 Found 178 hits with Last Name = 'kuramoto' and Initial = 's'
Found 178 hits with Last Name = 'kuramoto' and Initial = 's' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
High affinity nerve growth factor receptor
(Homo sapiens (Human)) | BDBM50593050
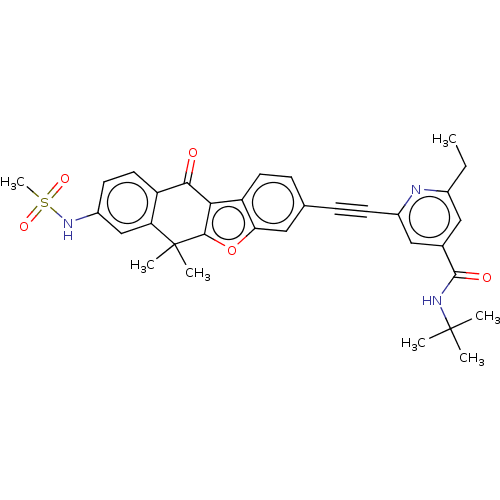
(CHEMBL5174525)Show SMILES CCc1cc(cc(n1)C#Cc1ccc2c3c(oc2c1)C(C)(C)c1cc(NS(C)(=O)=O)ccc1C3=O)C(=O)NC(C)(C)C | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01099
BindingDB Entry DOI: 10.7270/Q2FB56Z1 |
More data for this
Ligand-Target Pair | |
High affinity nerve growth factor receptor
(Homo sapiens (Human)) | BDBM50593047
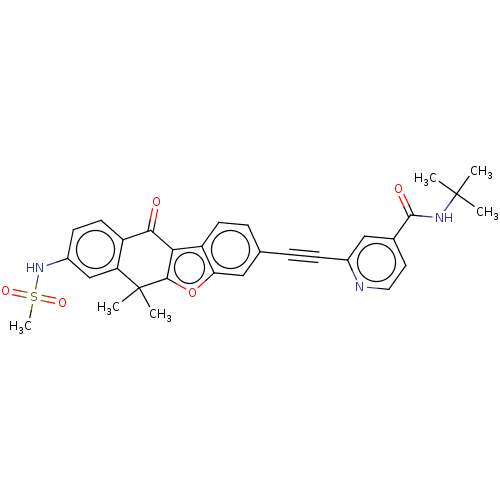
(CHEMBL5172448)Show SMILES CC(C)(C)NC(=O)c1ccnc(c1)C#Cc1ccc2c3c(oc2c1)C(C)(C)c1cc(NS(C)(=O)=O)ccc1C3=O | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01099
BindingDB Entry DOI: 10.7270/Q2FB56Z1 |
More data for this
Ligand-Target Pair | |
High affinity nerve growth factor receptor
(Homo sapiens (Human)) | BDBM50593051
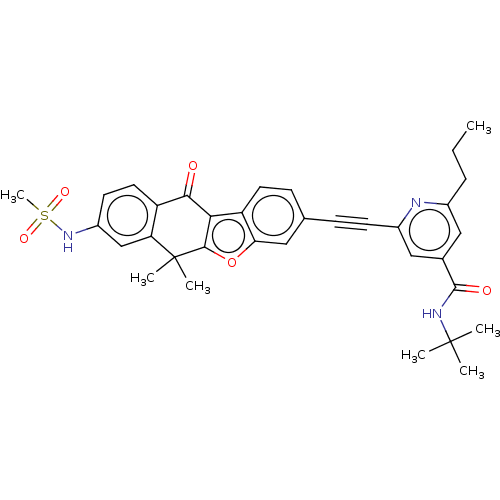
(CHEMBL5171942)Show SMILES CCCc1cc(cc(n1)C#Cc1ccc2c3c(oc2c1)C(C)(C)c1cc(NS(C)(=O)=O)ccc1C3=O)C(=O)NC(C)(C)C | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01099
BindingDB Entry DOI: 10.7270/Q2FB56Z1 |
More data for this
Ligand-Target Pair | |
High affinity nerve growth factor receptor
(Homo sapiens (Human)) | BDBM50525707

(CHEMBL4457566)Show SMILES Cc1cc(cc(n1)C#Cc1ccc2c3c(oc2c1)C(C)(C)c1cc(NS(C)(=O)=O)ccc1C3=O)C(=O)NC(C)(C)C Show InChI InChI=1S/C32H31N3O5S/c1-18-14-20(30(37)34-31(2,3)4)16-21(33-18)10-8-19-9-12-24-26(15-19)40-29-27(24)28(36)23-13-11-22(35-41(7,38)39)17-25(23)32(29,5)6/h9,11-17,35H,1-7H3,(H,34,37) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01099
BindingDB Entry DOI: 10.7270/Q2FB56Z1 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
High affinity nerve growth factor receptor
(Homo sapiens (Human)) | BDBM50593035

(CHEMBL5183149)Show SMILES CC1(C)c2oc3cc(ccc3c2C(=O)c2ccc(OCCN3CCOCC3)cc12)C#Cc1cc(ccn1)C(=O)NC1CC1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01099
BindingDB Entry DOI: 10.7270/Q2FB56Z1 |
More data for this
Ligand-Target Pair | |
High affinity nerve growth factor receptor
(Homo sapiens (Human)) | BDBM50593043
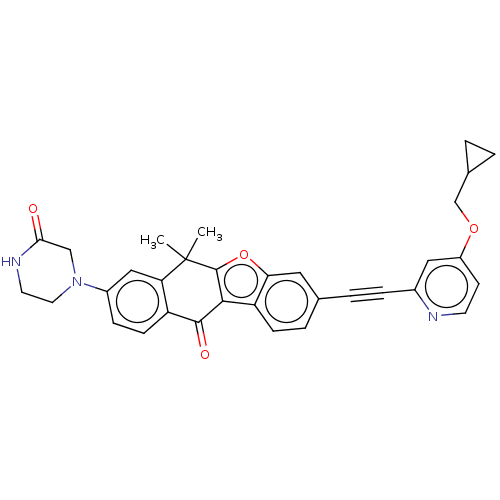
(CHEMBL5189763)Show SMILES CC1(C)c2oc3cc(ccc3c2C(=O)c2ccc(cc12)N1CCNC(=O)C1)C#Cc1cc(OCC2CC2)ccn1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01099
BindingDB Entry DOI: 10.7270/Q2FB56Z1 |
More data for this
Ligand-Target Pair | |
BDNF/NT-3 growth factors receptor
(Homo sapiens (Human)) | BDBM50525707

(CHEMBL4457566)Show SMILES Cc1cc(cc(n1)C#Cc1ccc2c3c(oc2c1)C(C)(C)c1cc(NS(C)(=O)=O)ccc1C3=O)C(=O)NC(C)(C)C Show InChI InChI=1S/C32H31N3O5S/c1-18-14-20(30(37)34-31(2,3)4)16-21(33-18)10-8-19-9-12-24-26(15-19)40-29-27(24)28(36)23-13-11-22(35-41(7,38)39)17-25(23)32(29,5)6/h9,11-17,35H,1-7H3,(H,34,37) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01099
BindingDB Entry DOI: 10.7270/Q2FB56Z1 |
More data for this
Ligand-Target Pair | |
High affinity nerve growth factor receptor
(Homo sapiens (Human)) | BDBM50593031

(CHEMBL5191460)Show SMILES CC1(C)c2oc3cc(ccc3c2C(=O)c2ccc(OCCN3CCOCC3)cc12)C#Cc1cc(OCCC2CC2)ccn1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01099
BindingDB Entry DOI: 10.7270/Q2FB56Z1 |
More data for this
Ligand-Target Pair | |
High affinity nerve growth factor receptor
(Homo sapiens (Human)) | BDBM50593029
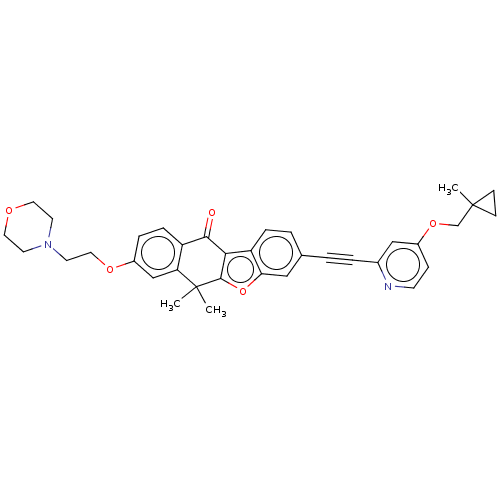
(CHEMBL5195158)Show SMILES CC1(COc2ccnc(c2)C#Cc2ccc3c4c(oc3c2)C(C)(C)c2cc(OCCN3CCOCC3)ccc2C4=O)CC1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01099
BindingDB Entry DOI: 10.7270/Q2FB56Z1 |
More data for this
Ligand-Target Pair | |
High affinity nerve growth factor receptor
(Homo sapiens (Human)) | BDBM50593039

(CHEMBL5199099)Show SMILES CC1(C)c2oc3cc(ccc3c2C(=O)c2ccc(OCCN3CCNC(=O)C3)cc12)C#Cc1cc(OCC2CC2)ccn1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01099
BindingDB Entry DOI: 10.7270/Q2FB56Z1 |
More data for this
Ligand-Target Pair | |
High affinity nerve growth factor receptor
(Homo sapiens (Human)) | BDBM50593045
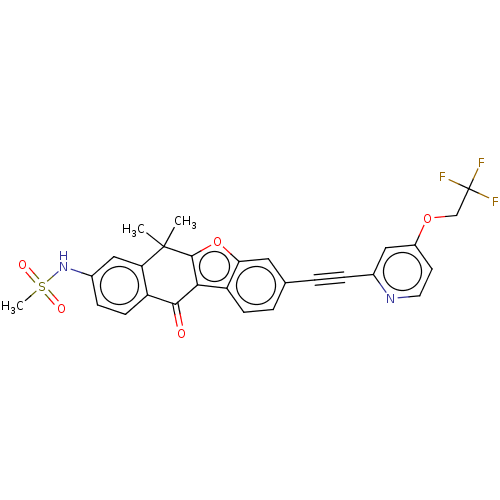
(CHEMBL5205778)Show SMILES CC1(C)c2oc3cc(ccc3c2C(=O)c2ccc(NS(C)(=O)=O)cc12)C#Cc1cc(OCC(F)(F)F)ccn1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01099
BindingDB Entry DOI: 10.7270/Q2FB56Z1 |
More data for this
Ligand-Target Pair | |
High affinity nerve growth factor receptor
(Homo sapiens (Human)) | BDBM50593053
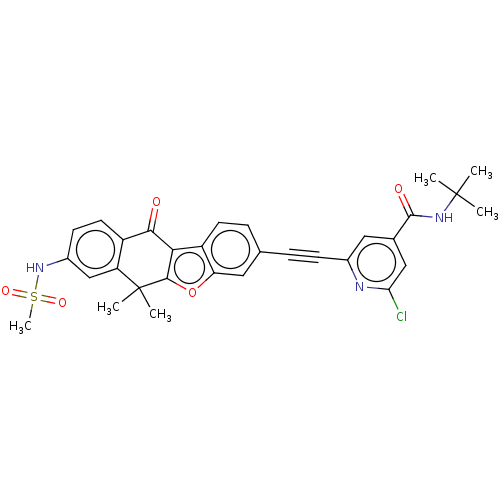
(CHEMBL5198357)Show SMILES CC(C)(C)NC(=O)c1cc(Cl)nc(c1)C#Cc1ccc2c3c(oc2c1)C(C)(C)c1cc(NS(C)(=O)=O)ccc1C3=O | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01099
BindingDB Entry DOI: 10.7270/Q2FB56Z1 |
More data for this
Ligand-Target Pair | |
High affinity nerve growth factor receptor
(Homo sapiens (Human)) | BDBM50593046

(CHEMBL5199353)Show SMILES CC1(C)c2oc3cc(ccc3c2C(=O)c2ccc(NS(C)(=O)=O)cc12)C#Cc1cc(ccn1)C(=O)NC1CC1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01099
BindingDB Entry DOI: 10.7270/Q2FB56Z1 |
More data for this
Ligand-Target Pair | |
High affinity nerve growth factor receptor
(Homo sapiens (Human)) | BDBM50593052
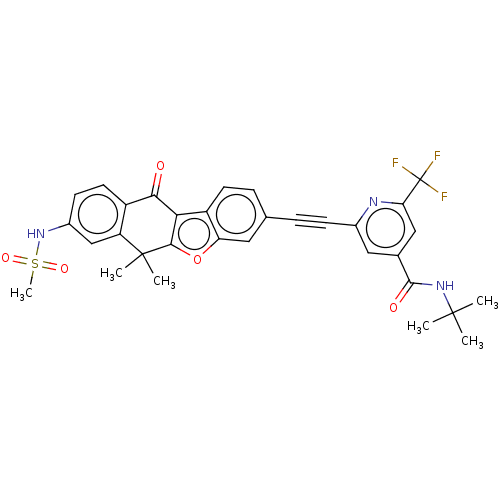
(CHEMBL5178903)Show SMILES CC(C)(C)NC(=O)c1cc(nc(c1)C(F)(F)F)C#Cc1ccc2c3c(oc2c1)C(C)(C)c1cc(NS(C)(=O)=O)ccc1C3=O | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01099
BindingDB Entry DOI: 10.7270/Q2FB56Z1 |
More data for this
Ligand-Target Pair | |
High affinity nerve growth factor receptor
(Homo sapiens (Human)) | BDBM50593030
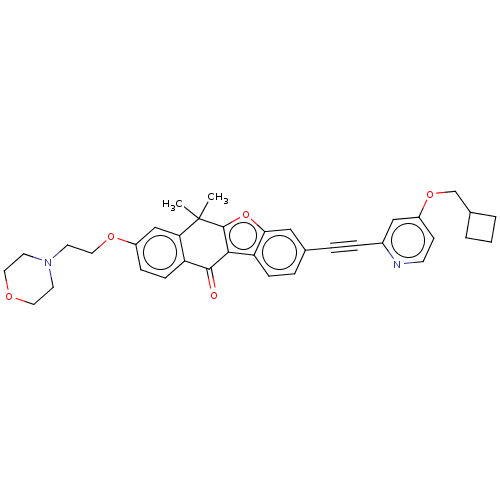
(CHEMBL5193231)Show SMILES CC1(C)c2oc3cc(ccc3c2C(=O)c2ccc(OCCN3CCOCC3)cc12)C#Cc1cc(OCC2CCC2)ccn1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01099
BindingDB Entry DOI: 10.7270/Q2FB56Z1 |
More data for this
Ligand-Target Pair | |
High affinity nerve growth factor receptor
(Homo sapiens (Human)) | BDBM50593054
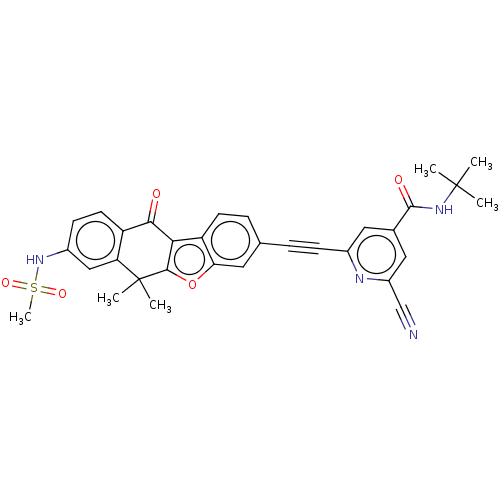
(CHEMBL5173139)Show SMILES CC(C)(C)NC(=O)c1cc(nc(c1)C#Cc1ccc2c3c(oc2c1)C(C)(C)c1cc(NS(C)(=O)=O)ccc1C3=O)C#N | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01099
BindingDB Entry DOI: 10.7270/Q2FB56Z1 |
More data for this
Ligand-Target Pair | |
High affinity nerve growth factor receptor
(Homo sapiens (Human)) | BDBM50593037

(CHEMBL5171199)Show SMILES CC1(C)c2oc3cc(ccc3c2C(=O)c2ccc(OCCN3CCCOCC3)cc12)C#Cc1cc(OCC2CC2)ccn1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01099
BindingDB Entry DOI: 10.7270/Q2FB56Z1 |
More data for this
Ligand-Target Pair | |
NT-3 growth factor receptor
(Homo sapiens (Human)) | BDBM50525707

(CHEMBL4457566)Show SMILES Cc1cc(cc(n1)C#Cc1ccc2c3c(oc2c1)C(C)(C)c1cc(NS(C)(=O)=O)ccc1C3=O)C(=O)NC(C)(C)C Show InChI InChI=1S/C32H31N3O5S/c1-18-14-20(30(37)34-31(2,3)4)16-21(33-18)10-8-19-9-12-24-26(15-19)40-29-27(24)28(36)23-13-11-22(35-41(7,38)39)17-25(23)32(29,5)6/h9,11-17,35H,1-7H3,(H,34,37) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01099
BindingDB Entry DOI: 10.7270/Q2FB56Z1 |
More data for this
Ligand-Target Pair | |
High affinity nerve growth factor receptor
(Homo sapiens (Human)) | BDBM50593042
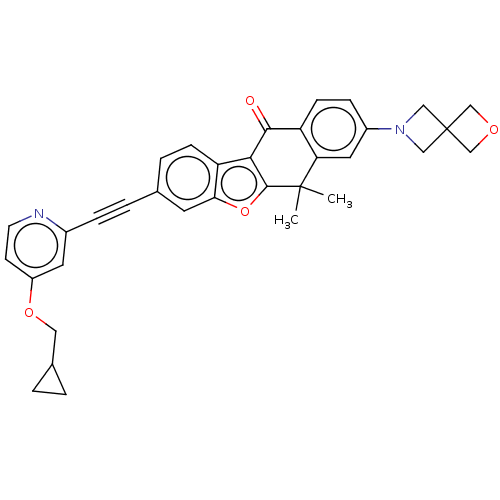
(CHEMBL5169941)Show SMILES CC1(C)c2oc3cc(ccc3c2C(=O)c2ccc(cc12)N1CC2(COC2)C1)C#Cc1cc(OCC2CC2)ccn1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01099
BindingDB Entry DOI: 10.7270/Q2FB56Z1 |
More data for this
Ligand-Target Pair | |
High affinity nerve growth factor receptor
(Homo sapiens (Human)) | BDBM50593033

(CHEMBL5173016)Show SMILES CC1(C)c2oc3cc(ccc3c2C(=O)c2ccc(OCCN3CCOCC3)cc12)C#Cc1cc(OCC2(CO)CC2)ccn1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01099
BindingDB Entry DOI: 10.7270/Q2FB56Z1 |
More data for this
Ligand-Target Pair | |
High affinity nerve growth factor receptor
(Homo sapiens (Human)) | BDBM50593032
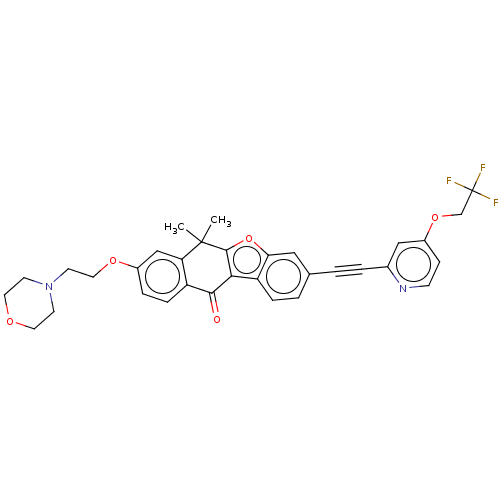
(CHEMBL5171966)Show SMILES CC1(C)c2oc3cc(ccc3c2C(=O)c2ccc(OCCN3CCOCC3)cc12)C#Cc1cc(OCC(F)(F)F)ccn1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01099
BindingDB Entry DOI: 10.7270/Q2FB56Z1 |
More data for this
Ligand-Target Pair | |
High affinity nerve growth factor receptor
(Homo sapiens (Human)) | BDBM50593041
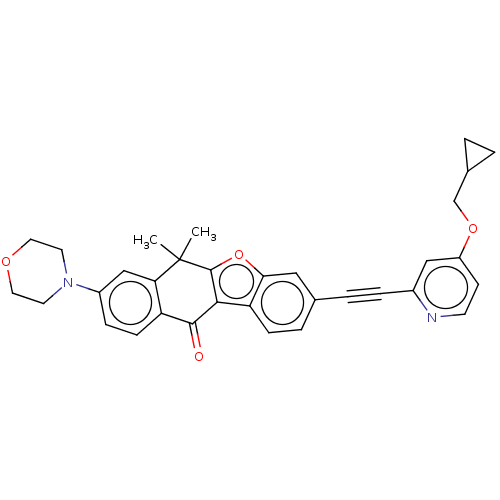
(CHEMBL5198427)Show SMILES CC1(C)c2oc3cc(ccc3c2C(=O)c2ccc(cc12)N1CCOCC1)C#Cc1cc(OCC2CC2)ccn1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01099
BindingDB Entry DOI: 10.7270/Q2FB56Z1 |
More data for this
Ligand-Target Pair | |
High affinity nerve growth factor receptor
(Homo sapiens (Human)) | BDBM50593049
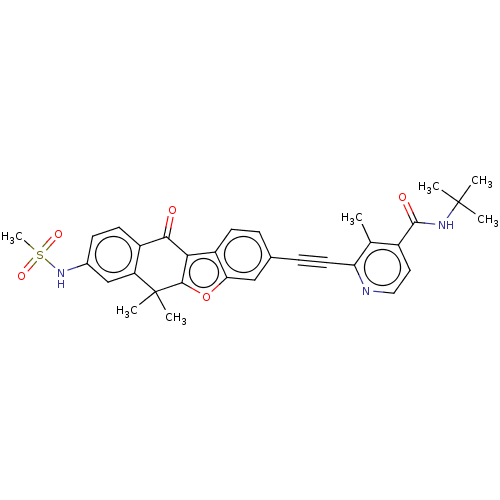
(CHEMBL5203355)Show SMILES Cc1c(ccnc1C#Cc1ccc2c3c(oc2c1)C(C)(C)c1cc(NS(C)(=O)=O)ccc1C3=O)C(=O)NC(C)(C)C | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01099
BindingDB Entry DOI: 10.7270/Q2FB56Z1 |
More data for this
Ligand-Target Pair | |
High affinity nerve growth factor receptor
(Homo sapiens (Human)) | BDBM50593048
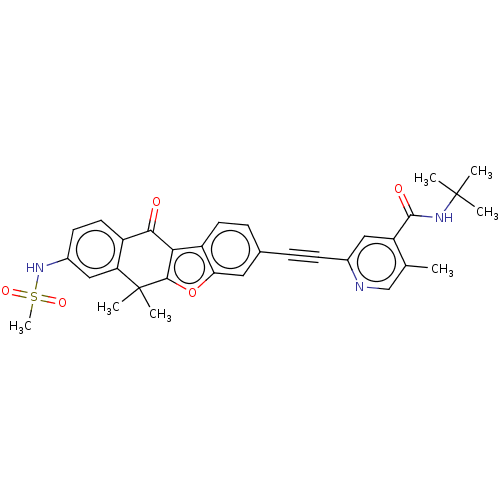
(CHEMBL5174514)Show SMILES Cc1cnc(cc1C(=O)NC(C)(C)C)C#Cc1ccc2c3c(oc2c1)C(C)(C)c1cc(NS(C)(=O)=O)ccc1C3=O | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01099
BindingDB Entry DOI: 10.7270/Q2FB56Z1 |
More data for this
Ligand-Target Pair | |
High affinity nerve growth factor receptor
(Homo sapiens (Human)) | BDBM50593044
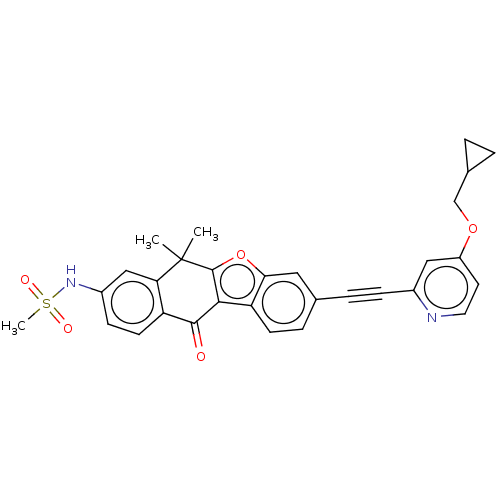
(CHEMBL5199002)Show SMILES CC1(C)c2oc3cc(ccc3c2C(=O)c2ccc(NS(C)(=O)=O)cc12)C#Cc1cc(OCC2CC2)ccn1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01099
BindingDB Entry DOI: 10.7270/Q2FB56Z1 |
More data for this
Ligand-Target Pair | |
High affinity nerve growth factor receptor
(Homo sapiens (Human)) | BDBM50593038
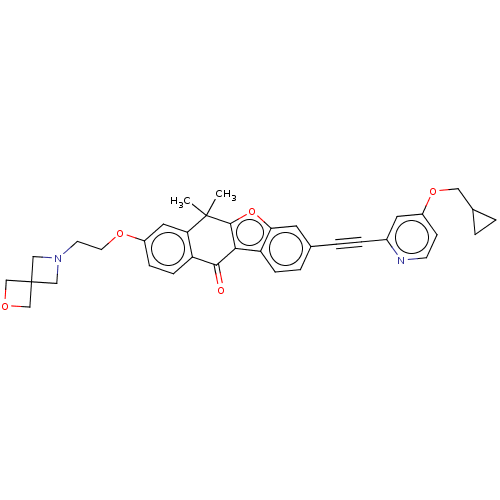
(CHEMBL5197535)Show SMILES CC1(C)c2oc3cc(ccc3c2C(=O)c2ccc(OCCN3CC4(COC4)C3)cc12)C#Cc1cc(OCC2CC2)ccn1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01099
BindingDB Entry DOI: 10.7270/Q2FB56Z1 |
More data for this
Ligand-Target Pair | |
High affinity nerve growth factor receptor
(Homo sapiens (Human)) | BDBM50593027
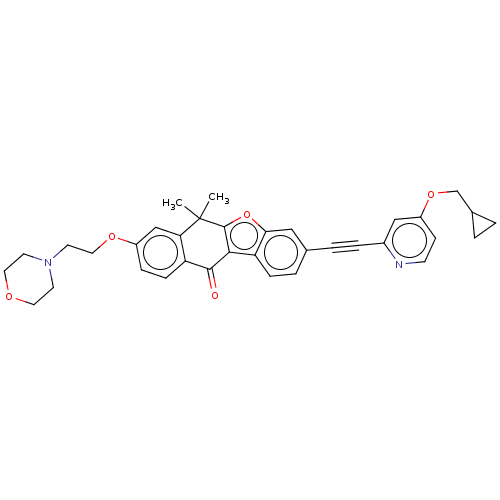
(CHEMBL5186340)Show SMILES CC1(C)c2oc3cc(ccc3c2C(=O)c2ccc(OCCN3CCOCC3)cc12)C#Cc1cc(OCC2CC2)ccn1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01099
BindingDB Entry DOI: 10.7270/Q2FB56Z1 |
More data for this
Ligand-Target Pair | |
NT-3 growth factor receptor
(Homo sapiens (Human)) | BDBM50593027

(CHEMBL5186340)Show SMILES CC1(C)c2oc3cc(ccc3c2C(=O)c2ccc(OCCN3CCOCC3)cc12)C#Cc1cc(OCC2CC2)ccn1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01099
BindingDB Entry DOI: 10.7270/Q2FB56Z1 |
More data for this
Ligand-Target Pair | |
BDNF/NT-3 growth factors receptor
(Homo sapiens (Human)) | BDBM50593027

(CHEMBL5186340)Show SMILES CC1(C)c2oc3cc(ccc3c2C(=O)c2ccc(OCCN3CCOCC3)cc12)C#Cc1cc(OCC2CC2)ccn1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 8.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01099
BindingDB Entry DOI: 10.7270/Q2FB56Z1 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50338198

(3-(2-morpholino-7-(pyridin-4-yl)-6,7-dihydro-5H-py...)Show SMILES Oc1cccc(c1)-c1nc(nc2N(CCc12)c1ccncc1)N1CCOCC1 Show InChI InChI=1S/C21H21N5O2/c27-17-3-1-2-15(14-17)19-18-6-9-26(16-4-7-22-8-5-16)20(18)24-21(23-19)25-10-12-28-13-11-25/h1-5,7-8,14,27H,6,9-13H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 8.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Chugai Pharmaceutical Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha |
Bioorg Med Chem Lett 21: 1767-72 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.065
BindingDB Entry DOI: 10.7270/Q23B60F3 |
More data for this
Ligand-Target Pair | |
High affinity nerve growth factor receptor
(Homo sapiens (Human)) | BDBM50593040
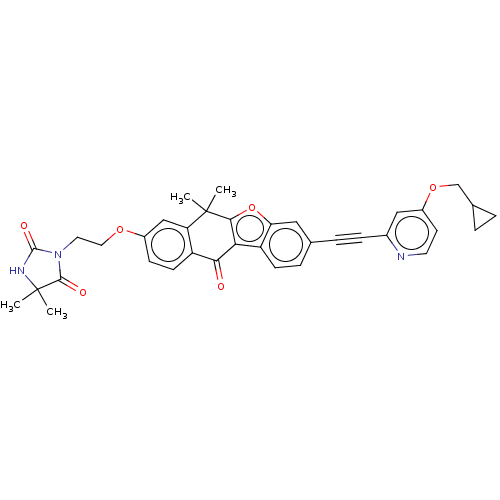
(CHEMBL5189733)Show SMILES CC1(C)NC(=O)N(CCOc2ccc3C(=O)c4c(oc5cc(ccc45)C#Cc4cc(OCC5CC5)ccn4)C(C)(C)c3c2)C1=O | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01099
BindingDB Entry DOI: 10.7270/Q2FB56Z1 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50338197
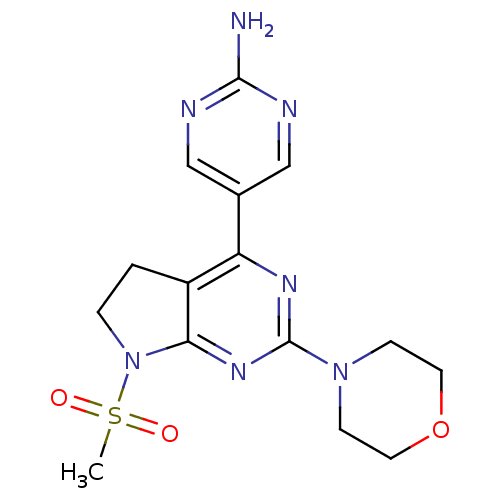
(5-(7-(methylsulfonyl)-2-morpholino-6,7-dihydro-5H-...)Show SMILES CS(=O)(=O)N1CCc2c1nc(nc2-c1cnc(N)nc1)N1CCOCC1 Show InChI InChI=1S/C15H19N7O3S/c1-26(23,24)22-3-2-11-12(10-8-17-14(16)18-9-10)19-15(20-13(11)22)21-4-6-25-7-5-21/h8-9H,2-7H2,1H3,(H2,16,17,18) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Chugai Pharmaceutical Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha |
Bioorg Med Chem Lett 21: 1767-72 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.065
BindingDB Entry DOI: 10.7270/Q23B60F3 |
More data for this
Ligand-Target Pair | |
High affinity nerve growth factor receptor
(Homo sapiens (Human)) | BDBM50593034

(CHEMBL5202910)Show SMILES COCCOc1ccnc(c1)C#Cc1ccc2c3c(oc2c1)C(C)(C)c1cc(OCCN2CCOCC2)ccc1C3=O | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01099
BindingDB Entry DOI: 10.7270/Q2FB56Z1 |
More data for this
Ligand-Target Pair | |
High affinity nerve growth factor receptor
(Homo sapiens (Human)) | BDBM50593036
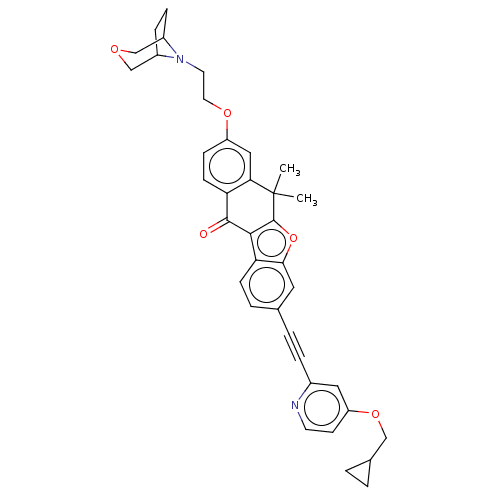
(CHEMBL5180301)Show SMILES CC1(C)c2oc3cc(ccc3c2C(=O)c2ccc(OCCN3C4CCC3COC4)cc12)C#Cc1cc(OCC2CC2)ccn1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01099
BindingDB Entry DOI: 10.7270/Q2FB56Z1 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lck
(Homo sapiens (Human)) | BDBM50593038

(CHEMBL5197535)Show SMILES CC1(C)c2oc3cc(ccc3c2C(=O)c2ccc(OCCN3CC4(COC4)C3)cc12)C#Cc1cc(OCC2CC2)ccn1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01099
BindingDB Entry DOI: 10.7270/Q2FB56Z1 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50338199

(5-(2-Morpholin-4-yl-7-pyridin-3-yl-6,7-dihydro-5H-...)Show SMILES Nc1ncc(cn1)-c1nc(nc2N(CCc12)c1cccnc1)N1CCOCC1 Show InChI InChI=1S/C19H20N8O/c20-18-22-10-13(11-23-18)16-15-3-5-27(14-2-1-4-21-12-14)17(15)25-19(24-16)26-6-8-28-9-7-26/h1-2,4,10-12H,3,5-9H2,(H2,20,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 33 | n/a | n/a | n/a | n/a | n/a | n/a |
Chugai Pharmaceutical Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha |
Bioorg Med Chem Lett 21: 1767-72 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.065
BindingDB Entry DOI: 10.7270/Q23B60F3 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50338197

(5-(7-(methylsulfonyl)-2-morpholino-6,7-dihydro-5H-...)Show SMILES CS(=O)(=O)N1CCc2c1nc(nc2-c1cnc(N)nc1)N1CCOCC1 Show InChI InChI=1S/C15H19N7O3S/c1-26(23,24)22-3-2-11-12(10-8-17-14(16)18-9-10)19-15(20-13(11)22)21-4-6-25-7-5-21/h8-9H,2-7H2,1H3,(H2,16,17,18) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
| PDB
Article
PubMed
| n/a | n/a | 36 | n/a | n/a | n/a | n/a | n/a | n/a |
Chugai Pharmaceutical Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kgamma |
Bioorg Med Chem Lett 21: 1767-72 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.065
BindingDB Entry DOI: 10.7270/Q23B60F3 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50338200

(3-(2-morpholino-7-(pyridin-3-yl)-6,7-dihydro-5H-py...)Show SMILES Oc1cccc(c1)-c1nc(nc2N(CCc12)c1cccnc1)N1CCOCC1 Show InChI InChI=1S/C21H21N5O2/c27-17-5-1-3-15(13-17)19-18-6-8-26(16-4-2-7-22-14-16)20(18)24-21(23-19)25-9-11-28-12-10-25/h1-5,7,13-14,27H,6,8-12H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 41 | n/a | n/a | n/a | n/a | n/a | n/a |
Chugai Pharmaceutical Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha |
Bioorg Med Chem Lett 21: 1767-72 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.065
BindingDB Entry DOI: 10.7270/Q23B60F3 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lck
(Homo sapiens (Human)) | BDBM50593043

(CHEMBL5189763)Show SMILES CC1(C)c2oc3cc(ccc3c2C(=O)c2ccc(cc12)N1CCNC(=O)C1)C#Cc1cc(OCC2CC2)ccn1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01099
BindingDB Entry DOI: 10.7270/Q2FB56Z1 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lck
(Homo sapiens (Human)) | BDBM50593030

(CHEMBL5193231)Show SMILES CC1(C)c2oc3cc(ccc3c2C(=O)c2ccc(OCCN3CCOCC3)cc12)C#Cc1cc(OCC2CCC2)ccn1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 53 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01099
BindingDB Entry DOI: 10.7270/Q2FB56Z1 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lck
(Homo sapiens (Human)) | BDBM50593054

(CHEMBL5173139)Show SMILES CC(C)(C)NC(=O)c1cc(nc(c1)C#Cc1ccc2c3c(oc2c1)C(C)(C)c1cc(NS(C)(=O)=O)ccc1C3=O)C#N | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 58 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01099
BindingDB Entry DOI: 10.7270/Q2FB56Z1 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lck
(Homo sapiens (Human)) | BDBM50593039

(CHEMBL5199099)Show SMILES CC1(C)c2oc3cc(ccc3c2C(=O)c2ccc(OCCN3CCNC(=O)C3)cc12)C#Cc1cc(OCC2CC2)ccn1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 68 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01099
BindingDB Entry DOI: 10.7270/Q2FB56Z1 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50338196

(3-(7-(1H-benzo[d]imidazol-6-yl)-2-morpholino-6,7-d...)Show SMILES Oc1cccc(c1)-c1nc(nc2N(CCc12)c1ccc2nc[nH]c2c1)N1CCOCC1 Show InChI InChI=1S/C23H22N6O2/c30-17-3-1-2-15(12-17)21-18-6-7-29(16-4-5-19-20(13-16)25-14-24-19)22(18)27-23(26-21)28-8-10-31-11-9-28/h1-5,12-14,30H,6-11H2,(H,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 77 | n/a | n/a | n/a | n/a | n/a | n/a |
Chugai Pharmaceutical Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha |
Bioorg Med Chem Lett 21: 1767-72 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.065
BindingDB Entry DOI: 10.7270/Q23B60F3 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lck
(Homo sapiens (Human)) | BDBM50593042

(CHEMBL5169941)Show SMILES CC1(C)c2oc3cc(ccc3c2C(=O)c2ccc(cc12)N1CC2(COC2)C1)C#Cc1cc(OCC2CC2)ccn1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 86 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01099
BindingDB Entry DOI: 10.7270/Q2FB56Z1 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lck
(Homo sapiens (Human)) | BDBM50593051

(CHEMBL5171942)Show SMILES CCCc1cc(cc(n1)C#Cc1ccc2c3c(oc2c1)C(C)(C)c1cc(NS(C)(=O)=O)ccc1C3=O)C(=O)NC(C)(C)C | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 96 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01099
BindingDB Entry DOI: 10.7270/Q2FB56Z1 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lck
(Homo sapiens (Human)) | BDBM50593037

(CHEMBL5171199)Show SMILES CC1(C)c2oc3cc(ccc3c2C(=O)c2ccc(OCCN3CCCOCC3)cc12)C#Cc1cc(OCC2CC2)ccn1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 96 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01099
BindingDB Entry DOI: 10.7270/Q2FB56Z1 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM50338197

(5-(7-(methylsulfonyl)-2-morpholino-6,7-dihydro-5H-...)Show SMILES CS(=O)(=O)N1CCc2c1nc(nc2-c1cnc(N)nc1)N1CCOCC1 Show InChI InChI=1S/C15H19N7O3S/c1-26(23,24)22-3-2-11-12(10-8-17-14(16)18-9-10)19-15(20-13(11)22)21-4-6-25-7-5-21/h8-9H,2-7H2,1H3,(H2,16,17,18) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
| Article
PubMed
| n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
Chugai Pharmaceutical Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kbeta |
Bioorg Med Chem Lett 21: 1767-72 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.065
BindingDB Entry DOI: 10.7270/Q23B60F3 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lck
(Homo sapiens (Human)) | BDBM50593052

(CHEMBL5178903)Show SMILES CC(C)(C)NC(=O)c1cc(nc(c1)C(F)(F)F)C#Cc1ccc2c3c(oc2c1)C(C)(C)c1cc(NS(C)(=O)=O)ccc1C3=O | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01099
BindingDB Entry DOI: 10.7270/Q2FB56Z1 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lck
(Homo sapiens (Human)) | BDBM50593047

(CHEMBL5172448)Show SMILES CC(C)(C)NC(=O)c1ccnc(c1)C#Cc1ccc2c3c(oc2c1)C(C)(C)c1cc(NS(C)(=O)=O)ccc1C3=O | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01099
BindingDB Entry DOI: 10.7270/Q2FB56Z1 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lck
(Homo sapiens (Human)) | BDBM50593040

(CHEMBL5189733)Show SMILES CC1(C)NC(=O)N(CCOc2ccc3C(=O)c4c(oc5cc(ccc45)C#Cc4cc(OCC5CC5)ccn4)C(C)(C)c3c2)C1=O | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01099
BindingDB Entry DOI: 10.7270/Q2FB56Z1 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data