

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Neuromedin-K receptor (Homo sapiens (Human)) | BDBM50291261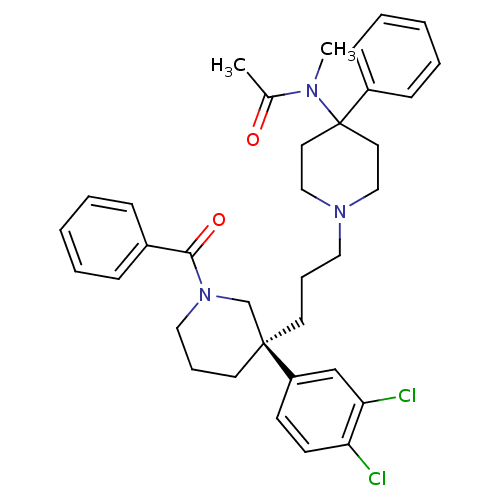 ((S)-(+)-N-((3-[1-Benzoyl-3-(3,4-dichlorophenyl)pip...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid UniChem Patents Similars | Article | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Tachykinin receptor 3 binding affinity was determined by incubation with CHO cells expressing human NK3 receptors | Bioorg Med Chem Lett 7: 555-560 (1997) Article DOI: 10.1016/S0960-894X(97)00064-4 BindingDB Entry DOI: 10.7270/Q2MK6CX0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sterol O-acyltransferase 1 (Rattus norvegicus) | BDBM50051461 (CHEMBL264861 | N-(2,6-Diisopropyl-phenyl)-2-(2-dod...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description In vitro inhibitory activity against acyl coenzyme A:cholesterol acyltransferase 1 of liver microsomes isolated from cholesterol-fed rats. | J Med Chem 39: 2354-66 (1996) Article DOI: 10.1021/jm960170f BindingDB Entry DOI: 10.7270/Q2W66JVV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sterol O-acyltransferase 1 (Rattus norvegicus) | BDBM50051466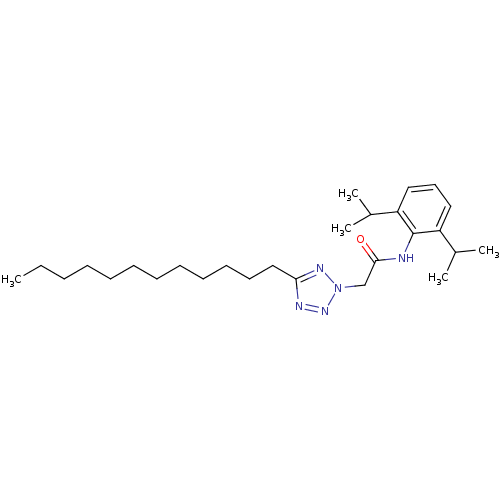 (CHEMBL175982 | N-(2,6-Diisopropyl-phenyl)-2-(5-dod...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description In vitro inhibitory activity against acyl coenzyme A:cholesterol acyltransferase 1 of liver microsomes isolated from cholesterol-fed rats. | J Med Chem 39: 2354-66 (1996) Article DOI: 10.1021/jm960170f BindingDB Entry DOI: 10.7270/Q2W66JVV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM112498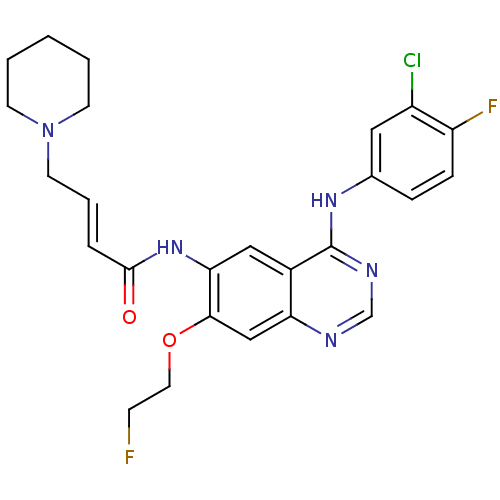 (US8623883, No. 1) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 6.44 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Warner-Lambert Company LLC US Patent | Assay Description Inhibition of erbB tyrosine kinase activity was assessed using an ELISA-based receptor tyrosine kinase assay. Kinase reactions (50 mM HEPES, pH 7.4, ... | US Patent US8623883 (2014) BindingDB Entry DOI: 10.7270/Q29885P7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM112499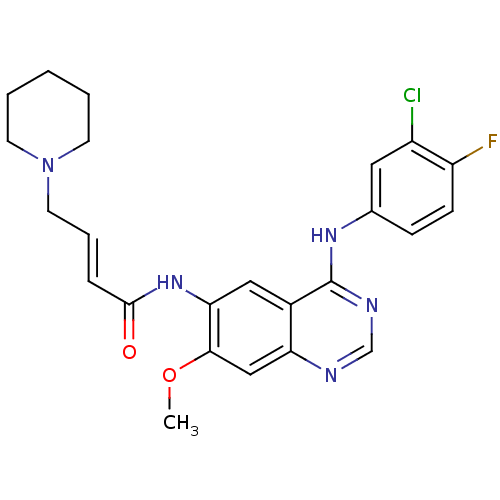 (DACOMITINIB | US8623883, No. 2 | WO2022090481, Exa...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Similars | PDB US Patent | n/a | n/a | 6.90 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Warner-Lambert Company LLC US Patent | Assay Description Inhibition of erbB tyrosine kinase activity was assessed using an ELISA-based receptor tyrosine kinase assay. Kinase reactions (50 mM HEPES, pH 7.4, ... | US Patent US8623883 (2014) BindingDB Entry DOI: 10.7270/Q29885P7 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Acyl-CoA:cholesterol acyltransferase (Oryctolagus cuniculus) | BDBM50069883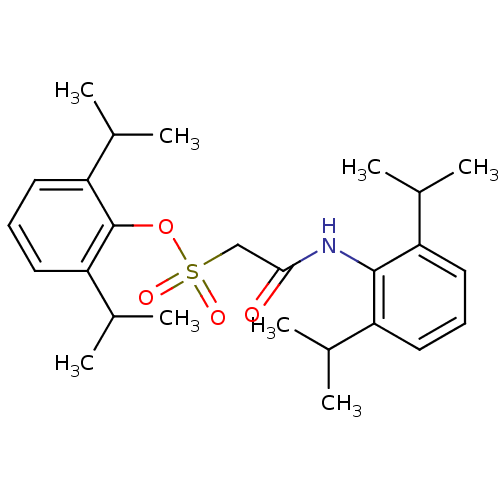 ((2,6-Diisopropyl-phenylcarbamoyl)-methanesulfonic ...) | PDB Reactome pathway KEGG UniProtKB/TrEMBL DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description In vitro inhibition of ACAT by incubation with [1-14C]-oleolyl-CoA and intestinal microsomes isolated from cholesterol-fed rabbits. | Bioorg Med Chem Lett 8: 289-94 (1999) BindingDB Entry DOI: 10.7270/Q2DN446R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acyl-CoA:cholesterol acyltransferase (Oryctolagus cuniculus) | BDBM50069886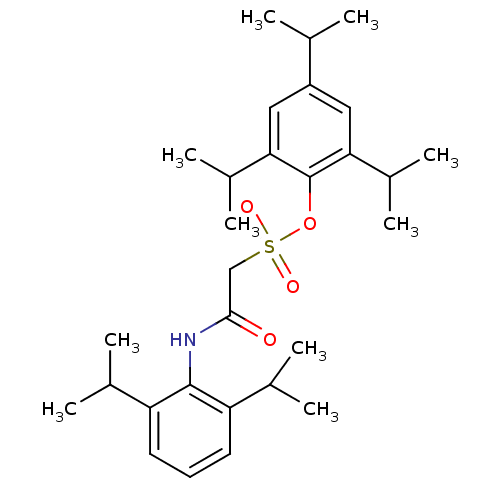 ((2,6-Diisopropyl-phenylcarbamoyl)-methanesulfonic ...) | PDB Reactome pathway KEGG UniProtKB/TrEMBL DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description In vitro inhibition of ACAT by incubation with [1-14C]-oleolyl-CoA and intestinal microsomes isolated from cholesterol-fed rabbits. | Bioorg Med Chem Lett 8: 289-94 (1999) BindingDB Entry DOI: 10.7270/Q2DN446R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acyl-CoA:cholesterol acyltransferase (Oryctolagus cuniculus) | BDBM50039427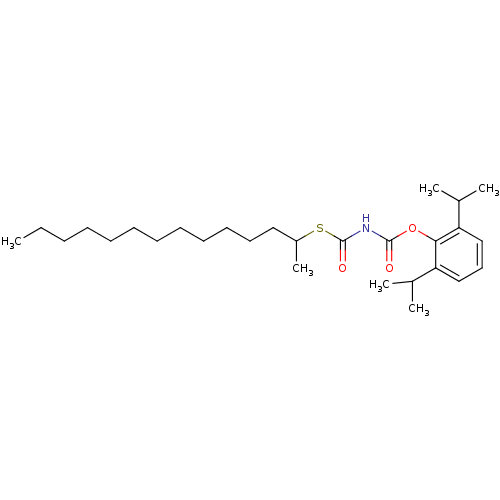 (+/-(1-Methyl-Tridecyl)Thiocarbonyl-carbamic acid 2...) | PDB Reactome pathway KEGG UniProtKB/TrEMBL DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description In vitro Acyl coenzyme A:cholesterol acyltransferase inhibitory activity (IAI) was determined by measuring the incorporation of [1-14C]oleolyl-CoA in... | J Med Chem 37: 2394-400 (1994) BindingDB Entry DOI: 10.7270/Q2XK8G5X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acyl-CoA:cholesterol acyltransferase (Oryctolagus cuniculus) | BDBM50051460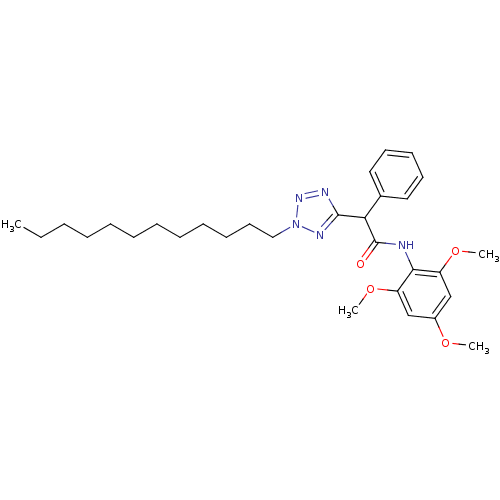 (2-(2-Dodecyl-2H-tetrazol-5-yl)-2-phenyl-N-(2,4,6-t...) | PDB Reactome pathway KEGG UniProtKB/TrEMBL DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Inhibitory concentration against Acyl coenzyme A:cholesterol acyltransferase 1 from rabbit intestine | J Med Chem 39: 2354-66 (1996) Article DOI: 10.1021/jm960170f BindingDB Entry DOI: 10.7270/Q2W66JVV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acyl-CoA:cholesterol acyltransferase (Oryctolagus cuniculus) | BDBM50039428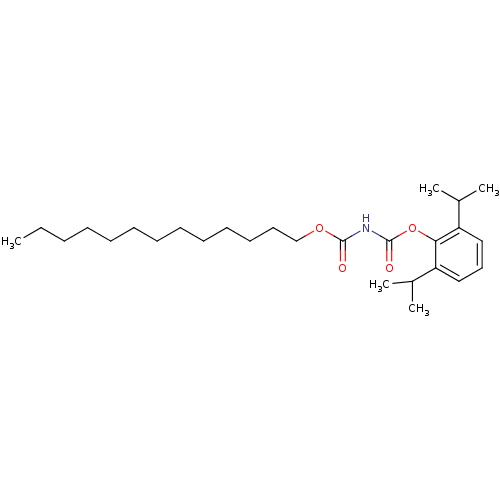 (CHEMBL77339 | Tridecyloxycarbonyl-carbamic acid 2,...) | PDB Reactome pathway KEGG UniProtKB/TrEMBL DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description In vitro Acyl coenzyme A:cholesterol acyltransferase inhibitory activity (IAI) was determined by measuring the incorporation of [1-14C]oleolyl-CoA in... | J Med Chem 37: 2394-400 (1994) BindingDB Entry DOI: 10.7270/Q2XK8G5X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM112500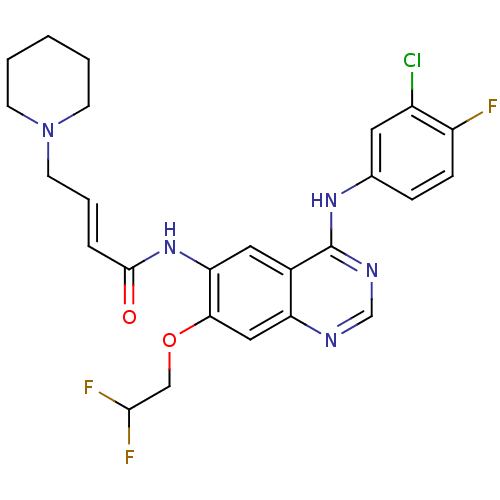 (US8623883, No. 6) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 9.87 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Warner-Lambert Company LLC US Patent | Assay Description Inhibition of erbB tyrosine kinase activity was assessed using an ELISA-based receptor tyrosine kinase assay. Kinase reactions (50 mM HEPES, pH 7.4, ... | US Patent US8623883 (2014) BindingDB Entry DOI: 10.7270/Q29885P7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sterol O-acyltransferase 1 (Rattus norvegicus) | BDBM50051456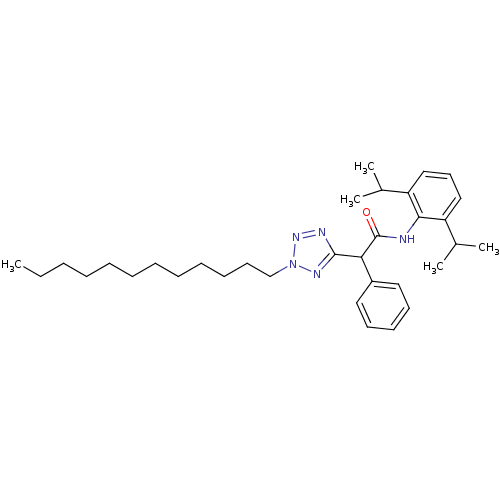 (CHEMBL306344 | N-(2,6-Diisopropyl-phenyl)-2-(2-dod...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description In vitro inhibitory activity against acyl coenzyme A:cholesterol acyltransferase 1 of liver microsomes isolated from cholesterol-fed rats. | J Med Chem 39: 2354-66 (1996) Article DOI: 10.1021/jm960170f BindingDB Entry DOI: 10.7270/Q2W66JVV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acyl-CoA:cholesterol acyltransferase (Oryctolagus cuniculus) | BDBM50039402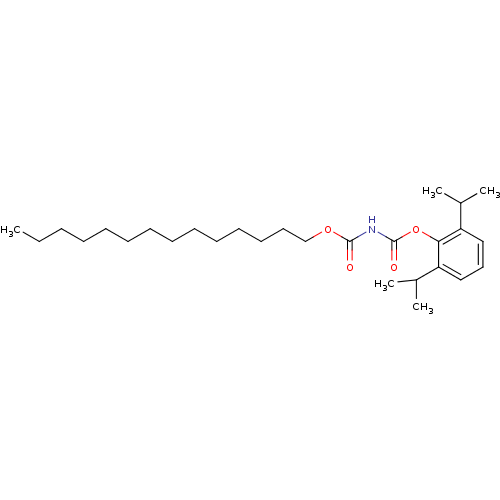 (CHEMBL80332 | Tetradecyloxycarbonyl-carbamic acid ...) | PDB Reactome pathway KEGG UniProtKB/TrEMBL DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description In vitro Acyl coenzyme A:cholesterol acyltransferase inhibitory activity (IAI) was determined by measuring the incorporation of [1-14C]oleolyl-CoA in... | J Med Chem 37: 2394-400 (1994) BindingDB Entry DOI: 10.7270/Q2XK8G5X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM112501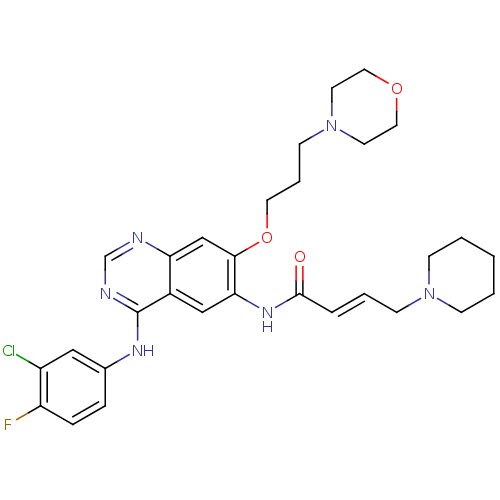 (US8623883, No. 7) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 11.3 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Warner-Lambert Company LLC US Patent | Assay Description Inhibition of erbB tyrosine kinase activity was assessed using an ELISA-based receptor tyrosine kinase assay. Kinase reactions (50 mM HEPES, pH 7.4, ... | US Patent US8623883 (2014) BindingDB Entry DOI: 10.7270/Q29885P7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM112504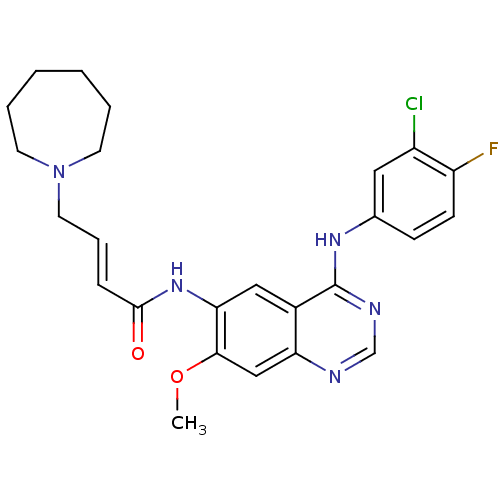 (US8623883, No. 10) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 12.1 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Warner-Lambert Company LLC US Patent | Assay Description Inhibition of erbB tyrosine kinase activity was assessed using an ELISA-based receptor tyrosine kinase assay. Kinase reactions (50 mM HEPES, pH 7.4, ... | US Patent US8623883 (2014) BindingDB Entry DOI: 10.7270/Q29885P7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sterol O-acyltransferase 1 (Rattus norvegicus) | BDBM50051474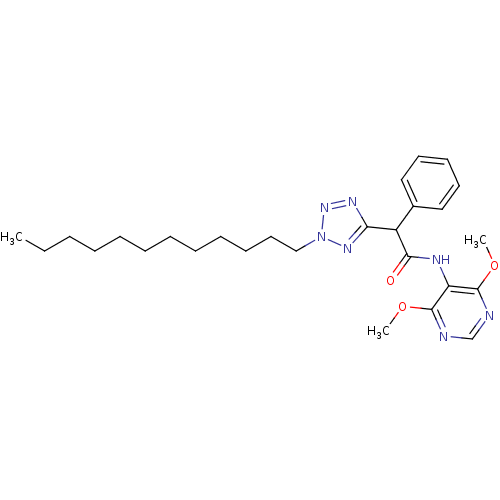 (CHEMBL78038 | N-(4,6-Dimethoxy-pyrimidin-5-yl)-2-(...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description In vitro inhibitory activity against acyl coenzyme A:cholesterol acyltransferase 1 of liver microsomes isolated from cholesterol-fed rats. | J Med Chem 39: 2354-66 (1996) Article DOI: 10.1021/jm960170f BindingDB Entry DOI: 10.7270/Q2W66JVV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sterol O-acyltransferase 1 (Rattus norvegicus) | BDBM50051478 (2-(2-Dodecyl-2H-tetrazol-5-yl)-2-phenyl-N-(1,3,5-t...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description In vitro inhibitory activity against acyl coenzyme A:cholesterol acyltransferase 1 of liver microsomes isolated from cholesterol-fed rats. | J Med Chem 39: 2354-66 (1996) Article DOI: 10.1021/jm960170f BindingDB Entry DOI: 10.7270/Q2W66JVV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sterol O-acyltransferase 1 (Rattus norvegicus) | BDBM50051452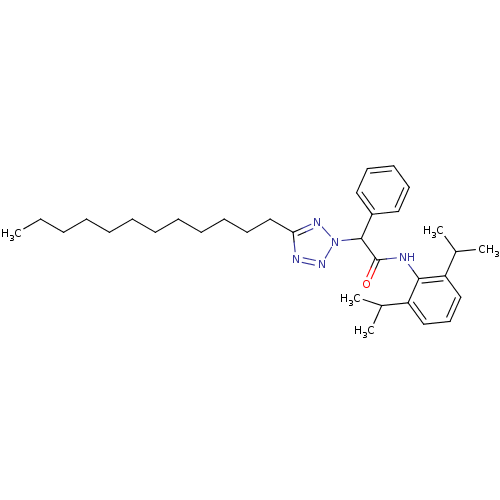 (CHEMBL80270 | N-(2,6-Diisopropyl-phenyl)-2-(5-dode...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description In vitro inhibitory activity against acyl coenzyme A:cholesterol acyltransferase 1 of liver microsomes isolated from cholesterol-fed rats. | J Med Chem 39: 2354-66 (1996) Article DOI: 10.1021/jm960170f BindingDB Entry DOI: 10.7270/Q2W66JVV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acyl-CoA:cholesterol acyltransferase (Oryctolagus cuniculus) | BDBM50039451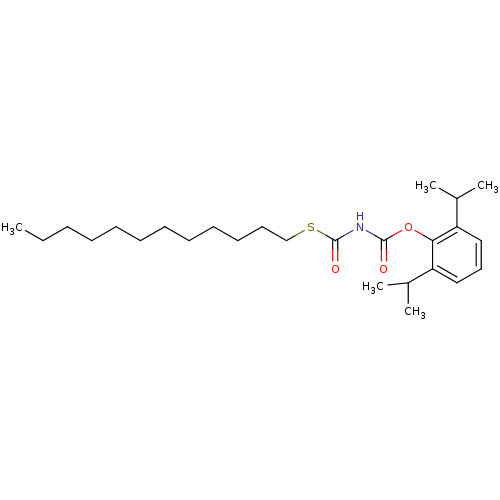 (CHEMBL78577 | Thiocarbomic S-dodecyl-carbamic acid...) | PDB Reactome pathway KEGG UniProtKB/TrEMBL DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description In vitro Acyl coenzyme A:cholesterol acyltransferase inhibitory activity (IAI) was determined by measuring the incorporation of [1-14C]oleolyl-CoA in... | J Med Chem 37: 2394-400 (1994) BindingDB Entry DOI: 10.7270/Q2XK8G5X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor tyrosine-protein kinase erbB-2 (Homo sapiens (Human)) | BDBM112499 (DACOMITINIB | US8623883, No. 2 | WO2022090481, Exa...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Similars | US Patent | n/a | n/a | 16.7 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Warner-Lambert Company LLC US Patent | Assay Description Inhibition of erbB tyrosine kinase activity was assessed using an ELISA-based receptor tyrosine kinase assay. Kinase reactions (50 mM HEPES, pH 7.4, ... | US Patent US8623883 (2014) BindingDB Entry DOI: 10.7270/Q29885P7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acyl-CoA:cholesterol acyltransferase (Oryctolagus cuniculus) | BDBM50039450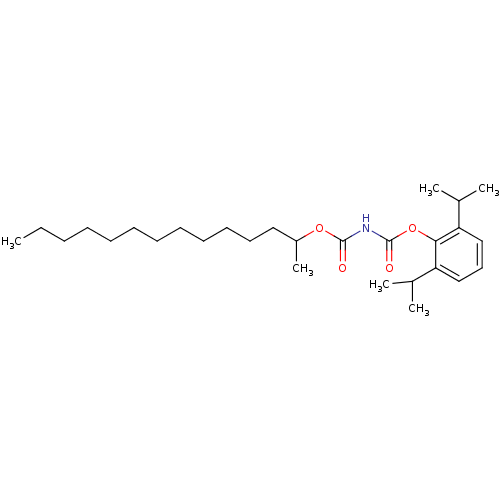 (+/-(1-Methyl-Tridecyloxycarbonyl)-carbamic acid 2,...) | PDB Reactome pathway KEGG UniProtKB/TrEMBL DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description In vitro Acyl coenzyme A:cholesterol acyltransferase inhibitory activity (IAI) was determined by measuring the incorporation of [1-14C]oleolyl-CoA in... | J Med Chem 37: 2394-400 (1994) BindingDB Entry DOI: 10.7270/Q2XK8G5X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM112503 (US8623883, No. 9) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 18.1 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Warner-Lambert Company LLC US Patent | Assay Description Inhibition of erbB tyrosine kinase activity was assessed using an ELISA-based receptor tyrosine kinase assay. Kinase reactions (50 mM HEPES, pH 7.4, ... | US Patent US8623883 (2014) BindingDB Entry DOI: 10.7270/Q29885P7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acyl-CoA:cholesterol acyltransferase (Oryctolagus cuniculus) | BDBM50039439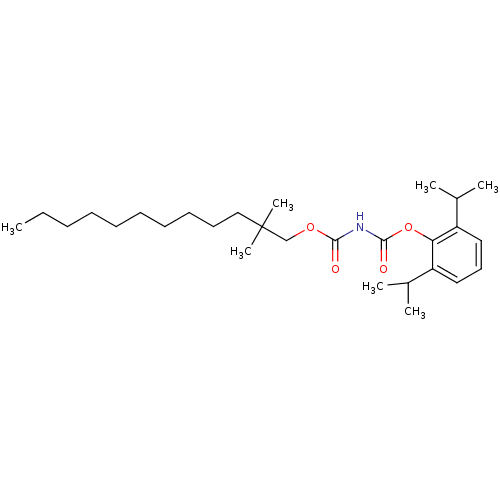 ((2,2-Dimethyl-dodecyloxycarbonyl)-carbamic acid 2,...) | PDB Reactome pathway KEGG UniProtKB/TrEMBL DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description In vitro Acyl coenzyme A:cholesterol acyltransferase inhibitory activity (IAI) was determined by measuring the incorporation of [1-14C]oleolyl-CoA in... | J Med Chem 37: 2394-400 (1994) BindingDB Entry DOI: 10.7270/Q2XK8G5X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acyl-CoA:cholesterol acyltransferase (Oryctolagus cuniculus) | BDBM50069893 ((2,6-Diisopropyl-phenylsulfamoyl)-acetic acid 1-me...) | PDB Reactome pathway KEGG UniProtKB/TrEMBL DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description In vitro inhibition of ACAT by incubation with [1-14C]-oleolyl-CoA and intestinal microsomes isolated from cholesterol-fed rabbits. | Bioorg Med Chem Lett 8: 289-94 (1999) BindingDB Entry DOI: 10.7270/Q2DN446R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acyl-CoA:cholesterol acyltransferase (Oryctolagus cuniculus) | BDBM50039423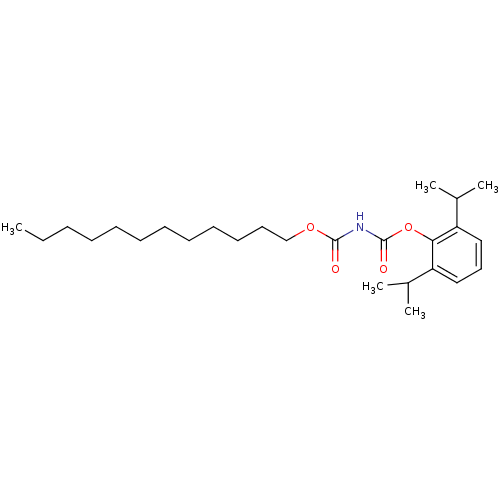 (CHEMBL80841 | Dodecyloxycarbonyl-carbamic acid 2,6...) | PDB Reactome pathway KEGG UniProtKB/TrEMBL DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description In vitro Acyl coenzyme A:cholesterol acyltransferase inhibitory activity (IAI) was determined by measuring the incorporation of [1-14C]oleolyl-CoA in... | J Med Chem 37: 2394-400 (1994) BindingDB Entry DOI: 10.7270/Q2XK8G5X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acyl-CoA:cholesterol acyltransferase (Oryctolagus cuniculus) | BDBM50039419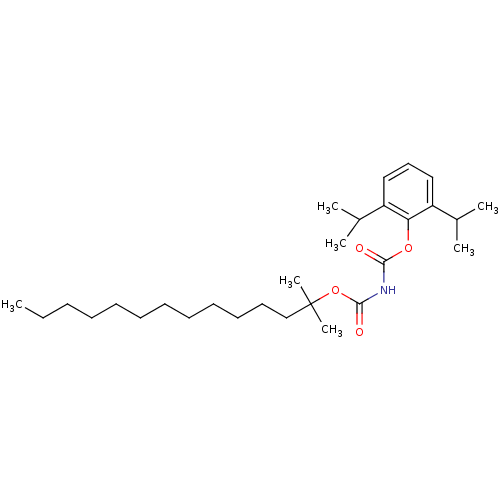 ((1,1-Dimethyl-Tridecyloxycarbonyll)-carbamic acid ...) | PDB Reactome pathway KEGG UniProtKB/TrEMBL DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description In vitro Acyl coenzyme A:cholesterol acyltransferase inhibitory activity (IAI) was determined by measuring the incorporation of [1-14C]oleolyl-CoA in... | J Med Chem 37: 2394-400 (1994) BindingDB Entry DOI: 10.7270/Q2XK8G5X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sterol O-acyltransferase 1 (Rattus norvegicus) | BDBM50051454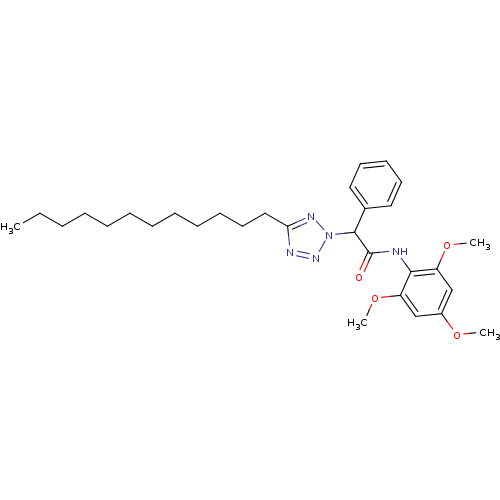 (2-(5-Dodecyl-tetrazol-2-yl)-2-phenyl-N-(2,4,6-trim...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description In vitro inhibitory activity against acyl coenzyme A:cholesterol acyltransferase 1 of liver microsomes isolated from cholesterol-fed rats. | J Med Chem 39: 2354-66 (1996) Article DOI: 10.1021/jm960170f BindingDB Entry DOI: 10.7270/Q2W66JVV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sterol O-acyltransferase 1 (Rattus norvegicus) | BDBM50051460 (2-(2-Dodecyl-2H-tetrazol-5-yl)-2-phenyl-N-(2,4,6-t...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Inhibitory concentration against Acyl coenzyme A:cholesterol acyltransferase 1 from rat liver | J Med Chem 39: 2354-66 (1996) Article DOI: 10.1021/jm960170f BindingDB Entry DOI: 10.7270/Q2W66JVV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sterol O-acyltransferase 1 (Rattus norvegicus) | BDBM50051460 (2-(2-Dodecyl-2H-tetrazol-5-yl)-2-phenyl-N-(2,4,6-t...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description In vitro inhibitory activity against acyl coenzyme A:cholesterol acyltransferase 1 of liver microsomes isolated from cholesterol-fed rats. | J Med Chem 39: 2354-66 (1996) Article DOI: 10.1021/jm960170f BindingDB Entry DOI: 10.7270/Q2W66JVV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sterol O-acyltransferase 1 (Rattus norvegicus) | BDBM50051445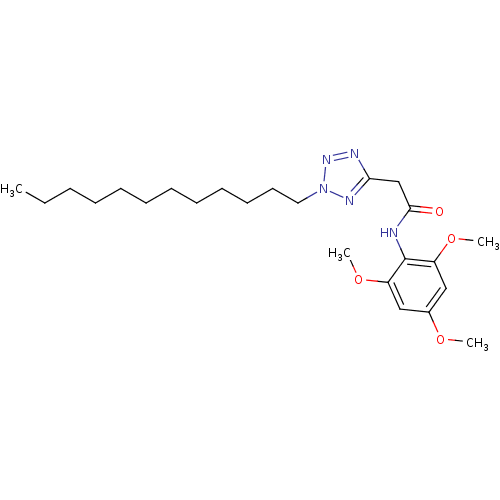 (2-(2-Dodecyl-2H-tetrazol-5-yl)-N-(2,4,6-trimethoxy...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description In vitro inhibitory activity against acyl coenzyme A:cholesterol acyltransferase 1 of liver microsomes isolated from cholesterol-fed rats. | J Med Chem 39: 2354-66 (1996) Article DOI: 10.1021/jm960170f BindingDB Entry DOI: 10.7270/Q2W66JVV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sterol O-acyltransferase 1 (Rattus norvegicus) | BDBM50051469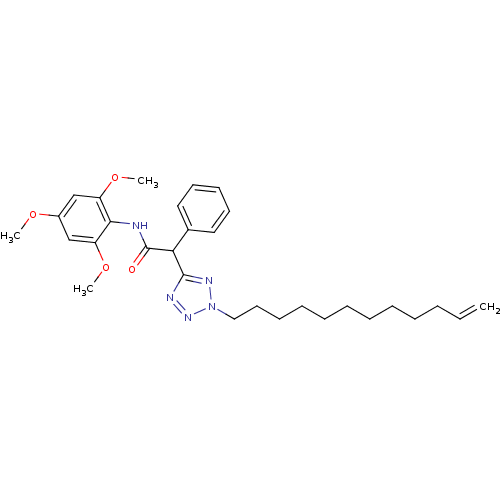 (2-(2-Dodec-11-enyl-2H-tetrazol-5-yl)-2-phenyl-N-(2...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description In vitro inhibitory activity against acyl coenzyme A:cholesterol acyltransferase 1 of liver microsomes isolated from cholesterol-fed rats. | J Med Chem 39: 2354-66 (1996) Article DOI: 10.1021/jm960170f BindingDB Entry DOI: 10.7270/Q2W66JVV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sterol O-acyltransferase 1 (Rattus norvegicus) | BDBM50051465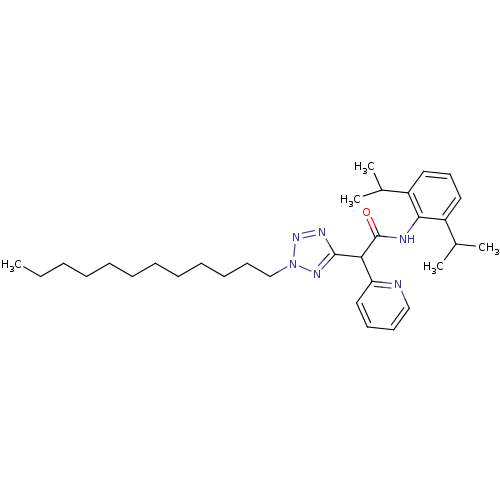 (CHEMBL78106 | N-(2,6-Diisopropyl-phenyl)-2-(2-dode...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description In vitro inhibitory activity against acyl coenzyme A:cholesterol acyltransferase 1 of liver microsomes isolated from cholesterol-fed rats. | J Med Chem 39: 2354-66 (1996) Article DOI: 10.1021/jm960170f BindingDB Entry DOI: 10.7270/Q2W66JVV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sterol O-acyltransferase 1 (Rattus norvegicus) | BDBM50051458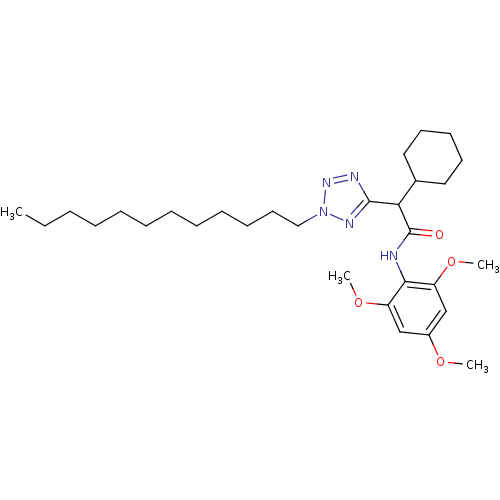 (2-Cyclohexyl-2-(2-dodecyl-2H-tetrazol-5-yl)-N-(2,4...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description In vitro inhibitory activity against acyl coenzyme A:cholesterol acyltransferase 1 of liver microsomes isolated from cholesterol-fed rats. | J Med Chem 39: 2354-66 (1996) Article DOI: 10.1021/jm960170f BindingDB Entry DOI: 10.7270/Q2W66JVV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sterol O-acyltransferase 1 (Rattus norvegicus) | BDBM50051459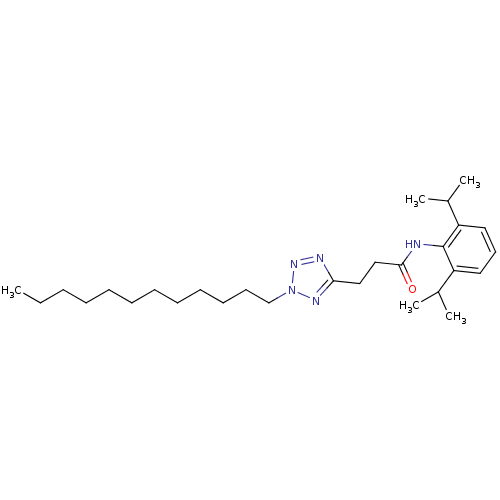 (CHEMBL77319 | N-(2,6-Diisopropyl-phenyl)-3-(2-dode...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description In vitro inhibitory activity against acyl coenzyme A:cholesterol acyltransferase 1 of liver microsomes isolated from cholesterol-fed rats. | J Med Chem 39: 2354-66 (1996) Article DOI: 10.1021/jm960170f BindingDB Entry DOI: 10.7270/Q2W66JVV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sterol O-acyltransferase 1 (Rattus norvegicus) | BDBM50051453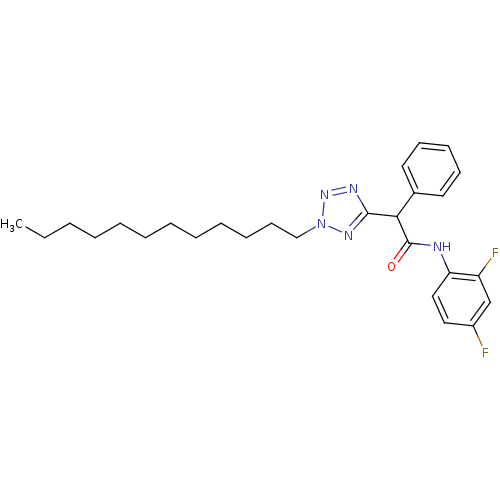 (CHEMBL78270 | N-(2,4-Difluoro-phenyl)-2-(2-dodecyl...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description In vitro inhibitory activity against acyl coenzyme A:cholesterol acyltransferase 1 of liver microsomes isolated from cholesterol-fed rats. | J Med Chem 39: 2354-66 (1996) Article DOI: 10.1021/jm960170f BindingDB Entry DOI: 10.7270/Q2W66JVV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sterol O-acyltransferase 1 (Rattus norvegicus) | BDBM50051455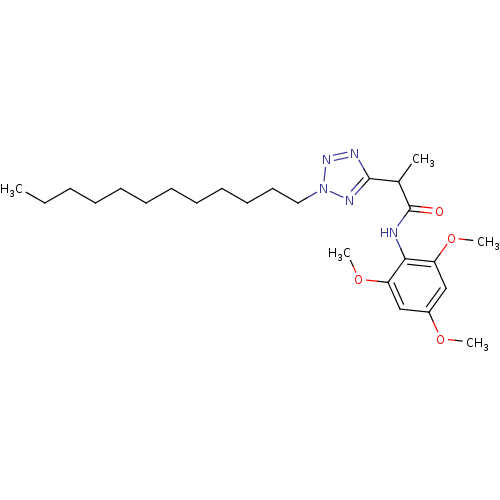 (2-(2-Dodecyl-2H-tetrazol-5-yl)-N-(2,4,6-trimethoxy...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description In vitro inhibitory activity against acyl coenzyme A:cholesterol acyltransferase 1 of liver microsomes isolated from cholesterol-fed rats. | J Med Chem 39: 2354-66 (1996) Article DOI: 10.1021/jm960170f BindingDB Entry DOI: 10.7270/Q2W66JVV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sterol O-acyltransferase 1 (Rattus norvegicus) | BDBM50051476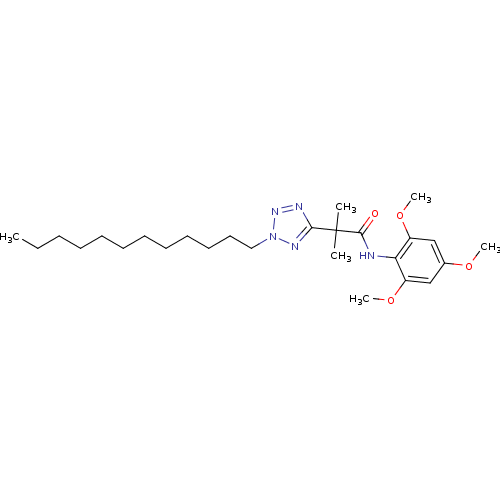 (2-(2-Dodecyl-2H-tetrazol-5-yl)-N-(2,4,6-trimethoxy...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 33 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description In vitro inhibitory activity against acyl coenzyme A:cholesterol acyltransferase 1 of liver microsomes isolated from cholesterol-fed rats. | J Med Chem 39: 2354-66 (1996) Article DOI: 10.1021/jm960170f BindingDB Entry DOI: 10.7270/Q2W66JVV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sterol O-acyltransferase 1 (Rattus norvegicus) | BDBM50051468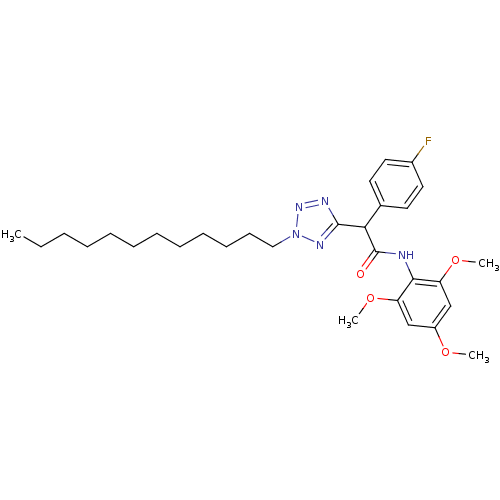 (2-(2-Dodecyl-2H-tetrazol-5-yl)-2-(4-fluoro-phenyl)...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 36 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description In vitro inhibitory activity against acyl coenzyme A:cholesterol acyltransferase 1 of liver microsomes isolated from cholesterol-fed rats. | J Med Chem 39: 2354-66 (1996) Article DOI: 10.1021/jm960170f BindingDB Entry DOI: 10.7270/Q2W66JVV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acyl-CoA:cholesterol acyltransferase (Oryctolagus cuniculus) | BDBM50069890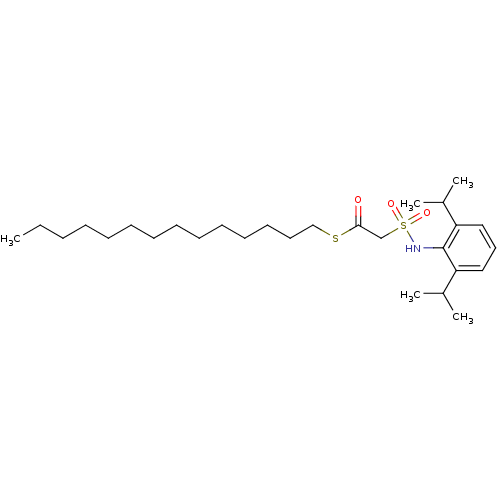 ((2,6-Diisopropyl-phenylsulfamoyl)-thioacetic acid ...) | PDB Reactome pathway KEGG UniProtKB/TrEMBL DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 36 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description In vitro inhibition of ACAT by incubation with [1-14C]-oleolyl-CoA and intestinal microsomes isolated from cholesterol-fed rabbits. | Bioorg Med Chem Lett 8: 289-94 (1999) BindingDB Entry DOI: 10.7270/Q2DN446R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor tyrosine-protein kinase erbB-4 (Homo sapiens (Human)) | BDBM112504 (US8623883, No. 10) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 41.3 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Warner-Lambert Company LLC US Patent | Assay Description Inhibition of erbB tyrosine kinase activity was assessed using an ELISA-based receptor tyrosine kinase assay. Kinase reactions (50 mM HEPES, pH 7.4, ... | US Patent US8623883 (2014) BindingDB Entry DOI: 10.7270/Q29885P7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM112502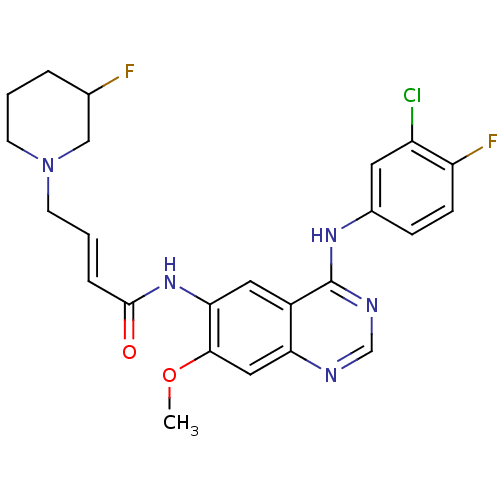 (US8623883, No. 8) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 45.3 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Warner-Lambert Company LLC US Patent | Assay Description Inhibition of erbB tyrosine kinase activity was assessed using an ELISA-based receptor tyrosine kinase assay. Kinase reactions (50 mM HEPES, pH 7.4, ... | US Patent US8623883 (2014) BindingDB Entry DOI: 10.7270/Q29885P7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acyl-CoA:cholesterol acyltransferase (Oryctolagus cuniculus) | BDBM50039401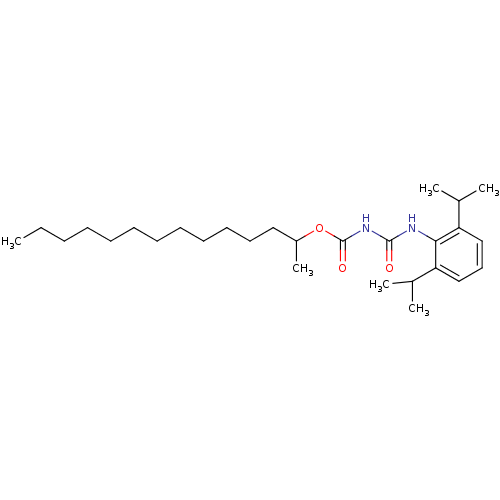 (CHEMBL309744 | [[[2,6-Bis(1-methylethyl)phenyl]-am...) | PDB Reactome pathway KEGG UniProtKB/TrEMBL DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 46 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description In vitro Acyl coenzyme A:cholesterol acyltransferase inhibitory activity (IAI) was determined by measuring the incorporation of [1-14C]oleolyl-CoA in... | J Med Chem 37: 2394-400 (1994) BindingDB Entry DOI: 10.7270/Q2XK8G5X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acyl-CoA:cholesterol acyltransferase (Oryctolagus cuniculus) | BDBM50039407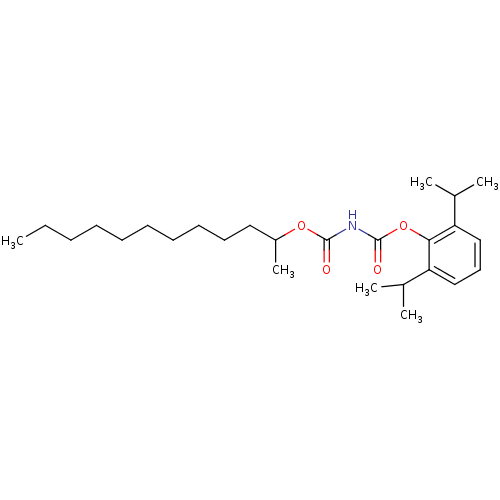 (+/-(1-Methyl-Undecyloxycarbonyl)-carbamic acid 2,6...) | PDB Reactome pathway KEGG UniProtKB/TrEMBL DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 49 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description In vitro Acyl coenzyme A:cholesterol acyltransferase inhibitory activity (IAI) was determined by measuring the incorporation of [1-14C]oleolyl-CoA in... | J Med Chem 37: 2394-400 (1994) BindingDB Entry DOI: 10.7270/Q2XK8G5X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acyl-CoA:cholesterol acyltransferase (Oryctolagus cuniculus) | BDBM50039415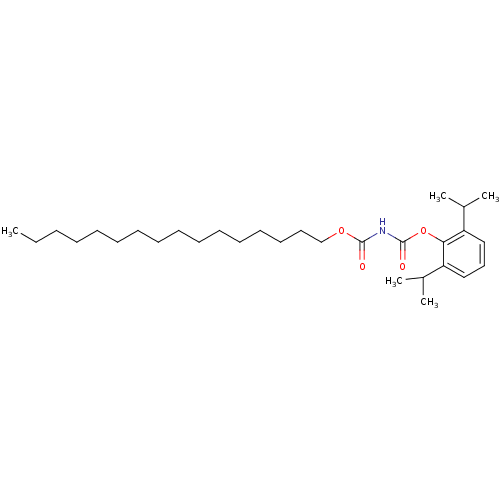 (CHEMBL76835 | Hexadecyloxycarbonyl-carbamic acid 2...) | PDB Reactome pathway KEGG UniProtKB/TrEMBL DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description In vitro Acyl coenzyme A:cholesterol acyltransferase inhibitory activity (IAI) was determined by measuring the incorporation of [1-14C]oleolyl-CoA in... | J Med Chem 37: 2394-400 (1994) BindingDB Entry DOI: 10.7270/Q2XK8G5X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acyl-CoA:cholesterol acyltransferase (Oryctolagus cuniculus) | BDBM50039411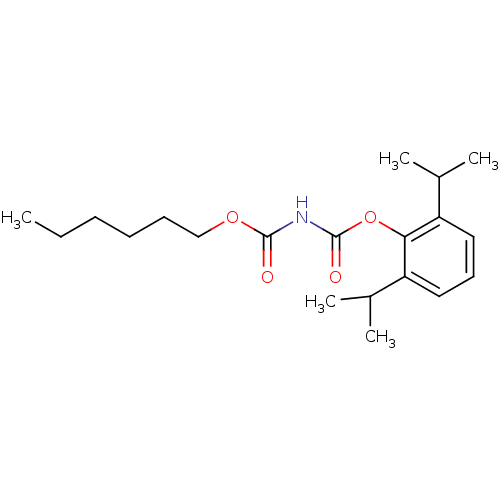 (CHEMBL79163 | hexyloxycarbonyl-carbamic acid 2,6-d...) | PDB Reactome pathway KEGG UniProtKB/TrEMBL DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 53 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description In vitro Acyl coenzyme A:cholesterol acyltransferase inhibitory activity (IAI) was determined by measuring the incorporation of [1-14C]oleolyl-CoA in... | J Med Chem 37: 2394-400 (1994) BindingDB Entry DOI: 10.7270/Q2XK8G5X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sterol O-acyltransferase 1 (Rattus norvegicus) | BDBM50051470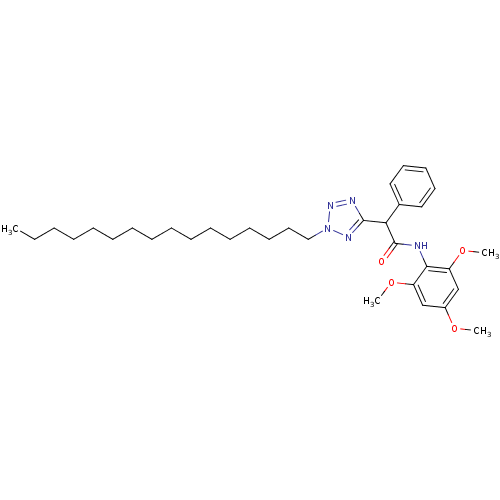 (2-(2-Hexadecyl-2H-tetrazol-5-yl)-2-phenyl-N-(2,4,6...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 53 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description In vitro inhibitory activity against acyl coenzyme A:cholesterol acyltransferase 1 of liver microsomes isolated from cholesterol-fed rats. | J Med Chem 39: 2354-66 (1996) Article DOI: 10.1021/jm960170f BindingDB Entry DOI: 10.7270/Q2W66JVV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sterol O-acyltransferase 1 (Rattus norvegicus) | BDBM50051462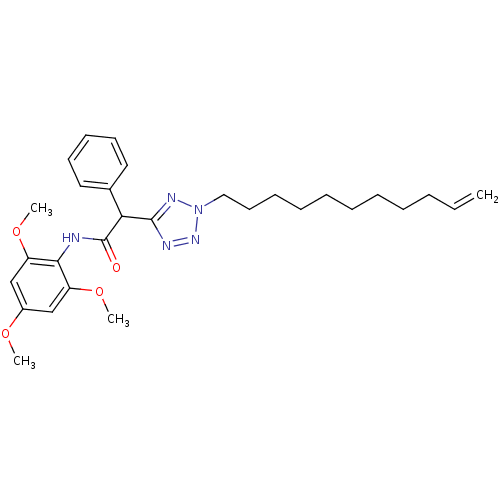 (2-Phenyl-N-(2,4,6-trimethoxy-phenyl)-2-(2-undec-10...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 54 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description In vitro inhibitory activity against acyl coenzyme A:cholesterol acyltransferase 1 of liver microsomes isolated from cholesterol-fed rats. | J Med Chem 39: 2354-66 (1996) Article DOI: 10.1021/jm960170f BindingDB Entry DOI: 10.7270/Q2W66JVV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sterol O-acyltransferase 1 (Rattus norvegicus) | BDBM50051440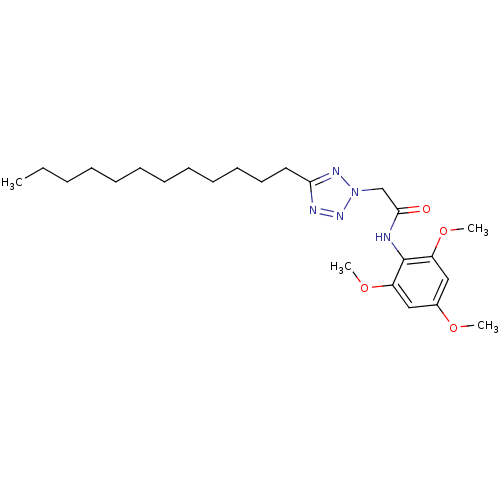 (2-(5-Dodecyl-tetrazol-2-yl)-N-(2,4,6-trimethoxy-ph...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 56 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description In vitro inhibitory activity against acyl coenzyme A:cholesterol acyltransferase 1 of liver microsomes isolated from cholesterol-fed rats. | J Med Chem 39: 2354-66 (1996) Article DOI: 10.1021/jm960170f BindingDB Entry DOI: 10.7270/Q2W66JVV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acyl-CoA:cholesterol acyltransferase (Oryctolagus cuniculus) | BDBM50039424 (+/-(1-Methyl-pentadecyloxycarbonyl)-carbamic acid ...) | PDB Reactome pathway KEGG UniProtKB/TrEMBL DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 58 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description In vitro Acyl coenzyme A:cholesterol acyltransferase inhibitory activity (IAI) was determined by measuring the incorporation of [1-14C]oleolyl-CoA in... | J Med Chem 37: 2394-400 (1994) BindingDB Entry DOI: 10.7270/Q2XK8G5X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor tyrosine-protein kinase erbB-4 (Homo sapiens (Human)) | BDBM112501 (US8623883, No. 7) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 61.5 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Warner-Lambert Company LLC US Patent | Assay Description Inhibition of erbB tyrosine kinase activity was assessed using an ELISA-based receptor tyrosine kinase assay. Kinase reactions (50 mM HEPES, pH 7.4, ... | US Patent US8623883 (2014) BindingDB Entry DOI: 10.7270/Q29885P7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 176 total ) | Next | Last >> |