 Found 63 hits with Last Name = 'lamarque' and Initial = 'l'
Found 63 hits with Last Name = 'lamarque' and Initial = 'l' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Vasopressin V1b receptor
(Homo sapiens (Human)) | BDBM35667

(AVP | CHEMBL373742 | US10131692, 44 (AVP) | [3H]Ar...)Show SMILES [#7]-[#6@H]-1-[#6]-[#16]-[#16]-[#6]-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#7])=O)-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6](-[#7])=O)-[#7]-[#6](=O)-[#6@H](-[#6]-c2ccccc2)-[#7]-[#6](=O)-[#6@H](-[#6]-c2ccc(-[#8])cc2)-[#7]-[#6]-1=O)-[#6](=O)-[#7]-1-[#6]-[#6]-[#6]-[#6@H]-1-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-[#6]-[#6](-[#7])=O Show InChI InChI=1S/C46H65N15O12S2/c47-27-22-74-75-23-33(45(73)61-17-5-9-34(61)44(72)56-28(8-4-16-53-46(51)52)39(67)54-21-37(50)65)60-43(71)32(20-36(49)64)59-40(68)29(14-15-35(48)63)55-41(69)31(18-24-6-2-1-3-7-24)58-42(70)30(57-38(27)66)19-25-10-12-26(62)13-11-25/h1-3,6-7,10-13,27-34,62H,4-5,8-9,14-23,47H2,(H2,48,63)(H2,49,64)(H2,50,65)(H,54,67)(H,55,69)(H,56,72)(H,57,66)(H,58,70)(H,59,68)(H,60,71)(H4,51,52,53)/t27-,28-,29-,30-,31-,32-,33-,34-/m0/s1 | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.490 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UMR7200 CNRS/Universit£ de Strasbourg
Curated by ChEMBL
| Assay Description
Displacement of [3H]-AVP from human vasopressin V1b receptor expressed in CHO cells after 30 mins |
J Med Chem 55: 8588-602 (2012)
Article DOI: 10.1021/jm3006146
BindingDB Entry DOI: 10.7270/Q28G8MT5 |
More data for this
Ligand-Target Pair | |
Vasopressin V2 receptor
(Homo sapiens (Human)) | BDBM50307117

(CHEMBL603708 | N-(4-(2,3,4,5-tetrahydro-1H-benzo[b...)Show SMILES O=C(Nc1ccc(cc1)C(=O)N1CCCCc2ccccc12)c1ccccc1-c1ccccc1 Show InChI InChI=1S/C30H26N2O2/c33-29(27-15-6-5-14-26(27)22-10-2-1-3-11-22)31-25-19-17-24(18-20-25)30(34)32-21-9-8-13-23-12-4-7-16-28(23)32/h1-7,10-12,14-20H,8-9,13,21H2,(H,31,33) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.570 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UMR7200 CNRS/Universit£ de Strasbourg
Curated by ChEMBL
| Assay Description
Displacement of [3H]-AVP from human vasopressin V2 receptor expressed in CHO cells after 30 mins |
J Med Chem 55: 8588-602 (2012)
Article DOI: 10.1021/jm3006146
BindingDB Entry DOI: 10.7270/Q28G8MT5 |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(Homo sapiens (Human)) | BDBM35667

(AVP | CHEMBL373742 | US10131692, 44 (AVP) | [3H]Ar...)Show SMILES [#7]-[#6@H]-1-[#6]-[#16]-[#16]-[#6]-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#7])=O)-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6](-[#7])=O)-[#7]-[#6](=O)-[#6@H](-[#6]-c2ccccc2)-[#7]-[#6](=O)-[#6@H](-[#6]-c2ccc(-[#8])cc2)-[#7]-[#6]-1=O)-[#6](=O)-[#7]-1-[#6]-[#6]-[#6]-[#6@H]-1-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-[#6]-[#6](-[#7])=O Show InChI InChI=1S/C46H65N15O12S2/c47-27-22-74-75-23-33(45(73)61-17-5-9-34(61)44(72)56-28(8-4-16-53-46(51)52)39(67)54-21-37(50)65)60-43(71)32(20-36(49)64)59-40(68)29(14-15-35(48)63)55-41(69)31(18-24-6-2-1-3-7-24)58-42(70)30(57-38(27)66)19-25-10-12-26(62)13-11-25/h1-3,6-7,10-13,27-34,62H,4-5,8-9,14-23,47H2,(H2,48,63)(H2,49,64)(H2,50,65)(H,54,67)(H,55,69)(H,56,72)(H,57,66)(H,58,70)(H,59,68)(H,60,71)(H4,51,52,53)/t27-,28-,29-,30-,31-,32-,33-,34-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UMR7200 CNRS/Universit£ de Strasbourg
Curated by ChEMBL
| Assay Description
Displacement of [3H]-AVP from human vasopressin V1a receptor expressed in CHO cells after 30 mins |
J Med Chem 55: 8588-602 (2012)
Article DOI: 10.1021/jm3006146
BindingDB Entry DOI: 10.7270/Q28G8MT5 |
More data for this
Ligand-Target Pair | |
Vasopressin V2 receptor
(Homo sapiens (Human)) | BDBM50397209

(CHEMBL2172291)Show SMILES CCN1\C(=C\C=C\C=C\C2=[N+](CC)c3ccc(cc3C2(C)C)S([O-])(=O)=O)C(C)(CCCC(=O)NCCOCCOCCOCCOCCOCCOCCn2cc(CNC(=O)CCC(=O)NC3CCCN(C(=O)c4ccc(NC(=O)c5ccccc5-c5ccccc5)cc4)c4ccccc34)nn2)c2cc(ccc12)S(O)(=O)=O |c:9| Show InChI InChI=1S/C83H100N10O17S2/c1-6-91-73-36-34-64(111(99,100)101)56-69(73)82(3,4)75(91)27-12-9-13-28-76-83(5,70-57-65(112(102,103)104)35-37-74(70)92(76)7-2)40-18-29-77(94)84-41-44-105-46-48-107-50-52-109-54-55-110-53-51-108-49-47-106-45-43-90-59-63(88-89-90)58-85-78(95)38-39-79(96)87-71-25-19-42-93(72-26-17-16-24-68(71)72)81(98)61-30-32-62(33-31-61)86-80(97)67-23-15-14-22-66(67)60-20-10-8-11-21-60/h8-17,20-24,26-28,30-37,56-57,59,71H,6-7,18-19,25,29,38-55,58H2,1-5H3,(H5-,84,85,86,87,94,95,96,97,98,99,100,101,102,103,104) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.830 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UMR7200 CNRS/Universit£ de Strasbourg
Curated by ChEMBL
| Assay Description
Displacement of [3H]-AVP from SNAP-tagged vasopressin V2 receptor expressed in HEK293 cells by FRET assay |
J Med Chem 55: 8588-602 (2012)
Article DOI: 10.1021/jm3006146
BindingDB Entry DOI: 10.7270/Q28G8MT5 |
More data for this
Ligand-Target Pair | |
Vasopressin V2 receptor
(Homo sapiens (Human)) | BDBM35667

(AVP | CHEMBL373742 | US10131692, 44 (AVP) | [3H]Ar...)Show SMILES [#7]-[#6@H]-1-[#6]-[#16]-[#16]-[#6]-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#7])=O)-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6](-[#7])=O)-[#7]-[#6](=O)-[#6@H](-[#6]-c2ccccc2)-[#7]-[#6](=O)-[#6@H](-[#6]-c2ccc(-[#8])cc2)-[#7]-[#6]-1=O)-[#6](=O)-[#7]-1-[#6]-[#6]-[#6]-[#6@H]-1-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-[#6]-[#6](-[#7])=O Show InChI InChI=1S/C46H65N15O12S2/c47-27-22-74-75-23-33(45(73)61-17-5-9-34(61)44(72)56-28(8-4-16-53-46(51)52)39(67)54-21-37(50)65)60-43(71)32(20-36(49)64)59-40(68)29(14-15-35(48)63)55-41(69)31(18-24-6-2-1-3-7-24)58-42(70)30(57-38(27)66)19-25-10-12-26(62)13-11-25/h1-3,6-7,10-13,27-34,62H,4-5,8-9,14-23,47H2,(H2,48,63)(H2,49,64)(H2,50,65)(H,54,67)(H,55,69)(H,56,72)(H,57,66)(H,58,70)(H,59,68)(H,60,71)(H4,51,52,53)/t27-,28-,29-,30-,31-,32-,33-,34-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.36 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UMR7200 CNRS/Universit£ de Strasbourg
Curated by ChEMBL
| Assay Description
Displacement of [3H]-AVP from human vasopressin V2 receptor expressed in CHO cells after 30 mins by saturation binding assay |
J Med Chem 55: 8588-602 (2012)
Article DOI: 10.1021/jm3006146
BindingDB Entry DOI: 10.7270/Q28G8MT5 |
More data for this
Ligand-Target Pair | |
Vasopressin V2 receptor
(Homo sapiens (Human)) | BDBM35667

(AVP | CHEMBL373742 | US10131692, 44 (AVP) | [3H]Ar...)Show SMILES [#7]-[#6@H]-1-[#6]-[#16]-[#16]-[#6]-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#7])=O)-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6](-[#7])=O)-[#7]-[#6](=O)-[#6@H](-[#6]-c2ccccc2)-[#7]-[#6](=O)-[#6@H](-[#6]-c2ccc(-[#8])cc2)-[#7]-[#6]-1=O)-[#6](=O)-[#7]-1-[#6]-[#6]-[#6]-[#6@H]-1-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-[#6]-[#6](-[#7])=O Show InChI InChI=1S/C46H65N15O12S2/c47-27-22-74-75-23-33(45(73)61-17-5-9-34(61)44(72)56-28(8-4-16-53-46(51)52)39(67)54-21-37(50)65)60-43(71)32(20-36(49)64)59-40(68)29(14-15-35(48)63)55-41(69)31(18-24-6-2-1-3-7-24)58-42(70)30(57-38(27)66)19-25-10-12-26(62)13-11-25/h1-3,6-7,10-13,27-34,62H,4-5,8-9,14-23,47H2,(H2,48,63)(H2,49,64)(H2,50,65)(H,54,67)(H,55,69)(H,56,72)(H,57,66)(H,58,70)(H,59,68)(H,60,71)(H4,51,52,53)/t27-,28-,29-,30-,31-,32-,33-,34-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.48 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UMR7200 CNRS/Universit£ de Strasbourg
Curated by ChEMBL
| Assay Description
Displacement of [3H]-AVP from human vasopressin V2 receptor expressed in CHO cells after 30 mins |
J Med Chem 55: 8588-602 (2012)
Article DOI: 10.1021/jm3006146
BindingDB Entry DOI: 10.7270/Q28G8MT5 |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM35667

(AVP | CHEMBL373742 | US10131692, 44 (AVP) | [3H]Ar...)Show SMILES [#7]-[#6@H]-1-[#6]-[#16]-[#16]-[#6]-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#7])=O)-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6](-[#7])=O)-[#7]-[#6](=O)-[#6@H](-[#6]-c2ccccc2)-[#7]-[#6](=O)-[#6@H](-[#6]-c2ccc(-[#8])cc2)-[#7]-[#6]-1=O)-[#6](=O)-[#7]-1-[#6]-[#6]-[#6]-[#6@H]-1-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-[#6]-[#6](-[#7])=O Show InChI InChI=1S/C46H65N15O12S2/c47-27-22-74-75-23-33(45(73)61-17-5-9-34(61)44(72)56-28(8-4-16-53-46(51)52)39(67)54-21-37(50)65)60-43(71)32(20-36(49)64)59-40(68)29(14-15-35(48)63)55-41(69)31(18-24-6-2-1-3-7-24)58-42(70)30(57-38(27)66)19-25-10-12-26(62)13-11-25/h1-3,6-7,10-13,27-34,62H,4-5,8-9,14-23,47H2,(H2,48,63)(H2,49,64)(H2,50,65)(H,54,67)(H,55,69)(H,56,72)(H,57,66)(H,58,70)(H,59,68)(H,60,71)(H4,51,52,53)/t27-,28-,29-,30-,31-,32-,33-,34-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.65 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UMR7200 CNRS/Universit£ de Strasbourg
Curated by ChEMBL
| Assay Description
Displacement of [3H]-AVP from human oxytocin receptor expressed in CHO cells after 30 mins |
J Med Chem 55: 8588-602 (2012)
Article DOI: 10.1021/jm3006146
BindingDB Entry DOI: 10.7270/Q28G8MT5 |
More data for this
Ligand-Target Pair | |
Vasopressin V2 receptor
(Homo sapiens (Human)) | BDBM50397210
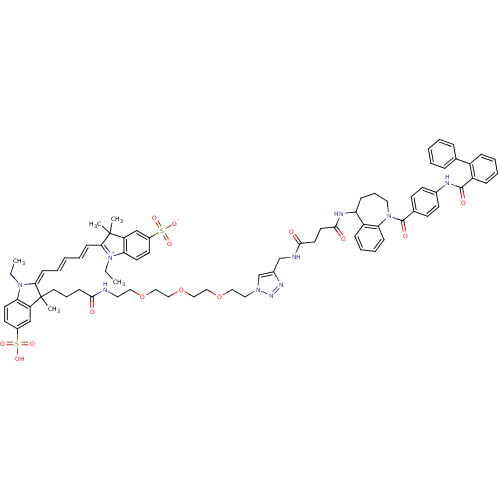
(CHEMBL2172290)Show SMILES CCN1\C(=C\C=C\C=C\C2=[N+](CC)c3ccc(cc3C2(C)C)S([O-])(=O)=O)C(C)(CCCC(=O)NCCOCCOCCOCCn2cc(CNC(=O)CCC(=O)NC3CCCN(C(=O)c4ccc(NC(=O)c5ccccc5-c5ccccc5)cc4)c4ccccc34)nn2)c2cc(ccc12)S(O)(=O)=O |c:9| Show InChI InChI=1S/C77H88N10O14S2/c1-6-85-67-36-34-58(102(93,94)95)50-63(67)76(3,4)69(85)27-12-9-13-28-70-77(5,64-51-59(103(96,97)98)35-37-68(64)86(70)7-2)40-18-29-71(88)78-41-44-99-46-48-101-49-47-100-45-43-84-53-57(82-83-84)52-79-72(89)38-39-73(90)81-65-25-19-42-87(66-26-17-16-24-62(65)66)75(92)55-30-32-56(33-31-55)80-74(91)61-23-15-14-22-60(61)54-20-10-8-11-21-54/h8-17,20-24,26-28,30-37,50-51,53,65H,6-7,18-19,25,29,38-49,52H2,1-5H3,(H5-,78,79,80,81,88,89,90,91,92,93,94,95,96,97,98) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.93 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UMR7200 CNRS/Universit£ de Strasbourg
Curated by ChEMBL
| Assay Description
Displacement of [3H]-AVP from SNAP-tagged vasopressin V2 receptor expressed in HEK293 cells by FRET assay |
J Med Chem 55: 8588-602 (2012)
Article DOI: 10.1021/jm3006146
BindingDB Entry DOI: 10.7270/Q28G8MT5 |
More data for this
Ligand-Target Pair | |
Vasopressin V2 receptor
(Homo sapiens (Human)) | BDBM50052954
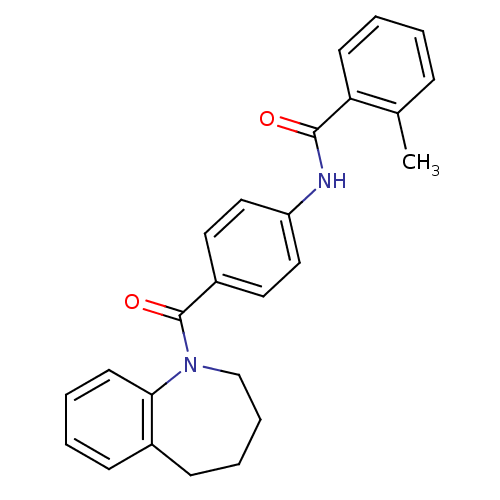
(2-Methyl-N-[4-(2,3,4,5-tetrahydro-benzo[b]azepine-...)Show SMILES Cc1ccccc1C(=O)Nc1ccc(cc1)C(=O)N1CCCCc2ccccc12 Show InChI InChI=1S/C25H24N2O2/c1-18-8-2-4-11-22(18)24(28)26-21-15-13-20(14-16-21)25(29)27-17-7-6-10-19-9-3-5-12-23(19)27/h2-5,8-9,11-16H,6-7,10,17H2,1H3,(H,26,28) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.68 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UMR7200 CNRS/Universit£ de Strasbourg
Curated by ChEMBL
| Assay Description
Displacement of [3H]-AVP from human vasopressin V2 receptor expressed in CHO cells after 30 mins |
J Med Chem 55: 8588-602 (2012)
Article DOI: 10.1021/jm3006146
BindingDB Entry DOI: 10.7270/Q28G8MT5 |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(Homo sapiens (Human)) | BDBM50307117

(CHEMBL603708 | N-(4-(2,3,4,5-tetrahydro-1H-benzo[b...)Show SMILES O=C(Nc1ccc(cc1)C(=O)N1CCCCc2ccccc12)c1ccccc1-c1ccccc1 Show InChI InChI=1S/C30H26N2O2/c33-29(27-15-6-5-14-26(27)22-10-2-1-3-11-22)31-25-19-17-24(18-20-25)30(34)32-21-9-8-13-23-12-4-7-16-28(23)32/h1-7,10-12,14-20H,8-9,13,21H2,(H,31,33) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.74 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UMR7200 CNRS/Universit£ de Strasbourg
Curated by ChEMBL
| Assay Description
Displacement of [3H]-AVP from human vasopressin V1a receptor expressed in CHO cells after 30 mins |
J Med Chem 55: 8588-602 (2012)
Article DOI: 10.1021/jm3006146
BindingDB Entry DOI: 10.7270/Q28G8MT5 |
More data for this
Ligand-Target Pair | |
Vasopressin V2 receptor
(Homo sapiens (Human)) | BDBM50397215

(CHEMBL2172295)Show SMILES CCN(CC)c1ccc2c(-c3ccc(cc3S([O-])(=O)=O)S(=O)(=O)NCCOCCOCCOCCn3cc(CNC(=O)CCC(=O)NC4CCCN(C(=O)c5ccc(NC(=O)c6ccccc6-c6ccccc6)cc5)c5ccccc45)nn3)c3ccc(cc3oc2c1)=[N+](CC)CC |(48.6,-20.67,;47.27,-21.45,;45.93,-20.68,;45.92,-19.14,;47.25,-18.37,;44.59,-21.45,;43.26,-20.69,;41.94,-21.46,;41.94,-22.99,;40.61,-23.75,;39.29,-22.97,;39.3,-21.42,;37.98,-20.64,;36.65,-21.39,;36.62,-22.93,;37.94,-23.72,;37.92,-25.26,;36.57,-26.02,;39.4,-24.87,;38.67,-26.59,;35.32,-20.6,;36.08,-19.26,;34.22,-19.5,;33.98,-21.36,;32.65,-20.58,;31.31,-21.33,;29.99,-20.54,;28.64,-21.29,;27.31,-20.5,;25.97,-21.25,;24.65,-20.47,;23.3,-21.22,;21.97,-20.44,;20.63,-21.19,;19.3,-20.4,;18,-21.14,;16.52,-20.66,;15.61,-21.92,;14.07,-21.9,;13.31,-20.56,;11.77,-20.54,;10.99,-21.87,;11.01,-19.19,;9.47,-19.18,;8.72,-17.84,;9.5,-16.52,;7.18,-17.83,;6.27,-19.07,;6.99,-20.43,;6.39,-21.83,;4.89,-22.24,;3.64,-21.35,;2.28,-22.08,;.97,-21.27,;2.24,-23.62,;.88,-24.35,;.83,-25.89,;2.16,-26.69,;2.11,-28.24,;3.43,-29.04,;4.78,-28.31,;3.38,-30.58,;2.01,-31.32,;1.97,-32.86,;3.29,-33.66,;4.65,-32.92,;4.69,-31.38,;6.04,-30.65,;7.35,-31.45,;8.7,-30.73,;8.75,-29.19,;7.42,-28.37,;6.07,-29.11,;3.51,-25.97,;3.55,-24.44,;3.58,-19.81,;2.12,-19.32,;1.81,-17.8,;2.98,-16.77,;4.45,-17.27,;4.75,-18.79,;16.53,-23.17,;18,-22.69,;40.62,-25.3,;39.29,-26.05,;39.27,-27.58,;40.61,-28.36,;41.93,-27.6,;41.94,-26.07,;43.27,-25.3,;43.27,-23.76,;44.6,-22.99,;40.61,-29.9,;41.94,-30.67,;41.94,-32.21,;39.26,-30.67,;39.26,-32.21,)| Show InChI InChI=1S/C72H80N10O13S2/c1-5-79(6-2)54-28-31-60-65(45-54)95-66-46-55(80(7-3)8-4)29-32-61(66)70(60)62-33-30-56(47-67(62)97(89,90)91)96(87,88)74-36-39-92-41-43-94-44-42-93-40-38-81-49-53(77-78-81)48-73-68(83)34-35-69(84)76-63-22-16-37-82(64-23-15-14-21-59(63)64)72(86)51-24-26-52(27-25-51)75-71(85)58-20-13-12-19-57(58)50-17-10-9-11-18-50/h9-15,17-21,23-33,45-47,49,63,74H,5-8,16,22,34-44,48H2,1-4H3,(H3-,73,75,76,83,84,85,86,89,90,91) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UMR7200 CNRS/Universit£ de Strasbourg
Curated by ChEMBL
| Assay Description
Displacement of [3H]-AVP from human vasopressin V2 receptor expressed in CHO cells after 30 mins |
J Med Chem 55: 8588-602 (2012)
Article DOI: 10.1021/jm3006146
BindingDB Entry DOI: 10.7270/Q28G8MT5 |
More data for this
Ligand-Target Pair | |
Vasopressin V2 receptor
(Homo sapiens (Human)) | BDBM50397206

(CHEMBL2172289)Show SMILES CCN1\C(=C\C=C\C=C\C2=[N+](CC)c3ccc(cc3C2(C)C)S([O-])(=O)=O)C(C)(CCCC(=O)NCCn2cc(CNC(=O)CCC(=O)NC3CCCN(C(=O)c4ccc(NC(=O)c5ccccc5-c5ccccc5)cc4)c4ccccc34)nn2)c2cc(ccc12)S(O)(=O)=O |c:9| Show InChI InChI=1S/C71H76N10O11S2/c1-6-79-61-36-34-52(93(87,88)89)44-57(61)70(3,4)63(79)27-12-9-13-28-64-71(5,58-45-53(94(90,91)92)35-37-62(58)80(64)7-2)40-18-29-65(82)72-41-43-78-47-51(76-77-78)46-73-66(83)38-39-67(84)75-59-25-19-42-81(60-26-17-16-24-56(59)60)69(86)49-30-32-50(33-31-49)74-68(85)55-23-15-14-22-54(55)48-20-10-8-11-21-48/h8-17,20-24,26-28,30-37,44-45,47,59H,6-7,18-19,25,29,38-43,46H2,1-5H3,(H5-,72,73,74,75,82,83,84,85,86,87,88,89,90,91,92) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.69 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UMR7200 CNRS/Universit£ de Strasbourg
Curated by ChEMBL
| Assay Description
Displacement of [3H]-AVP from human vasopressin V2 receptor expressed in CHO cells after 30 mins |
J Med Chem 55: 8588-602 (2012)
Article DOI: 10.1021/jm3006146
BindingDB Entry DOI: 10.7270/Q28G8MT5 |
More data for this
Ligand-Target Pair | |
Vasopressin V2 receptor
(Homo sapiens (Human)) | BDBM50052954

(2-Methyl-N-[4-(2,3,4,5-tetrahydro-benzo[b]azepine-...)Show SMILES Cc1ccccc1C(=O)Nc1ccc(cc1)C(=O)N1CCCCc2ccccc12 Show InChI InChI=1S/C25H24N2O2/c1-18-8-2-4-11-22(18)24(28)26-21-15-13-20(14-16-21)25(29)27-17-7-6-10-19-9-3-5-12-23(19)27/h2-5,8-9,11-16H,6-7,10,17H2,1H3,(H,26,28) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 7.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UMR7200 CNRS/Universit£ de Strasbourg
Curated by ChEMBL
| Assay Description
Binding affinity to vasopressin V2 receptor |
J Med Chem 55: 8588-602 (2012)
Article DOI: 10.1021/jm3006146
BindingDB Entry DOI: 10.7270/Q28G8MT5 |
More data for this
Ligand-Target Pair | |
Vasopressin V2 receptor
(Homo sapiens (Human)) | BDBM50307117

(CHEMBL603708 | N-(4-(2,3,4,5-tetrahydro-1H-benzo[b...)Show SMILES O=C(Nc1ccc(cc1)C(=O)N1CCCCc2ccccc12)c1ccccc1-c1ccccc1 Show InChI InChI=1S/C30H26N2O2/c33-29(27-15-6-5-14-26(27)22-10-2-1-3-11-22)31-25-19-17-24(18-20-25)30(34)32-21-9-8-13-23-12-4-7-16-28(23)32/h1-7,10-12,14-20H,8-9,13,21H2,(H,31,33) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UMR7200 CNRS/Universit£ de Strasbourg
Curated by ChEMBL
| Assay Description
Binding affinity to vasopressin V2 receptor |
J Med Chem 55: 8588-602 (2012)
Article DOI: 10.1021/jm3006146
BindingDB Entry DOI: 10.7270/Q28G8MT5 |
More data for this
Ligand-Target Pair | |
Vasopressin V2 receptor
(Homo sapiens (Human)) | BDBM50397213
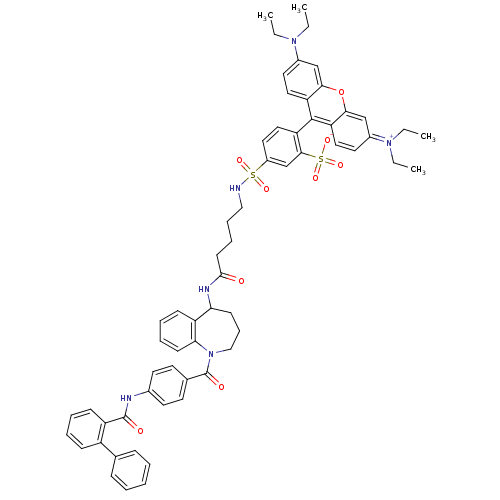
(CHEMBL2172391)Show SMILES CCN(CC)c1ccc2c(-c3ccc(cc3S([O-])(=O)=O)S(=O)(=O)NCCCCC(=O)NC3CCCN(C(=O)c4ccc(NC(=O)c5ccccc5-c5ccccc5)cc4)c4ccccc34)c3ccc(cc3oc2c1)=[N+](CC)CC |(39.65,-33.22,;38.86,-34.54,;39.62,-35.89,;41.16,-35.91,;41.94,-34.59,;38.82,-37.21,;39.57,-38.56,;38.78,-39.87,;37.26,-39.85,;36.57,-41.11,;37.34,-42.45,;36.56,-43.79,;37.33,-45.12,;38.86,-45.12,;39.64,-43.79,;38.88,-42.46,;39.65,-41.13,;41.19,-41.13,;40.04,-39.63,;38.31,-40.35,;39.62,-46.46,;40.95,-45.68,;40.95,-47.23,;38.86,-47.8,;37.32,-47.8,;36.55,-49.14,;35.01,-49.14,;34.25,-50.48,;32.71,-50.49,;31.93,-49.16,;31.94,-51.82,;32.7,-53.12,;34.24,-53.11,;35.22,-54.3,;34.89,-55.82,;33.49,-56.5,;33.47,-58.08,;32.14,-58.84,;34.8,-58.85,;34.8,-60.39,;36.13,-61.16,;37.46,-60.39,;38.79,-61.17,;40.13,-60.4,;40.13,-58.86,;41.46,-61.17,;41.45,-62.72,;42.78,-63.5,;44.12,-62.72,;44.12,-61.18,;42.78,-60.41,;42.8,-58.87,;44.13,-58.11,;44.13,-56.57,;42.8,-55.79,;41.45,-56.58,;41.46,-58.11,;37.48,-58.86,;36.15,-58.08,;32.1,-55.85,;30.99,-56.93,;29.5,-56.5,;29.12,-54.99,;30.24,-53.9,;31.73,-54.34,;34.93,-41.14,;34.16,-42.46,;32.63,-42.45,;31.88,-41.11,;32.65,-39.8,;34.18,-39.81,;34.96,-38.48,;36.51,-38.5,;37.29,-37.18,;30.34,-41.09,;29.58,-39.75,;28.04,-39.74,;29.55,-42.43,;28.01,-42.4,)| Show InChI InChI=1S/C62H64N6O9S2/c1-5-66(6-2)45-31-34-51-56(39-45)77-57-40-46(67(7-3)8-4)32-35-52(57)60(51)53-36-33-47(41-58(53)79(74,75)76)78(72,73)63-37-17-16-26-59(69)65-54-24-18-38-68(55-25-15-14-23-50(54)55)62(71)43-27-29-44(30-28-43)64-61(70)49-22-13-12-21-48(49)42-19-10-9-11-20-42/h9-15,19-23,25,27-36,39-41,54,63H,5-8,16-18,24,26,37-38H2,1-4H3,(H2-,64,65,69,70,71,74,75,76) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UMR7200 CNRS/Universit£ de Strasbourg
Curated by ChEMBL
| Assay Description
Displacement of [3H]-AVP from human vasopressin V2 receptor expressed in CHO cells after 30 mins |
J Med Chem 55: 8588-602 (2012)
Article DOI: 10.1021/jm3006146
BindingDB Entry DOI: 10.7270/Q28G8MT5 |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(Homo sapiens (Human)) | BDBM50052954

(2-Methyl-N-[4-(2,3,4,5-tetrahydro-benzo[b]azepine-...)Show SMILES Cc1ccccc1C(=O)Nc1ccc(cc1)C(=O)N1CCCCc2ccccc12 Show InChI InChI=1S/C25H24N2O2/c1-18-8-2-4-11-22(18)24(28)26-21-15-13-20(14-16-21)25(29)27-17-7-6-10-19-9-3-5-12-23(19)27/h2-5,8-9,11-16H,6-7,10,17H2,1H3,(H,26,28) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 8.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UMR7200 CNRS/Universit£ de Strasbourg
Curated by ChEMBL
| Assay Description
Binding affinity to vasopressin V1a receptor |
J Med Chem 55: 8588-602 (2012)
Article DOI: 10.1021/jm3006146
BindingDB Entry DOI: 10.7270/Q28G8MT5 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1
(Homo sapiens (Human)) | BDBM50397219
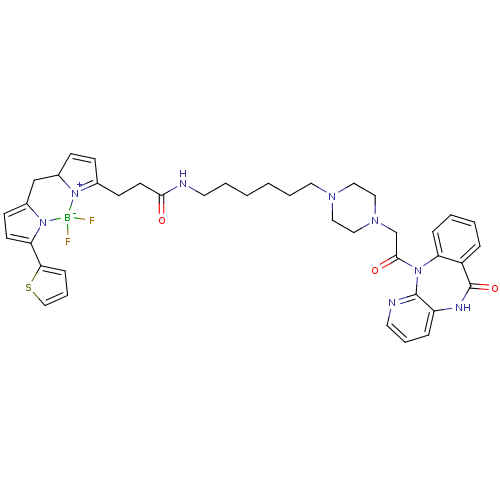
(CHEMBL2172285)Show SMILES F[B-]1(F)n2c(CC3C=CC(CCC(=O)NCCCCCCN4CCN(CC(=O)N5c6ccccc6C(=O)Nc6cccnc56)CC4)=[N+]13)ccc2-c1cccs1 |c:7,t:49| Show InChI InChI=1S/C40H45BF2N8O3S/c42-41(43)50-29(13-14-30(50)27-31-15-17-35(51(31)41)36-12-8-26-55-36)16-18-37(52)44-19-5-1-2-6-21-47-22-24-48(25-23-47)28-38(53)49-34-11-4-3-9-32(34)40(54)46-33-10-7-20-45-39(33)49/h3-4,7-15,17,20,26,30H,1-2,5-6,16,18-19,21-25,27-28H2,(H,44,52)(H,46,54) | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 8.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UMR7200 CNRS/Universit£ de Strasbourg
Curated by ChEMBL
| Assay Description
Binding affinity to human muscarinic M1 receptor |
J Med Chem 55: 8588-602 (2012)
Article DOI: 10.1021/jm3006146
BindingDB Entry DOI: 10.7270/Q28G8MT5 |
More data for this
Ligand-Target Pair | |
Vasopressin V2 receptor
(Homo sapiens (Human)) | BDBM50397210

(CHEMBL2172290)Show SMILES CCN1\C(=C\C=C\C=C\C2=[N+](CC)c3ccc(cc3C2(C)C)S([O-])(=O)=O)C(C)(CCCC(=O)NCCOCCOCCOCCn2cc(CNC(=O)CCC(=O)NC3CCCN(C(=O)c4ccc(NC(=O)c5ccccc5-c5ccccc5)cc4)c4ccccc34)nn2)c2cc(ccc12)S(O)(=O)=O |c:9| Show InChI InChI=1S/C77H88N10O14S2/c1-6-85-67-36-34-58(102(93,94)95)50-63(67)76(3,4)69(85)27-12-9-13-28-70-77(5,64-51-59(103(96,97)98)35-37-68(64)86(70)7-2)40-18-29-71(88)78-41-44-99-46-48-101-49-47-100-45-43-84-53-57(82-83-84)52-79-72(89)38-39-73(90)81-65-25-19-42-87(66-26-17-16-24-62(65)66)75(92)55-30-32-56(33-31-55)80-74(91)61-23-15-14-22-60(61)54-20-10-8-11-21-54/h8-17,20-24,26-28,30-37,50-51,53,65H,6-7,18-19,25,29,38-49,52H2,1-5H3,(H5-,78,79,80,81,88,89,90,91,92,93,94,95,96,97,98) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 9.02 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UMR7200 CNRS/Universit£ de Strasbourg
Curated by ChEMBL
| Assay Description
Displacement of [3H]-AVP from human vasopressin V2 receptor expressed in CHO cells after 30 mins |
J Med Chem 55: 8588-602 (2012)
Article DOI: 10.1021/jm3006146
BindingDB Entry DOI: 10.7270/Q28G8MT5 |
More data for this
Ligand-Target Pair | |
Vasopressin V2 receptor
(Homo sapiens (Human)) | BDBM35714

(CHEMBL420762 | Mozavaptan | N-[4-(5-Dimethylamino-...)Show SMILES CN(C)C1CCCN(C(=O)c2ccc(NC(=O)c3ccccc3C)cc2)c2ccccc12 Show InChI InChI=1S/C27H29N3O2/c1-19-9-4-5-10-22(19)26(31)28-21-16-14-20(15-17-21)27(32)30-18-8-13-24(29(2)3)23-11-6-7-12-25(23)30/h4-7,9-12,14-17,24H,8,13,18H2,1-3H3,(H,28,31) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 9.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UMR7200 CNRS/Universit£ de Strasbourg
Curated by ChEMBL
| Assay Description
Binding affinity to vasopressin V2 receptor |
J Med Chem 55: 8588-602 (2012)
Article DOI: 10.1021/jm3006146
BindingDB Entry DOI: 10.7270/Q28G8MT5 |
More data for this
Ligand-Target Pair | |
Vasopressin V2 receptor
(Homo sapiens (Human)) | BDBM50397212
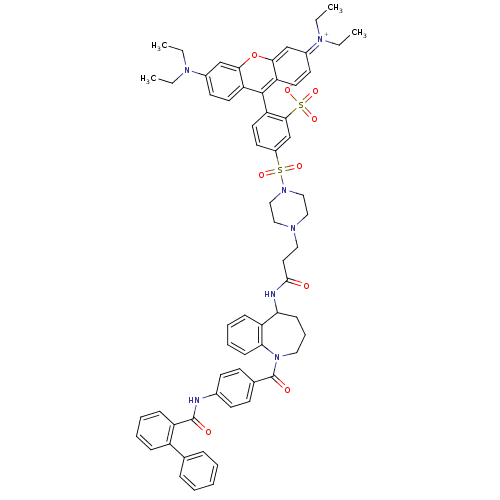
(CHEMBL2172392)Show SMILES CCN(CC)c1ccc2c(-c3ccc(cc3S([O-])(=O)=O)S(=O)(=O)N3CCN(CCC(=O)NC4CCCN(C(=O)c5ccc(NC(=O)c6ccccc6-c6ccccc6)cc5)c5ccccc45)CC3)c3ccc(cc3oc2c1)=[N+](CC)CC |(24.73,-1.99,;23.19,-1.95,;22.38,-3.27,;23.12,-4.62,;24.66,-4.66,;20.84,-3.22,;20.04,-4.53,;18.51,-4.48,;17.78,-3.15,;16.34,-3.17,;15.55,-4.49,;14,-4.47,;13.21,-5.79,;13.96,-7.13,;15.51,-7.16,;16.3,-5.84,;17.84,-5.86,;18.58,-7.21,;19.33,-5.47,;17.86,-4.32,;13.17,-8.45,;14.5,-9.22,;13.15,-9.98,;11.63,-8.43,;10.87,-9.77,;9.33,-9.77,;8.56,-8.44,;7.02,-8.45,;6.25,-9.79,;4.71,-9.79,;3.94,-8.46,;3.95,-11.13,;4.7,-12.43,;6.25,-12.42,;7.23,-13.61,;6.9,-15.12,;5.5,-15.81,;5.48,-17.38,;4.15,-18.15,;6.81,-18.16,;6.81,-19.7,;8.14,-20.47,;9.47,-19.7,;10.8,-20.47,;12.13,-19.71,;12.14,-18.17,;13.46,-20.48,;13.46,-22.03,;14.79,-22.8,;16.13,-22.03,;16.13,-20.49,;14.79,-19.72,;14.8,-18.18,;16.13,-17.41,;16.14,-15.87,;14.8,-15.1,;13.46,-15.88,;13.46,-17.41,;9.48,-18.17,;8.16,-17.39,;4.11,-15.16,;3,-16.24,;1.5,-15.81,;1.12,-14.29,;2.25,-13.21,;3.74,-13.64,;9.32,-7.1,;10.87,-7.1,;15.51,-1.75,;13.98,-1.73,;13.24,-.39,;14.04,.93,;15.56,.9,;16.3,-.45,;17.84,-.48,;18.58,-1.84,;20.12,-1.87,;13.3,2.28,;14.1,3.6,;13.36,4.94,;11.76,2.32,;11.02,3.66,)| Show InChI InChI=1S/C64H67N7O9S2/c1-5-68(6-2)47-28-31-53-58(41-47)80-59-42-48(69(7-3)8-4)29-32-54(59)62(53)55-33-30-49(43-60(55)82(77,78)79)81(75,76)70-39-37-67(38-40-70)36-34-61(72)66-56-22-16-35-71(57-23-15-14-21-52(56)57)64(74)45-24-26-46(27-25-45)65-63(73)51-20-13-12-19-50(51)44-17-10-9-11-18-44/h9-15,17-21,23-33,41-43,56H,5-8,16,22,34-40H2,1-4H3,(H2-,65,66,72,73,74,77,78,79) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 12.7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UMR7200 CNRS/Universit£ de Strasbourg
Curated by ChEMBL
| Assay Description
Displacement of [3H]-AVP from human vasopressin V2 receptor expressed in CHO cells after 30 mins |
J Med Chem 55: 8588-602 (2012)
Article DOI: 10.1021/jm3006146
BindingDB Entry DOI: 10.7270/Q28G8MT5 |
More data for this
Ligand-Target Pair | |
Vasopressin V2 receptor
(Homo sapiens (Human)) | BDBM50397216
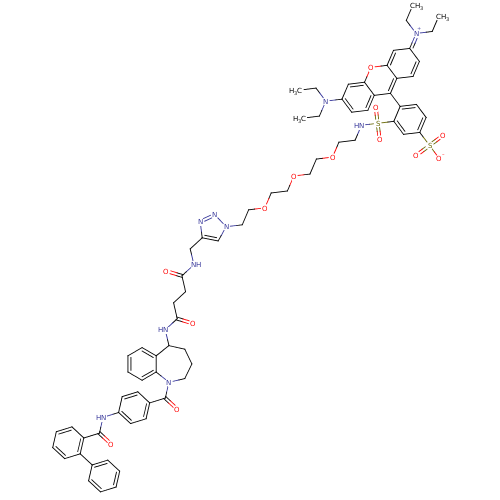
(CHEMBL2172294)Show SMILES CCN(CC)c1ccc2c(-c3ccc(cc3S(=O)(=O)NCCOCCOCCOCCn3cc(CNC(=O)CCC(=O)NC4CCCN(C(=O)c5ccc(NC(=O)c6ccccc6-c6ccccc6)cc5)c5ccccc45)nn3)S([O-])(=O)=O)c3ccc(cc3oc2c1)=[N+](CC)CC |(31.09,-9.94,;32.43,-10.72,;32.42,-12.26,;31.09,-13.02,;29.76,-12.25,;33.76,-13.04,;33.76,-14.58,;35.09,-15.33,;36.41,-14.58,;37.73,-15.35,;37.72,-16.89,;39.04,-17.68,;39.02,-19.23,;37.68,-19.97,;36.36,-19.19,;36.38,-17.65,;35.06,-16.86,;33.95,-15.76,;35.82,-15.52,;33.71,-17.61,;32.39,-16.82,;31.04,-17.58,;29.72,-16.79,;28.38,-17.54,;27.05,-16.75,;25.71,-17.51,;24.38,-16.72,;23.04,-17.48,;21.71,-16.69,;20.36,-17.44,;19.04,-16.65,;17.7,-17.4,;16.23,-16.95,;15.34,-18.21,;13.8,-18.23,;13.02,-16.9,;11.48,-16.92,;10.72,-18.26,;10.69,-15.59,;9.15,-15.61,;8.37,-14.28,;9.13,-12.94,;6.83,-14.3,;5.95,-15.57,;6.71,-16.9,;6.12,-18.32,;4.63,-18.76,;3.38,-17.89,;2.03,-18.65,;.71,-17.87,;2.02,-20.19,;3.35,-20.98,;3.33,-22.52,;2,-23.26,;1.98,-24.81,;3.31,-25.58,;4.65,-24.83,;3.3,-27.13,;1.95,-27.9,;1.95,-29.44,;3.28,-30.21,;4.61,-29.44,;4.62,-27.91,;5.97,-27.14,;7.3,-27.94,;8.65,-27.18,;8.67,-25.62,;7.32,-24.83,;5.98,-25.6,;.66,-22.49,;.68,-20.96,;3.28,-16.35,;1.81,-15.9,;1.47,-14.39,;2.62,-13.33,;4.09,-13.8,;4.43,-15.31,;16.27,-19.44,;17.72,-18.95,;37.66,-21.51,;36.32,-22.25,;38.98,-20.73,;38.98,-22.27,;39.07,-14.59,;40.38,-15.37,;41.71,-14.62,;41.73,-13.08,;40.41,-12.3,;39.09,-13.06,;37.75,-12.27,;36.41,-13.04,;35.09,-12.27,;43.07,-12.31,;44.39,-13.1,;45.74,-12.33,;43.08,-10.77,;44.41,-10.02,)| Show InChI InChI=1S/C72H80N10O13S2/c1-5-79(6-2)54-28-31-60-65(45-54)95-66-46-55(80(7-3)8-4)29-32-61(66)70(60)62-33-30-56(97(89,90)91)47-67(62)96(87,88)74-36-39-92-41-43-94-44-42-93-40-38-81-49-53(77-78-81)48-73-68(83)34-35-69(84)76-63-22-16-37-82(64-23-15-14-21-59(63)64)72(86)51-24-26-52(27-25-51)75-71(85)58-20-13-12-19-57(58)50-17-10-9-11-18-50/h9-15,17-21,23-33,45-47,49,63,74H,5-8,16,22,34-44,48H2,1-4H3,(H3-,73,75,76,83,84,85,86,89,90,91) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 14.1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UMR7200 CNRS/Universit£ de Strasbourg
Curated by ChEMBL
| Assay Description
Displacement of [3H]-AVP from human vasopressin V2 receptor expressed in CHO cells after 30 mins |
J Med Chem 55: 8588-602 (2012)
Article DOI: 10.1021/jm3006146
BindingDB Entry DOI: 10.7270/Q28G8MT5 |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(Homo sapiens (Human)) | BDBM50307117

(CHEMBL603708 | N-(4-(2,3,4,5-tetrahydro-1H-benzo[b...)Show SMILES O=C(Nc1ccc(cc1)C(=O)N1CCCCc2ccccc12)c1ccccc1-c1ccccc1 Show InChI InChI=1S/C30H26N2O2/c33-29(27-15-6-5-14-26(27)22-10-2-1-3-11-22)31-25-19-17-24(18-20-25)30(34)32-21-9-8-13-23-12-4-7-16-28(23)32/h1-7,10-12,14-20H,8-9,13,21H2,(H,31,33) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 14.1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UMR7200 CNRS/Universit£ de Strasbourg
Curated by ChEMBL
| Assay Description
Binding affinity to vasopressin V1a receptor |
J Med Chem 55: 8588-602 (2012)
Article DOI: 10.1021/jm3006146
BindingDB Entry DOI: 10.7270/Q28G8MT5 |
More data for this
Ligand-Target Pair | |
Vasopressin V2 receptor
(Homo sapiens (Human)) | BDBM50397209

(CHEMBL2172291)Show SMILES CCN1\C(=C\C=C\C=C\C2=[N+](CC)c3ccc(cc3C2(C)C)S([O-])(=O)=O)C(C)(CCCC(=O)NCCOCCOCCOCCOCCOCCOCCn2cc(CNC(=O)CCC(=O)NC3CCCN(C(=O)c4ccc(NC(=O)c5ccccc5-c5ccccc5)cc4)c4ccccc34)nn2)c2cc(ccc12)S(O)(=O)=O |c:9| Show InChI InChI=1S/C83H100N10O17S2/c1-6-91-73-36-34-64(111(99,100)101)56-69(73)82(3,4)75(91)27-12-9-13-28-76-83(5,70-57-65(112(102,103)104)35-37-74(70)92(76)7-2)40-18-29-77(94)84-41-44-105-46-48-107-50-52-109-54-55-110-53-51-108-49-47-106-45-43-90-59-63(88-89-90)58-85-78(95)38-39-79(96)87-71-25-19-42-93(72-26-17-16-24-68(71)72)81(98)61-30-32-62(33-31-61)86-80(97)67-23-15-14-22-66(67)60-20-10-8-11-21-60/h8-17,20-24,26-28,30-37,56-57,59,71H,6-7,18-19,25,29,38-55,58H2,1-5H3,(H5-,84,85,86,87,94,95,96,97,98,99,100,101,102,103,104) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 14.2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UMR7200 CNRS/Universit£ de Strasbourg
Curated by ChEMBL
| Assay Description
Displacement of [3H]-AVP from human vasopressin V2 receptor expressed in CHO cells after 30 mins |
J Med Chem 55: 8588-602 (2012)
Article DOI: 10.1021/jm3006146
BindingDB Entry DOI: 10.7270/Q28G8MT5 |
More data for this
Ligand-Target Pair | |
Vasopressin V2 receptor
(Homo sapiens (Human)) | BDBM50397211
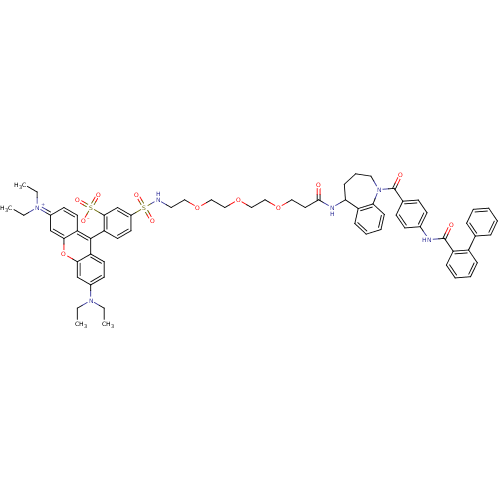
(CHEMBL2172393)Show SMILES CCN(CC)c1ccc2c(-c3ccc(cc3S([O-])(=O)=O)S(=O)(=O)NCCOCCOCCOCCC(=O)NC3CCCN(C(=O)c4ccc(NC(=O)c5ccccc5-c5ccccc5)cc4)c4ccccc34)c3ccc(cc3oc2c1)=[N+](CC)CC |(51.81,4.38,;50.27,4.42,;49.46,3.11,;50.2,1.76,;51.74,1.71,;47.92,3.16,;47.12,1.84,;45.58,1.88,;44.86,3.22,;43.41,3.2,;42.63,1.89,;41.08,1.9,;40.29,.58,;41.04,-.76,;42.59,-.79,;43.38,.53,;44.91,.51,;45.66,-.84,;46.41,.91,;44.93,2.06,;40.25,-2.08,;41.58,-2.86,;40.23,-3.62,;38.71,-2.09,;37.95,-3.42,;36.4,-3.43,;35.64,-4.77,;34.1,-4.77,;33.34,-6.11,;31.8,-6.12,;31.03,-7.45,;29.49,-7.46,;28.73,-8.79,;27.19,-8.8,;26.42,-10.14,;24.88,-10.14,;24.11,-8.81,;24.12,-11.48,;24.87,-12.78,;26.41,-12.77,;27.4,-13.96,;27.06,-15.47,;25.66,-16.16,;25.65,-17.73,;24.32,-18.5,;26.98,-18.51,;26.97,-20.05,;28.3,-20.82,;29.64,-20.05,;30.97,-20.82,;32.3,-20.06,;32.31,-18.52,;33.63,-20.83,;33.63,-22.38,;34.96,-23.15,;36.3,-22.38,;36.3,-20.84,;34.96,-20.07,;34.97,-18.53,;36.3,-17.76,;36.3,-16.22,;34.97,-15.45,;33.63,-16.23,;33.63,-17.76,;29.65,-18.52,;28.33,-17.74,;24.28,-15.51,;23.16,-16.59,;21.67,-16.16,;21.29,-14.64,;22.41,-13.56,;23.91,-14,;42.59,4.61,;41.06,4.65,;40.32,5.97,;41.12,7.29,;42.64,7.26,;43.38,5.92,;44.92,5.9,;45.66,4.54,;47.2,4.5,;40.38,8.65,;41.18,9.96,;40.44,11.31,;38.84,8.68,;38.1,10.02,)| Show InChI InChI=1S/C66H72N6O12S2/c1-5-70(6-2)49-28-31-55-60(43-49)84-61-44-50(71(7-3)8-4)29-32-56(61)64(55)57-33-30-51(45-62(57)86(78,79)80)85(76,77)67-35-38-82-40-42-83-41-39-81-37-34-63(73)69-58-22-16-36-72(59-23-15-14-21-54(58)59)66(75)47-24-26-48(27-25-47)68-65(74)53-20-13-12-19-52(53)46-17-10-9-11-18-46/h9-15,17-21,23-33,43-45,58,67H,5-8,16,22,34-42H2,1-4H3,(H2-,68,69,73,74,75,78,79,80) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 16.8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UMR7200 CNRS/Universit£ de Strasbourg
Curated by ChEMBL
| Assay Description
Displacement of [3H]-AVP from human vasopressin V2 receptor expressed in CHO cells after 30 mins |
J Med Chem 55: 8588-602 (2012)
Article DOI: 10.1021/jm3006146
BindingDB Entry DOI: 10.7270/Q28G8MT5 |
More data for this
Ligand-Target Pair | |
Vasopressin V2 receptor
(Homo sapiens (Human)) | BDBM50397214
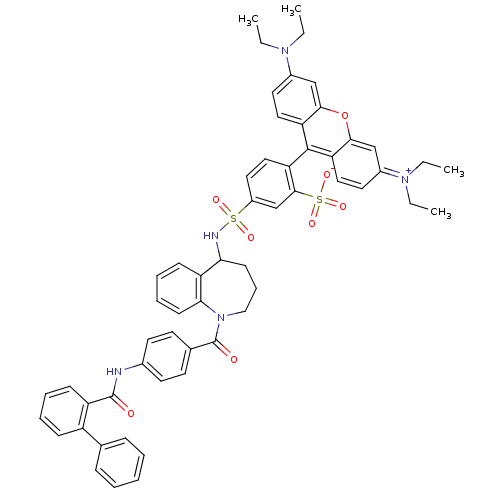
(CHEMBL2172390)Show SMILES CCN(CC)c1ccc2c(-c3ccc(cc3S([O-])(=O)=O)S(=O)(=O)NC3CCCN(C(=O)c4ccc(NC(=O)c5ccccc5-c5ccccc5)cc4)c4ccccc34)c3ccc(cc3oc2c1)=[N+](CC)CC |(14.53,-35.6,;13.74,-36.92,;14.49,-38.27,;16.03,-38.29,;16.82,-36.97,;13.7,-39.59,;14.45,-40.93,;13.66,-42.24,;12.14,-42.22,;11.45,-43.49,;12.21,-44.83,;11.44,-46.16,;12.21,-47.5,;13.74,-47.5,;14.52,-46.17,;13.76,-44.83,;14.53,-43.5,;16.07,-43.51,;14.92,-42,;13.19,-42.73,;14.5,-48.83,;15.83,-48.06,;15.83,-49.6,;13.62,-50.1,;14.24,-51.47,;15.78,-51.46,;16.77,-52.65,;16.43,-54.17,;15.03,-54.85,;15.02,-56.42,;13.68,-57.19,;16.35,-57.2,;16.34,-58.74,;17.67,-59.51,;19.01,-58.74,;20.34,-59.52,;21.67,-58.75,;21.68,-57.21,;23,-59.52,;23,-61.07,;24.33,-61.85,;25.67,-61.07,;25.67,-59.53,;24.33,-58.76,;24.34,-57.22,;25.67,-56.46,;25.67,-54.92,;24.34,-54.14,;23,-54.93,;23,-56.46,;19.02,-57.21,;17.7,-56.43,;13.65,-54.2,;12.53,-55.28,;11.04,-54.85,;10.66,-53.34,;11.78,-52.25,;13.28,-52.69,;9.81,-43.52,;9.04,-44.84,;7.51,-44.83,;6.75,-43.49,;7.53,-42.18,;9.06,-42.19,;9.84,-40.86,;11.38,-40.88,;12.17,-39.56,;5.21,-43.47,;4.46,-42.13,;2.92,-42.12,;4.43,-44.8,;2.89,-44.78,)| Show InChI InChI=1S/C57H55N5O8S2/c1-5-60(6-2)41-28-31-47-52(35-41)70-53-36-42(61(7-3)8-4)29-32-48(53)55(47)49-33-30-43(37-54(49)72(67,68)69)71(65,66)59-50-22-16-34-62(51-23-15-14-21-46(50)51)57(64)39-24-26-40(27-25-39)58-56(63)45-20-13-12-19-44(45)38-17-10-9-11-18-38/h9-15,17-21,23-33,35-37,50,59H,5-8,16,22,34H2,1-4H3,(H-,58,63,64,67,68,69) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 25.3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UMR7200 CNRS/Universit£ de Strasbourg
Curated by ChEMBL
| Assay Description
Displacement of [3H]-AVP from human vasopressin V2 receptor expressed in CHO cells after 30 mins |
J Med Chem 55: 8588-602 (2012)
Article DOI: 10.1021/jm3006146
BindingDB Entry DOI: 10.7270/Q28G8MT5 |
More data for this
Ligand-Target Pair | |
Vasopressin V2 receptor
(Homo sapiens (Human)) | BDBM50397207
(CHEMBL2172288)Show SMILES OC(=O)c1cc(NC(=S)NCCOCCOCCOCCn2cc(CNC(=O)CCC(=O)NC3CCCN(C(=O)c4ccc(NC(=O)c5ccccc5-c5ccccc5)cc4)c4ccccc34)nn2)ccc1-c1c2ccc(O)cc2oc2cc(=O)ccc12 |(30.96,-29.16,;30.97,-27.63,;29.62,-28.36,;31.01,-26.09,;29.7,-25.29,;29.75,-23.75,;28.43,-22.94,;28.48,-21.4,;29.83,-20.67,;27.16,-20.6,;27.2,-19.06,;25.89,-18.25,;25.93,-16.71,;24.62,-15.91,;24.66,-14.37,;23.34,-13.56,;23.38,-12.02,;22.07,-11.22,;22.11,-9.68,;20.8,-8.87,;19.48,-9.65,;19.48,-11.19,;18.26,-12.12,;18.78,-13.58,;17.91,-14.85,;16.37,-14.85,;15.6,-16.19,;16.38,-17.52,;14.06,-16.19,;13.3,-17.54,;11.74,-17.54,;10.97,-16.2,;10.98,-18.88,;11.73,-20.18,;13.29,-20.17,;14.27,-21.36,;13.94,-22.87,;12.54,-23.56,;12.52,-25.13,;11.18,-25.89,;13.86,-25.9,;15.21,-25.14,;16.53,-25.91,;16.52,-27.45,;17.84,-28.22,;19.18,-27.45,;19.18,-25.91,;20.51,-28.23,;20.5,-29.78,;21.83,-30.55,;23.18,-29.78,;23.18,-28.24,;21.83,-27.46,;21.84,-25.92,;23.18,-25.16,;23.18,-23.63,;21.85,-22.85,;20.5,-23.63,;20.5,-25.16,;15.18,-28.22,;13.85,-27.45,;11.14,-22.9,;10.03,-23.98,;8.54,-23.56,;8.15,-22.04,;9.28,-20.96,;10.77,-21.39,;20.31,-13.54,;20.75,-12.06,;31.09,-23.01,;32.41,-23.81,;32.37,-25.36,;33.68,-26.15,;34.98,-25.34,;34.93,-23.82,;36.22,-23,;37.58,-23.72,;38.89,-22.91,;37.63,-25.26,;36.33,-26.06,;36.4,-27.61,;35.08,-28.42,;35.13,-29.95,;33.83,-30.76,;33.88,-32.3,;32.47,-30.03,;32.43,-28.5,;33.73,-27.69,)| Show InChI InChI=1S/C66H63N9O12S/c76-47-21-24-53-58(38-47)87-59-39-48(77)22-25-54(59)62(53)50-23-20-45(37-55(50)65(82)83)70-66(88)67-28-31-84-33-35-86-36-34-85-32-30-74-41-46(72-73-74)40-68-60(78)26-27-61(79)71-56-14-8-29-75(57-15-7-6-13-52(56)57)64(81)43-16-18-44(19-17-43)69-63(80)51-12-5-4-11-49(51)42-9-2-1-3-10-42/h1-7,9-13,15-25,37-39,41,56,76H,8,14,26-36,40H2,(H,68,78)(H,69,80)(H,71,79)(H,82,83)(H2,67,70,88) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 26.1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UMR7200 CNRS/Universit£ de Strasbourg
Curated by ChEMBL
| Assay Description
Displacement of [3H]-AVP from human vasopressin V2 receptor expressed in CHO cells after 30 mins |
J Med Chem 55: 8588-602 (2012)
Article DOI: 10.1021/jm3006146
BindingDB Entry DOI: 10.7270/Q28G8MT5 |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50052954

(2-Methyl-N-[4-(2,3,4,5-tetrahydro-benzo[b]azepine-...)Show SMILES Cc1ccccc1C(=O)Nc1ccc(cc1)C(=O)N1CCCCc2ccccc12 Show InChI InChI=1S/C25H24N2O2/c1-18-8-2-4-11-22(18)24(28)26-21-15-13-20(14-16-21)25(29)27-17-7-6-10-19-9-3-5-12-23(19)27/h2-5,8-9,11-16H,6-7,10,17H2,1H3,(H,26,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UMR7200 CNRS/Universit£ de Strasbourg
Curated by ChEMBL
| Assay Description
Binding affinity to oxytocin receptor |
J Med Chem 55: 8588-602 (2012)
Article DOI: 10.1021/jm3006146
BindingDB Entry DOI: 10.7270/Q28G8MT5 |
More data for this
Ligand-Target Pair | |
Vasopressin V2 receptor
(Homo sapiens (Human)) | BDBM50397217
(CHEMBL2172293)Show SMILES CCN(CC)c1ccc2c(-c3ccc(cc3S([O-])(=O)=O)S(=O)(=O)NCCOCCOCCOCCn3cc(CNC(=O)CCC(=O)NC4CCCN(C(=O)c5ccc(NC(=O)c6ccccc6C)cc5)c5ccccc45)nn3)c3ccc(cc3oc2c1)=[N+](CC)CC |(48.61,-15.81,;47.28,-16.58,;45.94,-15.82,;45.93,-14.27,;47.27,-13.49,;44.6,-16.59,;43.27,-15.83,;41.95,-16.6,;41.95,-18.12,;40.62,-18.89,;39.3,-18.1,;39.31,-16.56,;37.99,-15.78,;36.66,-16.53,;36.62,-18.07,;37.95,-18.86,;37.93,-20.4,;36.58,-21.15,;39.41,-20,;38.68,-21.73,;35.33,-15.74,;36.09,-14.4,;34.23,-14.64,;33.98,-16.49,;32.66,-15.71,;31.32,-16.46,;29.99,-15.67,;28.65,-16.42,;27.32,-15.64,;25.98,-16.39,;24.66,-15.61,;23.31,-16.36,;21.98,-15.57,;20.64,-16.32,;19.32,-15.53,;17.97,-16.29,;16.49,-15.84,;15.62,-17.1,;14.08,-17.11,;13.3,-15.79,;11.75,-15.81,;11,-17.15,;10.97,-14.49,;9.42,-14.5,;8.63,-13.18,;9.39,-11.82,;7.09,-13.19,;6.22,-14.46,;6.98,-15.8,;6.4,-17.22,;4.91,-17.67,;3.64,-16.8,;2.31,-17.56,;.97,-16.78,;2.3,-19.1,;.95,-19.86,;.94,-21.41,;2.28,-22.17,;2.27,-23.71,;3.6,-24.49,;4.93,-23.73,;3.59,-26.03,;2.24,-26.81,;2.23,-28.34,;3.57,-29.12,;4.91,-28.35,;4.91,-26.81,;6.25,-26.04,;3.61,-21.43,;3.63,-19.89,;3.55,-15.26,;2.07,-14.8,;1.74,-13.28,;2.87,-12.23,;4.35,-12.69,;4.69,-14.21,;16.54,-18.33,;18,-17.83,;40.63,-20.43,;39.3,-21.18,;39.29,-22.72,;40.62,-23.49,;41.94,-22.73,;41.95,-21.21,;43.28,-20.44,;43.28,-18.89,;44.61,-18.12,;40.62,-25.03,;41.95,-25.81,;41.94,-27.35,;39.27,-25.8,;39.27,-27.34,)| Show InChI InChI=1S/C67H78N10O13S2/c1-6-74(7-2)50-24-27-55-60(41-50)90-61-42-51(75(8-3)9-4)25-28-56(61)65(55)57-29-26-52(43-62(57)92(84,85)86)91(82,83)69-32-35-87-37-39-89-40-38-88-36-34-76-45-49(72-73-76)44-68-63(78)30-31-64(79)71-58-18-14-33-77(59-19-13-12-17-54(58)59)67(81)47-20-22-48(23-21-47)70-66(80)53-16-11-10-15-46(53)5/h10-13,15-17,19-29,41-43,45,58,69H,6-9,14,18,30-40,44H2,1-5H3,(H3-,68,70,71,78,79,80,81,84,85,86) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 54.3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UMR7200 CNRS/Universit£ de Strasbourg
Curated by ChEMBL
| Assay Description
Displacement of [3H]-AVP from human vasopressin V2 receptor expressed in CHO cells after 30 mins |
J Med Chem 55: 8588-602 (2012)
Article DOI: 10.1021/jm3006146
BindingDB Entry DOI: 10.7270/Q28G8MT5 |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50307117

(CHEMBL603708 | N-(4-(2,3,4,5-tetrahydro-1H-benzo[b...)Show SMILES O=C(Nc1ccc(cc1)C(=O)N1CCCCc2ccccc12)c1ccccc1-c1ccccc1 Show InChI InChI=1S/C30H26N2O2/c33-29(27-15-6-5-14-26(27)22-10-2-1-3-11-22)31-25-19-17-24(18-20-25)30(34)32-21-9-8-13-23-12-4-7-16-28(23)32/h1-7,10-12,14-20H,8-9,13,21H2,(H,31,33) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 63 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UMR7200 CNRS/Universit£ de Strasbourg
Curated by ChEMBL
| Assay Description
Binding affinity to oxytocin receptor |
J Med Chem 55: 8588-602 (2012)
Article DOI: 10.1021/jm3006146
BindingDB Entry DOI: 10.7270/Q28G8MT5 |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50307117

(CHEMBL603708 | N-(4-(2,3,4,5-tetrahydro-1H-benzo[b...)Show SMILES O=C(Nc1ccc(cc1)C(=O)N1CCCCc2ccccc12)c1ccccc1-c1ccccc1 Show InChI InChI=1S/C30H26N2O2/c33-29(27-15-6-5-14-26(27)22-10-2-1-3-11-22)31-25-19-17-24(18-20-25)30(34)32-21-9-8-13-23-12-4-7-16-28(23)32/h1-7,10-12,14-20H,8-9,13,21H2,(H,31,33) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 109 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UMR7200 CNRS/Universit£ de Strasbourg
Curated by ChEMBL
| Assay Description
Displacement of [3H]-AVP from human oxytocin receptor expressed in CHO cells after 30 mins |
J Med Chem 55: 8588-602 (2012)
Article DOI: 10.1021/jm3006146
BindingDB Entry DOI: 10.7270/Q28G8MT5 |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50397206

(CHEMBL2172289)Show SMILES CCN1\C(=C\C=C\C=C\C2=[N+](CC)c3ccc(cc3C2(C)C)S([O-])(=O)=O)C(C)(CCCC(=O)NCCn2cc(CNC(=O)CCC(=O)NC3CCCN(C(=O)c4ccc(NC(=O)c5ccccc5-c5ccccc5)cc4)c4ccccc34)nn2)c2cc(ccc12)S(O)(=O)=O |c:9| Show InChI InChI=1S/C71H76N10O11S2/c1-6-79-61-36-34-52(93(87,88)89)44-57(61)70(3,4)63(79)27-12-9-13-28-64-71(5,58-45-53(94(90,91)92)35-37-62(58)80(64)7-2)40-18-29-65(82)72-41-43-78-47-51(76-77-78)46-73-66(83)38-39-67(84)75-59-25-19-42-81(60-26-17-16-24-56(59)60)69(86)49-30-32-50(33-31-49)74-68(85)55-23-15-14-22-54(55)48-20-10-8-11-21-48/h8-17,20-24,26-28,30-37,44-45,47,59H,6-7,18-19,25,29,38-43,46H2,1-5H3,(H5-,72,73,74,75,82,83,84,85,86,87,88,89,90,91,92) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 184 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UMR7200 CNRS/Universit£ de Strasbourg
Curated by ChEMBL
| Assay Description
Displacement of [3H]-AVP from human oxytocin receptor expressed in CHO cells after 30 mins |
J Med Chem 55: 8588-602 (2012)
Article DOI: 10.1021/jm3006146
BindingDB Entry DOI: 10.7270/Q28G8MT5 |
More data for this
Ligand-Target Pair | |
Vasopressin V2 receptor
(Homo sapiens (Human)) | BDBM50397218
(CHEMBL2172292)Show SMILES CCN(CC)c1ccc2c(-c3ccc(cc3S(=O)(=O)NCCOCCOCCOCCn3cc(CNC(=O)CCC(=O)NC4CCCN(C(=O)c5ccc(NC(=O)c6ccccc6C)cc5)c5ccccc45)nn3)S([O-])(=O)=O)c3ccc(cc3oc2c1)=[N+](CC)CC |(31.1,-9.95,;32.44,-10.72,;32.43,-12.26,;31.09,-13.03,;29.76,-12.25,;33.76,-13.04,;33.76,-14.58,;35.09,-15.34,;36.42,-14.58,;37.74,-15.36,;37.73,-16.9,;39.05,-17.68,;39.03,-19.23,;37.69,-19.98,;36.37,-19.19,;36.39,-17.65,;35.07,-16.86,;33.96,-15.76,;35.83,-15.52,;33.72,-17.62,;32.4,-16.82,;31.05,-17.59,;29.72,-16.79,;28.38,-17.55,;27.06,-16.76,;25.71,-17.51,;24.39,-16.73,;23.04,-17.48,;21.72,-16.69,;20.37,-17.44,;19.05,-16.65,;17.71,-17.41,;16.23,-16.96,;15.35,-18.22,;13.81,-18.23,;13.03,-16.91,;11.49,-16.92,;10.73,-18.27,;10.7,-15.6,;9.16,-15.61,;8.37,-14.28,;9.13,-12.95,;6.83,-14.31,;5.95,-15.57,;6.71,-16.9,;6.13,-18.33,;4.63,-18.77,;3.38,-17.9,;2.03,-18.66,;.71,-17.87,;2.02,-20.2,;3.35,-20.98,;3.33,-22.52,;2,-23.27,;1.98,-24.82,;3.31,-25.59,;4.65,-24.84,;3.3,-27.14,;1.95,-27.9,;1.95,-29.44,;3.28,-30.22,;4.61,-29.45,;4.62,-27.91,;5.97,-27.15,;.66,-22.5,;.68,-20.96,;3.28,-16.36,;1.81,-15.9,;1.47,-14.39,;2.62,-13.33,;4.09,-13.8,;4.44,-15.31,;16.27,-19.45,;17.73,-18.96,;37.67,-21.51,;36.33,-22.26,;38.99,-20.73,;38.99,-22.27,;39.07,-14.59,;40.39,-15.37,;41.72,-14.62,;41.74,-13.09,;40.42,-12.31,;39.1,-13.06,;37.76,-12.27,;36.42,-13.05,;35.1,-12.27,;43.08,-12.32,;44.4,-13.1,;45.75,-12.33,;43.09,-10.78,;44.42,-10.02,)| Show InChI InChI=1S/C67H78N10O13S2/c1-6-74(7-2)50-24-27-55-60(41-50)90-61-42-51(75(8-3)9-4)25-28-56(61)65(55)57-29-26-52(92(84,85)86)43-62(57)91(82,83)69-32-35-87-37-39-89-40-38-88-36-34-76-45-49(72-73-76)44-68-63(78)30-31-64(79)71-58-18-14-33-77(59-19-13-12-17-54(58)59)67(81)47-20-22-48(23-21-47)70-66(80)53-16-11-10-15-46(53)5/h10-13,15-17,19-29,41-43,45,58,69H,6-9,14,18,30-40,44H2,1-5H3,(H3-,68,70,71,78,79,80,81,84,85,86) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 188 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UMR7200 CNRS/Universit£ de Strasbourg
Curated by ChEMBL
| Assay Description
Displacement of [3H]-AVP from human vasopressin V2 receptor expressed in CHO cells after 30 mins |
J Med Chem 55: 8588-602 (2012)
Article DOI: 10.1021/jm3006146
BindingDB Entry DOI: 10.7270/Q28G8MT5 |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(Homo sapiens (Human)) | BDBM35714

(CHEMBL420762 | Mozavaptan | N-[4-(5-Dimethylamino-...)Show SMILES CN(C)C1CCCN(C(=O)c2ccc(NC(=O)c3ccccc3C)cc2)c2ccccc12 Show InChI InChI=1S/C27H29N3O2/c1-19-9-4-5-10-22(19)26(31)28-21-16-14-20(15-17-21)27(32)30-18-8-13-24(29(2)3)23-11-6-7-12-25(23)30/h4-7,9-12,14-17,24H,8,13,18H2,1-3H3,(H,28,31) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 195 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UMR7200 CNRS/Universit£ de Strasbourg
Curated by ChEMBL
| Assay Description
Binding affinity to vasopressin V1a receptor |
J Med Chem 55: 8588-602 (2012)
Article DOI: 10.1021/jm3006146
BindingDB Entry DOI: 10.7270/Q28G8MT5 |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50397218

(CHEMBL2172292)Show SMILES CCN(CC)c1ccc2c(-c3ccc(cc3S(=O)(=O)NCCOCCOCCOCCn3cc(CNC(=O)CCC(=O)NC4CCCN(C(=O)c5ccc(NC(=O)c6ccccc6C)cc5)c5ccccc45)nn3)S([O-])(=O)=O)c3ccc(cc3oc2c1)=[N+](CC)CC |(31.1,-9.95,;32.44,-10.72,;32.43,-12.26,;31.09,-13.03,;29.76,-12.25,;33.76,-13.04,;33.76,-14.58,;35.09,-15.34,;36.42,-14.58,;37.74,-15.36,;37.73,-16.9,;39.05,-17.68,;39.03,-19.23,;37.69,-19.98,;36.37,-19.19,;36.39,-17.65,;35.07,-16.86,;33.96,-15.76,;35.83,-15.52,;33.72,-17.62,;32.4,-16.82,;31.05,-17.59,;29.72,-16.79,;28.38,-17.55,;27.06,-16.76,;25.71,-17.51,;24.39,-16.73,;23.04,-17.48,;21.72,-16.69,;20.37,-17.44,;19.05,-16.65,;17.71,-17.41,;16.23,-16.96,;15.35,-18.22,;13.81,-18.23,;13.03,-16.91,;11.49,-16.92,;10.73,-18.27,;10.7,-15.6,;9.16,-15.61,;8.37,-14.28,;9.13,-12.95,;6.83,-14.31,;5.95,-15.57,;6.71,-16.9,;6.13,-18.33,;4.63,-18.77,;3.38,-17.9,;2.03,-18.66,;.71,-17.87,;2.02,-20.2,;3.35,-20.98,;3.33,-22.52,;2,-23.27,;1.98,-24.82,;3.31,-25.59,;4.65,-24.84,;3.3,-27.14,;1.95,-27.9,;1.95,-29.44,;3.28,-30.22,;4.61,-29.45,;4.62,-27.91,;5.97,-27.15,;.66,-22.5,;.68,-20.96,;3.28,-16.36,;1.81,-15.9,;1.47,-14.39,;2.62,-13.33,;4.09,-13.8,;4.44,-15.31,;16.27,-19.45,;17.73,-18.96,;37.67,-21.51,;36.33,-22.26,;38.99,-20.73,;38.99,-22.27,;39.07,-14.59,;40.39,-15.37,;41.72,-14.62,;41.74,-13.09,;40.42,-12.31,;39.1,-13.06,;37.76,-12.27,;36.42,-13.05,;35.1,-12.27,;43.08,-12.32,;44.4,-13.1,;45.75,-12.33,;43.09,-10.78,;44.42,-10.02,)| Show InChI InChI=1S/C67H78N10O13S2/c1-6-74(7-2)50-24-27-55-60(41-50)90-61-42-51(75(8-3)9-4)25-28-56(61)65(55)57-29-26-52(92(84,85)86)43-62(57)91(82,83)69-32-35-87-37-39-89-40-38-88-36-34-76-45-49(72-73-76)44-68-63(78)30-31-64(79)71-58-18-14-33-77(59-19-13-12-17-54(58)59)67(81)47-20-22-48(23-21-47)70-66(80)53-16-11-10-15-46(53)5/h10-13,15-17,19-29,41-43,45,58,69H,6-9,14,18,30-40,44H2,1-5H3,(H3-,68,70,71,78,79,80,81,84,85,86) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 307 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UMR7200 CNRS/Universit£ de Strasbourg
Curated by ChEMBL
| Assay Description
Displacement of [3H]-AVP from human oxytocin receptor expressed in CHO cells after 30 mins |
J Med Chem 55: 8588-602 (2012)
Article DOI: 10.1021/jm3006146
BindingDB Entry DOI: 10.7270/Q28G8MT5 |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50397217

(CHEMBL2172293)Show SMILES CCN(CC)c1ccc2c(-c3ccc(cc3S([O-])(=O)=O)S(=O)(=O)NCCOCCOCCOCCn3cc(CNC(=O)CCC(=O)NC4CCCN(C(=O)c5ccc(NC(=O)c6ccccc6C)cc5)c5ccccc45)nn3)c3ccc(cc3oc2c1)=[N+](CC)CC |(48.61,-15.81,;47.28,-16.58,;45.94,-15.82,;45.93,-14.27,;47.27,-13.49,;44.6,-16.59,;43.27,-15.83,;41.95,-16.6,;41.95,-18.12,;40.62,-18.89,;39.3,-18.1,;39.31,-16.56,;37.99,-15.78,;36.66,-16.53,;36.62,-18.07,;37.95,-18.86,;37.93,-20.4,;36.58,-21.15,;39.41,-20,;38.68,-21.73,;35.33,-15.74,;36.09,-14.4,;34.23,-14.64,;33.98,-16.49,;32.66,-15.71,;31.32,-16.46,;29.99,-15.67,;28.65,-16.42,;27.32,-15.64,;25.98,-16.39,;24.66,-15.61,;23.31,-16.36,;21.98,-15.57,;20.64,-16.32,;19.32,-15.53,;17.97,-16.29,;16.49,-15.84,;15.62,-17.1,;14.08,-17.11,;13.3,-15.79,;11.75,-15.81,;11,-17.15,;10.97,-14.49,;9.42,-14.5,;8.63,-13.18,;9.39,-11.82,;7.09,-13.19,;6.22,-14.46,;6.98,-15.8,;6.4,-17.22,;4.91,-17.67,;3.64,-16.8,;2.31,-17.56,;.97,-16.78,;2.3,-19.1,;.95,-19.86,;.94,-21.41,;2.28,-22.17,;2.27,-23.71,;3.6,-24.49,;4.93,-23.73,;3.59,-26.03,;2.24,-26.81,;2.23,-28.34,;3.57,-29.12,;4.91,-28.35,;4.91,-26.81,;6.25,-26.04,;3.61,-21.43,;3.63,-19.89,;3.55,-15.26,;2.07,-14.8,;1.74,-13.28,;2.87,-12.23,;4.35,-12.69,;4.69,-14.21,;16.54,-18.33,;18,-17.83,;40.63,-20.43,;39.3,-21.18,;39.29,-22.72,;40.62,-23.49,;41.94,-22.73,;41.95,-21.21,;43.28,-20.44,;43.28,-18.89,;44.61,-18.12,;40.62,-25.03,;41.95,-25.81,;41.94,-27.35,;39.27,-25.8,;39.27,-27.34,)| Show InChI InChI=1S/C67H78N10O13S2/c1-6-74(7-2)50-24-27-55-60(41-50)90-61-42-51(75(8-3)9-4)25-28-56(61)65(55)57-29-26-52(43-62(57)92(84,85)86)91(82,83)69-32-35-87-37-39-89-40-38-88-36-34-76-45-49(72-73-76)44-68-63(78)30-31-64(79)71-58-18-14-33-77(59-19-13-12-17-54(58)59)67(81)47-20-22-48(23-21-47)70-66(80)53-16-11-10-15-46(53)5/h10-13,15-17,19-29,41-43,45,58,69H,6-9,14,18,30-40,44H2,1-5H3,(H3-,68,70,71,78,79,80,81,84,85,86) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 495 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UMR7200 CNRS/Universit£ de Strasbourg
Curated by ChEMBL
| Assay Description
Displacement of [3H]-AVP from human oxytocin receptor expressed in CHO cells after 30 mins |
J Med Chem 55: 8588-602 (2012)
Article DOI: 10.1021/jm3006146
BindingDB Entry DOI: 10.7270/Q28G8MT5 |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(Homo sapiens (Human)) | BDBM50397206

(CHEMBL2172289)Show SMILES CCN1\C(=C\C=C\C=C\C2=[N+](CC)c3ccc(cc3C2(C)C)S([O-])(=O)=O)C(C)(CCCC(=O)NCCn2cc(CNC(=O)CCC(=O)NC3CCCN(C(=O)c4ccc(NC(=O)c5ccccc5-c5ccccc5)cc4)c4ccccc34)nn2)c2cc(ccc12)S(O)(=O)=O |c:9| Show InChI InChI=1S/C71H76N10O11S2/c1-6-79-61-36-34-52(93(87,88)89)44-57(61)70(3,4)63(79)27-12-9-13-28-64-71(5,58-45-53(94(90,91)92)35-37-62(58)80(64)7-2)40-18-29-65(82)72-41-43-78-47-51(76-77-78)46-73-66(83)38-39-67(84)75-59-25-19-42-81(60-26-17-16-24-56(59)60)69(86)49-30-32-50(33-31-49)74-68(85)55-23-15-14-22-54(55)48-20-10-8-11-21-48/h8-17,20-24,26-28,30-37,44-45,47,59H,6-7,18-19,25,29,38-43,46H2,1-5H3,(H5-,72,73,74,75,82,83,84,85,86,87,88,89,90,91,92) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 621 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UMR7200 CNRS/Universit£ de Strasbourg
Curated by ChEMBL
| Assay Description
Displacement of [3H]-AVP from human vasopressin V1a receptor expressed in CHO cells after 30 mins |
J Med Chem 55: 8588-602 (2012)
Article DOI: 10.1021/jm3006146
BindingDB Entry DOI: 10.7270/Q28G8MT5 |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM35714

(CHEMBL420762 | Mozavaptan | N-[4-(5-Dimethylamino-...)Show SMILES CN(C)C1CCCN(C(=O)c2ccc(NC(=O)c3ccccc3C)cc2)c2ccccc12 Show InChI InChI=1S/C27H29N3O2/c1-19-9-4-5-10-22(19)26(31)28-21-16-14-20(15-17-21)27(32)30-18-8-13-24(29(2)3)23-11-6-7-12-25(23)30/h4-7,9-12,14-17,24H,8,13,18H2,1-3H3,(H,28,31) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 660 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UMR7200 CNRS/Universit£ de Strasbourg
Curated by ChEMBL
| Assay Description
Binding affinity to oxytocin receptor |
J Med Chem 55: 8588-602 (2012)
Article DOI: 10.1021/jm3006146
BindingDB Entry DOI: 10.7270/Q28G8MT5 |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50397207

(CHEMBL2172288)Show SMILES OC(=O)c1cc(NC(=S)NCCOCCOCCOCCn2cc(CNC(=O)CCC(=O)NC3CCCN(C(=O)c4ccc(NC(=O)c5ccccc5-c5ccccc5)cc4)c4ccccc34)nn2)ccc1-c1c2ccc(O)cc2oc2cc(=O)ccc12 |(30.96,-29.16,;30.97,-27.63,;29.62,-28.36,;31.01,-26.09,;29.7,-25.29,;29.75,-23.75,;28.43,-22.94,;28.48,-21.4,;29.83,-20.67,;27.16,-20.6,;27.2,-19.06,;25.89,-18.25,;25.93,-16.71,;24.62,-15.91,;24.66,-14.37,;23.34,-13.56,;23.38,-12.02,;22.07,-11.22,;22.11,-9.68,;20.8,-8.87,;19.48,-9.65,;19.48,-11.19,;18.26,-12.12,;18.78,-13.58,;17.91,-14.85,;16.37,-14.85,;15.6,-16.19,;16.38,-17.52,;14.06,-16.19,;13.3,-17.54,;11.74,-17.54,;10.97,-16.2,;10.98,-18.88,;11.73,-20.18,;13.29,-20.17,;14.27,-21.36,;13.94,-22.87,;12.54,-23.56,;12.52,-25.13,;11.18,-25.89,;13.86,-25.9,;15.21,-25.14,;16.53,-25.91,;16.52,-27.45,;17.84,-28.22,;19.18,-27.45,;19.18,-25.91,;20.51,-28.23,;20.5,-29.78,;21.83,-30.55,;23.18,-29.78,;23.18,-28.24,;21.83,-27.46,;21.84,-25.92,;23.18,-25.16,;23.18,-23.63,;21.85,-22.85,;20.5,-23.63,;20.5,-25.16,;15.18,-28.22,;13.85,-27.45,;11.14,-22.9,;10.03,-23.98,;8.54,-23.56,;8.15,-22.04,;9.28,-20.96,;10.77,-21.39,;20.31,-13.54,;20.75,-12.06,;31.09,-23.01,;32.41,-23.81,;32.37,-25.36,;33.68,-26.15,;34.98,-25.34,;34.93,-23.82,;36.22,-23,;37.58,-23.72,;38.89,-22.91,;37.63,-25.26,;36.33,-26.06,;36.4,-27.61,;35.08,-28.42,;35.13,-29.95,;33.83,-30.76,;33.88,-32.3,;32.47,-30.03,;32.43,-28.5,;33.73,-27.69,)| Show InChI InChI=1S/C66H63N9O12S/c76-47-21-24-53-58(38-47)87-59-39-48(77)22-25-54(59)62(53)50-23-20-45(37-55(50)65(82)83)70-66(88)67-28-31-84-33-35-86-36-34-85-32-30-74-41-46(72-73-74)40-68-60(78)26-27-61(79)71-56-14-8-29-75(57-15-7-6-13-52(56)57)64(81)43-16-18-44(19-17-43)69-63(80)51-12-5-4-11-49(51)42-9-2-1-3-10-42/h1-7,9-13,15-25,37-39,41,56,76H,8,14,26-36,40H2,(H,68,78)(H,69,80)(H,71,79)(H,82,83)(H2,67,70,88) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 665 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UMR7200 CNRS/Universit£ de Strasbourg
Curated by ChEMBL
| Assay Description
Displacement of [3H]-AVP from human oxytocin receptor expressed in CHO cells after 30 mins |
J Med Chem 55: 8588-602 (2012)
Article DOI: 10.1021/jm3006146
BindingDB Entry DOI: 10.7270/Q28G8MT5 |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50397210

(CHEMBL2172290)Show SMILES CCN1\C(=C\C=C\C=C\C2=[N+](CC)c3ccc(cc3C2(C)C)S([O-])(=O)=O)C(C)(CCCC(=O)NCCOCCOCCOCCn2cc(CNC(=O)CCC(=O)NC3CCCN(C(=O)c4ccc(NC(=O)c5ccccc5-c5ccccc5)cc4)c4ccccc34)nn2)c2cc(ccc12)S(O)(=O)=O |c:9| Show InChI InChI=1S/C77H88N10O14S2/c1-6-85-67-36-34-58(102(93,94)95)50-63(67)76(3,4)69(85)27-12-9-13-28-70-77(5,64-51-59(103(96,97)98)35-37-68(64)86(70)7-2)40-18-29-71(88)78-41-44-99-46-48-101-49-47-100-45-43-84-53-57(82-83-84)52-79-72(89)38-39-73(90)81-65-25-19-42-87(66-26-17-16-24-62(65)66)75(92)55-30-32-56(33-31-55)80-74(91)61-23-15-14-22-60(61)54-20-10-8-11-21-54/h8-17,20-24,26-28,30-37,50-51,53,65H,6-7,18-19,25,29,38-49,52H2,1-5H3,(H5-,78,79,80,81,88,89,90,91,92,93,94,95,96,97,98) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 808 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UMR7200 CNRS/Universit£ de Strasbourg
Curated by ChEMBL
| Assay Description
Displacement of [3H]-AVP from human oxytocin receptor expressed in CHO cells after 30 mins |
J Med Chem 55: 8588-602 (2012)
Article DOI: 10.1021/jm3006146
BindingDB Entry DOI: 10.7270/Q28G8MT5 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1
(Homo sapiens (Human)) | BDBM50397220

(CHEMBL2172286)Show SMILES F[B-]1(F)n2c(CC3C=CC(CCC(=O)NCCN4CCN(CC(=O)N5c6ccccc6C(=O)Nc6cccnc56)CC4)=[N+]13)ccc2-c1cccs1 |c:7,t:45| Show InChI InChI=1S/C36H37BF2N8O3S/c38-37(39)46-25(9-10-26(46)23-27-11-13-31(47(27)37)32-8-4-22-51-32)12-14-33(48)40-16-17-43-18-20-44(21-19-43)24-34(49)45-30-7-2-1-5-28(30)36(50)42-29-6-3-15-41-35(29)45/h1-11,13,15,22,26H,12,14,16-21,23-24H2,(H,40,48)(H,42,50) | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UMR7200 CNRS/Universit£ de Strasbourg
Curated by ChEMBL
| Assay Description
Binding affinity to human muscarinic M1 receptor |
J Med Chem 55: 8588-602 (2012)
Article DOI: 10.1021/jm3006146
BindingDB Entry DOI: 10.7270/Q28G8MT5 |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(Homo sapiens (Human)) | BDBM50397210

(CHEMBL2172290)Show SMILES CCN1\C(=C\C=C\C=C\C2=[N+](CC)c3ccc(cc3C2(C)C)S([O-])(=O)=O)C(C)(CCCC(=O)NCCOCCOCCOCCn2cc(CNC(=O)CCC(=O)NC3CCCN(C(=O)c4ccc(NC(=O)c5ccccc5-c5ccccc5)cc4)c4ccccc34)nn2)c2cc(ccc12)S(O)(=O)=O |c:9| Show InChI InChI=1S/C77H88N10O14S2/c1-6-85-67-36-34-58(102(93,94)95)50-63(67)76(3,4)69(85)27-12-9-13-28-70-77(5,64-51-59(103(96,97)98)35-37-68(64)86(70)7-2)40-18-29-71(88)78-41-44-99-46-48-101-49-47-100-45-43-84-53-57(82-83-84)52-79-72(89)38-39-73(90)81-65-25-19-42-87(66-26-17-16-24-62(65)66)75(92)55-30-32-56(33-31-55)80-74(91)61-23-15-14-22-60(61)54-20-10-8-11-21-54/h8-17,20-24,26-28,30-37,50-51,53,65H,6-7,18-19,25,29,38-49,52H2,1-5H3,(H5-,78,79,80,81,88,89,90,91,92,93,94,95,96,97,98) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.75E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UMR7200 CNRS/Universit£ de Strasbourg
Curated by ChEMBL
| Assay Description
Displacement of [3H]-AVP from human vasopressin V1a receptor expressed in CHO cells after 30 mins |
J Med Chem 55: 8588-602 (2012)
Article DOI: 10.1021/jm3006146
BindingDB Entry DOI: 10.7270/Q28G8MT5 |
More data for this
Ligand-Target Pair | |
Vasopressin V1b receptor
(Homo sapiens (Human)) | BDBM50397218

(CHEMBL2172292)Show SMILES CCN(CC)c1ccc2c(-c3ccc(cc3S(=O)(=O)NCCOCCOCCOCCn3cc(CNC(=O)CCC(=O)NC4CCCN(C(=O)c5ccc(NC(=O)c6ccccc6C)cc5)c5ccccc45)nn3)S([O-])(=O)=O)c3ccc(cc3oc2c1)=[N+](CC)CC |(31.1,-9.95,;32.44,-10.72,;32.43,-12.26,;31.09,-13.03,;29.76,-12.25,;33.76,-13.04,;33.76,-14.58,;35.09,-15.34,;36.42,-14.58,;37.74,-15.36,;37.73,-16.9,;39.05,-17.68,;39.03,-19.23,;37.69,-19.98,;36.37,-19.19,;36.39,-17.65,;35.07,-16.86,;33.96,-15.76,;35.83,-15.52,;33.72,-17.62,;32.4,-16.82,;31.05,-17.59,;29.72,-16.79,;28.38,-17.55,;27.06,-16.76,;25.71,-17.51,;24.39,-16.73,;23.04,-17.48,;21.72,-16.69,;20.37,-17.44,;19.05,-16.65,;17.71,-17.41,;16.23,-16.96,;15.35,-18.22,;13.81,-18.23,;13.03,-16.91,;11.49,-16.92,;10.73,-18.27,;10.7,-15.6,;9.16,-15.61,;8.37,-14.28,;9.13,-12.95,;6.83,-14.31,;5.95,-15.57,;6.71,-16.9,;6.13,-18.33,;4.63,-18.77,;3.38,-17.9,;2.03,-18.66,;.71,-17.87,;2.02,-20.2,;3.35,-20.98,;3.33,-22.52,;2,-23.27,;1.98,-24.82,;3.31,-25.59,;4.65,-24.84,;3.3,-27.14,;1.95,-27.9,;1.95,-29.44,;3.28,-30.22,;4.61,-29.45,;4.62,-27.91,;5.97,-27.15,;.66,-22.5,;.68,-20.96,;3.28,-16.36,;1.81,-15.9,;1.47,-14.39,;2.62,-13.33,;4.09,-13.8,;4.44,-15.31,;16.27,-19.45,;17.73,-18.96,;37.67,-21.51,;36.33,-22.26,;38.99,-20.73,;38.99,-22.27,;39.07,-14.59,;40.39,-15.37,;41.72,-14.62,;41.74,-13.09,;40.42,-12.31,;39.1,-13.06,;37.76,-12.27,;36.42,-13.05,;35.1,-12.27,;43.08,-12.32,;44.4,-13.1,;45.75,-12.33,;43.09,-10.78,;44.42,-10.02,)| Show InChI InChI=1S/C67H78N10O13S2/c1-6-74(7-2)50-24-27-55-60(41-50)90-61-42-51(75(8-3)9-4)25-28-56(61)65(55)57-29-26-52(92(84,85)86)43-62(57)91(82,83)69-32-35-87-37-39-89-40-38-88-36-34-76-45-49(72-73-76)44-68-63(78)30-31-64(79)71-58-18-14-33-77(59-19-13-12-17-54(58)59)67(81)47-20-22-48(23-21-47)70-66(80)53-16-11-10-15-46(53)5/h10-13,15-17,19-29,41-43,45,58,69H,6-9,14,18,30-40,44H2,1-5H3,(H3-,68,70,71,78,79,80,81,84,85,86) | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UMR7200 CNRS/Universit£ de Strasbourg
Curated by ChEMBL
| Assay Description
Displacement of [3H]-AVP from human vasopressin V1b receptor expressed in CHO cells after 30 mins |
J Med Chem 55: 8588-602 (2012)
Article DOI: 10.1021/jm3006146
BindingDB Entry DOI: 10.7270/Q28G8MT5 |
More data for this
Ligand-Target Pair | |
Vasopressin V1b receptor
(Homo sapiens (Human)) | BDBM50397217

(CHEMBL2172293)Show SMILES CCN(CC)c1ccc2c(-c3ccc(cc3S([O-])(=O)=O)S(=O)(=O)NCCOCCOCCOCCn3cc(CNC(=O)CCC(=O)NC4CCCN(C(=O)c5ccc(NC(=O)c6ccccc6C)cc5)c5ccccc45)nn3)c3ccc(cc3oc2c1)=[N+](CC)CC |(48.61,-15.81,;47.28,-16.58,;45.94,-15.82,;45.93,-14.27,;47.27,-13.49,;44.6,-16.59,;43.27,-15.83,;41.95,-16.6,;41.95,-18.12,;40.62,-18.89,;39.3,-18.1,;39.31,-16.56,;37.99,-15.78,;36.66,-16.53,;36.62,-18.07,;37.95,-18.86,;37.93,-20.4,;36.58,-21.15,;39.41,-20,;38.68,-21.73,;35.33,-15.74,;36.09,-14.4,;34.23,-14.64,;33.98,-16.49,;32.66,-15.71,;31.32,-16.46,;29.99,-15.67,;28.65,-16.42,;27.32,-15.64,;25.98,-16.39,;24.66,-15.61,;23.31,-16.36,;21.98,-15.57,;20.64,-16.32,;19.32,-15.53,;17.97,-16.29,;16.49,-15.84,;15.62,-17.1,;14.08,-17.11,;13.3,-15.79,;11.75,-15.81,;11,-17.15,;10.97,-14.49,;9.42,-14.5,;8.63,-13.18,;9.39,-11.82,;7.09,-13.19,;6.22,-14.46,;6.98,-15.8,;6.4,-17.22,;4.91,-17.67,;3.64,-16.8,;2.31,-17.56,;.97,-16.78,;2.3,-19.1,;.95,-19.86,;.94,-21.41,;2.28,-22.17,;2.27,-23.71,;3.6,-24.49,;4.93,-23.73,;3.59,-26.03,;2.24,-26.81,;2.23,-28.34,;3.57,-29.12,;4.91,-28.35,;4.91,-26.81,;6.25,-26.04,;3.61,-21.43,;3.63,-19.89,;3.55,-15.26,;2.07,-14.8,;1.74,-13.28,;2.87,-12.23,;4.35,-12.69,;4.69,-14.21,;16.54,-18.33,;18,-17.83,;40.63,-20.43,;39.3,-21.18,;39.29,-22.72,;40.62,-23.49,;41.94,-22.73,;41.95,-21.21,;43.28,-20.44,;43.28,-18.89,;44.61,-18.12,;40.62,-25.03,;41.95,-25.81,;41.94,-27.35,;39.27,-25.8,;39.27,-27.34,)| Show InChI InChI=1S/C67H78N10O13S2/c1-6-74(7-2)50-24-27-55-60(41-50)90-61-42-51(75(8-3)9-4)25-28-56(61)65(55)57-29-26-52(43-62(57)92(84,85)86)91(82,83)69-32-35-87-37-39-89-40-38-88-36-34-76-45-49(72-73-76)44-68-63(78)30-31-64(79)71-58-18-14-33-77(59-19-13-12-17-54(58)59)67(81)47-20-22-48(23-21-47)70-66(80)53-16-11-10-15-46(53)5/h10-13,15-17,19-29,41-43,45,58,69H,6-9,14,18,30-40,44H2,1-5H3,(H3-,68,70,71,78,79,80,81,84,85,86) | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UMR7200 CNRS/Universit£ de Strasbourg
Curated by ChEMBL
| Assay Description
Displacement of [3H]-AVP from human vasopressin V1b receptor expressed in CHO cells after 30 mins |
J Med Chem 55: 8588-602 (2012)
Article DOI: 10.1021/jm3006146
BindingDB Entry DOI: 10.7270/Q28G8MT5 |
More data for this
Ligand-Target Pair | |
Vasopressin V1b receptor
(Homo sapiens (Human)) | BDBM50397215

(CHEMBL2172295)Show SMILES CCN(CC)c1ccc2c(-c3ccc(cc3S([O-])(=O)=O)S(=O)(=O)NCCOCCOCCOCCn3cc(CNC(=O)CCC(=O)NC4CCCN(C(=O)c5ccc(NC(=O)c6ccccc6-c6ccccc6)cc5)c5ccccc45)nn3)c3ccc(cc3oc2c1)=[N+](CC)CC |(48.6,-20.67,;47.27,-21.45,;45.93,-20.68,;45.92,-19.14,;47.25,-18.37,;44.59,-21.45,;43.26,-20.69,;41.94,-21.46,;41.94,-22.99,;40.61,-23.75,;39.29,-22.97,;39.3,-21.42,;37.98,-20.64,;36.65,-21.39,;36.62,-22.93,;37.94,-23.72,;37.92,-25.26,;36.57,-26.02,;39.4,-24.87,;38.67,-26.59,;35.32,-20.6,;36.08,-19.26,;34.22,-19.5,;33.98,-21.36,;32.65,-20.58,;31.31,-21.33,;29.99,-20.54,;28.64,-21.29,;27.31,-20.5,;25.97,-21.25,;24.65,-20.47,;23.3,-21.22,;21.97,-20.44,;20.63,-21.19,;19.3,-20.4,;18,-21.14,;16.52,-20.66,;15.61,-21.92,;14.07,-21.9,;13.31,-20.56,;11.77,-20.54,;10.99,-21.87,;11.01,-19.19,;9.47,-19.18,;8.72,-17.84,;9.5,-16.52,;7.18,-17.83,;6.27,-19.07,;6.99,-20.43,;6.39,-21.83,;4.89,-22.24,;3.64,-21.35,;2.28,-22.08,;.97,-21.27,;2.24,-23.62,;.88,-24.35,;.83,-25.89,;2.16,-26.69,;2.11,-28.24,;3.43,-29.04,;4.78,-28.31,;3.38,-30.58,;2.01,-31.32,;1.97,-32.86,;3.29,-33.66,;4.65,-32.92,;4.69,-31.38,;6.04,-30.65,;7.35,-31.45,;8.7,-30.73,;8.75,-29.19,;7.42,-28.37,;6.07,-29.11,;3.51,-25.97,;3.55,-24.44,;3.58,-19.81,;2.12,-19.32,;1.81,-17.8,;2.98,-16.77,;4.45,-17.27,;4.75,-18.79,;16.53,-23.17,;18,-22.69,;40.62,-25.3,;39.29,-26.05,;39.27,-27.58,;40.61,-28.36,;41.93,-27.6,;41.94,-26.07,;43.27,-25.3,;43.27,-23.76,;44.6,-22.99,;40.61,-29.9,;41.94,-30.67,;41.94,-32.21,;39.26,-30.67,;39.26,-32.21,)| Show InChI InChI=1S/C72H80N10O13S2/c1-5-79(6-2)54-28-31-60-65(45-54)95-66-46-55(80(7-3)8-4)29-32-61(66)70(60)62-33-30-56(47-67(62)97(89,90)91)96(87,88)74-36-39-92-41-43-94-44-42-93-40-38-81-49-53(77-78-81)48-73-68(83)34-35-69(84)76-63-22-16-37-82(64-23-15-14-21-59(63)64)72(86)51-24-26-52(27-25-51)75-71(85)58-20-13-12-19-57(58)50-17-10-9-11-18-50/h9-15,17-21,23-33,45-47,49,63,74H,5-8,16,22,34-44,48H2,1-4H3,(H3-,73,75,76,83,84,85,86,89,90,91) | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UMR7200 CNRS/Universit£ de Strasbourg
Curated by ChEMBL
| Assay Description
Displacement of [3H]-AVP from human vasopressin V1b receptor expressed in CHO cells after 30 mins |
J Med Chem 55: 8588-602 (2012)
Article DOI: 10.1021/jm3006146
BindingDB Entry DOI: 10.7270/Q28G8MT5 |
More data for this
Ligand-Target Pair | |
Vasopressin V1b receptor
(Homo sapiens (Human)) | BDBM50397207

(CHEMBL2172288)Show SMILES OC(=O)c1cc(NC(=S)NCCOCCOCCOCCn2cc(CNC(=O)CCC(=O)NC3CCCN(C(=O)c4ccc(NC(=O)c5ccccc5-c5ccccc5)cc4)c4ccccc34)nn2)ccc1-c1c2ccc(O)cc2oc2cc(=O)ccc12 |(30.96,-29.16,;30.97,-27.63,;29.62,-28.36,;31.01,-26.09,;29.7,-25.29,;29.75,-23.75,;28.43,-22.94,;28.48,-21.4,;29.83,-20.67,;27.16,-20.6,;27.2,-19.06,;25.89,-18.25,;25.93,-16.71,;24.62,-15.91,;24.66,-14.37,;23.34,-13.56,;23.38,-12.02,;22.07,-11.22,;22.11,-9.68,;20.8,-8.87,;19.48,-9.65,;19.48,-11.19,;18.26,-12.12,;18.78,-13.58,;17.91,-14.85,;16.37,-14.85,;15.6,-16.19,;16.38,-17.52,;14.06,-16.19,;13.3,-17.54,;11.74,-17.54,;10.97,-16.2,;10.98,-18.88,;11.73,-20.18,;13.29,-20.17,;14.27,-21.36,;13.94,-22.87,;12.54,-23.56,;12.52,-25.13,;11.18,-25.89,;13.86,-25.9,;15.21,-25.14,;16.53,-25.91,;16.52,-27.45,;17.84,-28.22,;19.18,-27.45,;19.18,-25.91,;20.51,-28.23,;20.5,-29.78,;21.83,-30.55,;23.18,-29.78,;23.18,-28.24,;21.83,-27.46,;21.84,-25.92,;23.18,-25.16,;23.18,-23.63,;21.85,-22.85,;20.5,-23.63,;20.5,-25.16,;15.18,-28.22,;13.85,-27.45,;11.14,-22.9,;10.03,-23.98,;8.54,-23.56,;8.15,-22.04,;9.28,-20.96,;10.77,-21.39,;20.31,-13.54,;20.75,-12.06,;31.09,-23.01,;32.41,-23.81,;32.37,-25.36,;33.68,-26.15,;34.98,-25.34,;34.93,-23.82,;36.22,-23,;37.58,-23.72,;38.89,-22.91,;37.63,-25.26,;36.33,-26.06,;36.4,-27.61,;35.08,-28.42,;35.13,-29.95,;33.83,-30.76,;33.88,-32.3,;32.47,-30.03,;32.43,-28.5,;33.73,-27.69,)| Show InChI InChI=1S/C66H63N9O12S/c76-47-21-24-53-58(38-47)87-59-39-48(77)22-25-54(59)62(53)50-23-20-45(37-55(50)65(82)83)70-66(88)67-28-31-84-33-35-86-36-34-85-32-30-74-41-46(72-73-74)40-68-60(78)26-27-61(79)71-56-14-8-29-75(57-15-7-6-13-52(56)57)64(81)43-16-18-44(19-17-43)69-63(80)51-12-5-4-11-49(51)42-9-2-1-3-10-42/h1-7,9-13,15-25,37-39,41,56,76H,8,14,26-36,40H2,(H,68,78)(H,69,80)(H,71,79)(H,82,83)(H2,67,70,88) | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UMR7200 CNRS/Universit£ de Strasbourg
Curated by ChEMBL
| Assay Description
Displacement of [3H]-AVP from human vasopressin V1b receptor expressed in CHO cells after 30 mins |
J Med Chem 55: 8588-602 (2012)
Article DOI: 10.1021/jm3006146
BindingDB Entry DOI: 10.7270/Q28G8MT5 |
More data for this
Ligand-Target Pair | |
Vasopressin V1b receptor
(Homo sapiens (Human)) | BDBM50397206

(CHEMBL2172289)Show SMILES CCN1\C(=C\C=C\C=C\C2=[N+](CC)c3ccc(cc3C2(C)C)S([O-])(=O)=O)C(C)(CCCC(=O)NCCn2cc(CNC(=O)CCC(=O)NC3CCCN(C(=O)c4ccc(NC(=O)c5ccccc5-c5ccccc5)cc4)c4ccccc34)nn2)c2cc(ccc12)S(O)(=O)=O |c:9| Show InChI InChI=1S/C71H76N10O11S2/c1-6-79-61-36-34-52(93(87,88)89)44-57(61)70(3,4)63(79)27-12-9-13-28-64-71(5,58-45-53(94(90,91)92)35-37-62(58)80(64)7-2)40-18-29-65(82)72-41-43-78-47-51(76-77-78)46-73-66(83)38-39-67(84)75-59-25-19-42-81(60-26-17-16-24-56(59)60)69(86)49-30-32-50(33-31-49)74-68(85)55-23-15-14-22-54(55)48-20-10-8-11-21-48/h8-17,20-24,26-28,30-37,44-45,47,59H,6-7,18-19,25,29,38-43,46H2,1-5H3,(H5-,72,73,74,75,82,83,84,85,86,87,88,89,90,91,92) | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UMR7200 CNRS/Universit£ de Strasbourg
Curated by ChEMBL
| Assay Description
Displacement of [3H]-AVP from human vasopressin V1b receptor expressed in CHO cells after 30 mins |
J Med Chem 55: 8588-602 (2012)
Article DOI: 10.1021/jm3006146
BindingDB Entry DOI: 10.7270/Q28G8MT5 |
More data for this
Ligand-Target Pair | |
Vasopressin V1b receptor
(Homo sapiens (Human)) | BDBM50397210

(CHEMBL2172290)Show SMILES CCN1\C(=C\C=C\C=C\C2=[N+](CC)c3ccc(cc3C2(C)C)S([O-])(=O)=O)C(C)(CCCC(=O)NCCOCCOCCOCCn2cc(CNC(=O)CCC(=O)NC3CCCN(C(=O)c4ccc(NC(=O)c5ccccc5-c5ccccc5)cc4)c4ccccc34)nn2)c2cc(ccc12)S(O)(=O)=O |c:9| Show InChI InChI=1S/C77H88N10O14S2/c1-6-85-67-36-34-58(102(93,94)95)50-63(67)76(3,4)69(85)27-12-9-13-28-70-77(5,64-51-59(103(96,97)98)35-37-68(64)86(70)7-2)40-18-29-71(88)78-41-44-99-46-48-101-49-47-100-45-43-84-53-57(82-83-84)52-79-72(89)38-39-73(90)81-65-25-19-42-87(66-26-17-16-24-62(65)66)75(92)55-30-32-56(33-31-55)80-74(91)61-23-15-14-22-60(61)54-20-10-8-11-21-54/h8-17,20-24,26-28,30-37,50-51,53,65H,6-7,18-19,25,29,38-49,52H2,1-5H3,(H5-,78,79,80,81,88,89,90,91,92,93,94,95,96,97,98) | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UMR7200 CNRS/Universit£ de Strasbourg
Curated by ChEMBL
| Assay Description
Displacement of [3H]-AVP from human vasopressin V1b receptor expressed in CHO cells after 30 mins |
J Med Chem 55: 8588-602 (2012)
Article DOI: 10.1021/jm3006146
BindingDB Entry DOI: 10.7270/Q28G8MT5 |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50397216

(CHEMBL2172294)Show SMILES CCN(CC)c1ccc2c(-c3ccc(cc3S(=O)(=O)NCCOCCOCCOCCn3cc(CNC(=O)CCC(=O)NC4CCCN(C(=O)c5ccc(NC(=O)c6ccccc6-c6ccccc6)cc5)c5ccccc45)nn3)S([O-])(=O)=O)c3ccc(cc3oc2c1)=[N+](CC)CC |(31.09,-9.94,;32.43,-10.72,;32.42,-12.26,;31.09,-13.02,;29.76,-12.25,;33.76,-13.04,;33.76,-14.58,;35.09,-15.33,;36.41,-14.58,;37.73,-15.35,;37.72,-16.89,;39.04,-17.68,;39.02,-19.23,;37.68,-19.97,;36.36,-19.19,;36.38,-17.65,;35.06,-16.86,;33.95,-15.76,;35.82,-15.52,;33.71,-17.61,;32.39,-16.82,;31.04,-17.58,;29.72,-16.79,;28.38,-17.54,;27.05,-16.75,;25.71,-17.51,;24.38,-16.72,;23.04,-17.48,;21.71,-16.69,;20.36,-17.44,;19.04,-16.65,;17.7,-17.4,;16.23,-16.95,;15.34,-18.21,;13.8,-18.23,;13.02,-16.9,;11.48,-16.92,;10.72,-18.26,;10.69,-15.59,;9.15,-15.61,;8.37,-14.28,;9.13,-12.94,;6.83,-14.3,;5.95,-15.57,;6.71,-16.9,;6.12,-18.32,;4.63,-18.76,;3.38,-17.89,;2.03,-18.65,;.71,-17.87,;2.02,-20.19,;3.35,-20.98,;3.33,-22.52,;2,-23.26,;1.98,-24.81,;3.31,-25.58,;4.65,-24.83,;3.3,-27.13,;1.95,-27.9,;1.95,-29.44,;3.28,-30.21,;4.61,-29.44,;4.62,-27.91,;5.97,-27.14,;7.3,-27.94,;8.65,-27.18,;8.67,-25.62,;7.32,-24.83,;5.98,-25.6,;.66,-22.49,;.68,-20.96,;3.28,-16.35,;1.81,-15.9,;1.47,-14.39,;2.62,-13.33,;4.09,-13.8,;4.43,-15.31,;16.27,-19.44,;17.72,-18.95,;37.66,-21.51,;36.32,-22.25,;38.98,-20.73,;38.98,-22.27,;39.07,-14.59,;40.38,-15.37,;41.71,-14.62,;41.73,-13.08,;40.41,-12.3,;39.09,-13.06,;37.75,-12.27,;36.41,-13.04,;35.09,-12.27,;43.07,-12.31,;44.39,-13.1,;45.74,-12.33,;43.08,-10.77,;44.41,-10.02,)| Show InChI InChI=1S/C72H80N10O13S2/c1-5-79(6-2)54-28-31-60-65(45-54)95-66-46-55(80(7-3)8-4)29-32-61(66)70(60)62-33-30-56(97(89,90)91)47-67(62)96(87,88)74-36-39-92-41-43-94-44-42-93-40-38-81-49-53(77-78-81)48-73-68(83)34-35-69(84)76-63-22-16-37-82(64-23-15-14-21-59(63)64)72(86)51-24-26-52(27-25-51)75-71(85)58-20-13-12-19-57(58)50-17-10-9-11-18-50/h9-15,17-21,23-33,45-47,49,63,74H,5-8,16,22,34-44,48H2,1-4H3,(H3-,73,75,76,83,84,85,86,89,90,91) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UMR7200 CNRS/Universit£ de Strasbourg
Curated by ChEMBL
| Assay Description
Displacement of [3H]-AVP from human oxytocin receptor expressed in CHO cells after 30 mins |
J Med Chem 55: 8588-602 (2012)
Article DOI: 10.1021/jm3006146
BindingDB Entry DOI: 10.7270/Q28G8MT5 |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50397215

(CHEMBL2172295)Show SMILES CCN(CC)c1ccc2c(-c3ccc(cc3S([O-])(=O)=O)S(=O)(=O)NCCOCCOCCOCCn3cc(CNC(=O)CCC(=O)NC4CCCN(C(=O)c5ccc(NC(=O)c6ccccc6-c6ccccc6)cc5)c5ccccc45)nn3)c3ccc(cc3oc2c1)=[N+](CC)CC |(48.6,-20.67,;47.27,-21.45,;45.93,-20.68,;45.92,-19.14,;47.25,-18.37,;44.59,-21.45,;43.26,-20.69,;41.94,-21.46,;41.94,-22.99,;40.61,-23.75,;39.29,-22.97,;39.3,-21.42,;37.98,-20.64,;36.65,-21.39,;36.62,-22.93,;37.94,-23.72,;37.92,-25.26,;36.57,-26.02,;39.4,-24.87,;38.67,-26.59,;35.32,-20.6,;36.08,-19.26,;34.22,-19.5,;33.98,-21.36,;32.65,-20.58,;31.31,-21.33,;29.99,-20.54,;28.64,-21.29,;27.31,-20.5,;25.97,-21.25,;24.65,-20.47,;23.3,-21.22,;21.97,-20.44,;20.63,-21.19,;19.3,-20.4,;18,-21.14,;16.52,-20.66,;15.61,-21.92,;14.07,-21.9,;13.31,-20.56,;11.77,-20.54,;10.99,-21.87,;11.01,-19.19,;9.47,-19.18,;8.72,-17.84,;9.5,-16.52,;7.18,-17.83,;6.27,-19.07,;6.99,-20.43,;6.39,-21.83,;4.89,-22.24,;3.64,-21.35,;2.28,-22.08,;.97,-21.27,;2.24,-23.62,;.88,-24.35,;.83,-25.89,;2.16,-26.69,;2.11,-28.24,;3.43,-29.04,;4.78,-28.31,;3.38,-30.58,;2.01,-31.32,;1.97,-32.86,;3.29,-33.66,;4.65,-32.92,;4.69,-31.38,;6.04,-30.65,;7.35,-31.45,;8.7,-30.73,;8.75,-29.19,;7.42,-28.37,;6.07,-29.11,;3.51,-25.97,;3.55,-24.44,;3.58,-19.81,;2.12,-19.32,;1.81,-17.8,;2.98,-16.77,;4.45,-17.27,;4.75,-18.79,;16.53,-23.17,;18,-22.69,;40.62,-25.3,;39.29,-26.05,;39.27,-27.58,;40.61,-28.36,;41.93,-27.6,;41.94,-26.07,;43.27,-25.3,;43.27,-23.76,;44.6,-22.99,;40.61,-29.9,;41.94,-30.67,;41.94,-32.21,;39.26,-30.67,;39.26,-32.21,)| Show InChI InChI=1S/C72H80N10O13S2/c1-5-79(6-2)54-28-31-60-65(45-54)95-66-46-55(80(7-3)8-4)29-32-61(66)70(60)62-33-30-56(47-67(62)97(89,90)91)96(87,88)74-36-39-92-41-43-94-44-42-93-40-38-81-49-53(77-78-81)48-73-68(83)34-35-69(84)76-63-22-16-37-82(64-23-15-14-21-59(63)64)72(86)51-24-26-52(27-25-51)75-71(85)58-20-13-12-19-57(58)50-17-10-9-11-18-50/h9-15,17-21,23-33,45-47,49,63,74H,5-8,16,22,34-44,48H2,1-4H3,(H3-,73,75,76,83,84,85,86,89,90,91) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UMR7200 CNRS/Universit£ de Strasbourg
Curated by ChEMBL
| Assay Description
Displacement of [3H]-AVP from human oxytocin receptor expressed in CHO cells after 30 mins |
J Med Chem 55: 8588-602 (2012)
Article DOI: 10.1021/jm3006146
BindingDB Entry DOI: 10.7270/Q28G8MT5 |
More data for this
Ligand-Target Pair | |
Vasopressin V1b receptor
(Homo sapiens (Human)) | BDBM50397216

(CHEMBL2172294)Show SMILES CCN(CC)c1ccc2c(-c3ccc(cc3S(=O)(=O)NCCOCCOCCOCCn3cc(CNC(=O)CCC(=O)NC4CCCN(C(=O)c5ccc(NC(=O)c6ccccc6-c6ccccc6)cc5)c5ccccc45)nn3)S([O-])(=O)=O)c3ccc(cc3oc2c1)=[N+](CC)CC |(31.09,-9.94,;32.43,-10.72,;32.42,-12.26,;31.09,-13.02,;29.76,-12.25,;33.76,-13.04,;33.76,-14.58,;35.09,-15.33,;36.41,-14.58,;37.73,-15.35,;37.72,-16.89,;39.04,-17.68,;39.02,-19.23,;37.68,-19.97,;36.36,-19.19,;36.38,-17.65,;35.06,-16.86,;33.95,-15.76,;35.82,-15.52,;33.71,-17.61,;32.39,-16.82,;31.04,-17.58,;29.72,-16.79,;28.38,-17.54,;27.05,-16.75,;25.71,-17.51,;24.38,-16.72,;23.04,-17.48,;21.71,-16.69,;20.36,-17.44,;19.04,-16.65,;17.7,-17.4,;16.23,-16.95,;15.34,-18.21,;13.8,-18.23,;13.02,-16.9,;11.48,-16.92,;10.72,-18.26,;10.69,-15.59,;9.15,-15.61,;8.37,-14.28,;9.13,-12.94,;6.83,-14.3,;5.95,-15.57,;6.71,-16.9,;6.12,-18.32,;4.63,-18.76,;3.38,-17.89,;2.03,-18.65,;.71,-17.87,;2.02,-20.19,;3.35,-20.98,;3.33,-22.52,;2,-23.26,;1.98,-24.81,;3.31,-25.58,;4.65,-24.83,;3.3,-27.13,;1.95,-27.9,;1.95,-29.44,;3.28,-30.21,;4.61,-29.44,;4.62,-27.91,;5.97,-27.14,;7.3,-27.94,;8.65,-27.18,;8.67,-25.62,;7.32,-24.83,;5.98,-25.6,;.66,-22.49,;.68,-20.96,;3.28,-16.35,;1.81,-15.9,;1.47,-14.39,;2.62,-13.33,;4.09,-13.8,;4.43,-15.31,;16.27,-19.44,;17.72,-18.95,;37.66,-21.51,;36.32,-22.25,;38.98,-20.73,;38.98,-22.27,;39.07,-14.59,;40.38,-15.37,;41.71,-14.62,;41.73,-13.08,;40.41,-12.3,;39.09,-13.06,;37.75,-12.27,;36.41,-13.04,;35.09,-12.27,;43.07,-12.31,;44.39,-13.1,;45.74,-12.33,;43.08,-10.77,;44.41,-10.02,)| Show InChI InChI=1S/C72H80N10O13S2/c1-5-79(6-2)54-28-31-60-65(45-54)95-66-46-55(80(7-3)8-4)29-32-61(66)70(60)62-33-30-56(97(89,90)91)47-67(62)96(87,88)74-36-39-92-41-43-94-44-42-93-40-38-81-49-53(77-78-81)48-73-68(83)34-35-69(84)76-63-22-16-37-82(64-23-15-14-21-59(63)64)72(86)51-24-26-52(27-25-51)75-71(85)58-20-13-12-19-57(58)50-17-10-9-11-18-50/h9-15,17-21,23-33,45-47,49,63,74H,5-8,16,22,34-44,48H2,1-4H3,(H3-,73,75,76,83,84,85,86,89,90,91) | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UMR7200 CNRS/Universit£ de Strasbourg
Curated by ChEMBL
| Assay Description
Displacement of [3H]-AVP from human vasopressin V1b receptor expressed in CHO cells after 30 mins |
J Med Chem 55: 8588-602 (2012)
Article DOI: 10.1021/jm3006146
BindingDB Entry DOI: 10.7270/Q28G8MT5 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

















































