 Found 132 hits with Last Name = 'ku?era' and Initial = 't'
Found 132 hits with Last Name = 'ku?era' and Initial = 't' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50004000

((3aS,8aR)-1,3a,8-trimethyl-1,2,3,3a,8,8a-hexahydro...)Show SMILES CNC(=O)Oc1ccc2N(C)[C@H]3N(C)CC[C@@]3(C)c2c1 |r| Show InChI InChI=1S/C15H21N3O2/c1-15-7-8-17(3)13(15)18(4)12-6-5-10(9-11(12)15)20-14(19)16-2/h5-6,9,13H,7-8H2,1-4H3,(H,16,19)/t13-,15+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| n/a | n/a | 63 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Defence
Curated by ChEMBL
| Assay Description
Inhibition of human AChE using acetylthiocholine iodide as substrate measured for 1 min by spectrophotometric based Ellman's method |
J Nat Prod 83: 1359-1367 (2020)
Article DOI: 10.1021/acs.jnatprod.9b00561
BindingDB Entry DOI: 10.7270/Q2XG9VQ0 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50579850
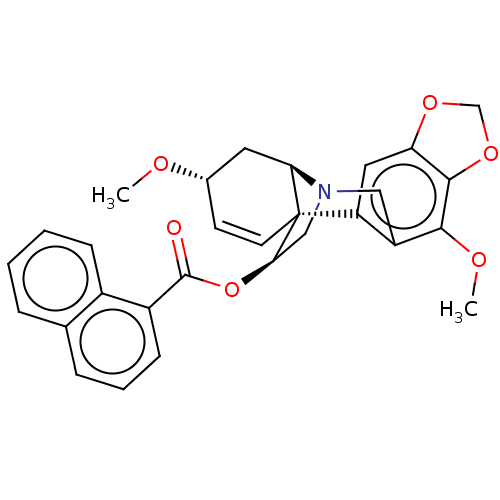
(CHEMBL5088077)Show SMILES [H][C@@]12C[C@@H](OC)C=C[C@]11[C@@H](CN2Cc2c(OC)c3OCOc3cc12)OC(=O)c1cccc2ccccc12 |r,c:6,TLB:14:13:1:10.9,22:23:1:10.9| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human BuChE using butyrylthiocholine chloride as substrate by Ellman's method |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128374
BindingDB Entry DOI: 10.7270/Q2QJ7N4Z |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50541552
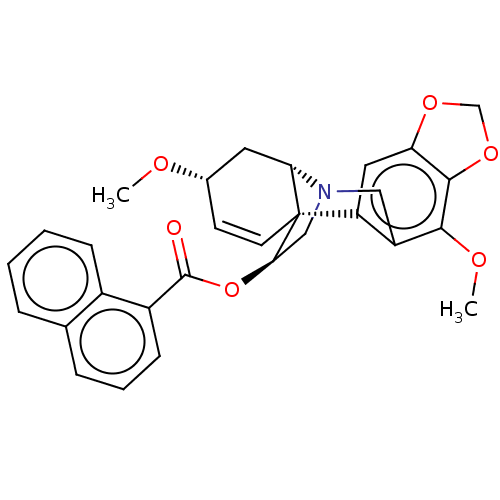
(CHEMBL4648132)Show SMILES [H][C@]12C[C@@H](OC)C=C[C@]11[C@@H](CN2Cc2c(OC)c3OCOc3cc12)OC(=O)c1cccc2ccccc12 |r,c:6,@:11,TLB:24:9:23.13.12:1| Show InChI InChI=1S/C29H27NO6/c1-32-18-10-11-29-22-13-23-27(35-16-34-23)26(33-2)21(22)14-30(24(29)12-18)15-25(29)36-28(31)20-9-5-7-17-6-3-4-8-19(17)20/h3-11,13,18,24-25H,12,14-16H2,1-2H3/t18-,24-,25+,29+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Defence
Curated by ChEMBL
| Assay Description
Inhibition of human BuChE using butyrylthiocholine iodide as substrate measured for 1 min by spectrophotometric based Ellman's method |
J Nat Prod 83: 1359-1367 (2020)
Article DOI: 10.1021/acs.jnatprod.9b00561
BindingDB Entry DOI: 10.7270/Q2XG9VQ0 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50004000

((3aS,8aR)-1,3a,8-trimethyl-1,2,3,3a,8,8a-hexahydro...)Show SMILES CNC(=O)Oc1ccc2N(C)[C@H]3N(C)CC[C@@]3(C)c2c1 |r| Show InChI InChI=1S/C15H21N3O2/c1-15-7-8-17(3)13(15)18(4)12-6-5-10(9-11(12)15)20-14(19)16-2/h5-6,9,13H,7-8H2,1-4H3,(H,16,19)/t13-,15+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Defence
Curated by ChEMBL
| Assay Description
Inhibition of human BuChE using butyrylthiocholine iodide as substrate measured for 1 min by spectrophotometric based Ellman's method |
J Nat Prod 83: 1359-1367 (2020)
Article DOI: 10.1021/acs.jnatprod.9b00561
BindingDB Entry DOI: 10.7270/Q2XG9VQ0 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50004000

((3aS,8aR)-1,3a,8-trimethyl-1,2,3,3a,8,8a-hexahydro...)Show SMILES CNC(=O)Oc1ccc2N(C)[C@H]3N(C)CC[C@@]3(C)c2c1 |r| Show InChI InChI=1S/C15H21N3O2/c1-15-7-8-17(3)13(15)18(4)12-6-5-10(9-11(12)15)20-14(19)16-2/h5-6,9,13H,7-8H2,1-4H3,(H,16,19)/t13-,15+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human AChE using acetylthiocholine as substrate by Ellman's method |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128374
BindingDB Entry DOI: 10.7270/Q2QJ7N4Z |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50541562
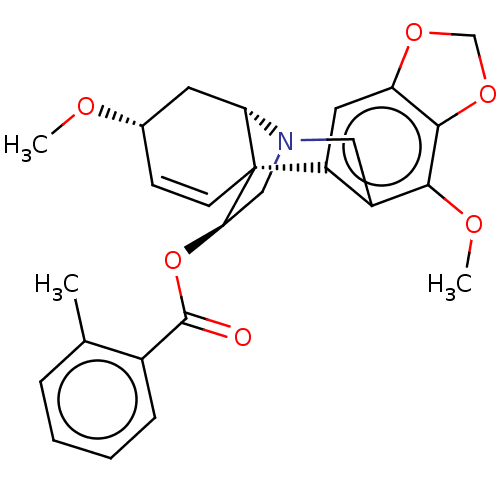
(CHEMBL4640945)Show SMILES [H][C@]12C[C@@H](OC)C=C[C@]11[C@@H](CN2Cc2c(OC)c3OCOc3cc12)OC(=O)c1ccccc1C |r,c:6,@:11,TLB:24:9:23.13.12:1| Show InChI InChI=1S/C26H27NO6/c1-15-6-4-5-7-17(15)25(28)33-22-13-27-12-18-19(11-20-24(23(18)30-3)32-14-31-20)26(22)9-8-16(29-2)10-21(26)27/h4-9,11,16,21-22H,10,12-14H2,1-3H3/t16-,21-,22+,26+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 280 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Defence
Curated by ChEMBL
| Assay Description
Inhibition of human BuChE using butyrylthiocholine iodide as substrate measured for 1 min by spectrophotometric based Ellman's method |
J Nat Prod 83: 1359-1367 (2020)
Article DOI: 10.1021/acs.jnatprod.9b00561
BindingDB Entry DOI: 10.7270/Q2XG9VQ0 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50004000

((3aS,8aR)-1,3a,8-trimethyl-1,2,3,3a,8,8a-hexahydro...)Show SMILES CNC(=O)Oc1ccc2N(C)[C@H]3N(C)CC[C@@]3(C)c2c1 |r| Show InChI InChI=1S/C15H21N3O2/c1-15-7-8-17(3)13(15)18(4)12-6-5-10(9-11(12)15)20-14(19)16-2/h5-6,9,13H,7-8H2,1-4H3,(H,16,19)/t13-,15+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human BuChE using butyrylthiocholine chloride as substrate by Ellman's method |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128374
BindingDB Entry DOI: 10.7270/Q2QJ7N4Z |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50541559
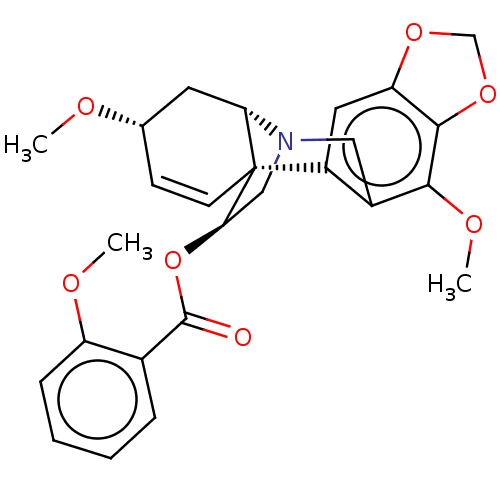
(CHEMBL4644410)Show SMILES [H][C@]12C[C@@H](OC)C=C[C@]11[C@@H](CN2Cc2c(OC)c3OCOc3cc12)OC(=O)c1ccccc1OC |r,c:6,@:11,TLB:24:9:23.13.12:1| Show InChI InChI=1S/C26H27NO7/c1-29-15-8-9-26-18-11-20-24(33-14-32-20)23(31-3)17(18)12-27(21(26)10-15)13-22(26)34-25(28)16-6-4-5-7-19(16)30-2/h4-9,11,15,21-22H,10,12-14H2,1-3H3/t15-,21-,22+,26+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 430 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Defence
Curated by ChEMBL
| Assay Description
Inhibition of human BuChE using butyrylthiocholine iodide as substrate measured for 1 min by spectrophotometric based Ellman's method |
J Nat Prod 83: 1359-1367 (2020)
Article DOI: 10.1021/acs.jnatprod.9b00561
BindingDB Entry DOI: 10.7270/Q2XG9VQ0 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50541561
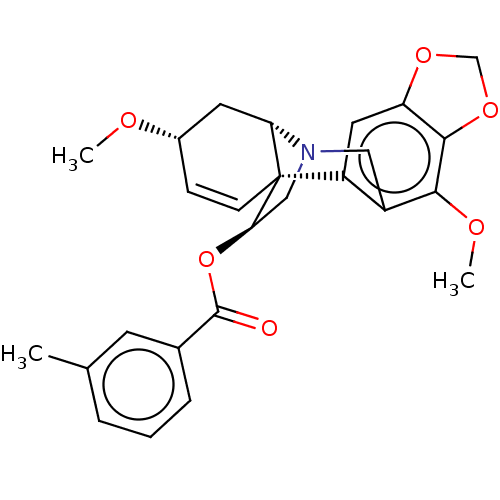
(CHEMBL4640168)Show SMILES [H][C@]12C[C@@H](OC)C=C[C@]11[C@@H](CN2Cc2c(OC)c3OCOc3cc12)OC(=O)c1cccc(C)c1 |r,c:6,@:11,TLB:24:9:23.13.12:1| Show InChI InChI=1S/C26H27NO6/c1-15-5-4-6-16(9-15)25(28)33-22-13-27-12-18-19(11-20-24(23(18)30-3)32-14-31-20)26(22)8-7-17(29-2)10-21(26)27/h4-9,11,17,21-22H,10,12-14H2,1-3H3/t17-,21-,22+,26+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 860 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Defence
Curated by ChEMBL
| Assay Description
Inhibition of human BuChE using butyrylthiocholine iodide as substrate measured for 1 min by spectrophotometric based Ellman's method |
J Nat Prod 83: 1359-1367 (2020)
Article DOI: 10.1021/acs.jnatprod.9b00561
BindingDB Entry DOI: 10.7270/Q2XG9VQ0 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Equus caballus (Horse)) | BDBM50541569

(CHEMBL4638699)Show SMILES [H][C@]1([C@H](C)N(C)C)[C@H](O)C[C@@]2(C)[C@]3([H])CC[C@]4([H])[C@]5(C[C@@]35CC[C@]12C)C=CC(=O)C4(C)C |r,c:29| Show InChI InChI=1S/C26H41NO2/c1-16(27(6)7)21-17(28)14-24(5)19-9-8-18-22(2,3)20(29)10-11-25(18)15-26(19,25)13-12-23(21,24)4/h10-11,16-19,21,28H,8-9,12-15H2,1-7H3/t16-,17+,18-,19-,21-,23+,24-,25+,26-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Defence
Curated by ChEMBL
| Assay Description
Inhibition of horse serum BuChE using butyrylthiocholine chloride as substrate by spectrophotometric based Ellman's method |
J Nat Prod 83: 1359-1367 (2020)
Article DOI: 10.1021/acs.jnatprod.9b00561
BindingDB Entry DOI: 10.7270/Q2XG9VQ0 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50579848
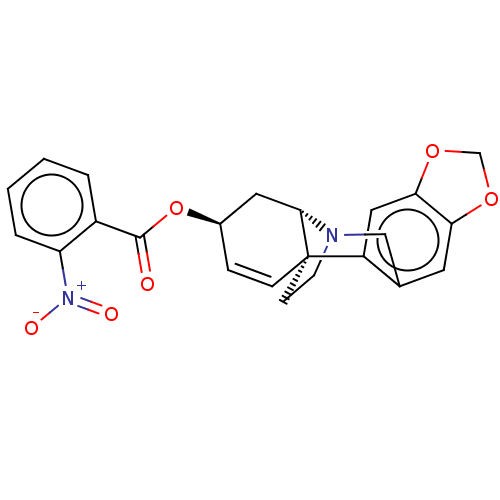
(CHEMBL5086177)Show SMILES [H][C@]12C[C@H](OC(=O)c3ccccc3[N+]([O-])=O)C=C[C@@]11CCN2Cc2cc3OCOc3cc12 |r,c:17| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human BuChE using butyrylthiocholine chloride as substrate by Ellman's method |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128374
BindingDB Entry DOI: 10.7270/Q2QJ7N4Z |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50579829
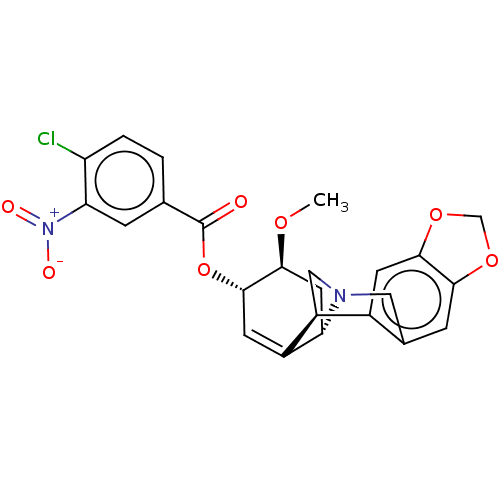
(CHEMBL5087321)Show SMILES [H][C@]12C[C@H](OC)[C@@H](OC(=O)c3ccc(Cl)c(c3)[N+]([O-])=O)C=C1[C@@]1([H])CN2Cc2cc3OCOc3cc12 |r,c:21| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.61E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human AChE using acetylthiocholine as substrate by Ellman's method |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128374
BindingDB Entry DOI: 10.7270/Q2QJ7N4Z |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Equus caballus (Horse)) | BDBM50541570
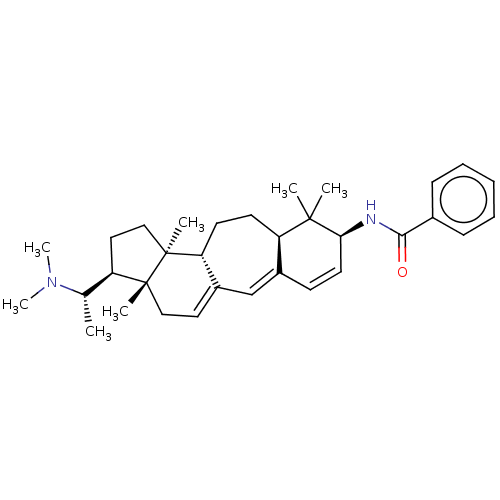
(CHEMBL4638459)Show SMILES [H][C@@]1(CC[C@@]2(C)[C@]3([H])CC[C@]4([H])C(C=C[C@H](NC(=O)c5ccccc5)C4(C)C)=CC3=CC[C@]12C)[C@H](C)N(C)C |r,wU:15.15,32.37,6.6,wD:34.40,1.0,4.4,10.10,c:13,29,32,(30.59,-13.08,;29.04,-13.08,;29.96,-14.32,;29.04,-15.56,;27.59,-15.09,;27.59,-16.63,;26.25,-15.86,;26.25,-14.32,;26.25,-17.4,;24.92,-18.17,;23.59,-17.4,;23.99,-18.89,;23.59,-15.86,;22.25,-15.09,;20.92,-15.86,;20.92,-17.4,;19.59,-18.17,;18.26,-17.4,;18.26,-15.86,;16.92,-18.17,;16.92,-19.71,;15.59,-20.48,;14.26,-19.71,;14.26,-18.17,;15.59,-17.39,;22.25,-18.17,;21.48,-19.51,;23.02,-19.51,;23.63,-14.32,;24.92,-15.09,;24.92,-13.55,;26.25,-12.78,;27.59,-13.55,;27.59,-12.01,;29.44,-11.59,;28.36,-10.5,;30.93,-11.19,;31.33,-9.71,;32.02,-12.28,)| Show InChI InChI=1S/C33H46N2O/c1-22(35(6)7)26-18-20-33(5)28-15-14-27-24(21-25(28)17-19-32(26,33)4)13-16-29(31(27,2)3)34-30(36)23-11-9-8-10-12-23/h8-13,16-17,21-22,26-29H,14-15,18-20H2,1-7H3,(H,34,36)/t22-,26+,27+,28+,29-,32+,33-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Defence
Curated by ChEMBL
| Assay Description
Inhibition of horse serum BuChE using butyrylthiocholine chloride as substrate by spectrophotometric based Ellman's method |
J Nat Prod 83: 1359-1367 (2020)
Article DOI: 10.1021/acs.jnatprod.9b00561
BindingDB Entry DOI: 10.7270/Q2XG9VQ0 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM10404

((1S,12S,14R)-9-methoxy-4-methyl-11-oxa-4-azatetrac...)Show SMILES COc1ccc2CN(C)CC[C@@]34C=C[C@H](O)C[C@@H]3Oc1c24 |r,c:12| Show InChI InChI=1S/C17H21NO3/c1-18-8-7-17-6-5-12(19)9-14(17)21-16-13(20-2)4-3-11(10-18)15(16)17/h3-6,12,14,19H,7-10H2,1-2H3/t12-,14-,17-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Defence
Curated by ChEMBL
| Assay Description
Inhibition of human AChE using acetylthiocholine iodide as substrate measured for 1 min by spectrophotometric based Ellman's method |
J Nat Prod 83: 1359-1367 (2020)
Article DOI: 10.1021/acs.jnatprod.9b00561
BindingDB Entry DOI: 10.7270/Q2XG9VQ0 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cholinesterase
(Homo sapiens (Human)) | BDBM50579837
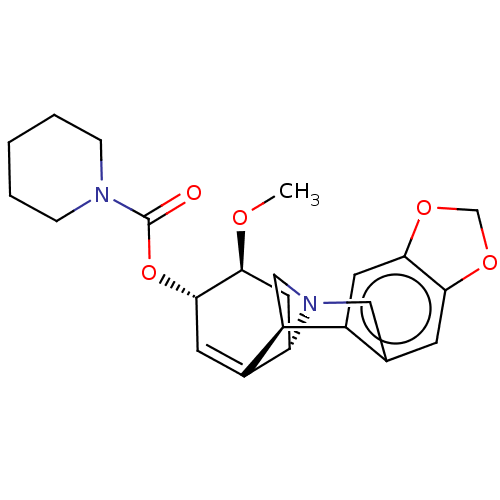
(CHEMBL5079347)Show SMILES [H][C@]12C[C@H](OC)[C@@H](OC(=O)N3CCCCC3)C=C1[C@@]1([H])CN2Cc2cc3OCOc3cc12 |r,c:17| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.73E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human BuChE using butyrylthiocholine chloride as substrate by Ellman's method |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128374
BindingDB Entry DOI: 10.7270/Q2QJ7N4Z |
More data for this
Ligand-Target Pair | |
DNA topoisomerase 2-alpha
(Homo sapiens (Human)) | BDBM50586362
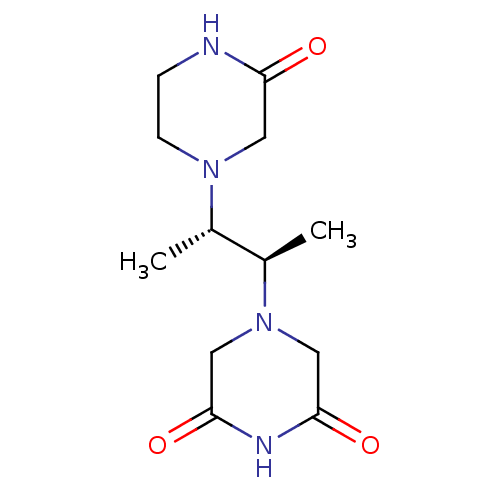
(CHEMBL5083245)Show SMILES C[C@@H]([C@@H](C)N1CC(=O)NC(=O)C1)N1CCNC(=O)C1 |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human TOP2A assessed as reduction in relaxation of supercoiled DNA using kDNA as substrate incubated for 30 min |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c02157
BindingDB Entry DOI: 10.7270/Q2QJ7N6V |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM10404

((1S,12S,14R)-9-methoxy-4-methyl-11-oxa-4-azatetrac...)Show SMILES COc1ccc2CN(C)CC[C@@]34C=C[C@H](O)C[C@@H]3Oc1c24 |r,c:12| Show InChI InChI=1S/C17H21NO3/c1-18-8-7-17-6-5-12(19)9-14(17)21-16-13(20-2)4-3-11(10-18)15(16)17/h3-6,12,14,19H,7-10H2,1-2H3/t12-,14-,17-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 2.01E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human AChE using acetylthiocholine as substrate by Ellman's method |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128374
BindingDB Entry DOI: 10.7270/Q2QJ7N4Z |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cholinesterase
(Homo sapiens (Human)) | BDBM50541556
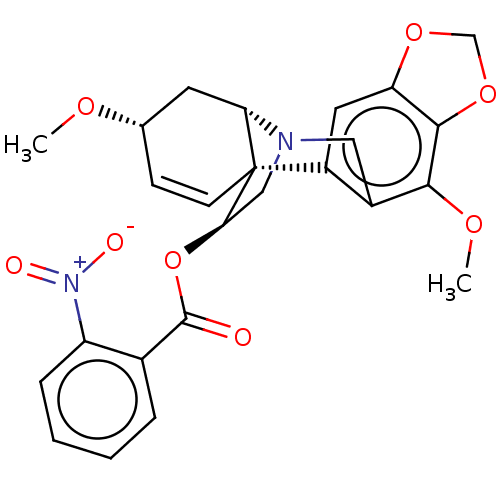
(CHEMBL4634465)Show SMILES [H][C@]12C[C@@H](OC)C=C[C@]11[C@@H](CN2Cc2c(OC)c3OCOc3cc12)OC(=O)c1ccccc1[N+]([O-])=O |r,c:6,@:11,TLB:24:9:23.13.12:1| Show InChI InChI=1S/C25H24N2O8/c1-31-14-7-8-25-17-10-19-23(34-13-33-19)22(32-2)16(17)11-26(20(25)9-14)12-21(25)35-24(28)15-5-3-4-6-18(15)27(29)30/h3-8,10,14,20-21H,9,11-13H2,1-2H3/t14-,20-,21+,25+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Defence
Curated by ChEMBL
| Assay Description
Inhibition of human BuChE using butyrylthiocholine iodide as substrate measured for 1 min by spectrophotometric based Ellman's method |
J Nat Prod 83: 1359-1367 (2020)
Article DOI: 10.1021/acs.jnatprod.9b00561
BindingDB Entry DOI: 10.7270/Q2XG9VQ0 |
More data for this
Ligand-Target Pair | |
DNA topoisomerase 2-beta
(Homo sapiens (Human)) | BDBM50586362

(CHEMBL5083245)Show SMILES C[C@@H]([C@@H](C)N1CC(=O)NC(=O)C1)N1CCNC(=O)C1 |r| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human TOP2B assessed as reduction in relaxation of supercoiled DNA using kDNA as substrate incubated for 30 min |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c02157
BindingDB Entry DOI: 10.7270/Q2QJ7N6V |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50579831
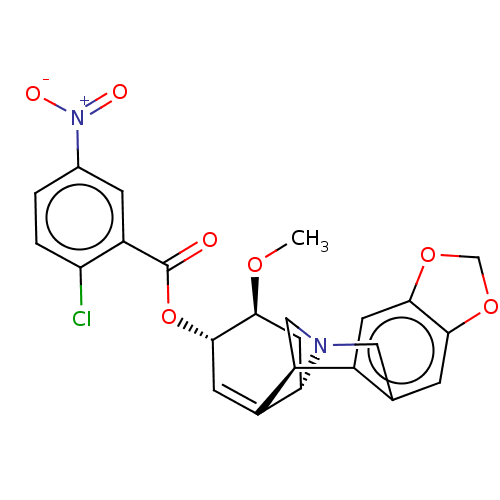
(CHEMBL5091842)Show SMILES [H][C@]12C[C@H](OC)[C@@H](OC(=O)c3cc(ccc3Cl)[N+]([O-])=O)C=C1[C@@]1([H])CN2Cc2cc3OCOc3cc12 |r,c:21| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.18E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human AChE using acetylthiocholine as substrate by Ellman's method |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128374
BindingDB Entry DOI: 10.7270/Q2QJ7N4Z |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50579847
(CHEMBL5090336)Show SMILES [H][C@]12C[C@H](O)C=C[C@@]11[C@H](CN2Cc2cc3OCOc3cc12)OC(=O)c1ccccc1OC |r,c:5,TLB:13:12:1:9.8,19:20:1:9.8| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human BuChE using butyrylthiocholine chloride as substrate by Ellman's method |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128374
BindingDB Entry DOI: 10.7270/Q2QJ7N4Z |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50541563
(CHEMBL4633343)Show SMILES [H][C@]12C[C@@H](OC)C=C[C@]11[C@@H](CN2Cc2c(OC)c3OCOc3cc12)OC(=O)c1ccccc1 |r,c:6,@:11,TLB:24:9:23.13.12:1| Show InChI InChI=1S/C25H25NO6/c1-28-16-8-9-25-18-11-19-23(31-14-30-19)22(29-2)17(18)12-26(20(25)10-16)13-21(25)32-24(27)15-6-4-3-5-7-15/h3-9,11,16,20-21H,10,12-14H2,1-2H3/t16-,20-,21+,25+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Defence
Curated by ChEMBL
| Assay Description
Inhibition of human BuChE using butyrylthiocholine iodide as substrate measured for 1 min by spectrophotometric based Ellman's method |
J Nat Prod 83: 1359-1367 (2020)
Article DOI: 10.1021/acs.jnatprod.9b00561
BindingDB Entry DOI: 10.7270/Q2XG9VQ0 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50579845
(CHEMBL5074625)Show SMILES [H][C@]12C[C@H](O)C=C[C@@]11[C@H](CN2Cc2cc3OCOc3cc12)OC(=O)c1cccc(c1)[N+]([O-])=O |r,c:5,TLB:13:12:1:9.8,19:20:1:9.8| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human AChE using acetylthiocholine as substrate by Ellman's method |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128374
BindingDB Entry DOI: 10.7270/Q2QJ7N4Z |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50579830
(CHEMBL5075459)Show SMILES [H][C@]12C[C@H](OC)[C@@H](OC(=O)c3ccc(C)c(c3)[N+]([O-])=O)C=C1[C@@]1([H])CN2Cc2cc3OCOc3cc12 |r,c:21| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.32E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human AChE using acetylthiocholine as substrate by Ellman's method |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128374
BindingDB Entry DOI: 10.7270/Q2QJ7N4Z |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50579846
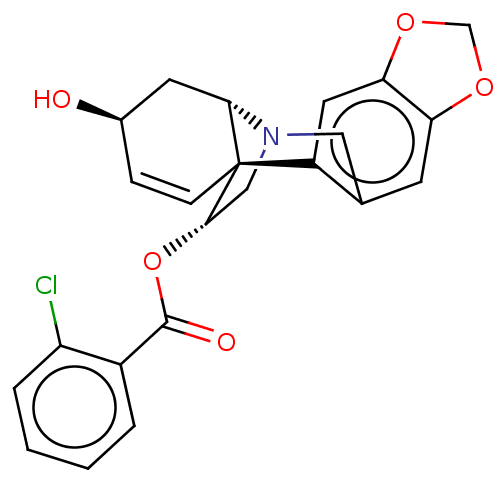
(CHEMBL5089535)Show SMILES [H][C@]12C[C@H](O)C=C[C@@]11[C@H](CN2Cc2cc3OCOc3cc12)OC(=O)c1ccccc1Cl |r,c:5,TLB:13:12:1:9.8,19:20:1:9.8| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human BuChE using butyrylthiocholine chloride as substrate by Ellman's method |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128374
BindingDB Entry DOI: 10.7270/Q2QJ7N4Z |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50541558
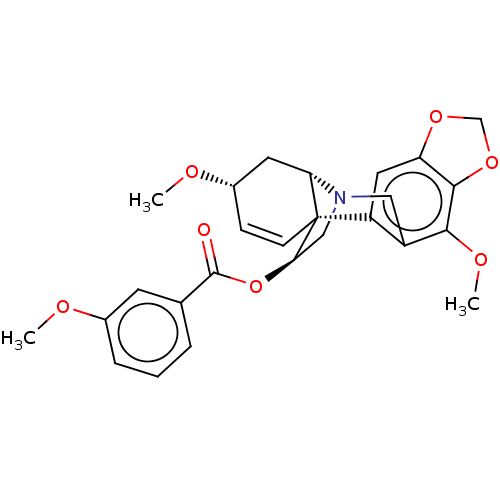
(CHEMBL4635404)Show SMILES [H][C@]12C[C@@H](OC)C=C[C@]11[C@@H](CN2Cc2c(OC)c3OCOc3cc12)OC(=O)c1cccc(OC)c1 |r,c:6,@:11,TLB:24:9:23.13.12:1| Show InChI InChI=1S/C26H27NO7/c1-29-16-6-4-5-15(9-16)25(28)34-22-13-27-12-18-19(11-20-24(23(18)31-3)33-14-32-20)26(22)8-7-17(30-2)10-21(26)27/h4-9,11,17,21-22H,10,12-14H2,1-3H3/t17-,21-,22+,26+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Defence
Curated by ChEMBL
| Assay Description
Inhibition of human BuChE using butyrylthiocholine iodide as substrate measured for 1 min by spectrophotometric based Ellman's method |
J Nat Prod 83: 1359-1367 (2020)
Article DOI: 10.1021/acs.jnatprod.9b00561
BindingDB Entry DOI: 10.7270/Q2XG9VQ0 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50579844
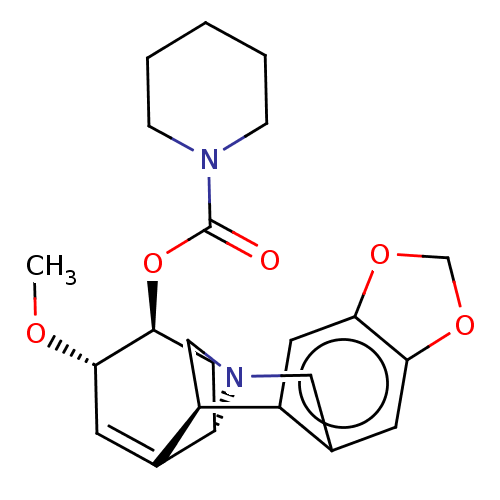
(CHEMBL5070022)Show SMILES [H][C@]12C[C@H](OC(=O)N3CCCCC3)[C@@H](OC)C=C1[C@@]1([H])CN2Cc2cc3OCOc3cc12 |r,c:17| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6.54E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human BuChE using butyrylthiocholine chloride as substrate by Ellman's method |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128374
BindingDB Entry DOI: 10.7270/Q2QJ7N4Z |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50579849
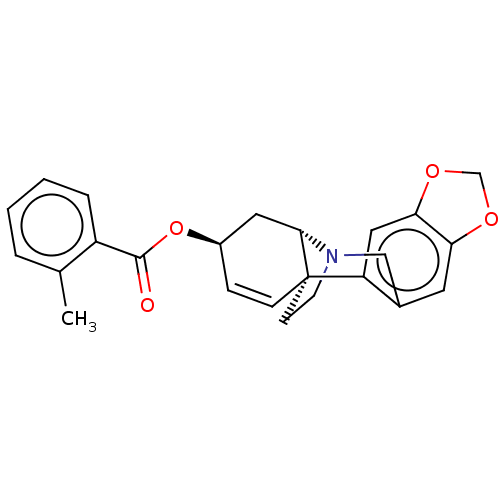
(CHEMBL5079168)Show SMILES [H][C@]12C[C@H](OC(=O)c3ccccc3C)C=C[C@@]11CCN2Cc2cc3OCOc3cc12 |r,c:15| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 8.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human BuChE using butyrylthiocholine chloride as substrate by Ellman's method |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128374
BindingDB Entry DOI: 10.7270/Q2QJ7N4Z |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50579846

(CHEMBL5089535)Show SMILES [H][C@]12C[C@H](O)C=C[C@@]11[C@H](CN2Cc2cc3OCOc3cc12)OC(=O)c1ccccc1Cl |r,c:5,TLB:13:12:1:9.8,19:20:1:9.8| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human AChE using acetylthiocholine as substrate by Ellman's method |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128374
BindingDB Entry DOI: 10.7270/Q2QJ7N4Z |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50579828
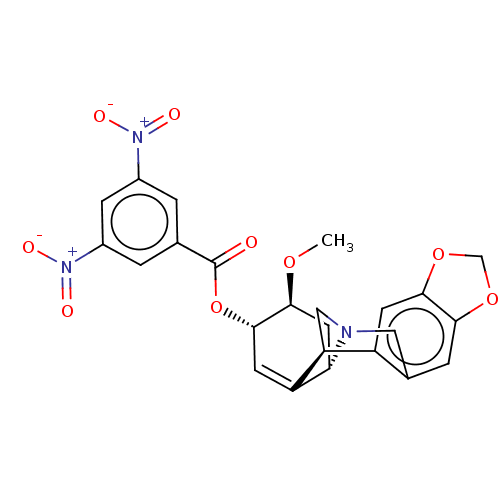
(CHEMBL5083797)Show SMILES [H][C@]12C[C@H](OC)[C@@H](OC(=O)c3cc(cc(c3)[N+]([O-])=O)[N+]([O-])=O)C=C1[C@@]1([H])CN2Cc2cc3OCOc3cc12 |r,c:23| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.31E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human AChE using acetylthiocholine as substrate by Ellman's method |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128374
BindingDB Entry DOI: 10.7270/Q2QJ7N4Z |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50541553
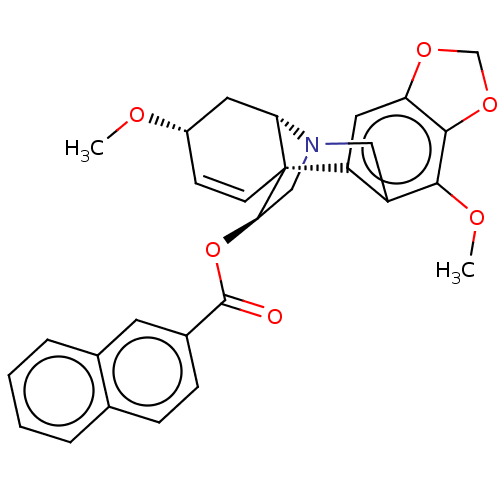
(CHEMBL4636736)Show SMILES [H][C@]12C[C@@H](OC)C=C[C@]11[C@@H](CN2Cc2c(OC)c3OCOc3cc12)OC(=O)c1ccc2ccccc2c1 |r,c:6,@:11,TLB:24:9:23.13.12:1| Show InChI InChI=1S/C29H27NO6/c1-32-20-9-10-29-22-13-23-27(35-16-34-23)26(33-2)21(22)14-30(24(29)12-20)15-25(29)36-28(31)19-8-7-17-5-3-4-6-18(17)11-19/h3-11,13,20,24-25H,12,14-16H2,1-2H3/t20-,24-,25+,29+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Defence
Curated by ChEMBL
| Assay Description
Inhibition of human BuChE using butyrylthiocholine iodide as substrate measured for 1 min by spectrophotometric based Ellman's method |
J Nat Prod 83: 1359-1367 (2020)
Article DOI: 10.1021/acs.jnatprod.9b00561
BindingDB Entry DOI: 10.7270/Q2XG9VQ0 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50579842
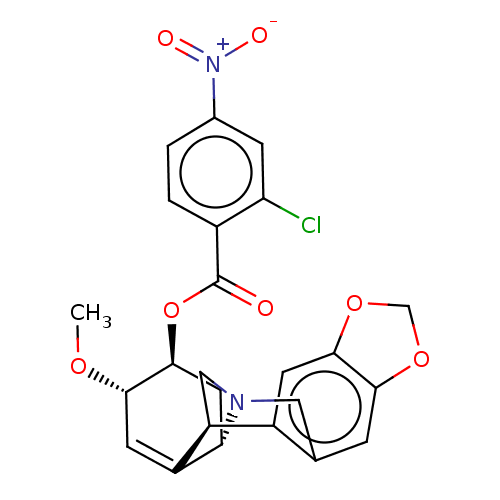
(CHEMBL5090352)Show SMILES [H][C@]12C[C@H](OC(=O)c3ccc(cc3Cl)[N+]([O-])=O)[C@@H](OC)C=C1[C@@]1([H])CN2Cc2cc3OCOc3cc12 |r,c:21| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human BuChE using butyrylthiocholine chloride as substrate by Ellman's method |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128374
BindingDB Entry DOI: 10.7270/Q2QJ7N4Z |
More data for this
Ligand-Target Pair | |
Cholinesterase
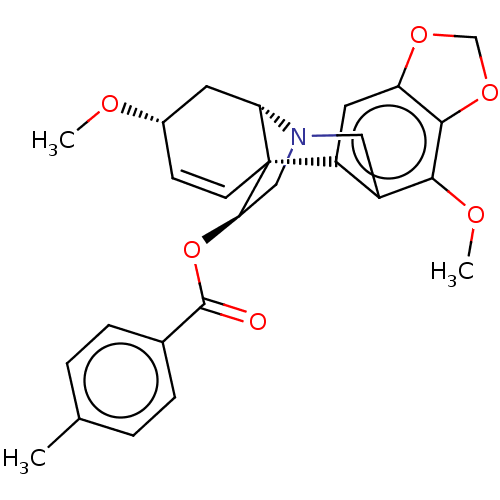
(Homo sapiens (Human)) | BDBM50541560
(CHEMBL4638682)Show SMILES [H][C@]12C[C@@H](OC)C=C[C@]11[C@@H](CN2Cc2c(OC)c3OCOc3cc12)OC(=O)c1ccc(C)cc1 |r,c:6,@:11,TLB:24:9:23.13.12:1| Show InChI InChI=1S/C26H27NO6/c1-15-4-6-16(7-5-15)25(28)33-22-13-27-12-18-19(11-20-24(23(18)30-3)32-14-31-20)26(22)9-8-17(29-2)10-21(26)27/h4-9,11,17,21-22H,10,12-14H2,1-3H3/t17-,21-,22+,26+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Defence
Curated by ChEMBL
| Assay Description
Inhibition of human BuChE using butyrylthiocholine iodide as substrate measured for 1 min by spectrophotometric based Ellman's method |
J Nat Prod 83: 1359-1367 (2020)
Article DOI: 10.1021/acs.jnatprod.9b00561
BindingDB Entry DOI: 10.7270/Q2XG9VQ0 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM10404

((1S,12S,14R)-9-methoxy-4-methyl-11-oxa-4-azatetrac...)Show SMILES COc1ccc2CN(C)CC[C@@]34C=C[C@H](O)C[C@@H]3Oc1c24 |r,c:12| Show InChI InChI=1S/C17H21NO3/c1-18-8-7-17-6-5-12(19)9-14(17)21-16-13(20-2)4-3-11(10-18)15(16)17/h3-6,12,14,19H,7-10H2,1-2H3/t12-,14-,17-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| n/a | n/a | 2.93E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human BuChE using butyrylthiocholine chloride as substrate by Ellman's method |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128374
BindingDB Entry DOI: 10.7270/Q2QJ7N4Z |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50541550
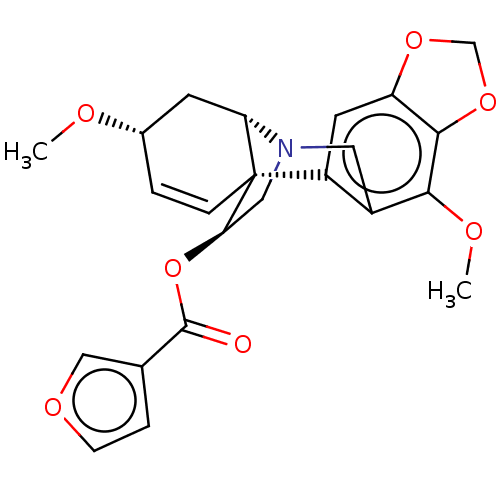
(CHEMBL4644284)Show SMILES [H][C@]12C[C@@H](OC)C=C[C@]11[C@@H](CN2Cc2c(OC)c3OCOc3cc12)OC(=O)c1ccoc1 |r,c:6,@:11,TLB:24:9:23.13.12:1| Show InChI InChI=1S/C23H23NO7/c1-26-14-3-5-23-16-8-17-21(30-12-29-17)20(27-2)15(16)9-24(18(23)7-14)10-19(23)31-22(25)13-4-6-28-11-13/h3-6,8,11,14,18-19H,7,9-10,12H2,1-2H3/t14-,18-,19+,23+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Defence
Curated by ChEMBL
| Assay Description
Inhibition of human BuChE using butyrylthiocholine iodide as substrate measured for 1 min by spectrophotometric based Ellman's method |
J Nat Prod 83: 1359-1367 (2020)
Article DOI: 10.1021/acs.jnatprod.9b00561
BindingDB Entry DOI: 10.7270/Q2XG9VQ0 |
More data for this
Ligand-Target Pair | |
DNA topoisomerase 2-alpha
(Homo sapiens (Human)) | BDBM50586360
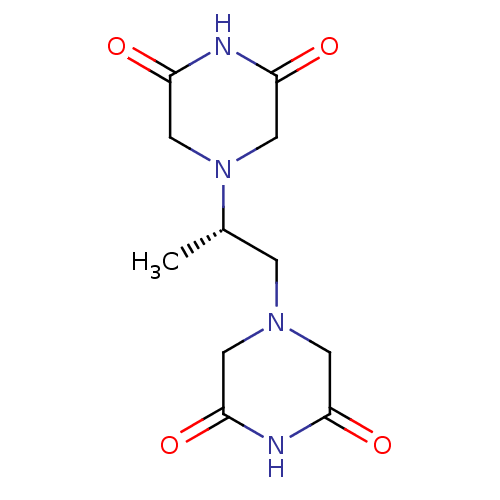
(ADR-529 | CHEBI:50223 | Cardioxane | DEXRAZOXANE |...) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human TOP2A assessed as reduction in relaxation of supercoiled DNA using kDNA as substrate incubated for 30 min |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c02157
BindingDB Entry DOI: 10.7270/Q2QJ7N6V |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM10404

((1S,12S,14R)-9-methoxy-4-methyl-11-oxa-4-azatetrac...)Show SMILES COc1ccc2CN(C)CC[C@@]34C=C[C@H](O)C[C@@H]3Oc1c24 |r,c:12| Show InChI InChI=1S/C17H21NO3/c1-18-8-7-17-6-5-12(19)9-14(17)21-16-13(20-2)4-3-11(10-18)15(16)17/h3-6,12,14,19H,7-10H2,1-2H3/t12-,14-,17-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| n/a | n/a | 4.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Defence
Curated by ChEMBL
| Assay Description
Inhibition of human BuChE using butyrylthiocholine iodide as substrate measured for 1 min by spectrophotometric based Ellman's method |
J Nat Prod 83: 1359-1367 (2020)
Article DOI: 10.1021/acs.jnatprod.9b00561
BindingDB Entry DOI: 10.7270/Q2XG9VQ0 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50541555
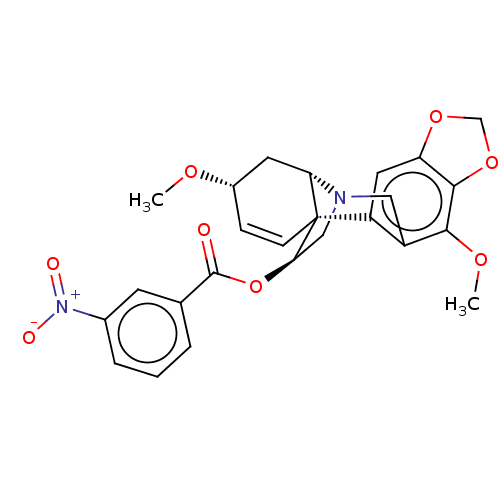
(CHEMBL4646747)Show SMILES [H][C@]12C[C@@H](OC)C=C[C@]11[C@@H](CN2Cc2c(OC)c3OCOc3cc12)OC(=O)c1cccc(c1)[N+]([O-])=O |r,c:6,@:11,TLB:24:9:23.13.12:1| Show InChI InChI=1S/C25H24N2O8/c1-31-16-6-7-25-18-10-19-23(34-13-33-19)22(32-2)17(18)11-26(20(25)9-16)12-21(25)35-24(28)14-4-3-5-15(8-14)27(29)30/h3-8,10,16,20-21H,9,11-13H2,1-2H3/t16-,20-,21+,25+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Defence
Curated by ChEMBL
| Assay Description
Inhibition of human AChE using acetylthiocholine iodide as substrate measured for 1 min by spectrophotometric based Ellman's method |
J Nat Prod 83: 1359-1367 (2020)
Article DOI: 10.1021/acs.jnatprod.9b00561
BindingDB Entry DOI: 10.7270/Q2XG9VQ0 |
More data for this
Ligand-Target Pair | |
DNA topoisomerase 2-beta
(Homo sapiens (Human)) | BDBM50586360

(ADR-529 | CHEBI:50223 | Cardioxane | DEXRAZOXANE |...) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human TOP2B assessed as reduction in relaxation of supercoiled DNA using kDNA as substrate incubated for 30 min |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c02157
BindingDB Entry DOI: 10.7270/Q2QJ7N6V |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50541556

(CHEMBL4634465)Show SMILES [H][C@]12C[C@@H](OC)C=C[C@]11[C@@H](CN2Cc2c(OC)c3OCOc3cc12)OC(=O)c1ccccc1[N+]([O-])=O |r,c:6,@:11,TLB:24:9:23.13.12:1| Show InChI InChI=1S/C25H24N2O8/c1-31-14-7-8-25-17-10-19-23(34-13-33-19)22(32-2)16(17)11-26(20(25)9-14)12-21(25)35-24(28)15-5-3-4-6-18(15)27(29)30/h3-8,10,14,20-21H,9,11-13H2,1-2H3/t14-,20-,21+,25+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Defence
Curated by ChEMBL
| Assay Description
Inhibition of human AChE using acetylthiocholine iodide as substrate measured for 1 min by spectrophotometric based Ellman's method |
J Nat Prod 83: 1359-1367 (2020)
Article DOI: 10.1021/acs.jnatprod.9b00561
BindingDB Entry DOI: 10.7270/Q2XG9VQ0 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50541562

(CHEMBL4640945)Show SMILES [H][C@]12C[C@@H](OC)C=C[C@]11[C@@H](CN2Cc2c(OC)c3OCOc3cc12)OC(=O)c1ccccc1C |r,c:6,@:11,TLB:24:9:23.13.12:1| Show InChI InChI=1S/C26H27NO6/c1-15-6-4-5-7-17(15)25(28)33-22-13-27-12-18-19(11-20-24(23(18)30-3)32-14-31-20)26(22)9-8-16(29-2)10-21(26)27/h4-9,11,16,21-22H,10,12-14H2,1-3H3/t16-,21-,22+,26+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Defence
Curated by ChEMBL
| Assay Description
Inhibition of human AChE using acetylthiocholine iodide as substrate measured for 1 min by spectrophotometric based Ellman's method |
J Nat Prod 83: 1359-1367 (2020)
Article DOI: 10.1021/acs.jnatprod.9b00561
BindingDB Entry DOI: 10.7270/Q2XG9VQ0 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50579848

(CHEMBL5086177)Show SMILES [H][C@]12C[C@H](OC(=O)c3ccccc3[N+]([O-])=O)C=C[C@@]11CCN2Cc2cc3OCOc3cc12 |r,c:17| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human AChE using acetylthiocholine as substrate by Ellman's method |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128374
BindingDB Entry DOI: 10.7270/Q2QJ7N4Z |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50541555

(CHEMBL4646747)Show SMILES [H][C@]12C[C@@H](OC)C=C[C@]11[C@@H](CN2Cc2c(OC)c3OCOc3cc12)OC(=O)c1cccc(c1)[N+]([O-])=O |r,c:6,@:11,TLB:24:9:23.13.12:1| Show InChI InChI=1S/C25H24N2O8/c1-31-16-6-7-25-18-10-19-23(34-13-33-19)22(32-2)17(18)11-26(20(25)9-16)12-21(25)35-24(28)14-4-3-5-15(8-14)27(29)30/h3-8,10,16,20-21H,9,11-13H2,1-2H3/t16-,20-,21+,25+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 8.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Defence
Curated by ChEMBL
| Assay Description
Inhibition of human BuChE using butyrylthiocholine iodide as substrate measured for 1 min by spectrophotometric based Ellman's method |
J Nat Prod 83: 1359-1367 (2020)
Article DOI: 10.1021/acs.jnatprod.9b00561
BindingDB Entry DOI: 10.7270/Q2XG9VQ0 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50579847

(CHEMBL5090336)Show SMILES [H][C@]12C[C@H](O)C=C[C@@]11[C@H](CN2Cc2cc3OCOc3cc12)OC(=O)c1ccccc1OC |r,c:5,TLB:13:12:1:9.8,19:20:1:9.8| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 9.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human AChE using acetylthiocholine as substrate by Ellman's method |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128374
BindingDB Entry DOI: 10.7270/Q2QJ7N4Z |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50579827
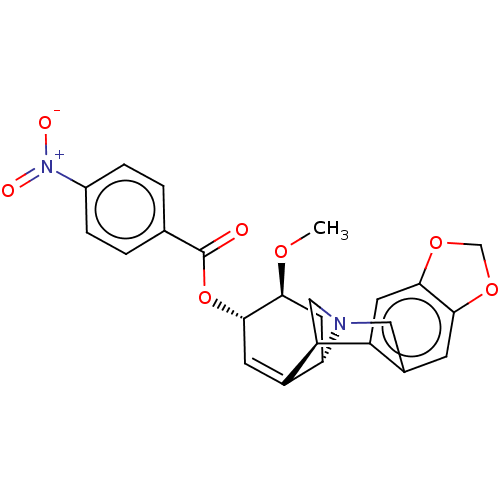
(CHEMBL5078705)Show SMILES [H][C@]12C[C@H](OC)[C@@H](OC(=O)c3ccc(cc3)[N+]([O-])=O)C=C1[C@@]1([H])CN2Cc2cc3OCOc3cc12 |r,c:20| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human AChE using acetylthiocholine as substrate by Ellman's method |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128374
BindingDB Entry DOI: 10.7270/Q2QJ7N4Z |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50579832
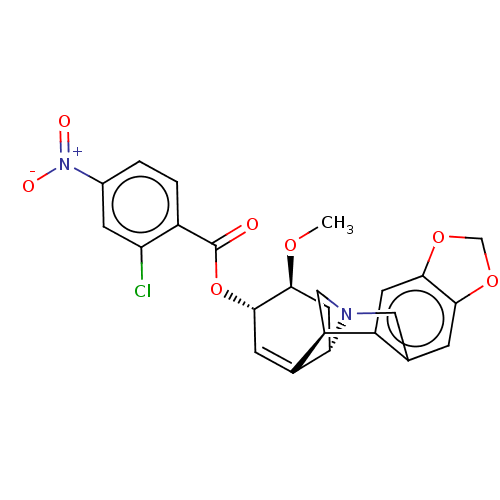
(CHEMBL5078153)Show SMILES [H][C@]12C[C@H](OC)[C@@H](OC(=O)c3ccc(cc3Cl)[N+]([O-])=O)C=C1[C@@]1([H])CN2Cc2cc3OCOc3cc12 |r,c:21| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human AChE using acetylthiocholine as substrate by Ellman's method |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128374
BindingDB Entry DOI: 10.7270/Q2QJ7N4Z |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50579833
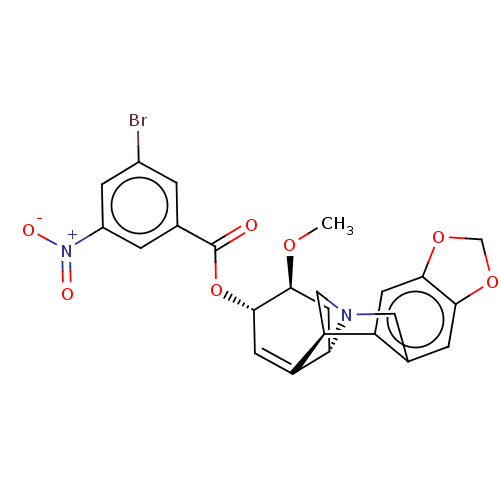
(CHEMBL5084173)Show SMILES [H][C@]12C[C@H](OC)[C@@H](OC(=O)c3cc(Br)cc(c3)[N+]([O-])=O)C=C1[C@@]1([H])CN2Cc2cc3OCOc3cc12 |r,c:21| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human AChE using acetylthiocholine as substrate by Ellman's method |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128374
BindingDB Entry DOI: 10.7270/Q2QJ7N4Z |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50579834
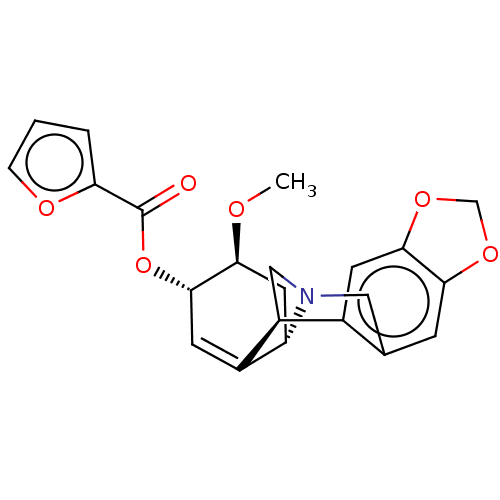
(CHEMBL5087001)Show SMILES [H][C@]12C[C@H](OC)[C@@H](OC(=O)c3ccco3)C=C1[C@@]1([H])CN2Cc2cc3OCOc3cc12 |r,c:16| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human AChE using acetylthiocholine as substrate by Ellman's method |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128374
BindingDB Entry DOI: 10.7270/Q2QJ7N4Z |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50579835
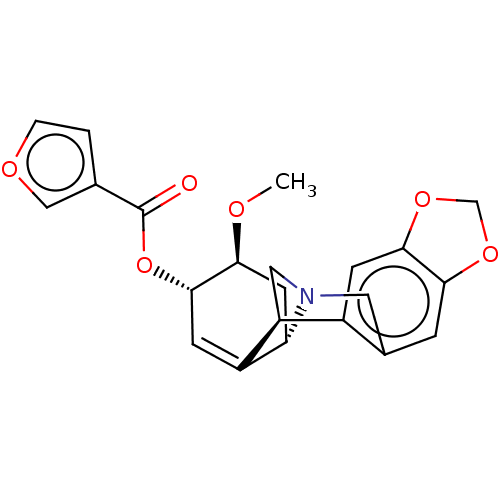
(CHEMBL5077463)Show SMILES [H][C@]12C[C@H](OC)[C@@H](OC(=O)c3ccoc3)C=C1[C@@]1([H])CN2Cc2cc3OCOc3cc12 |r,c:16| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human AChE using acetylthiocholine as substrate by Ellman's method |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128374
BindingDB Entry DOI: 10.7270/Q2QJ7N4Z |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50579836
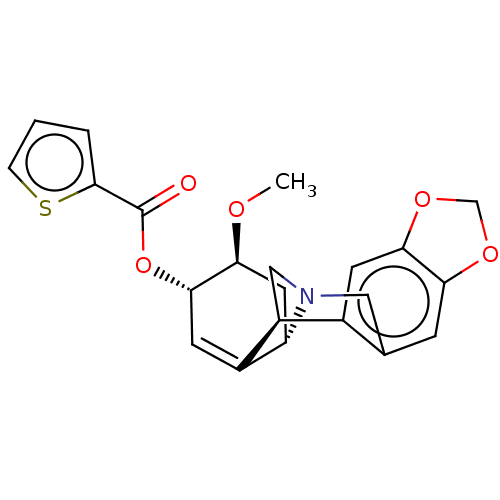
(CHEMBL5077388)Show SMILES [H][C@]12C[C@H](OC)[C@@H](OC(=O)c3cccs3)C=C1[C@@]1([H])CN2Cc2cc3OCOc3cc12 |r,c:16| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human AChE using acetylthiocholine as substrate by Ellman's method |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128374
BindingDB Entry DOI: 10.7270/Q2QJ7N4Z |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data