

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50143951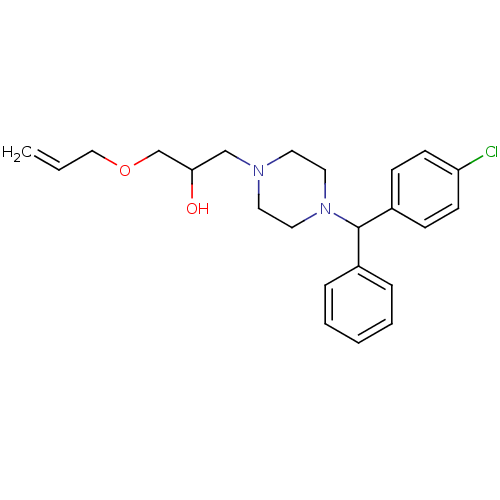 (1-Allyloxy-3-{4-[(4-chloro-phenyl)-phenyl-methyl]-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ArQule Inc Curated by ChEMBL | Assay Description Predictive competitive inhibition of cytochrome P450 2D6 was determined in silico | Bioorg Med Chem Lett 14: 2025-30 (2004) Article DOI: 10.1016/j.bmcl.2004.02.078 BindingDB Entry DOI: 10.7270/Q22J6B8R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50143948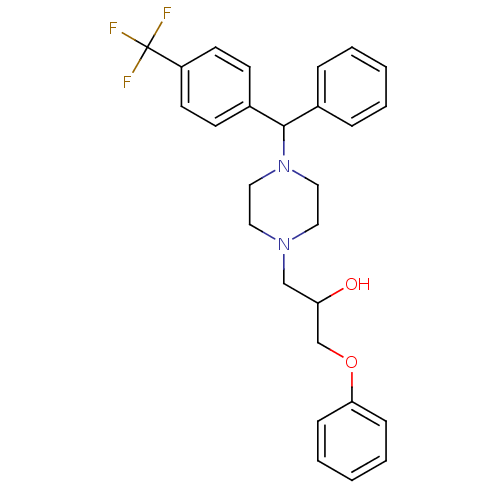 (1-Phenoxy-3-{4-[phenyl-(4-trifluoromethyl-phenyl)-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ArQule Inc Curated by ChEMBL | Assay Description Predictive competitive inhibition of cytochrome P450 2D6 was determined in silico | Bioorg Med Chem Lett 14: 2025-30 (2004) Article DOI: 10.1016/j.bmcl.2004.02.078 BindingDB Entry DOI: 10.7270/Q22J6B8R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50143952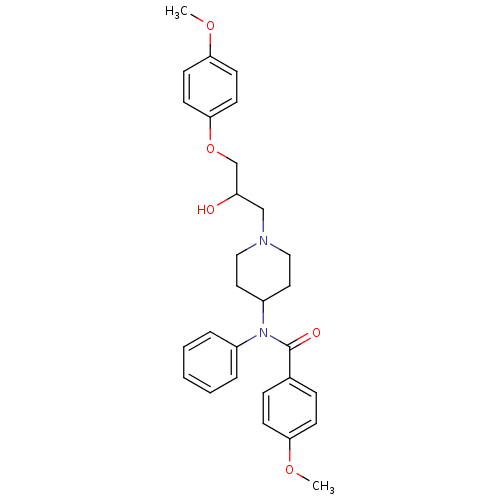 (CHEMBL59712 | N-{1-[2-Hydroxy-3-(4-methoxy-phenoxy...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ArQule Inc Curated by ChEMBL | Assay Description Predictive competitive inhibition of cytochrome P450 2D6 was determined in silico | Bioorg Med Chem Lett 14: 2025-30 (2004) Article DOI: 10.1016/j.bmcl.2004.02.078 BindingDB Entry DOI: 10.7270/Q22J6B8R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50143958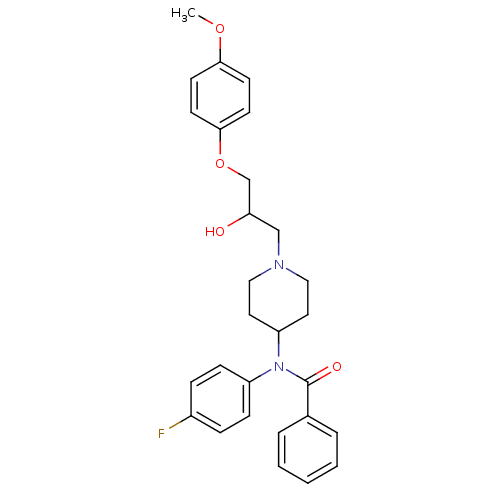 (CHEMBL63060 | N-(4-Fluoro-phenyl)-N-{1-[2-hydroxy-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ArQule Inc Curated by ChEMBL | Assay Description Predictive competitive inhibition of cytochrome P450 2D6 was determined in silico | Bioorg Med Chem Lett 14: 2025-30 (2004) Article DOI: 10.1016/j.bmcl.2004.02.078 BindingDB Entry DOI: 10.7270/Q22J6B8R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50143949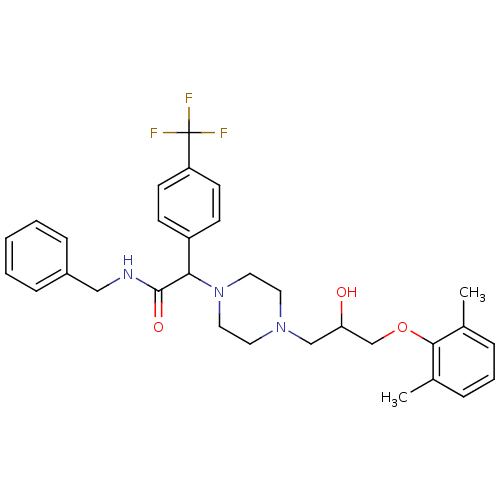 (CHEMBL60843 | N-Benzyl-2-{4-[3-(2,6-dimethyl-pheno...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ArQule Inc Curated by ChEMBL | Assay Description Predictive competitive inhibition of cytochrome P450 2D6 was determined in silico | Bioorg Med Chem Lett 14: 2025-30 (2004) Article DOI: 10.1016/j.bmcl.2004.02.078 BindingDB Entry DOI: 10.7270/Q22J6B8R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50143962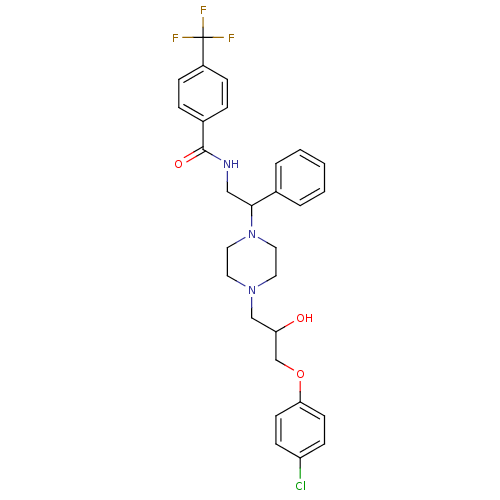 (CHEMBL62878 | N-(2-{4-[3-(4-Chloro-phenoxy)-2-hydr...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ArQule Inc Curated by ChEMBL | Assay Description Predictive competitive inhibition of cytochrome P450 2D6 was determined in silico | Bioorg Med Chem Lett 14: 2025-30 (2004) Article DOI: 10.1016/j.bmcl.2004.02.078 BindingDB Entry DOI: 10.7270/Q22J6B8R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50143945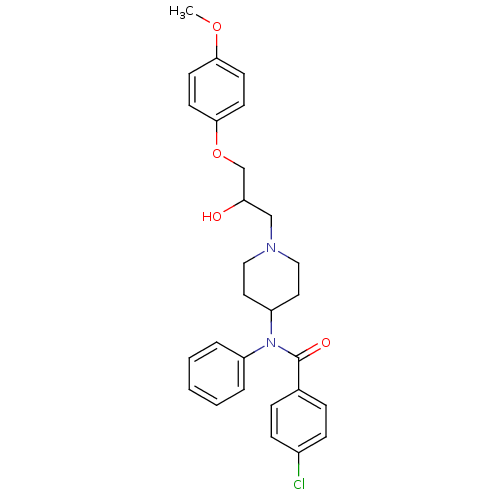 (4-Chloro-N-{1-[2-hydroxy-3-(4-methoxy-phenoxy)-pro...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ArQule Inc Curated by ChEMBL | Assay Description Predictive competitive inhibition of cytochrome P450 2D6 was determined in silico | Bioorg Med Chem Lett 14: 2025-30 (2004) Article DOI: 10.1016/j.bmcl.2004.02.078 BindingDB Entry DOI: 10.7270/Q22J6B8R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50143961 (CHEMBL62240 | N-{1-[2-Hydroxy-3-(4-methoxy-phenoxy...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ArQule Inc Curated by ChEMBL | Assay Description Predictive competitive inhibition of cytochrome P450 2D6 was determined in silico | Bioorg Med Chem Lett 14: 2025-30 (2004) Article DOI: 10.1016/j.bmcl.2004.02.078 BindingDB Entry DOI: 10.7270/Q22J6B8R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50143974 (2-{4-[3-(2,6-Dimethyl-phenoxy)-2-hydroxy-propyl]-p...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ArQule Inc Curated by ChEMBL | Assay Description Predictive competitive inhibition of cytochrome P450 2D6 was determined in silico | Bioorg Med Chem Lett 14: 2025-30 (2004) Article DOI: 10.1016/j.bmcl.2004.02.078 BindingDB Entry DOI: 10.7270/Q22J6B8R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50143950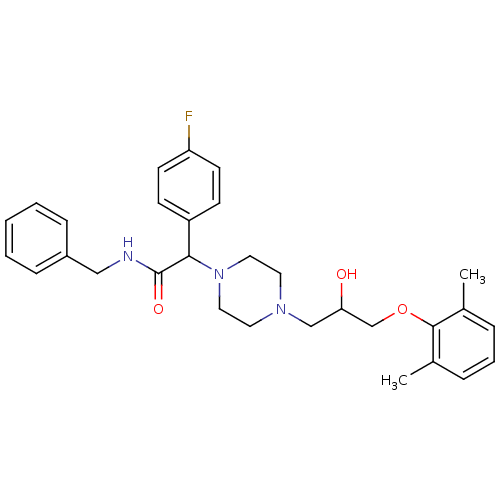 (CHEMBL305591 | N-Benzyl-2-{4-[3-(2,6-dimethyl-phen...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ArQule Inc Curated by ChEMBL | Assay Description Predictive competitive inhibition of cytochrome P450 2D6 was determined in silico | Bioorg Med Chem Lett 14: 2025-30 (2004) Article DOI: 10.1016/j.bmcl.2004.02.078 BindingDB Entry DOI: 10.7270/Q22J6B8R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50143964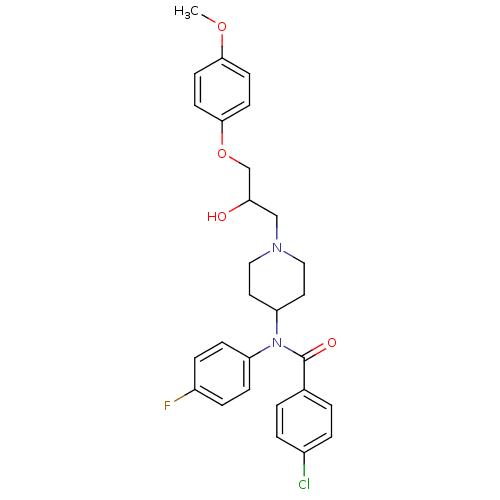 (4-Chloro-N-(4-fluoro-phenyl)-N-{1-[2-hydroxy-3-(4-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ArQule Inc Curated by ChEMBL | Assay Description Predictive competitive inhibition of cytochrome P450 2D6 was determined in silico | Bioorg Med Chem Lett 14: 2025-30 (2004) Article DOI: 10.1016/j.bmcl.2004.02.078 BindingDB Entry DOI: 10.7270/Q22J6B8R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50143967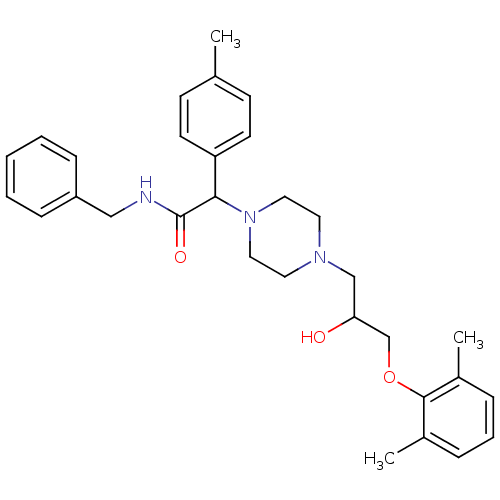 (CHEMBL60568 | N-Benzyl-2-{4-[3-(2,6-dimethyl-pheno...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ArQule Inc Curated by ChEMBL | Assay Description Predictive competitive inhibition of cytochrome P450 2D6 was determined in silico | Bioorg Med Chem Lett 14: 2025-30 (2004) Article DOI: 10.1016/j.bmcl.2004.02.078 BindingDB Entry DOI: 10.7270/Q22J6B8R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50143971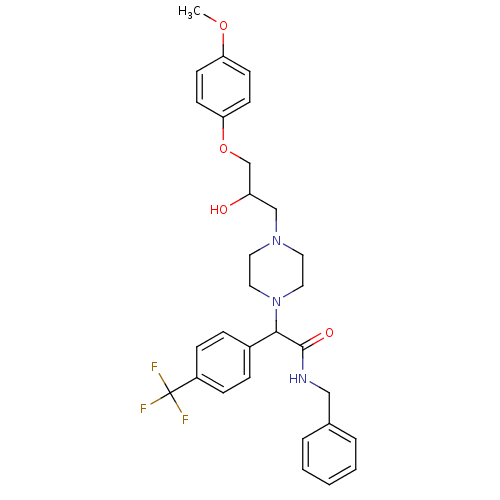 (CHEMBL59644 | N-Benzyl-2-{4-[2-hydroxy-3-(4-methox...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ArQule Inc Curated by ChEMBL | Assay Description Predictive competitive inhibition of cytochrome P450 2D6 was determined in silico | Bioorg Med Chem Lett 14: 2025-30 (2004) Article DOI: 10.1016/j.bmcl.2004.02.078 BindingDB Entry DOI: 10.7270/Q22J6B8R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50143954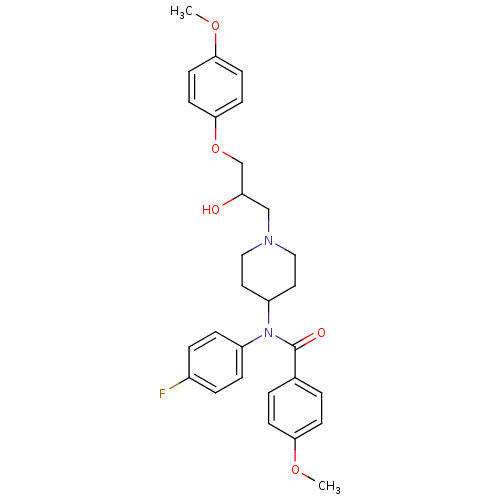 (CHEMBL433100 | N-(4-Fluoro-phenyl)-N-{1-[2-hydroxy...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ArQule Inc Curated by ChEMBL | Assay Description Predictive competitive inhibition of cytochrome P450 2D6 was determined in silico | Bioorg Med Chem Lett 14: 2025-30 (2004) Article DOI: 10.1016/j.bmcl.2004.02.078 BindingDB Entry DOI: 10.7270/Q22J6B8R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50143956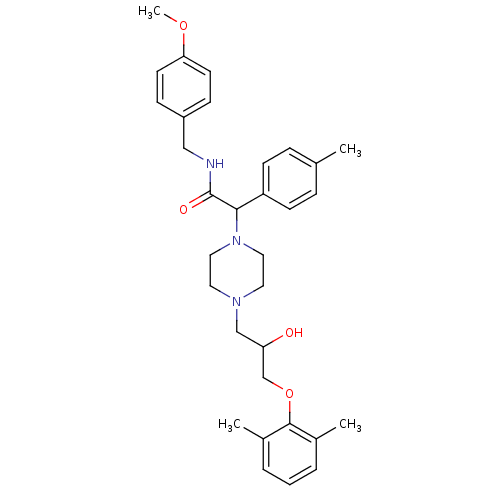 (2-{4-[3-(2,6-Dimethyl-phenoxy)-2-hydroxy-propyl]-p...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ArQule Inc Curated by ChEMBL | Assay Description Predictive competitive inhibition of cytochrome P450 2D6 was determined in silico | Bioorg Med Chem Lett 14: 2025-30 (2004) Article DOI: 10.1016/j.bmcl.2004.02.078 BindingDB Entry DOI: 10.7270/Q22J6B8R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50143976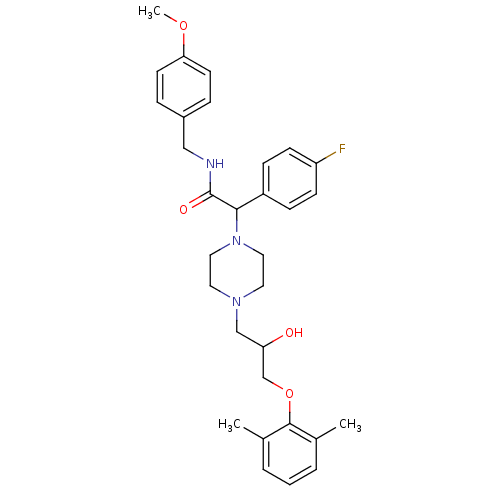 (2-{4-[3-(2,6-Dimethyl-phenoxy)-2-hydroxy-propyl]-p...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ArQule Inc Curated by ChEMBL | Assay Description Predictive competitive inhibition of cytochrome P450 2D6 was determined in silico | Bioorg Med Chem Lett 14: 2025-30 (2004) Article DOI: 10.1016/j.bmcl.2004.02.078 BindingDB Entry DOI: 10.7270/Q22J6B8R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50143970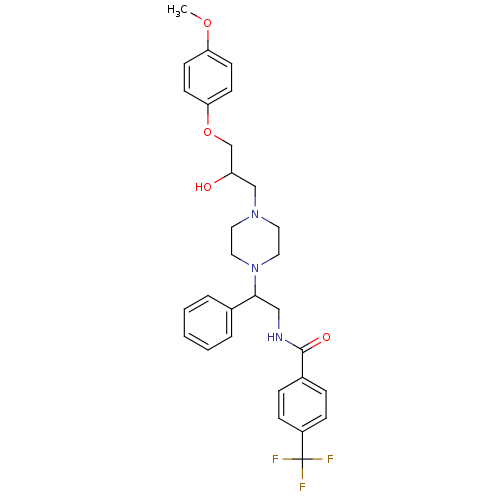 (CHEMBL61336 | N-(2-{4-[2-Hydroxy-3-(4-methoxy-phen...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ArQule Inc Curated by ChEMBL | Assay Description Predictive competitive inhibition of cytochrome P450 2D6 was determined in silico | Bioorg Med Chem Lett 14: 2025-30 (2004) Article DOI: 10.1016/j.bmcl.2004.02.078 BindingDB Entry DOI: 10.7270/Q22J6B8R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50143968 (4-Fluoro-N-(2-{4-[2-hydroxy-3-(4-methoxy-phenoxy)-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ArQule Inc Curated by ChEMBL | Assay Description Predictive competitive inhibition of cytochrome P450 2D6 was determined in silico | Bioorg Med Chem Lett 14: 2025-30 (2004) Article DOI: 10.1016/j.bmcl.2004.02.078 BindingDB Entry DOI: 10.7270/Q22J6B8R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50143946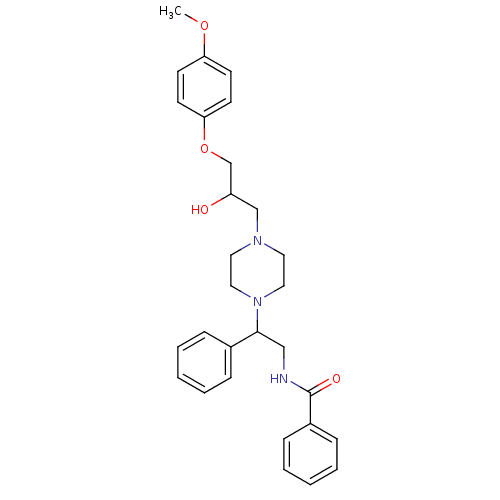 (CHEMBL64177 | N-(2-{4-[2-Hydroxy-3-(4-methoxy-phen...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ArQule Inc Curated by ChEMBL | Assay Description Predictive competitive inhibition of cytochrome P450 2D6 was determined in silico | Bioorg Med Chem Lett 14: 2025-30 (2004) Article DOI: 10.1016/j.bmcl.2004.02.078 BindingDB Entry DOI: 10.7270/Q22J6B8R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50143973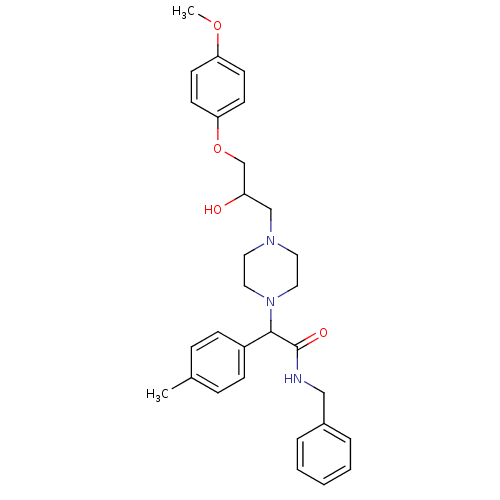 (CHEMBL291629 | N-Benzyl-2-{4-[2-hydroxy-3-(4-metho...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ArQule Inc Curated by ChEMBL | Assay Description Predictive competitive inhibition of cytochrome P450 2D6 was determined in silico | Bioorg Med Chem Lett 14: 2025-30 (2004) Article DOI: 10.1016/j.bmcl.2004.02.078 BindingDB Entry DOI: 10.7270/Q22J6B8R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50143955 (2-{4-[2-Hydroxy-3-(4-methoxy-phenoxy)-propyl]-pipe...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ArQule Inc Curated by ChEMBL | Assay Description Predictive competitive inhibition of cytochrome P450 2D6 was determined in silico | Bioorg Med Chem Lett 14: 2025-30 (2004) Article DOI: 10.1016/j.bmcl.2004.02.078 BindingDB Entry DOI: 10.7270/Q22J6B8R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50143959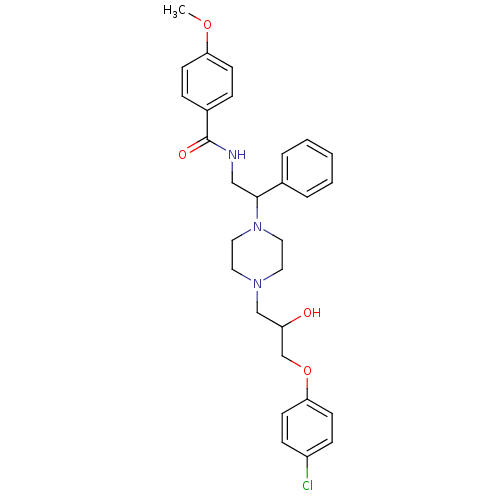 (CHEMBL61283 | N-(2-{4-[3-(4-Chloro-phenoxy)-2-hydr...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ArQule Inc Curated by ChEMBL | Assay Description Predictive competitive inhibition of cytochrome P450 2D6 was determined in silico | Bioorg Med Chem Lett 14: 2025-30 (2004) Article DOI: 10.1016/j.bmcl.2004.02.078 BindingDB Entry DOI: 10.7270/Q22J6B8R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50143957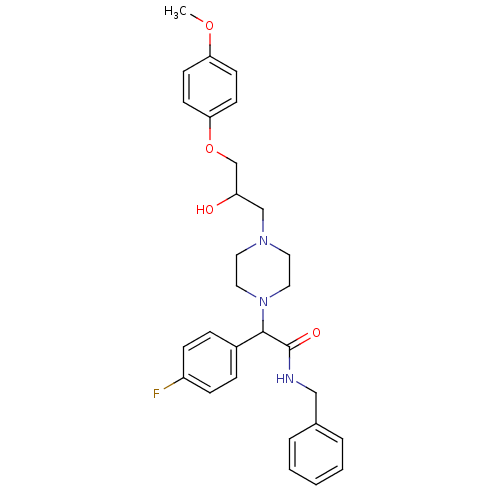 (CHEMBL60847 | N-Benzyl-2-(4-fluoro-phenyl)-2-{4-[2...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ArQule Inc Curated by ChEMBL | Assay Description Predictive competitive inhibition of cytochrome P450 2D6 was determined in silico | Bioorg Med Chem Lett 14: 2025-30 (2004) Article DOI: 10.1016/j.bmcl.2004.02.078 BindingDB Entry DOI: 10.7270/Q22J6B8R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50143972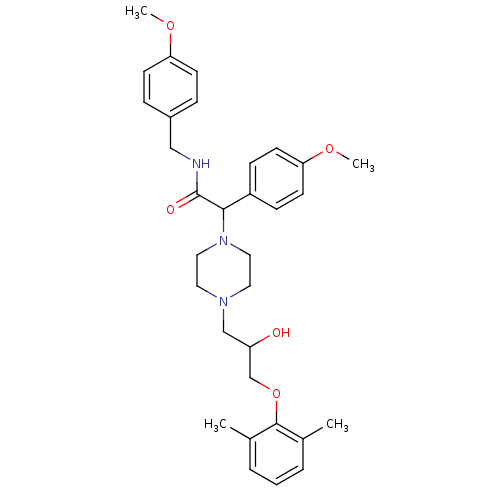 (2-{4-[3-(2,6-Dimethyl-phenoxy)-2-hydroxy-propyl]-p...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ArQule Inc Curated by ChEMBL | Assay Description Predictive competitive inhibition of cytochrome P450 2D6 was determined in silico | Bioorg Med Chem Lett 14: 2025-30 (2004) Article DOI: 10.1016/j.bmcl.2004.02.078 BindingDB Entry DOI: 10.7270/Q22J6B8R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50143975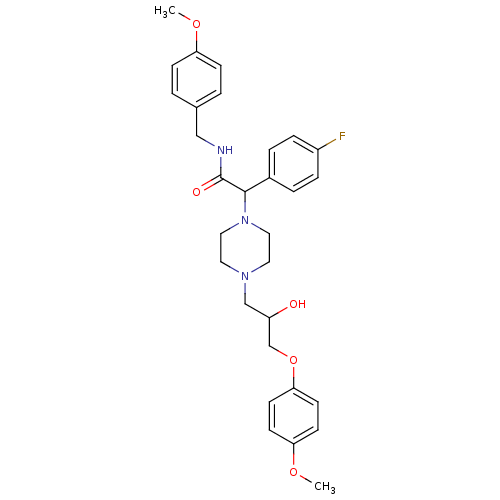 (2-(4-Fluoro-phenyl)-2-{4-[2-hydroxy-3-(4-methoxy-p...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ArQule Inc Curated by ChEMBL | Assay Description Predictive competitive inhibition of cytochrome P450 2D6 was determined in silico | Bioorg Med Chem Lett 14: 2025-30 (2004) Article DOI: 10.1016/j.bmcl.2004.02.078 BindingDB Entry DOI: 10.7270/Q22J6B8R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50143947 (4-Fluoro-N-{2-[4-(2-hydroxy-3-phenoxy-propyl)-pipe...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ArQule Inc Curated by ChEMBL | Assay Description Predictive competitive inhibition of cytochrome P450 2D6 was determined in silico | Bioorg Med Chem Lett 14: 2025-30 (2004) Article DOI: 10.1016/j.bmcl.2004.02.078 BindingDB Entry DOI: 10.7270/Q22J6B8R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50143965 (CHEMBL60666 | N-(2-{4-[3-(4-Chloro-phenoxy)-2-hydr...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ArQule Inc Curated by ChEMBL | Assay Description Predictive competitive inhibition of cytochrome P450 2D6 was determined in silico | Bioorg Med Chem Lett 14: 2025-30 (2004) Article DOI: 10.1016/j.bmcl.2004.02.078 BindingDB Entry DOI: 10.7270/Q22J6B8R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50143960 (CHEMBL64688 | N-(2-{4-[2-Hydroxy-3-(4-methoxy-phen...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 8.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ArQule Inc Curated by ChEMBL | Assay Description Predictive competitive inhibition of cytochrome P450 2D6 was determined in silico | Bioorg Med Chem Lett 14: 2025-30 (2004) Article DOI: 10.1016/j.bmcl.2004.02.078 BindingDB Entry DOI: 10.7270/Q22J6B8R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50143953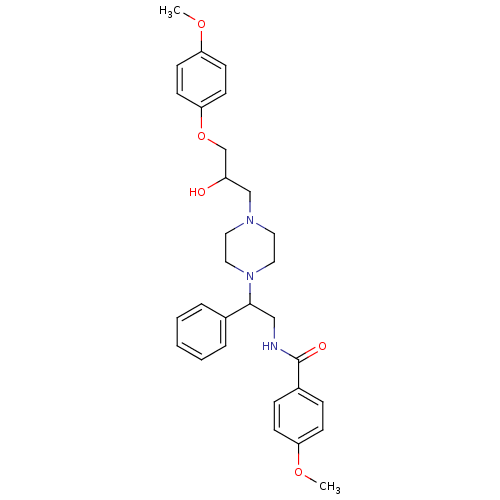 (CHEMBL64831 | N-(2-{4-[2-Hydroxy-3-(4-methoxy-phen...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 8.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ArQule Inc Curated by ChEMBL | Assay Description Predictive competitive inhibition of cytochrome P450 2D6 was determined in silico | Bioorg Med Chem Lett 14: 2025-30 (2004) Article DOI: 10.1016/j.bmcl.2004.02.078 BindingDB Entry DOI: 10.7270/Q22J6B8R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50143966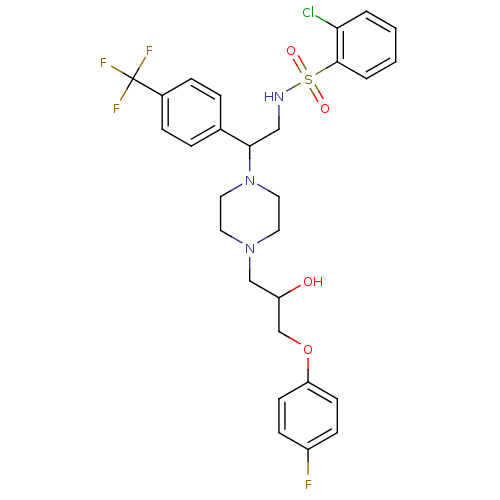 (2-Chloro-N-[2-{4-[3-(4-fluoro-phenoxy)-2-hydroxy-p...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ArQule Inc Curated by ChEMBL | Assay Description Predictive competitive inhibition of cytochrome P450 2D6 was determined in silico | Bioorg Med Chem Lett 14: 2025-30 (2004) Article DOI: 10.1016/j.bmcl.2004.02.078 BindingDB Entry DOI: 10.7270/Q22J6B8R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50143963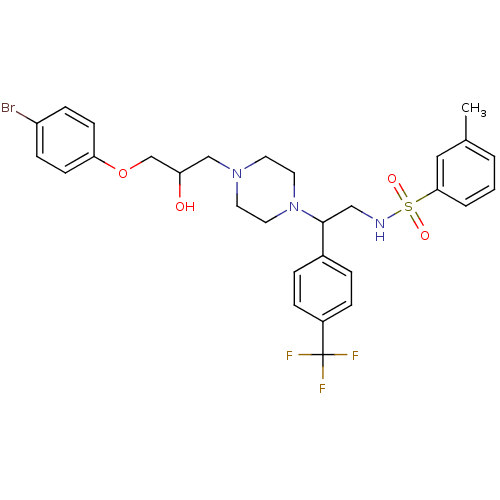 (CHEMBL64869 | N-[2-{4-[3-(4-Bromo-phenoxy)-2-hydro...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ArQule Inc Curated by ChEMBL | Assay Description Predictive competitive inhibition of cytochrome P450 2D6 was determined in silico | Bioorg Med Chem Lett 14: 2025-30 (2004) Article DOI: 10.1016/j.bmcl.2004.02.078 BindingDB Entry DOI: 10.7270/Q22J6B8R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steryl-sulfatase (Homo sapiens (Human)) | BDBM50223216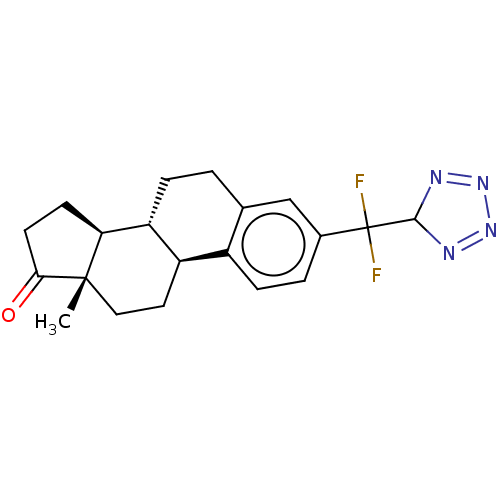 (CHEMBL3348562) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Waterloo Curated by ChEMBL | Assay Description Inhibitory activity against steroid sulfatase (STS) | Bioorg Med Chem Lett 14: 151-5 (2003) BindingDB Entry DOI: 10.7270/Q28K79NV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50143969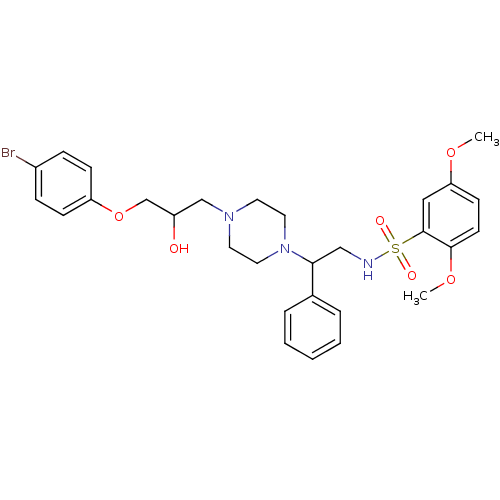 (CHEMBL64583 | N-(2-{4-[3-(4-Bromo-phenoxy)-2-hydro...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ArQule Inc Curated by ChEMBL | Assay Description Predictive competitive inhibition of cytochrome P450 2D6 was determined in silico | Bioorg Med Chem Lett 14: 2025-30 (2004) Article DOI: 10.1016/j.bmcl.2004.02.078 BindingDB Entry DOI: 10.7270/Q22J6B8R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steryl-sulfatase (Homo sapiens (Human)) | BDBM50366887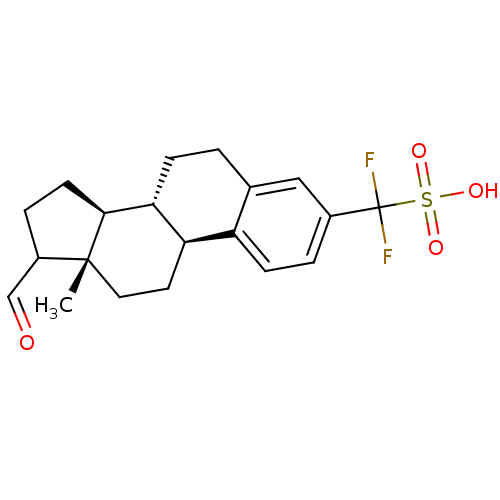 (CHEMBL1627329) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 3.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Waterloo Curated by ChEMBL | Assay Description Inhibitory activity against steroid sulfatase (STS) | Bioorg Med Chem Lett 14: 151-5 (2003) BindingDB Entry DOI: 10.7270/Q28K79NV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steryl-sulfatase (Homo sapiens (Human)) | BDBM50366886 (CHEMBL1628110) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 5.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Waterloo Curated by ChEMBL | Assay Description Competitive inhibitory activity against steroid sulfatase (STS) | Bioorg Med Chem Lett 14: 151-5 (2003) BindingDB Entry DOI: 10.7270/Q28K79NV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steryl-sulfatase (Homo sapiens (Human)) | BDBM50366524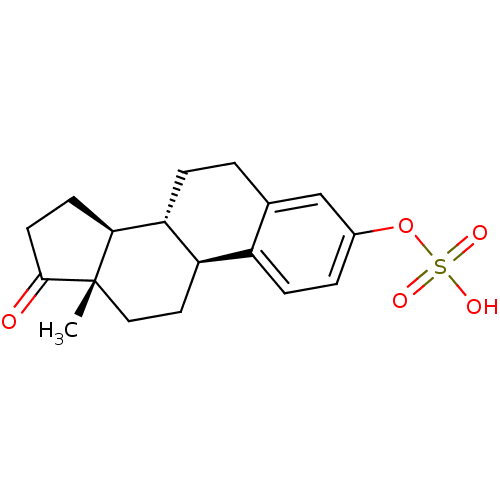 (ESTRONE | ESTROPIPATE | Estrone 3-sulfate | Estron...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid UniChem Similars | PubMed | 6.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Waterloo Curated by ChEMBL | Assay Description Inhibitory activity against steroid sulfatase (STS) desulfation of 4-MUS | Bioorg Med Chem Lett 14: 151-5 (2003) BindingDB Entry DOI: 10.7270/Q28K79NV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steryl-sulfatase (Homo sapiens (Human)) | BDBM50366888 (CHEMBL1627836) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 7.27E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Waterloo Curated by ChEMBL | Assay Description Inhibitory activity against steroid sulfatase (STS) | Bioorg Med Chem Lett 14: 151-5 (2003) BindingDB Entry DOI: 10.7270/Q28K79NV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steryl-sulfatase (Homo sapiens (Human)) | BDBM50366884 (CHEMBL1628109) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | 1.40E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Waterloo Curated by ChEMBL | Assay Description Inhibitory activity against steroid sulfatase (STS) | Bioorg Med Chem Lett 14: 151-5 (2003) BindingDB Entry DOI: 10.7270/Q28K79NV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steryl-sulfatase (Homo sapiens (Human)) | BDBM50366885 (CHEMBL1628114) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | 1.40E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Waterloo Curated by ChEMBL | Assay Description Inhibitory activity against steroid sulfatase (STS) | Bioorg Med Chem Lett 14: 151-5 (2003) BindingDB Entry DOI: 10.7270/Q28K79NV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steryl-sulfatase (Homo sapiens (Human)) | BDBM50366884 (CHEMBL1628109) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | 6.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Waterloo Curated by ChEMBL | Assay Description Inhibitory activity against steroid sulfatase (STS) using methodology of Li et al. | Bioorg Med Chem Lett 14: 151-5 (2003) BindingDB Entry DOI: 10.7270/Q28K79NV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK [C481S] (Homo sapiens (Human)) | BDBM499813 ((2-chloro-4-phenoxyphenyl)(4- (((3R,6R)-6-(hydroxy...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.370 | n/a | n/a | n/a | n/a | n/a | n/a |
ArQule, Inc. US Patent | Assay Description Enzyme assay using full length recombinant active form of wild-type BTK and BTK-C481S was measured as described previously (Anastassiadis T, et al., ... | US Patent US11020398 (2021) BindingDB Entry DOI: 10.7270/Q2DZ0CFC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK [C481S] (Homo sapiens (Human)) | BDBM499811 (US11020398, Compound I-123 | US20230364079, Exampl...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | PDB US Patent | n/a | n/a | 0.390 | n/a | n/a | n/a | n/a | n/a | n/a |
ArQule, Inc. US Patent | Assay Description Enzyme assay using full length recombinant active form of wild-type BTK and BTK-C481S was measured as described previously (Anastassiadis T, et al., ... | US Patent US11020398 (2021) BindingDB Entry DOI: 10.7270/Q2DZ0CFC | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM499813 ((2-chloro-4-phenoxyphenyl)(4- (((3R,6R)-6-(hydroxy...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.560 | n/a | n/a | n/a | n/a | n/a | n/a |
ArQule, Inc. US Patent | Assay Description Enzyme assay using full length recombinant active form of wild-type BTK and BTK-C481S was measured as described previously (Anastassiadis T, et al., ... | US Patent US11020398 (2021) BindingDB Entry DOI: 10.7270/Q2DZ0CFC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM499813 ((2-chloro-4-phenoxyphenyl)(4- (((3R,6R)-6-(hydroxy...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.560 | n/a | n/a | n/a | n/a | n/a | n/a |
ArQule, Inc. US Patent | Assay Description Enzyme assay using full length recombinant active form of wild-type BTK and BTK-C481S was measured as described previously (Anastassiadis T, et al., ... | US Patent US11020398 (2021) BindingDB Entry DOI: 10.7270/Q2DZ0CFC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM499810 (N-((S)-1-((2S,5R)-5-((5-(2-chloro-4- phenoxybenzoy...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.770 | n/a | n/a | n/a | n/a | n/a | n/a |
ArQule, Inc. US Patent | Assay Description Full length unphosphorylated form of BTK expressed in Sf9 cells was employed to test inhibitory activity in the inactive BTK assay. The assay was mea... | US Patent US11020398 (2021) BindingDB Entry DOI: 10.7270/Q2DZ0CFC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK [C481S] (Homo sapiens (Human)) | BDBM499814 ((2-chloro-4-phenoxyphenyl)(4- (((3S,6S)-6-(hydroxy...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.790 | n/a | n/a | n/a | n/a | n/a | n/a |
ArQule, Inc. US Patent | Assay Description Enzyme assay using full length recombinant active form of wild-type BTK and BTK-C481S was measured as described previously (Anastassiadis T, et al., ... | US Patent US11020398 (2021) BindingDB Entry DOI: 10.7270/Q2DZ0CFC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM499811 (US11020398, Compound I-123 | US20230364079, Exampl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | PDB US Patent | n/a | n/a | 0.850 | n/a | n/a | n/a | n/a | n/a | n/a |
ArQule, Inc. US Patent | Assay Description Enzyme assay using full length recombinant active form of wild-type BTK and BTK-C481S was measured as described previously (Anastassiadis T, et al., ... | US Patent US11020398 (2021) BindingDB Entry DOI: 10.7270/Q2DZ0CFC | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM499811 (US11020398, Compound I-123 | US20230364079, Exampl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | PDB US Patent | n/a | n/a | 0.850 | n/a | n/a | n/a | n/a | n/a | n/a |
ArQule, Inc. US Patent | Assay Description Enzyme assay using full length recombinant active form of wild-type BTK and BTK-C481S was measured as described previously (Anastassiadis T, et al., ... | US Patent US11020398 (2021) BindingDB Entry DOI: 10.7270/Q2DZ0CFC | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM499838 ((2-chloro-4-phenoxyphenyl)(4- (((3R,6S)-6-((R)-1- ...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.930 | n/a | n/a | n/a | n/a | n/a | n/a |
ArQule, Inc. US Patent | Assay Description Full length unphosphorylated form of BTK expressed in Sf9 cells was employed to test inhibitory activity in the inactive BTK assay. The assay was mea... | US Patent US11020398 (2021) BindingDB Entry DOI: 10.7270/Q2DZ0CFC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM499976 ((racemic)-1-(2-(((5-(2-chloro-4- phenoxybenzoyl)-7...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.980 | n/a | n/a | n/a | n/a | n/a | n/a |
ArQule, Inc. US Patent | Assay Description Purified full-length inactive BTK (wild type and C481 mutant, N-terminal 6XHIS tagged BTK, Mwt=78.2 kDa) were activated using soluble inositol hexaki... | US Patent US11020398 (2021) BindingDB Entry DOI: 10.7270/Q2DZ0CFC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 1102 total ) | Next | Last >> |