 Found 166 hits with Last Name = 'kwan' and Initial = 'jc'
Found 166 hits with Last Name = 'kwan' and Initial = 'jc' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Cathepsin D
(Homo sapiens (Human)) | BDBM912

((3S,4S)-3-hydroxy-4-[(2S)-2-[(3S,4S)-3-hydroxy-6-m...)Show SMILES CC(C)C[C@H](NC(=O)[C@H](C)NC(=O)C[C@H](O)[C@H](CC(C)C)NC(=O)[C@@H](NC(=O)[C@@H](NC(=O)CC(C)C)C(C)C)C(C)C)[C@@H](O)CC(O)=O |r| Show InChI InChI=1S/C34H63N5O9/c1-17(2)12-23(37-33(47)31(21(9)10)39-34(48)30(20(7)8)38-27(42)14-19(5)6)25(40)15-28(43)35-22(11)32(46)36-24(13-18(3)4)26(41)16-29(44)45/h17-26,30-31,40-41H,12-16H2,1-11H3,(H,35,43)(H,36,46)(H,37,47)(H,38,42)(H,39,48)(H,44,45)/t22-,23-,24-,25-,26-,30-,31-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
Article
PubMed
| n/a | n/a | 0.173 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida
Curated by ChEMBL
| Assay Description
Inhibition of cathepsin D |
J Med Chem 52: 5732-47 (2009)
Article DOI: 10.1021/jm9009394
BindingDB Entry DOI: 10.7270/Q2BG2PXP |
More data for this
Ligand-Target Pair | |
Cathepsin E
(Homo sapiens (Human)) | BDBM912

((3S,4S)-3-hydroxy-4-[(2S)-2-[(3S,4S)-3-hydroxy-6-m...)Show SMILES CC(C)C[C@H](NC(=O)[C@H](C)NC(=O)C[C@H](O)[C@H](CC(C)C)NC(=O)[C@@H](NC(=O)[C@@H](NC(=O)CC(C)C)C(C)C)C(C)C)[C@@H](O)CC(O)=O |r| Show InChI InChI=1S/C34H63N5O9/c1-17(2)12-23(37-33(47)31(21(9)10)39-34(48)30(20(7)8)38-27(42)14-19(5)6)25(40)15-28(43)35-22(11)32(46)36-24(13-18(3)4)26(41)16-29(44)45/h17-26,30-31,40-41H,12-16H2,1-11H3,(H,35,43)(H,36,46)(H,37,47)(H,38,42)(H,39,48)(H,44,45)/t22-,23-,24-,25-,26-,30-,31-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.181 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida
Curated by ChEMBL
| Assay Description
Inhibition of cathepsin E |
J Med Chem 52: 5732-47 (2009)
Article DOI: 10.1021/jm9009394
BindingDB Entry DOI: 10.7270/Q2BG2PXP |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50055512
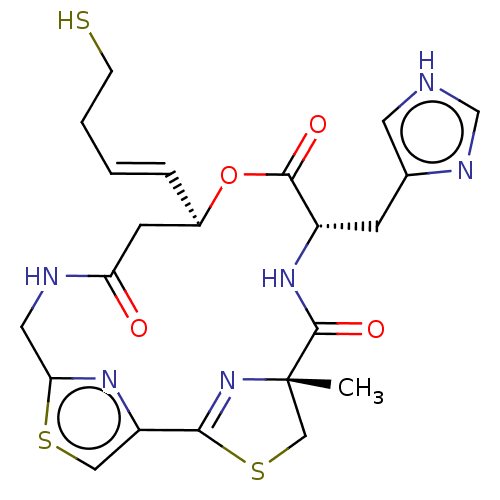
(CHEMBL3317812)Show SMILES C[C@@]12CSC(=N1)c1csc(CNC(=O)C[C@H](OC(=O)[C@H](Cc3c[nH]cn3)NC2=O)\C=C\CCS)n1 |r,c:4| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Duke University
Curated by ChEMBL
| Assay Description
Inhibition of HDAC1 (unknown origin) incubated at 37 degC for 30 mins |
Bioorg Med Chem Lett 24: 3728-31 (2014)
Article DOI: 10.1016/j.bmcl.2014.07.006
BindingDB Entry DOI: 10.7270/Q25Q4XRZ |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50055513
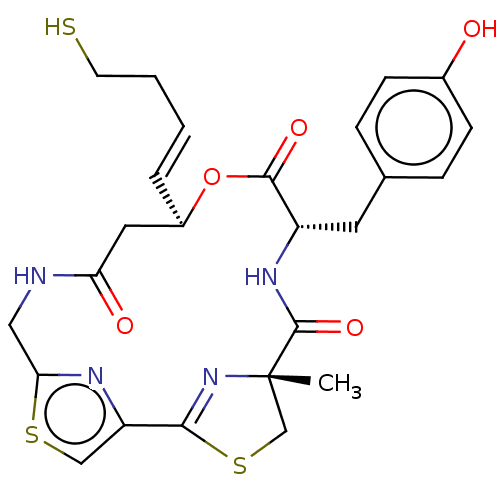
(CHEMBL3317811)Show SMILES C[C@@]12CSC(=N1)c1csc(CNC(=O)C[C@H](OC(=O)[C@H](Cc3ccc(O)cc3)NC2=O)\C=C\CCS)n1 |r,c:4| Show InChI InChI=1S/C25H28N4O5S3/c1-25-14-37-22(29-25)19-13-36-21(27-19)12-26-20(31)11-17(4-2-3-9-35)34-23(32)18(28-24(25)33)10-15-5-7-16(30)8-6-15/h2,4-8,13,17-18,30,35H,3,9-12,14H2,1H3,(H,26,31)(H,28,33)/b4-2+/t17-,18+,25+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.210 | n/a | n/a | n/a | n/a | n/a | n/a |
Duke University
Curated by ChEMBL
| Assay Description
Inhibition of HDAC1 (unknown origin) incubated at 37 degC for 30 mins |
Bioorg Med Chem Lett 24: 3728-31 (2014)
Article DOI: 10.1016/j.bmcl.2014.07.006
BindingDB Entry DOI: 10.7270/Q25Q4XRZ |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50055514
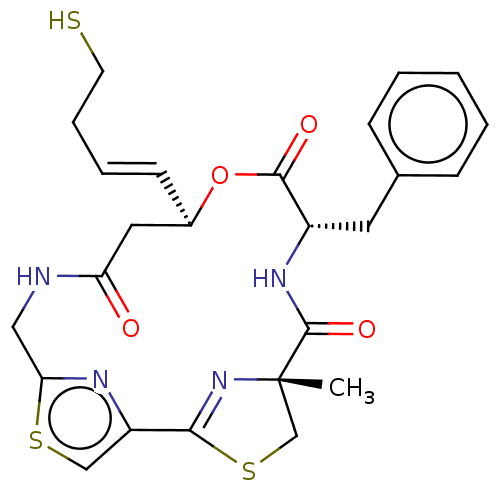
(CHEMBL3317810)Show SMILES C[C@@]12CSC(=N1)c1csc(CNC(=O)C[C@H](OC(=O)[C@H](Cc3ccccc3)NC2=O)\C=C\CCS)n1 |r,c:4| Show InChI InChI=1S/C25H28N4O4S3/c1-25-15-36-22(29-25)19-14-35-21(27-19)13-26-20(30)12-17(9-5-6-10-34)33-23(31)18(28-24(25)32)11-16-7-3-2-4-8-16/h2-5,7-9,14,17-18,34H,6,10-13,15H2,1H3,(H,26,30)(H,28,32)/b9-5+/t17-,18+,25+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.290 | n/a | n/a | n/a | n/a | n/a | n/a |
Duke University
Curated by ChEMBL
| Assay Description
Inhibition of HDAC1 (unknown origin) incubated at 37 degC for 30 mins |
Bioorg Med Chem Lett 24: 3728-31 (2014)
Article DOI: 10.1016/j.bmcl.2014.07.006
BindingDB Entry DOI: 10.7270/Q25Q4XRZ |
More data for this
Ligand-Target Pair | |
Cathepsin E
(Homo sapiens (Human)) | BDBM50302109

(CHEMBL568553 | Grassystatin B)Show SMILES CC[C@H](NC(=O)[C@@H](NC(=O)C[C@H](O)[C@H](CC(C)C)NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](OC(=O)[C@@H](OC(=O)[C@H](C(C)C)N(C)C)C(C)C)C(C)C)[C@@H](C)O)C(=O)N(C)[C@H](Cc1ccccc1)C(=O)N1CCC[C@H]1C(=O)OC |r| Show InChI InChI=1S/C59H97N9O16/c1-17-38(55(77)67(15)43(28-37-22-19-18-20-23-37)56(78)68-25-21-24-42(68)57(79)82-16)61-53(75)47(36(12)69)65-46(72)30-44(70)39(26-31(2)3)62-52(74)41(29-45(60)71)63-51(73)40(27-32(4)5)64-54(76)49(34(8)9)83-59(81)50(35(10)11)84-58(80)48(33(6)7)66(13)14/h18-20,22-23,31-36,38-44,47-50,69-70H,17,21,24-30H2,1-16H3,(H2,60,71)(H,61,75)(H,62,74)(H,63,73)(H,64,76)(H,65,72)/t36-,38+,39+,40+,41+,42+,43-,44+,47+,48+,49-,50+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.354 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida
Curated by ChEMBL
| Assay Description
Inhibition of cathepsin E |
J Med Chem 52: 5732-47 (2009)
Article DOI: 10.1021/jm9009394
BindingDB Entry DOI: 10.7270/Q2BG2PXP |
More data for this
Ligand-Target Pair | |
Histone deacetylase 3
(Homo sapiens (Human)) | BDBM50055513

(CHEMBL3317811)Show SMILES C[C@@]12CSC(=N1)c1csc(CNC(=O)C[C@H](OC(=O)[C@H](Cc3ccc(O)cc3)NC2=O)\C=C\CCS)n1 |r,c:4| Show InChI InChI=1S/C25H28N4O5S3/c1-25-14-37-22(29-25)19-13-36-21(27-19)12-26-20(31)11-17(4-2-3-9-35)34-23(32)18(28-24(25)33)10-15-5-7-16(30)8-6-15/h2,4-8,13,17-18,30,35H,3,9-12,14H2,1H3,(H,26,31)(H,28,33)/b4-2+/t17-,18+,25+/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.380 | n/a | n/a | n/a | n/a | n/a | n/a |
Duke University
Curated by ChEMBL
| Assay Description
Inhibition of HDAC3 (unknown origin) incubated at 37 degC for 30 mins |
Bioorg Med Chem Lett 24: 3728-31 (2014)
Article DOI: 10.1016/j.bmcl.2014.07.006
BindingDB Entry DOI: 10.7270/Q25Q4XRZ |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50020912

(Largazole Thiol)Show SMILES [H][C@]1(CC(=O)NCc2nc(cs2)C2=N[C@@](C)(CS2)C(=O)N[C@H](C(C)C)C(=O)O1)\C=C\CCS |r,t:13| Show InChI InChI=1S/C21H28N4O4S3/c1-12(2)17-19(27)29-13(6-4-5-7-30)8-15(26)22-9-16-23-14(10-31-16)18-25-21(3,11-32-18)20(28)24-17/h4,6,10,12-13,17,30H,5,7-9,11H2,1-3H3,(H,22,26)(H,24,28)/b6-4+/t13-,17-,21+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Duke University
Curated by ChEMBL
| Assay Description
Inhibition of HDAC1 (unknown origin) incubated at 37 degC for 30 mins |
Bioorg Med Chem Lett 24: 3728-31 (2014)
Article DOI: 10.1016/j.bmcl.2014.07.006
BindingDB Entry DOI: 10.7270/Q25Q4XRZ |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50055515

(CHEMBL3317818)Show SMILES CC(C)(C)OC(=O)n1cnc(C[C@@H]2NC(=O)[C@]3(C)CSC(=N3)c3csc(CNC(=O)C[C@H](OC2=O)\C=C\CCS)n3)c1 |r,c:20| Show InChI InChI=1S/C27H34N6O6S3/c1-26(2,3)39-25(37)33-12-16(29-15-33)9-18-23(35)38-17(7-5-6-8-40)10-20(34)28-11-21-30-19(13-41-21)22-32-27(4,14-42-22)24(36)31-18/h5,7,12-13,15,17-18,40H,6,8-11,14H2,1-4H3,(H,28,34)(H,31,36)/b7-5+/t17-,18+,27+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.620 | n/a | n/a | n/a | n/a | n/a | n/a |
Duke University
Curated by ChEMBL
| Assay Description
Inhibition of HDAC1 (unknown origin) incubated at 37 degC for 30 mins |
Bioorg Med Chem Lett 24: 3728-31 (2014)
Article DOI: 10.1016/j.bmcl.2014.07.006
BindingDB Entry DOI: 10.7270/Q25Q4XRZ |
More data for this
Ligand-Target Pair | |
Histone deacetylase 3
(Homo sapiens (Human)) | BDBM50055514

(CHEMBL3317810)Show SMILES C[C@@]12CSC(=N1)c1csc(CNC(=O)C[C@H](OC(=O)[C@H](Cc3ccccc3)NC2=O)\C=C\CCS)n1 |r,c:4| Show InChI InChI=1S/C25H28N4O4S3/c1-25-15-36-22(29-25)19-14-35-21(27-19)13-26-20(30)12-17(9-5-6-10-34)33-23(31)18(28-24(25)32)11-16-7-3-2-4-8-16/h2-5,7-9,14,17-18,34H,6,10-13,15H2,1H3,(H,26,30)(H,28,32)/b9-5+/t17-,18+,25+/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.680 | n/a | n/a | n/a | n/a | n/a | n/a |
Duke University
Curated by ChEMBL
| Assay Description
Inhibition of HDAC3 (unknown origin) incubated at 37 degC for 30 mins |
Bioorg Med Chem Lett 24: 3728-31 (2014)
Article DOI: 10.1016/j.bmcl.2014.07.006
BindingDB Entry DOI: 10.7270/Q25Q4XRZ |
More data for this
Ligand-Target Pair | |
Histone deacetylase 3
(Homo sapiens (Human)) | BDBM50020912

(Largazole Thiol)Show SMILES [H][C@]1(CC(=O)NCc2nc(cs2)C2=N[C@@](C)(CS2)C(=O)N[C@H](C(C)C)C(=O)O1)\C=C\CCS |r,t:13| Show InChI InChI=1S/C21H28N4O4S3/c1-12(2)17-19(27)29-13(6-4-5-7-30)8-15(26)22-9-16-23-14(10-31-16)18-25-21(3,11-32-18)20(28)24-17/h4,6,10,12-13,17,30H,5,7-9,11H2,1-3H3,(H,22,26)(H,24,28)/b6-4+/t13-,17-,21+/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Duke University
Curated by ChEMBL
| Assay Description
Inhibition of HDAC3 (unknown origin) incubated at 37 degC for 30 mins |
Bioorg Med Chem Lett 24: 3728-31 (2014)
Article DOI: 10.1016/j.bmcl.2014.07.006
BindingDB Entry DOI: 10.7270/Q25Q4XRZ |
More data for this
Ligand-Target Pair | |
Cathepsin E
(Homo sapiens (Human)) | BDBM50302107
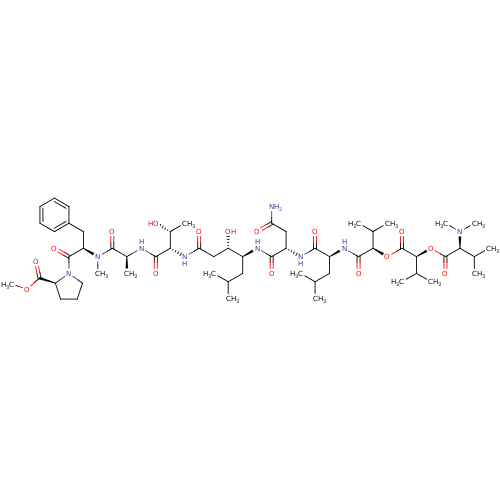
(CHEMBL567893 | Grassystatin A)Show SMILES COC(=O)[C@@H]1CCCN1C(=O)[C@@H](Cc1ccccc1)N(C)C(=O)[C@H](C)NC(=O)[C@@H](NC(=O)C[C@H](O)[C@H](CC(C)C)NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](OC(=O)[C@@H](OC(=O)[C@H](C(C)C)N(C)C)C(C)C)C(C)C)[C@@H](C)O |r| Show InChI InChI=1S/C58H95N9O16/c1-30(2)25-38(43(69)29-45(71)64-46(36(12)68)52(74)60-35(11)54(76)66(15)42(27-37-21-18-17-19-22-37)55(77)67-24-20-23-41(67)56(78)81-16)61-51(73)40(28-44(59)70)62-50(72)39(26-31(3)4)63-53(75)48(33(7)8)82-58(80)49(34(9)10)83-57(79)47(32(5)6)65(13)14/h17-19,21-22,30-36,38-43,46-49,68-69H,20,23-29H2,1-16H3,(H2,59,70)(H,60,74)(H,61,73)(H,62,72)(H,63,75)(H,64,71)/t35-,36+,38-,39-,40-,41-,42+,43-,46-,47-,48+,49-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.886 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida
Curated by ChEMBL
| Assay Description
Inhibition of cathepsin E |
J Med Chem 52: 5732-47 (2009)
Article DOI: 10.1021/jm9009394
BindingDB Entry DOI: 10.7270/Q2BG2PXP |
More data for this
Ligand-Target Pair | |
Histone deacetylase 2
(Homo sapiens (Human)) | BDBM50020912

(Largazole Thiol)Show SMILES [H][C@]1(CC(=O)NCc2nc(cs2)C2=N[C@@](C)(CS2)C(=O)N[C@H](C(C)C)C(=O)O1)\C=C\CCS |r,t:13| Show InChI InChI=1S/C21H28N4O4S3/c1-12(2)17-19(27)29-13(6-4-5-7-30)8-15(26)22-9-16-23-14(10-31-16)18-25-21(3,11-32-18)20(28)24-17/h4,6,10,12-13,17,30H,5,7-9,11H2,1-3H3,(H,22,26)(H,24,28)/b6-4+/t13-,17-,21+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Duke University
Curated by ChEMBL
| Assay Description
Inhibition of HDAC2 (unknown origin) incubated at 37 degC for 30 mins |
Bioorg Med Chem Lett 24: 3728-31 (2014)
Article DOI: 10.1016/j.bmcl.2014.07.006
BindingDB Entry DOI: 10.7270/Q25Q4XRZ |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50055516
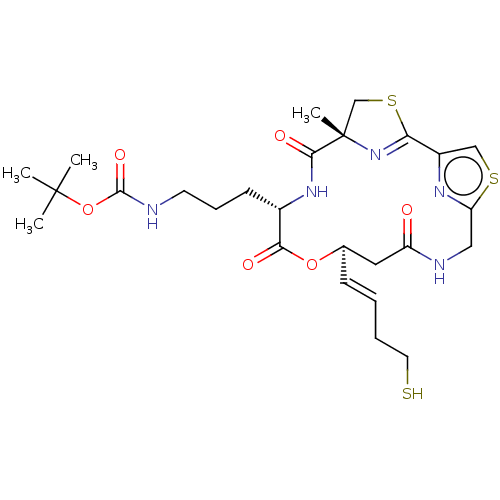
(CHEMBL3317817)Show SMILES CC(C)(C)OC(=O)NCCC[C@@H]1NC(=O)[C@]2(C)CSC(=N2)c2csc(CNC(=O)C[C@H](OC1=O)\C=C\CCS)n2 |r,c:19| Show InChI InChI=1S/C26H37N5O6S3/c1-25(2,3)37-24(35)27-10-7-9-17-22(33)36-16(8-5-6-11-38)12-19(32)28-13-20-29-18(14-39-20)21-31-26(4,15-40-21)23(34)30-17/h5,8,14,16-17,38H,6-7,9-13,15H2,1-4H3,(H,27,35)(H,28,32)(H,30,34)/b8-5+/t16-,17+,26+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.960 | n/a | n/a | n/a | n/a | n/a | n/a |
Duke University
Curated by ChEMBL
| Assay Description
Inhibition of HDAC1 (unknown origin) incubated at 37 degC for 30 mins |
Bioorg Med Chem Lett 24: 3728-31 (2014)
Article DOI: 10.1016/j.bmcl.2014.07.006
BindingDB Entry DOI: 10.7270/Q25Q4XRZ |
More data for this
Ligand-Target Pair | |
Histone deacetylase 2
(Homo sapiens (Human)) | BDBM50055515

(CHEMBL3317818)Show SMILES CC(C)(C)OC(=O)n1cnc(C[C@@H]2NC(=O)[C@]3(C)CSC(=N3)c3csc(CNC(=O)C[C@H](OC2=O)\C=C\CCS)n3)c1 |r,c:20| Show InChI InChI=1S/C27H34N6O6S3/c1-26(2,3)39-25(37)33-12-16(29-15-33)9-18-23(35)38-17(7-5-6-8-40)10-20(34)28-11-21-30-19(13-41-21)22-32-27(4,14-42-22)24(36)31-18/h5,7,12-13,15,17-18,40H,6,8-11,14H2,1-4H3,(H,28,34)(H,31,36)/b7-5+/t17-,18+,27+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Duke University
Curated by ChEMBL
| Assay Description
Inhibition of HDAC2 (unknown origin) incubated at 37 degC for 30 mins |
Bioorg Med Chem Lett 24: 3728-31 (2014)
Article DOI: 10.1016/j.bmcl.2014.07.006
BindingDB Entry DOI: 10.7270/Q25Q4XRZ |
More data for this
Ligand-Target Pair | |
Histone deacetylase 3
(Homo sapiens (Human)) | BDBM50055515

(CHEMBL3317818)Show SMILES CC(C)(C)OC(=O)n1cnc(C[C@@H]2NC(=O)[C@]3(C)CSC(=N3)c3csc(CNC(=O)C[C@H](OC2=O)\C=C\CCS)n3)c1 |r,c:20| Show InChI InChI=1S/C27H34N6O6S3/c1-26(2,3)39-25(37)33-12-16(29-15-33)9-18-23(35)38-17(7-5-6-8-40)10-20(34)28-11-21-30-19(13-41-21)22-32-27(4,14-42-22)24(36)31-18/h5,7,12-13,15,17-18,40H,6,8-11,14H2,1-4H3,(H,28,34)(H,31,36)/b7-5+/t17-,18+,27+/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Duke University
Curated by ChEMBL
| Assay Description
Inhibition of HDAC3 (unknown origin) incubated at 37 degC for 30 mins |
Bioorg Med Chem Lett 24: 3728-31 (2014)
Article DOI: 10.1016/j.bmcl.2014.07.006
BindingDB Entry DOI: 10.7270/Q25Q4XRZ |
More data for this
Ligand-Target Pair | |
Histone deacetylase 2
(Homo sapiens (Human)) | BDBM50055513

(CHEMBL3317811)Show SMILES C[C@@]12CSC(=N1)c1csc(CNC(=O)C[C@H](OC(=O)[C@H](Cc3ccc(O)cc3)NC2=O)\C=C\CCS)n1 |r,c:4| Show InChI InChI=1S/C25H28N4O5S3/c1-25-14-37-22(29-25)19-13-36-21(27-19)12-26-20(31)11-17(4-2-3-9-35)34-23(32)18(28-24(25)33)10-15-5-7-16(30)8-6-15/h2,4-8,13,17-18,30,35H,3,9-12,14H2,1H3,(H,26,31)(H,28,33)/b4-2+/t17-,18+,25+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Duke University
Curated by ChEMBL
| Assay Description
Inhibition of HDAC2 (unknown origin) incubated at 37 degC for 30 mins |
Bioorg Med Chem Lett 24: 3728-31 (2014)
Article DOI: 10.1016/j.bmcl.2014.07.006
BindingDB Entry DOI: 10.7270/Q25Q4XRZ |
More data for this
Ligand-Target Pair | |
Histone deacetylase 3
(Homo sapiens (Human)) | BDBM50055512

(CHEMBL3317812)Show SMILES C[C@@]12CSC(=N1)c1csc(CNC(=O)C[C@H](OC(=O)[C@H](Cc3c[nH]cn3)NC2=O)\C=C\CCS)n1 |r,c:4| | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Duke University
Curated by ChEMBL
| Assay Description
Inhibition of HDAC3 (unknown origin) incubated at 37 degC for 30 mins |
Bioorg Med Chem Lett 24: 3728-31 (2014)
Article DOI: 10.1016/j.bmcl.2014.07.006
BindingDB Entry DOI: 10.7270/Q25Q4XRZ |
More data for this
Ligand-Target Pair | |
Histone deacetylase 2
(Homo sapiens (Human)) | BDBM50055512

(CHEMBL3317812)Show SMILES C[C@@]12CSC(=N1)c1csc(CNC(=O)C[C@H](OC(=O)[C@H](Cc3c[nH]cn3)NC2=O)\C=C\CCS)n1 |r,c:4| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Duke University
Curated by ChEMBL
| Assay Description
Inhibition of HDAC2 (unknown origin) incubated at 37 degC for 30 mins |
Bioorg Med Chem Lett 24: 3728-31 (2014)
Article DOI: 10.1016/j.bmcl.2014.07.006
BindingDB Entry DOI: 10.7270/Q25Q4XRZ |
More data for this
Ligand-Target Pair | |
Histone deacetylase 2
(Homo sapiens (Human)) | BDBM50055516

(CHEMBL3317817)Show SMILES CC(C)(C)OC(=O)NCCC[C@@H]1NC(=O)[C@]2(C)CSC(=N2)c2csc(CNC(=O)C[C@H](OC1=O)\C=C\CCS)n2 |r,c:19| Show InChI InChI=1S/C26H37N5O6S3/c1-25(2,3)37-24(35)27-10-7-9-17-22(33)36-16(8-5-6-11-38)12-19(32)28-13-20-29-18(14-39-20)21-31-26(4,15-40-21)23(34)30-17/h5,8,14,16-17,38H,6-7,9-13,15H2,1-4H3,(H,27,35)(H,28,32)(H,30,34)/b8-5+/t16-,17+,26+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Duke University
Curated by ChEMBL
| Assay Description
Inhibition of HDAC2 (unknown origin) incubated at 37 degC for 30 mins |
Bioorg Med Chem Lett 24: 3728-31 (2014)
Article DOI: 10.1016/j.bmcl.2014.07.006
BindingDB Entry DOI: 10.7270/Q25Q4XRZ |
More data for this
Ligand-Target Pair | |
Histone deacetylase 2
(Homo sapiens (Human)) | BDBM50055514

(CHEMBL3317810)Show SMILES C[C@@]12CSC(=N1)c1csc(CNC(=O)C[C@H](OC(=O)[C@H](Cc3ccccc3)NC2=O)\C=C\CCS)n1 |r,c:4| Show InChI InChI=1S/C25H28N4O4S3/c1-25-15-36-22(29-25)19-14-35-21(27-19)13-26-20(30)12-17(9-5-6-10-34)33-23(31)18(28-24(25)32)11-16-7-3-2-4-8-16/h2-5,7-9,14,17-18,34H,6,10-13,15H2,1H3,(H,26,30)(H,28,32)/b9-5+/t17-,18+,25+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Duke University
Curated by ChEMBL
| Assay Description
Inhibition of HDAC2 (unknown origin) incubated at 37 degC for 30 mins |
Bioorg Med Chem Lett 24: 3728-31 (2014)
Article DOI: 10.1016/j.bmcl.2014.07.006
BindingDB Entry DOI: 10.7270/Q25Q4XRZ |
More data for this
Ligand-Target Pair | |
Histone deacetylase 3
(Homo sapiens (Human)) | BDBM50055516

(CHEMBL3317817)Show SMILES CC(C)(C)OC(=O)NCCC[C@@H]1NC(=O)[C@]2(C)CSC(=N2)c2csc(CNC(=O)C[C@H](OC1=O)\C=C\CCS)n2 |r,c:19| Show InChI InChI=1S/C26H37N5O6S3/c1-25(2,3)37-24(35)27-10-7-9-17-22(33)36-16(8-5-6-11-38)12-19(32)28-13-20-29-18(14-39-20)21-31-26(4,15-40-21)23(34)30-17/h5,8,14,16-17,38H,6-7,9-13,15H2,1-4H3,(H,27,35)(H,28,32)(H,30,34)/b8-5+/t16-,17+,26+/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Duke University
Curated by ChEMBL
| Assay Description
Inhibition of HDAC3 (unknown origin) incubated at 37 degC for 30 mins |
Bioorg Med Chem Lett 24: 3728-31 (2014)
Article DOI: 10.1016/j.bmcl.2014.07.006
BindingDB Entry DOI: 10.7270/Q25Q4XRZ |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50055517
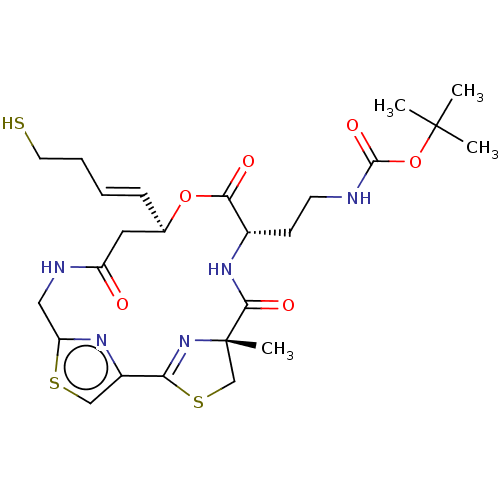
(CHEMBL3317816)Show SMILES CC(C)(C)OC(=O)NCC[C@@H]1NC(=O)[C@]2(C)CSC(=N2)c2csc(CNC(=O)C[C@H](OC1=O)\C=C\CCS)n2 |r,c:18| Show InChI InChI=1S/C25H35N5O6S3/c1-24(2,3)36-23(34)26-9-8-16-21(32)35-15(7-5-6-10-37)11-18(31)27-12-19-28-17(13-38-19)20-30-25(4,14-39-20)22(33)29-16/h5,7,13,15-16,37H,6,8-12,14H2,1-4H3,(H,26,34)(H,27,31)(H,29,33)/b7-5+/t15-,16+,25+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Duke University
Curated by ChEMBL
| Assay Description
Inhibition of HDAC1 (unknown origin) incubated at 37 degC for 30 mins |
Bioorg Med Chem Lett 24: 3728-31 (2014)
Article DOI: 10.1016/j.bmcl.2014.07.006
BindingDB Entry DOI: 10.7270/Q25Q4XRZ |
More data for this
Ligand-Target Pair | |
Histone deacetylase 2
(Homo sapiens (Human)) | BDBM50055517

(CHEMBL3317816)Show SMILES CC(C)(C)OC(=O)NCC[C@@H]1NC(=O)[C@]2(C)CSC(=N2)c2csc(CNC(=O)C[C@H](OC1=O)\C=C\CCS)n2 |r,c:18| Show InChI InChI=1S/C25H35N5O6S3/c1-24(2,3)36-23(34)26-9-8-16-21(32)35-15(7-5-6-10-37)11-18(31)27-12-19-28-17(13-38-19)20-30-25(4,14-39-20)22(33)29-16/h5,7,13,15-16,37H,6,8-12,14H2,1-4H3,(H,26,34)(H,27,31)(H,29,33)/b7-5+/t15-,16+,25+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Duke University
Curated by ChEMBL
| Assay Description
Inhibition of HDAC2 (unknown origin) incubated at 37 degC for 30 mins |
Bioorg Med Chem Lett 24: 3728-31 (2014)
Article DOI: 10.1016/j.bmcl.2014.07.006
BindingDB Entry DOI: 10.7270/Q25Q4XRZ |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50055518

(CHEMBL3317815)Show SMILES C[C@@]12CSC(=N1)c1csc(CNC(=O)C[C@H](OC(=O)[C@H](CCCN)NC2=O)\C=C\CCS)n1 |r,c:4| Show InChI InChI=1S/C21H29N5O4S3/c1-21-12-33-18(26-21)15-11-32-17(24-15)10-23-16(27)9-13(5-2-3-8-31)30-19(28)14(6-4-7-22)25-20(21)29/h2,5,11,13-14,31H,3-4,6-10,12,22H2,1H3,(H,23,27)(H,25,29)/b5-2+/t13-,14+,21+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Duke University
Curated by ChEMBL
| Assay Description
Inhibition of HDAC1 (unknown origin) incubated at 37 degC for 30 mins |
Bioorg Med Chem Lett 24: 3728-31 (2014)
Article DOI: 10.1016/j.bmcl.2014.07.006
BindingDB Entry DOI: 10.7270/Q25Q4XRZ |
More data for this
Ligand-Target Pair | |
Histone deacetylase 3
(Homo sapiens (Human)) | BDBM50055517

(CHEMBL3317816)Show SMILES CC(C)(C)OC(=O)NCC[C@@H]1NC(=O)[C@]2(C)CSC(=N2)c2csc(CNC(=O)C[C@H](OC1=O)\C=C\CCS)n2 |r,c:18| Show InChI InChI=1S/C25H35N5O6S3/c1-24(2,3)36-23(34)26-9-8-16-21(32)35-15(7-5-6-10-37)11-18(31)27-12-19-28-17(13-38-19)20-30-25(4,14-39-20)22(33)29-16/h5,7,13,15-16,37H,6,8-12,14H2,1-4H3,(H,26,34)(H,27,31)(H,29,33)/b7-5+/t15-,16+,25+/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Duke University
Curated by ChEMBL
| Assay Description
Inhibition of HDAC3 (unknown origin) incubated at 37 degC for 30 mins |
Bioorg Med Chem Lett 24: 3728-31 (2014)
Article DOI: 10.1016/j.bmcl.2014.07.006
BindingDB Entry DOI: 10.7270/Q25Q4XRZ |
More data for this
Ligand-Target Pair | |
Cathepsin D
(Homo sapiens (Human)) | BDBM912

((3S,4S)-3-hydroxy-4-[(2S)-2-[(3S,4S)-3-hydroxy-6-m...)Show SMILES CC(C)C[C@H](NC(=O)[C@H](C)NC(=O)C[C@H](O)[C@H](CC(C)C)NC(=O)[C@@H](NC(=O)[C@@H](NC(=O)CC(C)C)C(C)C)C(C)C)[C@@H](O)CC(O)=O |r| Show InChI InChI=1S/C34H63N5O9/c1-17(2)12-23(37-33(47)31(21(9)10)39-34(48)30(20(7)8)38-27(42)14-19(5)6)25(40)15-28(43)35-22(11)32(46)36-24(13-18(3)4)26(41)16-29(44)45/h17-26,30-31,40-41H,12-16H2,1-11H3,(H,35,43)(H,36,46)(H,37,47)(H,38,42)(H,39,48)(H,44,45)/t22-,23-,24-,25-,26-,30-,31-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida
Curated by ChEMBL
| Assay Description
Inhibition of cathepsin D in human MCF7 cells by fluorescence assay |
J Med Chem 52: 5732-47 (2009)
Article DOI: 10.1021/jm9009394
BindingDB Entry DOI: 10.7270/Q2BG2PXP |
More data for this
Ligand-Target Pair | |
Cathepsin E
(Homo sapiens (Human)) | BDBM912

((3S,4S)-3-hydroxy-4-[(2S)-2-[(3S,4S)-3-hydroxy-6-m...)Show SMILES CC(C)C[C@H](NC(=O)[C@H](C)NC(=O)C[C@H](O)[C@H](CC(C)C)NC(=O)[C@@H](NC(=O)[C@@H](NC(=O)CC(C)C)C(C)C)C(C)C)[C@@H](O)CC(O)=O |r| Show InChI InChI=1S/C34H63N5O9/c1-17(2)12-23(37-33(47)31(21(9)10)39-34(48)30(20(7)8)38-27(42)14-19(5)6)25(40)15-28(43)35-22(11)32(46)36-24(13-18(3)4)26(41)16-29(44)45/h17-26,30-31,40-41H,12-16H2,1-11H3,(H,35,43)(H,36,46)(H,37,47)(H,38,42)(H,39,48)(H,44,45)/t22-,23-,24-,25-,26-,30-,31-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida
Curated by ChEMBL
| Assay Description
Inhibition of cathepsin E in human MCF7 cells by fluorescence assay |
J Med Chem 52: 5732-47 (2009)
Article DOI: 10.1021/jm9009394
BindingDB Entry DOI: 10.7270/Q2BG2PXP |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50055510

(CHEMBL3317814)Show SMILES C[C@@]12CSC(=N1)c1csc(CNC(=O)C[C@H](OC(=O)[C@H](CCN)NC2=O)\C=C\CCS)n1 |r,c:4| Show InChI InChI=1S/C20H27N5O4S3/c1-20-11-32-17(25-20)14-10-31-16(23-14)9-22-15(26)8-12(4-2-3-7-30)29-18(27)13(5-6-21)24-19(20)28/h2,4,10,12-13,30H,3,5-9,11,21H2,1H3,(H,22,26)(H,24,28)/b4-2+/t12-,13+,20+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Duke University
Curated by ChEMBL
| Assay Description
Inhibition of HDAC1 (unknown origin) incubated at 37 degC for 30 mins |
Bioorg Med Chem Lett 24: 3728-31 (2014)
Article DOI: 10.1016/j.bmcl.2014.07.006
BindingDB Entry DOI: 10.7270/Q25Q4XRZ |
More data for this
Ligand-Target Pair | |
Histone deacetylase 3
(Homo sapiens (Human)) | BDBM50055518

(CHEMBL3317815)Show SMILES C[C@@]12CSC(=N1)c1csc(CNC(=O)C[C@H](OC(=O)[C@H](CCCN)NC2=O)\C=C\CCS)n1 |r,c:4| Show InChI InChI=1S/C21H29N5O4S3/c1-21-12-33-18(26-21)15-11-32-17(24-15)10-23-16(27)9-13(5-2-3-8-31)30-19(28)14(6-4-7-22)25-20(21)29/h2,5,11,13-14,31H,3-4,6-10,12,22H2,1H3,(H,23,27)(H,25,29)/b5-2+/t13-,14+,21+/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Duke University
Curated by ChEMBL
| Assay Description
Inhibition of HDAC3 (unknown origin) incubated at 37 degC for 30 mins |
Bioorg Med Chem Lett 24: 3728-31 (2014)
Article DOI: 10.1016/j.bmcl.2014.07.006
BindingDB Entry DOI: 10.7270/Q25Q4XRZ |
More data for this
Ligand-Target Pair | |
Histone deacetylase 2
(Homo sapiens (Human)) | BDBM50055518

(CHEMBL3317815)Show SMILES C[C@@]12CSC(=N1)c1csc(CNC(=O)C[C@H](OC(=O)[C@H](CCCN)NC2=O)\C=C\CCS)n1 |r,c:4| Show InChI InChI=1S/C21H29N5O4S3/c1-21-12-33-18(26-21)15-11-32-17(24-15)10-23-16(27)9-13(5-2-3-8-31)30-19(28)14(6-4-7-22)25-20(21)29/h2,5,11,13-14,31H,3-4,6-10,12,22H2,1H3,(H,23,27)(H,25,29)/b5-2+/t13-,14+,21+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Duke University
Curated by ChEMBL
| Assay Description
Inhibition of HDAC2 (unknown origin) incubated at 37 degC for 30 mins |
Bioorg Med Chem Lett 24: 3728-31 (2014)
Article DOI: 10.1016/j.bmcl.2014.07.006
BindingDB Entry DOI: 10.7270/Q25Q4XRZ |
More data for this
Ligand-Target Pair | |
Histone deacetylase 3
(Homo sapiens (Human)) | BDBM50055510

(CHEMBL3317814)Show SMILES C[C@@]12CSC(=N1)c1csc(CNC(=O)C[C@H](OC(=O)[C@H](CCN)NC2=O)\C=C\CCS)n1 |r,c:4| Show InChI InChI=1S/C20H27N5O4S3/c1-20-11-32-17(25-20)14-10-31-16(23-14)9-22-15(26)8-12(4-2-3-7-30)29-18(27)13(5-6-21)24-19(20)28/h2,4,10,12-13,30H,3,5-9,11,21H2,1H3,(H,22,26)(H,24,28)/b4-2+/t12-,13+,20+/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Duke University
Curated by ChEMBL
| Assay Description
Inhibition of HDAC3 (unknown origin) incubated at 37 degC for 30 mins |
Bioorg Med Chem Lett 24: 3728-31 (2014)
Article DOI: 10.1016/j.bmcl.2014.07.006
BindingDB Entry DOI: 10.7270/Q25Q4XRZ |
More data for this
Ligand-Target Pair | |
Chymase
(Homo sapiens (Human)) | BDBM50288847
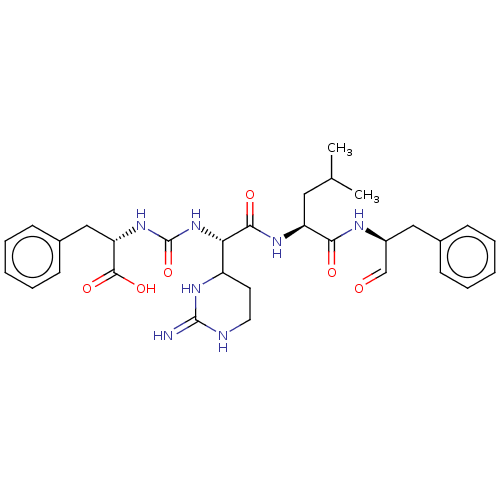
(Chymostatin)Show SMILES CC(C)C[C@H](NC(=O)[C@@H](NC(=O)N[C@@H](Cc1ccccc1)C(O)=O)C1CCNC(=N)N1)C(=O)N[C@@H](Cc1ccccc1)C=O |r| Show InChI InChI=1S/C9H13N3O6/c13-6(3-12-5-10-4-11-12)1-2-18-7(8(14)15)9(16)17/h4-7,13H,1-3H2,(H,14,15)(H,16,17) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 9.40 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida
Curated by ChEMBL
| Assay Description
Inhibition of chymase (unknown origin) by fluorescence assay |
J Med Chem 61: 6364-6378 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00885
BindingDB Entry DOI: 10.7270/Q2N87D9X |
More data for this
Ligand-Target Pair | |
Disintegrin and metalloproteinase domain-containing protein 17
(Homo sapiens (Human)) | BDBM50062351

((R)-N*4*-Hydroxy-N*1*-[(S)-2-(1H-indol-3-yl)-1-met...)Show SMILES CNC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](CC(C)C)CC(=O)NO |r| Show InChI InChI=1S/C20H28N4O4/c1-12(2)8-13(10-18(25)24-28)19(26)23-17(20(27)21-3)9-14-11-22-16-7-5-4-6-15(14)16/h4-7,11-13,17,22,28H,8-10H2,1-3H3,(H,21,27)(H,23,26)(H,24,25)/t13-,17+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida
Curated by ChEMBL
| Assay Description
Inhibition of TACE after 10 to 15 mins by fluorescence assay |
J Med Chem 52: 5732-47 (2009)
Article DOI: 10.1021/jm9009394
BindingDB Entry DOI: 10.7270/Q2BG2PXP |
More data for this
Ligand-Target Pair | |
Histone deacetylase 2
(Homo sapiens (Human)) | BDBM50055510

(CHEMBL3317814)Show SMILES C[C@@]12CSC(=N1)c1csc(CNC(=O)C[C@H](OC(=O)[C@H](CCN)NC2=O)\C=C\CCS)n1 |r,c:4| Show InChI InChI=1S/C20H27N5O4S3/c1-20-11-32-17(25-20)14-10-31-16(23-14)9-22-15(26)8-12(4-2-3-7-30)29-18(27)13(5-6-21)24-19(20)28/h2,4,10,12-13,30H,3,5-9,11,21H2,1H3,(H,22,26)(H,24,28)/b4-2+/t12-,13+,20+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Duke University
Curated by ChEMBL
| Assay Description
Inhibition of HDAC2 (unknown origin) incubated at 37 degC for 30 mins |
Bioorg Med Chem Lett 24: 3728-31 (2014)
Article DOI: 10.1016/j.bmcl.2014.07.006
BindingDB Entry DOI: 10.7270/Q25Q4XRZ |
More data for this
Ligand-Target Pair | |
Cathepsin D
(Homo sapiens (Human)) | BDBM50302107

(CHEMBL567893 | Grassystatin A)Show SMILES COC(=O)[C@@H]1CCCN1C(=O)[C@@H](Cc1ccccc1)N(C)C(=O)[C@H](C)NC(=O)[C@@H](NC(=O)C[C@H](O)[C@H](CC(C)C)NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](OC(=O)[C@@H](OC(=O)[C@H](C(C)C)N(C)C)C(C)C)C(C)C)[C@@H](C)O |r| Show InChI InChI=1S/C58H95N9O16/c1-30(2)25-38(43(69)29-45(71)64-46(36(12)68)52(74)60-35(11)54(76)66(15)42(27-37-21-18-17-19-22-37)55(77)67-24-20-23-41(67)56(78)81-16)61-51(73)40(28-44(59)70)62-50(72)39(26-31(3)4)63-53(75)48(33(7)8)82-58(80)49(34(9)10)83-57(79)47(32(5)6)65(13)14/h17-19,21-22,30-36,38-43,46-49,68-69H,20,23-29H2,1-16H3,(H2,59,70)(H,60,74)(H,61,73)(H,62,72)(H,63,75)(H,64,71)/t35-,36+,38-,39-,40-,41-,42+,43-,46-,47-,48+,49-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida
Curated by ChEMBL
| Assay Description
Inhibition of cathepsin D |
J Med Chem 52: 5732-47 (2009)
Article DOI: 10.1021/jm9009394
BindingDB Entry DOI: 10.7270/Q2BG2PXP |
More data for this
Ligand-Target Pair | |
Chymotrypsin-like elastase family member 1
(Sus scrofa (Pig)) | BDBM50312532
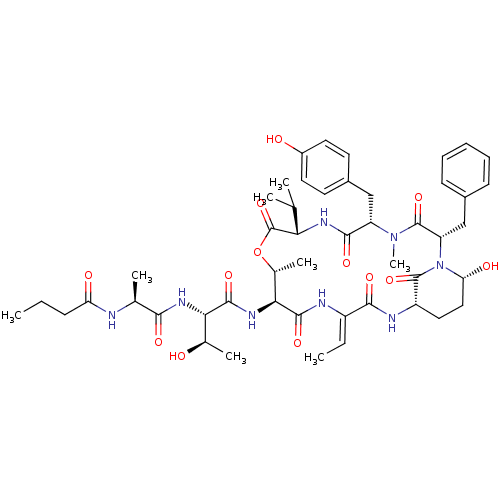
(CHEMBL1077010 | molassamide)Show SMILES CCCC(=O)N[C@@H](C)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@H]1[C@@H](C)OC(=O)[C@@H](NC(=O)[C@H](Cc2ccc(O)cc2)N(C)C(=O)[C@H](Cc2ccccc2)N2[C@H](O)CC[C@H](NC(=O)\C(NC1=O)=C\C)C2=O)C(C)C |r| Show InChI InChI=1S/C48H66N8O13/c1-9-14-36(59)49-26(5)41(61)53-39(27(6)57)44(64)54-40-28(7)69-48(68)38(25(3)4)52-43(63)34(23-30-17-19-31(58)20-18-30)55(8)47(67)35(24-29-15-12-11-13-16-29)56-37(60)22-21-33(46(56)66)51-42(62)32(10-2)50-45(40)65/h10-13,15-20,25-28,33-35,37-40,57-58,60H,9,14,21-24H2,1-8H3,(H,49,59)(H,50,65)(H,51,62)(H,52,63)(H,53,61)(H,54,64)/b32-10-/t26-,27+,28+,33-,34-,35-,37+,38-,39-,40-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
Smithsonian Marine Station at Ft. Pierce
Curated by ChEMBL
| Assay Description
Inhibition of pig pancreatic elastase preincubated for 15 mins by spectrophotometry |
J Nat Prod 73: 459-62 (2010)
Article DOI: 10.1021/np900603f
BindingDB Entry DOI: 10.7270/Q2GH9J4Z |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50055511

(CHEMBL3317813)Show SMILES C[C@@]12CSC(=N1)c1csc(CNC(=O)C[C@H](OC(=O)[C@H](CC(O)=O)NC2=O)\C=C\CCS)n1 |r,c:4| Show InChI InChI=1S/C20H24N4O6S3/c1-20-10-33-17(24-20)13-9-32-15(22-13)8-21-14(25)6-11(4-2-3-5-31)30-18(28)12(7-16(26)27)23-19(20)29/h2,4,9,11-12,31H,3,5-8,10H2,1H3,(H,21,25)(H,23,29)(H,26,27)/b4-2+/t11-,12+,20+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 39 | n/a | n/a | n/a | n/a | n/a | n/a |
Duke University
Curated by ChEMBL
| Assay Description
Inhibition of HDAC1 (unknown origin) incubated at 37 degC for 30 mins |
Bioorg Med Chem Lett 24: 3728-31 (2014)
Article DOI: 10.1016/j.bmcl.2014.07.006
BindingDB Entry DOI: 10.7270/Q25Q4XRZ |
More data for this
Ligand-Target Pair | |
Cathepsin E
(Homo sapiens (Human)) | BDBM50302108
(CHEMBL569445 | Grassystatin C)Show SMILES CC[C@H](C)[C@@H](O)C(=O)N[C@@H](CC(C)C)C(=O)N(C)[C@@H](CCC(N)=O)C(=O)N[C@@H](CC(C)C)[C@@H](O)CC(=O)N[C@@H]([C@@H](C)CC)C(=O)NCC(=O)N(C)[C@H](Cc1ccccc1)C(=O)N1CCC[C@H]1C(=O)OC |r| Show InChI InChI=1S/C50H82N8O12/c1-12-31(7)43(46(65)52-28-42(62)56(9)38(26-33-18-15-14-16-19-33)49(68)58-23-17-20-37(58)50(69)70-11)55-41(61)27-39(59)34(24-29(3)4)53-45(64)36(21-22-40(51)60)57(10)48(67)35(25-30(5)6)54-47(66)44(63)32(8)13-2/h14-16,18-19,29-32,34-39,43-44,59,63H,12-13,17,20-28H2,1-11H3,(H2,51,60)(H,52,65)(H,53,64)(H,54,66)(H,55,61)/t31-,32-,34-,35-,36-,37-,38+,39-,43-,44+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 43 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida
Curated by ChEMBL
| Assay Description
Inhibition of cathepsin E |
J Med Chem 52: 5732-47 (2009)
Article DOI: 10.1021/jm9009394
BindingDB Entry DOI: 10.7270/Q2BG2PXP |
More data for this
Ligand-Target Pair | |
Disintegrin and metalloproteinase domain-containing protein 9
(Homo sapiens (Human)) | BDBM50062351

((R)-N*4*-Hydroxy-N*1*-[(S)-2-(1H-indol-3-yl)-1-met...)Show SMILES CNC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](CC(C)C)CC(=O)NO |r| Show InChI InChI=1S/C20H28N4O4/c1-12(2)8-13(10-18(25)24-28)19(26)23-17(20(27)21-3)9-14-11-22-16-7-5-4-6-15(14)16/h4-7,11-13,17,22,28H,8-10H2,1-3H3,(H,21,27)(H,23,26)(H,24,25)/t13-,17+/m1/s1 | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 56 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida
Curated by ChEMBL
| Assay Description
Inhibition of ADAM9 after 10 to 15 mins by fluorescence assay |
J Med Chem 52: 5732-47 (2009)
Article DOI: 10.1021/jm9009394
BindingDB Entry DOI: 10.7270/Q2BG2PXP |
More data for this
Ligand-Target Pair | |
Disintegrin and metalloproteinase domain-containing protein 9
(Homo sapiens (Human)) | BDBM912

((3S,4S)-3-hydroxy-4-[(2S)-2-[(3S,4S)-3-hydroxy-6-m...)Show SMILES CC(C)C[C@H](NC(=O)[C@H](C)NC(=O)C[C@H](O)[C@H](CC(C)C)NC(=O)[C@@H](NC(=O)[C@@H](NC(=O)CC(C)C)C(C)C)C(C)C)[C@@H](O)CC(O)=O |r| Show InChI InChI=1S/C34H63N5O9/c1-17(2)12-23(37-33(47)31(21(9)10)39-34(48)30(20(7)8)38-27(42)14-19(5)6)25(40)15-28(43)35-22(11)32(46)36-24(13-18(3)4)26(41)16-29(44)45/h17-26,30-31,40-41H,12-16H2,1-11H3,(H,35,43)(H,36,46)(H,37,47)(H,38,42)(H,39,48)(H,44,45)/t22-,23-,24-,25-,26-,30-,31-/m0/s1 | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 56 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida
Curated by ChEMBL
| Assay Description
Inhibition of ADAM9 after 10 to 15 mins by fluorescence assay |
J Med Chem 52: 5732-47 (2009)
Article DOI: 10.1021/jm9009394
BindingDB Entry DOI: 10.7270/Q2BG2PXP |
More data for this
Ligand-Target Pair | |
Histone deacetylase 3
(Homo sapiens (Human)) | BDBM50055511

(CHEMBL3317813)Show SMILES C[C@@]12CSC(=N1)c1csc(CNC(=O)C[C@H](OC(=O)[C@H](CC(O)=O)NC2=O)\C=C\CCS)n1 |r,c:4| Show InChI InChI=1S/C20H24N4O6S3/c1-20-10-33-17(24-20)13-9-32-15(22-13)8-21-14(25)6-11(4-2-3-5-31)30-18(28)12(7-16(26)27)23-19(20)29/h2,4,9,11-12,31H,3,5-8,10H2,1H3,(H,21,25)(H,23,29)(H,26,27)/b4-2+/t11-,12+,20+/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Duke University
Curated by ChEMBL
| Assay Description
Inhibition of HDAC3 (unknown origin) incubated at 37 degC for 30 mins |
Bioorg Med Chem Lett 24: 3728-31 (2014)
Article DOI: 10.1016/j.bmcl.2014.07.006
BindingDB Entry DOI: 10.7270/Q25Q4XRZ |
More data for this
Ligand-Target Pair | |
Histone deacetylase 8
(Homo sapiens (Human)) | BDBM50020912

(Largazole Thiol)Show SMILES [H][C@]1(CC(=O)NCc2nc(cs2)C2=N[C@@](C)(CS2)C(=O)N[C@H](C(C)C)C(=O)O1)\C=C\CCS |r,t:13| Show InChI InChI=1S/C21H28N4O4S3/c1-12(2)17-19(27)29-13(6-4-5-7-30)8-15(26)22-9-16-23-14(10-31-16)18-25-21(3,11-32-18)20(28)24-17/h4,6,10,12-13,17,30H,5,7-9,11H2,1-3H3,(H,22,26)(H,24,28)/b6-4+/t13-,17-,21+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 102 | n/a | n/a | n/a | n/a | n/a | n/a |
Duke University
Curated by ChEMBL
| Assay Description
Inhibition of HDAC8 (unknown origin) incubated at 37 degC for 30 mins |
Bioorg Med Chem Lett 24: 3728-31 (2014)
Article DOI: 10.1016/j.bmcl.2014.07.006
BindingDB Entry DOI: 10.7270/Q25Q4XRZ |
More data for this
Ligand-Target Pair | |
Histone deacetylase 2
(Homo sapiens (Human)) | BDBM50055511

(CHEMBL3317813)Show SMILES C[C@@]12CSC(=N1)c1csc(CNC(=O)C[C@H](OC(=O)[C@H](CC(O)=O)NC2=O)\C=C\CCS)n1 |r,c:4| Show InChI InChI=1S/C20H24N4O6S3/c1-20-10-33-17(24-20)13-9-32-15(22-13)8-21-14(25)6-11(4-2-3-5-31)30-18(28)12(7-16(26)27)23-19(20)29/h2,4,9,11-12,31H,3,5-8,10H2,1H3,(H,21,25)(H,23,29)(H,26,27)/b4-2+/t11-,12+,20+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
Duke University
Curated by ChEMBL
| Assay Description
Inhibition of HDAC2 (unknown origin) incubated at 37 degC for 30 mins |
Bioorg Med Chem Lett 24: 3728-31 (2014)
Article DOI: 10.1016/j.bmcl.2014.07.006
BindingDB Entry DOI: 10.7270/Q25Q4XRZ |
More data for this
Ligand-Target Pair | |
Chymotrypsinogen A
(Bos taurus (bovine)) | BDBM50312532

(CHEMBL1077010 | molassamide)Show SMILES CCCC(=O)N[C@@H](C)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@H]1[C@@H](C)OC(=O)[C@@H](NC(=O)[C@H](Cc2ccc(O)cc2)N(C)C(=O)[C@H](Cc2ccccc2)N2[C@H](O)CC[C@H](NC(=O)\C(NC1=O)=C\C)C2=O)C(C)C |r| Show InChI InChI=1S/C48H66N8O13/c1-9-14-36(59)49-26(5)41(61)53-39(27(6)57)44(64)54-40-28(7)69-48(68)38(25(3)4)52-43(63)34(23-30-17-19-31(58)20-18-30)55(8)47(67)35(24-29-15-12-11-13-16-29)56-37(60)22-21-33(46(56)66)51-42(62)32(10-2)50-45(40)65/h10-13,15-20,25-28,33-35,37-40,57-58,60H,9,14,21-24H2,1-8H3,(H,49,59)(H,50,65)(H,51,62)(H,52,63)(H,53,61)(H,54,64)/b32-10-/t26-,27+,28+,33-,34-,35-,37+,38-,39-,40-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 234 | n/a | n/a | n/a | n/a | n/a | n/a |
Smithsonian Marine Station at Ft. Pierce
Curated by ChEMBL
| Assay Description
Inhibition of bovine pancreas alpha-chymotrypsin preincubated for 10 mins by spectrophotometry |
J Nat Prod 73: 459-62 (2010)
Article DOI: 10.1021/np900603f
BindingDB Entry DOI: 10.7270/Q2GH9J4Z |
More data for this
Ligand-Target Pair | |
Disintegrin and metalloproteinase domain-containing protein 10
(Homo sapiens (Human)) | BDBM50062351

((R)-N*4*-Hydroxy-N*1*-[(S)-2-(1H-indol-3-yl)-1-met...)Show SMILES CNC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](CC(C)C)CC(=O)NO |r| Show InChI InChI=1S/C20H28N4O4/c1-12(2)8-13(10-18(25)24-28)19(26)23-17(20(27)21-3)9-14-11-22-16-7-5-4-6-15(14)16/h4-7,11-13,17,22,28H,8-10H2,1-3H3,(H,21,27)(H,23,26)(H,24,25)/t13-,17+/m1/s1 | PDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 263 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida
Curated by ChEMBL
| Assay Description
Inhibition of ADAM10 after 10 to 15 mins by fluorescence assay |
J Med Chem 52: 5732-47 (2009)
Article DOI: 10.1021/jm9009394
BindingDB Entry DOI: 10.7270/Q2BG2PXP |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 10
(Homo sapiens (Human)) | BDBM50288844

(CHEMBL4168948)Show SMILES CC[C@H](C)[C@@H](OC(=O)\C=C\c1ccccc1)C(=O)N1CCC[C@H]1C(=O)NCC(=O)N(C)[C@@H](CC(C)C)C(=O)N[C@@H](C)C(=O)N(C)[C@H](Cc1ccccc1)C(=O)N1CCC[C@H]1C(=O)OC |r| Show InChI InChI=1S/C7H9N3O5/c11-4(5-8-2-9-10-5)1-3(6(12)13)7(14)15/h2-4,11H,1H2,(H,12,13)(H,14,15)(H,8,9,10) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 440 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida
Curated by ChEMBL
| Assay Description
Antagonist activity at human CCR10 expressed in human U2OS cells assessed as inhibition of CCL27-induced beta-arrestin recruitment pre-incubated for ... |
J Med Chem 61: 6364-6378 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00885
BindingDB Entry DOI: 10.7270/Q2N87D9X |
More data for this
Ligand-Target Pair | |
Histone deacetylase 8
(Homo sapiens (Human)) | BDBM50055515

(CHEMBL3317818)Show SMILES CC(C)(C)OC(=O)n1cnc(C[C@@H]2NC(=O)[C@]3(C)CSC(=N3)c3csc(CNC(=O)C[C@H](OC2=O)\C=C\CCS)n3)c1 |r,c:20| Show InChI InChI=1S/C27H34N6O6S3/c1-26(2,3)39-25(37)33-12-16(29-15-33)9-18-23(35)38-17(7-5-6-8-40)10-20(34)28-11-21-30-19(13-41-21)22-32-27(4,14-42-22)24(36)31-18/h5,7,12-13,15,17-18,40H,6,8-11,14H2,1-4H3,(H,28,34)(H,31,36)/b7-5+/t17-,18+,27+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 540 | n/a | n/a | n/a | n/a | n/a | n/a |
Duke University
Curated by ChEMBL
| Assay Description
Inhibition of HDAC8 (unknown origin) incubated at 37 degC for 30 mins |
Bioorg Med Chem Lett 24: 3728-31 (2014)
Article DOI: 10.1016/j.bmcl.2014.07.006
BindingDB Entry DOI: 10.7270/Q25Q4XRZ |
More data for this
Ligand-Target Pair | |
Histone deacetylase 8
(Homo sapiens (Human)) | BDBM50055516

(CHEMBL3317817)Show SMILES CC(C)(C)OC(=O)NCCC[C@@H]1NC(=O)[C@]2(C)CSC(=N2)c2csc(CNC(=O)C[C@H](OC1=O)\C=C\CCS)n2 |r,c:19| Show InChI InChI=1S/C26H37N5O6S3/c1-25(2,3)37-24(35)27-10-7-9-17-22(33)36-16(8-5-6-11-38)12-19(32)28-13-20-29-18(14-39-20)21-31-26(4,15-40-21)23(34)30-17/h5,8,14,16-17,38H,6-7,9-13,15H2,1-4H3,(H,27,35)(H,28,32)(H,30,34)/b8-5+/t16-,17+,26+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 790 | n/a | n/a | n/a | n/a | n/a | n/a |
Duke University
Curated by ChEMBL
| Assay Description
Inhibition of HDAC8 (unknown origin) incubated at 37 degC for 30 mins |
Bioorg Med Chem Lett 24: 3728-31 (2014)
Article DOI: 10.1016/j.bmcl.2014.07.006
BindingDB Entry DOI: 10.7270/Q25Q4XRZ |
More data for this
Ligand-Target Pair | |
Cathepsin D
(Homo sapiens (Human)) | BDBM50302107

(CHEMBL567893 | Grassystatin A)Show SMILES COC(=O)[C@@H]1CCCN1C(=O)[C@@H](Cc1ccccc1)N(C)C(=O)[C@H](C)NC(=O)[C@@H](NC(=O)C[C@H](O)[C@H](CC(C)C)NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](OC(=O)[C@@H](OC(=O)[C@H](C(C)C)N(C)C)C(C)C)C(C)C)[C@@H](C)O |r| Show InChI InChI=1S/C58H95N9O16/c1-30(2)25-38(43(69)29-45(71)64-46(36(12)68)52(74)60-35(11)54(76)66(15)42(27-37-21-18-17-19-22-37)55(77)67-24-20-23-41(67)56(78)81-16)61-51(73)40(28-44(59)70)62-50(72)39(26-31(3)4)63-53(75)48(33(7)8)82-58(80)49(34(9)10)83-57(79)47(32(5)6)65(13)14/h17-19,21-22,30-36,38-43,46-49,68-69H,20,23-29H2,1-16H3,(H2,59,70)(H,60,74)(H,61,73)(H,62,72)(H,63,75)(H,64,71)/t35-,36+,38-,39-,40-,41-,42+,43-,46-,47-,48+,49-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida
Curated by ChEMBL
| Assay Description
Inhibition of cathepsin D in human MCF7 cells by fluorescence assay |
J Med Chem 52: 5732-47 (2009)
Article DOI: 10.1021/jm9009394
BindingDB Entry DOI: 10.7270/Q2BG2PXP |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data