChE (unknown origin) using butyrylthiocholine chloride as substrate preincubated for 30 mins before substrate addition measured after ... | Bioorg Med Chem 23: 6014-24 (2015) Article DOI: 10.1016/j.bmc.2015.06.051 BindingDB Entry DOI: 10.7270/Q2TT4SQS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oligo-1,6-glucosidase IMA1 (Saccharomyces cerevisiae S288c (Baker's yeast)) | BDBM50445975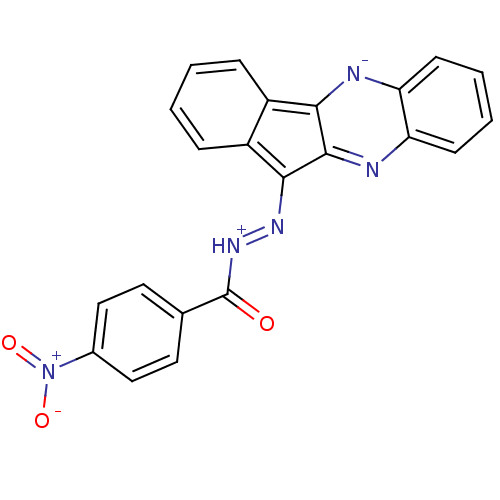 (CHEMBL3103092) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.95E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of the Punjab Curated by ChEMBL | Assay Description Inhibition of Saccharomyces cerevisiae alpha-glucosidase using p-nitrophenyl-alpha-D-glucopyranoside as substrate preincubated for 10 mins measured a... | Bioorg Med Chem 22: 1195-200 (2014) Article DOI: 10.1016/j.bmc.2013.12.024 BindingDB Entry DOI: 10.7270/Q2F1916H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oligo-1,6-glucosidase IMA1 (Saccharomyces cerevisiae S288c (Baker's yeast)) | BDBM50445979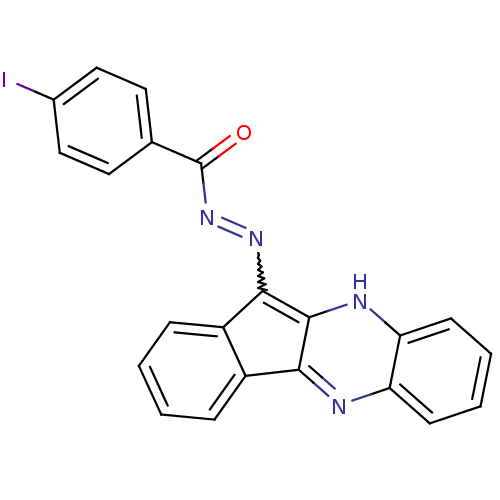 (CHEMBL3103088) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.77E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of the Punjab Curated by ChEMBL | Assay Description Inhibition of Saccharomyces cerevisiae alpha-glucosidase using p-nitrophenyl-alpha-D-glucopyranoside as substrate preincubated for 10 mins measured a... | Bioorg Med Chem 22: 1195-200 (2014) Article DOI: 10.1016/j.bmc.2013.12.024 BindingDB Entry DOI: 10.7270/Q2F1916H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oligo-1,6-glucosidase IMA1 (Saccharomyces cerevisiae S288c (Baker's yeast)) | BDBM50333465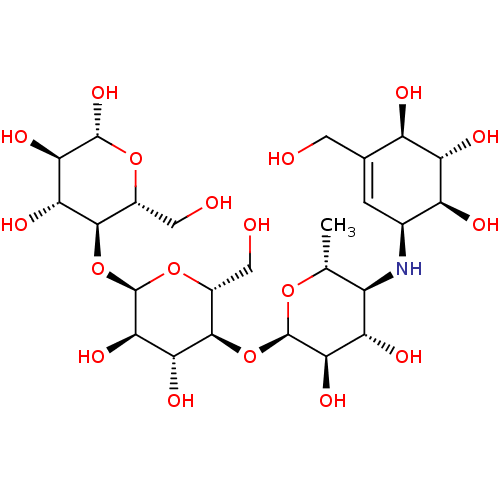 ((2R,3R,4R,5R,6R)-5-((2R,3R,4R,5S,6R)-5-((2R,3R,4S,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 3.83E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of the Punjab Curated by ChEMBL | Assay Description Inhibition of Saccharomyces cerevisiae alpha-glucosidase using p-nitrophenyl-alpha-D-glucopyranoside as substrate preincubated for 10 mins measured a... | Bioorg Med Chem 22: 1195-200 (2014) Article DOI: 10.1016/j.bmc.2013.12.024 BindingDB Entry DOI: 10.7270/Q2F1916H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50110323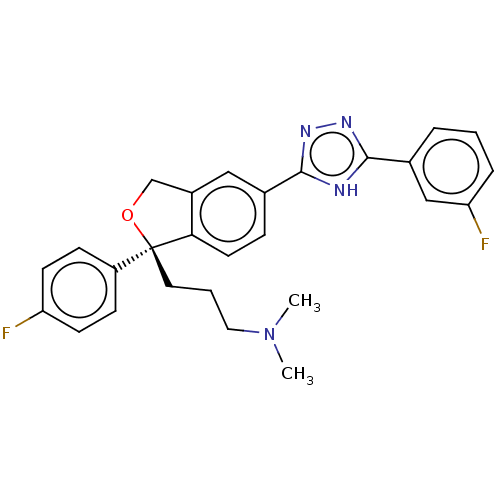 (CHEMBL3605425) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.48E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of the Punjab Curated by ChEMBL | Assay Description Inhibition of BChE (unknown origin) using butyrylthiocholine chloride as substrate preincubated for 30 mins before substrate addition measured after ... | Bioorg Med Chem 23: 6014-24 (2015) Article DOI: 10.1016/j.bmc.2015.06.051 BindingDB Entry DOI: 10.7270/Q2TT4SQS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oligo-1,6-glucosidase IMA1 (Saccharomyces cerevisiae S288c (Baker's yeast)) | BDBM50445971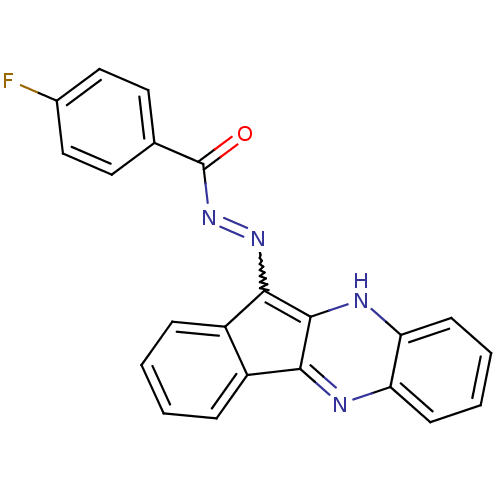 (CHEMBL3103155) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.34E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of the Punjab Curated by ChEMBL | Assay Description Inhibition of Saccharomyces cerevisiae alpha-glucosidase using p-nitrophenyl-alpha-D-glucopyranoside as substrate preincubated for 10 mins measured a... | Bioorg Med Chem 22: 1195-200 (2014) Article DOI: 10.1016/j.bmc.2013.12.024 BindingDB Entry DOI: 10.7270/Q2F1916H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oligo-1,6-glucosidase IMA1 (Saccharomyces cerevisiae S288c (Baker's yeast)) | BDBM50445977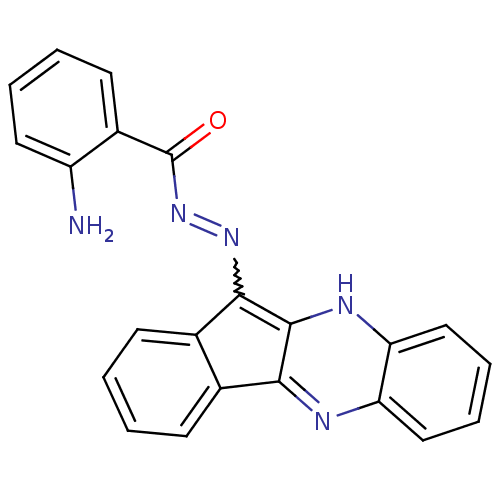 (CHEMBL3103090) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.23E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of the Punjab Curated by ChEMBL | Assay Description Inhibition of Saccharomyces cerevisiae alpha-glucosidase using p-nitrophenyl-alpha-D-glucopyranoside as substrate preincubated for 10 mins measured a... | Bioorg Med Chem 22: 1195-200 (2014) Article DOI: 10.1016/j.bmc.2013.12.024 BindingDB Entry DOI: 10.7270/Q2F1916H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oligo-1,6-glucosidase IMA1 (Saccharomyces cerevisiae S288c (Baker's yeast)) | BDBM75463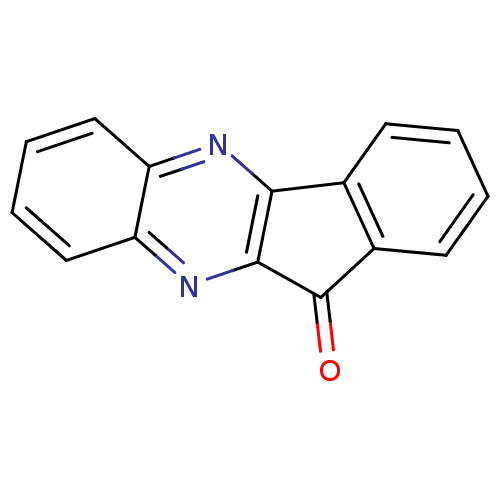 (11-indeno[1,2-b]quinoxalinone | MLS000067615 | SMR...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6.25E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of the Punjab Curated by ChEMBL | Assay Description Inhibition of Saccharomyces cerevisiae alpha-glucosidase using p-nitrophenyl-alpha-D-glucopyranoside as substrate preincubated for 10 mins measured a... | Bioorg Med Chem 22: 1195-200 (2014) Article DOI: 10.1016/j.bmc.2013.12.024 BindingDB Entry DOI: 10.7270/Q2F1916H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50110314 (CHEMBL3605437) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.59E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of the Punjab Curated by ChEMBL | Assay Description Inhibition of BChE (unknown origin) using butyrylthiocholine chloride as substrate preincubated for 30 mins before substrate addition measured after ... | Bioorg Med Chem 23: 6014-24 (2015) Article DOI: 10.1016/j.bmc.2015.06.051 BindingDB Entry DOI: 10.7270/Q2TT4SQS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oligo-1,6-glucosidase IMA1 (Saccharomyces cerevisiae S288c (Baker's yeast)) | BDBM50445980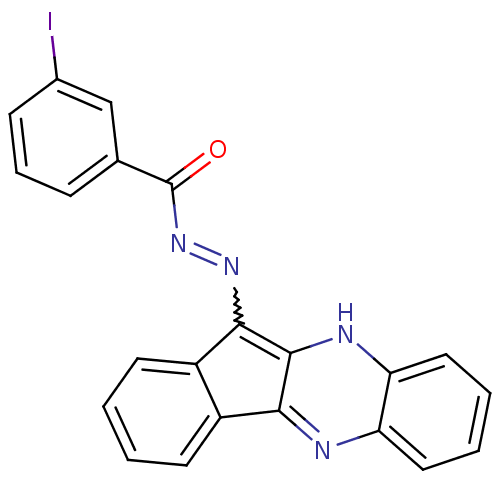 (CHEMBL3103162) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.75E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of the Punjab Curated by ChEMBL | Assay Description Inhibition of Saccharomyces cerevisiae alpha-glucosidase using p-nitrophenyl-alpha-D-glucopyranoside as substrate preincubated for 10 mins measured a... | Bioorg Med Chem 22: 1195-200 (2014) Article DOI: 10.1016/j.bmc.2013.12.024 BindingDB Entry DOI: 10.7270/Q2F1916H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50110321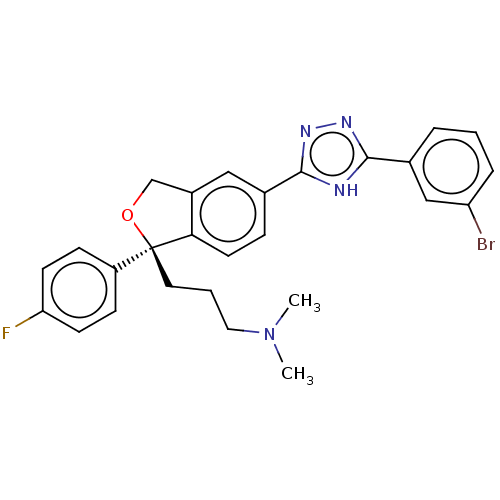 (CHEMBL3605428) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of the Punjab Curated by ChEMBL | Assay Description Inhibition of BChE (unknown origin) using butyrylthiocholine chloride as substrate preincubated for 30 mins before substrate addition measured after ... | Bioorg Med Chem 23: 6014-24 (2015) Article DOI: 10.1016/j.bmc.2015.06.051 BindingDB Entry DOI: 10.7270/Q2TT4SQS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50110280 (CHEMBL3605421) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 8.14E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of the Punjab Curated by ChEMBL | Assay Description Inhibition of BChE (unknown origin) using butyrylthiocholine chloride as substrate preincubated for 30 mins before substrate addition measured after ... | Bioorg Med Chem 23: 6014-24 (2015) Article DOI: 10.1016/j.bmc.2015.06.051 BindingDB Entry DOI: 10.7270/Q2TT4SQS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50110252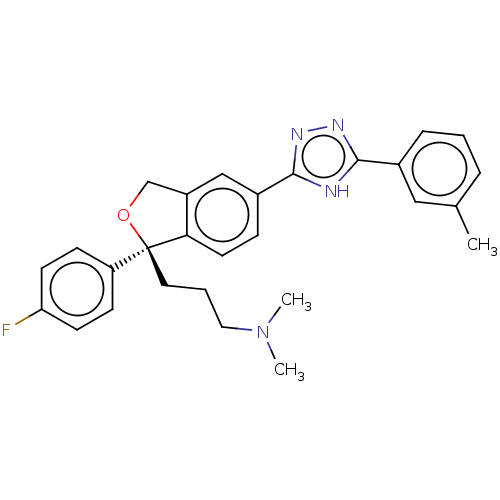 (CHEMBL3605419) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.92E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of the Punjab Curated by ChEMBL | Assay Description Inhibition of BChE (unknown origin) using butyrylthiocholine chloride as substrate preincubated for 30 mins before substrate addition measured after ... | Bioorg Med Chem 23: 6014-24 (2015) Article DOI: 10.1016/j.bmc.2015.06.051 BindingDB Entry DOI: 10.7270/Q2TT4SQS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oligo-1,6-glucosidase IMA1 (Saccharomyces cerevisiae S288c (Baker's yeast)) | BDBM50445972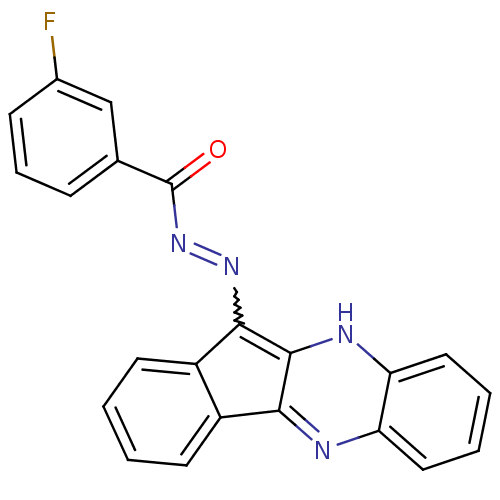 (CHEMBL3103154) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.09E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of the Punjab Curated by ChEMBL | Assay Description Inhibition of Saccharomyces cerevisiae alpha-glucosidase using p-nitrophenyl-alpha-D-glucopyranoside as substrate preincubated for 10 mins measured a... | Bioorg Med Chem 22: 1195-200 (2014) Article DOI: 10.1016/j.bmc.2013.12.024 BindingDB Entry DOI: 10.7270/Q2F1916H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50110253 (CHEMBL3605418) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.97E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of the Punjab Curated by ChEMBL | Assay Description Inhibition of BChE (unknown origin) using butyrylthiocholine chloride as substrate preincubated for 30 mins before substrate addition measured after ... | Bioorg Med Chem 23: 6014-24 (2015) Article DOI: 10.1016/j.bmc.2015.06.051 BindingDB Entry DOI: 10.7270/Q2TT4SQS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oligo-1,6-glucosidase IMA1 (Saccharomyces cerevisiae S288c (Baker's yeast)) | BDBM50445981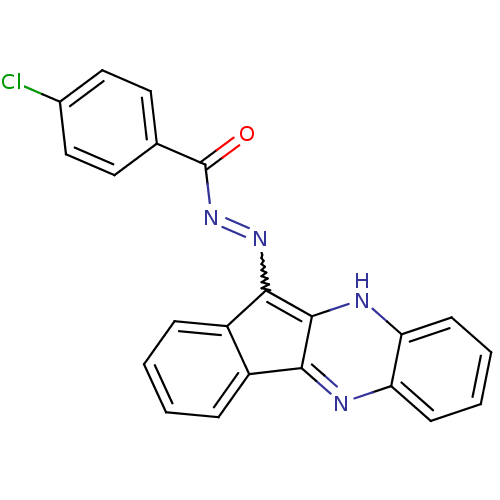 (CHEMBL3103161) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.99E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of the Punjab Curated by ChEMBL | Assay Description Inhibition of Saccharomyces cerevisiae alpha-glucosidase using p-nitrophenyl-alpha-D-glucopyranoside as substrate preincubated for 10 mins measured a... | Bioorg Med Chem 22: 1195-200 (2014) Article DOI: 10.1016/j.bmc.2013.12.024 BindingDB Entry DOI: 10.7270/Q2F1916H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50110269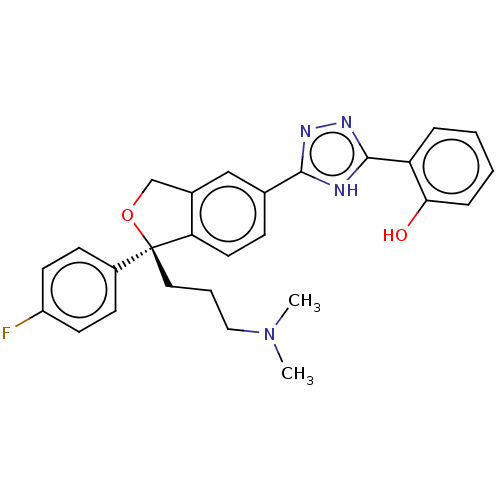 (CHEMBL3605312) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.02E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of the Punjab Curated by ChEMBL | Assay Description Inhibition of BChE (unknown origin) using butyrylthiocholine chloride as substrate preincubated for 30 mins before substrate addition measured after ... | Bioorg Med Chem 23: 6014-24 (2015) Article DOI: 10.1016/j.bmc.2015.06.051 BindingDB Entry DOI: 10.7270/Q2TT4SQS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oligo-1,6-glucosidase IMA1 (Saccharomyces cerevisiae S288c (Baker's yeast)) | BDBM50445974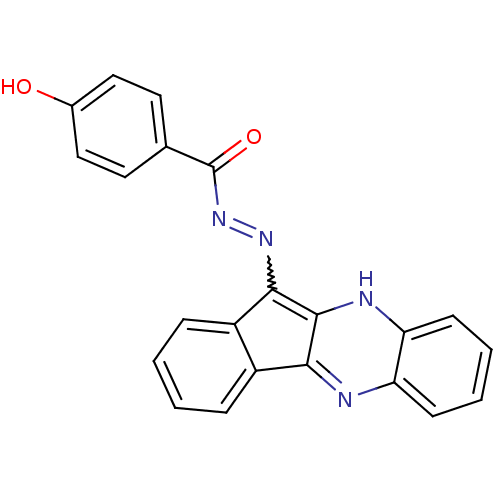 (CHEMBL3103094) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.02E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of the Punjab Curated by ChEMBL | Assay Description Inhibition of Saccharomyces cerevisiae alpha-glucosidase using p-nitrophenyl-alpha-D-glucopyranoside as substrate preincubated for 10 mins measured a... | Bioorg Med Chem 22: 1195-200 (2014) Article DOI: 10.1016/j.bmc.2013.12.024 BindingDB Entry DOI: 10.7270/Q2F1916H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50110316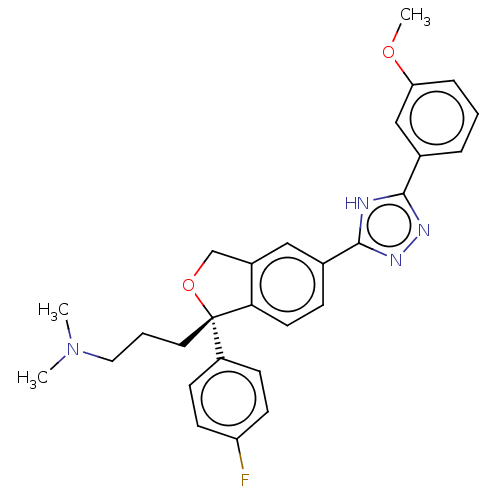 (CHEMBL3605434) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.03E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of the Punjab Curated by ChEMBL | Assay Description Inhibition of BChE (unknown origin) using butyrylthiocholine chloride as substrate preincubated for 30 mins before substrate addition measured after ... | Bioorg Med Chem 23: 6014-24 (2015) Article DOI: 10.1016/j.bmc.2015.06.051 BindingDB Entry DOI: 10.7270/Q2TT4SQS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50110255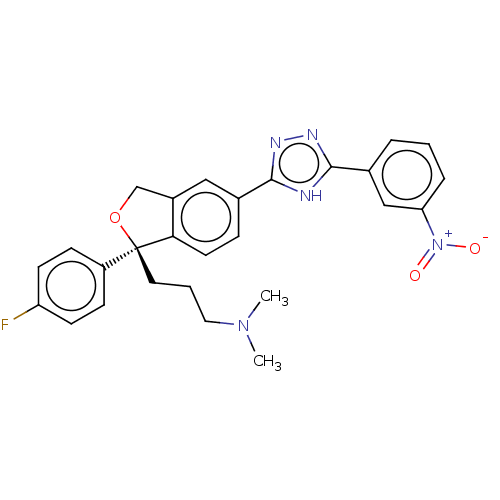 (CHEMBL3605316) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.05E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of the Punjab Curated by ChEMBL | Assay Description Inhibition of BChE (unknown origin) using butyrylthiocholine chloride as substrate preincubated for 30 mins before substrate addition measured after ... | Bioorg Med Chem 23: 6014-24 (2015) Article DOI: 10.1016/j.bmc.2015.06.051 BindingDB Entry DOI: 10.7270/Q2TT4SQS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oligo-1,6-glucosidase IMA1 (Saccharomyces cerevisiae S288c (Baker's yeast)) | BDBM50445982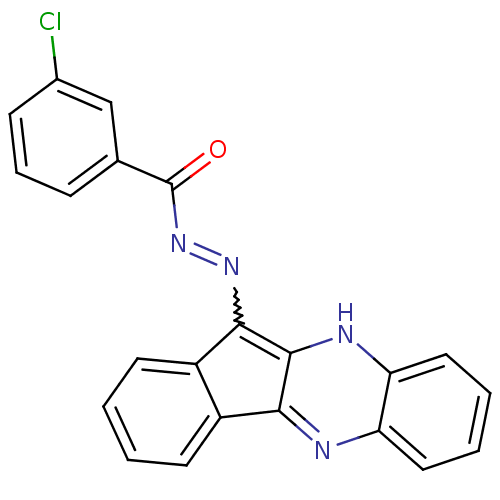 (CHEMBL3103160) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.05E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of the Punjab Curated by ChEMBL | Assay Description Inhibition of Saccharomyces cerevisiae alpha-glucosidase using p-nitrophenyl-alpha-D-glucopyranoside as substrate preincubated for 10 mins measured a... | Bioorg Med Chem 22: 1195-200 (2014) Article DOI: 10.1016/j.bmc.2013.12.024 BindingDB Entry DOI: 10.7270/Q2F1916H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50110254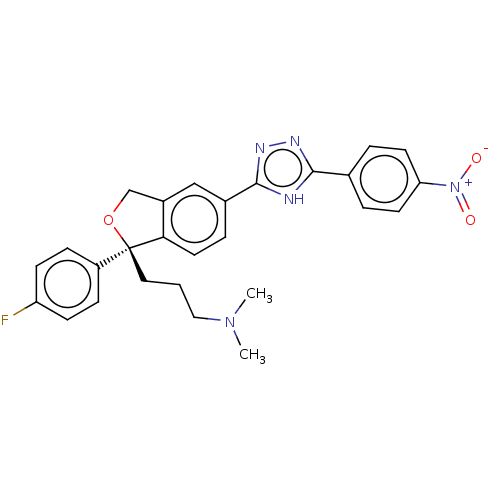 (CHEMBL3605317) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.06E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of the Punjab Curated by ChEMBL | Assay Description Inhibition of BChE (unknown origin) using butyrylthiocholine chloride as substrate preincubated for 30 mins before substrate addition measured after ... | Bioorg Med Chem 23: 6014-24 (2015) Article DOI: 10.1016/j.bmc.2015.06.051 BindingDB Entry DOI: 10.7270/Q2TT4SQS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50110278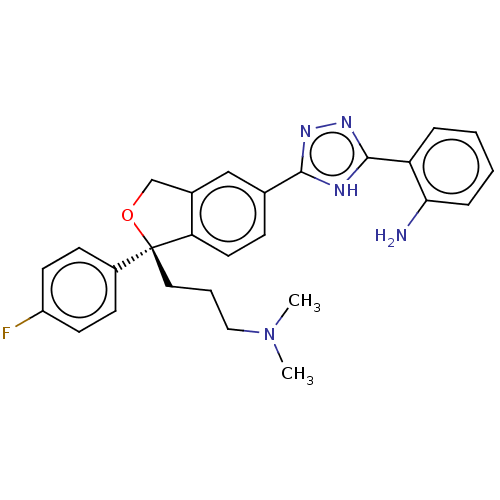 (CHEMBL3605309) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.11E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of the Punjab Curated by ChEMBL | Assay Description Inhibition of BChE (unknown origin) using butyrylthiocholine chloride as substrate preincubated for 30 mins before substrate addition measured after ... | Bioorg Med Chem 23: 6014-24 (2015) Article DOI: 10.1016/j.bmc.2015.06.051 BindingDB Entry DOI: 10.7270/Q2TT4SQS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oligo-1,6-glucosidase IMA1 (Saccharomyces cerevisiae S288c (Baker's yeast)) | BDBM50445985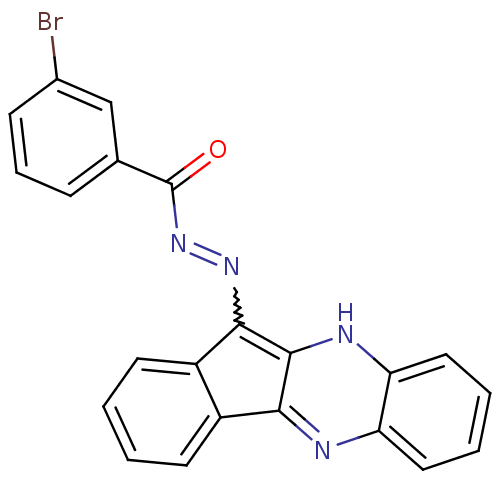 (CHEMBL3103157) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.13E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of the Punjab Curated by ChEMBL | Assay Description Inhibition of Saccharomyces cerevisiae alpha-glucosidase using p-nitrophenyl-alpha-D-glucopyranoside as substrate preincubated for 10 mins measured a... | Bioorg Med Chem 22: 1195-200 (2014) Article DOI: 10.1016/j.bmc.2013.12.024 BindingDB Entry DOI: 10.7270/Q2F1916H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50110261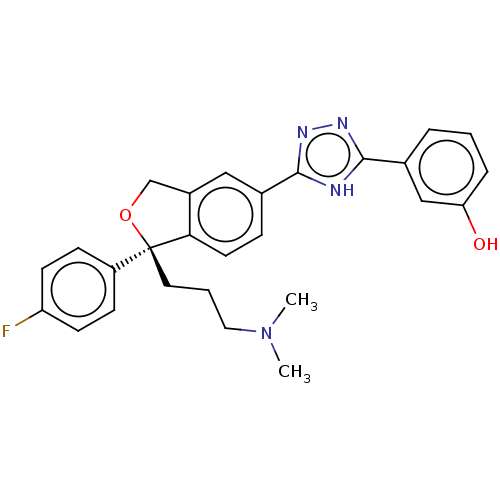 (CHEMBL3605313) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.19E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of the Punjab Curated by ChEMBL | Assay Description Inhibition of BChE (unknown origin) using butyrylthiocholine chloride as substrate preincubated for 30 mins before substrate addition measured after ... | Bioorg Med Chem 23: 6014-24 (2015) Article DOI: 10.1016/j.bmc.2015.06.051 BindingDB Entry DOI: 10.7270/Q2TT4SQS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50110270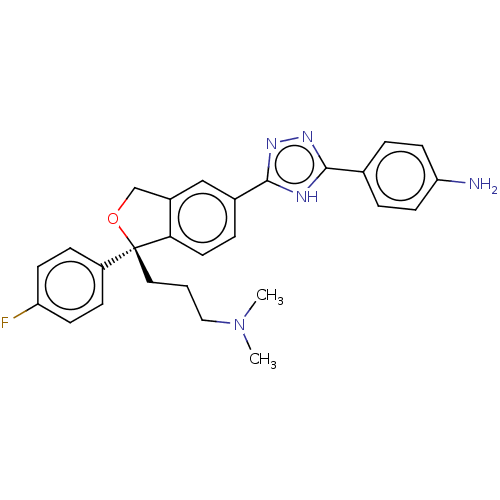 (CHEMBL3605311) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.19E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of the Punjab Curated by ChEMBL | Assay Description Inhibition of BChE (unknown origin) using butyrylthiocholine chloride as substrate preincubated for 30 mins before substrate addition measured after ... | Bioorg Med Chem 23: 6014-24 (2015) Article DOI: 10.1016/j.bmc.2015.06.051 BindingDB Entry DOI: 10.7270/Q2TT4SQS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50110316 (CHEMBL3605434) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.24E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of the Punjab Curated by ChEMBL | Assay Description Inhibition of AChE (unknown origin) using acetylthiocholine iodide as substrate preincubated for 30 mins before substrate addition measured after 30 ... | Bioorg Med Chem 23: 6014-24 (2015) Article DOI: 10.1016/j.bmc.2015.06.051 BindingDB Entry DOI: 10.7270/Q2TT4SQS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50110279 (CHEMBL3605308) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.29E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of the Punjab Curated by ChEMBL | Assay Description Inhibition of BChE (unknown origin) using butyrylthiocholine chloride as substrate preincubated for 30 mins before substrate addition measured after ... | Bioorg Med Chem 23: 6014-24 (2015) Article DOI: 10.1016/j.bmc.2015.06.051 BindingDB Entry DOI: 10.7270/Q2TT4SQS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50110252 (CHEMBL3605419) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.33E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of the Punjab Curated by ChEMBL | Assay Description Inhibition of AChE (unknown origin) using acetylthiocholine iodide as substrate preincubated for 30 mins before substrate addition measured after 30 ... | Bioorg Med Chem 23: 6014-24 (2015) Article DOI: 10.1016/j.bmc.2015.06.051 BindingDB Entry DOI: 10.7270/Q2TT4SQS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50110255 (CHEMBL3605316) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.38E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of the Punjab Curated by ChEMBL | Assay Description Inhibition of AChE (unknown origin) using acetylthiocholine iodide as substrate preincubated for 30 mins before substrate addition measured after 30 ... | Bioorg Med Chem 23: 6014-24 (2015) Article DOI: 10.1016/j.bmc.2015.06.051 BindingDB Entry DOI: 10.7270/Q2TT4SQS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50110257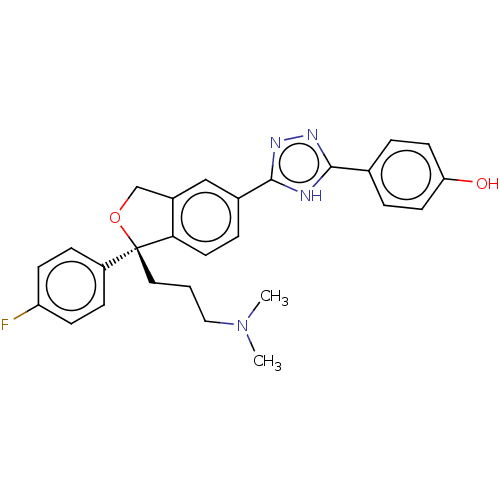 (CHEMBL3605314) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.40E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of the Punjab Curated by ChEMBL | Assay Description Inhibition of BChE (unknown origin) using butyrylthiocholine chloride as substrate preincubated for 30 mins before substrate addition measured after ... | Bioorg Med Chem 23: 6014-24 (2015) Article DOI: 10.1016/j.bmc.2015.06.051 BindingDB Entry DOI: 10.7270/Q2TT4SQS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50110329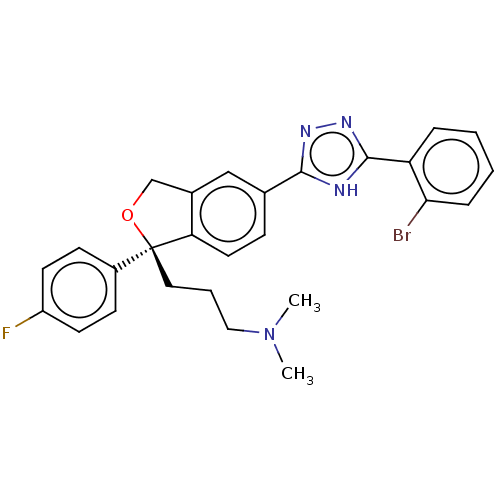 (CHEMBL3605427) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.41E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of the Punjab Curated by ChEMBL | Assay Description Inhibition of BChE (unknown origin) using butyrylthiocholine chloride as substrate preincubated for 30 mins before substrate addition measured after ... | Bioorg Med Chem 23: 6014-24 (2015) Article DOI: 10.1016/j.bmc.2015.06.051 BindingDB Entry DOI: 10.7270/Q2TT4SQS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50110325 (CHEMBL3605423) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.51E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of the Punjab Curated by ChEMBL | Assay Description Inhibition of BChE (unknown origin) using butyrylthiocholine chloride as substrate preincubated for 30 mins before substrate addition measured after ... | Bioorg Med Chem 23: 6014-24 (2015) Article DOI: 10.1016/j.bmc.2015.06.051 BindingDB Entry DOI: 10.7270/Q2TT4SQS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50110251 (CHEMBL3605420) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.52E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of the Punjab Curated by ChEMBL | Assay Description Inhibition of BChE (unknown origin) using butyrylthiocholine chloride as substrate preincubated for 30 mins before substrate addition measured after ... | Bioorg Med Chem 23: 6014-24 (2015) Article DOI: 10.1016/j.bmc.2015.06.051 BindingDB Entry DOI: 10.7270/Q2TT4SQS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50110318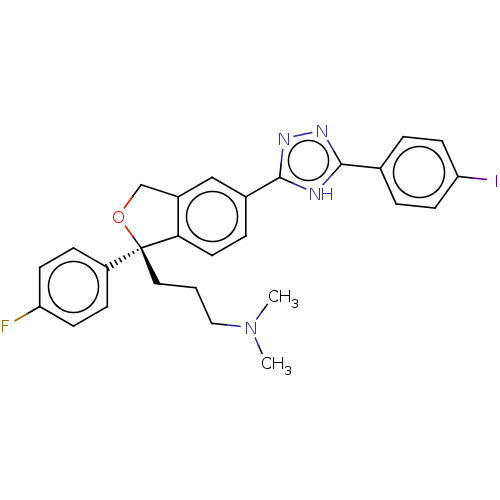 (CHEMBL3605432) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.61E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of the Punjab Curated by ChEMBL | Assay Description Inhibition of BChE (unknown origin) using butyrylthiocholine chloride as substrate preincubated for 30 mins before substrate addition measured after ... | Bioorg Med Chem 23: 6014-24 (2015) Article DOI: 10.1016/j.bmc.2015.06.051 BindingDB Entry DOI: 10.7270/Q2TT4SQS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50110317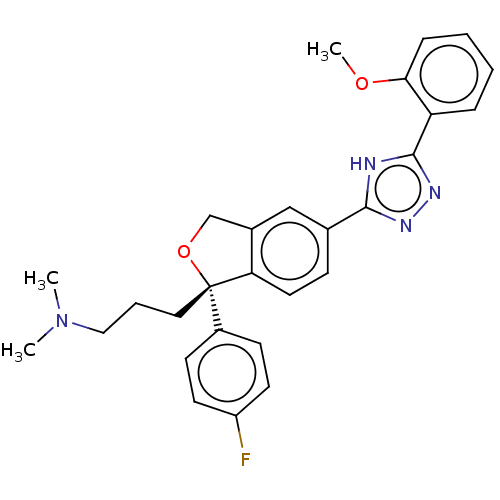 (CHEMBL3605433) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.61E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of the Punjab Curated by ChEMBL | Assay Description Inhibition of AChE (unknown origin) using acetylthiocholine iodide as substrate preincubated for 30 mins before substrate addition measured after 30 ... | Bioorg Med Chem 23: 6014-24 (2015) Article DOI: 10.1016/j.bmc.2015.06.051 BindingDB Entry DOI: 10.7270/Q2TT4SQS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50110327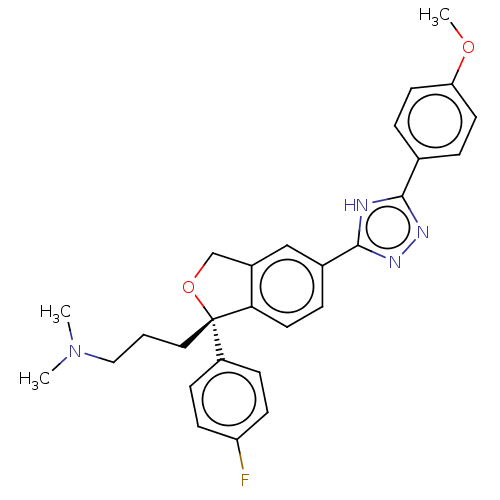 (CHEMBL3605435) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.68E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of the Punjab Curated by ChEMBL | Assay Description Inhibition of BChE (unknown origin) using butyrylthiocholine chloride as substrate preincubated for 30 mins before substrate addition measured after ... | Bioorg Med Chem 23: 6014-24 (2015) Article DOI: 10.1016/j.bmc.2015.06.051 BindingDB Entry DOI: 10.7270/Q2TT4SQS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 70 total ) | Next | Last >> |