 Found 104 hits with Last Name = 'lafleur' and Initial = 'k'
Found 104 hits with Last Name = 'lafleur' and Initial = 'k' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Tyrosine-protein kinase ABL1
(Homo sapiens (Human)) | BDBM50299218

(8-(2-Methoxyphenyl)-1-methyl-7-(2'-methyl-5'-hydro...)Show SMILES COc1ccccc1-n1c(cn2c1nc1n(C)c(=O)[nH]c(=O)c21)-c1cc(O)ccc1C |(16.89,-42.99,;16.89,-41.45,;18.23,-40.68,;19.55,-41.45,;20.88,-40.68,;20.88,-39.14,;19.55,-38.38,;18.23,-39.15,;16.9,-38.38,;16.91,-36.84,;15.44,-36.35,;14.53,-37.6,;15.43,-38.85,;14.53,-40.09,;13.06,-39.61,;11.74,-40.37,;11.73,-41.91,;10.41,-39.61,;9.07,-40.38,;10.41,-38.07,;11.74,-37.29,;11.74,-35.75,;13.06,-38.07,;18.24,-36.06,;19.58,-36.83,;20.9,-36.05,;22.24,-36.81,;20.9,-34.51,;19.55,-33.75,;18.23,-34.53,;16.89,-33.77,)| Show InChI InChI=1S/C22H19N5O4/c1-12-8-9-13(28)10-14(12)16-11-26-18-19(25(2)22(30)24-20(18)29)23-21(26)27(16)15-6-4-5-7-17(15)31-3/h4-11,28H,1-3H3,(H,24,29,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.920 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Zurich
Curated by ChEMBL
| Assay Description
Inhibition of Abl (unknown origin) using [gamma-33P]ATP after 30 mins by radiometric assay |
J Med Chem 56: 84-96 (2013)
Article DOI: 10.1021/jm301187e
BindingDB Entry DOI: 10.7270/Q2WW7K1S |
More data for this
Ligand-Target Pair | |
Ephrin type-B receptor 1
(Homo sapiens (Human)) | BDBM50299218

(8-(2-Methoxyphenyl)-1-methyl-7-(2'-methyl-5'-hydro...)Show SMILES COc1ccccc1-n1c(cn2c1nc1n(C)c(=O)[nH]c(=O)c21)-c1cc(O)ccc1C |(16.89,-42.99,;16.89,-41.45,;18.23,-40.68,;19.55,-41.45,;20.88,-40.68,;20.88,-39.14,;19.55,-38.38,;18.23,-39.15,;16.9,-38.38,;16.91,-36.84,;15.44,-36.35,;14.53,-37.6,;15.43,-38.85,;14.53,-40.09,;13.06,-39.61,;11.74,-40.37,;11.73,-41.91,;10.41,-39.61,;9.07,-40.38,;10.41,-38.07,;11.74,-37.29,;11.74,-35.75,;13.06,-38.07,;18.24,-36.06,;19.58,-36.83,;20.9,-36.05,;22.24,-36.81,;20.9,-34.51,;19.55,-33.75,;18.23,-34.53,;16.89,-33.77,)| Show InChI InChI=1S/C22H19N5O4/c1-12-8-9-13(28)10-14(12)16-11-26-18-19(25(2)22(30)24-20(18)29)23-21(26)27(16)15-6-4-5-7-17(15)31-3/h4-11,28H,1-3H3,(H,24,29,30) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Zurich
Curated by ChEMBL
| Assay Description
Inhibition of EphB1 by [gamma33-P]ATP based assay |
J Med Chem 52: 6433-46 (2009)
Article DOI: 10.1021/jm9009444
BindingDB Entry DOI: 10.7270/Q2BZ663G |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM50299218

(8-(2-Methoxyphenyl)-1-methyl-7-(2'-methyl-5'-hydro...)Show SMILES COc1ccccc1-n1c(cn2c1nc1n(C)c(=O)[nH]c(=O)c21)-c1cc(O)ccc1C |(16.89,-42.99,;16.89,-41.45,;18.23,-40.68,;19.55,-41.45,;20.88,-40.68,;20.88,-39.14,;19.55,-38.38,;18.23,-39.15,;16.9,-38.38,;16.91,-36.84,;15.44,-36.35,;14.53,-37.6,;15.43,-38.85,;14.53,-40.09,;13.06,-39.61,;11.74,-40.37,;11.73,-41.91,;10.41,-39.61,;9.07,-40.38,;10.41,-38.07,;11.74,-37.29,;11.74,-35.75,;13.06,-38.07,;18.24,-36.06,;19.58,-36.83,;20.9,-36.05,;22.24,-36.81,;20.9,-34.51,;19.55,-33.75,;18.23,-34.53,;16.89,-33.77,)| Show InChI InChI=1S/C22H19N5O4/c1-12-8-9-13(28)10-14(12)16-11-26-18-19(25(2)22(30)24-20(18)29)23-21(26)27(16)15-6-4-5-7-17(15)31-3/h4-11,28H,1-3H3,(H,24,29,30) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Zurich
Curated by ChEMBL
| Assay Description
Inhibition of Src (unknown origin) using [gamma-33P]ATP after 30 mins by radiometric assay |
J Med Chem 56: 84-96 (2013)
Article DOI: 10.1021/jm301187e
BindingDB Entry DOI: 10.7270/Q2WW7K1S |
More data for this
Ligand-Target Pair | |
Ephrin type-B receptor 2
(Homo sapiens (Human)) | BDBM50299218

(8-(2-Methoxyphenyl)-1-methyl-7-(2'-methyl-5'-hydro...)Show SMILES COc1ccccc1-n1c(cn2c1nc1n(C)c(=O)[nH]c(=O)c21)-c1cc(O)ccc1C |(16.89,-42.99,;16.89,-41.45,;18.23,-40.68,;19.55,-41.45,;20.88,-40.68,;20.88,-39.14,;19.55,-38.38,;18.23,-39.15,;16.9,-38.38,;16.91,-36.84,;15.44,-36.35,;14.53,-37.6,;15.43,-38.85,;14.53,-40.09,;13.06,-39.61,;11.74,-40.37,;11.73,-41.91,;10.41,-39.61,;9.07,-40.38,;10.41,-38.07,;11.74,-37.29,;11.74,-35.75,;13.06,-38.07,;18.24,-36.06,;19.58,-36.83,;20.9,-36.05,;22.24,-36.81,;20.9,-34.51,;19.55,-33.75,;18.23,-34.53,;16.89,-33.77,)| Show InChI InChI=1S/C22H19N5O4/c1-12-8-9-13(28)10-14(12)16-11-26-18-19(25(2)22(30)24-20(18)29)23-21(26)27(16)15-6-4-5-7-17(15)31-3/h4-11,28H,1-3H3,(H,24,29,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Zurich
Curated by ChEMBL
| Assay Description
Inhibition of EphB2 by [gamma33-P]ATP based assay |
J Med Chem 52: 6433-46 (2009)
Article DOI: 10.1021/jm9009444
BindingDB Entry DOI: 10.7270/Q2BZ663G |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lck
(Homo sapiens (Human)) | BDBM50299218

(8-(2-Methoxyphenyl)-1-methyl-7-(2'-methyl-5'-hydro...)Show SMILES COc1ccccc1-n1c(cn2c1nc1n(C)c(=O)[nH]c(=O)c21)-c1cc(O)ccc1C |(16.89,-42.99,;16.89,-41.45,;18.23,-40.68,;19.55,-41.45,;20.88,-40.68,;20.88,-39.14,;19.55,-38.38,;18.23,-39.15,;16.9,-38.38,;16.91,-36.84,;15.44,-36.35,;14.53,-37.6,;15.43,-38.85,;14.53,-40.09,;13.06,-39.61,;11.74,-40.37,;11.73,-41.91,;10.41,-39.61,;9.07,-40.38,;10.41,-38.07,;11.74,-37.29,;11.74,-35.75,;13.06,-38.07,;18.24,-36.06,;19.58,-36.83,;20.9,-36.05,;22.24,-36.81,;20.9,-34.51,;19.55,-33.75,;18.23,-34.53,;16.89,-33.77,)| Show InChI InChI=1S/C22H19N5O4/c1-12-8-9-13(28)10-14(12)16-11-26-18-19(25(2)22(30)24-20(18)29)23-21(26)27(16)15-6-4-5-7-17(15)31-3/h4-11,28H,1-3H3,(H,24,29,30) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Zurich
Curated by ChEMBL
| Assay Description
Inhibition of Lck (unknown origin) using [gamma-33P]ATP after 30 mins by radiometric assay |
J Med Chem 56: 84-96 (2013)
Article DOI: 10.1021/jm301187e
BindingDB Entry DOI: 10.7270/Q2WW7K1S |
More data for this
Ligand-Target Pair | |
Ephrin type-B receptor 4
(Homo sapiens (Human)) | BDBM50299218

(8-(2-Methoxyphenyl)-1-methyl-7-(2'-methyl-5'-hydro...)Show SMILES COc1ccccc1-n1c(cn2c1nc1n(C)c(=O)[nH]c(=O)c21)-c1cc(O)ccc1C |(16.89,-42.99,;16.89,-41.45,;18.23,-40.68,;19.55,-41.45,;20.88,-40.68,;20.88,-39.14,;19.55,-38.38,;18.23,-39.15,;16.9,-38.38,;16.91,-36.84,;15.44,-36.35,;14.53,-37.6,;15.43,-38.85,;14.53,-40.09,;13.06,-39.61,;11.74,-40.37,;11.73,-41.91,;10.41,-39.61,;9.07,-40.38,;10.41,-38.07,;11.74,-37.29,;11.74,-35.75,;13.06,-38.07,;18.24,-36.06,;19.58,-36.83,;20.9,-36.05,;22.24,-36.81,;20.9,-34.51,;19.55,-33.75,;18.23,-34.53,;16.89,-33.77,)| Show InChI InChI=1S/C22H19N5O4/c1-12-8-9-13(28)10-14(12)16-11-26-18-19(25(2)22(30)24-20(18)29)23-21(26)27(16)15-6-4-5-7-17(15)31-3/h4-11,28H,1-3H3,(H,24,29,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Zurich
Curated by ChEMBL
| Assay Description
Inhibition of EphB4 (unknown origin) using [gamma-33P]ATP by radiometric assay |
J Med Chem 56: 84-96 (2013)
Article DOI: 10.1021/jm301187e
BindingDB Entry DOI: 10.7270/Q2WW7K1S |
More data for this
Ligand-Target Pair | |
Ephrin type-B receptor 4
(Homo sapiens (Human)) | BDBM50299218

(8-(2-Methoxyphenyl)-1-methyl-7-(2'-methyl-5'-hydro...)Show SMILES COc1ccccc1-n1c(cn2c1nc1n(C)c(=O)[nH]c(=O)c21)-c1cc(O)ccc1C |(16.89,-42.99,;16.89,-41.45,;18.23,-40.68,;19.55,-41.45,;20.88,-40.68,;20.88,-39.14,;19.55,-38.38,;18.23,-39.15,;16.9,-38.38,;16.91,-36.84,;15.44,-36.35,;14.53,-37.6,;15.43,-38.85,;14.53,-40.09,;13.06,-39.61,;11.74,-40.37,;11.73,-41.91,;10.41,-39.61,;9.07,-40.38,;10.41,-38.07,;11.74,-37.29,;11.74,-35.75,;13.06,-38.07,;18.24,-36.06,;19.58,-36.83,;20.9,-36.05,;22.24,-36.81,;20.9,-34.51,;19.55,-33.75,;18.23,-34.53,;16.89,-33.77,)| Show InChI InChI=1S/C22H19N5O4/c1-12-8-9-13(28)10-14(12)16-11-26-18-19(25(2)22(30)24-20(18)29)23-21(26)27(16)15-6-4-5-7-17(15)31-3/h4-11,28H,1-3H3,(H,24,29,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Zurich
Curated by ChEMBL
| Assay Description
Inhibition of EphB4 by [gamma33-P]ATP based assay |
J Med Chem 52: 6433-46 (2009)
Article DOI: 10.1021/jm9009444
BindingDB Entry DOI: 10.7270/Q2BZ663G |
More data for this
Ligand-Target Pair | |
Ephrin type-A receptor 2
(Homo sapiens (Human)) | BDBM50299218

(8-(2-Methoxyphenyl)-1-methyl-7-(2'-methyl-5'-hydro...)Show SMILES COc1ccccc1-n1c(cn2c1nc1n(C)c(=O)[nH]c(=O)c21)-c1cc(O)ccc1C |(16.89,-42.99,;16.89,-41.45,;18.23,-40.68,;19.55,-41.45,;20.88,-40.68,;20.88,-39.14,;19.55,-38.38,;18.23,-39.15,;16.9,-38.38,;16.91,-36.84,;15.44,-36.35,;14.53,-37.6,;15.43,-38.85,;14.53,-40.09,;13.06,-39.61,;11.74,-40.37,;11.73,-41.91,;10.41,-39.61,;9.07,-40.38,;10.41,-38.07,;11.74,-37.29,;11.74,-35.75,;13.06,-38.07,;18.24,-36.06,;19.58,-36.83,;20.9,-36.05,;22.24,-36.81,;20.9,-34.51,;19.55,-33.75,;18.23,-34.53,;16.89,-33.77,)| Show InChI InChI=1S/C22H19N5O4/c1-12-8-9-13(28)10-14(12)16-11-26-18-19(25(2)22(30)24-20(18)29)23-21(26)27(16)15-6-4-5-7-17(15)31-3/h4-11,28H,1-3H3,(H,24,29,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Zurich
Curated by ChEMBL
| Assay Description
Inhibition of EphA2 by [gamma33-P]ATP based assay |
J Med Chem 52: 6433-46 (2009)
Article DOI: 10.1021/jm9009444
BindingDB Entry DOI: 10.7270/Q2BZ663G |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Ephrin type-A receptor 1
(Homo sapiens (Human)) | BDBM50299218

(8-(2-Methoxyphenyl)-1-methyl-7-(2'-methyl-5'-hydro...)Show SMILES COc1ccccc1-n1c(cn2c1nc1n(C)c(=O)[nH]c(=O)c21)-c1cc(O)ccc1C |(16.89,-42.99,;16.89,-41.45,;18.23,-40.68,;19.55,-41.45,;20.88,-40.68,;20.88,-39.14,;19.55,-38.38,;18.23,-39.15,;16.9,-38.38,;16.91,-36.84,;15.44,-36.35,;14.53,-37.6,;15.43,-38.85,;14.53,-40.09,;13.06,-39.61,;11.74,-40.37,;11.73,-41.91,;10.41,-39.61,;9.07,-40.38,;10.41,-38.07,;11.74,-37.29,;11.74,-35.75,;13.06,-38.07,;18.24,-36.06,;19.58,-36.83,;20.9,-36.05,;22.24,-36.81,;20.9,-34.51,;19.55,-33.75,;18.23,-34.53,;16.89,-33.77,)| Show InChI InChI=1S/C22H19N5O4/c1-12-8-9-13(28)10-14(12)16-11-26-18-19(25(2)22(30)24-20(18)29)23-21(26)27(16)15-6-4-5-7-17(15)31-3/h4-11,28H,1-3H3,(H,24,29,30) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Zurich
Curated by ChEMBL
| Assay Description
Inhibition of EphA1 by [gamma33-P]ATP based assay |
J Med Chem 52: 6433-46 (2009)
Article DOI: 10.1021/jm9009444
BindingDB Entry DOI: 10.7270/Q2BZ663G |
More data for this
Ligand-Target Pair | |
Ephrin type-A receptor 5
(Homo sapiens (Human)) | BDBM50299218

(8-(2-Methoxyphenyl)-1-methyl-7-(2'-methyl-5'-hydro...)Show SMILES COc1ccccc1-n1c(cn2c1nc1n(C)c(=O)[nH]c(=O)c21)-c1cc(O)ccc1C |(16.89,-42.99,;16.89,-41.45,;18.23,-40.68,;19.55,-41.45,;20.88,-40.68,;20.88,-39.14,;19.55,-38.38,;18.23,-39.15,;16.9,-38.38,;16.91,-36.84,;15.44,-36.35,;14.53,-37.6,;15.43,-38.85,;14.53,-40.09,;13.06,-39.61,;11.74,-40.37,;11.73,-41.91,;10.41,-39.61,;9.07,-40.38,;10.41,-38.07,;11.74,-37.29,;11.74,-35.75,;13.06,-38.07,;18.24,-36.06,;19.58,-36.83,;20.9,-36.05,;22.24,-36.81,;20.9,-34.51,;19.55,-33.75,;18.23,-34.53,;16.89,-33.77,)| Show InChI InChI=1S/C22H19N5O4/c1-12-8-9-13(28)10-14(12)16-11-26-18-19(25(2)22(30)24-20(18)29)23-21(26)27(16)15-6-4-5-7-17(15)31-3/h4-11,28H,1-3H3,(H,24,29,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Zurich
Curated by ChEMBL
| Assay Description
Inhibition of EphA5 by [gamma33-P]ATP based assay |
J Med Chem 52: 6433-46 (2009)
Article DOI: 10.1021/jm9009444
BindingDB Entry DOI: 10.7270/Q2BZ663G |
More data for this
Ligand-Target Pair | |
Ephrin type-A receptor 4
(Homo sapiens (Human)) | BDBM50299218

(8-(2-Methoxyphenyl)-1-methyl-7-(2'-methyl-5'-hydro...)Show SMILES COc1ccccc1-n1c(cn2c1nc1n(C)c(=O)[nH]c(=O)c21)-c1cc(O)ccc1C |(16.89,-42.99,;16.89,-41.45,;18.23,-40.68,;19.55,-41.45,;20.88,-40.68,;20.88,-39.14,;19.55,-38.38,;18.23,-39.15,;16.9,-38.38,;16.91,-36.84,;15.44,-36.35,;14.53,-37.6,;15.43,-38.85,;14.53,-40.09,;13.06,-39.61,;11.74,-40.37,;11.73,-41.91,;10.41,-39.61,;9.07,-40.38,;10.41,-38.07,;11.74,-37.29,;11.74,-35.75,;13.06,-38.07,;18.24,-36.06,;19.58,-36.83,;20.9,-36.05,;22.24,-36.81,;20.9,-34.51,;19.55,-33.75,;18.23,-34.53,;16.89,-33.77,)| Show InChI InChI=1S/C22H19N5O4/c1-12-8-9-13(28)10-14(12)16-11-26-18-19(25(2)22(30)24-20(18)29)23-21(26)27(16)15-6-4-5-7-17(15)31-3/h4-11,28H,1-3H3,(H,24,29,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Zurich
Curated by ChEMBL
| Assay Description
Inhibition of EphA4 by [gamma33-P]ATP based assay |
J Med Chem 52: 6433-46 (2009)
Article DOI: 10.1021/jm9009444
BindingDB Entry DOI: 10.7270/Q2BZ663G |
More data for this
Ligand-Target Pair | |
Ephrin type-A receptor 8
(Homo sapiens (Human)) | BDBM50299218

(8-(2-Methoxyphenyl)-1-methyl-7-(2'-methyl-5'-hydro...)Show SMILES COc1ccccc1-n1c(cn2c1nc1n(C)c(=O)[nH]c(=O)c21)-c1cc(O)ccc1C |(16.89,-42.99,;16.89,-41.45,;18.23,-40.68,;19.55,-41.45,;20.88,-40.68,;20.88,-39.14,;19.55,-38.38,;18.23,-39.15,;16.9,-38.38,;16.91,-36.84,;15.44,-36.35,;14.53,-37.6,;15.43,-38.85,;14.53,-40.09,;13.06,-39.61,;11.74,-40.37,;11.73,-41.91,;10.41,-39.61,;9.07,-40.38,;10.41,-38.07,;11.74,-37.29,;11.74,-35.75,;13.06,-38.07,;18.24,-36.06,;19.58,-36.83,;20.9,-36.05,;22.24,-36.81,;20.9,-34.51,;19.55,-33.75,;18.23,-34.53,;16.89,-33.77,)| Show InChI InChI=1S/C22H19N5O4/c1-12-8-9-13(28)10-14(12)16-11-26-18-19(25(2)22(30)24-20(18)29)23-21(26)27(16)15-6-4-5-7-17(15)31-3/h4-11,28H,1-3H3,(H,24,29,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Zurich
Curated by ChEMBL
| Assay Description
Inhibition of EphA8 by [gamma33-P]ATP based assay |
J Med Chem 52: 6433-46 (2009)
Article DOI: 10.1021/jm9009444
BindingDB Entry DOI: 10.7270/Q2BZ663G |
More data for this
Ligand-Target Pair | |
Ephrin type-B receptor 4
(Homo sapiens (Human)) | BDBM50299218

(8-(2-Methoxyphenyl)-1-methyl-7-(2'-methyl-5'-hydro...)Show SMILES COc1ccccc1-n1c(cn2c1nc1n(C)c(=O)[nH]c(=O)c21)-c1cc(O)ccc1C |(16.89,-42.99,;16.89,-41.45,;18.23,-40.68,;19.55,-41.45,;20.88,-40.68,;20.88,-39.14,;19.55,-38.38,;18.23,-39.15,;16.9,-38.38,;16.91,-36.84,;15.44,-36.35,;14.53,-37.6,;15.43,-38.85,;14.53,-40.09,;13.06,-39.61,;11.74,-40.37,;11.73,-41.91,;10.41,-39.61,;9.07,-40.38,;10.41,-38.07,;11.74,-37.29,;11.74,-35.75,;13.06,-38.07,;18.24,-36.06,;19.58,-36.83,;20.9,-36.05,;22.24,-36.81,;20.9,-34.51,;19.55,-33.75,;18.23,-34.53,;16.89,-33.77,)| Show InChI InChI=1S/C22H19N5O4/c1-12-8-9-13(28)10-14(12)16-11-26-18-19(25(2)22(30)24-20(18)29)23-21(26)27(16)15-6-4-5-7-17(15)31-3/h4-11,28H,1-3H3,(H,24,29,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Zurich
Curated by ChEMBL
| Assay Description
Inhibition of EphB4 assessed as blockade of synthetic substrate phosphorylation by FRET assay |
J Med Chem 52: 6433-46 (2009)
Article DOI: 10.1021/jm9009444
BindingDB Entry DOI: 10.7270/Q2BZ663G |
More data for this
Ligand-Target Pair | |
Ephrin type-B receptor 4
(Homo sapiens (Human)) | BDBM50299218

(8-(2-Methoxyphenyl)-1-methyl-7-(2'-methyl-5'-hydro...)Show SMILES COc1ccccc1-n1c(cn2c1nc1n(C)c(=O)[nH]c(=O)c21)-c1cc(O)ccc1C |(16.89,-42.99,;16.89,-41.45,;18.23,-40.68,;19.55,-41.45,;20.88,-40.68,;20.88,-39.14,;19.55,-38.38,;18.23,-39.15,;16.9,-38.38,;16.91,-36.84,;15.44,-36.35,;14.53,-37.6,;15.43,-38.85,;14.53,-40.09,;13.06,-39.61,;11.74,-40.37,;11.73,-41.91,;10.41,-39.61,;9.07,-40.38,;10.41,-38.07,;11.74,-37.29,;11.74,-35.75,;13.06,-38.07,;18.24,-36.06,;19.58,-36.83,;20.9,-36.05,;22.24,-36.81,;20.9,-34.51,;19.55,-33.75,;18.23,-34.53,;16.89,-33.77,)| Show InChI InChI=1S/C22H19N5O4/c1-12-8-9-13(28)10-14(12)16-11-26-18-19(25(2)22(30)24-20(18)29)23-21(26)27(16)15-6-4-5-7-17(15)31-3/h4-11,28H,1-3H3,(H,24,29,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Zurich
Curated by ChEMBL
| Assay Description
Inhibition of EphB4 (unknown origin) using Z'-LYTE TYR-1 peptide as substrate after 2 hrs by FRET assay |
J Med Chem 56: 84-96 (2013)
Article DOI: 10.1021/jm301187e
BindingDB Entry DOI: 10.7270/Q2WW7K1S |
More data for this
Ligand-Target Pair | |
Ephrin type-B receptor 4
(Homo sapiens (Human)) | BDBM50100316
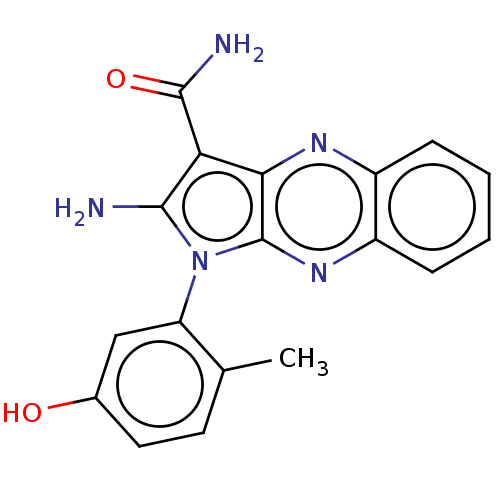
(CHEMBL3321809)Show SMILES Cc1ccc(O)cc1-n1c(N)c(C(N)=O)c2nc3ccccc3nc12 |(9.45,-34.75,;10.95,-35.07,;11.43,-36.54,;12.94,-36.86,;13.97,-35.71,;15.47,-36.03,;13.49,-34.25,;11.98,-33.93,;11.51,-32.46,;12.41,-31.22,;13.95,-31.22,;11.51,-29.97,;11.98,-28.51,;13.47,-28.11,;10.95,-27.36,;10.04,-30.45,;8.71,-29.68,;7.38,-30.45,;6.04,-29.68,;4.71,-30.45,;4.71,-31.99,;6.04,-32.76,;7.38,-31.99,;8.71,-32.76,;10.04,-31.99,)| Show InChI InChI=1S/C18H15N5O2/c1-9-6-7-10(24)8-13(9)23-16(19)14(17(20)25)15-18(23)22-12-5-3-2-4-11(12)21-15/h2-8,24H,19H2,1H3,(H2,20,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.40 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Z£rich
Curated by ChEMBL
| Assay Description
Inhibition of phosphorylation of full-length myc-tagged human EphB4 overexpressed in mouse embryonic fibroblasts after 90 mins by sandwich ELISA |
J Med Chem 57: 6834-44 (2014)
Article DOI: 10.1021/jm5009242
BindingDB Entry DOI: 10.7270/Q2TQ638D |
More data for this
Ligand-Target Pair | |
Ephrin type-B receptor 4
(Homo sapiens (Human)) | BDBM50428741

(CHEMBL2333602)Show SMILES CCCCn1c(cn2c1nc1n(C)c(=O)[nH]c(=O)c21)-c1cc(ccc1C)-c1cccc2[nH]ncc12 Show InChI InChI=1S/C26H25N7O2/c1-4-5-11-32-21(14-33-22-23(28-25(32)33)31(3)26(35)29-24(22)34)18-12-16(10-9-15(18)2)17-7-6-8-20-19(17)13-27-30-20/h6-10,12-14H,4-5,11H2,1-3H3,(H,27,30)(H,29,34,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Zurich
Curated by ChEMBL
| Assay Description
Inhibition of EphB4 (unknown origin) transfected in CHO cells using Z'-LYTE TYR-1 peptide as substrate after 2 hrs by FRET assay |
J Med Chem 56: 84-96 (2013)
Article DOI: 10.1021/jm301187e
BindingDB Entry DOI: 10.7270/Q2WW7K1S |
More data for this
Ligand-Target Pair | |
Ephrin type-B receptor 4
(Homo sapiens (Human)) | BDBM50100326
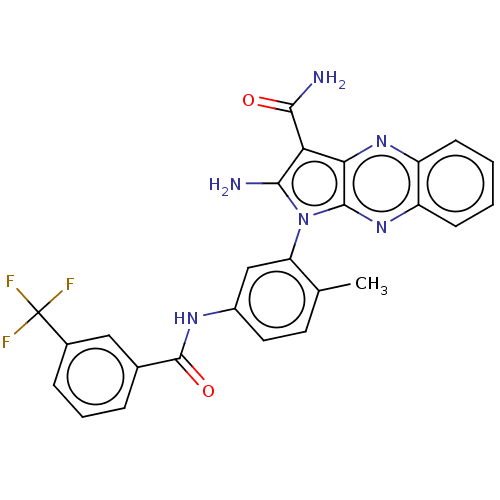
(CHEMBL3321812)Show SMILES Cc1ccc(NC(=O)c2cccc(c2)C(F)(F)F)cc1-n1c(N)c(C(N)=O)c2nc3ccccc3nc12 |(4.06,-42.16,;5.56,-42.48,;6.04,-43.95,;7.55,-44.27,;8.58,-43.13,;10.08,-43.45,;10.56,-44.91,;9.53,-46.05,;12.06,-45.23,;13.08,-44.09,;14.59,-44.4,;15.07,-45.87,;14.04,-47.02,;12.53,-46.7,;14.51,-48.48,;13.48,-49.63,;16.02,-48.8,;15.28,-49.81,;8.1,-41.66,;6.59,-41.34,;6.12,-39.88,;7.02,-38.63,;8.56,-38.63,;6.12,-37.38,;6.59,-35.92,;8.08,-35.52,;5.56,-34.77,;4.65,-37.86,;3.32,-37.09,;1.99,-37.86,;.65,-37.09,;-.69,-37.86,;-.69,-39.4,;.65,-40.17,;1.99,-39.4,;3.32,-40.17,;4.65,-39.4,)| Show InChI InChI=1S/C26H19F3N6O2/c1-13-9-10-16(32-25(37)14-5-4-6-15(11-14)26(27,28)29)12-19(13)35-22(30)20(23(31)36)21-24(35)34-18-8-3-2-7-17(18)33-21/h2-12H,30H2,1H3,(H2,31,36)(H,32,37) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Z£rich
Curated by ChEMBL
| Assay Description
Inhibition of phosphorylation of full-length myc-tagged human EphB4 overexpressed in mouse embryonic fibroblasts after 90 mins by sandwich ELISA |
J Med Chem 57: 6834-44 (2014)
Article DOI: 10.1021/jm5009242
BindingDB Entry DOI: 10.7270/Q2TQ638D |
More data for this
Ligand-Target Pair | |
Ephrin type-B receptor 4
(Homo sapiens (Human)) | BDBM50100327
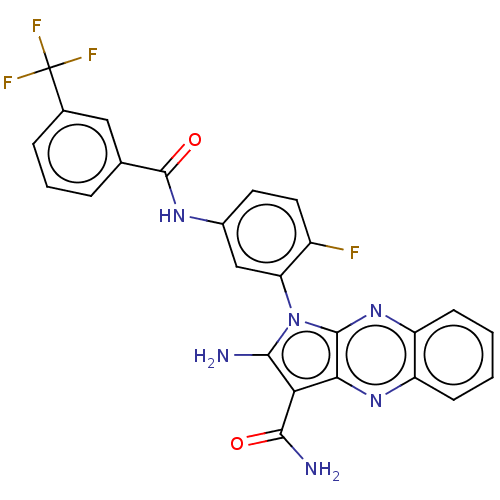
(CHEMBL3321813)Show SMILES NC(=O)c1c(N)n(-c2cc(NC(=O)c3cccc(c3)C(F)(F)F)ccc2F)c2nc3ccccc3nc12 |(24.7,-37.03,;23.21,-37.43,;22.18,-36.28,;22.74,-38.89,;23.64,-40.14,;25.18,-40.14,;22.74,-41.38,;23.21,-42.85,;24.72,-43.17,;25.2,-44.63,;26.7,-44.95,;27.18,-46.42,;26.15,-47.56,;28.69,-46.74,;29.71,-45.6,;31.21,-45.91,;31.69,-47.38,;30.66,-48.53,;29.16,-48.21,;31.14,-49.99,;30.1,-51.13,;32.64,-50.31,;31.9,-51.32,;24.17,-45.78,;22.66,-45.46,;22.18,-43.99,;20.68,-43.67,;21.27,-40.91,;19.94,-41.68,;18.61,-40.91,;17.27,-41.68,;15.94,-40.91,;15.94,-39.37,;17.27,-38.6,;18.61,-39.37,;19.94,-38.6,;21.27,-39.37,)| Show InChI InChI=1S/C25H16F4N6O2/c26-15-9-8-14(32-24(37)12-4-3-5-13(10-12)25(27,28)29)11-18(15)35-21(30)19(22(31)36)20-23(35)34-17-7-2-1-6-16(17)33-20/h1-11H,30H2,(H2,31,36)(H,32,37) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Z£rich
Curated by ChEMBL
| Assay Description
Inhibition of phosphorylation of full-length myc-tagged human EphB4 overexpressed in mouse embryonic fibroblasts after 90 mins by sandwich ELISA |
J Med Chem 57: 6834-44 (2014)
Article DOI: 10.1021/jm5009242
BindingDB Entry DOI: 10.7270/Q2TQ638D |
More data for this
Ligand-Target Pair | |
Ephrin type-B receptor 3
(Homo sapiens (Human)) | BDBM50299218

(8-(2-Methoxyphenyl)-1-methyl-7-(2'-methyl-5'-hydro...)Show SMILES COc1ccccc1-n1c(cn2c1nc1n(C)c(=O)[nH]c(=O)c21)-c1cc(O)ccc1C |(16.89,-42.99,;16.89,-41.45,;18.23,-40.68,;19.55,-41.45,;20.88,-40.68,;20.88,-39.14,;19.55,-38.38,;18.23,-39.15,;16.9,-38.38,;16.91,-36.84,;15.44,-36.35,;14.53,-37.6,;15.43,-38.85,;14.53,-40.09,;13.06,-39.61,;11.74,-40.37,;11.73,-41.91,;10.41,-39.61,;9.07,-40.38,;10.41,-38.07,;11.74,-37.29,;11.74,-35.75,;13.06,-38.07,;18.24,-36.06,;19.58,-36.83,;20.9,-36.05,;22.24,-36.81,;20.9,-34.51,;19.55,-33.75,;18.23,-34.53,;16.89,-33.77,)| Show InChI InChI=1S/C22H19N5O4/c1-12-8-9-13(28)10-14(12)16-11-26-18-19(25(2)22(30)24-20(18)29)23-21(26)27(16)15-6-4-5-7-17(15)31-3/h4-11,28H,1-3H3,(H,24,29,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Zurich
Curated by ChEMBL
| Assay Description
Inhibition of EphB3 by [gamma33-P]ATP based assay |
J Med Chem 52: 6433-46 (2009)
Article DOI: 10.1021/jm9009444
BindingDB Entry DOI: 10.7270/Q2BZ663G |
More data for this
Ligand-Target Pair | |
Ephrin type-B receptor 4
(Homo sapiens (Human)) | BDBM50100325
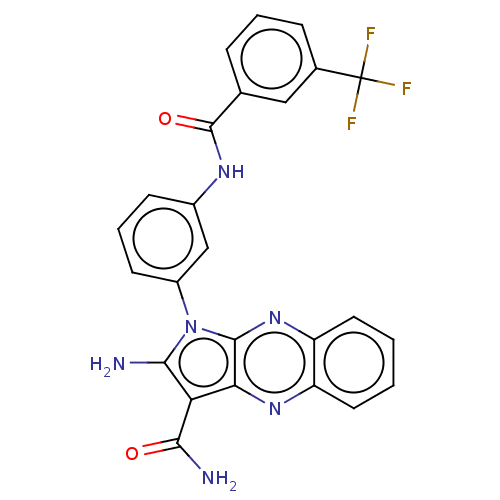
(CHEMBL3321811)Show SMILES NC(=O)c1c(N)n(-c2cccc(NC(=O)c3cccc(c3)C(F)(F)F)c2)c2nc3ccccc3nc12 Show InChI InChI=1S/C25H17F3N6O2/c26-25(27,28)14-6-3-5-13(11-14)24(36)31-15-7-4-8-16(12-15)34-21(29)19(22(30)35)20-23(34)33-18-10-2-1-9-17(18)32-20/h1-12H,29H2,(H2,30,35)(H,31,36) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Z£rich
Curated by ChEMBL
| Assay Description
Inhibition of phosphorylation of full-length myc-tagged human EphB4 overexpressed in mouse embryonic fibroblasts after 90 mins by sandwich ELISA |
J Med Chem 57: 6834-44 (2014)
Article DOI: 10.1021/jm5009242
BindingDB Entry DOI: 10.7270/Q2TQ638D |
More data for this
Ligand-Target Pair | |
Ephrin type-B receptor 4
(Homo sapiens (Human)) | BDBM50100340
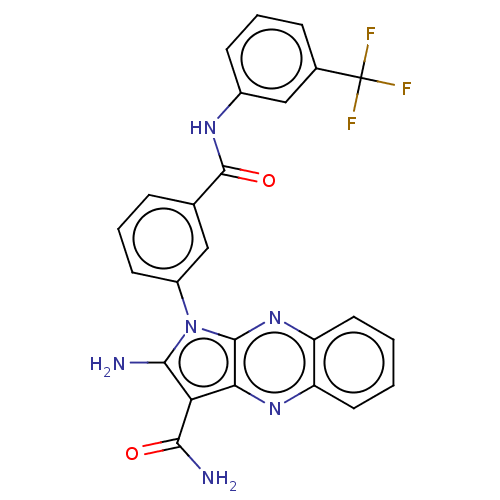
(CHEMBL3321816)Show SMILES NC(=O)c1c(N)n(-c2cccc(c2)C(=O)Nc2cccc(c2)C(F)(F)F)c2nc3ccccc3nc12 Show InChI InChI=1S/C25H17F3N6O2/c26-25(27,28)14-6-4-7-15(12-14)31-24(36)13-5-3-8-16(11-13)34-21(29)19(22(30)35)20-23(34)33-18-10-2-1-9-17(18)32-20/h1-12H,29H2,(H2,30,35)(H,31,36) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Z£rich
Curated by ChEMBL
| Assay Description
Inhibition of phosphorylation of full-length myc-tagged human EphB4 overexpressed in mouse embryonic fibroblasts after 90 mins by sandwich ELISA |
J Med Chem 57: 6834-44 (2014)
Article DOI: 10.1021/jm5009242
BindingDB Entry DOI: 10.7270/Q2TQ638D |
More data for this
Ligand-Target Pair | |
Ephrin type-B receptor 4
(Homo sapiens (Human)) | BDBM50100382

(CHEMBL3321817)Show SMILES Cc1ccc(cc1-n1c(N)c(C(N)=O)c2nc3ccccc3nc12)C(=O)Nc1cccc(c1)C(F)(F)F |(1.14,-21.09,;2.65,-21.41,;3.12,-22.87,;4.63,-23.19,;5.66,-22.05,;5.18,-20.58,;3.68,-20.26,;3.2,-18.8,;4.11,-17.55,;5.65,-17.55,;3.2,-16.31,;3.68,-14.84,;5.16,-14.44,;2.65,-13.7,;1.74,-16.78,;.4,-16.01,;-.94,-16.78,;-2.28,-16.01,;-3.6,-16.78,;-3.6,-18.32,;-2.28,-19.09,;-.94,-18.32,;.4,-19.09,;1.74,-18.32,;7.17,-22.37,;8.2,-21.22,;7.64,-23.83,;9.15,-24.15,;10.17,-23.01,;11.67,-23.32,;12.15,-24.79,;11.12,-25.94,;9.62,-25.62,;11.6,-27.4,;10.57,-28.55,;13.1,-27.72,;11.99,-28.89,)| Show InChI InChI=1S/C26H19F3N6O2/c1-13-9-10-14(25(37)32-16-6-4-5-15(12-16)26(27,28)29)11-19(13)35-22(30)20(23(31)36)21-24(35)34-18-8-3-2-7-17(18)33-21/h2-12H,30H2,1H3,(H2,31,36)(H,32,37) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Z£rich
Curated by ChEMBL
| Assay Description
Inhibition of phosphorylation of full-length myc-tagged human EphB4 overexpressed in mouse embryonic fibroblasts after 90 mins by sandwich ELISA |
J Med Chem 57: 6834-44 (2014)
Article DOI: 10.1021/jm5009242
BindingDB Entry DOI: 10.7270/Q2TQ638D |
More data for this
Ligand-Target Pair | |
Ephrin type-B receptor 4
(Homo sapiens (Human)) | BDBM50100383
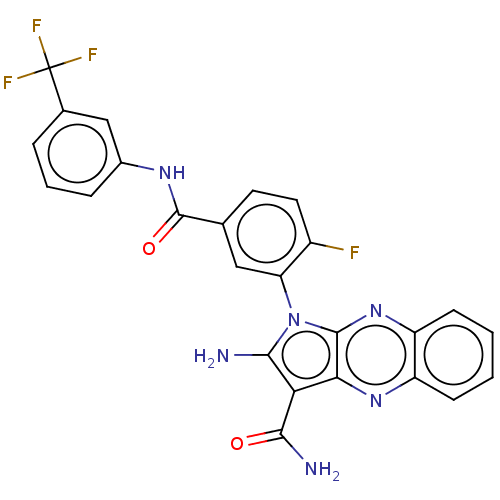
(CHEMBL3321818)Show SMILES NC(=O)c1c(N)n(-c2cc(ccc2F)C(=O)Nc2cccc(c2)C(F)(F)F)c2nc3ccccc3nc12 |(23.12,-14.22,;21.64,-14.62,;20.61,-13.47,;21.16,-16.08,;22.06,-17.33,;23.6,-17.33,;21.16,-18.57,;21.64,-20.04,;23.14,-20.36,;23.62,-21.82,;22.59,-22.97,;21.08,-22.65,;20.61,-21.18,;19.1,-20.86,;25.12,-22.14,;26.15,-21,;25.6,-23.61,;27.11,-23.93,;28.13,-22.78,;29.63,-23.1,;30.11,-24.57,;29.08,-25.71,;27.58,-25.39,;29.56,-27.18,;28.53,-28.32,;31.06,-27.5,;29.95,-28.66,;19.7,-18.1,;18.36,-18.87,;17.03,-18.1,;15.69,-18.87,;14.36,-18.1,;14.36,-16.56,;15.69,-15.79,;17.03,-16.56,;18.36,-15.79,;19.7,-16.56,)| Show InChI InChI=1S/C25H16F4N6O2/c26-15-9-8-12(24(37)32-14-5-3-4-13(11-14)25(27,28)29)10-18(15)35-21(30)19(22(31)36)20-23(35)34-17-7-2-1-6-16(17)33-20/h1-11H,30H2,(H2,31,36)(H,32,37) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Z£rich
Curated by ChEMBL
| Assay Description
Inhibition of phosphorylation of full-length myc-tagged human EphB4 overexpressed in mouse embryonic fibroblasts after 90 mins by sandwich ELISA |
J Med Chem 57: 6834-44 (2014)
Article DOI: 10.1021/jm5009242
BindingDB Entry DOI: 10.7270/Q2TQ638D |
More data for this
Ligand-Target Pair | |
Ephrin type-A receptor 3
(Homo sapiens (Human)) | BDBM50299218

(8-(2-Methoxyphenyl)-1-methyl-7-(2'-methyl-5'-hydro...)Show SMILES COc1ccccc1-n1c(cn2c1nc1n(C)c(=O)[nH]c(=O)c21)-c1cc(O)ccc1C |(16.89,-42.99,;16.89,-41.45,;18.23,-40.68,;19.55,-41.45,;20.88,-40.68,;20.88,-39.14,;19.55,-38.38,;18.23,-39.15,;16.9,-38.38,;16.91,-36.84,;15.44,-36.35,;14.53,-37.6,;15.43,-38.85,;14.53,-40.09,;13.06,-39.61,;11.74,-40.37,;11.73,-41.91,;10.41,-39.61,;9.07,-40.38,;10.41,-38.07,;11.74,-37.29,;11.74,-35.75,;13.06,-38.07,;18.24,-36.06,;19.58,-36.83,;20.9,-36.05,;22.24,-36.81,;20.9,-34.51,;19.55,-33.75,;18.23,-34.53,;16.89,-33.77,)| Show InChI InChI=1S/C22H19N5O4/c1-12-8-9-13(28)10-14(12)16-11-26-18-19(25(2)22(30)24-20(18)29)23-21(26)27(16)15-6-4-5-7-17(15)31-3/h4-11,28H,1-3H3,(H,24,29,30) | PDB
MMDB
Reactome pathway
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Zurich
Curated by ChEMBL
| Assay Description
Inhibition of EphA3 by [gamma33-P]ATP based assay |
J Med Chem 52: 6433-46 (2009)
Article DOI: 10.1021/jm9009444
BindingDB Entry DOI: 10.7270/Q2BZ663G |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM50428741

(CHEMBL2333602)Show SMILES CCCCn1c(cn2c1nc1n(C)c(=O)[nH]c(=O)c21)-c1cc(ccc1C)-c1cccc2[nH]ncc12 Show InChI InChI=1S/C26H25N7O2/c1-4-5-11-32-21(14-33-22-23(28-25(32)33)31(3)26(35)29-24(22)34)18-12-16(10-9-15(18)2)17-7-6-8-20-19(17)13-27-30-20/h6-10,12-14H,4-5,11H2,1-3H3,(H,27,30)(H,29,34,35) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 46 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Zurich
Curated by ChEMBL
| Assay Description
Inhibition of Src (unknown origin) using [gamma-33P]ATP after 30 mins by radiometric assay |
J Med Chem 56: 84-96 (2013)
Article DOI: 10.1021/jm301187e
BindingDB Entry DOI: 10.7270/Q2WW7K1S |
More data for this
Ligand-Target Pair | |
Ephrin type-B receptor 4
(Homo sapiens (Human)) | BDBM50299219

(8-(2-Methoxyphenyl)-1-methyl-7-o-methylphenyl-1H-i...)Show SMILES COc1ccccc1-n1c(cn2c1nc1n(C)c(=O)[nH]c(=O)c21)-c1ccccc1C |(28.6,-45.77,;28.6,-44.23,;29.94,-43.46,;31.26,-44.23,;32.59,-43.46,;32.59,-41.92,;31.26,-41.16,;29.94,-41.93,;28.61,-41.16,;28.62,-39.62,;27.15,-39.13,;26.24,-40.38,;27.14,-41.63,;26.24,-42.87,;24.77,-42.4,;23.44,-43.16,;23.44,-44.7,;22.11,-42.39,;20.78,-43.17,;22.11,-40.85,;23.44,-40.08,;23.44,-38.54,;24.77,-40.85,;29.95,-38.84,;31.29,-39.61,;32.61,-38.83,;32.61,-37.29,;31.26,-36.53,;29.93,-37.31,;28.59,-36.55,)| Show InChI InChI=1S/C22H19N5O3/c1-13-8-4-5-9-14(13)16-12-26-18-19(25(2)22(29)24-20(18)28)23-21(26)27(16)15-10-6-7-11-17(15)30-3/h4-12H,1-3H3,(H,24,28,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 47 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Zurich
Curated by ChEMBL
| Assay Description
Inhibition of EphB4 by [gamma33-P]ATP based assay |
J Med Chem 52: 6433-46 (2009)
Article DOI: 10.1021/jm9009444
BindingDB Entry DOI: 10.7270/Q2BZ663G |
More data for this
Ligand-Target Pair | |
Ephrin type-B receptor 4
(Homo sapiens (Human)) | BDBM50428743

(CHEMBL2333603)Show SMILES CCCCn1c(cn2c1nc1n(C)c(=O)[nH]c(=O)c21)-c1ccccc1C Show InChI InChI=1S/C19H21N5O2/c1-4-5-10-23-14(13-9-7-6-8-12(13)2)11-24-15-16(20-18(23)24)22(3)19(26)21-17(15)25/h6-9,11H,4-5,10H2,1-3H3,(H,21,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Zurich
Curated by ChEMBL
| Assay Description
Inhibition of human myc-tagged full length EphB4 expressed in MEF cells assessed as inhibition of ephrinB2-Fc-induced autophosphorylation incubated f... |
J Med Chem 56: 84-96 (2013)
Article DOI: 10.1021/jm301187e
BindingDB Entry DOI: 10.7270/Q2WW7K1S |
More data for this
Ligand-Target Pair | |
Ephrin type-B receptor 4
(Homo sapiens (Human)) | BDBM50428743

(CHEMBL2333603)Show SMILES CCCCn1c(cn2c1nc1n(C)c(=O)[nH]c(=O)c21)-c1ccccc1C Show InChI InChI=1S/C19H21N5O2/c1-4-5-10-23-14(13-9-7-6-8-12(13)2)11-24-15-16(20-18(23)24)22(3)19(26)21-17(15)25/h6-9,11H,4-5,10H2,1-3H3,(H,21,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 56 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Zurich
Curated by ChEMBL
| Assay Description
Inhibition of EphB4 (unknown origin) transfected in CHO cells using Z'-LYTE TYR-1 peptide as substrate after 2 hrs by FRET assay |
J Med Chem 56: 84-96 (2013)
Article DOI: 10.1021/jm301187e
BindingDB Entry DOI: 10.7270/Q2WW7K1S |
More data for this
Ligand-Target Pair | |
Ephrin type-B receptor 4
(Homo sapiens (Human)) | BDBM50428742
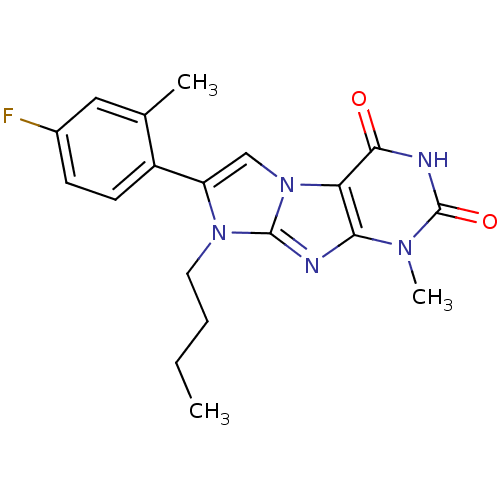
(CHEMBL2333595)Show SMILES CCCCn1c(cn2c1nc1n(C)c(=O)[nH]c(=O)c21)-c1ccc(F)cc1C Show InChI InChI=1S/C19H20FN5O2/c1-4-5-8-24-14(13-7-6-12(20)9-11(13)2)10-25-15-16(21-18(24)25)23(3)19(27)22-17(15)26/h6-7,9-10H,4-5,8H2,1-3H3,(H,22,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 68 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Zurich
Curated by ChEMBL
| Assay Description
Inhibition of human myc-tagged full length EphB4 expressed in MEF cells assessed as inhibition of ephrinB2-Fc-induced autophosphorylation incubated f... |
J Med Chem 56: 84-96 (2013)
Article DOI: 10.1021/jm301187e
BindingDB Entry DOI: 10.7270/Q2WW7K1S |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM50428743

(CHEMBL2333603)Show SMILES CCCCn1c(cn2c1nc1n(C)c(=O)[nH]c(=O)c21)-c1ccccc1C Show InChI InChI=1S/C19H21N5O2/c1-4-5-10-23-14(13-9-7-6-8-12(13)2)11-24-15-16(20-18(23)24)22(3)19(26)21-17(15)25/h6-9,11H,4-5,10H2,1-3H3,(H,21,25,26) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 71 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Zurich
Curated by ChEMBL
| Assay Description
Inhibition of Src (unknown origin) using [gamma-33P]ATP after 30 mins by radiometric assay |
J Med Chem 56: 84-96 (2013)
Article DOI: 10.1021/jm301187e
BindingDB Entry DOI: 10.7270/Q2WW7K1S |
More data for this
Ligand-Target Pair | |
Ephrin type-B receptor 4
(Homo sapiens (Human)) | BDBM50428743

(CHEMBL2333603)Show SMILES CCCCn1c(cn2c1nc1n(C)c(=O)[nH]c(=O)c21)-c1ccccc1C Show InChI InChI=1S/C19H21N5O2/c1-4-5-10-23-14(13-9-7-6-8-12(13)2)11-24-15-16(20-18(23)24)22(3)19(26)21-17(15)25/h6-9,11H,4-5,10H2,1-3H3,(H,21,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 82 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Zurich
Curated by ChEMBL
| Assay Description
Inhibition of EphB4 (unknown origin) transfected in CHO cells using [gamma-33P]ATP by radiometric assay |
J Med Chem 56: 84-96 (2013)
Article DOI: 10.1021/jm301187e
BindingDB Entry DOI: 10.7270/Q2WW7K1S |
More data for this
Ligand-Target Pair | |
Ephrin type-B receptor 4
(Homo sapiens (Human)) | BDBM50100317

(CHEMBL3321822)Show SMILES NC(=O)c1c(N)n(-c2ccc(NC(=O)Nc3cccc(c3)C(F)(F)F)cc2)c2nc3ccccc3nc12 Show InChI InChI=1S/C25H18F3N7O2/c26-25(27,28)13-4-3-5-15(12-13)32-24(37)31-14-8-10-16(11-9-14)35-21(29)19(22(30)36)20-23(35)34-18-7-2-1-6-17(18)33-20/h1-12H,29H2,(H2,30,36)(H2,31,32,37) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 89 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Z£rich
Curated by ChEMBL
| Assay Description
Inhibition of phosphorylation of full-length myc-tagged human EphB4 overexpressed in mouse embryonic fibroblasts after 90 mins by sandwich ELISA |
J Med Chem 57: 6834-44 (2014)
Article DOI: 10.1021/jm5009242
BindingDB Entry DOI: 10.7270/Q2TQ638D |
More data for this
Ligand-Target Pair | |
Ephrin type-B receptor 4
(Homo sapiens (Human)) | BDBM50428742

(CHEMBL2333595)Show SMILES CCCCn1c(cn2c1nc1n(C)c(=O)[nH]c(=O)c21)-c1ccc(F)cc1C Show InChI InChI=1S/C19H20FN5O2/c1-4-5-8-24-14(13-7-6-12(20)9-11(13)2)10-25-15-16(21-18(24)25)23(3)19(27)22-17(15)26/h6-7,9-10H,4-5,8H2,1-3H3,(H,22,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 91 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Zurich
Curated by ChEMBL
| Assay Description
Inhibition of EphB4 (unknown origin) transfected in CHO cells using Z'-LYTE TYR-1 peptide as substrate after 2 hrs by FRET assay |
J Med Chem 56: 84-96 (2013)
Article DOI: 10.1021/jm301187e
BindingDB Entry DOI: 10.7270/Q2WW7K1S |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM50428742

(CHEMBL2333595)Show SMILES CCCCn1c(cn2c1nc1n(C)c(=O)[nH]c(=O)c21)-c1ccc(F)cc1C Show InChI InChI=1S/C19H20FN5O2/c1-4-5-8-24-14(13-7-6-12(20)9-11(13)2)10-25-15-16(21-18(24)25)23(3)19(27)22-17(15)26/h6-7,9-10H,4-5,8H2,1-3H3,(H,22,26,27) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Zurich
Curated by ChEMBL
| Assay Description
Inhibition of Src (unknown origin) using [gamma-33P]ATP after 30 mins by radiometric assay |
J Med Chem 56: 84-96 (2013)
Article DOI: 10.1021/jm301187e
BindingDB Entry DOI: 10.7270/Q2WW7K1S |
More data for this
Ligand-Target Pair | |
Ephrin type-B receptor 4
(Homo sapiens (Human)) | BDBM50299218

(8-(2-Methoxyphenyl)-1-methyl-7-(2'-methyl-5'-hydro...)Show SMILES COc1ccccc1-n1c(cn2c1nc1n(C)c(=O)[nH]c(=O)c21)-c1cc(O)ccc1C |(16.89,-42.99,;16.89,-41.45,;18.23,-40.68,;19.55,-41.45,;20.88,-40.68,;20.88,-39.14,;19.55,-38.38,;18.23,-39.15,;16.9,-38.38,;16.91,-36.84,;15.44,-36.35,;14.53,-37.6,;15.43,-38.85,;14.53,-40.09,;13.06,-39.61,;11.74,-40.37,;11.73,-41.91,;10.41,-39.61,;9.07,-40.38,;10.41,-38.07,;11.74,-37.29,;11.74,-35.75,;13.06,-38.07,;18.24,-36.06,;19.58,-36.83,;20.9,-36.05,;22.24,-36.81,;20.9,-34.51,;19.55,-33.75,;18.23,-34.53,;16.89,-33.77,)| Show InChI InChI=1S/C22H19N5O4/c1-12-8-9-13(28)10-14(12)16-11-26-18-19(25(2)22(30)24-20(18)29)23-21(26)27(16)15-6-4-5-7-17(15)31-3/h4-11,28H,1-3H3,(H,24,29,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Zurich
Curated by ChEMBL
| Assay Description
Inhibition of human myc-tagged full length EphB4 expressed in MEF cells assessed as inhibition of ephrinB2-Fc-induced autophosphorylation incubated f... |
J Med Chem 56: 84-96 (2013)
Article DOI: 10.1021/jm301187e
BindingDB Entry DOI: 10.7270/Q2WW7K1S |
More data for this
Ligand-Target Pair | |
Ephrin type-B receptor 4
(Homo sapiens (Human)) | BDBM50428741

(CHEMBL2333602)Show SMILES CCCCn1c(cn2c1nc1n(C)c(=O)[nH]c(=O)c21)-c1cc(ccc1C)-c1cccc2[nH]ncc12 Show InChI InChI=1S/C26H25N7O2/c1-4-5-11-32-21(14-33-22-23(28-25(32)33)31(3)26(35)29-24(22)34)18-12-16(10-9-15(18)2)17-7-6-8-20-19(17)13-27-30-20/h6-10,12-14H,4-5,11H2,1-3H3,(H,27,30)(H,29,34,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 133 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Zurich
Curated by ChEMBL
| Assay Description
Inhibition of EphB4 (unknown origin) transfected in CHO cells using [gamma-33P]ATP by radiometric assay |
J Med Chem 56: 84-96 (2013)
Article DOI: 10.1021/jm301187e
BindingDB Entry DOI: 10.7270/Q2WW7K1S |
More data for this
Ligand-Target Pair | |
Ephrin type-B receptor 4
(Homo sapiens (Human)) | BDBM50428742

(CHEMBL2333595)Show SMILES CCCCn1c(cn2c1nc1n(C)c(=O)[nH]c(=O)c21)-c1ccc(F)cc1C Show InChI InChI=1S/C19H20FN5O2/c1-4-5-8-24-14(13-7-6-12(20)9-11(13)2)10-25-15-16(21-18(24)25)23(3)19(27)22-17(15)26/h6-7,9-10H,4-5,8H2,1-3H3,(H,22,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 139 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Zurich
Curated by ChEMBL
| Assay Description
Inhibition of EphB4 (unknown origin) transfected in CHO cells using [gamma-33P]ATP by radiometric assay |
J Med Chem 56: 84-96 (2013)
Article DOI: 10.1021/jm301187e
BindingDB Entry DOI: 10.7270/Q2WW7K1S |
More data for this
Ligand-Target Pair | |
Ephrin type-B receptor 4
(Homo sapiens (Human)) | BDBM50428741

(CHEMBL2333602)Show SMILES CCCCn1c(cn2c1nc1n(C)c(=O)[nH]c(=O)c21)-c1cc(ccc1C)-c1cccc2[nH]ncc12 Show InChI InChI=1S/C26H25N7O2/c1-4-5-11-32-21(14-33-22-23(28-25(32)33)31(3)26(35)29-24(22)34)18-12-16(10-9-15(18)2)17-7-6-8-20-19(17)13-27-30-20/h6-10,12-14H,4-5,11H2,1-3H3,(H,27,30)(H,29,34,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Zurich
Curated by ChEMBL
| Assay Description
Inhibition of human myc-tagged full length EphB4 expressed in MEF cells assessed as inhibition of ephrinB2-Fc-induced autophosphorylation incubated f... |
J Med Chem 56: 84-96 (2013)
Article DOI: 10.1021/jm301187e
BindingDB Entry DOI: 10.7270/Q2WW7K1S |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Yes
(Homo sapiens (Human)) | BDBM50428741

(CHEMBL2333602)Show SMILES CCCCn1c(cn2c1nc1n(C)c(=O)[nH]c(=O)c21)-c1cc(ccc1C)-c1cccc2[nH]ncc12 Show InChI InChI=1S/C26H25N7O2/c1-4-5-11-32-21(14-33-22-23(28-25(32)33)31(3)26(35)29-24(22)34)18-12-16(10-9-15(18)2)17-7-6-8-20-19(17)13-27-30-20/h6-10,12-14H,4-5,11H2,1-3H3,(H,27,30)(H,29,34,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 160 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Zurich
Curated by ChEMBL
| Assay Description
Inhibition of Yes1 (unknown origin) using [gamma-33P]ATP after 30 mins by radiometric assay |
J Med Chem 56: 84-96 (2013)
Article DOI: 10.1021/jm301187e
BindingDB Entry DOI: 10.7270/Q2WW7K1S |
More data for this
Ligand-Target Pair | |
Ephrin type-B receptor 4
(Homo sapiens (Human)) | BDBM50100324
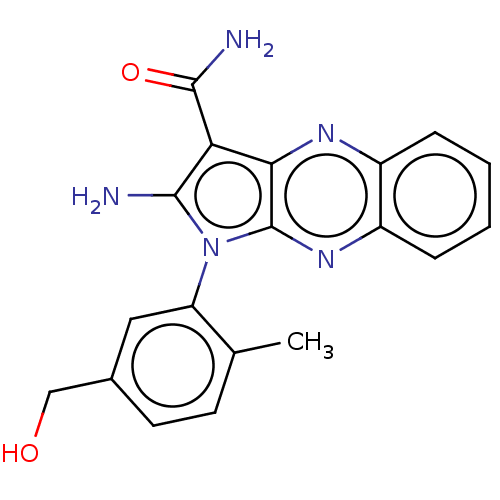
(CHEMBL3321804)Show SMILES Cc1ccc(CO)cc1-n1c(N)c(C(N)=O)c2nc3ccccc3nc12 |(17.1,-32.34,;18.6,-32.66,;19.08,-34.12,;20.58,-34.45,;21.62,-33.3,;23.13,-33.62,;23.61,-35.08,;21.14,-31.84,;19.63,-31.52,;19.16,-30.05,;20.06,-28.81,;21.6,-28.81,;19.16,-27.56,;19.63,-26.09,;21.12,-25.7,;18.6,-24.95,;17.69,-28.04,;16.36,-27.27,;15.02,-28.04,;13.69,-27.27,;12.36,-28.04,;12.36,-29.58,;13.69,-30.35,;15.02,-29.58,;16.36,-30.35,;17.69,-29.58,)| Show InChI InChI=1S/C19H17N5O2/c1-10-6-7-11(9-25)8-14(10)24-17(20)15(18(21)26)16-19(24)23-13-5-3-2-4-12(13)22-16/h2-8,25H,9,20H2,1H3,(H2,21,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 160 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Z£rich
Curated by ChEMBL
| Assay Description
Inhibition of phosphorylation of full-length myc-tagged human EphB4 overexpressed in mouse embryonic fibroblasts after 90 mins by sandwich ELISA |
J Med Chem 57: 6834-44 (2014)
Article DOI: 10.1021/jm5009242
BindingDB Entry DOI: 10.7270/Q2TQ638D |
More data for this
Ligand-Target Pair | |
Ephrin type-B receptor 4
(Homo sapiens (Human)) | BDBM50393034

(CHEMBL2152704)Show SMILES Cc1ccc(O)cc1-n1c2ncnc(N)c2c2CCCCc2c1=O |(21.85,-7.48,;23.19,-8.25,;24.52,-7.48,;25.87,-8.25,;25.86,-9.8,;27.2,-10.57,;24.52,-10.56,;23.19,-9.78,;21.86,-10.55,;20.52,-9.78,;20.52,-8.25,;19.2,-7.49,;17.87,-8.26,;17.88,-9.78,;16.54,-10.55,;19.19,-10.54,;19.18,-12.07,;17.85,-12.85,;17.85,-14.39,;19.18,-15.15,;20.52,-14.39,;20.51,-12.85,;21.85,-12.09,;23.18,-12.87,)| Show InChI InChI=1S/C18H18N4O2/c1-10-6-7-11(23)8-14(10)22-17-15(16(19)20-9-21-17)12-4-2-3-5-13(12)18(22)24/h6-9,23H,2-5H2,1H3,(H2,19,20,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 160 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of EphB4 in presence of 1 uM radiolabeled ATP |
ACS Med Chem Lett 3: 834-838 (2012)
Article DOI: 10.1021/ml3001984
BindingDB Entry DOI: 10.7270/Q28S4R0N |
More data for this
Ligand-Target Pair | |
Ephrin type-B receptor 4
(Homo sapiens (Human)) | BDBM50100328
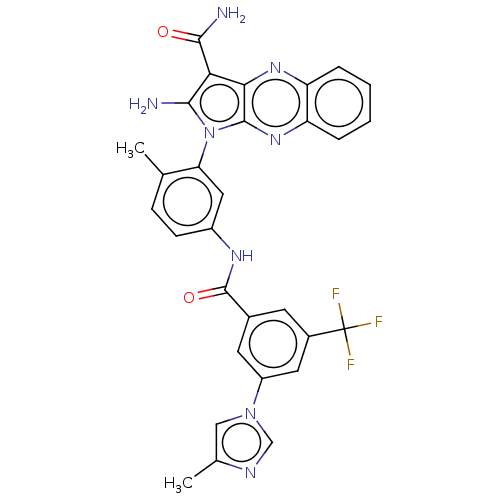
(CHEMBL3321814)Show SMILES Cc1cn(cn1)-c1cc(cc(c1)C(F)(F)F)C(=O)Nc1ccc(C)c(c1)-n1c(N)c(C(N)=O)c2nc3ccccc3nc12 |(12.67,-33.82,;12.52,-35.36,;11.19,-36.13,;11.51,-37.64,;13.04,-37.79,;13.66,-36.38,;10.49,-38.79,;8.99,-38.47,;7.97,-39.61,;8.44,-41.08,;9.94,-41.4,;10.97,-40.26,;10.42,-42.87,;9.38,-44.01,;11.92,-43.19,;11.18,-44.19,;6.46,-39.29,;5.43,-40.44,;5.98,-37.83,;4.48,-37.51,;3.45,-38.65,;1.94,-38.33,;1.46,-36.87,;-.05,-36.55,;2.49,-35.72,;4,-36.04,;2.02,-34.26,;2.92,-33.01,;4.46,-33.01,;2.02,-31.77,;2.49,-30.3,;3.98,-29.91,;1.46,-29.16,;.55,-32.24,;-.79,-31.47,;-2.12,-32.24,;-3.46,-31.47,;-4.79,-32.24,;-4.79,-33.78,;-3.46,-34.55,;-2.12,-33.78,;-.79,-34.55,;.55,-33.78,)| Show InChI InChI=1S/C30H23F3N8O2/c1-15-7-8-19(37-29(43)17-9-18(30(31,32)33)11-20(10-17)40-13-16(2)36-14-40)12-23(15)41-26(34)24(27(35)42)25-28(41)39-22-6-4-3-5-21(22)38-25/h3-14H,34H2,1-2H3,(H2,35,42)(H,37,43) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 170 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Z£rich
Curated by ChEMBL
| Assay Description
Inhibition of phosphorylation of full-length myc-tagged human EphB4 overexpressed in mouse embryonic fibroblasts after 90 mins by sandwich ELISA |
J Med Chem 57: 6834-44 (2014)
Article DOI: 10.1021/jm5009242
BindingDB Entry DOI: 10.7270/Q2TQ638D |
More data for this
Ligand-Target Pair | |
Ephrin type-B receptor 4
(Homo sapiens (Human)) | BDBM50428744
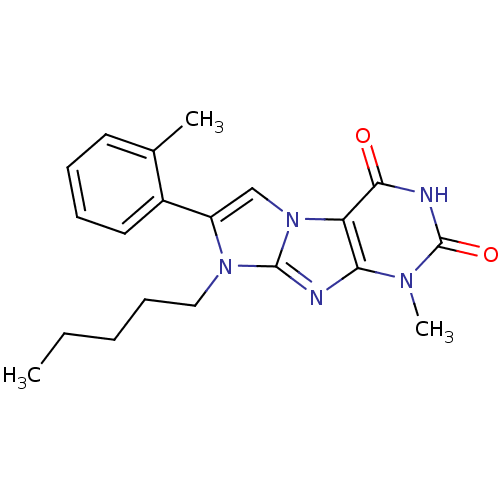
(CHEMBL2333593)Show SMILES CCCCCn1c(cn2c1nc1n(C)c(=O)[nH]c(=O)c21)-c1ccccc1C Show InChI InChI=1S/C20H23N5O2/c1-4-5-8-11-24-15(14-10-7-6-9-13(14)2)12-25-16-17(21-19(24)25)23(3)20(27)22-18(16)26/h6-7,9-10,12H,4-5,8,11H2,1-3H3,(H,22,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 177 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Zurich
Curated by ChEMBL
| Assay Description
Inhibition of EphB4 (unknown origin) transfected in CHO cells using Z'-LYTE TYR-1 peptide as substrate after 2 hrs by FRET assay |
J Med Chem 56: 84-96 (2013)
Article DOI: 10.1021/jm301187e
BindingDB Entry DOI: 10.7270/Q2WW7K1S |
More data for this
Ligand-Target Pair | |
Ephrin type-B receptor 4
(Homo sapiens (Human)) | BDBM50299219

(8-(2-Methoxyphenyl)-1-methyl-7-o-methylphenyl-1H-i...)Show SMILES COc1ccccc1-n1c(cn2c1nc1n(C)c(=O)[nH]c(=O)c21)-c1ccccc1C |(28.6,-45.77,;28.6,-44.23,;29.94,-43.46,;31.26,-44.23,;32.59,-43.46,;32.59,-41.92,;31.26,-41.16,;29.94,-41.93,;28.61,-41.16,;28.62,-39.62,;27.15,-39.13,;26.24,-40.38,;27.14,-41.63,;26.24,-42.87,;24.77,-42.4,;23.44,-43.16,;23.44,-44.7,;22.11,-42.39,;20.78,-43.17,;22.11,-40.85,;23.44,-40.08,;23.44,-38.54,;24.77,-40.85,;29.95,-38.84,;31.29,-39.61,;32.61,-38.83,;32.61,-37.29,;31.26,-36.53,;29.93,-37.31,;28.59,-36.55,)| Show InChI InChI=1S/C22H19N5O3/c1-13-8-4-5-9-14(13)16-12-26-18-19(25(2)22(29)24-20(18)28)23-21(26)27(16)15-10-6-7-11-17(15)30-3/h4-12H,1-3H3,(H,24,28,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 180 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Zurich
Curated by ChEMBL
| Assay Description
Inhibition of EphB4 assessed as blockade of synthetic substrate phosphorylation by FRET assay |
J Med Chem 52: 6433-46 (2009)
Article DOI: 10.1021/jm9009444
BindingDB Entry DOI: 10.7270/Q2BZ663G |
More data for this
Ligand-Target Pair | |
Ephrin type-B receptor 4
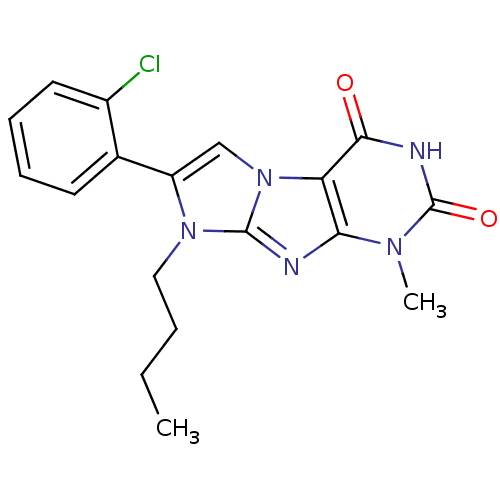
(Homo sapiens (Human)) | BDBM50428746
(CHEMBL2333597)Show SMILES CCCCn1c(cn2c1nc1n(C)c(=O)[nH]c(=O)c21)-c1ccccc1Cl Show InChI InChI=1S/C18H18ClN5O2/c1-3-4-9-23-13(11-7-5-6-8-12(11)19)10-24-14-15(20-17(23)24)22(2)18(26)21-16(14)25/h5-8,10H,3-4,9H2,1-2H3,(H,21,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 182 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Zurich
Curated by ChEMBL
| Assay Description
Inhibition of EphB4 (unknown origin) transfected in CHO cells using Z'-LYTE TYR-1 peptide as substrate after 2 hrs by FRET assay |
J Med Chem 56: 84-96 (2013)
Article DOI: 10.1021/jm301187e
BindingDB Entry DOI: 10.7270/Q2WW7K1S |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ABL1
(Homo sapiens (Human)) | BDBM50428741

(CHEMBL2333602)Show SMILES CCCCn1c(cn2c1nc1n(C)c(=O)[nH]c(=O)c21)-c1cc(ccc1C)-c1cccc2[nH]ncc12 Show InChI InChI=1S/C26H25N7O2/c1-4-5-11-32-21(14-33-22-23(28-25(32)33)31(3)26(35)29-24(22)34)18-12-16(10-9-15(18)2)17-7-6-8-20-19(17)13-27-30-20/h6-10,12-14H,4-5,11H2,1-3H3,(H,27,30)(H,29,34,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 203 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Zurich
Curated by ChEMBL
| Assay Description
Inhibition of Abl (unknown origin) using [gamma-33P]ATP after 30 mins by radiometric assay |
J Med Chem 56: 84-96 (2013)
Article DOI: 10.1021/jm301187e
BindingDB Entry DOI: 10.7270/Q2WW7K1S |
More data for this
Ligand-Target Pair | |
Ephrin type-B receptor 4
(Homo sapiens (Human)) | BDBM50299220
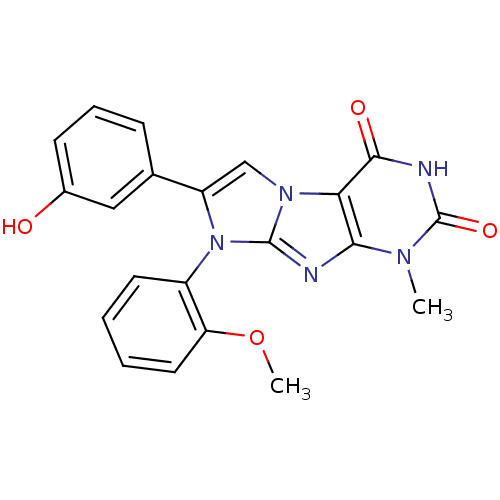
(8-(2-Methoxyphenyl)-1-methyl-7-m-hydroxyphenyl-1H-...)Show SMILES COc1ccccc1-n1c(cn2c1nc1n(C)c(=O)[nH]c(=O)c21)-c1cccc(O)c1 |(15.4,-17.54,;15.41,-16,;16.74,-15.23,;18.07,-16,;19.4,-15.23,;19.39,-13.69,;18.06,-12.93,;16.75,-13.7,;15.42,-12.93,;15.42,-11.39,;13.96,-10.9,;13.05,-12.15,;13.95,-13.4,;13.05,-14.64,;11.58,-14.17,;10.25,-14.93,;10.25,-16.47,;8.92,-14.16,;7.59,-14.94,;8.92,-12.62,;10.25,-11.85,;10.25,-10.31,;11.58,-12.62,;16.75,-10.61,;16.74,-9.08,;18.06,-8.3,;19.41,-9.06,;19.42,-10.6,;20.76,-11.36,;18.09,-11.38,)| Show InChI InChI=1S/C21H17N5O4/c1-24-18-17(19(28)23-21(24)29)25-11-15(12-6-5-7-13(27)10-12)26(20(25)22-18)14-8-3-4-9-16(14)30-2/h3-11,27H,1-2H3,(H,23,28,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 213 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Zurich
Curated by ChEMBL
| Assay Description
Inhibition of EphB4 by [gamma33-P]ATP based assay |
J Med Chem 52: 6433-46 (2009)
Article DOI: 10.1021/jm9009444
BindingDB Entry DOI: 10.7270/Q2BZ663G |
More data for this
Ligand-Target Pair | |
Ephrin type-B receptor 4
(Homo sapiens (Human)) | BDBM50428744

(CHEMBL2333593)Show SMILES CCCCCn1c(cn2c1nc1n(C)c(=O)[nH]c(=O)c21)-c1ccccc1C Show InChI InChI=1S/C20H23N5O2/c1-4-5-8-11-24-15(14-10-7-6-9-13(14)2)12-25-16-17(21-19(24)25)23(3)20(27)22-18(16)26/h6-7,9-10,12H,4-5,8,11H2,1-3H3,(H,22,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 230 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Zurich
Curated by ChEMBL
| Assay Description
Inhibition of human myc-tagged full length EphB4 expressed in MEF cells assessed as inhibition of ephrinB2-Fc-induced autophosphorylation incubated f... |
J Med Chem 56: 84-96 (2013)
Article DOI: 10.1021/jm301187e
BindingDB Entry DOI: 10.7270/Q2WW7K1S |
More data for this
Ligand-Target Pair | |
Ephrin type-B receptor 4
(Homo sapiens (Human)) | BDBM50100319
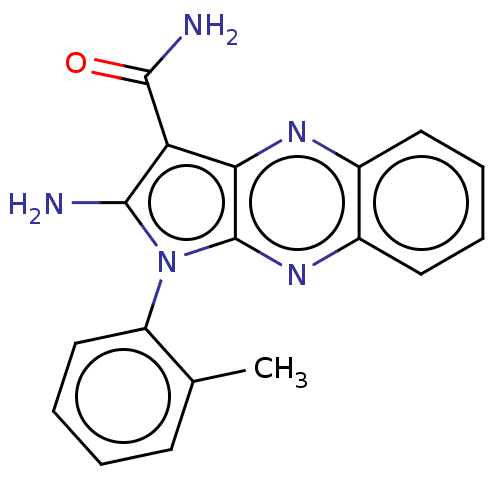
(CHEMBL3321800)Show SMILES Cc1ccccc1-n1c(N)c(C(N)=O)c2nc3ccccc3nc12 |(9.69,-38.66,;11.2,-38.98,;11.68,-40.44,;13.18,-40.76,;14.22,-39.62,;13.73,-38.16,;12.23,-37.83,;11.75,-36.37,;12.66,-35.13,;14.2,-35.13,;11.75,-33.88,;12.23,-32.41,;11.2,-31.27,;13.72,-32.02,;10.29,-34.35,;8.95,-33.59,;7.62,-34.35,;6.29,-33.59,;4.96,-34.35,;4.96,-35.89,;6.29,-36.67,;7.62,-35.89,;8.95,-36.67,;10.29,-35.89,)| Show InChI InChI=1S/C18H15N5O/c1-10-6-2-5-9-13(10)23-16(19)14(17(20)24)15-18(23)22-12-8-4-3-7-11(12)21-15/h2-9H,19H2,1H3,(H2,20,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 230 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Z£rich
Curated by ChEMBL
| Assay Description
Inhibition of phosphorylation of full-length myc-tagged human EphB4 overexpressed in mouse embryonic fibroblasts after 90 mins by sandwich ELISA |
J Med Chem 57: 6834-44 (2014)
Article DOI: 10.1021/jm5009242
BindingDB Entry DOI: 10.7270/Q2TQ638D |
More data for this
Ligand-Target Pair | |
Ephrin type-B receptor 4
(Homo sapiens (Human)) | BDBM50299221

(8-(2-Methoxyphenyl)-1-methyl-7-(2'-methyl-4'-hydro...)Show SMILES COc1ccccc1-n1c(cn2c1nc1n(C)c(=O)[nH]c(=O)c21)-c1ccc(O)cc1C |(-1.58,-43.46,;-1.58,-41.92,;-.25,-41.15,;1.08,-41.91,;2.41,-41.15,;2.41,-39.61,;1.08,-38.85,;-.24,-39.61,;-1.57,-38.85,;-1.56,-37.3,;-3.03,-36.82,;-3.94,-38.06,;-3.04,-39.32,;-3.94,-40.56,;-5.41,-40.08,;-6.74,-40.84,;-6.74,-42.38,;-8.07,-40.08,;-9.4,-40.85,;-8.07,-38.54,;-6.74,-37.76,;-6.74,-36.22,;-5.41,-38.54,;-.23,-36.52,;1.1,-37.29,;2.43,-36.52,;2.42,-34.97,;3.75,-34.19,;1.08,-34.21,;-.25,-34.99,;-1.59,-34.24,)| Show InChI InChI=1S/C22H19N5O4/c1-12-10-13(28)8-9-14(12)16-11-26-18-19(25(2)22(30)24-20(18)29)23-21(26)27(16)15-6-4-5-7-17(15)31-3/h4-11,28H,1-3H3,(H,24,29,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 236 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Zurich
Curated by ChEMBL
| Assay Description
Inhibition of EphB4 assessed as blockade of synthetic substrate phosphorylation by FRET assay |
J Med Chem 52: 6433-46 (2009)
Article DOI: 10.1021/jm9009444
BindingDB Entry DOI: 10.7270/Q2BZ663G |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data