

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Farnesyl pyrophosphate synthase (Homo sapiens (Human)) | BDBM25308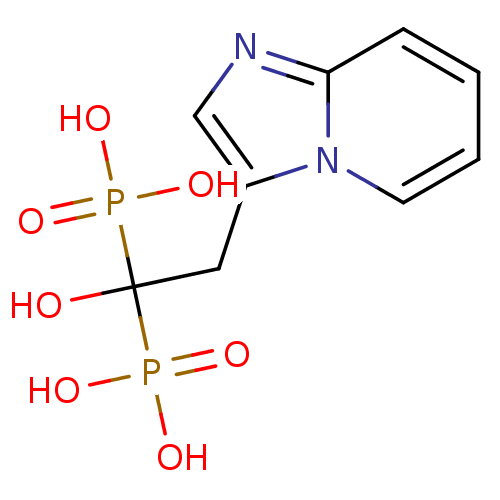 ((1-hydroxy-2-{imidazo[1,2-a]pyridin-3-yl}-1-phosph...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
McGill University Curated by ChEMBL | Assay Description Inhibition of human FPPS | Bioorg Med Chem Lett 25: 1117-23 (2015) Article DOI: 10.1016/j.bmcl.2014.12.089 BindingDB Entry DOI: 10.7270/Q25X2BMF | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Farnesyl pyrophosphate synthase (Homo sapiens (Human)) | BDBM50098378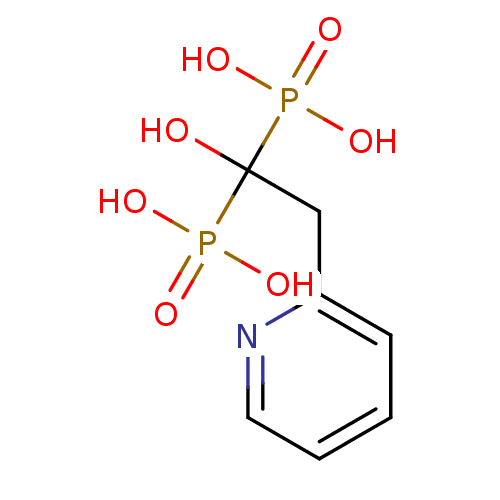 ((1-Hydroxy-1-phosphono-2-pyridin-2-yl-ethyl)-phosp...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human FPPS expressed in Escherichia coli BL21 (DE3) preincubated for 10 mins in presence compound relative to control | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01913 BindingDB Entry DOI: 10.7270/Q28K7F00 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Farnesyl pyrophosphate synthase (Homo sapiens (Human)) | BDBM50098378 ((1-Hydroxy-1-phosphono-2-pyridin-2-yl-ethyl)-phosp...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
McGill University Curated by ChEMBL | Assay Description Inhibition of human FPPS using [14C]-IPP and FPP as substrates after 10 mins by scintillation counting | J Med Chem 61: 6904-6917 (2018) Article DOI: 10.1021/acs.jmedchem.8b00886 BindingDB Entry DOI: 10.7270/Q2CJ8HWF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Farnesyl pyrophosphate synthase (Homo sapiens (Human)) | BDBM12578 (2-(imidazol-1-yl)-1-hydroxyethylidene-1,1-bisphosp...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank MMDB PDB Article PubMed | n/a | n/a | 4.10 | n/a | n/a | n/a | n/a | n/a | n/a |
McGill University Curated by ChEMBL | Assay Description Inhibition of human FPPS | Bioorg Med Chem Lett 25: 1117-23 (2015) Article DOI: 10.1016/j.bmcl.2014.12.089 BindingDB Entry DOI: 10.7270/Q25X2BMF | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Farnesyl pyrophosphate synthase (Homo sapiens (Human)) | BDBM12576 (Bisphosphonate 1 | CHEMBL923 | JMC515594 Compound ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank MMDB PDB Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
McGill University Curated by ChEMBL | Assay Description Inhibition of human FPPS | Bioorg Med Chem Lett 25: 1117-23 (2015) Article DOI: 10.1016/j.bmcl.2014.12.089 BindingDB Entry DOI: 10.7270/Q25X2BMF | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Geranylgeranyl pyrophosphate synthase (Homo sapiens (Human)) | BDBM50585603 (CHEMBL5087030) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human N- terminal His-tagged /TEV cleavage site fused GGPPS (1 to 300 residues) expressed in Escherichia coli BL21 (DE3) us... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01913 BindingDB Entry DOI: 10.7270/Q28K7F00 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Geranylgeranyl pyrophosphate synthase (Homo sapiens (Human)) | BDBM50585613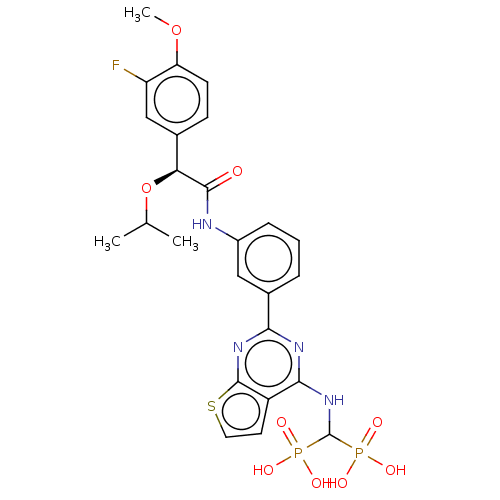 (CHEMBL5088349) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human N- terminal His-tagged /TEV cleavage site fused GGPPS (1 to 300 residues) expressed in Escherichia coli BL21 (DE3) us... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01913 BindingDB Entry DOI: 10.7270/Q28K7F00 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Geranylgeranyl pyrophosphate synthase (Homo sapiens (Human)) | BDBM544004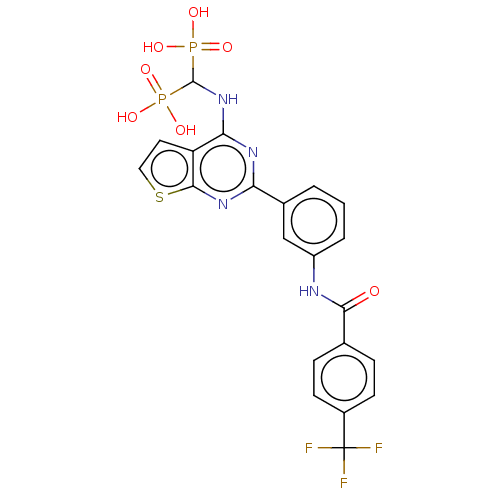 (US11279719, Example I-36) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human N- terminal His-tagged /TEV cleavage site fused GGPPS (1 to 300 residues) expressed in Escherichia coli BL21 (DE3) us... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01913 BindingDB Entry DOI: 10.7270/Q28K7F00 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Geranylgeranyl pyrophosphate synthase (Homo sapiens (Human)) | BDBM50520641 (CHEMBL4455060 | US11279719, Example I-13) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human N- terminal His-tagged /TEV cleavage site fused GGPPS (1 to 300 residues) expressed in Escherichia coli BL21 (DE3) us... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01913 BindingDB Entry DOI: 10.7270/Q28K7F00 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Geranylgeranyl pyrophosphate synthase (Homo sapiens (Human)) | BDBM50585614 (CHEMBL5082193) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human N- terminal His-tagged /TEV cleavage site fused GGPPS (1 to 300 residues) expressed in Escherichia coli BL21 (DE3) us... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01913 BindingDB Entry DOI: 10.7270/Q28K7F00 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Geranylgeranyl pyrophosphate synthase (Homo sapiens (Human)) | BDBM50585601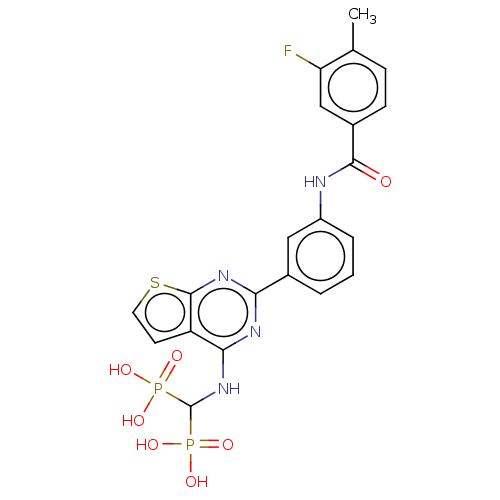 (CHEMBL5077349) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human N- terminal His-tagged /TEV cleavage site fused GGPPS (1 to 300 residues) expressed in Escherichia coli BL21 (DE3) us... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01913 BindingDB Entry DOI: 10.7270/Q28K7F00 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Geranylgeranyl pyrophosphate synthase (Homo sapiens (Human)) | BDBM50585611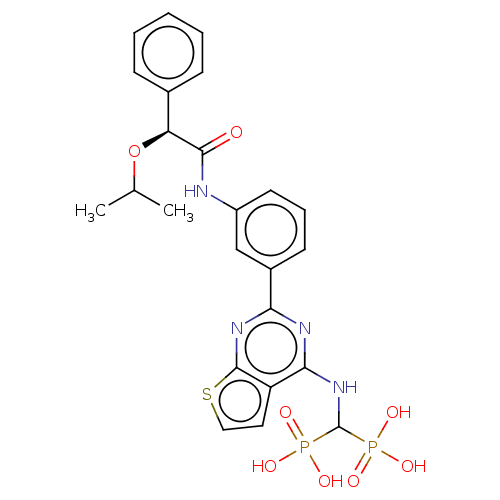 (CHEMBL5077117) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human N- terminal His-tagged /TEV cleavage site fused GGPPS (1 to 300 residues) expressed in Escherichia coli BL21 (DE3) us... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01913 BindingDB Entry DOI: 10.7270/Q28K7F00 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Farnesyl pyrophosphate synthase (Homo sapiens (Human)) | BDBM50443054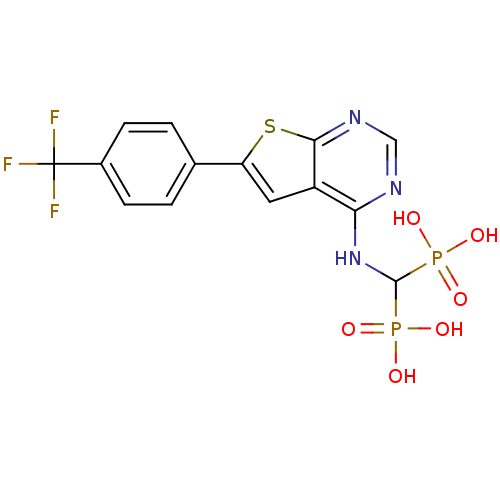 (CHEMBL3087934 | US11279719, Example C-12) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
McGill University Curated by ChEMBL | Assay Description Inhibition of human FPPS using [14C]-IPP and FPP as substrates after 10 mins by scintillation counting | J Med Chem 61: 6904-6917 (2018) Article DOI: 10.1021/acs.jmedchem.8b00886 BindingDB Entry DOI: 10.7270/Q2CJ8HWF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Geranylgeranyl pyrophosphate synthase (Homo sapiens (Human)) | BDBM50585608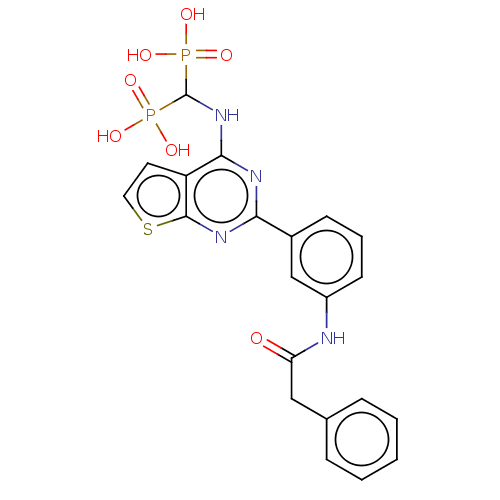 (CHEMBL5075557) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human N- terminal His-tagged /TEV cleavage site fused GGPPS (1 to 300 residues) expressed in Escherichia coli BL21 (DE3) us... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01913 BindingDB Entry DOI: 10.7270/Q28K7F00 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Geranylgeranyl pyrophosphate synthase (Homo sapiens (Human)) | BDBM50585606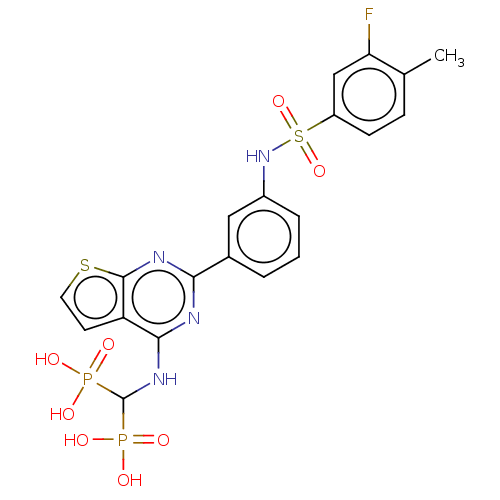 (CHEMBL5085126) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human N- terminal His-tagged /TEV cleavage site fused GGPPS (1 to 300 residues) expressed in Escherichia coli BL21 (DE3) us... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01913 BindingDB Entry DOI: 10.7270/Q28K7F00 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Geranylgeranyl pyrophosphate synthase (Homo sapiens (Human)) | BDBM50585604 (CHEMBL5083916) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human N- terminal His-tagged /TEV cleavage site fused GGPPS (1 to 300 residues) expressed in Escherichia coli BL21 (DE3) us... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01913 BindingDB Entry DOI: 10.7270/Q28K7F00 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Geranylgeranyl pyrophosphate synthase (Homo sapiens (Human)) | BDBM50585612 (CHEMBL5089506) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human N- terminal His-tagged /TEV cleavage site fused GGPPS (1 to 300 residues) expressed in Escherichia coli BL21 (DE3) us... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01913 BindingDB Entry DOI: 10.7270/Q28K7F00 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Geranylgeranyl pyrophosphate synthase (Homo sapiens (Human)) | BDBM50585605 (CHEMBL5080782) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human N- terminal His-tagged /TEV cleavage site fused GGPPS (1 to 300 residues) expressed in Escherichia coli BL21 (DE3) us... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01913 BindingDB Entry DOI: 10.7270/Q28K7F00 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Geranylgeranyl pyrophosphate synthase (Homo sapiens (Human)) | BDBM50520640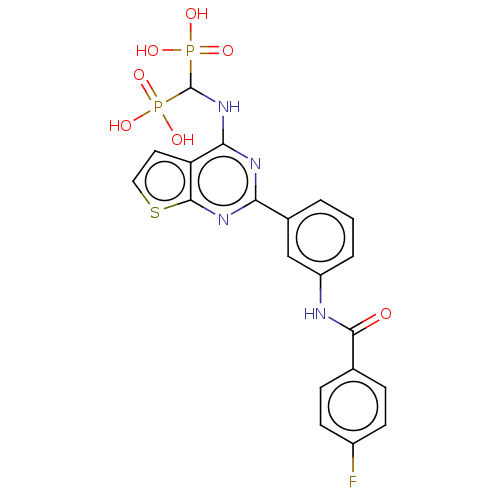 (CHEMBL4472025 | US11279719, Example I-34) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human N- terminal His-tagged /TEV cleavage site fused GGPPS (1 to 300 residues) expressed in Escherichia coli BL21 (DE3) us... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01913 BindingDB Entry DOI: 10.7270/Q28K7F00 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Geranylgeranyl pyrophosphate synthase (Homo sapiens (Human)) | BDBM50520637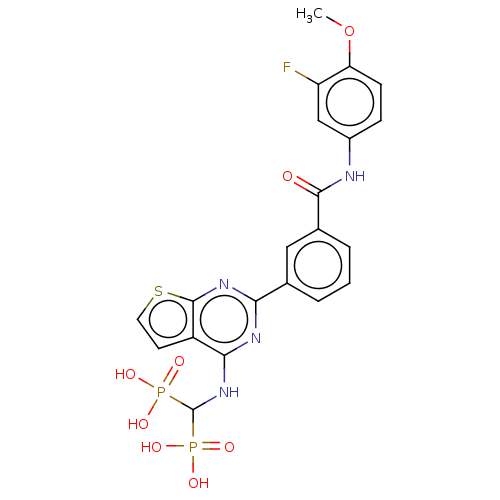 (CHEMBL4577077 | US11279719, Example I-37) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human N- terminal His-tagged /TEV cleavage site fused GGPPS (1 to 300 residues) expressed in Escherichia coli BL21 (DE3) us... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01913 BindingDB Entry DOI: 10.7270/Q28K7F00 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Geranylgeranyl pyrophosphate synthase (Homo sapiens (Human)) | BDBM50585599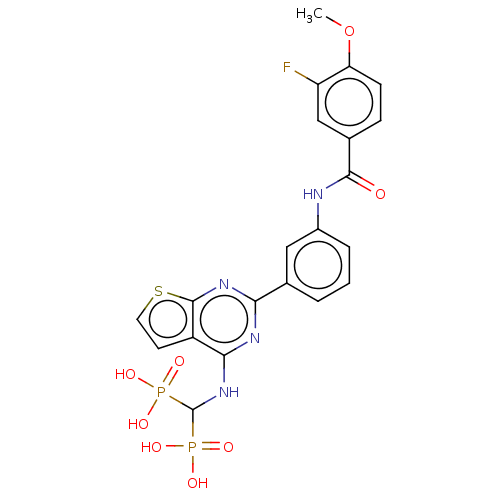 (CHEMBL5088555) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human N- terminal His-tagged /TEV cleavage site fused GGPPS (1 to 300 residues) expressed in Escherichia coli BL21 (DE3) us... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01913 BindingDB Entry DOI: 10.7270/Q28K7F00 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Geranylgeranyl pyrophosphate synthase (Homo sapiens (Human)) | BDBM50585602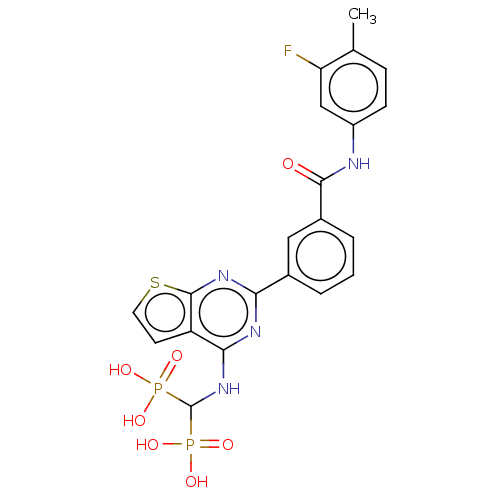 (CHEMBL5075029) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human N- terminal His-tagged /TEV cleavage site fused GGPPS (1 to 300 residues) expressed in Escherichia coli BL21 (DE3) us... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01913 BindingDB Entry DOI: 10.7270/Q28K7F00 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Geranylgeranyl pyrophosphate synthase (Homo sapiens (Human)) | BDBM50585607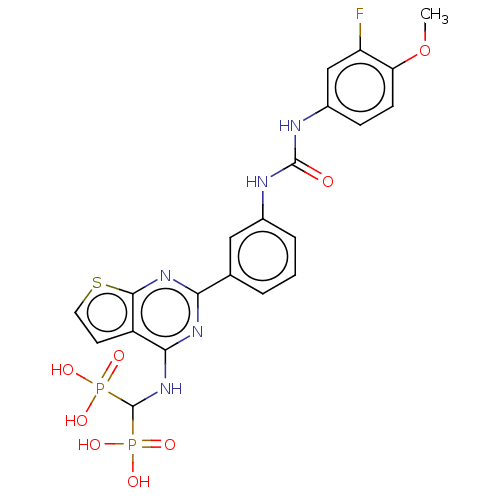 (CHEMBL5080121) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 34 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human N- terminal His-tagged /TEV cleavage site fused GGPPS (1 to 300 residues) expressed in Escherichia coli BL21 (DE3) us... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01913 BindingDB Entry DOI: 10.7270/Q28K7F00 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Geranylgeranyl pyrophosphate synthase (Homo sapiens (Human)) | BDBM50585600 (CHEMBL5092247) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 36 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human N- terminal His-tagged /TEV cleavage site fused GGPPS (1 to 300 residues) expressed in Escherichia coli BL21 (DE3) us... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01913 BindingDB Entry DOI: 10.7270/Q28K7F00 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Geranylgeranyl pyrophosphate synthase (Homo sapiens (Human)) | BDBM50520645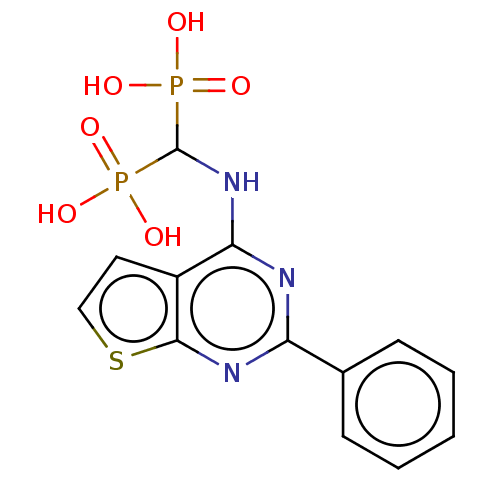 (CHEMBL2347859 | US11279719, Example C-5) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 37 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human N- terminal His-tagged /TEV cleavage site fused GGPPS (1 to 300 residues) expressed in Escherichia coli BL21 (DE3) us... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01913 BindingDB Entry DOI: 10.7270/Q28K7F00 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Geranylgeranyl pyrophosphate synthase (Homo sapiens (Human)) | BDBM50520638 (CHEMBL4471037 | US11279719, Example I-18) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 37 | n/a | n/a | n/a | n/a | n/a | n/a |
McGill University Curated by ChEMBL | Assay Description Inhibition of human N-terminal His6-tagged GGPPS Y246D mutant expressed in Escherichia coli BL21(DE3) using [14C]-IPP and FPP as substrates after 10 ... | J Med Chem 61: 6904-6917 (2018) Article DOI: 10.1021/acs.jmedchem.8b00886 BindingDB Entry DOI: 10.7270/Q2CJ8HWF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Geranylgeranyl pyrophosphate synthase (Homo sapiens (Human)) | BDBM50585609 (CHEMBL5082291) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 38 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human N- terminal His-tagged /TEV cleavage site fused GGPPS (1 to 300 residues) expressed in Escherichia coli BL21 (DE3) us... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01913 BindingDB Entry DOI: 10.7270/Q28K7F00 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Geranylgeranyl pyrophosphate synthase (Homo sapiens (Human)) | BDBM50585610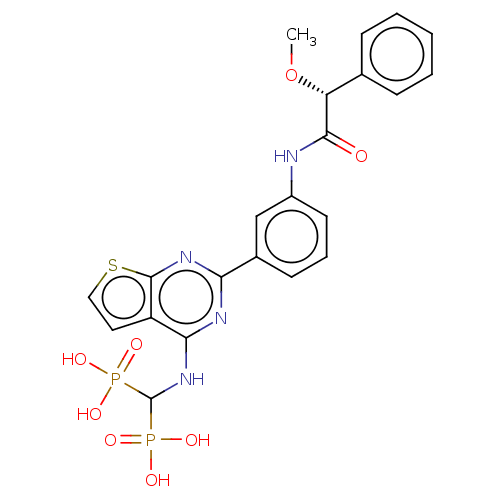 (CHEMBL5081807) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 39 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human N- terminal His-tagged /TEV cleavage site fused GGPPS (1 to 300 residues) expressed in Escherichia coli BL21 (DE3) us... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01913 BindingDB Entry DOI: 10.7270/Q28K7F00 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Geranylgeranyl pyrophosphate synthase (Homo sapiens (Human)) | BDBM50520641 (CHEMBL4455060 | US11279719, Example I-13) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 39 | n/a | n/a | n/a | n/a | n/a | n/a |
McGill University Curated by ChEMBL | Assay Description Inhibition of human N-terminal His6-tagged GGPPS Y246D mutant expressed in Escherichia coli BL21(DE3) using [14C]-IPP and FPP as substrates after 10 ... | J Med Chem 61: 6904-6917 (2018) Article DOI: 10.1021/acs.jmedchem.8b00886 BindingDB Entry DOI: 10.7270/Q2CJ8HWF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Geranylgeranyl pyrophosphate synthase (Homo sapiens (Human)) | BDBM50520639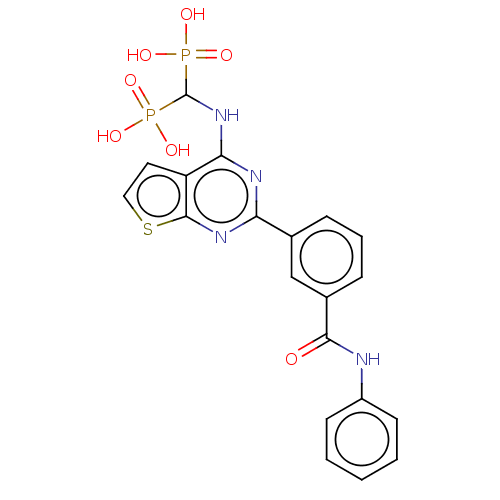 (CHEMBL4465832 | US11279719, Example I-6) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 41 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human N- terminal His-tagged /TEV cleavage site fused GGPPS (1 to 300 residues) expressed in Escherichia coli BL21 (DE3) us... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01913 BindingDB Entry DOI: 10.7270/Q28K7F00 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Geranylgeranyl pyrophosphate synthase (Homo sapiens (Human)) | BDBM543975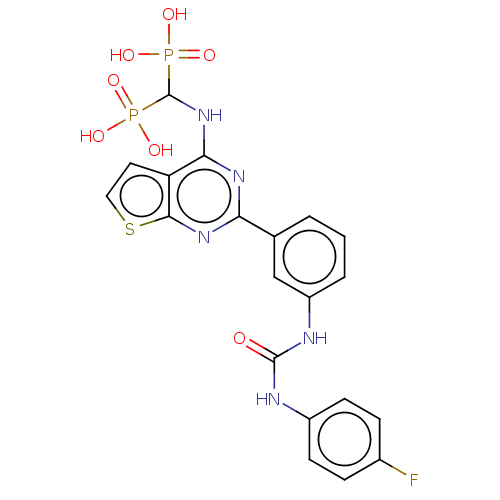 (US11279719, Example I-7) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 41 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human N- terminal His-tagged /TEV cleavage site fused GGPPS (1 to 300 residues) expressed in Escherichia coli BL21 (DE3) us... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01913 BindingDB Entry DOI: 10.7270/Q28K7F00 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Geranylgeranyl pyrophosphate synthase (Homo sapiens (Human)) | BDBM50520640 (CHEMBL4472025 | US11279719, Example I-34) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 42 | n/a | n/a | n/a | n/a | n/a | n/a |
McGill University Curated by ChEMBL | Assay Description Inhibition of human N-terminal His6-tagged GGPPS expressed in Escherichia coli BL21(DE3) using [14C]-IPP and FPP as substrates after 10 mins by scint... | J Med Chem 61: 6904-6917 (2018) Article DOI: 10.1021/acs.jmedchem.8b00886 BindingDB Entry DOI: 10.7270/Q2CJ8HWF | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Geranylgeranyl pyrophosphate synthase (Homo sapiens (Human)) | BDBM50520639 (CHEMBL4465832 | US11279719, Example I-6) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 49 | n/a | n/a | n/a | n/a | n/a | n/a |
McGill University Curated by ChEMBL | Assay Description Inhibition of human N-terminal His6-tagged GGPPS expressed in Escherichia coli BL21(DE3) using [14C]-IPP and FPP as substrates after 10 mins by scint... | J Med Chem 61: 6904-6917 (2018) Article DOI: 10.1021/acs.jmedchem.8b00886 BindingDB Entry DOI: 10.7270/Q2CJ8HWF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Geranylgeranyl pyrophosphate synthase (Homo sapiens (Human)) | BDBM50520639 (CHEMBL4465832 | US11279719, Example I-6) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
McGill University Curated by ChEMBL | Assay Description Inhibition of human N-terminal His6-tagged GGPPS Y246D mutant expressed in Escherichia coli BL21(DE3) using [14C]-IPP and FPP as substrates after 10 ... | J Med Chem 61: 6904-6917 (2018) Article DOI: 10.1021/acs.jmedchem.8b00886 BindingDB Entry DOI: 10.7270/Q2CJ8HWF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Geranylgeranyl pyrophosphate synthase (Homo sapiens (Human)) | BDBM544003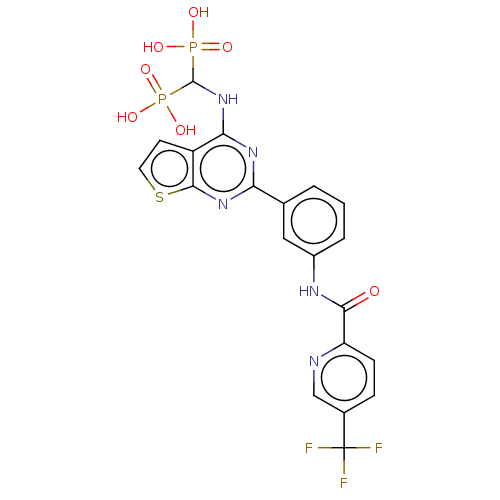 (US11279719, Example I-35) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 52 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human N- terminal His-tagged /TEV cleavage site fused GGPPS (1 to 300 residues) expressed in Escherichia coli BL21 (DE3) us... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01913 BindingDB Entry DOI: 10.7270/Q28K7F00 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Geranylgeranyl pyrophosphate synthase (Homo sapiens (Human)) | BDBM50520641 (CHEMBL4455060 | US11279719, Example I-13) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 64 | n/a | n/a | n/a | n/a | n/a | n/a |
McGill University Curated by ChEMBL | Assay Description Inhibition of human N-terminal His6-tagged GGPPS expressed in Escherichia coli BL21(DE3) using [14C]-IPP and FPP as substrates after 10 mins by scint... | J Med Chem 61: 6904-6917 (2018) Article DOI: 10.1021/acs.jmedchem.8b00886 BindingDB Entry DOI: 10.7270/Q2CJ8HWF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Geranylgeranyl pyrophosphate synthase (Homo sapiens (Human)) | BDBM544000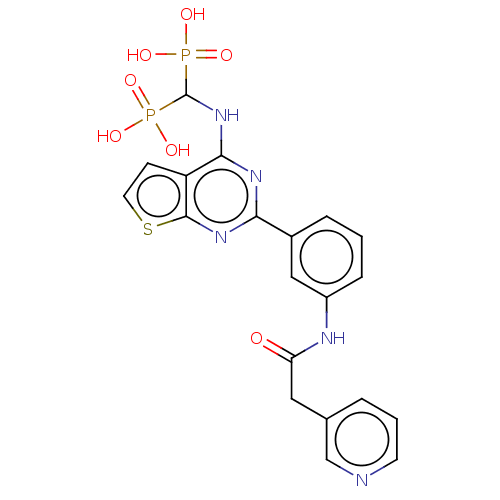 (US11279719, Example I-32) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 71 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human N- terminal His-tagged /TEV cleavage site fused GGPPS (1 to 300 residues) expressed in Escherichia coli BL21 (DE3) us... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01913 BindingDB Entry DOI: 10.7270/Q28K7F00 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Geranylgeranyl pyrophosphate synthase (Homo sapiens (Human)) | BDBM50520643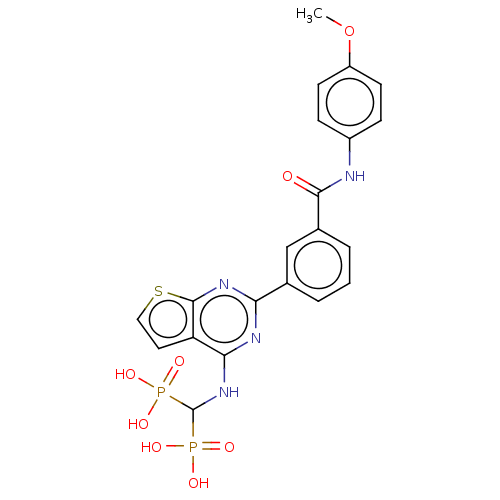 (CHEMBL4538679 | US11279719, Example I-39) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 75 | n/a | n/a | n/a | n/a | n/a | n/a |
McGill University Curated by ChEMBL | Assay Description Inhibition of human N-terminal His6-tagged GGPPS expressed in Escherichia coli BL21(DE3) using [14C]-IPP and FPP as substrates after 10 mins by scint... | J Med Chem 61: 6904-6917 (2018) Article DOI: 10.1021/acs.jmedchem.8b00886 BindingDB Entry DOI: 10.7270/Q2CJ8HWF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Geranylgeranyl pyrophosphate synthase (Homo sapiens (Human)) | BDBM50520637 (CHEMBL4577077 | US11279719, Example I-37) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 75 | n/a | n/a | n/a | n/a | n/a | n/a |
McGill University Curated by ChEMBL | Assay Description Inhibition of human N-terminal His6-tagged GGPPS Y246D mutant expressed in Escherichia coli BL21(DE3) using [14C]-IPP and FPP as substrates after 10 ... | J Med Chem 61: 6904-6917 (2018) Article DOI: 10.1021/acs.jmedchem.8b00886 BindingDB Entry DOI: 10.7270/Q2CJ8HWF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Geranylgeranyl pyrophosphate synthase (Homo sapiens (Human)) | BDBM50520645 (CHEMBL2347859 | US11279719, Example C-5) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 82 | n/a | n/a | n/a | n/a | n/a | n/a |
McGill University Curated by ChEMBL | Assay Description Inhibition of human N-terminal His6-tagged GGPPS expressed in Escherichia coli BL21(DE3) using [14C]-IPP and FPP as substrates after 10 mins by scint... | J Med Chem 61: 6904-6917 (2018) Article DOI: 10.1021/acs.jmedchem.8b00886 BindingDB Entry DOI: 10.7270/Q2CJ8HWF | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Geranylgeranyl pyrophosphate synthase (Homo sapiens (Human)) | BDBM50520646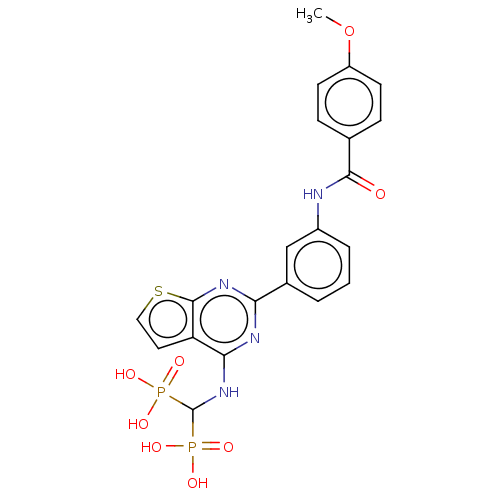 (CHEMBL4564934 | US11279719, Example I-5) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 85 | n/a | n/a | n/a | n/a | n/a | n/a |
McGill University Curated by ChEMBL | Assay Description Inhibition of human N-terminal His6-tagged GGPPS expressed in Escherichia coli BL21(DE3) using [14C]-IPP and FPP as substrates after 10 mins by scint... | J Med Chem 61: 6904-6917 (2018) Article DOI: 10.1021/acs.jmedchem.8b00886 BindingDB Entry DOI: 10.7270/Q2CJ8HWF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Geranylgeranyl pyrophosphate synthase (Homo sapiens (Human)) | BDBM50520637 (CHEMBL4577077 | US11279719, Example I-37) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 86 | n/a | n/a | n/a | n/a | n/a | n/a |
McGill University Curated by ChEMBL | Assay Description Inhibition of human N-terminal His6-tagged GGPPS expressed in Escherichia coli BL21(DE3) using [14C]-IPP and FPP as substrates after 10 mins by scint... | J Med Chem 61: 6904-6917 (2018) Article DOI: 10.1021/acs.jmedchem.8b00886 BindingDB Entry DOI: 10.7270/Q2CJ8HWF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Geranylgeranyl pyrophosphate synthase (Homo sapiens (Human)) | BDBM50520646 (CHEMBL4564934 | US11279719, Example I-5) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 92 | n/a | n/a | n/a | n/a | n/a | n/a |
McGill University Curated by ChEMBL | Assay Description Inhibition of human N-terminal His6-tagged GGPPS Y246D mutant expressed in Escherichia coli BL21(DE3) using [14C]-IPP and FPP as substrates after 10 ... | J Med Chem 61: 6904-6917 (2018) Article DOI: 10.1021/acs.jmedchem.8b00886 BindingDB Entry DOI: 10.7270/Q2CJ8HWF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Geranylgeranyl pyrophosphate synthase (Homo sapiens (Human)) | BDBM50520642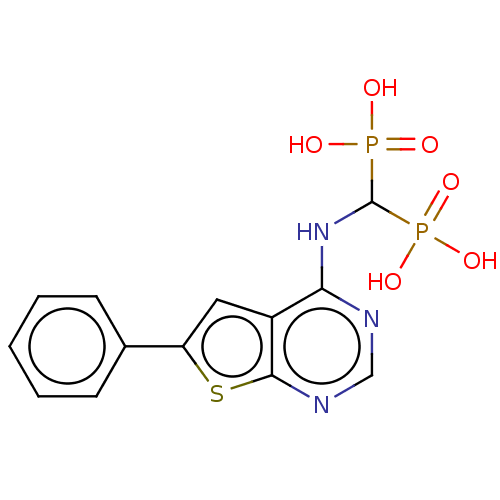 (CHEMBL2347861 | US11279719, Example C-10) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 94 | n/a | n/a | n/a | n/a | n/a | n/a |
McGill University Curated by ChEMBL | Assay Description Inhibition of human N-terminal His6-tagged GGPPS expressed in Escherichia coli BL21(DE3) using [14C]-IPP and FPP as substrates after 10 mins by scint... | J Med Chem 61: 6904-6917 (2018) Article DOI: 10.1021/acs.jmedchem.8b00886 BindingDB Entry DOI: 10.7270/Q2CJ8HWF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Geranylgeranyl pyrophosphate synthase (Homo sapiens (Human)) | BDBM50520638 (CHEMBL4471037 | US11279719, Example I-18) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
McGill University Curated by ChEMBL | Assay Description Inhibition of human N-terminal His6-tagged GGPPS expressed in Escherichia coli BL21(DE3) using [14C]-IPP and FPP as substrates after 10 mins by scint... | J Med Chem 61: 6904-6917 (2018) Article DOI: 10.1021/acs.jmedchem.8b00886 BindingDB Entry DOI: 10.7270/Q2CJ8HWF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Farnesyl pyrophosphate synthase (Homo sapiens (Human)) | BDBM50520642 (CHEMBL2347861 | US11279719, Example C-10) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
McGill University Curated by ChEMBL | Assay Description Inhibition of human FPPS using [14C]-IPP and FPP as substrates after 10 mins by scintillation counting | J Med Chem 61: 6904-6917 (2018) Article DOI: 10.1021/acs.jmedchem.8b00886 BindingDB Entry DOI: 10.7270/Q2CJ8HWF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Geranylgeranyl pyrophosphate synthase (Homo sapiens (Human)) | BDBM25270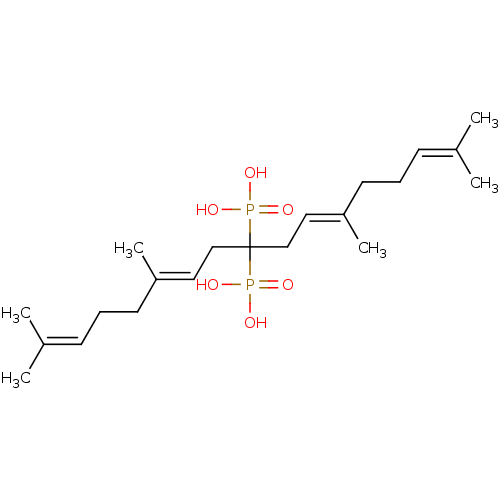 ([(6E,11E)-2,6,12,16-tetramethyl-9-phosphonoheptade...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 430 | n/a | n/a | n/a | n/a | n/a | n/a |
McGill University Curated by ChEMBL | Assay Description Inhibition of human N-terminal His6-tagged GGPPS expressed in Escherichia coli BL21(DE3) using [14C]-IPP and FPP as substrates after 10 mins by scint... | J Med Chem 61: 6904-6917 (2018) Article DOI: 10.1021/acs.jmedchem.8b00886 BindingDB Entry DOI: 10.7270/Q2CJ8HWF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Farnesyl pyrophosphate synthase (Homo sapiens (Human)) | BDBM50585613 (CHEMBL5088349) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 460 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human FPPS expressed in Escherichia coli BL21 (DE3) preincubated for 10 mins in presence compound relative to control | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01913 BindingDB Entry DOI: 10.7270/Q28K7F00 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Farnesyl pyrophosphate synthase (Homo sapiens (Human)) | BDBM50520645 (CHEMBL2347859 | US11279719, Example C-5) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 550 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human FPPS expressed in Escherichia coli BL21 (DE3) preincubated for 10 mins in presence compound relative to control | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01913 BindingDB Entry DOI: 10.7270/Q28K7F00 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Farnesyl pyrophosphate synthase (Homo sapiens (Human)) | BDBM50520645 (CHEMBL2347859 | US11279719, Example C-5) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 550 | n/a | n/a | n/a | n/a | n/a | n/a |
McGill University Curated by ChEMBL | Assay Description Inhibition of human FPPS using [14C]-IPP and FPP as substrates after 10 mins by scintillation counting | J Med Chem 61: 6904-6917 (2018) Article DOI: 10.1021/acs.jmedchem.8b00886 BindingDB Entry DOI: 10.7270/Q2CJ8HWF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 156 total ) | Next | Last >> |