 Found 83 hits with Last Name = 'lee' and Initial = 'ym'
Found 83 hits with Last Name = 'lee' and Initial = 'ym' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM25737

(12-[(adamantan-1-ylcarbamoyl)amino]dodecanoic acid...)Show SMILES OC(=O)CCCCCCCCCCCNC(=O)NC12CC3CC(CC(C3)C1)C2 |TLB:25:20:27:24.23.26,25:24:20.21.19:27,THB:23:22:19:24.25.26,23:24:19:22.21.27| Show InChI InChI=1S/C23H40N2O3/c26-21(27)10-8-6-4-2-1-3-5-7-9-11-24-22(28)25-23-15-18-12-19(16-23)14-20(13-18)17-23/h18-20H,1-17H2,(H,26,27)(H2,24,25,28) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Chungnam National University
Curated by ChEMBL
| Assay Description
Inhibition of sEH (unknown origin) assessed as substrate PHOME hydrolysis after 1 hr by fluorescence method |
Bioorg Med Chem Lett 24: 1895-900 (2014)
Article DOI: 10.1016/j.bmcl.2014.03.014
BindingDB Entry DOI: 10.7270/Q22Z1722 |
More data for this
Ligand-Target Pair | |
Endothelial PAS domain-containing protein 1/Hypoxia-inducible factor 1-alpha
(Homo sapiens (Human)) | BDBM50498799
(Manassantin A | Manassantin B1)Show SMILES COc1ccc(cc1OC)[C@@H](O)[C@@H](C)Oc1ccc(cc1O)[C@H]1O[C@@H]([C@H](C)[C@H]1C)c1ccc(O[C@H](C)[C@H](O)c2ccc(OC)c(OC)c2)c(OC)c1 |r| Show InChI InChI=1S/C41H50O11/c1-22-23(2)41(29-13-17-34(37(21-29)49-9)51-25(4)39(44)27-11-16-33(46-6)36(20-27)48-8)52-40(22)28-12-14-31(30(42)18-28)50-24(3)38(43)26-10-15-32(45-5)35(19-26)47-7/h10-25,38-44H,1-9H3/t22-,23-,24-,25-,38+,39+,40+,41+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Duke University
Curated by ChEMBL
| Assay Description
Inhibition of HIF1 in human HEK293T cells transfected with pGL3-5HRE-VEGF and pRL-SV40 after 24 hrs by dual luciferase reporter assay |
J Med Chem 58: 7659-71 (2015)
Article DOI: 10.1021/acs.jmedchem.5b01220
BindingDB Entry DOI: 10.7270/Q2T156N9 |
More data for this
Ligand-Target Pair | |
Aldo-keto reductase family 1 member B1
(Rattus norvegicus) | BDBM50049730
(2-(5-(2-methyl-3-phenylallylidene)-4-oxo-2-thioxot...)Show InChI InChI=1S/C15H13NO3S2/c1-10(7-11-5-3-2-4-6-11)8-12-14(19)16(9-13(17)18)15(20)21-12/h2-8H,9H2,1H3,(H,17,18)/b10-7+,12-8- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 67 | n/a | n/a | n/a | n/a | n/a | n/a |
Korean Institute of Oriental Medicine (KIOM)
Curated by ChEMBL
| Assay Description
Inhibition of Sprague-Dawley rat lens aldose reductase |
J Nat Prod 75: 267-70 (2012)
Article DOI: 10.1021/np200646e
BindingDB Entry DOI: 10.7270/Q2XS5WCH |
More data for this
Ligand-Target Pair | |
Aldo-keto reductase family 1 member B1
(Rattus norvegicus) | BDBM50049730

(2-(5-(2-methyl-3-phenylallylidene)-4-oxo-2-thioxot...)Show InChI InChI=1S/C15H13NO3S2/c1-10(7-11-5-3-2-4-6-11)8-12-14(19)16(9-13(17)18)15(20)21-12/h2-8H,9H2,1H3,(H,17,18)/b10-7+,12-8- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Oriental Medicine
Curated by ChEMBL
| Assay Description
Inhibition of rat lens aldose reductase |
J Nat Prod 71: 713-5 (2008)
Article DOI: 10.1021/np070489a
BindingDB Entry DOI: 10.7270/Q2RN38SR |
More data for this
Ligand-Target Pair | |
Aldo-keto reductase family 1 member B1
(Rattus norvegicus) | BDBM84978

(Quercitrin | cid_5280459)Show SMILES C[C@@H]1O[C@@H](Oc2c(O)c3c(cc(O)cc3=O)oc2-c2ccc(O)c(O)c2)[C@H](O)[C@H](O)[C@H]1O Show InChI InChI=1S/C21H20O11/c1-7-15(26)17(28)18(29)21(30-7)32-20-16(27)14-12(25)5-9(22)6-13(14)31-19(20)8-2-3-10(23)11(24)4-8/h2-7,15,17-18,21-24,26-29H,1H3/t7-,15-,17+,18+,21-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| KEGG
MMDB
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 160 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Oriental Medicine
Curated by ChEMBL
| Assay Description
Inhibition of rat lens aldose reductase |
J Nat Prod 71: 713-5 (2008)
Article DOI: 10.1021/np070489a
BindingDB Entry DOI: 10.7270/Q2RN38SR |
More data for this
Ligand-Target Pair | |
Aldo-keto reductase family 1 member B1
(Rattus norvegicus) | BDBM50362839
(CHEMBL249447)Show SMILES O[C@H]1[C@@H](C[C@@](O)(C[C@H]1OC(=O)\C=C\c1ccc(O)c(O)c1)C(O)=O)OC(=O)\C=C\c1ccc(O)c(O)c1 |r,wU:2.25,4.22,wD:1.0,7.8,4.4,(-1.06,-23.53,;-1.06,-21.99,;.29,-21.22,;.29,-19.66,;-1.06,-18.89,;.22,-18.03,;-2.39,-19.66,;-2.39,-21.22,;-3.73,-21.99,;-3.73,-23.53,;-2.39,-24.3,;-5.06,-24.3,;-5.06,-25.84,;-6.39,-26.61,;-7.73,-25.84,;-9.06,-26.61,;-9.06,-28.15,;-10.39,-28.92,;-7.72,-28.92,;-7.71,-30.46,;-6.39,-28.15,;-1.91,-17.6,;-.95,-16.4,;-3.45,-17.53,;1.62,-21.99,;1.61,-23.53,;.28,-24.3,;2.95,-24.3,;2.94,-25.84,;4.27,-26.62,;4.26,-28.16,;5.59,-28.93,;6.93,-28.16,;8.26,-28.93,;6.93,-26.61,;8.26,-25.84,;5.6,-25.85,)| Show InChI InChI=1S/C25H24O12/c26-15-5-1-13(9-17(15)28)3-7-21(30)36-19-11-25(35,24(33)34)12-20(23(19)32)37-22(31)8-4-14-2-6-16(27)18(29)10-14/h1-10,19-20,23,26-29,32,35H,11-12H2,(H,33,34)/b7-3+,8-4+/t19-,20-,23-,25+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
Korean Institute of Oriental Medicine (KIOM)
Curated by ChEMBL
| Assay Description
Inhibition of Sprague-Dawley rat lens aldose reductase |
J Nat Prod 75: 267-70 (2012)
Article DOI: 10.1021/np200646e
BindingDB Entry DOI: 10.7270/Q2XS5WCH |
More data for this
Ligand-Target Pair | |
Aldo-keto reductase family 1 member B1
(Rattus norvegicus) | BDBM50248650
(CHEMBL464507 | Guajaverin)Show SMILES O[C@H]1CO[C@@H](Oc2c(O)c3c(cc(O)cc3=O)oc2-c2ccc(O)c(O)c2)[C@H](O)[C@H]1O |r| Show InChI InChI=1S/C20H18O11/c21-8-4-11(24)14-13(5-8)30-18(7-1-2-9(22)10(23)3-7)19(16(14)27)31-20-17(28)15(26)12(25)6-29-20/h1-5,12,15,17,20-23,25-28H,6H2/t12-,15-,17+,20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 320 | n/a | n/a | n/a | n/a | n/a | n/a |
Korean Institute of Oriental Medicine (KIOM)
Curated by ChEMBL
| Assay Description
Inhibition of Sprague-Dawley rat lens aldose reductase |
J Nat Prod 75: 267-70 (2012)
Article DOI: 10.1021/np200646e
BindingDB Entry DOI: 10.7270/Q2XS5WCH |
More data for this
Ligand-Target Pair | |
Endothelial PAS domain-containing protein 1/Hypoxia-inducible factor 1-alpha
(Homo sapiens (Human)) | BDBM50498797

(CHEMBL3105545)Show SMILES COc1ccc(cc1OC)[C@@H](O)[C@@H](C)Oc1ccc(cc1OC)[C@H]1O[C@@H]([C@H](C)[C@H]1C)c1ccc(OC)c(OC)c1 |r| Show InChI InChI=1S/C32H40O8/c1-18-19(2)32(40-31(18)22-10-13-25(35-5)28(16-22)37-7)23-11-14-26(29(17-23)38-8)39-20(3)30(33)21-9-12-24(34-4)27(15-21)36-6/h9-20,30-33H,1-8H3/t18-,19-,20-,30+,31+,32+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 320 | n/a | n/a | n/a | n/a | n/a | n/a |
Duke University
Curated by ChEMBL
| Assay Description
Inhibition of HIF1 in human HEK293T cells transfected with pGL3-5HRE-VEGF and pRL-SV40 after 24 hrs by dual luciferase reporter assay |
J Med Chem 58: 7659-71 (2015)
Article DOI: 10.1021/acs.jmedchem.5b01220
BindingDB Entry DOI: 10.7270/Q2T156N9 |
More data for this
Ligand-Target Pair | |
Endothelial PAS domain-containing protein 1/Hypoxia-inducible factor 1-alpha
(Homo sapiens (Human)) | BDBM50498805
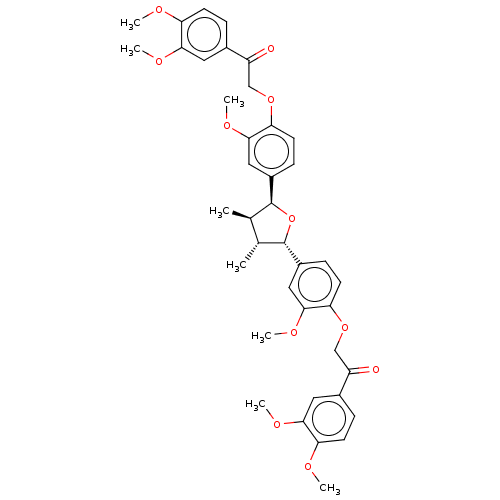
(CHEMBL3623807)Show SMILES COc1ccc(cc1OC)C(=O)COc1ccc(cc1OC)[C@H]1O[C@@H]([C@H](C)[C@H]1C)c1ccc(OCC(=O)c2ccc(OC)c(OC)c2)c(OC)c1 |r| Show InChI InChI=1S/C40H44O11/c1-23-24(2)40(28-12-16-34(38(20-28)48-8)50-22-30(42)26-10-14-32(44-4)36(18-26)46-6)51-39(23)27-11-15-33(37(19-27)47-7)49-21-29(41)25-9-13-31(43-3)35(17-25)45-5/h9-20,23-24,39-40H,21-22H2,1-8H3/t23-,24-,39+,40+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 350 | n/a | n/a | n/a | n/a | n/a | n/a |
Duke University
Curated by ChEMBL
| Assay Description
Inhibition of HIF1 in human HEK293T cells transfected with pGL3-5HRE-VEGF and pRL-SV40 after 24 hrs by dual luciferase reporter assay |
J Med Chem 58: 7659-71 (2015)
Article DOI: 10.1021/acs.jmedchem.5b01220
BindingDB Entry DOI: 10.7270/Q2T156N9 |
More data for this
Ligand-Target Pair | |
Endothelial PAS domain-containing protein 1/Hypoxia-inducible factor 1-alpha
(Homo sapiens (Human)) | BDBM50498796

(CHEMBL3623808)Show SMILES COc1ccc(cc1OC)[C@@H](O)COc1ccc(cc1OC)[C@H]1O[C@@H]([C@H](C)[C@H]1C)c1ccc(OC[C@H](O)c2ccc(OC)c(OC)c2)c(OC)c1 |r| Show InChI InChI=1S/C40H48O11/c1-23-24(2)40(28-12-16-34(38(20-28)48-8)50-22-30(42)26-10-14-32(44-4)36(18-26)46-6)51-39(23)27-11-15-33(37(19-27)47-7)49-21-29(41)25-9-13-31(43-3)35(17-25)45-5/h9-20,23-24,29-30,39-42H,21-22H2,1-8H3/t23-,24-,29+,30+,39+,40+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 380 | n/a | n/a | n/a | n/a | n/a | n/a |
Duke University
Curated by ChEMBL
| Assay Description
Inhibition of HIF1 in human HEK293T cells transfected with pGL3-5HRE-VEGF and pRL-SV40 after 24 hrs by dual luciferase reporter assay |
J Med Chem 58: 7659-71 (2015)
Article DOI: 10.1021/acs.jmedchem.5b01220
BindingDB Entry DOI: 10.7270/Q2T156N9 |
More data for this
Ligand-Target Pair | |
Endothelial PAS domain-containing protein 1/Hypoxia-inducible factor 1-alpha
(Homo sapiens (Human)) | BDBM50498801
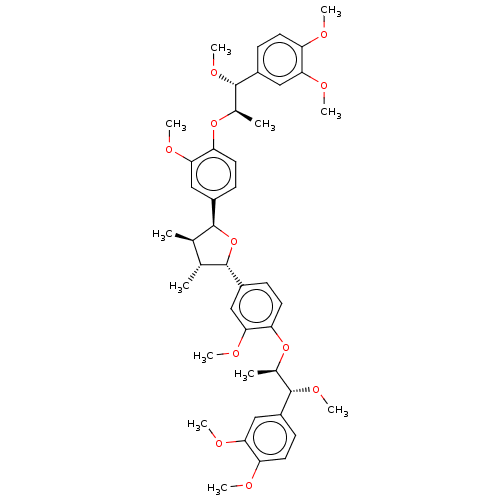
(CHEMBL3623806)Show SMILES CO[C@@H]([C@@H](C)Oc1ccc(cc1OC)[C@H]1O[C@@H]([C@H](C)[C@H]1C)c1ccc(O[C@H](C)[C@H](OC)c2ccc(OC)c(OC)c2)c(OC)c1)c1ccc(OC)c(OC)c1 |r| Show InChI InChI=1S/C44H56O11/c1-25-26(2)42(30-16-20-36(40(22-30)50-10)54-28(4)44(52-12)32-14-18-34(46-6)38(24-32)48-8)55-41(25)29-15-19-35(39(21-29)49-9)53-27(3)43(51-11)31-13-17-33(45-5)37(23-31)47-7/h13-28,41-44H,1-12H3/t25-,26-,27-,28-,41+,42+,43+,44+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 430 | n/a | n/a | n/a | n/a | n/a | n/a |
Duke University
Curated by ChEMBL
| Assay Description
Inhibition of HIF1 in human HEK293T cells transfected with pGL3-5HRE-VEGF and pRL-SV40 after 24 hrs by dual luciferase reporter assay |
J Med Chem 58: 7659-71 (2015)
Article DOI: 10.1021/acs.jmedchem.5b01220
BindingDB Entry DOI: 10.7270/Q2T156N9 |
More data for this
Ligand-Target Pair | |
Endothelial PAS domain-containing protein 1/Hypoxia-inducible factor 1-alpha
(Homo sapiens (Human)) | BDBM50498794
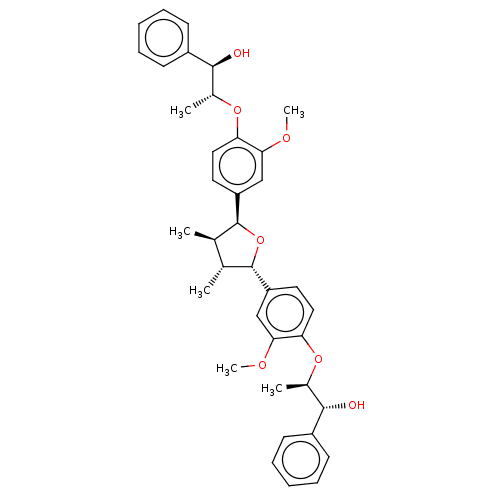
(CHEMBL3623812)Show SMILES COc1cc(ccc1O[C@H](C)[C@H](O)c1ccccc1)[C@H]1O[C@@H]([C@H](C)[C@H]1C)c1ccc(O[C@H](C)[C@H](O)c2ccccc2)c(OC)c1 |r| Show InChI InChI=1S/C38H44O7/c1-23-24(2)38(30-18-20-32(34(22-30)42-6)44-26(4)36(40)28-15-11-8-12-16-28)45-37(23)29-17-19-31(33(21-29)41-5)43-25(3)35(39)27-13-9-7-10-14-27/h7-26,35-40H,1-6H3/t23-,24-,25-,26-,35+,36+,37+,38+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 590 | n/a | n/a | n/a | n/a | n/a | n/a |
Duke University
Curated by ChEMBL
| Assay Description
Inhibition of HIF1 in human HEK293T cells transfected with pGL3-5HRE-VEGF and pRL-SV40 after 24 hrs by dual luciferase reporter assay |
J Med Chem 58: 7659-71 (2015)
Article DOI: 10.1021/acs.jmedchem.5b01220
BindingDB Entry DOI: 10.7270/Q2T156N9 |
More data for this
Ligand-Target Pair | |
Endothelial PAS domain-containing protein 1/Hypoxia-inducible factor 1-alpha
(Homo sapiens (Human)) | BDBM50498800
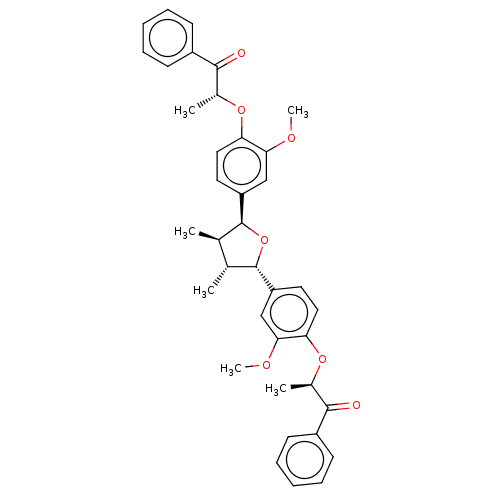
(CHEMBL3623811)Show SMILES COc1cc(ccc1O[C@H](C)C(=O)c1ccccc1)[C@H]1O[C@@H]([C@H](C)[C@H]1C)c1ccc(O[C@H](C)C(=O)c2ccccc2)c(OC)c1 |r| Show InChI InChI=1S/C38H40O7/c1-23-24(2)38(30-18-20-32(34(22-30)42-6)44-26(4)36(40)28-15-11-8-12-16-28)45-37(23)29-17-19-31(33(21-29)41-5)43-25(3)35(39)27-13-9-7-10-14-27/h7-26,37-38H,1-6H3/t23-,24-,25-,26-,37+,38+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 690 | n/a | n/a | n/a | n/a | n/a | n/a |
Duke University
Curated by ChEMBL
| Assay Description
Inhibition of HIF1 in human HEK293T cells transfected with pGL3-5HRE-VEGF and pRL-SV40 after 24 hrs by dual luciferase reporter assay |
J Med Chem 58: 7659-71 (2015)
Article DOI: 10.1021/acs.jmedchem.5b01220
BindingDB Entry DOI: 10.7270/Q2T156N9 |
More data for this
Ligand-Target Pair | |
Endothelial PAS domain-containing protein 1/Hypoxia-inducible factor 1-alpha
(Homo sapiens (Human)) | BDBM50498804
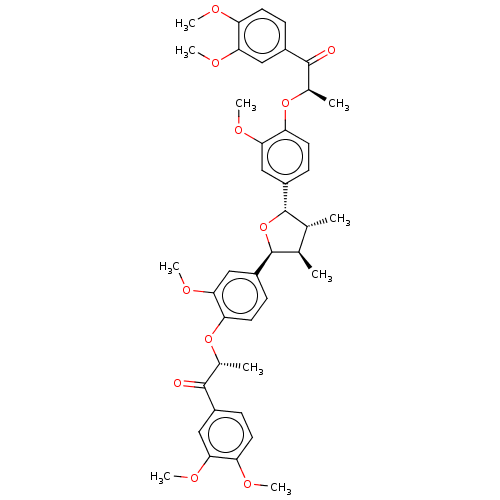
(CHEMBL3623805)Show SMILES COc1ccc(cc1OC)C(=O)[C@@H](C)Oc1ccc(cc1OC)[C@H]1O[C@@H]([C@H](C)[C@H]1C)c1ccc(O[C@H](C)C(=O)c2ccc(OC)c(OC)c2)c(OC)c1 |r| Show InChI InChI=1S/C42H48O11/c1-23-24(2)42(30-14-18-34(38(22-30)50-10)52-26(4)40(44)28-12-16-32(46-6)36(20-28)48-8)53-41(23)29-13-17-33(37(21-29)49-9)51-25(3)39(43)27-11-15-31(45-5)35(19-27)47-7/h11-26,41-42H,1-10H3/t23-,24-,25-,26-,41+,42+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 910 | n/a | n/a | n/a | n/a | n/a | n/a |
Duke University
Curated by ChEMBL
| Assay Description
Inhibition of HIF1 in human HEK293T cells transfected with pGL3-5HRE-VEGF and pRL-SV40 after 24 hrs by dual luciferase reporter assay |
J Med Chem 58: 7659-71 (2015)
Article DOI: 10.1021/acs.jmedchem.5b01220
BindingDB Entry DOI: 10.7270/Q2T156N9 |
More data for this
Ligand-Target Pair | |
Aldo-keto reductase family 1 member B1
(Rattus norvegicus) | BDBM50327036

((1R,3S,4S,5S)-3-[(E)-3-(3,4-Dihydroxy-phenyl)-acry...)Show SMILES O[C@@H]1C[C@](O)(C[C@@H](OC(=O)\C=C\c2ccc(O)c(O)c2)[C@@H]1O)C(O)=O |r| Show InChI InChI=1S/C16H18O9/c17-9-3-1-8(5-10(9)18)2-4-13(20)25-12-7-16(24,15(22)23)6-11(19)14(12)21/h1-5,11-12,14,17-19,21,24H,6-7H2,(H,22,23)/b4-2+/t11-,12-,14-,16+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 950 | n/a | n/a | n/a | n/a | n/a | n/a |
Korean Institute of Oriental Medicine (KIOM)
Curated by ChEMBL
| Assay Description
Inhibition of Sprague-Dawley rat lens aldose reductase |
J Nat Prod 75: 267-70 (2012)
Article DOI: 10.1021/np200646e
BindingDB Entry DOI: 10.7270/Q2XS5WCH |
More data for this
Ligand-Target Pair | |
Aldo-keto reductase family 1 member B1
(Rattus norvegicus) | BDBM50241345
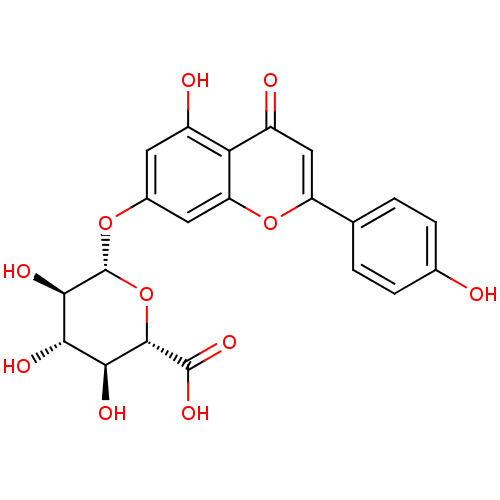
(Apigenin 7-O-β-D-glucuronide (9) | CHEMBL2542...)Show SMILES O[C@H]1[C@H](Oc2cc(O)c3c(c2)oc(cc3=O)-c2ccc(O)cc2)O[C@@H]([C@@H](O)[C@@H]1O)C(O)=O |r| Show InChI InChI=1S/C21H18O11/c22-9-3-1-8(2-4-9)13-7-12(24)15-11(23)5-10(6-14(15)31-13)30-21-18(27)16(25)17(26)19(32-21)20(28)29/h1-7,16-19,21-23,25-27H,(H,28,29)/t16-,17-,18+,19-,21+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.28E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Oriental Medicine
Curated by ChEMBL
| Assay Description
Inhibition of rat lens aldose reductase |
J Nat Prod 71: 713-5 (2008)
Article DOI: 10.1021/np070489a
BindingDB Entry DOI: 10.7270/Q2RN38SR |
More data for this
Ligand-Target Pair | |
Aldo-keto reductase family 1 member B1
(Rattus norvegicus) | BDBM7462

(3,5,7-trihydroxy-2-(4-hydroxyphenyl)-4H-chromen-4-...)Show InChI InChI=1S/C15H10O6/c16-8-3-1-7(2-4-8)15-14(20)13(19)12-10(18)5-9(17)6-11(12)21-15/h1-6,16-18,20H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.33E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Oriental Medicine
Curated by ChEMBL
| Assay Description
Inhibition of rat lens aldose reductase |
J Nat Prod 71: 713-5 (2008)
Article DOI: 10.1021/np070489a
BindingDB Entry DOI: 10.7270/Q2RN38SR |
More data for this
Ligand-Target Pair | |
Endothelial PAS domain-containing protein 1/Hypoxia-inducible factor 1-alpha
(Homo sapiens (Human)) | BDBM50498803

(CHEMBL3623810)Show SMILES COc1ccc(CCOc2ccc(cc2OC)[C@H]2O[C@@H]([C@H](C)[C@H]2C)c2ccc(OCCc3ccc(OC)c(OC)c3)c(OC)c2)cc1OC |r| Show InChI InChI=1S/C40H48O9/c1-25-26(2)40(30-12-16-34(38(24-30)46-8)48-20-18-28-10-14-32(42-4)36(22-28)44-6)49-39(25)29-11-15-33(37(23-29)45-7)47-19-17-27-9-13-31(41-3)35(21-27)43-5/h9-16,21-26,39-40H,17-20H2,1-8H3/t25-,26-,39+,40+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.44E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Duke University
Curated by ChEMBL
| Assay Description
Inhibition of HIF1 in human HEK293T cells transfected with pGL3-5HRE-VEGF and pRL-SV40 after 24 hrs by dual luciferase reporter assay |
J Med Chem 58: 7659-71 (2015)
Article DOI: 10.1021/acs.jmedchem.5b01220
BindingDB Entry DOI: 10.7270/Q2T156N9 |
More data for this
Ligand-Target Pair | |
Aldo-keto reductase family 1 member B1
(Rattus norvegicus) | BDBM7459

(2-(3,4-dihydroxyphenyl)-5,7-dihydroxy-4H-chromen-4...)Show InChI InChI=1S/C15H10O6/c16-8-4-11(19)15-12(20)6-13(21-14(15)5-8)7-1-2-9(17)10(18)3-7/h1-6,16-19H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.54E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Oriental Medicine
Curated by ChEMBL
| Assay Description
Inhibition of rat lens aldose reductase |
J Nat Prod 71: 713-5 (2008)
Article DOI: 10.1021/np070489a
BindingDB Entry DOI: 10.7270/Q2RN38SR |
More data for this
Ligand-Target Pair | |
Endothelial PAS domain-containing protein 1/Hypoxia-inducible factor 1-alpha
(Homo sapiens (Human)) | BDBM50498795
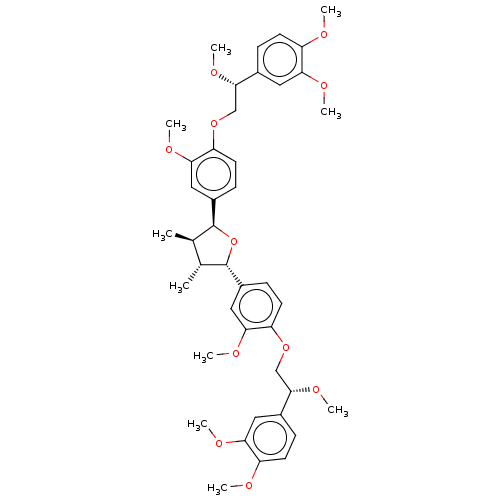
(CHEMBL3623809)Show SMILES CO[C@@H](COc1ccc(cc1OC)[C@H]1O[C@@H]([C@H](C)[C@H]1C)c1ccc(OC[C@H](OC)c2ccc(OC)c(OC)c2)c(OC)c1)c1ccc(OC)c(OC)c1 |r| Show InChI InChI=1S/C42H52O11/c1-25-26(2)42(30-14-18-34(38(22-30)48-8)52-24-40(50-10)28-12-16-32(44-4)36(20-28)46-6)53-41(25)29-13-17-33(37(21-29)47-7)51-23-39(49-9)27-11-15-31(43-3)35(19-27)45-5/h11-22,25-26,39-42H,23-24H2,1-10H3/t25-,26-,39+,40+,41+,42+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.93E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Duke University
Curated by ChEMBL
| Assay Description
Inhibition of HIF1 in human HEK293T cells transfected with pGL3-5HRE-VEGF and pRL-SV40 after 24 hrs by dual luciferase reporter assay |
J Med Chem 58: 7659-71 (2015)
Article DOI: 10.1021/acs.jmedchem.5b01220
BindingDB Entry DOI: 10.7270/Q2T156N9 |
More data for this
Ligand-Target Pair | |
Endothelial PAS domain-containing protein 1/Hypoxia-inducible factor 1-alpha
(Homo sapiens (Human)) | BDBM50498793
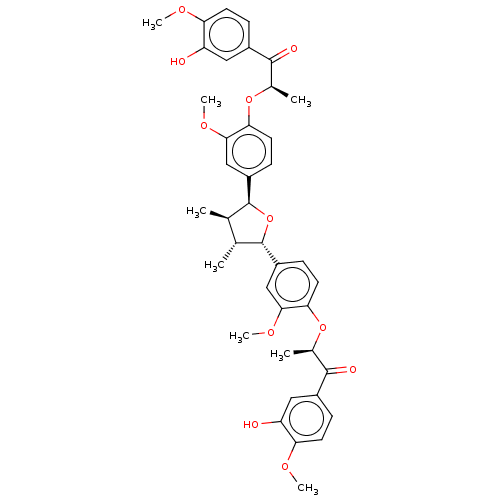
(CHEMBL3623803)Show SMILES COc1ccc(cc1O)C(=O)[C@@H](C)Oc1ccc(cc1OC)[C@H]1O[C@@H]([C@H](C)[C@H]1C)c1ccc(O[C@H](C)C(=O)c2ccc(OC)c(O)c2)c(OC)c1 |r| Show InChI InChI=1S/C40H44O11/c1-21-22(2)40(28-12-16-34(36(20-28)48-8)50-24(4)38(44)26-10-14-32(46-6)30(42)18-26)51-39(21)27-11-15-33(35(19-27)47-7)49-23(3)37(43)25-9-13-31(45-5)29(41)17-25/h9-24,39-42H,1-8H3/t21-,22-,23-,24-,39+,40+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.11E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Duke University
Curated by ChEMBL
| Assay Description
Inhibition of HIF1 in human HEK293T cells transfected with pGL3-5HRE-VEGF and pRL-SV40 after 24 hrs by dual luciferase reporter assay |
J Med Chem 58: 7659-71 (2015)
Article DOI: 10.1021/acs.jmedchem.5b01220
BindingDB Entry DOI: 10.7270/Q2T156N9 |
More data for this
Ligand-Target Pair | |
Aldo-keto reductase family 1 member B1
(Rattus norvegicus) | BDBM50056908
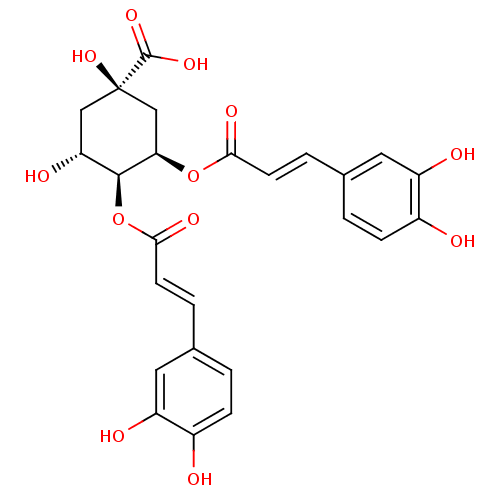
((1R,3R,4S,5R)-3,4-Bis-[(E)-3-(3,4-dihydroxy-phenyl...)Show SMILES O[C@@H]1C[C@@](O)(C[C@@H](OC(=O)\C=C\c2ccc(O)c(O)c2)[C@H]1OC(=O)\C=C\c1ccc(O)c(O)c1)C(O)=O |r| Show InChI InChI=1S/C25H24O12/c26-15-5-1-13(9-17(15)28)3-7-21(31)36-20-12-25(35,24(33)34)11-19(30)23(20)37-22(32)8-4-14-2-6-16(27)18(29)10-14/h1-10,19-20,23,26-30,35H,11-12H2,(H,33,34)/b7-3+,8-4+/t19-,20-,23+,25-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Korean Institute of Oriental Medicine (KIOM)
Curated by ChEMBL
| Assay Description
Inhibition of Sprague-Dawley rat lens aldose reductase |
J Nat Prod 75: 267-70 (2012)
Article DOI: 10.1021/np200646e
BindingDB Entry DOI: 10.7270/Q2XS5WCH |
More data for this
Ligand-Target Pair | |
Endothelial PAS domain-containing protein 1/Hypoxia-inducible factor 1-alpha
(Homo sapiens (Human)) | BDBM50498802

(CHEMBL3621232)Show SMILES COc1ccc(cc1OC)[C@H]1O[C@@H]([C@H](C)[C@H]1C)c1ccc(O[C@H](C)C(=O)c2ccc(OC)c(OC)c2)c(OC)c1 |r| Show InChI InChI=1S/C32H38O8/c1-18-19(2)32(40-31(18)22-10-13-25(35-5)28(16-22)37-7)23-11-14-26(29(17-23)38-8)39-20(3)30(33)21-9-12-24(34-4)27(15-21)36-6/h9-20,31-32H,1-8H3/t18-,19-,20-,31+,32+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Duke University
Curated by ChEMBL
| Assay Description
Inhibition of HIF1 in human HEK293T cells transfected with pGL3-5HRE-VEGF and pRL-SV40 after 24 hrs by dual luciferase reporter assay |
J Med Chem 58: 7659-71 (2015)
Article DOI: 10.1021/acs.jmedchem.5b01220
BindingDB Entry DOI: 10.7270/Q2T156N9 |
More data for this
Ligand-Target Pair | |
Aldo-keto reductase family 1 member B1
(Rattus norvegicus) | BDBM50115820
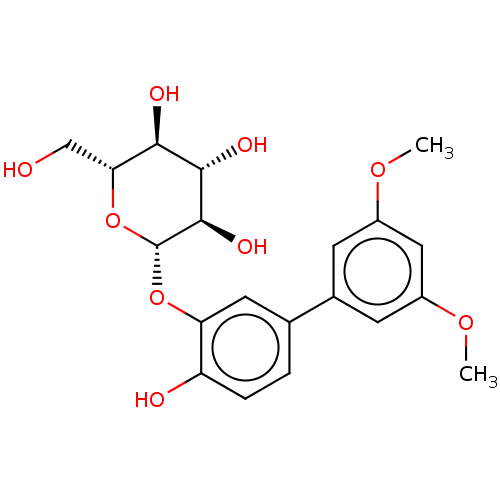
(CHEMBL3608478)Show SMILES COc1cc(OC)cc(c1)-c1ccc(O)c(O[C@@H]2O[C@H](CO)[C@@H](O)[C@H](O)[C@H]2O)c1 |r| Show InChI InChI=1S/C20H24O9/c1-26-12-5-11(6-13(8-12)27-2)10-3-4-14(22)15(7-10)28-20-19(25)18(24)17(23)16(9-21)29-20/h3-8,16-25H,9H2,1-2H3/t16-,17-,18+,19-,20-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Oriental Medicine
Curated by ChEMBL
| Assay Description
Inhibition of Sprague-Dawley rat lense aldose reductase using DL-glyceraldehyde as substrate by spectrofluorometric analysis |
J Nat Prod 78: 2249-54 (2015)
Article DOI: 10.1021/acs.jnatprod.5b00469
BindingDB Entry DOI: 10.7270/Q2Z321FB |
More data for this
Ligand-Target Pair | |
Aldo-keto reductase family 1 member B1
(Rattus norvegicus) | BDBM50241354
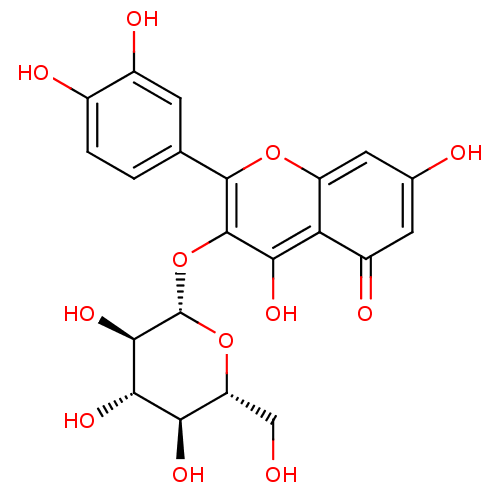
(2-(3,4-Dihydroxy-phenyl)-5,7-dihydroxy-3-(3,4,5-tr...)Show SMILES OC[C@H]1O[C@@H](Oc2c(O)c3c(cc(O)cc3=O)oc2-c2ccc(O)c(O)c2)[C@H](O)[C@@H](O)[C@@H]1O |r| Show InChI InChI=1S/C21H20O12/c22-6-13-15(27)17(29)18(30)21(32-13)33-20-16(28)14-11(26)4-8(23)5-12(14)31-19(20)7-1-2-9(24)10(25)3-7/h1-5,13,15,17-18,21-25,27-30H,6H2/t13-,15-,17+,18-,21+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Korean Institute of Oriental Medicine (KIOM)
Curated by ChEMBL
| Assay Description
Inhibition of Sprague-Dawley rat lens aldose reductase |
J Nat Prod 75: 267-70 (2012)
Article DOI: 10.1021/np200646e
BindingDB Entry DOI: 10.7270/Q2XS5WCH |
More data for this
Ligand-Target Pair | |
Aldo-keto reductase family 1 member B1
(Rattus norvegicus) | BDBM50241243

(3,4',5,7-Tetrahydroxyflavone-3-glucoside | 3-(beta...)Show SMILES OC[C@H]1O[C@@H](Oc2c(O)c3c(cc(O)cc3=O)oc2-c2ccc(O)cc2)[C@H](O)[C@@H](O)[C@@H]1O |r| Show InChI InChI=1S/C21H20O11/c22-7-13-15(26)17(28)18(29)21(31-13)32-20-16(27)14-11(25)5-10(24)6-12(14)30-19(20)8-1-3-9(23)4-2-8/h1-6,13,15,17-18,21-24,26-29H,7H2/t13-,15-,17+,18-,21+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.09E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Oriental Medicine
Curated by ChEMBL
| Assay Description
Inhibition of rat lens aldose reductase |
J Nat Prod 71: 713-5 (2008)
Article DOI: 10.1021/np070489a
BindingDB Entry DOI: 10.7270/Q2RN38SR |
More data for this
Ligand-Target Pair | |
Aldo-keto reductase family 1 member B1
(Rattus norvegicus) | BDBM50115821

(CHEMBL3608477)Show SMILES COc1cc(O[C@@H]2O[C@H](CO)[C@@H](O)[C@H](O)[C@H]2O)cc(c1)-c1ccc(O)c(O)c1 |r| Show InChI InChI=1S/C19H22O9/c1-26-11-4-10(9-2-3-13(21)14(22)6-9)5-12(7-11)27-19-18(25)17(24)16(23)15(8-20)28-19/h2-7,15-25H,8H2,1H3/t15-,16-,17+,18-,19-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Oriental Medicine
Curated by ChEMBL
| Assay Description
Inhibition of Sprague-Dawley rat lense aldose reductase using DL-glyceraldehyde as substrate by spectrofluorometric analysis |
J Nat Prod 78: 2249-54 (2015)
Article DOI: 10.1021/acs.jnatprod.5b00469
BindingDB Entry DOI: 10.7270/Q2Z321FB |
More data for this
Ligand-Target Pair | |
Aldo-keto reductase family 1 member B1
(Rattus norvegicus) | BDBM7458

(5,7-dihydroxy-2-(4-hydroxyphenyl)-4H-chromen-4-one...)Show InChI InChI=1S/C15H10O5/c16-9-3-1-8(2-4-9)13-7-12(19)15-11(18)5-10(17)6-14(15)20-13/h1-7,16-18H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 6.67E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Oriental Medicine
Curated by ChEMBL
| Assay Description
Inhibition of rat lens aldose reductase |
J Nat Prod 71: 713-5 (2008)
Article DOI: 10.1021/np070489a
BindingDB Entry DOI: 10.7270/Q2RN38SR |
More data for this
Ligand-Target Pair | |
Aldo-keto reductase family 1 member B1
(Rattus norvegicus) | BDBM7460

(2-(3,4-dihydroxyphenyl)-3,5,7-trihydroxy-4H-chrome...)Show InChI InChI=1S/C15H10O7/c16-7-4-10(19)12-11(5-7)22-15(14(21)13(12)20)6-1-2-8(17)9(18)3-6/h1-5,16-19,21H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 7.21E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Oriental Medicine
Curated by ChEMBL
| Assay Description
Inhibition of rat lens aldose reductase |
J Nat Prod 71: 713-5 (2008)
Article DOI: 10.1021/np070489a
BindingDB Entry DOI: 10.7270/Q2RN38SR |
More data for this
Ligand-Target Pair | |
Aldo-keto reductase family 1 member B1
(Rattus norvegicus) | BDBM50251270

(CHEMBL464868 | apigenin-7-O-beta-D-glucuronide met...)Show SMILES COC(=O)[C@H]1O[C@@H](Oc2cc(O)c3c(c2)oc(cc3=O)-c2ccc(O)cc2)[C@H](O)[C@@H](O)[C@@H]1O |r| Show InChI InChI=1S/C22H20O11/c1-30-21(29)20-18(27)17(26)19(28)22(33-20)31-11-6-12(24)16-13(25)8-14(32-15(16)7-11)9-2-4-10(23)5-3-9/h2-8,17-20,22-24,26-28H,1H3/t17-,18-,19+,20-,22+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9.02E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Oriental Medicine
Curated by ChEMBL
| Assay Description
Inhibition of rat lens aldose reductase |
J Nat Prod 71: 713-5 (2008)
Article DOI: 10.1021/np070489a
BindingDB Entry DOI: 10.7270/Q2RN38SR |
More data for this
Ligand-Target Pair | |
Aldo-keto reductase family 1 member B1
(Rattus norvegicus) | BDBM50379283
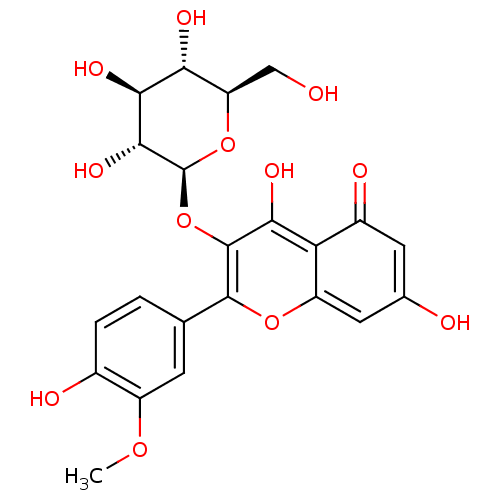
(CHEMBL234316)Show SMILES COc1cc(ccc1O)-c1oc2cc(O)cc(=O)c2c(O)c1O[C@@H]1O[C@H](CO)[C@@H](O)[C@H](O)[C@H]1O |r| Show InChI InChI=1S/C22H22O12/c1-31-12-4-8(2-3-10(12)25)20-21(17(28)15-11(26)5-9(24)6-13(15)32-20)34-22-19(30)18(29)16(27)14(7-23)33-22/h2-6,14,16,18-19,22-25,27-30H,7H2,1H3/t14-,16-,18+,19-,22+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Korean Institute of Oriental Medicine (KIOM)
Curated by ChEMBL
| Assay Description
Inhibition of Sprague-Dawley rat lens aldose reductase |
J Nat Prod 75: 267-70 (2012)
Article DOI: 10.1021/np200646e
BindingDB Entry DOI: 10.7270/Q2XS5WCH |
More data for this
Ligand-Target Pair | |
Aldo-keto reductase family 1 member B1
(Rattus norvegicus) | BDBM50378581

(NICOTIFLOROSIDE)Show SMILES C[C@@H]1O[C@@H](OC[C@H]2O[C@@H](Oc3c(O)c4c(cc(O)cc4=O)oc3-c3ccc(O)cc3)[C@H](O)[C@@H](O)[C@@H]2O)[C@H](O)[C@H](O)[C@H]1O |r| Show InChI InChI=1S/C27H30O15/c1-9-17(31)20(34)22(36)26(39-9)38-8-15-18(32)21(35)23(37)27(41-15)42-25-19(33)16-13(30)6-12(29)7-14(16)40-24(25)10-2-4-11(28)5-3-10/h2-7,9,15,17-18,20-23,26-29,31-37H,8H2,1H3/t9-,15+,17-,18+,20+,21-,22+,23+,26+,27-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.13E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Korean Institute of Oriental Medicine (KIOM)
Curated by ChEMBL
| Assay Description
Inhibition of Sprague-Dawley rat lens aldose reductase |
J Nat Prod 75: 267-70 (2012)
Article DOI: 10.1021/np200646e
BindingDB Entry DOI: 10.7270/Q2XS5WCH |
More data for this
Ligand-Target Pair | |
Aldo-keto reductase family 1 member B1
(Rattus norvegicus) | BDBM50310722
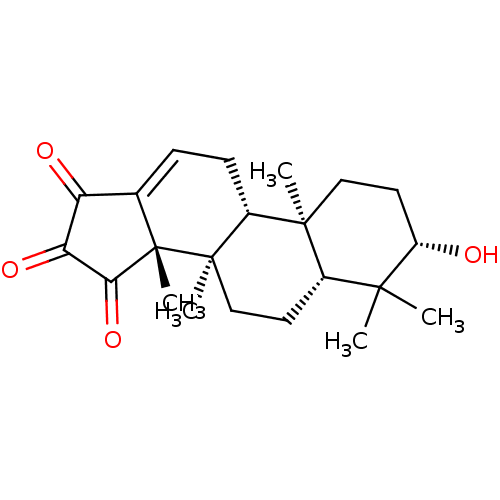
(CHEMBL575924 | palbinone)Show SMILES C[C@@]12C(=O)C(=O)C(=O)C1=CC[C@@H]1[C@@]3(C)CC[C@H](O)C(C)(C)[C@@H]3CC[C@@]21C |r,c:9| Show InChI InChI=1S/C22H30O4/c1-19(2)13-8-11-21(4)14(20(13,3)10-9-15(19)23)7-6-12-16(24)17(25)18(26)22(12,21)5/h6,13-15,23H,7-11H2,1-5H3/t13-,14+,15-,20-,21+,22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.14E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Chungnam National University
Curated by ChEMBL
| Assay Description
Inhibition of Sprague-Dawley rat lens alodse reductase by spectrofluorimetry |
J Nat Prod 72: 1465-70 (2009)
Article DOI: 10.1021/np9002004
BindingDB Entry DOI: 10.7270/Q2WS8V57 |
More data for this
Ligand-Target Pair | |
Aldo-keto reductase family 1 member B1
(Rattus norvegicus) | BDBM50379284
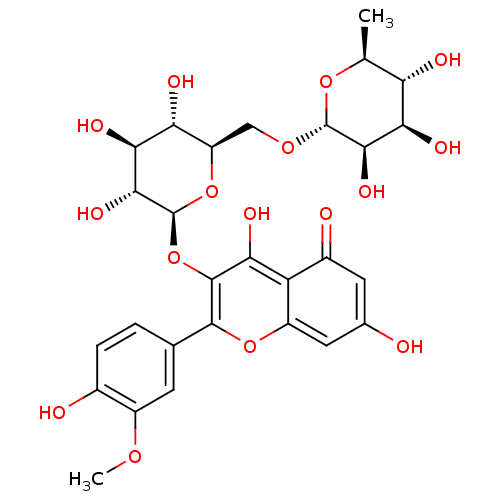
(NARCISSIN)Show SMILES COc1cc(ccc1O)-c1oc2cc(O)cc(=O)c2c(O)c1O[C@@H]1O[C@H](CO[C@@H]2O[C@@H](C)[C@H](O)[C@@H](O)[C@H]2O)[C@@H](O)[C@H](O)[C@H]1O |r| Show InChI InChI=1S/C28H32O16/c1-9-18(32)21(35)23(37)27(41-9)40-8-16-19(33)22(36)24(38)28(43-16)44-26-20(34)17-13(31)6-11(29)7-15(17)42-25(26)10-3-4-12(30)14(5-10)39-2/h3-7,9,16,18-19,21-24,27-30,32-38H,8H2,1-2H3/t9-,16+,18-,19+,21+,22-,23+,24+,27+,28-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.21E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Korean Institute of Oriental Medicine (KIOM)
Curated by ChEMBL
| Assay Description
Inhibition of Sprague-Dawley rat lens aldose reductase |
J Nat Prod 75: 267-70 (2012)
Article DOI: 10.1021/np200646e
BindingDB Entry DOI: 10.7270/Q2XS5WCH |
More data for this
Ligand-Target Pair | |
Aldo-keto reductase family 1 member B1
(Rattus norvegicus) | BDBM50115822

(CHEMBL3608476)Show InChI InChI=1S/C13H12O4/c1-17-11-5-9(4-10(14)7-11)8-2-3-12(15)13(16)6-8/h2-7,14-16H,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.38E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Oriental Medicine
Curated by ChEMBL
| Assay Description
Inhibition of Sprague-Dawley rat lense aldose reductase using DL-glyceraldehyde as substrate by spectrofluorometric analysis |
J Nat Prod 78: 2249-54 (2015)
Article DOI: 10.1021/acs.jnatprod.5b00469
BindingDB Entry DOI: 10.7270/Q2Z321FB |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50004202

(CHEBI:75813 | CHEMBL1221722)Show SMILES OC[C@H]1O[C@@H](Oc2c(oc3cc(O)cc(O)c3c2=O)-c2cc(O)c(O)c(O)c2)[C@H](O)[C@@H](O)[C@@H]1O |r| Show InChI InChI=1S/C21H20O13/c22-5-12-15(28)17(30)18(31)21(33-12)34-20-16(29)13-8(24)3-7(23)4-11(13)32-19(20)6-1-9(25)14(27)10(26)2-6/h1-4,12,15,17-18,21-28,30-31H,5H2/t12-,15-,17+,18-,21+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.72E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Chungnam National University
Curated by ChEMBL
| Assay Description
Inhibition of sEH (unknown origin) assessed as substrate PHOME hydrolysis after 1 hr by fluorescence method |
Bioorg Med Chem Lett 24: 1895-900 (2014)
Article DOI: 10.1016/j.bmcl.2014.03.014
BindingDB Entry DOI: 10.7270/Q22Z1722 |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50004200

(CHEMBL459260)Show SMILES OC[C@H]1O[C@@H](Oc2c(oc3cc(O)cc(O)c3c2=O)-c2ccc(O)c(O)c2)[C@H](OC(=O)c2cc(O)c(O)c(O)c2)[C@@H](O)[C@@H]1O |r| Show InChI InChI=1S/C28H24O16/c29-8-18-21(37)23(39)26(43-27(40)10-4-15(34)20(36)16(35)5-10)28(42-18)44-25-22(38)19-14(33)6-11(30)7-17(19)41-24(25)9-1-2-12(31)13(32)3-9/h1-7,18,21,23,26,28-37,39H,8H2/t18-,21-,23+,26-,28+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.81E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Chungnam National University
Curated by ChEMBL
| Assay Description
Inhibition of sEH (unknown origin) assessed as substrate PHOME hydrolysis after 1 hr by fluorescence method |
Bioorg Med Chem Lett 24: 1895-900 (2014)
Article DOI: 10.1016/j.bmcl.2014.03.014
BindingDB Entry DOI: 10.7270/Q22Z1722 |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50004201

(CHEMBL3236511)Show SMILES C[C@@H]1O[C@@H](Oc2c(oc3cc(O)cc(O)c3c2=O)-c2ccc(O)c(O)c2)[C@H](OC(=O)c2cc(O)c(O)c(O)c2)[C@H](O)[C@H]1O |r| Show InChI InChI=1S/C28H24O15/c1-9-20(35)23(38)26(42-27(39)11-5-16(33)21(36)17(34)6-11)28(40-9)43-25-22(37)19-15(32)7-12(29)8-18(19)41-24(25)10-2-3-13(30)14(31)4-10/h2-9,20,23,26,28-36,38H,1H3/t9-,20-,23+,26+,28-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.86E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Chungnam National University
Curated by ChEMBL
| Assay Description
Inhibition of sEH (unknown origin) assessed as substrate PHOME hydrolysis after 1 hr by fluorescence method |
Bioorg Med Chem Lett 24: 1895-900 (2014)
Article DOI: 10.1016/j.bmcl.2014.03.014
BindingDB Entry DOI: 10.7270/Q22Z1722 |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM4078
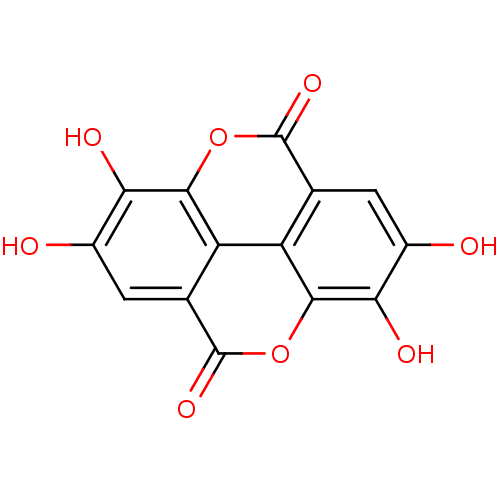
(6,7,13,14-tetrahydroxy-2,9-dioxatetracyclo[6.6.2.0...)Show InChI InChI=1S/C14H6O8/c15-5-1-3-7-8-4(14(20)22-11(7)9(5)17)2-6(16)10(18)12(8)21-13(3)19/h1-2,15-18H | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.07E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Chungnam National University
Curated by ChEMBL
| Assay Description
Inhibition of sEH (unknown origin) assessed as substrate PHOME hydrolysis after 1 hr by fluorescence method |
Bioorg Med Chem Lett 24: 1895-900 (2014)
Article DOI: 10.1016/j.bmcl.2014.03.014
BindingDB Entry DOI: 10.7270/Q22Z1722 |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50241367
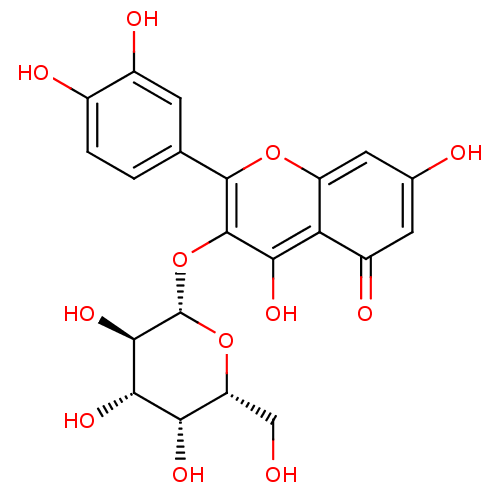
(2-(3,4-Dihydroxy-phenyl)-5,7-dihydroxy-3-((2S,4R,5...)Show SMILES OC[C@H]1O[C@@H](Oc2c(O)c3c(cc(O)cc3=O)oc2-c2ccc(O)c(O)c2)[C@H](O)[C@@H](O)[C@H]1O |r| Show InChI InChI=1S/C21H20O12/c22-6-13-15(27)17(29)18(30)21(32-13)33-20-16(28)14-11(26)4-8(23)5-12(14)31-19(20)7-1-2-9(24)10(25)3-7/h1-5,13,15,17-18,21-25,27-30H,6H2/t13-,15+,17+,18-,21+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.18E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Chungnam National University
Curated by ChEMBL
| Assay Description
Inhibition of sEH (unknown origin) assessed as substrate PHOME hydrolysis after 1 hr by fluorescence method |
Bioorg Med Chem Lett 24: 1895-900 (2014)
Article DOI: 10.1016/j.bmcl.2014.03.014
BindingDB Entry DOI: 10.7270/Q22Z1722 |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50004198

(CHEMBL3236510)Show SMILES OC[C@H]1O[C@@H](Oc2c(oc3cc(O)cc(O)c3c2=O)-c2ccc(O)cc2)[C@H](OC(=O)c2cc(O)c(O)c(O)c2)[C@@H](OC(=O)c2cc(O)c(O)c(O)c2)[C@@H]1O |r| Show InChI InChI=1S/C35H28O19/c36-11-23-27(46)30(52-33(48)13-5-18(40)25(44)19(41)6-13)32(53-34(49)14-7-20(42)26(45)21(43)8-14)35(51-23)54-31-28(47)24-17(39)9-16(38)10-22(24)50-29(31)12-1-3-15(37)4-2-12/h1-10,23,27,30,32,35-46H,11H2/t23-,27-,30+,32-,35+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.38E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Chungnam National University
Curated by ChEMBL
| Assay Description
Inhibition of sEH (unknown origin) assessed as substrate PHOME hydrolysis after 1 hr by fluorescence method |
Bioorg Med Chem Lett 24: 1895-900 (2014)
Article DOI: 10.1016/j.bmcl.2014.03.014
BindingDB Entry DOI: 10.7270/Q22Z1722 |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50217942
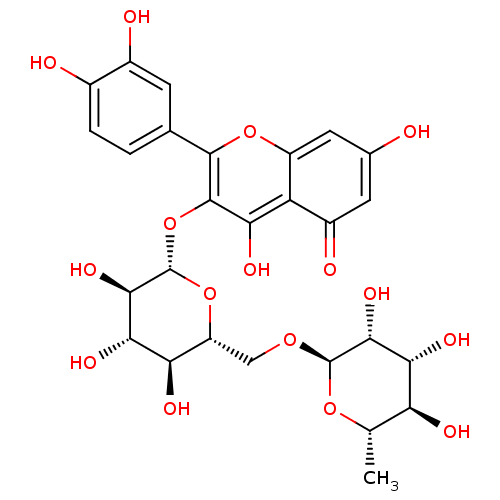
(2-(3,4-dihydroxyphenyl)-5,7-dihydroxy-4-oxo-4H-chr...)Show SMILES C[C@@H]1O[C@@H](OC[C@H]2O[C@@H](Oc3c(O)c4c(cc(O)cc4=O)oc3-c3ccc(O)c(O)c3)[C@H](O)[C@@H](O)[C@@H]2O)[C@H](O)[C@H](O)[C@H]1O |r| Show InChI InChI=1S/C27H30O16/c1-8-17(32)20(35)22(37)26(40-8)39-7-15-18(33)21(36)23(38)27(42-15)43-25-19(34)16-13(31)5-10(28)6-14(16)41-24(25)9-2-3-11(29)12(30)4-9/h2-6,8,15,17-18,20-23,26-30,32-38H,7H2,1H3/t8-,15+,17-,18+,20+,21-,22+,23+,26+,27-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.43E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Chungnam National University
Curated by ChEMBL
| Assay Description
Inhibition of sEH (unknown origin) assessed as substrate PHOME hydrolysis after 1 hr by fluorescence method |
Bioorg Med Chem Lett 24: 1895-900 (2014)
Article DOI: 10.1016/j.bmcl.2014.03.014
BindingDB Entry DOI: 10.7270/Q22Z1722 |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50004199
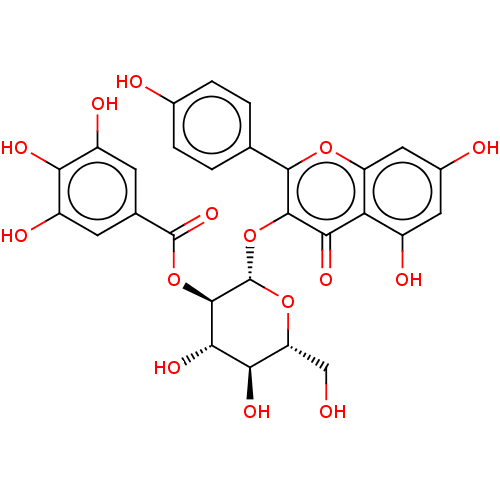
(CHEMBL444191)Show SMILES OC[C@H]1O[C@@H](Oc2c(oc3cc(O)cc(O)c3c2=O)-c2ccc(O)cc2)[C@H](OC(=O)c2cc(O)c(O)c(O)c2)[C@@H](O)[C@@H]1O |r| Show InChI InChI=1S/C28H24O15/c29-9-18-21(36)23(38)26(42-27(39)11-5-15(33)20(35)16(34)6-11)28(41-18)43-25-22(37)19-14(32)7-13(31)8-17(19)40-24(25)10-1-3-12(30)4-2-10/h1-8,18,21,23,26,28-36,38H,9H2/t18-,21-,23+,26-,28+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.74E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Chungnam National University
Curated by ChEMBL
| Assay Description
Inhibition of sEH (unknown origin) assessed as substrate PHOME hydrolysis after 1 hr by fluorescence method |
Bioorg Med Chem Lett 24: 1895-900 (2014)
Article DOI: 10.1016/j.bmcl.2014.03.014
BindingDB Entry DOI: 10.7270/Q22Z1722 |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50241354

(2-(3,4-Dihydroxy-phenyl)-5,7-dihydroxy-3-(3,4,5-tr...)Show SMILES OC[C@H]1O[C@@H](Oc2c(O)c3c(cc(O)cc3=O)oc2-c2ccc(O)c(O)c2)[C@H](O)[C@@H](O)[C@@H]1O |r| Show InChI InChI=1S/C21H20O12/c22-6-13-15(27)17(29)18(30)21(32-13)33-20-16(28)14-11(26)4-8(23)5-12(14)31-19(20)7-1-2-9(24)10(25)3-7/h1-5,13,15,17-18,21-25,27-30H,6H2/t13-,15-,17+,18-,21+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.84E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Chungnam National University
Curated by ChEMBL
| Assay Description
Inhibition of sEH (unknown origin) assessed as substrate PHOME hydrolysis after 1 hr by fluorescence method |
Bioorg Med Chem Lett 24: 1895-900 (2014)
Article DOI: 10.1016/j.bmcl.2014.03.014
BindingDB Entry DOI: 10.7270/Q22Z1722 |
More data for this
Ligand-Target Pair | |
Aldo-keto reductase family 1 member B1
(Rattus norvegicus) | BDBM50022418
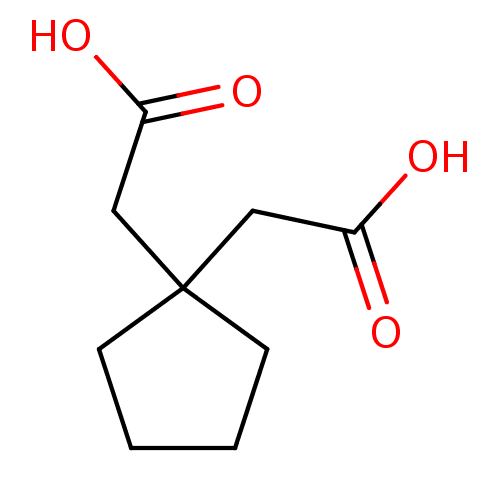
((1-Carboxymethyl-cyclopentyl)-acetic acid(TMG) | 3...)Show InChI InChI=1S/C9H14O4/c10-7(11)5-9(6-8(12)13)3-1-2-4-9/h1-6H2,(H,10,11)(H,12,13) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.87E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Oriental Medicine
Curated by ChEMBL
| Assay Description
Inhibition of Sprague-Dawley rat lense aldose reductase using DL-glyceraldehyde as substrate by spectrofluorometric analysis |
J Nat Prod 78: 2249-54 (2015)
Article DOI: 10.1021/acs.jnatprod.5b00469
BindingDB Entry DOI: 10.7270/Q2Z321FB |
More data for this
Ligand-Target Pair | |
Aldo-keto reductase family 1 member B1
(Rattus norvegicus) | BDBM50310723
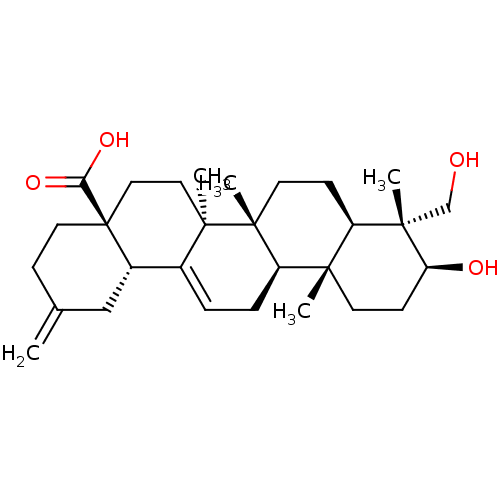
(30-norhederagenin | CHEMBL1081326)Show SMILES C[C@@]1(CO)[C@@H](O)CC[C@@]2(C)[C@H]1CC[C@]1(C)[C@@H]2CC=C2[C@@H]3CC(=C)CC[C@@]3(CC[C@@]12C)C(O)=O |r,t:19| Show InChI InChI=1S/C29H44O4/c1-18-8-13-29(24(32)33)15-14-27(4)19(20(29)16-18)6-7-22-25(2)11-10-23(31)26(3,17-30)21(25)9-12-28(22,27)5/h6,20-23,30-31H,1,7-17H2,2-5H3,(H,32,33)/t20-,21+,22+,23-,25-,26-,27+,28+,29-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.88E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Chungnam National University
Curated by ChEMBL
| Assay Description
Inhibition of Sprague-Dawley rat lens alodse reductase by spectrofluorimetry |
J Nat Prod 72: 1465-70 (2009)
Article DOI: 10.1021/np9002004
BindingDB Entry DOI: 10.7270/Q2WS8V57 |
More data for this
Ligand-Target Pair | |
Aldo-keto reductase family 1 member B1
(Rattus norvegicus) | BDBM50275507

(5-alpha-stigmasta-7,22-diene-3beta-ol | CHEMBL4877...)Show SMILES CC[C@H](\C=C\[C@@H](C)[C@H]1CC[C@H]2C3=CC[C@H]4C[C@@H](O)CC[C@]4(C)[C@H]3CC[C@]12C)C(C)C |r,t:11| Show InChI InChI=1S/C29H48O/c1-7-21(19(2)3)9-8-20(4)25-12-13-26-24-11-10-22-18-23(30)14-16-28(22,5)27(24)15-17-29(25,26)6/h8-9,11,19-23,25-27,30H,7,10,12-18H2,1-6H3/b9-8+/t20-,21-,22+,23+,25-,26+,27+,28+,29-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Korean Institute of Oriental Medicine (KIOM)
Curated by ChEMBL
| Assay Description
Inhibition of Sprague-Dawley rat lens aldose reductase |
J Nat Prod 75: 267-70 (2012)
Article DOI: 10.1021/np200646e
BindingDB Entry DOI: 10.7270/Q2XS5WCH |
More data for this
Ligand-Target Pair | |
Aldo-keto reductase family 1 member B1
(Rattus norvegicus) | BDBM50257635

((-)-BETA-SITOSTEROL-BETA-D-GLUCOPYRANOSIDE | (2R,3...)Show SMILES CC[C@H](CC[C@@H](C)[C@H]1CC[C@H]2[C@@H]3CC=C4C[C@H](CC[C@]4(C)[C@H]3CC[C@]12C)O[C@@H]1O[C@H](CO)[C@@H](O)[C@H](O)[C@H]1O)C(C)C |r,t:13| Show InChI InChI=1S/C35H60O6/c1-7-22(20(2)3)9-8-21(4)26-12-13-27-25-11-10-23-18-24(14-16-34(23,5)28(25)15-17-35(26,27)6)40-33-32(39)31(38)30(37)29(19-36)41-33/h10,20-22,24-33,36-39H,7-9,11-19H2,1-6H3/t21-,22-,24+,25+,26-,27+,28+,29-,30-,31+,32-,33-,34+,35-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Korean Institute of Oriental Medicine (KIOM)
Curated by ChEMBL
| Assay Description
Inhibition of Sprague-Dawley rat lens aldose reductase |
J Nat Prod 75: 267-70 (2012)
Article DOI: 10.1021/np200646e
BindingDB Entry DOI: 10.7270/Q2XS5WCH |
More data for this
Ligand-Target Pair | |
Aldo-keto reductase family 1 member B1
(Rattus norvegicus) | BDBM50379286

(CHEMBL2011519)Show SMILES C\C=C\C#CC#CCCCO[C@@H]1O[C@H](CO)[C@@H](O)[C@H](O)[C@H]1O |r| Show InChI InChI=1S/C16H22O6/c1-2-3-4-5-6-7-8-9-10-21-16-15(20)14(19)13(18)12(11-17)22-16/h2-3,12-20H,8-11H2,1H3/b3-2+/t12-,13-,14+,15-,16-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Korean Institute of Oriental Medicine (KIOM)
Curated by ChEMBL
| Assay Description
Inhibition of Sprague-Dawley rat lens aldose reductase |
J Nat Prod 75: 267-70 (2012)
Article DOI: 10.1021/np200646e
BindingDB Entry DOI: 10.7270/Q2XS5WCH |
More data for this
Ligand-Target Pair | |
Aldo-keto reductase family 1 member B1
(Rattus norvegicus) | BDBM50207159
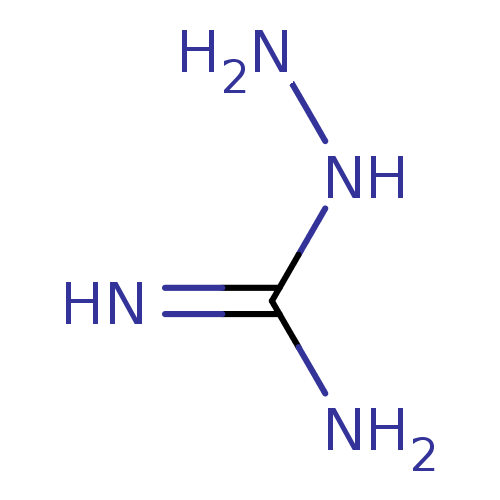
(2-aminoguanidine | 2-azanylguanidine | AMINOGUANID...)Show InChI InChI=1S/CH6N4/c2-1(3)5-4/h4H2,(H4,2,3,5) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Korean Institute of Oriental Medicine (KIOM)
Curated by ChEMBL
| Assay Description
Inhibition of Sprague-Dawley rat lens aldose reductase |
J Nat Prod 75: 267-70 (2012)
Article DOI: 10.1021/np200646e
BindingDB Entry DOI: 10.7270/Q2XS5WCH |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data