 Found 67 hits with Last Name = 'lópez-vallejo' and Initial = 'f'
Found 67 hits with Last Name = 'lópez-vallejo' and Initial = 'f' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Furin
(Homo sapiens (Human)) | BDBM50386999
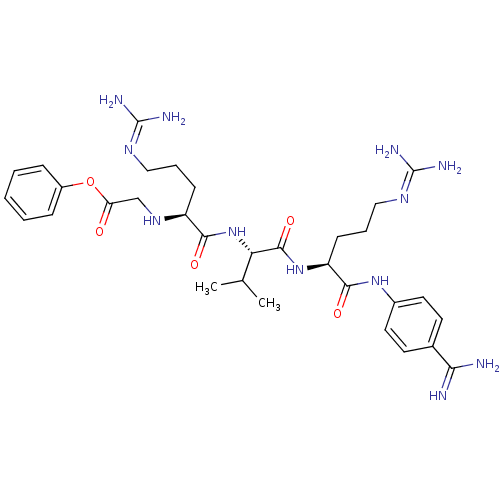
(CHEMBL2049152)Show SMILES [#6]-[#6](-[#6])-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#7]-[#6]-[#6](=O)-[#8]-c1ccccc1)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-c1ccc(cc1)-[#6](-[#7])=[#7] |r| Show InChI InChI=1S/C32H48N12O5/c1-19(2)26(44-28(46)23(10-6-16-39-31(35)36)41-18-25(45)49-22-8-4-3-5-9-22)30(48)43-24(11-7-17-40-32(37)38)29(47)42-21-14-12-20(13-15-21)27(33)34/h3-5,8-9,12-15,19,23-24,26,41H,6-7,10-11,16-18H2,1-2H3,(H3,33,34)(H,42,47)(H,43,48)(H,44,46)(H4,35,36,39)(H4,37,38,40)/t23-,24-,26-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.810 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Torrey Pines Institute for Molecular Studies
Curated by ChEMBL
| Assay Description
Inhibition of furin |
Bioorg Med Chem 20: 4462-71 (2012)
Article DOI: 10.1016/j.bmc.2012.05.029
BindingDB Entry DOI: 10.7270/Q2514075 |
More data for this
Ligand-Target Pair | |
3-hydroxy-3-methylglutaryl-coenzyme A reductase
(Homo sapiens (Human)) | BDBM18372
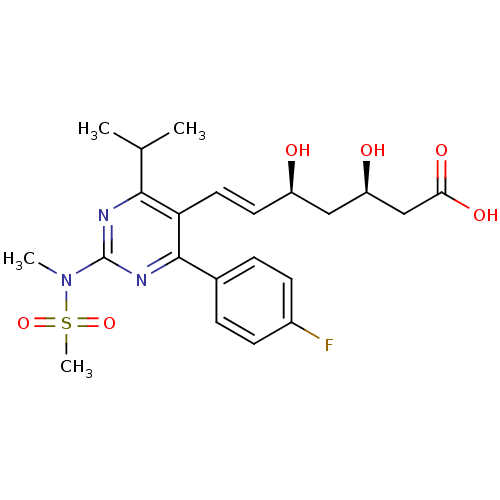
((3R,5S,6E)-7-[4-(4-fluorophenyl)-2-(N-methylmethan...)Show SMILES CC(C)c1nc(nc(-c2ccc(F)cc2)c1\C=C\[C@@H](O)C[C@@H](O)CC(O)=O)N(C)S(C)(=O)=O |r| Show InChI InChI=1S/C22H28FN3O6S/c1-13(2)20-18(10-9-16(27)11-17(28)12-19(29)30)21(14-5-7-15(23)8-6-14)25-22(24-20)26(3)33(4,31)32/h5-10,13,16-17,27-28H,11-12H2,1-4H3,(H,29,30)/b10-9+/t16-,17-/m1/s1 | PDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Nacional Autónoma de México
Curated by ChEMBL
| Assay Description
Inhibitory constant against HMG-CoA reductase |
Bioorg Med Chem Lett 15: 989-94 (2005)
Article DOI: 10.1016/j.bmcl.2004.12.046
BindingDB Entry DOI: 10.7270/Q2BC3Z1P |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Furin
(Homo sapiens (Human)) | BDBM50387001
(CHEMBL2049154)Show SMILES [#6]-[#6](-[#6])-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#7]-[#6](-[#6])=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-c1ccc(cc1)-[#6](-[#7])=[#7] |r| Show InChI InChI=1S/C26H44N12O4/c1-14(2)20(38-23(41)18(35-15(3)39)6-4-12-33-25(29)30)24(42)37-19(7-5-13-34-26(31)32)22(40)36-17-10-8-16(9-11-17)21(27)28/h8-11,14,18-20H,4-7,12-13H2,1-3H3,(H3,27,28)(H,35,39)(H,36,40)(H,37,42)(H,38,41)(H4,29,30,33)(H4,31,32,34)/t18-,19-,20-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Torrey Pines Institute for Molecular Studies
Curated by ChEMBL
| Assay Description
Inhibition of furin |
Bioorg Med Chem 20: 4462-71 (2012)
Article DOI: 10.1016/j.bmc.2012.05.029
BindingDB Entry DOI: 10.7270/Q2514075 |
More data for this
Ligand-Target Pair | |
Furin
(Homo sapiens (Human)) | BDBM50387000
(CHEMBL2049153)Show SMILES [#6]-[#6]-[#6]-[#6]-[#6]-[#6]-[#6]-[#6]-[#6]-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6](-[#6])-[#6])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-c1ccc(cc1)-[#6](-[#7])=[#7] |r| Show InChI InChI=1S/C34H60N12O4/c1-4-5-6-7-8-9-10-15-27(47)44-25(13-11-20-41-33(37)38)31(49)46-28(22(2)3)32(50)45-26(14-12-21-42-34(39)40)30(48)43-24-18-16-23(17-19-24)29(35)36/h16-19,22,25-26,28H,4-15,20-21H2,1-3H3,(H3,35,36)(H,43,48)(H,44,47)(H,45,50)(H,46,49)(H4,37,38,41)(H4,39,40,42)/t25-,26-,28-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Torrey Pines Institute for Molecular Studies
Curated by ChEMBL
| Assay Description
Inhibition of furin |
Bioorg Med Chem 20: 4462-71 (2012)
Article DOI: 10.1016/j.bmc.2012.05.029
BindingDB Entry DOI: 10.7270/Q2514075 |
More data for this
Ligand-Target Pair | |
3-hydroxy-3-methylglutaryl-coenzyme A reductase
(Homo sapiens (Human)) | BDBM50139181

((1S,3R,7S,8S,8aR)-8-{2-[(2R,4R)-4-hydroxy-6-oxotet...)Show SMILES CCC(C)(C)C(=O)O[C@H]1C[C@@H](C)C=C2C=C[C@H](C)[C@H](CC[C@@H]3C[C@@H](O)CC(=O)O3)[C@@H]12 |r,c:14,t:12| Show InChI InChI=1S/C25H38O5/c1-6-25(4,5)24(28)30-21-12-15(2)11-17-8-7-16(3)20(23(17)21)10-9-19-13-18(26)14-22(27)29-19/h7-8,11,15-16,18-21,23,26H,6,9-10,12-14H2,1-5H3/t15-,16-,18+,19+,20-,21-,23-/m0/s1 | PDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Nacional Autónoma de México
Curated by ChEMBL
| Assay Description
Inhibitory constant against HMG-CoA reductase |
Bioorg Med Chem Lett 15: 989-94 (2005)
Article DOI: 10.1016/j.bmcl.2004.12.046
BindingDB Entry DOI: 10.7270/Q2BC3Z1P |
More data for this
Ligand-Target Pair | |
Furin
(Homo sapiens (Human)) | BDBM50387002

(CHEMBL2049155)Show SMILES [#6]-[#6]-[#6]-[#6]-[#6]-[#6]-[#6]-[#6]-[#6]-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6](-[#6])-[#6])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#6](=O)-[#7]-c1ccc(cc1)-[#6](-[#7])=[#7] |r| Show InChI InChI=1S/C34H60N10O4/c1-4-5-6-7-8-9-10-16-28(45)42-26(15-13-22-40-34(38)39)32(47)44-29(23(2)3)33(48)43-27(14-11-12-21-35)31(46)41-25-19-17-24(18-20-25)30(36)37/h17-20,23,26-27,29H,4-16,21-22,35H2,1-3H3,(H3,36,37)(H,41,46)(H,42,45)(H,43,48)(H,44,47)(H4,38,39,40)/t26-,27-,29-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Torrey Pines Institute for Molecular Studies
Curated by ChEMBL
| Assay Description
Inhibition of furin |
Bioorg Med Chem 20: 4462-71 (2012)
Article DOI: 10.1016/j.bmc.2012.05.029
BindingDB Entry DOI: 10.7270/Q2514075 |
More data for this
Ligand-Target Pair | |
3-hydroxy-3-methylglutaryl-coenzyme A reductase
(Homo sapiens (Human)) | BDBM50160720
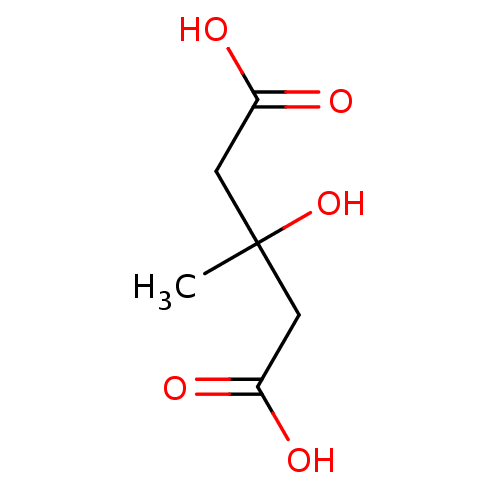
(3-hydroxy-3-methylglutaric acid | 3-hydroxy-3-meth...)Show InChI InChI=1S/C6H10O5/c1-6(11,2-4(7)8)3-5(9)10/h11H,2-3H2,1H3,(H,7,8)(H,9,10) | PDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 23.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Nacional Autónoma de México
Curated by ChEMBL
| Assay Description
Inhibitory constant against HMG-CoA reductase |
Bioorg Med Chem Lett 15: 989-94 (2005)
Article DOI: 10.1016/j.bmcl.2004.12.046
BindingDB Entry DOI: 10.7270/Q2BC3Z1P |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Furin
(Homo sapiens (Human)) | BDBM50386998

(CHEMBL2049151)Show SMILES [#6]-[#6](-[#6])-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#7]-[#6]-[#6](=O)-[#8]-c1ccccc1)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-[#6]-1-[#6]-[#6]-[#7](-[#6]-[#6]-1)-[#6](-[#7])=[#7] |r| Show InChI InChI=1S/C31H53N13O5/c1-19(2)25(43-26(46)22(10-6-14-38-29(32)33)40-18-24(45)49-21-8-4-3-5-9-21)28(48)42-23(11-7-15-39-30(34)35)27(47)41-20-12-16-44(17-13-20)31(36)37/h3-5,8-9,19-20,22-23,25,40H,6-7,10-18H2,1-2H3,(H3,36,37)(H,41,47)(H,42,48)(H,43,46)(H4,32,33,38)(H4,34,35,39)/t22-,23-,25-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 53 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Torrey Pines Institute for Molecular Studies
Curated by ChEMBL
| Assay Description
Inhibition of furin |
Bioorg Med Chem 20: 4462-71 (2012)
Article DOI: 10.1016/j.bmc.2012.05.029
BindingDB Entry DOI: 10.7270/Q2514075 |
More data for this
Ligand-Target Pair | |
Furin
(Homo sapiens (Human)) | BDBM50386989
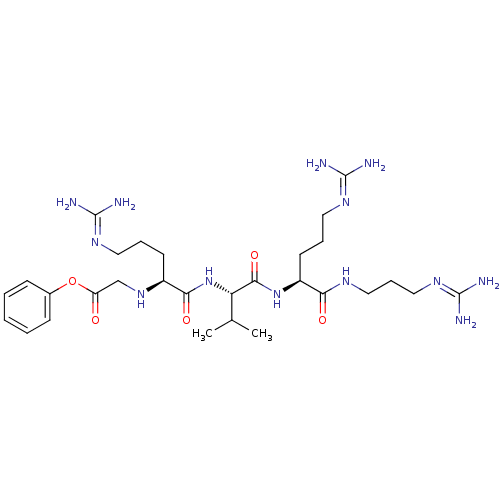
(CHEMBL2049013)Show SMILES [#6]-[#6](-[#6])-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#7]-[#6]-[#6](=O)-[#8]-c1ccccc1)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7] |r| Show InChI InChI=1S/C29H51N13O5/c1-18(2)23(26(46)41-21(12-7-14-38-28(32)33)24(44)36-15-8-16-39-29(34)35)42-25(45)20(11-6-13-37-27(30)31)40-17-22(43)47-19-9-4-3-5-10-19/h3-5,9-10,18,20-21,23,40H,6-8,11-17H2,1-2H3,(H,36,44)(H,41,46)(H,42,45)(H4,30,31,37)(H4,32,33,38)(H4,34,35,39)/t20-,21-,23-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 63 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Torrey Pines Institute for Molecular Studies
Curated by ChEMBL
| Assay Description
Inhibition of furin |
Bioorg Med Chem 20: 4462-71 (2012)
Article DOI: 10.1016/j.bmc.2012.05.029
BindingDB Entry DOI: 10.7270/Q2514075 |
More data for this
Ligand-Target Pair | |
Furin
(Homo sapiens (Human)) | BDBM50386987
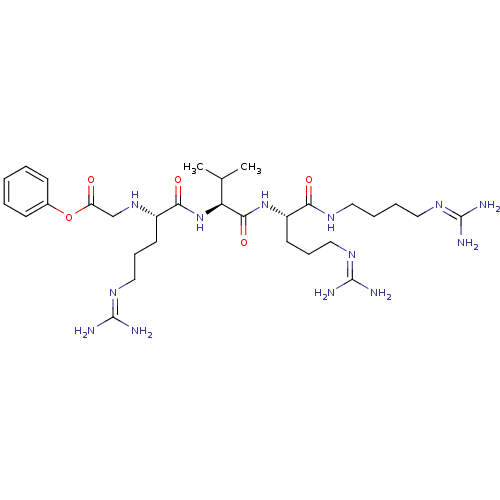
(CHEMBL2049015)Show SMILES [#6]-[#6](-[#6])-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#7]-[#6]-[#6](=O)-[#8]-c1ccccc1)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-[#6]-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7] |r| Show InChI InChI=1S/C30H53N13O5/c1-19(2)24(27(47)42-22(13-9-17-40-30(35)36)25(45)37-14-6-7-15-38-28(31)32)43-26(46)21(12-8-16-39-29(33)34)41-18-23(44)48-20-10-4-3-5-11-20/h3-5,10-11,19,21-22,24,41H,6-9,12-18H2,1-2H3,(H,37,45)(H,42,47)(H,43,46)(H4,31,32,38)(H4,33,34,39)(H4,35,36,40)/t21-,22-,24-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 78 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Torrey Pines Institute for Molecular Studies
Curated by ChEMBL
| Assay Description
Inhibition of furin |
Bioorg Med Chem 20: 4462-71 (2012)
Article DOI: 10.1016/j.bmc.2012.05.029
BindingDB Entry DOI: 10.7270/Q2514075 |
More data for this
Ligand-Target Pair | |
Furin
(Homo sapiens (Human)) | BDBM50387012
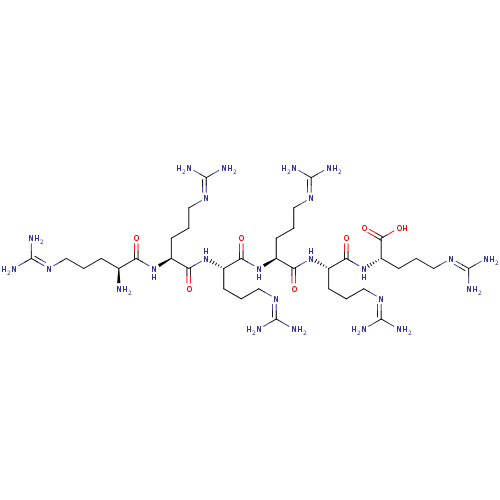
(CHEMBL2049164)Show SMILES [#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#6](-[#8])=O |r| Show InChI InChI=1S/C36H74N24O7/c37-19(7-1-13-50-31(38)39)25(61)56-20(8-2-14-51-32(40)41)26(62)57-21(9-3-15-52-33(42)43)27(63)58-22(10-4-16-53-34(44)45)28(64)59-23(11-5-17-54-35(46)47)29(65)60-24(30(66)67)12-6-18-55-36(48)49/h19-24H,1-18,37H2,(H,56,61)(H,57,62)(H,58,63)(H,59,64)(H,60,65)(H,66,67)(H4,38,39,50)(H4,40,41,51)(H4,42,43,52)(H4,44,45,53)(H4,46,47,54)(H4,48,49,55)/t19-,20-,21-,22-,23-,24-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 114 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Torrey Pines Institute for Molecular Studies
Curated by ChEMBL
| Assay Description
Inhibition of furin |
Bioorg Med Chem 20: 4462-71 (2012)
Article DOI: 10.1016/j.bmc.2012.05.029
BindingDB Entry DOI: 10.7270/Q2514075 |
More data for this
Ligand-Target Pair | |
Furin
(Homo sapiens (Human)) | BDBM50387005
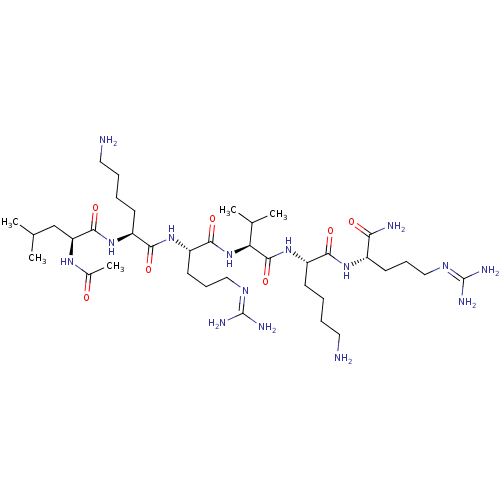
(CHEMBL2049158)Show SMILES [#6]-[#6](-[#6])-[#6]-[#6@H](-[#7]-[#6](-[#6])=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6](-[#6])-[#6])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#6](-[#7])=O |r| Show InChI InChI=1S/C37H73N15O7/c1-21(2)20-28(47-23(5)53)34(58)50-25(12-6-8-16-38)32(56)49-27(15-11-19-46-37(43)44)33(57)52-29(22(3)4)35(59)51-26(13-7-9-17-39)31(55)48-24(30(40)54)14-10-18-45-36(41)42/h21-22,24-29H,6-20,38-39H2,1-5H3,(H2,40,54)(H,47,53)(H,48,55)(H,49,56)(H,50,58)(H,51,59)(H,52,57)(H4,41,42,45)(H4,43,44,46)/t24-,25-,26-,27-,28-,29-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Torrey Pines Institute for Molecular Studies
Curated by ChEMBL
| Assay Description
Inhibition of furin |
Bioorg Med Chem 20: 4462-71 (2012)
Article DOI: 10.1016/j.bmc.2012.05.029
BindingDB Entry DOI: 10.7270/Q2514075 |
More data for this
Ligand-Target Pair | |
Furin
(Homo sapiens (Human)) | BDBM50387009
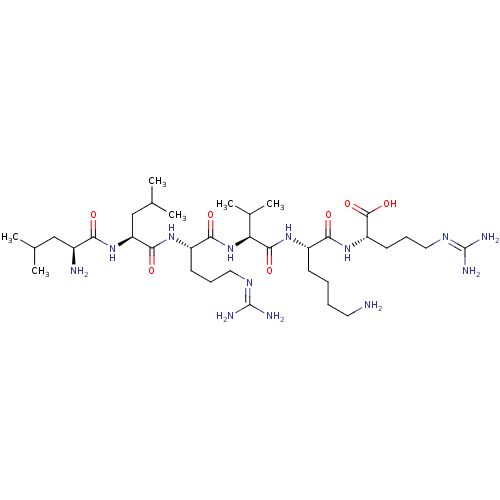
(CHEMBL2049161)Show SMILES [#6]-[#6](-[#6])-[#6]-[#6@H](-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#6])-[#6])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6](-[#6])-[#6])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#6](-[#8])=O |r| Show InChI InChI=1S/C35H69N13O7/c1-19(2)17-22(37)28(49)47-26(18-20(3)4)31(52)44-24(12-9-15-42-34(38)39)30(51)48-27(21(5)6)32(53)45-23(11-7-8-14-36)29(50)46-25(33(54)55)13-10-16-43-35(40)41/h19-27H,7-18,36-37H2,1-6H3,(H,44,52)(H,45,53)(H,46,50)(H,47,49)(H,48,51)(H,54,55)(H4,38,39,42)(H4,40,41,43)/t22-,23-,24-,25-,26-,27-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 420 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Torrey Pines Institute for Molecular Studies
Curated by ChEMBL
| Assay Description
Inhibition of furin |
Bioorg Med Chem 20: 4462-71 (2012)
Article DOI: 10.1016/j.bmc.2012.05.029
BindingDB Entry DOI: 10.7270/Q2514075 |
More data for this
Ligand-Target Pair | |
Furin
(Homo sapiens (Human)) | BDBM50386991
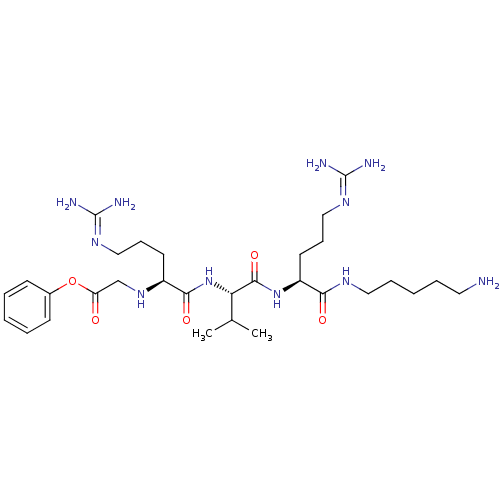
(CHEMBL2049016)Show SMILES [#6]-[#6](-[#6])-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#7]-[#6]-[#6](=O)-[#8]-c1ccccc1)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-[#6]-[#6]-[#6]-[#6]-[#6]-[#7] |r| Show InChI InChI=1S/C30H53N11O5/c1-20(2)25(28(45)40-23(14-10-18-38-30(34)35)26(43)36-16-8-4-7-15-31)41-27(44)22(13-9-17-37-29(32)33)39-19-24(42)46-21-11-5-3-6-12-21/h3,5-6,11-12,20,22-23,25,39H,4,7-10,13-19,31H2,1-2H3,(H,36,43)(H,40,45)(H,41,44)(H4,32,33,37)(H4,34,35,38)/t22-,23-,25-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 553 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Torrey Pines Institute for Molecular Studies
Curated by ChEMBL
| Assay Description
Inhibition of furin |
Bioorg Med Chem 20: 4462-71 (2012)
Article DOI: 10.1016/j.bmc.2012.05.029
BindingDB Entry DOI: 10.7270/Q2514075 |
More data for this
Ligand-Target Pair | |
Furin
(Homo sapiens (Human)) | BDBM50386993

(CHEMBL2049018)Show SMILES [#6]-[#6]-c1ccc(-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#7]-[#6](=O)-[#6@@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#7]-[#6]-[#6](=O)-[#8]-c2ccccc2)-[#6](-[#6])-[#6])cc1 |r| Show InChI InChI=1S/C33H50N10O5/c1-4-22-14-16-23(17-15-22)41-30(46)26(13-9-19-39-33(36)37)42-31(47)28(21(2)3)43-29(45)25(12-8-18-38-32(34)35)40-20-27(44)48-24-10-6-5-7-11-24/h5-7,10-11,14-17,21,25-26,28,40H,4,8-9,12-13,18-20H2,1-3H3,(H,41,46)(H,42,47)(H,43,45)(H4,34,35,38)(H4,36,37,39)/t25-,26-,28-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 627 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Torrey Pines Institute for Molecular Studies
Curated by ChEMBL
| Assay Description
Inhibition of furin |
Bioorg Med Chem 20: 4462-71 (2012)
Article DOI: 10.1016/j.bmc.2012.05.029
BindingDB Entry DOI: 10.7270/Q2514075 |
More data for this
Ligand-Target Pair | |
Furin
(Homo sapiens (Human)) | BDBM50387007
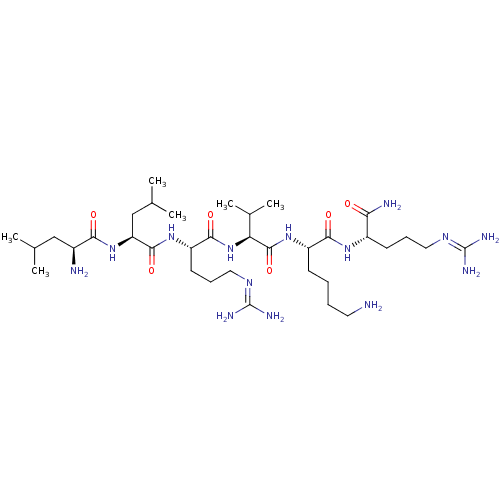
(CHEMBL2049160)Show SMILES [#6]-[#6](-[#6])-[#6]-[#6@H](-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#6])-[#6])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6](-[#6])-[#6])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#6](-[#7])=O |r| Show InChI InChI=1S/C35H70N14O6/c1-19(2)17-22(37)29(51)48-26(18-20(3)4)32(54)46-25(13-10-16-44-35(41)42)31(53)49-27(21(5)6)33(55)47-24(11-7-8-14-36)30(52)45-23(28(38)50)12-9-15-43-34(39)40/h19-27H,7-18,36-37H2,1-6H3,(H2,38,50)(H,45,52)(H,46,54)(H,47,55)(H,48,51)(H,49,53)(H4,39,40,43)(H4,41,42,44)/t22-,23-,24-,25-,26-,27-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Torrey Pines Institute for Molecular Studies
Curated by ChEMBL
| Assay Description
Inhibition of furin |
Bioorg Med Chem 20: 4462-71 (2012)
Article DOI: 10.1016/j.bmc.2012.05.029
BindingDB Entry DOI: 10.7270/Q2514075 |
More data for this
Ligand-Target Pair | |
Furin
(Homo sapiens (Human)) | BDBM50387011
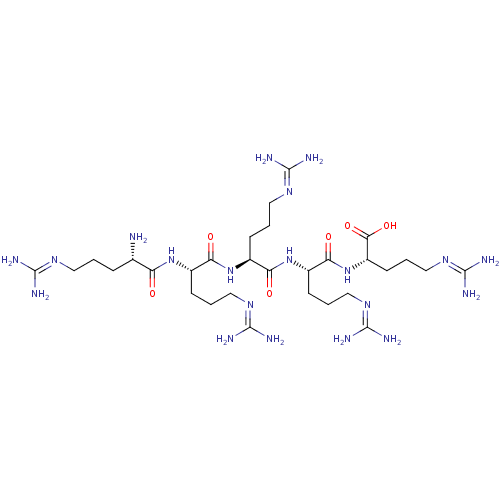
(CHEMBL2049163)Show SMILES [#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#6](-[#8])=O |r| Show InChI InChI=1S/C30H62N20O6/c31-16(6-1-11-42-26(32)33)21(51)47-17(7-2-12-43-27(34)35)22(52)48-18(8-3-13-44-28(36)37)23(53)49-19(9-4-14-45-29(38)39)24(54)50-20(25(55)56)10-5-15-46-30(40)41/h16-20H,1-15,31H2,(H,47,51)(H,48,52)(H,49,53)(H,50,54)(H,55,56)(H4,32,33,42)(H4,34,35,43)(H4,36,37,44)(H4,38,39,45)(H4,40,41,46)/t16-,17-,18-,19-,20-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 990 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Torrey Pines Institute for Molecular Studies
Curated by ChEMBL
| Assay Description
Inhibition of furin |
Bioorg Med Chem 20: 4462-71 (2012)
Article DOI: 10.1016/j.bmc.2012.05.029
BindingDB Entry DOI: 10.7270/Q2514075 |
More data for this
Ligand-Target Pair | |
Furin
(Homo sapiens (Human)) | BDBM50386992

(CHEMBL2049017)Show SMILES [#6]-[#6](-[#6])-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#7]-[#6]-[#6](=O)-[#8]-c1ccccc1)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-[#6]-[#6]-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7] |r| Show InChI InChI=1S/C31H55N13O5/c1-20(2)25(28(48)43-23(14-10-18-41-31(36)37)26(46)38-15-7-4-8-16-39-29(32)33)44-27(47)22(13-9-17-40-30(34)35)42-19-24(45)49-21-11-5-3-6-12-21/h3,5-6,11-12,20,22-23,25,42H,4,7-10,13-19H2,1-2H3,(H,38,46)(H,43,48)(H,44,47)(H4,32,33,39)(H4,34,35,40)(H4,36,37,41)/t22-,23-,25-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.07E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Torrey Pines Institute for Molecular Studies
Curated by ChEMBL
| Assay Description
Inhibition of furin |
Bioorg Med Chem 20: 4462-71 (2012)
Article DOI: 10.1016/j.bmc.2012.05.029
BindingDB Entry DOI: 10.7270/Q2514075 |
More data for this
Ligand-Target Pair | |
Furin
(Homo sapiens (Human)) | BDBM50386995
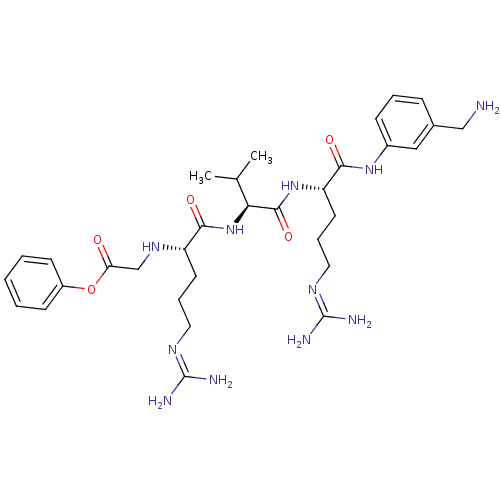
(CHEMBL2049020)Show SMILES [#6]-[#6](-[#6])-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#7]-[#6]-[#6](=O)-[#8]-c1ccccc1)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-c1cccc(-[#6]-[#7])c1 |r| Show InChI InChI=1S/C32H49N11O5/c1-20(2)27(30(47)42-25(14-8-16-39-32(36)37)29(46)41-22-10-6-9-21(17-22)18-33)43-28(45)24(13-7-15-38-31(34)35)40-19-26(44)48-23-11-4-3-5-12-23/h3-6,9-12,17,20,24-25,27,40H,7-8,13-16,18-19,33H2,1-2H3,(H,41,46)(H,42,47)(H,43,45)(H4,34,35,38)(H4,36,37,39)/t24-,25-,27-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.32E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Torrey Pines Institute for Molecular Studies
Curated by ChEMBL
| Assay Description
Inhibition of furin |
Bioorg Med Chem 20: 4462-71 (2012)
Article DOI: 10.1016/j.bmc.2012.05.029
BindingDB Entry DOI: 10.7270/Q2514075 |
More data for this
Ligand-Target Pair | |
Furin
(Homo sapiens (Human)) | BDBM50387003
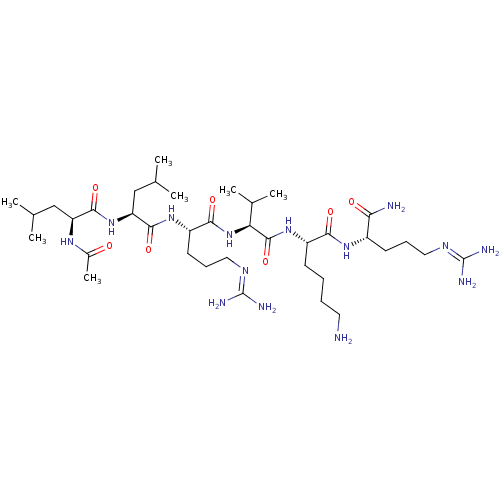
(CHEMBL2049156)Show SMILES [#6]-[#6](-[#6])-[#6]-[#6@H](-[#7]-[#6](-[#6])=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#6])-[#6])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6](-[#6])-[#6])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#6](-[#7])=O |r| Show InChI InChI=1S/C37H72N14O7/c1-20(2)18-27(46-23(7)52)33(56)50-28(19-21(3)4)34(57)48-26(14-11-17-45-37(42)43)32(55)51-29(22(5)6)35(58)49-25(12-8-9-15-38)31(54)47-24(30(39)53)13-10-16-44-36(40)41/h20-22,24-29H,8-19,38H2,1-7H3,(H2,39,53)(H,46,52)(H,47,54)(H,48,57)(H,49,58)(H,50,56)(H,51,55)(H4,40,41,44)(H4,42,43,45)/t24-,25-,26-,27-,28-,29-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Torrey Pines Institute for Molecular Studies
Curated by ChEMBL
| Assay Description
Inhibition of furin |
Bioorg Med Chem 20: 4462-71 (2012)
Article DOI: 10.1016/j.bmc.2012.05.029
BindingDB Entry DOI: 10.7270/Q2514075 |
More data for this
Ligand-Target Pair | |
Furin
(Homo sapiens (Human)) | BDBM50386994

(CHEMBL2049019)Show SMILES [#6]-[#6](-[#6])-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#7]-[#6]-[#6](=O)-[#8]-c1ccccc1)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-c1ccc(-[#6]\[#7]=[#6](\[#7])-[#7])cc1 |r| Show InChI InChI=1S/C33H51N13O5/c1-20(2)27(46-28(48)24(10-6-16-40-31(34)35)42-19-26(47)51-23-8-4-3-5-9-23)30(50)45-25(11-7-17-41-32(36)37)29(49)44-22-14-12-21(13-15-22)18-43-33(38)39/h3-5,8-9,12-15,20,24-25,27,42H,6-7,10-11,16-19H2,1-2H3,(H,44,49)(H,45,50)(H,46,48)(H4,34,35,40)(H4,36,37,41)(H4,38,39,43)/t24-,25-,27-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.43E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Torrey Pines Institute for Molecular Studies
Curated by ChEMBL
| Assay Description
Inhibition of furin |
Bioorg Med Chem 20: 4462-71 (2012)
Article DOI: 10.1016/j.bmc.2012.05.029
BindingDB Entry DOI: 10.7270/Q2514075 |
More data for this
Ligand-Target Pair | |
Furin
(Homo sapiens (Human)) | BDBM50387004

(CHEMBL2049157)Show SMILES [#6]-[#16]-[#6]-[#6]-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#6])-[#6])-[#7]-[#6](-[#6])=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6](-[#6])-[#6])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#6](-[#7])=O |r| Show InChI InChI=1S/C36H70N14O7S/c1-20(2)19-27(45-22(5)51)33(56)48-26(14-18-58-6)31(54)47-25(13-10-17-44-36(41)42)32(55)50-28(21(3)4)34(57)49-24(11-7-8-15-37)30(53)46-23(29(38)52)12-9-16-43-35(39)40/h20-21,23-28H,7-19,37H2,1-6H3,(H2,38,52)(H,45,51)(H,46,53)(H,47,54)(H,48,56)(H,49,57)(H,50,55)(H4,39,40,43)(H4,41,42,44)/t23-,24-,25-,26-,27-,28-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Torrey Pines Institute for Molecular Studies
Curated by ChEMBL
| Assay Description
Inhibition of furin |
Bioorg Med Chem 20: 4462-71 (2012)
Article DOI: 10.1016/j.bmc.2012.05.029
BindingDB Entry DOI: 10.7270/Q2514075 |
More data for this
Ligand-Target Pair | |
Furin
(Homo sapiens (Human)) | BDBM50386996
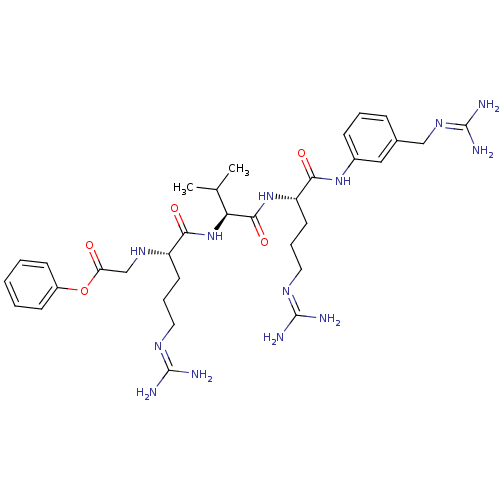
(CHEMBL2049149)Show SMILES [#6]-[#6](-[#6])-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#7]-[#6]-[#6](=O)-[#8]-c1ccccc1)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-c1cccc(-[#6]\[#7]=[#6](\[#7])-[#7])c1 |r| Show InChI InChI=1S/C33H51N13O5/c1-20(2)27(46-28(48)24(13-7-15-40-31(34)35)42-19-26(47)51-23-11-4-3-5-12-23)30(50)45-25(14-8-16-41-32(36)37)29(49)44-22-10-6-9-21(17-22)18-43-33(38)39/h3-6,9-12,17,20,24-25,27,42H,7-8,13-16,18-19H2,1-2H3,(H,44,49)(H,45,50)(H,46,48)(H4,34,35,40)(H4,36,37,41)(H4,38,39,43)/t24-,25-,27-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.73E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Torrey Pines Institute for Molecular Studies
Curated by ChEMBL
| Assay Description
Inhibition of furin |
Bioorg Med Chem 20: 4462-71 (2012)
Article DOI: 10.1016/j.bmc.2012.05.029
BindingDB Entry DOI: 10.7270/Q2514075 |
More data for this
Ligand-Target Pair | |
Furin
(Homo sapiens (Human)) | BDBM50386988

(CHEMBL2049012)Show SMILES [#6]-[#6](-[#6])-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#7]-[#6]-[#6](=O)-[#8]-c1ccccc1)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-[#6]-[#6]-[#6]-[#7] |r| Show InChI InChI=1S/C28H49N11O5/c1-18(2)23(26(43)38-21(12-7-15-36-28(32)33)24(41)34-16-8-13-29)39-25(42)20(11-6-14-35-27(30)31)37-17-22(40)44-19-9-4-3-5-10-19/h3-5,9-10,18,20-21,23,37H,6-8,11-17,29H2,1-2H3,(H,34,41)(H,38,43)(H,39,42)(H4,30,31,35)(H4,32,33,36)/t20-,21-,23-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.02E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Torrey Pines Institute for Molecular Studies
Curated by ChEMBL
| Assay Description
Inhibition of furin |
Bioorg Med Chem 20: 4462-71 (2012)
Article DOI: 10.1016/j.bmc.2012.05.029
BindingDB Entry DOI: 10.7270/Q2514075 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50148911

((3beta)-3-hydroxyurs-12-en-28-oic acid | 3beta-hyd...)Show SMILES C[C@@H]1CC[C@@]2(CC[C@]3(C)C(=CC[C@@H]4[C@@]5(C)CC[C@H](O)C(C)(C)[C@@H]5CC[C@@]34C)[C@@H]2[C@H]1C)C(O)=O |r,c:9| Show InChI InChI=1S/C30H48O3/c1-18-10-15-30(25(32)33)17-16-28(6)20(24(30)19(18)2)8-9-22-27(5)13-12-23(31)26(3,4)21(27)11-14-29(22,28)7/h8,18-19,21-24,31H,9-17H2,1-7H3,(H,32,33)/t18-,19+,21+,22-,23+,24+,27+,28-,29-,30+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 3.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Aut£noma del Estado de Morelos
Curated by ChEMBL
| Assay Description
Mixed type inhibition of human recombinant PTP-1B expressed in Escherichia coli TB1 using p-nitrophenyl phosphate as substrate |
Eur J Med Chem 46: 2243-51 (2011)
Article DOI: 10.1016/j.ejmech.2011.03.005
BindingDB Entry DOI: 10.7270/Q2FT8N4M |
More data for this
Ligand-Target Pair | |
Furin
(Homo sapiens (Human)) | BDBM50387006
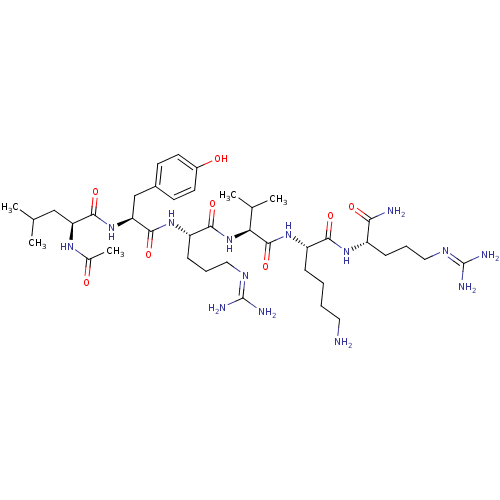
(CHEMBL2049159)Show SMILES [#6]-[#6](-[#6])-[#6]-[#6@H](-[#7]-[#6](-[#6])=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-c1ccc(-[#8])cc1)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6](-[#6])-[#6])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#6](-[#7])=O |r| Show InChI InChI=1S/C40H70N14O8/c1-22(2)20-30(49-24(5)55)36(60)53-31(21-25-13-15-26(56)16-14-25)37(61)51-29(12-9-19-48-40(45)46)35(59)54-32(23(3)4)38(62)52-28(10-6-7-17-41)34(58)50-27(33(42)57)11-8-18-47-39(43)44/h13-16,22-23,27-32,56H,6-12,17-21,41H2,1-5H3,(H2,42,57)(H,49,55)(H,50,58)(H,51,61)(H,52,62)(H,53,60)(H,54,59)(H4,43,44,47)(H4,45,46,48)/t27-,28-,29-,30-,31-,32-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Torrey Pines Institute for Molecular Studies
Curated by ChEMBL
| Assay Description
Inhibition of furin |
Bioorg Med Chem 20: 4462-71 (2012)
Article DOI: 10.1016/j.bmc.2012.05.029
BindingDB Entry DOI: 10.7270/Q2514075 |
More data for this
Ligand-Target Pair | |
Furin
(Homo sapiens (Human)) | BDBM50387008

(CHEMBL2046471)Show SMILES [#6]-[#6](-[#6])-[#6]-[#6@H](-[#7]-[#6](-[#6])=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#6])-[#6])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6](-[#6])-[#6])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#6](-[#8])=O |r| Show InChI InChI=1S/C37H71N13O8/c1-20(2)18-27(45-23(7)51)32(54)49-28(19-21(3)4)33(55)46-25(13-10-16-43-36(39)40)31(53)50-29(22(5)6)34(56)47-24(12-8-9-15-38)30(52)48-26(35(57)58)14-11-17-44-37(41)42/h20-22,24-29H,8-19,38H2,1-7H3,(H,45,51)(H,46,55)(H,47,56)(H,48,52)(H,49,54)(H,50,53)(H,57,58)(H4,39,40,43)(H4,41,42,44)/t24-,25-,26-,27-,28-,29-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Torrey Pines Institute for Molecular Studies
Curated by ChEMBL
| Assay Description
Inhibition of furin |
Bioorg Med Chem 20: 4462-71 (2012)
Article DOI: 10.1016/j.bmc.2012.05.029
BindingDB Entry DOI: 10.7270/Q2514075 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50346601

(NSC-114945 | OLEANOLIC_ACID | Oleanolic acid | Ole...)Show SMILES CC1(C)CC[C@@]2(CC[C@]3(C)C(=CC[C@@H]4[C@@]5(C)CC[C@H](O)C(C)(C)[C@@H]5CC[C@@]34C)[C@@H]2C1)C(O)=O |r,c:10| Show InChI InChI=1S/C30H48O3/c1-25(2)14-16-30(24(32)33)17-15-28(6)19(20(30)18-25)8-9-22-27(5)12-11-23(31)26(3,4)21(27)10-13-29(22,28)7/h8,20-23,31H,9-18H2,1-7H3,(H,32,33)/t20-,21-,22+,23-,27-,28+,29+,30-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 6.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Aut£noma del Estado de Morelos
Curated by ChEMBL
| Assay Description
Mixed type inhibition of human recombinant PTP-1B expressed in Escherichia coli TB1 using p-nitrophenyl phosphate as substrate |
Eur J Med Chem 46: 2243-51 (2011)
Article DOI: 10.1016/j.ejmech.2011.03.005
BindingDB Entry DOI: 10.7270/Q2FT8N4M |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50385306

(CHEMBL2002784)Show InChI InChI=1S/C12H12N2O3S/c1-3-17-11(16)10(15)14-12-13-8-5-4-7(2)6-9(8)18-12/h4-6H,3H2,1-2H3,(H,13,14,15) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Aut£noma del Estado de Morelos
Curated by ChEMBL
| Assay Description
Mixed-type inhibition of human recombinant GST-tagged PTP1B expressed in Escherichia coli TB1 cells using p-NPP as substrate by double reciprocal plo... |
Eur J Med Chem 53: 346-55 (2012)
Article DOI: 10.1016/j.ejmech.2012.04.025
BindingDB Entry DOI: 10.7270/Q2KP836R |
More data for this
Ligand-Target Pair | |
Furin
(Homo sapiens (Human)) | BDBM50387010
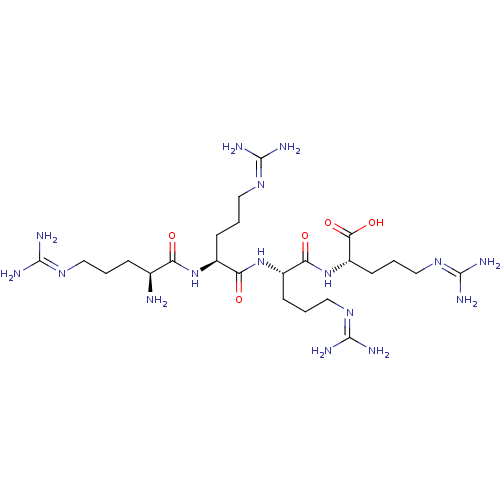
(CHEMBL2049162)Show SMILES [#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#6](-[#8])=O |r| Show InChI InChI=1S/C24H50N16O5/c25-13(5-1-9-34-21(26)27)17(41)38-14(6-2-10-35-22(28)29)18(42)39-15(7-3-11-36-23(30)31)19(43)40-16(20(44)45)8-4-12-37-24(32)33/h13-16H,1-12,25H2,(H,38,41)(H,39,42)(H,40,43)(H,44,45)(H4,26,27,34)(H4,28,29,35)(H4,30,31,36)(H4,32,33,37)/t13-,14-,15-,16-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Torrey Pines Institute for Molecular Studies
Curated by ChEMBL
| Assay Description
Inhibition of furin |
Bioorg Med Chem 20: 4462-71 (2012)
Article DOI: 10.1016/j.bmc.2012.05.029
BindingDB Entry DOI: 10.7270/Q2514075 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50391854

(CHEMBL463665)Show SMILES CC1(C)CC[C@@]2(CC[C@]3(C)[C@H](CC[C@@H]4[C@@]5(C)CC[C@H](O)C(C)(C)[C@@H]5CC[C@@]34C)C2=C1)C(O)=O |r,c:32| Show InChI InChI=1S/C30H48O3/c1-25(2)14-16-30(24(32)33)17-15-28(6)19(20(30)18-25)8-9-22-27(5)12-11-23(31)26(3,4)21(27)10-13-29(22,28)7/h18-19,21-23,31H,8-17H2,1-7H3,(H,32,33)/t19-,21+,22-,23+,27+,28-,29-,30+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Aut£noma del Estado de Morelos
Curated by ChEMBL
| Assay Description
Non-competitive inhibition of human recombinant PTP-1B expressed in Escherichia coli TB1 using p-nitrophenyl phosphate as substrate |
Eur J Med Chem 46: 2243-51 (2011)
Article DOI: 10.1016/j.ejmech.2011.03.005
BindingDB Entry DOI: 10.7270/Q2FT8N4M |
More data for this
Ligand-Target Pair | |
Furin
(Homo sapiens (Human)) | BDBM50386990
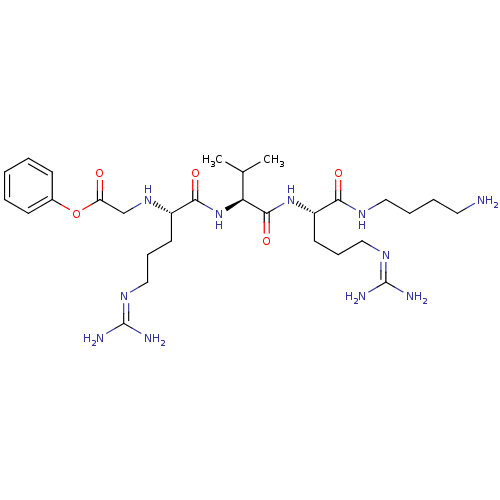
(CHEMBL2049014)Show SMILES [#6]-[#6](-[#6])-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#7]-[#6]-[#6](=O)-[#8]-c1ccccc1)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-[#6]-[#6]-[#6]-[#6]-[#7] |r| Show InChI InChI=1S/C29H51N11O5/c1-19(2)24(27(44)39-22(13-9-17-37-29(33)34)25(42)35-15-7-6-14-30)40-26(43)21(12-8-16-36-28(31)32)38-18-23(41)45-20-10-4-3-5-11-20/h3-5,10-11,19,21-22,24,38H,6-9,12-18,30H2,1-2H3,(H,35,42)(H,39,44)(H,40,43)(H4,31,32,36)(H4,33,34,37)/t21-,22-,24-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7.49E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Torrey Pines Institute for Molecular Studies
Curated by ChEMBL
| Assay Description
Inhibition of furin |
Bioorg Med Chem 20: 4462-71 (2012)
Article DOI: 10.1016/j.bmc.2012.05.029
BindingDB Entry DOI: 10.7270/Q2514075 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50385308

(CHEMBL2035962)Show InChI InChI=1S/C11H9FN2O3S/c1-2-17-10(16)9(15)14-11-13-7-4-3-6(12)5-8(7)18-11/h3-5H,2H2,1H3,(H,13,14,15) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Aut£noma del Estado de Morelos
Curated by ChEMBL
| Assay Description
Mixed-type inhibition of human recombinant GST-tagged PTP1B expressed in Escherichia coli TB1 cells using p-NPP as substrate by double reciprocal plo... |
Eur J Med Chem 53: 346-55 (2012)
Article DOI: 10.1016/j.ejmech.2012.04.025
BindingDB Entry DOI: 10.7270/Q2KP836R |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50385307

(CHEMBL2035961)Show InChI InChI=1S/C11H9ClN2O3S/c1-2-17-10(16)9(15)14-11-13-7-4-3-6(12)5-8(7)18-11/h3-5H,2H2,1H3,(H,13,14,15) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Aut£noma del Estado de Morelos
Curated by ChEMBL
| Assay Description
Mixed-type inhibition of human recombinant GST-tagged PTP1B expressed in Escherichia coli TB1 cells using p-NPP as substrate by double reciprocal plo... |
Eur J Med Chem 53: 346-55 (2012)
Article DOI: 10.1016/j.ejmech.2012.04.025
BindingDB Entry DOI: 10.7270/Q2KP836R |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM54263

((4aS,6aR,6aR,6bR,8aR,12aR,14aS)-10-keto-2,2,6a,6b,...)Show SMILES CC1(C)CC[C@@]2(CC[C@]3(C)[C@H](CC[C@@H]4[C@@]5(C)CCC(=O)C(C)(C)[C@@H]5CC[C@@]34C)C2=C1)C(O)=O |c:32| Show InChI InChI=1S/C30H46O3/c1-25(2)14-16-30(24(32)33)17-15-28(6)19(20(30)18-25)8-9-22-27(5)12-11-23(31)26(3,4)21(27)10-13-29(22,28)7/h18-19,21-22H,8-17H2,1-7H3,(H,32,33)/t19-,21+,22-,27+,28-,29-,30+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 8.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Aut£noma del Estado de Morelos
Curated by ChEMBL
| Assay Description
Mixed type inhibition of human recombinant PTP-1B expressed in Escherichia coli TB1 using p-nitrophenyl phosphate as substrate |
Eur J Med Chem 46: 2243-51 (2011)
Article DOI: 10.1016/j.ejmech.2011.03.005
BindingDB Entry DOI: 10.7270/Q2FT8N4M |
More data for this
Ligand-Target Pair | |
Furin
(Homo sapiens (Human)) | BDBM50386997
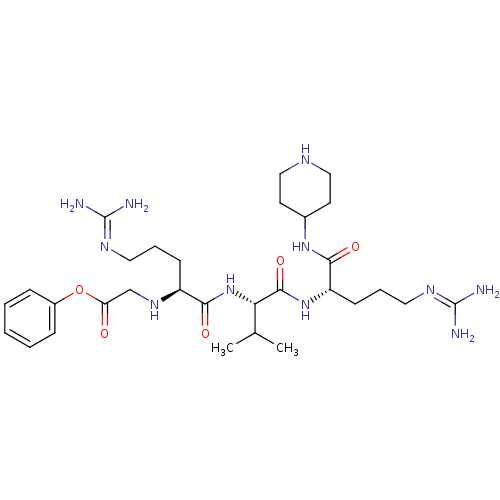
(CHEMBL2049150)Show SMILES [#6]-[#6](-[#6])-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#7]-[#6]-[#6](=O)-[#8]-c1ccccc1)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-[#6]-1-[#6]-[#6]-[#7]-[#6]-[#6]-1 |r| Show InChI InChI=1S/C30H51N11O5/c1-19(2)25(28(45)40-23(11-7-15-37-30(33)34)27(44)39-20-12-16-35-17-13-20)41-26(43)22(10-6-14-36-29(31)32)38-18-24(42)46-21-8-4-3-5-9-21/h3-5,8-9,19-20,22-23,25,35,38H,6-7,10-18H2,1-2H3,(H,39,44)(H,40,45)(H,41,43)(H4,31,32,36)(H4,33,34,37)/t22-,23-,25-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 9.71E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Torrey Pines Institute for Molecular Studies
Curated by ChEMBL
| Assay Description
Inhibition of furin |
Bioorg Med Chem 20: 4462-71 (2012)
Article DOI: 10.1016/j.bmc.2012.05.029
BindingDB Entry DOI: 10.7270/Q2514075 |
More data for this
Ligand-Target Pair | |
3-hydroxy-3-methylglutaryl-coenzyme A reductase
(Homo sapiens (Human)) | BDBM50139181

((1S,3R,7S,8S,8aR)-8-{2-[(2R,4R)-4-hydroxy-6-oxotet...)Show SMILES CCC(C)(C)C(=O)O[C@H]1C[C@@H](C)C=C2C=C[C@H](C)[C@H](CC[C@@H]3C[C@@H](O)CC(=O)O3)[C@@H]12 |r,c:14,t:12| Show InChI InChI=1S/C25H38O5/c1-6-25(4,5)24(28)30-21-12-15(2)11-17-8-7-16(3)20(23(17)21)10-9-19-13-18(26)14-22(27)29-19/h7-8,11,15-16,18-21,23,26H,6,9-10,12-14H2,1-5H3/t15-,16-,18+,19+,20-,21-,23-/m0/s1 | PDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 6.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Nacional Autónoma de México
Curated by ChEMBL
| Assay Description
Inhibitory constant against HMG-CoA reductase with alpha asarone |
Bioorg Med Chem Lett 15: 989-94 (2005)
Article DOI: 10.1016/j.bmcl.2004.12.046
BindingDB Entry DOI: 10.7270/Q2BC3Z1P |
More data for this
Ligand-Target Pair | |
3-hydroxy-3-methylglutaryl-coenzyme A reductase
(Homo sapiens (Human)) | BDBM18372

((3R,5S,6E)-7-[4-(4-fluorophenyl)-2-(N-methylmethan...)Show SMILES CC(C)c1nc(nc(-c2ccc(F)cc2)c1\C=C\[C@@H](O)C[C@@H](O)CC(O)=O)N(C)S(C)(=O)=O |r| Show InChI InChI=1S/C22H28FN3O6S/c1-13(2)20-18(10-9-16(27)11-17(28)12-19(29)30)21(14-5-7-15(23)8-6-14)25-22(24-20)26(3)33(4,31)32/h5-10,13,16-17,27-28H,11-12H2,1-4H3,(H,29,30)/b10-9+/t16-,17-/m1/s1 | PDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 7.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Nacional Autónoma de México
Curated by ChEMBL
| Assay Description
Inhibitory constant against HMG-CoA reductase with alpha asarone |
Bioorg Med Chem Lett 15: 989-94 (2005)
Article DOI: 10.1016/j.bmcl.2004.12.046
BindingDB Entry DOI: 10.7270/Q2BC3Z1P |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
3-hydroxy-3-methylglutaryl-coenzyme A reductase
(Homo sapiens (Human)) | BDBM18372

((3R,5S,6E)-7-[4-(4-fluorophenyl)-2-(N-methylmethan...)Show SMILES CC(C)c1nc(nc(-c2ccc(F)cc2)c1\C=C\[C@@H](O)C[C@@H](O)CC(O)=O)N(C)S(C)(=O)=O |r| Show InChI InChI=1S/C22H28FN3O6S/c1-13(2)20-18(10-9-16(27)11-17(28)12-19(29)30)21(14-5-7-15(23)8-6-14)25-22(24-20)26(3)33(4,31)32/h5-10,13,16-17,27-28H,11-12H2,1-4H3,(H,29,30)/b10-9+/t16-,17-/m1/s1 | PDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 5.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Nacional Autónoma de México
Curated by ChEMBL
| Assay Description
Inhibitory concentration against HMG-CoA reductase |
Bioorg Med Chem Lett 15: 989-94 (2005)
Article DOI: 10.1016/j.bmcl.2004.12.046
BindingDB Entry DOI: 10.7270/Q2BC3Z1P |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
3-hydroxy-3-methylglutaryl-coenzyme A reductase
(Homo sapiens (Human)) | BDBM50139181

((1S,3R,7S,8S,8aR)-8-{2-[(2R,4R)-4-hydroxy-6-oxotet...)Show SMILES CCC(C)(C)C(=O)O[C@H]1C[C@@H](C)C=C2C=C[C@H](C)[C@H](CC[C@@H]3C[C@@H](O)CC(=O)O3)[C@@H]12 |r,c:14,t:12| Show InChI InChI=1S/C25H38O5/c1-6-25(4,5)24(28)30-21-12-15(2)11-17-8-7-16(3)20(23(17)21)10-9-19-13-18(26)14-22(27)29-19/h7-8,11,15-16,18-21,23,26H,6,9-10,12-14H2,1-5H3/t15-,16-,18+,19+,20-,21-,23-/m0/s1 | PDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 11.2 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Nacional Autónoma de México
Curated by ChEMBL
| Assay Description
Inhibitory concentration against HMG-CoA reductase |
Bioorg Med Chem Lett 15: 989-94 (2005)
Article DOI: 10.1016/j.bmcl.2004.12.046
BindingDB Entry DOI: 10.7270/Q2BC3Z1P |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50148911

((3beta)-3-hydroxyurs-12-en-28-oic acid | 3beta-hyd...)Show SMILES C[C@@H]1CC[C@@]2(CC[C@]3(C)C(=CC[C@@H]4[C@@]5(C)CC[C@H](O)C(C)(C)[C@@H]5CC[C@@]34C)[C@@H]2[C@H]1C)C(O)=O |r,c:9| Show InChI InChI=1S/C30H48O3/c1-18-10-15-30(25(32)33)17-16-28(6)20(24(30)19(18)2)8-9-22-27(5)13-12-23(31)26(3,4)21(27)11-14-29(22,28)7/h8,18-19,21-24,31H,9-17H2,1-7H3,(H,32,33)/t18-,19+,21+,22-,23+,24+,27+,28-,29-,30+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Aut£noma del Estado de Morelos
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PTP-1B expressed in Escherichia coli TB1 using p-nitrophenyl phosphate as substrate after 1 hr |
Eur J Med Chem 46: 2243-51 (2011)
Article DOI: 10.1016/j.ejmech.2011.03.005
BindingDB Entry DOI: 10.7270/Q2FT8N4M |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein phosphatase F
(Homo sapiens (Human)) | BDBM50148911

((3beta)-3-hydroxyurs-12-en-28-oic acid | 3beta-hyd...)Show SMILES C[C@@H]1CC[C@@]2(CC[C@]3(C)C(=CC[C@@H]4[C@@]5(C)CC[C@H](O)C(C)(C)[C@@H]5CC[C@@]34C)[C@@H]2[C@H]1C)C(O)=O |r,c:9| Show InChI InChI=1S/C30H48O3/c1-18-10-15-30(25(32)33)17-16-28(6)20(24(30)19(18)2)8-9-22-27(5)13-12-23(31)26(3,4)21(27)11-14-29(22,28)7/h8,18-19,21-24,31H,9-17H2,1-7H3,(H,32,33)/t18-,19+,21+,22-,23+,24+,27+,28-,29-,30+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Aut£noma del Estado de Morelos
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant LAR expressed in Escherichia coli TB1 using p-nitrophenyl phosphate as substrate after 1 hr |
Eur J Med Chem 46: 2243-51 (2011)
Article DOI: 10.1016/j.ejmech.2011.03.005
BindingDB Entry DOI: 10.7270/Q2FT8N4M |
More data for this
Ligand-Target Pair | |
3-hydroxy-3-methylglutaryl-coenzyme A reductase
(Homo sapiens (Human)) | BDBM50160720

(3-hydroxy-3-methylglutaric acid | 3-hydroxy-3-meth...)Show InChI InChI=1S/C6H10O5/c1-6(11,2-4(7)8)3-5(9)10/h11H,2-3H2,1H3,(H,7,8)(H,9,10) | PDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Nacional Autónoma de México
Curated by ChEMBL
| Assay Description
Inhibitory concentration against HMG-CoA reductase |
Bioorg Med Chem Lett 15: 989-94 (2005)
Article DOI: 10.1016/j.bmcl.2004.12.046
BindingDB Entry DOI: 10.7270/Q2BC3Z1P |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
DNA (cytosine-5)-methyltransferase 1
(Homo sapiens (Human)) | BDBM50009672

(AdoHcy | CHEMBL418052 | S-(5'-adenosyl)-L-homocyst...)Show SMILES N[C@@H](CCSC[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(N)ncnc12)C(O)=O Show InChI InChI=1S/C14H20N6O5S/c15-6(14(23)24)1-2-26-3-7-9(21)10(22)13(25-7)20-5-19-8-11(16)17-4-18-12(8)20/h4-7,9-10,13,21-22H,1-3,15H2,(H,23,24)(H2,16,17,18)/t6-,7+,9+,10+,13+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Salerno
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant DNMT1 expressed in Sf9 cells assessed as incorporation of [3H]S-adenosyl methionine into hemimethylated oligonucleoti... |
J Med Chem 54: 7663-77 (2011)
Article DOI: 10.1021/jm2010404
BindingDB Entry DOI: 10.7270/Q28S4Q98 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Low molecular weight phosphotyrosine protein phosphatase
(Homo sapiens (Human)) | BDBM50148911

((3beta)-3-hydroxyurs-12-en-28-oic acid | 3beta-hyd...)Show SMILES C[C@@H]1CC[C@@]2(CC[C@]3(C)C(=CC[C@@H]4[C@@]5(C)CC[C@H](O)C(C)(C)[C@@H]5CC[C@@]34C)[C@@H]2[C@H]1C)C(O)=O |r,c:9| Show InChI InChI=1S/C30H48O3/c1-18-10-15-30(25(32)33)17-16-28(6)20(24(30)19(18)2)8-9-22-27(5)13-12-23(31)26(3,4)21(27)11-14-29(22,28)7/h8,18-19,21-24,31H,9-17H2,1-7H3,(H,32,33)/t18-,19+,21+,22-,23+,24+,27+,28-,29-,30+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 7.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Aut£noma del Estado de Morelos
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant LMW-PTP1 expressed in Escherichia coli TB1 using p-nitrophenyl phosphate as substrate after 1 hr |
Eur J Med Chem 46: 2243-51 (2011)
Article DOI: 10.1016/j.ejmech.2011.03.005
BindingDB Entry DOI: 10.7270/Q2FT8N4M |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50385308

(CHEMBL2035962)Show InChI InChI=1S/C11H9FN2O3S/c1-2-17-10(16)9(15)14-11-13-7-4-3-6(12)5-8(7)18-11/h3-5H,2H2,1H3,(H,13,14,15) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Aut£noma del Estado de Morelos
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant GST-tagged PTP1B expressed in Escherichia coli TB1 cells using p-NPP as substrate at 40 uM |
Eur J Med Chem 53: 346-55 (2012)
Article DOI: 10.1016/j.ejmech.2012.04.025
BindingDB Entry DOI: 10.7270/Q2KP836R |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50391854

(CHEMBL463665)Show SMILES CC1(C)CC[C@@]2(CC[C@]3(C)[C@H](CC[C@@H]4[C@@]5(C)CC[C@H](O)C(C)(C)[C@@H]5CC[C@@]34C)C2=C1)C(O)=O |r,c:32| Show InChI InChI=1S/C30H48O3/c1-25(2)14-16-30(24(32)33)17-15-28(6)19(20(30)18-25)8-9-22-27(5)12-11-23(31)26(3,4)21(27)10-13-29(22,28)7/h18-19,21-23,31H,8-17H2,1-7H3,(H,32,33)/t19-,21+,22-,23+,27+,28-,29-,30+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Aut£noma del Estado de Morelos
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PTP-1B expressed in Escherichia coli TB1 using p-nitrophenyl phosphate as substrate after 1 hr |
Eur J Med Chem 46: 2243-51 (2011)
Article DOI: 10.1016/j.ejmech.2011.03.005
BindingDB Entry DOI: 10.7270/Q2FT8N4M |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50346601

(NSC-114945 | OLEANOLIC_ACID | Oleanolic acid | Ole...)Show SMILES CC1(C)CC[C@@]2(CC[C@]3(C)C(=CC[C@@H]4[C@@]5(C)CC[C@H](O)C(C)(C)[C@@H]5CC[C@@]34C)[C@@H]2C1)C(O)=O |r,c:10| Show InChI InChI=1S/C30H48O3/c1-25(2)14-16-30(24(32)33)17-15-28(6)19(20(30)18-25)8-9-22-27(5)12-11-23(31)26(3,4)21(27)10-13-29(22,28)7/h8,20-23,31H,9-18H2,1-7H3,(H,32,33)/t20-,21-,22+,23-,27-,28+,29+,30-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 9.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Aut£noma del Estado de Morelos
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PTP-1B expressed in Escherichia coli TB1 using p-nitrophenyl phosphate as substrate after 1 hr |
Eur J Med Chem 46: 2243-51 (2011)
Article DOI: 10.1016/j.ejmech.2011.03.005
BindingDB Entry DOI: 10.7270/Q2FT8N4M |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50385307

(CHEMBL2035961)Show InChI InChI=1S/C11H9ClN2O3S/c1-2-17-10(16)9(15)14-11-13-7-4-3-6(12)5-8(7)18-11/h3-5H,2H2,1H3,(H,13,14,15) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.01E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Aut£noma del Estado de Morelos
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant GST-tagged PTP1B expressed in Escherichia coli TB1 cells using p-NPP as substrate at 40 uM |
Eur J Med Chem 53: 346-55 (2012)
Article DOI: 10.1016/j.ejmech.2012.04.025
BindingDB Entry DOI: 10.7270/Q2KP836R |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50385306

(CHEMBL2002784)Show InChI InChI=1S/C12H12N2O3S/c1-3-17-11(16)10(15)14-12-13-8-5-4-7(2)6-9(8)18-12/h4-6H,3H2,1-2H3,(H,13,14,15) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Aut£noma del Estado de Morelos
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant GST-tagged PTP1B expressed in Escherichia coli TB1 cells using p-NPP as substrate at 40 uM |
Eur J Med Chem 53: 346-55 (2012)
Article DOI: 10.1016/j.ejmech.2012.04.025
BindingDB Entry DOI: 10.7270/Q2KP836R |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data