

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Beta-1 adrenergic receptor (Rattus norvegicus (Rat)) | BDBM25392 (4-[1-hydroxy-2-(isopropylamino)ethyl]pyrocatechol;...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | 5.30 | n/a | n/a | n/a | n/a | n/a | n/a |
American Cyanamid Company Curated by ChEMBL | Assay Description Compound was evaluated in vitro for beta-adrenergic activity against beta-1 adrenergic receptor by the inhibition of insulin stimulated [14C]- glucos... | J Med Chem 35: 3081-4 (1992) BindingDB Entry DOI: 10.7270/Q2PR7TWN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-2 adrenergic receptor (Rattus norvegicus) | BDBM50002132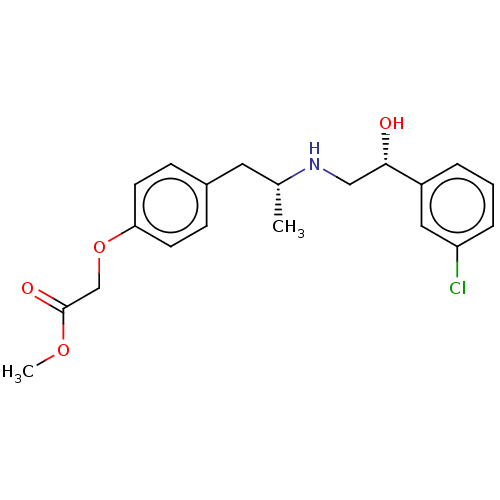 ((4-{(R)-2-[(R)-2-(3-Chloro-phenyl)-2-hydroxy-ethyl...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
American Cyanamid Company Curated by ChEMBL | Assay Description Compound was evaluated for its binding affinity towards Beta-2 adrenergic receptor in rat soleus membrane by displacing (-)-isoproterenol (50 microM) | J Med Chem 35: 3081-4 (1992) BindingDB Entry DOI: 10.7270/Q2PR7TWN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-1 adrenergic receptor (Rattus norvegicus (Rat)) | BDBM50002132 ((4-{(R)-2-[(R)-2-(3-Chloro-phenyl)-2-hydroxy-ethyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 59 | n/a | n/a | n/a | n/a | n/a | n/a |
American Cyanamid Company Curated by ChEMBL | Assay Description Compound was evaluated for its binding affinity towards Beta-1 adrenergic receptor in rat heart membrane by displacing [125I]- iodocyanopindolol | J Med Chem 35: 3081-4 (1992) BindingDB Entry DOI: 10.7270/Q2PR7TWN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sterol O-acyltransferase 1 (Homo sapiens (Human)) | BDBM50227933 (CHEMBL77761) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
American Cyanamid Company Curated by ChEMBL | Assay Description In vitro inhibitory concentration against Muscarinic acetylcholine receptor derived from smooth muscle cells from thoracic aorta of monkeys | J Med Chem 32: 2318-25 (1989) BindingDB Entry DOI: 10.7270/Q2QZ2D5R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sterol O-acyltransferase 1 (Homo sapiens (Human)) | BDBM50022279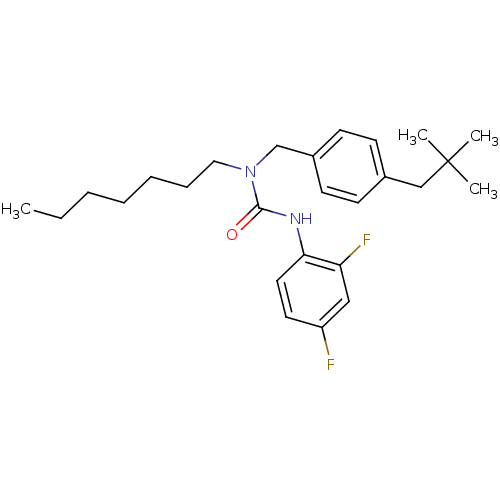 (3-(2,4-Difluoro-phenyl)-1-[4-(2,2-dimethyl-propyl)...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of smooth muscle cell ACAT activity for cells stimulated by cationized LDL. | J Med Chem 29: 1131-3 (1987) BindingDB Entry DOI: 10.7270/Q2V69HKP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-1 adrenergic receptor (Rattus norvegicus (Rat)) | BDBM50002133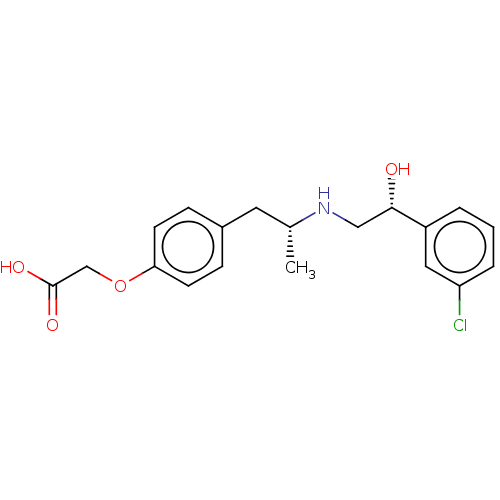 ((+/-)-2-(4-((R)-2-((R)-2-(3-chlorophenyl)-2-hydrox...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 190 | n/a | n/a | n/a | n/a | n/a | n/a |
American Cyanamid Company Curated by ChEMBL | Assay Description Compound was evaluated in vitro for beta-adrenergic activity against beta-1 adrenergic receptor by the inhibition of insulin stimulated [14C]- glucos... | J Med Chem 35: 3081-4 (1992) BindingDB Entry DOI: 10.7270/Q2PR7TWN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sterol O-acyltransferase 1 (Homo sapiens (Human)) | BDBM50227908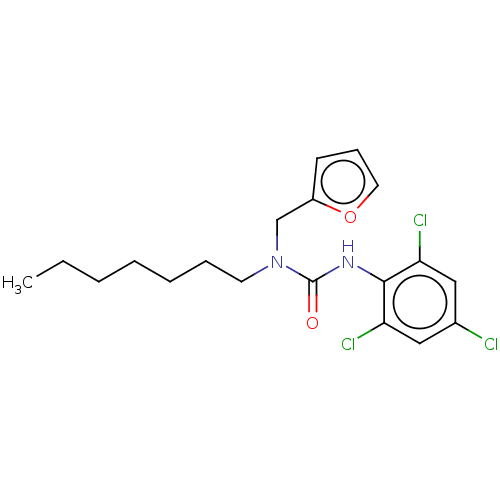 (CHEMBL77988) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 311 | n/a | n/a | n/a | n/a | n/a | n/a |
American Cyanamid Company Curated by ChEMBL | Assay Description In vitro inhibitory concentration against Acyl coenzyme A:cholesterol acyltransferase derived from smooth muscle cells from thoracic aorta of monkeys | J Med Chem 32: 2318-25 (1989) BindingDB Entry DOI: 10.7270/Q2QZ2D5R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sterol O-acyltransferase 1 (Rattus norvegicus) | BDBM50227935 (CHEMBL75708) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 330 | n/a | n/a | n/a | n/a | n/a | n/a |
American Cyanamid Company Curated by ChEMBL | Assay Description In vitro inhibitory concentration against Acyl coenzyme A:cholesterol acyltransferase derived from rat adrenals | J Med Chem 32: 2318-25 (1989) BindingDB Entry DOI: 10.7270/Q2QZ2D5R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sterol O-acyltransferase 1 (Rattus norvegicus) | BDBM50227908 (CHEMBL77988) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 406 | n/a | n/a | n/a | n/a | n/a | n/a |
American Cyanamid Company Curated by ChEMBL | Assay Description In vitro inhibitory concentration against Acyl coenzyme A:cholesterol acyltransferase derived from rat adrenals | J Med Chem 32: 2318-25 (1989) BindingDB Entry DOI: 10.7270/Q2QZ2D5R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sterol O-acyltransferase 1 (Rattus norvegicus) | BDBM50227910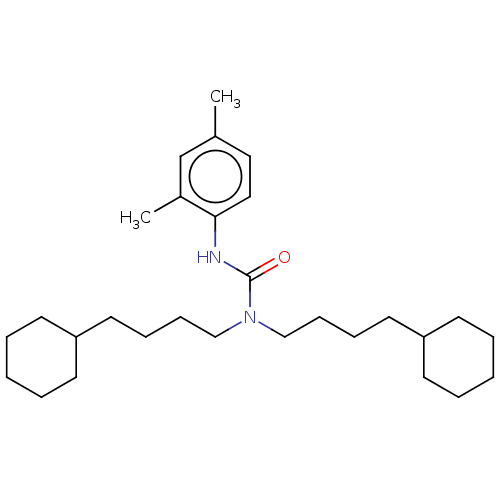 (CHEMBL305937) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 431 | n/a | n/a | n/a | n/a | n/a | n/a |
American Cyanamid Company Curated by ChEMBL | Assay Description In vitro inhibitory concentration against Acyl coenzyme A:cholesterol acyltransferase derived from smooth muscle cells from thoracic aorta of monkeys | J Med Chem 32: 2318-25 (1989) BindingDB Entry DOI: 10.7270/Q2QZ2D5R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sterol O-acyltransferase 1 (Rattus norvegicus) | BDBM50227918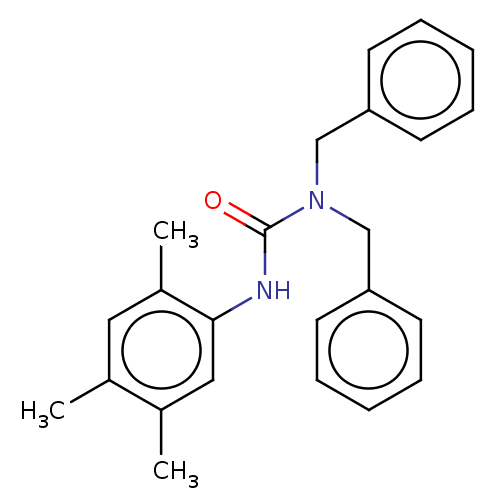 (CHEMBL75224) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 446 | n/a | n/a | n/a | n/a | n/a | n/a |
American Cyanamid Company Curated by ChEMBL | Assay Description In vitro inhibitory concentration against Acyl coenzyme A:cholesterol acyltransferase derived from rat adrenals | J Med Chem 32: 2318-25 (1989) BindingDB Entry DOI: 10.7270/Q2QZ2D5R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sterol O-acyltransferase 1 (Rattus norvegicus) | BDBM50227912 (CHEMBL75204) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 484 | n/a | n/a | n/a | n/a | n/a | n/a |
American Cyanamid Company Curated by ChEMBL | Assay Description In vitro inhibitory concentration against Acyl coenzyme A:cholesterol acyltransferase derived from rat adrenals | J Med Chem 32: 2318-25 (1989) BindingDB Entry DOI: 10.7270/Q2QZ2D5R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-1 adrenergic receptor (Rattus norvegicus (Rat)) | BDBM50002132 ((4-{(R)-2-[(R)-2-(3-Chloro-phenyl)-2-hydroxy-ethyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
American Cyanamid Company Curated by ChEMBL | Assay Description Compound was evaluated in vitro for beta-adrenergic activity against beta-1 adrenergic receptor by the inhibition of insulin stimulated [14C]- glucos... | J Med Chem 35: 3081-4 (1992) BindingDB Entry DOI: 10.7270/Q2PR7TWN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-1 adrenergic receptor (Rattus norvegicus (Rat)) | BDBM25392 (4-[1-hydroxy-2-(isopropylamino)ethyl]pyrocatechol;...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
American Cyanamid Company Curated by ChEMBL | Assay Description Compound was evaluated for its binding affinity towards beta-1 adrenergic receptor in rat heart membrane by displacing [125I]- iodocyanopindolol | J Med Chem 35: 3081-4 (1992) BindingDB Entry DOI: 10.7270/Q2PR7TWN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sterol O-acyltransferase 1 (Rattus norvegicus) | BDBM50227931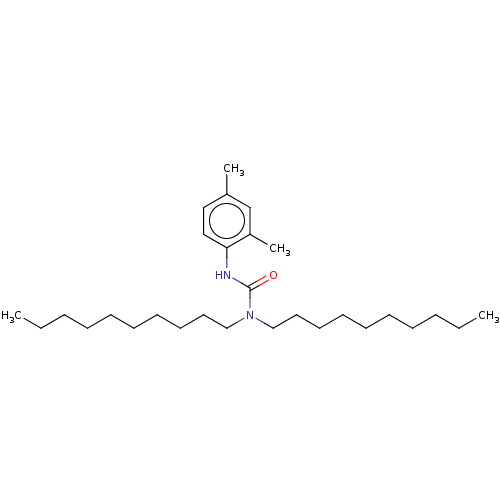 (CHEMBL76938) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 629 | n/a | n/a | n/a | n/a | n/a | n/a |
American Cyanamid Company Curated by ChEMBL | Assay Description In vitro inhibitory concentration against Acyl coenzyme A:cholesterol acyltransferase derived from rat adrenals | J Med Chem 32: 2318-25 (1989) BindingDB Entry DOI: 10.7270/Q2QZ2D5R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sterol O-acyltransferase 1 (Rattus norvegicus) | BDBM50227921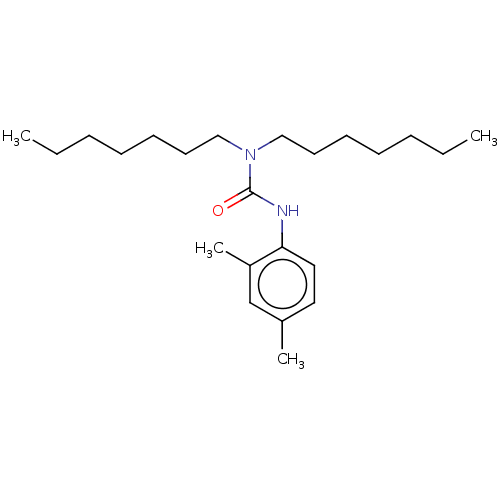 (CHEMBL75709) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 721 | n/a | n/a | n/a | n/a | n/a | n/a |
American Cyanamid Company Curated by ChEMBL | Assay Description In vitro inhibitory concentration against Acyl coenzyme A:cholesterol acyltransferase derived from rat adrenals | J Med Chem 32: 2318-25 (1989) BindingDB Entry DOI: 10.7270/Q2QZ2D5R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sterol O-acyltransferase 1 (Homo sapiens (Human)) | BDBM50022279 (3-(2,4-Difluoro-phenyl)-1-[4-(2,2-dimethyl-propyl)...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | n/a | n/a | 740 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for the effect on Cholesterol O-Acyltransferase (ACAT) in Liver microsomes | J Med Chem 29: 1131-3 (1987) BindingDB Entry DOI: 10.7270/Q2V69HKP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sterol O-acyltransferase 1 (Homo sapiens (Human)) | BDBM50022279 (3-(2,4-Difluoro-phenyl)-1-[4-(2,2-dimethyl-propyl)...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | n/a | n/a | 810 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for the effect on Cholesterol O-Acyltransferase (ACAT) in intestinal microsomes | J Med Chem 29: 1131-3 (1987) BindingDB Entry DOI: 10.7270/Q2V69HKP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sterol O-acyltransferase 1 (Rattus norvegicus) | BDBM50227943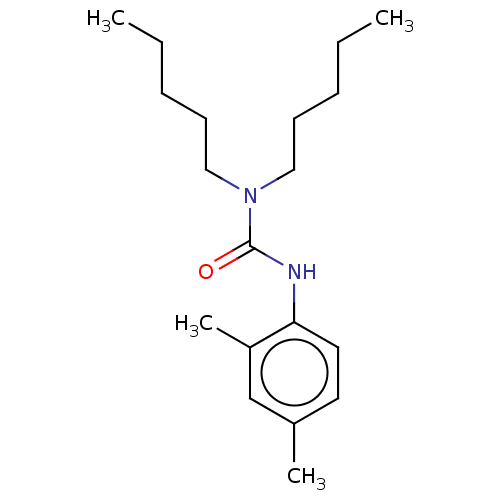 (CHEMBL76935) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 952 | n/a | n/a | n/a | n/a | n/a | n/a |
American Cyanamid Company Curated by ChEMBL | Assay Description In vitro inhibitory concentration against ACAT derived from rat adrenals | J Med Chem 32: 2318-25 (1989) BindingDB Entry DOI: 10.7270/Q2QZ2D5R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sterol O-acyltransferase 1 (Rattus norvegicus) | BDBM50227909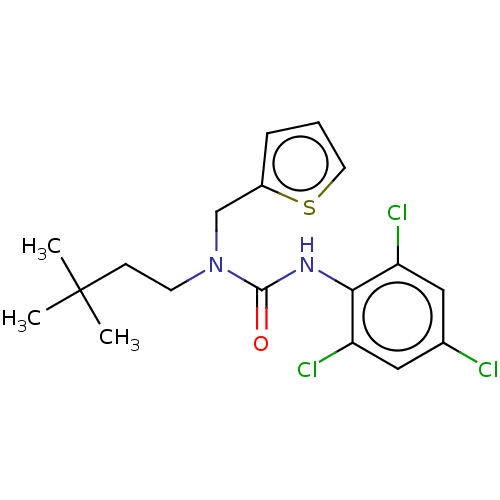 (CHEMBL307505) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 976 | n/a | n/a | n/a | n/a | n/a | n/a |
American Cyanamid Company Curated by ChEMBL | Assay Description In vitro inhibitory concentration against acyl coenzyme A:cholesterol acyltransferase derived from rat adrenals | J Med Chem 32: 2318-25 (1989) BindingDB Entry DOI: 10.7270/Q2QZ2D5R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-2 adrenergic receptor (Canis familiaris) | BDBM25392 (4-[1-hydroxy-2-(isopropylamino)ethyl]pyrocatechol;...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
American Cyanamid Company Curated by ChEMBL | Assay Description Compound was evaluated for its binding affinity towards beta-2 adrenergic receptor in rat soleus membrane by displacing(-)-isoproterenol (50 microM) | J Med Chem 35: 3081-4 (1992) BindingDB Entry DOI: 10.7270/Q2PR7TWN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sterol O-acyltransferase 1 (Homo sapiens (Human)) | BDBM50227918 (CHEMBL75224) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1.08E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
American Cyanamid Company Curated by ChEMBL | Assay Description In vitro inhibitory concentration against ACAT derived from smooth muscle cells from thoracic aorta of monkeys | J Med Chem 32: 2318-25 (1989) BindingDB Entry DOI: 10.7270/Q2QZ2D5R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sterol O-acyltransferase 1 (Homo sapiens (Human)) | BDBM50022279 (3-(2,4-Difluoro-phenyl)-1-[4-(2,2-dimethyl-propyl)...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | n/a | n/a | 1.09E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for the effect on Cholesterol O-Acyltransferase (ACAT) in Aorta (homogenate Low-density lipoproteins (LDL) | J Med Chem 29: 1131-3 (1987) BindingDB Entry DOI: 10.7270/Q2V69HKP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-2 adrenergic receptor (Rattus norvegicus) | BDBM50002133 ((+/-)-2-(4-((R)-2-((R)-2-(3-chlorophenyl)-2-hydrox...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
American Cyanamid Company Curated by ChEMBL | Assay Description Compound was evaluated for its binding affinity towards Beta-2 adrenergic receptor in rat soleus membrane by displacing (-)-isoproterenol (50 microM) | J Med Chem 35: 3081-4 (1992) BindingDB Entry DOI: 10.7270/Q2PR7TWN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sterol O-acyltransferase 1 (Homo sapiens (Human)) | BDBM50227936 (CHEMBL73918) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1.31E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
American Cyanamid Company Curated by ChEMBL | Assay Description In vitro inhibitory concentration against Acyl coenzyme A:cholesterol acyltransferase derived from smooth muscle cells from thoracic aorta of monkeys | J Med Chem 32: 2318-25 (1989) BindingDB Entry DOI: 10.7270/Q2QZ2D5R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sterol O-acyltransferase 1 (Rattus norvegicus) | BDBM50227936 (CHEMBL73918) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1.41E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
American Cyanamid Company Curated by ChEMBL | Assay Description In vitro inhibitory concentration against Acyl coenzyme A:cholesterol acyltransferase derived from smooth muscle cells from thoracic aorta of monkeys | J Med Chem 32: 2318-25 (1989) BindingDB Entry DOI: 10.7270/Q2QZ2D5R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sterol O-acyltransferase 1 (Rattus norvegicus) | BDBM50227907 (CHEMBL441803) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1.44E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
American Cyanamid Company Curated by ChEMBL | Assay Description In vitro inhibitory concentration against ACAT derived from rat adrenals | J Med Chem 32: 2318-25 (1989) BindingDB Entry DOI: 10.7270/Q2QZ2D5R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sterol O-acyltransferase 1 (Rattus norvegicus) | BDBM50227902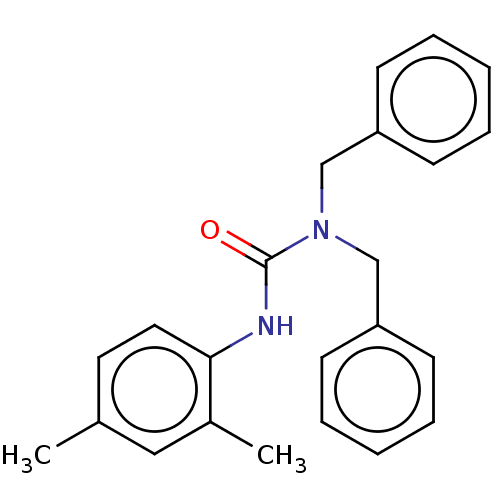 (CHEMBL76706) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.71E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
American Cyanamid Company Curated by ChEMBL | Assay Description In vitro inhibitory concentration against Acyl coenzyme A:cholesterol acyltransferase derived from smooth muscle cells from thoracic aorta of monkeys | J Med Chem 32: 2318-25 (1989) BindingDB Entry DOI: 10.7270/Q2QZ2D5R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sterol O-acyltransferase 1 (Homo sapiens (Human)) | BDBM50227915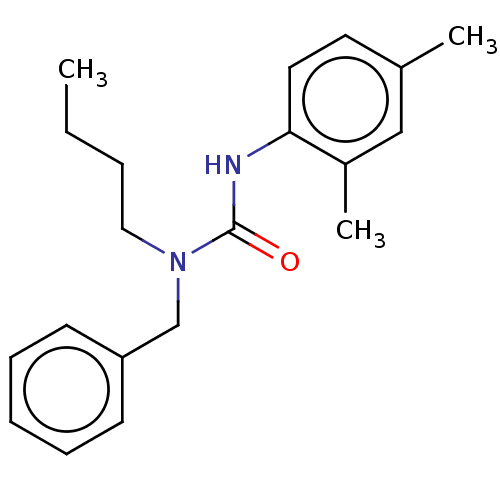 (CHEMBL421876) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1.83E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
American Cyanamid Company Curated by ChEMBL | Assay Description In vitro inhibitory concentration against acyl coenzyme A:cholesterol acyltransferase derived from smooth muscle cells from thoracic aorta of monkeys | J Med Chem 32: 2318-25 (1989) BindingDB Entry DOI: 10.7270/Q2QZ2D5R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sterol O-acyltransferase 1 (Rattus norvegicus) | BDBM50227933 (CHEMBL77761) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 2.24E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
American Cyanamid Company Curated by ChEMBL | Assay Description In vitro inhibitory concentration against Muscarinic acetylcholine receptor derived from rat adrenals | J Med Chem 32: 2318-25 (1989) BindingDB Entry DOI: 10.7270/Q2QZ2D5R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sterol O-acyltransferase 1 (Homo sapiens (Human)) | BDBM50227909 (CHEMBL307505) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.62E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
American Cyanamid Company Curated by ChEMBL | Assay Description In vitro inhibitory concentration against acyl coenzyme A:cholesterol acyltransferase derived from smooth muscle cells from thoracic aorta of monkeys | J Med Chem 32: 2318-25 (1989) BindingDB Entry DOI: 10.7270/Q2QZ2D5R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sterol O-acyltransferase 1 (Rattus norvegicus) | BDBM50227932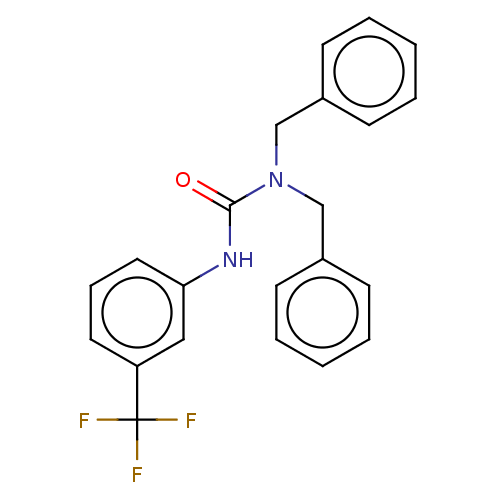 (CHEMBL77164) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 2.86E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
American Cyanamid Company Curated by ChEMBL | Assay Description In vitro inhibitory concentration against Acyl coenzyme A:cholesterol acyltransferase derived from rat adrenals | J Med Chem 32: 2318-25 (1989) BindingDB Entry DOI: 10.7270/Q2QZ2D5R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sterol O-acyltransferase 1 (Homo sapiens (Human)) | BDBM50227937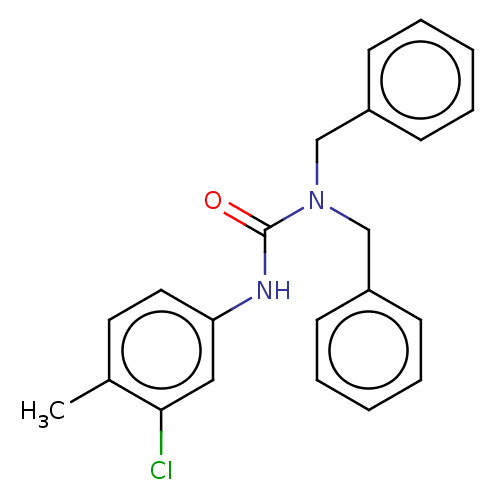 (CHEMBL76258) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents | PubMed | n/a | n/a | 3.01E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
American Cyanamid Company Curated by ChEMBL | Assay Description In vitro inhibitory concentration against Acyl coenzyme A:cholesterol acyltransferase derived from smooth muscle cells from thoracic aorta of monkeys | J Med Chem 32: 2318-25 (1989) BindingDB Entry DOI: 10.7270/Q2QZ2D5R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sterol O-acyltransferase 1 (Rattus norvegicus) | BDBM50227948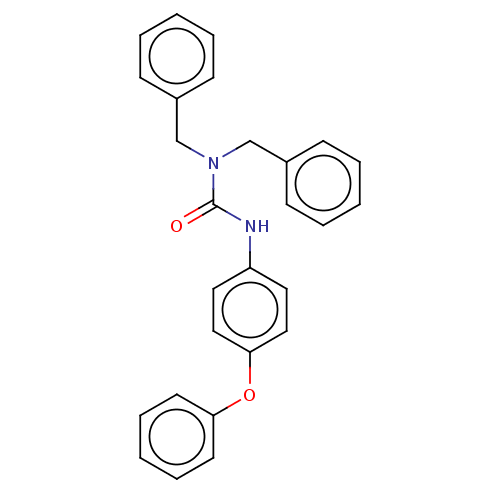 (CHEMBL75596) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.18E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
American Cyanamid Company Curated by ChEMBL | Assay Description In vitro inhibitory concentration against acyl coenzyme A:cholesterol acyltransferase derived from rat adrenals | J Med Chem 32: 2318-25 (1989) BindingDB Entry DOI: 10.7270/Q2QZ2D5R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sterol O-acyltransferase 1 (Rattus norvegicus) | BDBM50227944 (CHEMBL77942) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.28E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
American Cyanamid Company Curated by ChEMBL | Assay Description In vitro inhibitory concentration against Acyl coenzyme A:cholesterol acyltransferase derived from rat adrenals | J Med Chem 32: 2318-25 (1989) BindingDB Entry DOI: 10.7270/Q2QZ2D5R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-1 adrenergic receptor (Rattus norvegicus (Rat)) | BDBM50002133 ((+/-)-2-(4-((R)-2-((R)-2-(3-chlorophenyl)-2-hydrox...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 3.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
American Cyanamid Company Curated by ChEMBL | Assay Description Compound was evaluated for its binding affinity towards Beta-1 adrenergic receptor in rat heart membrane by displacing [125I]- iodocyanopindolol | J Med Chem 35: 3081-4 (1992) BindingDB Entry DOI: 10.7270/Q2PR7TWN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sterol O-acyltransferase 1 (Rattus norvegicus) | BDBM50227929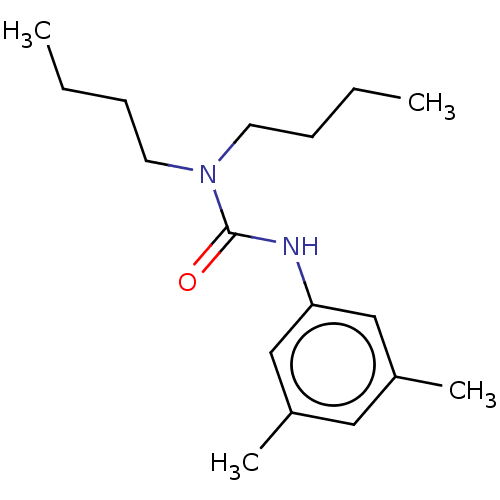 (CHEMBL419639) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.54E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
American Cyanamid Company Curated by ChEMBL | Assay Description In vitro inhibitory concentration against acyl coenzyme A:cholesterol acyltransferase derived from rat adrenals | J Med Chem 32: 2318-25 (1989) BindingDB Entry DOI: 10.7270/Q2QZ2D5R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sterol O-acyltransferase 1 (Rattus norvegicus) | BDBM50227934 (CHEMBL75371) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 3.62E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
American Cyanamid Company Curated by ChEMBL | Assay Description In vitro inhibitory concentration against Acyl coenzyme A:cholesterol acyltransferase derived from rat adrenals | J Med Chem 32: 2318-25 (1989) BindingDB Entry DOI: 10.7270/Q2QZ2D5R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sterol O-acyltransferase 1 (Homo sapiens (Human)) | BDBM50227906 (CHEMBL74091) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.62E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
American Cyanamid Company Curated by ChEMBL | Assay Description In vitro inhibitory concentration against acyl coenzyme A:cholesterol acyltransferase derived from smooth muscle cells from thoracic aorta of monkeys | J Med Chem 32: 2318-25 (1989) BindingDB Entry DOI: 10.7270/Q2QZ2D5R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sterol O-acyltransferase 1 (Homo sapiens (Human)) | BDBM50227902 (CHEMBL76706) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.77E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
American Cyanamid Company Curated by ChEMBL | Assay Description In vitro inhibitory concentration against Acyl coenzyme A:cholesterol acyltransferase derived from smooth muscle cells from thoracic aorta of monkeys | J Med Chem 32: 2318-25 (1989) BindingDB Entry DOI: 10.7270/Q2QZ2D5R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sterol O-acyltransferase 1 (Rattus norvegicus) | BDBM50227917 (CHEMBL77694) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 3.89E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
American Cyanamid Company Curated by ChEMBL | Assay Description In vitro inhibitory concentration against Acyl coenzyme A:cholesterol acyltransferase derived from rat adrenals | J Med Chem 32: 2318-25 (1989) BindingDB Entry DOI: 10.7270/Q2QZ2D5R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sterol O-acyltransferase 1 (Rattus norvegicus) | BDBM50227925 (CHEMBL75331) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 4.04E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
American Cyanamid Company Curated by ChEMBL | Assay Description In vitro inhibitory concentration against acyl coenzyme A:cholesterol acyltransferase derived from rat adrenals | J Med Chem 32: 2318-25 (1989) BindingDB Entry DOI: 10.7270/Q2QZ2D5R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sterol O-acyltransferase 1 (Rattus norvegicus) | BDBM50227927 (CHEMBL442178) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4.41E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
American Cyanamid Company Curated by ChEMBL | Assay Description In vitro inhibitory concentration against acyl coenzyme A:cholesterol acyltransferase derived from rat adrenals | J Med Chem 32: 2318-25 (1989) BindingDB Entry DOI: 10.7270/Q2QZ2D5R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sterol O-acyltransferase 1 (Homo sapiens (Human)) | BDBM50227910 (CHEMBL305937) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 4.53E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
American Cyanamid Company Curated by ChEMBL | Assay Description In vitro inhibitory concentration against Acyl coenzyme A:cholesterol acyltransferase derived from smooth muscle cells from thoracic aorta of monkeys | J Med Chem 32: 2318-25 (1989) BindingDB Entry DOI: 10.7270/Q2QZ2D5R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sterol O-acyltransferase 1 (Rattus norvegicus) | BDBM50227923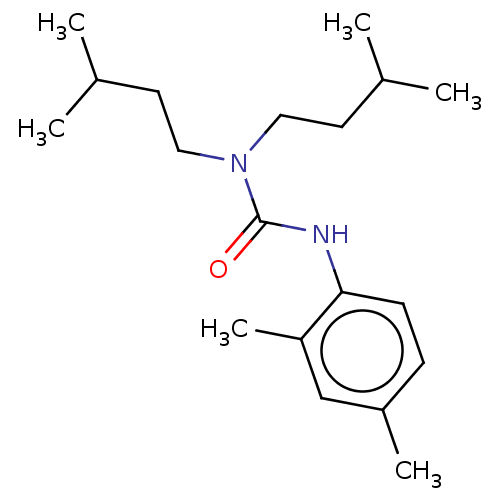 (CHEMBL305913) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 4.59E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
American Cyanamid Company Curated by ChEMBL | Assay Description In vitro inhibitory concentration against acyl coenzyme A:cholesterol acyltransferase derived from rat adrenals | J Med Chem 32: 2318-25 (1989) BindingDB Entry DOI: 10.7270/Q2QZ2D5R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sterol O-acyltransferase 1 (Rattus norvegicus) | BDBM50227915 (CHEMBL421876) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 4.83E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
American Cyanamid Company Curated by ChEMBL | Assay Description In vitro inhibitory concentration against acyl coenzyme A:cholesterol acyltransferase derived from rat adrenals | J Med Chem 32: 2318-25 (1989) BindingDB Entry DOI: 10.7270/Q2QZ2D5R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sterol O-acyltransferase 1 (Rattus norvegicus) | BDBM50227924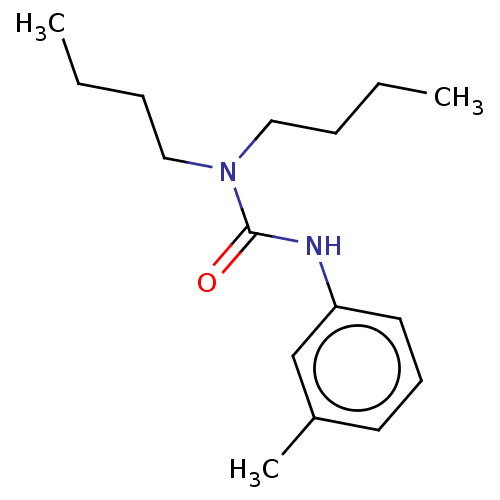 (CHEMBL307709) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 4.95E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
American Cyanamid Company Curated by ChEMBL | Assay Description In vitro inhibitory concentration against acyl coenzyme A:cholesterol acyltransferase derived from rat adrenals | J Med Chem 32: 2318-25 (1989) BindingDB Entry DOI: 10.7270/Q2QZ2D5R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sterol O-acyltransferase 1 (Rattus norvegicus) | BDBM50227928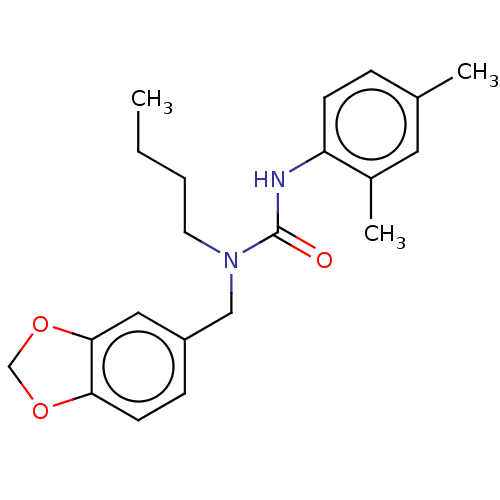 (CHEMBL75354) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 5.64E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
American Cyanamid Company Curated by ChEMBL | Assay Description In vitro inhibitory concentration against acyl coenzyme A:cholesterol acyltransferase derived from rat adrenals | J Med Chem 32: 2318-25 (1989) BindingDB Entry DOI: 10.7270/Q2QZ2D5R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sterol O-acyltransferase 1 (Rattus norvegicus) | BDBM50227930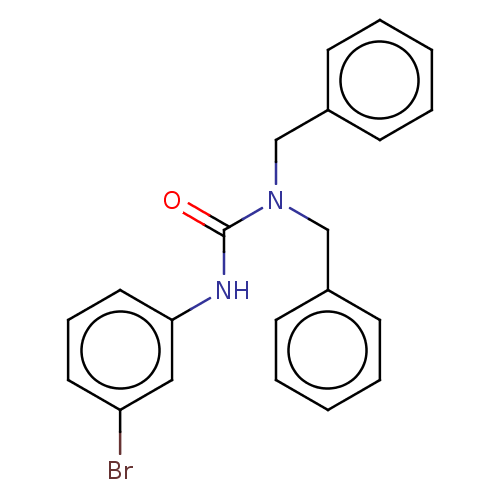 (CHEMBL77943) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 5.81E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
American Cyanamid Company Curated by ChEMBL | Assay Description In vitro inhibitory concentration against acyl coenzyme A:cholesterol acyltransferase derived from rat adrenals | J Med Chem 32: 2318-25 (1989) BindingDB Entry DOI: 10.7270/Q2QZ2D5R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sterol O-acyltransferase 1 (Homo sapiens (Human)) | BDBM50022279 (3-(2,4-Difluoro-phenyl)-1-[4-(2,2-dimethyl-propyl)...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | n/a | n/a | 6.06E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of smooth muscle cell ACAT activity for cells stimulated by native LDL. | J Med Chem 29: 1131-3 (1987) BindingDB Entry DOI: 10.7270/Q2V69HKP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 84 total ) | Next | Last >> |