

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 5-hydroxytryptamine receptor 1F (Homo sapiens (Human)) | BDBM50106483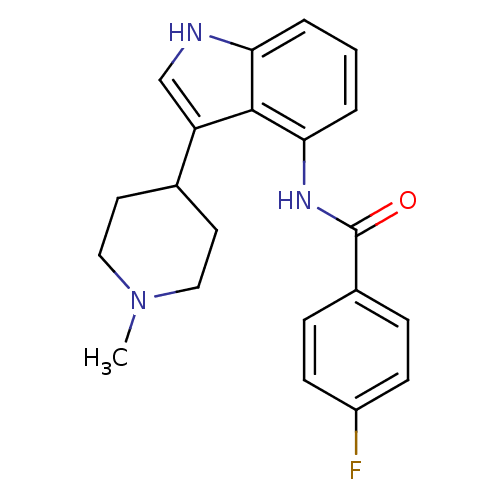 (4-Fluoro-N-[3-(1-methyl-piperidin-4-yl)-1H-indol-4...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Binding affinity towards human 5-hydroxytryptamine 1F receptor | J Med Chem 44: 4031-4 (2001) BindingDB Entry DOI: 10.7270/Q22Z168B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1F (Homo sapiens (Human)) | BDBM50106484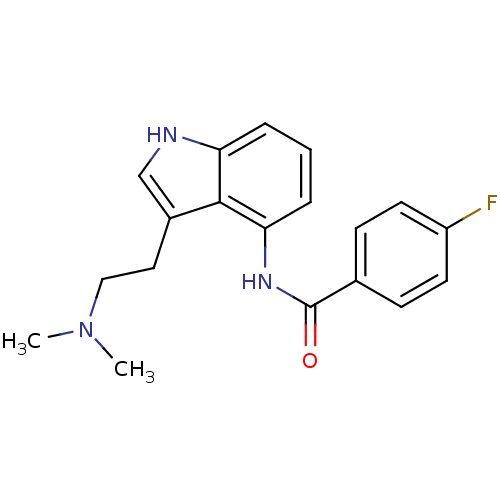 (CHEMBL421287 | N-[3-(2-Dimethylamino-ethyl)-1H-ind...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 3.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Binding affinity towards human 5-hydroxytryptamine 1F receptor | J Med Chem 44: 4031-4 (2001) BindingDB Entry DOI: 10.7270/Q22Z168B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1F (Homo sapiens (Human)) | BDBM50106482 (CHEMBL339980 | N-[3-(2-Dimethylamino-ethyl)-2-meth...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 8.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Binding affinity towards human 5-hydroxytryptamine 1F receptor using [3H]-5-HT radioligand | J Med Chem 44: 4031-4 (2001) BindingDB Entry DOI: 10.7270/Q22Z168B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1F (Homo sapiens (Human)) | BDBM50005835 ((3-[2-(dimethylamino)ethyl]-1H-indol-5-yl)-N-methy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Patents | DrugBank PubMed | 25.7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Binding affinity towards human 5-hydroxytryptamine 1F receptor was determined | J Med Chem 44: 4031-4 (2001) BindingDB Entry DOI: 10.7270/Q22Z168B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50106482 (CHEMBL339980 | N-[3-(2-Dimethylamino-ethyl)-2-meth...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 265 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Binding affinity towards human 5-hydroxytryptamine 1A receptor was determined using [3H]-5-HT as radioligand | J Med Chem 44: 4031-4 (2001) BindingDB Entry DOI: 10.7270/Q22Z168B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 4 (Homo sapiens (Human)) | BDBM50106482 (CHEMBL339980 | N-[3-(2-Dimethylamino-ethyl)-2-meth...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 380 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Binding affinity towards human 5-hydroxytryptamine 4 receptor using [3H]5-HT as radioligand | J Med Chem 44: 4031-4 (2001) BindingDB Entry DOI: 10.7270/Q22Z168B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2B (Homo sapiens (Human)) | BDBM50106482 (CHEMBL339980 | N-[3-(2-Dimethylamino-ethyl)-2-meth...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 676 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Binding affinity towards human 5-hydroxytryptamine 2B receptor using [3H]5-HT as radioligand | J Med Chem 44: 4031-4 (2001) BindingDB Entry DOI: 10.7270/Q22Z168B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM50106482 (CHEMBL339980 | N-[3-(2-Dimethylamino-ethyl)-2-meth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Binding affinity towards human 5-hydroxytryptamine 2A receptor using [125I]DOI as radioligand | J Med Chem 44: 4031-4 (2001) BindingDB Entry DOI: 10.7270/Q22Z168B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1B (Homo sapiens (Human)) | BDBM50106482 (CHEMBL339980 | N-[3-(2-Dimethylamino-ethyl)-2-meth...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.06E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Binding affinity towards human 5-hydroxytryptamine 1B receptor using [3H]5-HT radioligand | J Med Chem 44: 4031-4 (2001) BindingDB Entry DOI: 10.7270/Q22Z168B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 7 (Homo sapiens (Human)) | BDBM50106482 (CHEMBL339980 | N-[3-(2-Dimethylamino-ethyl)-2-meth...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.46E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Binding affinity towards human 5-hydroxytryptamine 7 receptor using [3H]5-HT as radioligand | J Med Chem 44: 4031-4 (2001) BindingDB Entry DOI: 10.7270/Q22Z168B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1D (Homo sapiens (Human)) | BDBM50106482 (CHEMBL339980 | N-[3-(2-Dimethylamino-ethyl)-2-meth...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.62E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Binding affinity towards human 5-hydroxytryptamine 1D receptor was determined using [3H]5-HT as radioligand | J Med Chem 44: 4031-4 (2001) BindingDB Entry DOI: 10.7270/Q22Z168B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM50106482 (CHEMBL339980 | N-[3-(2-Dimethylamino-ethyl)-2-meth...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.77E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Binding affinity towards human 5-hydroxytryptamine 6 receptor using [3H]LSD as radioligand | J Med Chem 44: 4031-4 (2001) BindingDB Entry DOI: 10.7270/Q22Z168B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2C (Homo sapiens (Human)) | BDBM50106482 (CHEMBL339980 | N-[3-(2-Dimethylamino-ethyl)-2-meth...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Binding affinity towards human 5-HT2C receptor was determined using [125I]- DOI as radioligand | J Med Chem 44: 4031-4 (2001) BindingDB Entry DOI: 10.7270/Q22Z168B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1E (Homo sapiens (Human)) | BDBM50106482 (CHEMBL339980 | N-[3-(2-Dimethylamino-ethyl)-2-meth...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | >4.83E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Binding affinity towards human 5-hydroxytryptamine 1E receptor was determined using [3H]5-HT as radioligand | J Med Chem 44: 4031-4 (2001) BindingDB Entry DOI: 10.7270/Q22Z168B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (RAT) | BDBM50106482 (CHEMBL339980 | N-[3-(2-Dimethylamino-ethyl)-2-meth...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Binding affinity towards rat histamine H1 receptor was determined using [3H]pyrilamine as radioligand | J Med Chem 44: 4031-4 (2001) BindingDB Entry DOI: 10.7270/Q22Z168B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50106482 (CHEMBL339980 | N-[3-(2-Dimethylamino-ethyl)-2-meth...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 3.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Binding affinity towards rat dopamine D2 receptor was determined using [3H]raclopride as radioligand | J Med Chem 44: 4031-4 (2001) BindingDB Entry DOI: 10.7270/Q22Z168B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A) dopamine receptor (RAT) | BDBM50106482 (CHEMBL339980 | N-[3-(2-Dimethylamino-ethyl)-2-meth...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Binding affinity towards rat dopamine receptor D1 was determined using [3H]SCH-23390 radioligand | J Med Chem 44: 4031-4 (2001) BindingDB Entry DOI: 10.7270/Q22Z168B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-2A adrenergic receptor [16-465]/Alpha-2B adrenergic receptor/Alpha-2C adrenergic receptor (RAT-NEONATAL RAT-Rattus norvegicus (rat)) | BDBM29568 (CHEMBL2 | PRAZOSIN | PRAZOSIN HYDROCHLORIDE | [3H]...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of Angiotensin I converting enzyme | J Med Chem 28: 1142-5 (1985) BindingDB Entry DOI: 10.7270/Q21V5H5W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A/Alpha-1B/Alpha-1D adrenergic receptor (Rattus norvegicus (rat)-Rattus norvegicus (Rat)) | BDBM50225742 (CHEMBL537973) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of Angiotensin I converting enzyme | J Med Chem 28: 1142-5 (1985) BindingDB Entry DOI: 10.7270/Q21V5H5W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A/Alpha-1B/Alpha-1D adrenergic receptor (Rattus norvegicus (rat)-Rattus norvegicus (Rat)) | BDBM50225741 (CHEMBL15359) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 45 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of Angiotensin I converting enzyme | J Med Chem 28: 1142-5 (1985) BindingDB Entry DOI: 10.7270/Q21V5H5W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A/Alpha-1B/Alpha-1D adrenergic receptor (Rattus norvegicus (rat)-Rattus norvegicus (Rat)) | BDBM50225740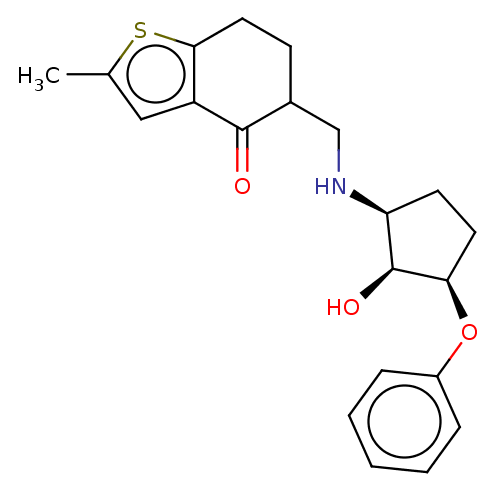 (CHEMBL15201) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 55 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of Angiotensin I converting enzyme | J Med Chem 28: 1142-5 (1985) BindingDB Entry DOI: 10.7270/Q21V5H5W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A/Alpha-1B/Alpha-1D adrenergic receptor (Rattus norvegicus (rat)-Rattus norvegicus (Rat)) | BDBM50225743 (CHEMBL14987) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of Angiotensin I converting enzyme | J Med Chem 28: 1142-5 (1985) BindingDB Entry DOI: 10.7270/Q21V5H5W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-2A adrenergic receptor [16-465]/Alpha-2B adrenergic receptor/Alpha-2C adrenergic receptor (RAT-NEONATAL RAT-Rattus norvegicus (rat)) | BDBM50225741 (CHEMBL15359) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of Angiotensin I converting enzyme | J Med Chem 28: 1142-5 (1985) BindingDB Entry DOI: 10.7270/Q21V5H5W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-2A adrenergic receptor [16-465]/Alpha-2B adrenergic receptor/Alpha-2C adrenergic receptor (RAT-NEONATAL RAT-Rattus norvegicus (rat)) | BDBM50225740 (CHEMBL15201) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of Angiotensin I converting enzyme | J Med Chem 28: 1142-5 (1985) BindingDB Entry DOI: 10.7270/Q21V5H5W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-2A adrenergic receptor [16-465]/Alpha-2B adrenergic receptor/Alpha-2C adrenergic receptor (RAT-NEONATAL RAT-Rattus norvegicus (rat)) | BDBM50225742 (CHEMBL537973) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 800 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of Angiotensin I converting enzyme | J Med Chem 28: 1142-5 (1985) BindingDB Entry DOI: 10.7270/Q21V5H5W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-2A adrenergic receptor [16-465]/Alpha-2B adrenergic receptor/Alpha-2C adrenergic receptor (RAT-NEONATAL RAT-Rattus norvegicus (rat)) | BDBM50225743 (CHEMBL14987) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of Angiotensin I converting enzyme | J Med Chem 28: 1142-5 (1985) BindingDB Entry DOI: 10.7270/Q21V5H5W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-2A adrenergic receptor [16-465]/Alpha-2B adrenergic receptor/Alpha-2C adrenergic receptor (RAT-NEONATAL RAT-Rattus norvegicus (rat)) | BDBM29568 (CHEMBL2 | PRAZOSIN | PRAZOSIN HYDROCHLORIDE | [3H]...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of Angiotensin I converting enzyme | J Med Chem 28: 1142-5 (1985) BindingDB Entry DOI: 10.7270/Q21V5H5W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1F (Homo sapiens (Human)) | BDBM50106482 (CHEMBL339980 | N-[3-(2-Dimethylamino-ethyl)-2-meth...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 6 | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description In vitro effective concentration for inhibition of skolin-stimulated adenylate cyclase in cell line expressing human 5-hydroxytryptamine 1F receptor | J Med Chem 44: 4031-4 (2001) BindingDB Entry DOI: 10.7270/Q22Z168B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1F (Homo sapiens (Human)) | BDBM10755 (14C-5-hydroxy tryptamine creatinine disulfate | 2-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 7.90 | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description In vitro effective concentration for inhibition of skolin-stimulated adenylate cyclase in cell line expressing human 5-hydroxytryptamine 1F receptor | J Med Chem 44: 4031-4 (2001) BindingDB Entry DOI: 10.7270/Q22Z168B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1F (Homo sapiens (Human)) | BDBM50005835 ((3-[2-(dimethylamino)ethyl]-1H-indol-5-yl)-N-methy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Patents | DrugBank PubMed | n/a | n/a | n/a | n/a | 35 | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description In vitro effective concentration for inhibition of skolin-stimulated adenylate cyclase in cell line expressing human 5-hydroxytryptamine 1F receptor | J Med Chem 44: 4031-4 (2001) BindingDB Entry DOI: 10.7270/Q22Z168B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||