 Found 573 hits with Last Name = 'leinert' and Initial = 'h'
Found 573 hits with Last Name = 'leinert' and Initial = 'h' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Prothrombin
(Homo sapiens (Human)) | BDBM50037996
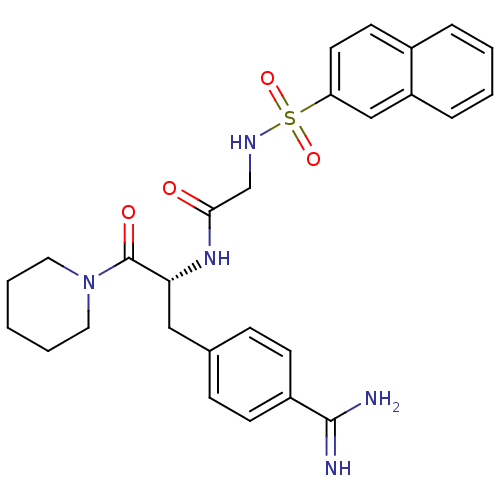
(1-[N-(naphthalen-2-ylsulfonyl)glycyl-4-carbamimido...)Show SMILES NC(=N)c1ccc(C[C@@H](NC(=O)CNS(=O)(=O)c2ccc3ccccc3c2)C(=O)N2CCCCC2)cc1 Show InChI InChI=1S/C27H31N5O4S/c28-26(29)21-10-8-19(9-11-21)16-24(27(34)32-14-4-1-5-15-32)31-25(33)18-30-37(35,36)23-13-12-20-6-2-3-7-22(20)17-23/h2-3,6-13,17,24,30H,1,4-5,14-16,18H2,(H3,28,29)(H,31,33)/t24-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
| 6 | n/a | n/a | n/a | n/a | n/a | n/a | 7.5 | n/a |
TBA
Curated by ChEMBL
| Assay Description
Tested for the Inhibition of the catalytic activity of human Thrombin at 25 degree C and pH 7.5. |
Bioorg Med Chem Lett 7: 1283-1288 (1997)
Article DOI: 10.1016/S0960-894X(97)00210-2
BindingDB Entry DOI: 10.7270/Q29W0FZ9 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50068485
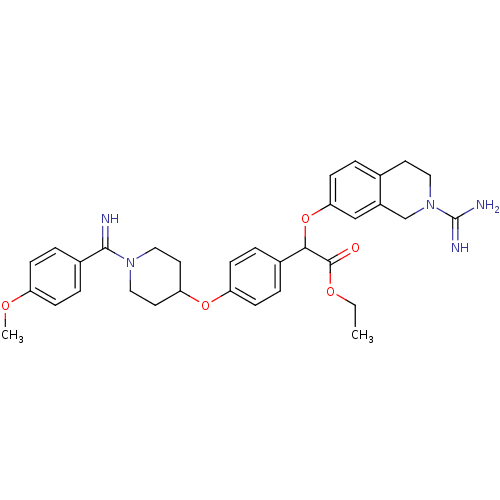
((2-Carbamimidoyl-1,2,3,4-tetrahydro-isoquinolin-7-...)Show SMILES CCOC(=O)C(Oc1ccc2CCN(Cc2c1)C(N)=N)c1ccc(OC2CCN(CC2)C(=N)c2ccc(OC)cc2)cc1 Show InChI InChI=1S/C33H39N5O5/c1-3-41-32(39)30(43-29-13-4-22-14-17-38(33(35)36)21-25(22)20-29)23-5-11-27(12-6-23)42-28-15-18-37(19-16-28)31(34)24-7-9-26(40-2)10-8-24/h4-13,20,28,30,34H,3,14-19,21H2,1-2H3,(H3,35,36) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 21 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Mannheim GmbH
Curated by ChEMBL
| Assay Description
The inhibition constant against human Coagulation factor X |
J Med Chem 41: 4983-94 (1999)
Article DOI: 10.1021/jm9800402
BindingDB Entry DOI: 10.7270/Q2611112 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50070629
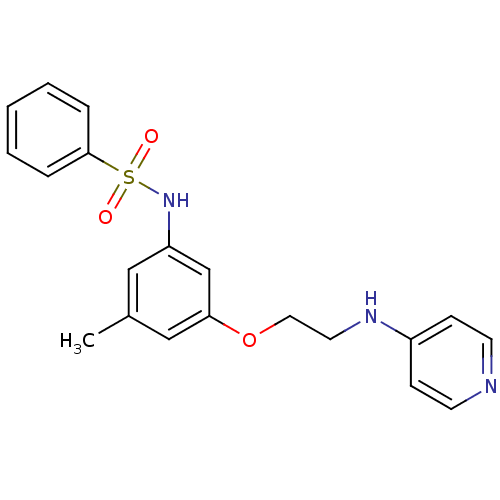
(4-[2-(3-Benzenesulfonylamino-5-methyl-phenoxy)-eth...)Show SMILES Cc1cc(NS(=O)(=O)c2ccccc2)cc(OCCNc2ccncc2)c1 Show InChI InChI=1S/C20H21N3O3S/c1-16-13-18(23-27(24,25)20-5-3-2-4-6-20)15-19(14-16)26-12-11-22-17-7-9-21-10-8-17/h2-10,13-15,23H,11-12H2,1H3,(H,21,22) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 23 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmazeutisch-Chemisches Institut der Universität
Curated by ChEMBL
| Assay Description
Inhibitory activity against thrombin |
Bioorg Med Chem Lett 8: 1613-8 (1999)
BindingDB Entry DOI: 10.7270/Q2VH5N09 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50068501
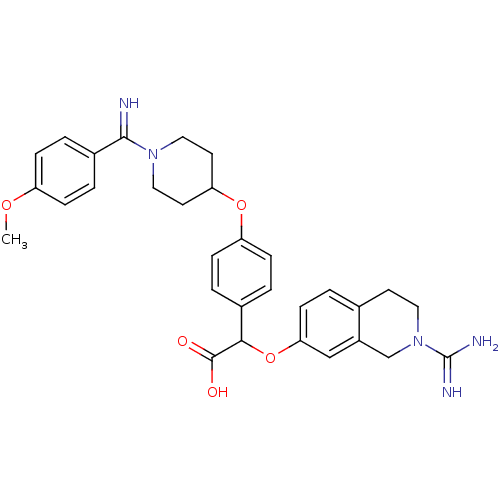
((2-Carbamimidoyl-1,2,3,4-tetrahydro-isoquinolin-7-...)Show SMILES COc1ccc(cc1)C(=N)N1CCC(CC1)Oc1ccc(cc1)C(Oc1ccc2CCN(Cc2c1)C(N)=N)C(O)=O Show InChI InChI=1S/C31H35N5O5/c1-39-24-7-5-22(6-8-24)29(32)35-16-13-26(14-17-35)40-25-9-3-21(4-10-25)28(30(37)38)41-27-11-2-20-12-15-36(31(33)34)19-23(20)18-27/h2-11,18,26,28,32H,12-17,19H2,1H3,(H3,33,34)(H,37,38) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 26 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Mannheim GmbH
Curated by ChEMBL
| Assay Description
The inhibition constant against human Coagulation factor X |
J Med Chem 41: 4983-94 (1999)
Article DOI: 10.1021/jm9800402
BindingDB Entry DOI: 10.7270/Q2611112 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50068490
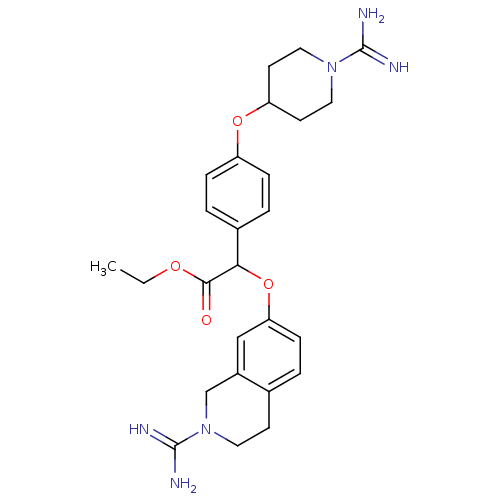
(CHEMBL146940 | [4-(1-Carbamimidoyl-piperidin-4-ylo...)Show SMILES CCOC(=O)C(Oc1ccc2CCN(Cc2c1)C(N)=N)c1ccc(OC2CCN(CC2)C(N)=N)cc1 Show InChI InChI=1S/C26H34N6O4/c1-2-34-24(33)23(36-22-8-3-17-9-12-32(26(29)30)16-19(17)15-22)18-4-6-20(7-5-18)35-21-10-13-31(14-11-21)25(27)28/h3-8,15,21,23H,2,9-14,16H2,1H3,(H3,27,28)(H3,29,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 28 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Mannheim GmbH
Curated by ChEMBL
| Assay Description
The inhibition constant against human Coagulation factor X |
J Med Chem 41: 4983-94 (1999)
Article DOI: 10.1021/jm9800402
BindingDB Entry DOI: 10.7270/Q2611112 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50068493
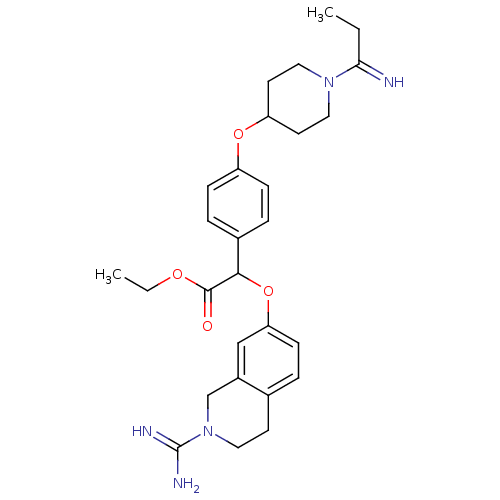
((2-Carbamimidoyl-1,2,3,4-tetrahydro-isoquinolin-7-...)Show SMILES CCOC(=O)C(Oc1ccc2CCN(Cc2c1)C(N)=N)c1ccc(OC2CCN(CC2)C(=N)CC)cc1 Show InChI InChI=1S/C28H37N5O4/c1-3-25(29)32-15-12-23(13-16-32)36-22-8-6-20(7-9-22)26(27(34)35-4-2)37-24-10-5-19-11-14-33(28(30)31)18-21(19)17-24/h5-10,17,23,26,29H,3-4,11-16,18H2,1-2H3,(H3,30,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Mannheim GmbH
Curated by ChEMBL
| Assay Description
The inhibition constant against human Coagulation factor X |
J Med Chem 41: 4983-94 (1999)
Article DOI: 10.1021/jm9800402
BindingDB Entry DOI: 10.7270/Q2611112 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50068494
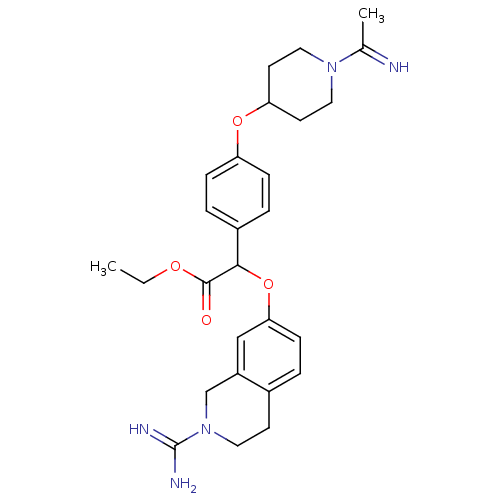
((2-Carbamimidoyl-1,2,3,4-tetrahydro-isoquinolin-7-...)Show SMILES CCOC(=O)C(Oc1ccc2CCN(Cc2c1)C(N)=N)c1ccc(OC2CCN(CC2)C(C)=N)cc1 Show InChI InChI=1S/C27H35N5O4/c1-3-34-26(33)25(36-24-9-4-19-10-13-32(27(29)30)17-21(19)16-24)20-5-7-22(8-6-20)35-23-11-14-31(15-12-23)18(2)28/h4-9,16,23,25,28H,3,10-15,17H2,1-2H3,(H3,29,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Mannheim GmbH
Curated by ChEMBL
| Assay Description
The inhibition constant against human Coagulation factor X |
J Med Chem 41: 4983-94 (1999)
Article DOI: 10.1021/jm9800402
BindingDB Entry DOI: 10.7270/Q2611112 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50068488
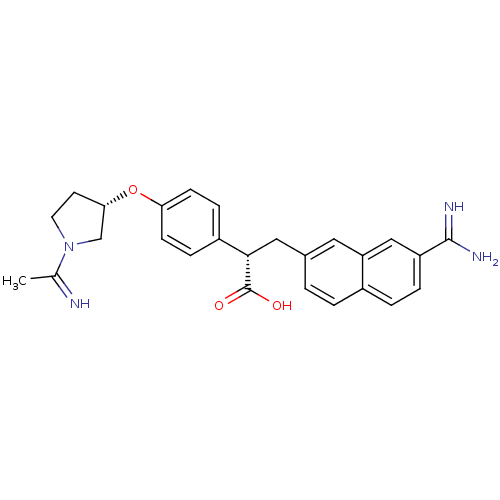
((S)-3-(7-Carbamimidoyl-naphthalen-2-yl)-2-{4-[(S)-...)Show SMILES CC(=N)N1CC[C@@H](C1)Oc1ccc(cc1)[C@H](Cc1ccc2ccc(cc2c1)C(N)=N)C(O)=O Show InChI InChI=1S/C26H28N4O3/c1-16(27)30-11-10-23(15-30)33-22-8-6-19(7-9-22)24(26(31)32)13-17-2-3-18-4-5-20(25(28)29)14-21(18)12-17/h2-9,12,14,23-24,27H,10-11,13,15H2,1H3,(H3,28,29)(H,31,32)/t23-,24-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Mannheim GmbH
Curated by ChEMBL
| Assay Description
The inhibition constant against human Coagulation factor X |
J Med Chem 41: 4983-94 (1999)
Article DOI: 10.1021/jm9800402
BindingDB Entry DOI: 10.7270/Q2611112 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50068487
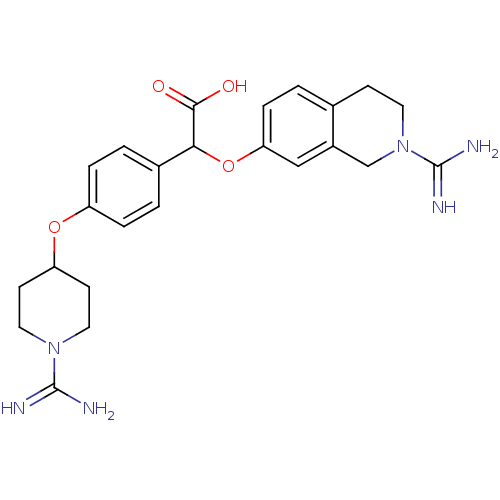
(CHEMBL148357 | [4-(1-Carbamimidoyl-piperidin-4-ylo...)Show SMILES NC(=N)N1CCC(CC1)Oc1ccc(cc1)C(Oc1ccc2CCN(Cc2c1)C(N)=N)C(O)=O Show InChI InChI=1S/C24H30N6O4/c25-23(26)29-11-8-19(9-12-29)33-18-4-2-16(3-5-18)21(22(31)32)34-20-6-1-15-7-10-30(24(27)28)14-17(15)13-20/h1-6,13,19,21H,7-12,14H2,(H3,25,26)(H3,27,28)(H,31,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 44 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Mannheim GmbH
Curated by ChEMBL
| Assay Description
The inhibition constant against human Coagulation factor X |
J Med Chem 41: 4983-94 (1999)
Article DOI: 10.1021/jm9800402
BindingDB Entry DOI: 10.7270/Q2611112 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50068506
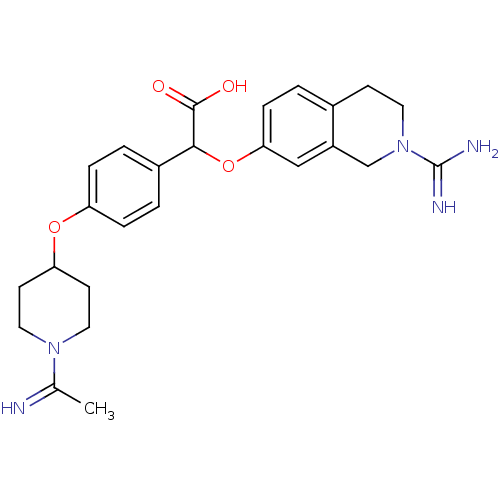
((2-Carbamimidoyl-1,2,3,4-tetrahydro-isoquinolin-7-...)Show SMILES CC(=N)N1CCC(CC1)Oc1ccc(cc1)C(Oc1ccc2CCN(Cc2c1)C(N)=N)C(O)=O Show InChI InChI=1S/C25H31N5O4/c1-16(26)29-12-9-21(10-13-29)33-20-5-3-18(4-6-20)23(24(31)32)34-22-7-2-17-8-11-30(25(27)28)15-19(17)14-22/h2-7,14,21,23,26H,8-13,15H2,1H3,(H3,27,28)(H,31,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 55 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Mannheim GmbH
Curated by ChEMBL
| Assay Description
The inhibition constant against human Coagulation factor X |
J Med Chem 41: 4983-94 (1999)
Article DOI: 10.1021/jm9800402
BindingDB Entry DOI: 10.7270/Q2611112 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50070596

(CHEMBL282549 | N-Methyl-N-{3-[2-(pyridin-4-ylamino...)Show SMILES CN(c1cccc(OCCNc2ccncc2)c1)S(=O)(=O)c1ccccc1 Show InChI InChI=1S/C20H21N3O3S/c1-23(27(24,25)20-8-3-2-4-9-20)18-6-5-7-19(16-18)26-15-14-22-17-10-12-21-13-11-17/h2-13,16H,14-15H2,1H3,(H,21,22) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| 70 | n/a | n/a | n/a | n/a | n/a | n/a | 7.5 | n/a |
TBA
Curated by ChEMBL
| Assay Description
Tested for the Inhibition of the catalytic activity of human Thrombin at 25 degree C and pH 7.5. |
Bioorg Med Chem Lett 7: 1283-1288 (1997)
Article DOI: 10.1016/S0960-894X(97)00210-2
BindingDB Entry DOI: 10.7270/Q29W0FZ9 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50068499
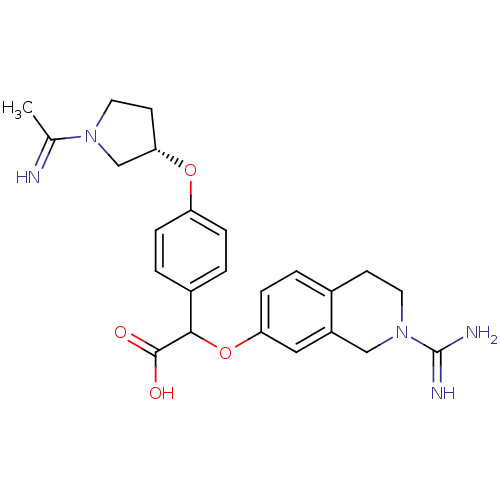
((2-Carbamimidoyl-1,2,3,4-tetrahydro-isoquinolin-7-...)Show SMILES CC(=N)N1CC[C@@H](C1)Oc1ccc(cc1)C(Oc1ccc2CCN(Cc2c1)C(N)=N)C(O)=O Show InChI InChI=1S/C24H29N5O4/c1-15(25)28-11-9-21(14-28)32-19-5-3-17(4-6-19)22(23(30)31)33-20-7-2-16-8-10-29(24(26)27)13-18(16)12-20/h2-7,12,21-22,25H,8-11,13-14H2,1H3,(H3,26,27)(H,30,31)/t21-,22?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Mannheim GmbH
Curated by ChEMBL
| Assay Description
The inhibition constant against human Coagulation factor X |
J Med Chem 41: 4983-94 (1999)
Article DOI: 10.1021/jm9800402
BindingDB Entry DOI: 10.7270/Q2611112 |
More data for this
Ligand-Target Pair | |
Serine protease 1/Trypsin-2
(Homo sapiens (Human)) | BDBM50068493

((2-Carbamimidoyl-1,2,3,4-tetrahydro-isoquinolin-7-...)Show SMILES CCOC(=O)C(Oc1ccc2CCN(Cc2c1)C(N)=N)c1ccc(OC2CCN(CC2)C(=N)CC)cc1 Show InChI InChI=1S/C28H37N5O4/c1-3-25(29)32-15-12-23(13-16-32)36-22-8-6-20(7-9-22)26(27(34)35-4-2)37-24-10-5-19-11-14-33(28(30)31)18-21(19)17-24/h5-10,17,23,26,29H,3-4,11-16,18H2,1-2H3,(H3,30,31) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Mannheim GmbH
Curated by ChEMBL
| Assay Description
The inhibition constant against human trypsin |
J Med Chem 41: 4983-94 (1999)
Article DOI: 10.1021/jm9800402
BindingDB Entry DOI: 10.7270/Q2611112 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50068502

((2-Carbamimidoyl-1,2,3,4-tetrahydro-isoquinolin-7-...)Show SMILES CCOC(=O)C(Oc1ccc2CCN(Cc2c1)C(N)=N)c1ccc(O[C@H]2CCN(C2)C(C)=N)cc1 Show InChI InChI=1S/C26H33N5O4/c1-3-33-25(32)24(35-22-9-4-18-10-12-31(26(28)29)15-20(18)14-22)19-5-7-21(8-6-19)34-23-11-13-30(16-23)17(2)27/h4-9,14,23-24,27H,3,10-13,15-16H2,1-2H3,(H3,28,29)/t23-,24?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Mannheim GmbH
Curated by ChEMBL
| Assay Description
The inhibition constant against human Coagulation factor X |
J Med Chem 41: 4983-94 (1999)
Article DOI: 10.1021/jm9800402
BindingDB Entry DOI: 10.7270/Q2611112 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50070633
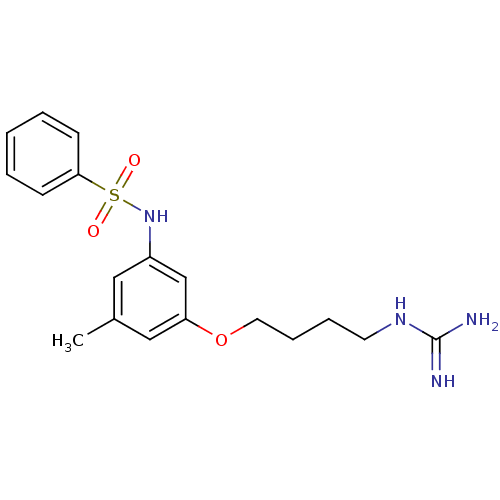
(CHEMBL289790 | N-[3-(4-Guanidino-butoxy)-5-methyl-...)Show SMILES Cc1cc(NS(=O)(=O)c2ccccc2)cc(OCCCCNC(N)=N)c1 Show InChI InChI=1S/C18H24N4O3S/c1-14-11-15(22-26(23,24)17-7-3-2-4-8-17)13-16(12-14)25-10-6-5-9-21-18(19)20/h2-4,7-8,11-13,22H,5-6,9-10H2,1H3,(H4,19,20,21) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmazeutisch-Chemisches Institut der Universität
Curated by ChEMBL
| Assay Description
Inhibitory activity against thrombin |
Bioorg Med Chem Lett 8: 1613-8 (1999)
BindingDB Entry DOI: 10.7270/Q2VH5N09 |
More data for this
Ligand-Target Pair | |
Serine protease 1/Trypsin-2
(Homo sapiens (Human)) | BDBM50068487

(CHEMBL148357 | [4-(1-Carbamimidoyl-piperidin-4-ylo...)Show SMILES NC(=N)N1CCC(CC1)Oc1ccc(cc1)C(Oc1ccc2CCN(Cc2c1)C(N)=N)C(O)=O Show InChI InChI=1S/C24H30N6O4/c25-23(26)29-11-8-19(9-12-29)33-18-4-2-16(3-5-18)21(22(31)32)34-20-6-1-15-7-10-30(24(27)28)14-17(15)13-20/h1-6,13,19,21H,7-12,14H2,(H3,25,26)(H3,27,28)(H,31,32) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Mannheim GmbH
Curated by ChEMBL
| Assay Description
The inhibition constant against human trypsin |
J Med Chem 41: 4983-94 (1999)
Article DOI: 10.1021/jm9800402
BindingDB Entry DOI: 10.7270/Q2611112 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50068486

(CHEMBL147988 | [4-((S)-1-Carbamimidoyl-pyrrolidin-...)Show SMILES NC(=N)N1CC[C@@H](C1)Oc1ccc(cc1)C(Oc1ccc2CCN(Cc2c1)C(N)=N)C(O)=O Show InChI InChI=1S/C23H28N6O4/c24-22(25)28-9-7-14-1-6-18(11-16(14)12-28)33-20(21(30)31)15-2-4-17(5-3-15)32-19-8-10-29(13-19)23(26)27/h1-6,11,19-20H,7-10,12-13H2,(H3,24,25)(H3,26,27)(H,30,31)/t19-,20?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Mannheim GmbH
Curated by ChEMBL
| Assay Description
The inhibition constant against human Coagulation factor X |
J Med Chem 41: 4983-94 (1999)
Article DOI: 10.1021/jm9800402
BindingDB Entry DOI: 10.7270/Q2611112 |
More data for this
Ligand-Target Pair | |
Serine protease 1/Trypsin-2
(Homo sapiens (Human)) | BDBM50068501

((2-Carbamimidoyl-1,2,3,4-tetrahydro-isoquinolin-7-...)Show SMILES COc1ccc(cc1)C(=N)N1CCC(CC1)Oc1ccc(cc1)C(Oc1ccc2CCN(Cc2c1)C(N)=N)C(O)=O Show InChI InChI=1S/C31H35N5O5/c1-39-24-7-5-22(6-8-24)29(32)35-16-13-26(14-17-35)40-25-9-3-21(4-10-25)28(30(37)38)41-27-11-2-20-12-15-36(31(33)34)19-23(20)18-27/h2-11,18,26,28,32H,12-17,19H2,1H3,(H3,33,34)(H,37,38) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Mannheim GmbH
Curated by ChEMBL
| Assay Description
The inhibition constant against human trypsin |
J Med Chem 41: 4983-94 (1999)
Article DOI: 10.1021/jm9800402
BindingDB Entry DOI: 10.7270/Q2611112 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50068491
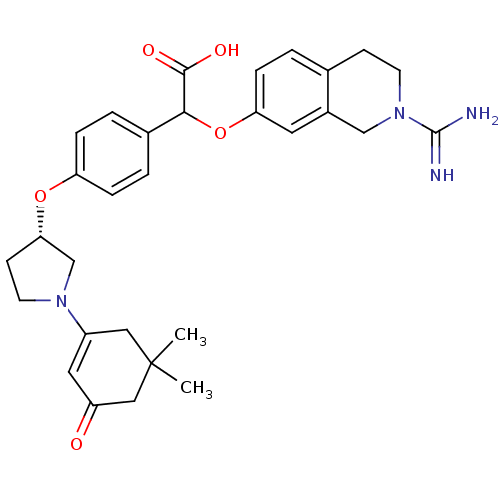
((2-Carbamimidoyl-1,2,3,4-tetrahydro-isoquinolin-7-...)Show SMILES CC1(C)CC(=O)C=C(C1)N1CC[C@@H](C1)Oc1ccc(cc1)C(Oc1ccc2CCN(Cc2c1)C(N)=N)C(O)=O |c:6| Show InChI InChI=1S/C30H36N4O5/c1-30(2)15-22(14-23(35)16-30)33-12-10-26(18-33)38-24-6-4-20(5-7-24)27(28(36)37)39-25-8-3-19-9-11-34(29(31)32)17-21(19)13-25/h3-8,13-14,26-27H,9-12,15-18H2,1-2H3,(H3,31,32)(H,36,37)/t26-,27?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Mannheim GmbH
Curated by ChEMBL
| Assay Description
The inhibition constant against human Coagulation factor X |
J Med Chem 41: 4983-94 (1999)
Article DOI: 10.1021/jm9800402
BindingDB Entry DOI: 10.7270/Q2611112 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50068484
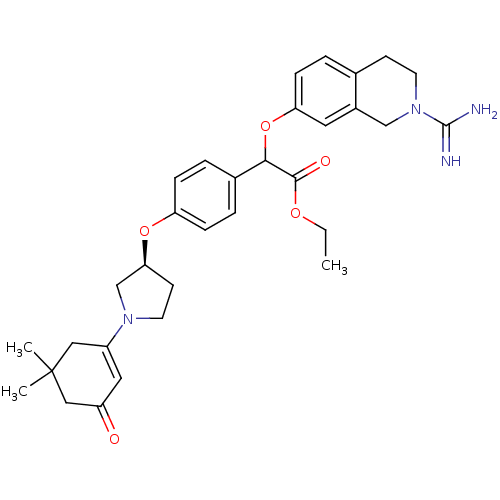
((2-Carbamimidoyl-1,2,3,4-tetrahydro-isoquinolin-7-...)Show SMILES CCOC(=O)C(Oc1ccc2CCN(Cc2c1)C(N)=N)c1ccc(O[C@H]2CCN(C2)C2=CC(=O)CC(C)(C)C2)cc1 |t:33| Show InChI InChI=1S/C32H40N4O5/c1-4-39-30(38)29(41-27-10-5-21-11-13-36(31(33)34)19-23(21)15-27)22-6-8-26(9-7-22)40-28-12-14-35(20-28)24-16-25(37)18-32(2,3)17-24/h5-10,15-16,28-29H,4,11-14,17-20H2,1-3H3,(H3,33,34)/t28-,29?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Mannheim GmbH
Curated by ChEMBL
| Assay Description
The inhibition constant against human Coagulation factor X |
J Med Chem 41: 4983-94 (1999)
Article DOI: 10.1021/jm9800402
BindingDB Entry DOI: 10.7270/Q2611112 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50289376
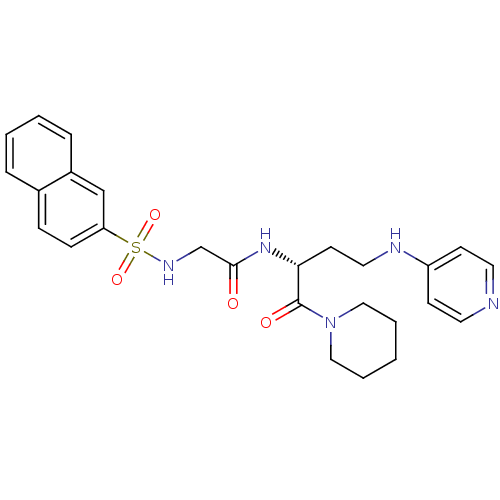
(2-(Naphthalene-2-sulfonylamino)-N-[(R)-1-(piperidi...)Show SMILES O=C(CNS(=O)(=O)c1ccc2ccccc2c1)N[C@H](CCNc1ccncc1)C(=O)N1CCCCC1 Show InChI InChI=1S/C26H31N5O4S/c32-25(19-29-36(34,35)23-9-8-20-6-2-3-7-21(20)18-23)30-24(26(33)31-16-4-1-5-17-31)12-15-28-22-10-13-27-14-11-22/h2-3,6-11,13-14,18,24,29H,1,4-5,12,15-17,19H2,(H,27,28)(H,30,32)/t24-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 200 | n/a | n/a | n/a | n/a | n/a | n/a | 7.5 | n/a |
TBA
Curated by ChEMBL
| Assay Description
Tested for the Inhibition of the catalytic activity of human Thrombin at 25 degree C and pH 7.5. |
Bioorg Med Chem Lett 7: 1283-1288 (1997)
Article DOI: 10.1016/S0960-894X(97)00210-2
BindingDB Entry DOI: 10.7270/Q29W0FZ9 |
More data for this
Ligand-Target Pair | |
Serine protease 1/Trypsin-2
(Homo sapiens (Human)) | BDBM50068485

((2-Carbamimidoyl-1,2,3,4-tetrahydro-isoquinolin-7-...)Show SMILES CCOC(=O)C(Oc1ccc2CCN(Cc2c1)C(N)=N)c1ccc(OC2CCN(CC2)C(=N)c2ccc(OC)cc2)cc1 Show InChI InChI=1S/C33H39N5O5/c1-3-41-32(39)30(43-29-13-4-22-14-17-38(33(35)36)21-25(22)20-29)23-5-11-27(12-6-23)42-28-15-18-37(19-16-28)31(34)24-7-9-26(40-2)10-8-24/h4-13,20,28,30,34H,3,14-19,21H2,1-2H3,(H3,35,36) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Mannheim GmbH
Curated by ChEMBL
| Assay Description
The inhibition constant against human trypsin |
J Med Chem 41: 4983-94 (1999)
Article DOI: 10.1021/jm9800402
BindingDB Entry DOI: 10.7270/Q2611112 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50068500
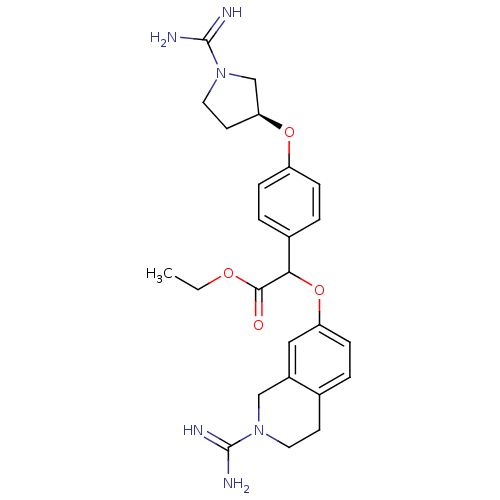
(CHEMBL344309 | [4-((S)-1-Carbamimidoyl-pyrrolidin-...)Show SMILES CCOC(=O)C(Oc1ccc2CCN(Cc2c1)C(N)=N)c1ccc(O[C@H]2CCN(C2)C(N)=N)cc1 Show InChI InChI=1S/C25H32N6O4/c1-2-33-23(32)22(35-20-8-3-16-9-11-30(24(26)27)14-18(16)13-20)17-4-6-19(7-5-17)34-21-10-12-31(15-21)25(28)29/h3-8,13,21-22H,2,9-12,14-15H2,1H3,(H3,26,27)(H3,28,29)/t21-,22?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Mannheim GmbH
Curated by ChEMBL
| Assay Description
The inhibition constant against human Coagulation factor X |
J Med Chem 41: 4983-94 (1999)
Article DOI: 10.1021/jm9800402
BindingDB Entry DOI: 10.7270/Q2611112 |
More data for this
Ligand-Target Pair | |
Serine protease 1/Trypsin-2
(Homo sapiens (Human)) | BDBM50068490

(CHEMBL146940 | [4-(1-Carbamimidoyl-piperidin-4-ylo...)Show SMILES CCOC(=O)C(Oc1ccc2CCN(Cc2c1)C(N)=N)c1ccc(OC2CCN(CC2)C(N)=N)cc1 Show InChI InChI=1S/C26H34N6O4/c1-2-34-24(33)23(36-22-8-3-17-9-12-32(26(29)30)16-19(17)15-22)18-4-6-20(7-5-18)35-21-10-13-31(14-11-21)25(27)28/h3-8,15,21,23H,2,9-14,16H2,1H3,(H3,27,28)(H3,29,30) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Mannheim GmbH
Curated by ChEMBL
| Assay Description
The inhibition constant against human trypsin |
J Med Chem 41: 4983-94 (1999)
Article DOI: 10.1021/jm9800402
BindingDB Entry DOI: 10.7270/Q2611112 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50068507
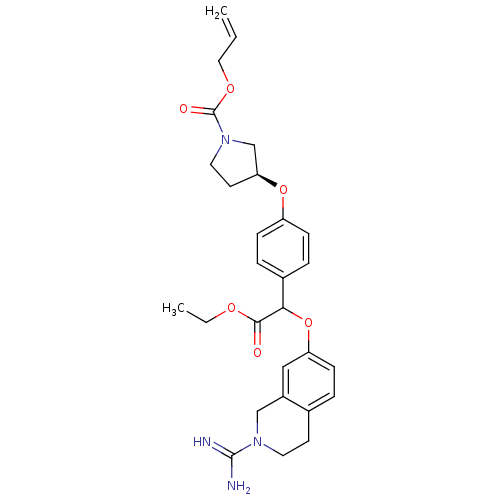
((S)-3-{4-[(2-Carbamimidoyl-1,2,3,4-tetrahydro-isoq...)Show SMILES CCOC(=O)C(Oc1ccc2CCN(Cc2c1)C(N)=N)c1ccc(O[C@H]2CCN(C2)C(=O)OCC=C)cc1 Show InChI InChI=1S/C28H34N4O6/c1-3-15-36-28(34)32-14-12-24(18-32)37-22-8-6-20(7-9-22)25(26(33)35-4-2)38-23-10-5-19-11-13-31(27(29)30)17-21(19)16-23/h3,5-10,16,24-25H,1,4,11-15,17-18H2,2H3,(H3,29,30)/t24-,25?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Mannheim GmbH
Curated by ChEMBL
| Assay Description
The inhibition constant against human Coagulation factor X |
J Med Chem 41: 4983-94 (1999)
Article DOI: 10.1021/jm9800402
BindingDB Entry DOI: 10.7270/Q2611112 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50070632
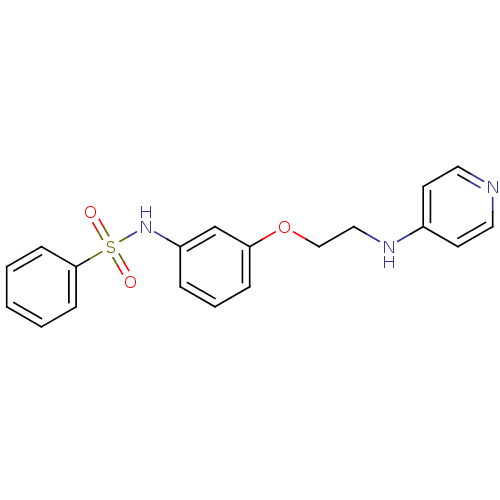
(4-[2-(3-Benzenesulfonylamino-phenoxy)-ethylamino]-...)Show InChI InChI=1S/C19H19N3O3S/c23-26(24,19-7-2-1-3-8-19)22-17-5-4-6-18(15-17)25-14-13-21-16-9-11-20-12-10-16/h1-12,15,22H,13-14H2,(H,20,21) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| 300 | n/a | n/a | n/a | n/a | n/a | n/a | 7.5 | n/a |
TBA
Curated by ChEMBL
| Assay Description
Tested for the Inhibition of the catalytic activity of human Thrombin at 25 degree C and pH 7.5. |
Bioorg Med Chem Lett 7: 1283-1288 (1997)
Article DOI: 10.1016/S0960-894X(97)00210-2
BindingDB Entry DOI: 10.7270/Q29W0FZ9 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50070632

(4-[2-(3-Benzenesulfonylamino-phenoxy)-ethylamino]-...)Show InChI InChI=1S/C19H19N3O3S/c23-26(24,19-7-2-1-3-8-19)22-17-5-4-6-18(15-17)25-14-13-21-16-9-11-20-12-10-16/h1-12,15,22H,13-14H2,(H,20,21) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmazeutisch-Chemisches Institut der Universität
Curated by ChEMBL
| Assay Description
Inhibitory activity against thrombin |
Bioorg Med Chem Lett 8: 1613-8 (1999)
BindingDB Entry DOI: 10.7270/Q2VH5N09 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50068505

((2-Carbamimidoyl-1,2,3,4-tetrahydro-isoquinolin-7-...)Show SMILES CCOC(=O)C(Oc1ccc2CCN(Cc2c1)C(N)=N)c1ccc(O[C@H]2CCNC2)cc1 Show InChI InChI=1S/C24H30N4O4/c1-2-30-23(29)22(17-4-6-19(7-5-17)31-21-9-11-27-14-21)32-20-8-3-16-10-12-28(24(25)26)15-18(16)13-20/h3-8,13,21-22,27H,2,9-12,14-15H2,1H3,(H3,25,26)/t21-,22?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Mannheim GmbH
Curated by ChEMBL
| Assay Description
The inhibition constant against human Coagulation factor X |
J Med Chem 41: 4983-94 (1999)
Article DOI: 10.1021/jm9800402
BindingDB Entry DOI: 10.7270/Q2611112 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50068495

(CHEMBL148972 | [4-(1-Acetyl-piperidin-4-yloxy)-phe...)Show SMILES CC(=O)N1CCC(CC1)Oc1ccc(cc1)C(Oc1ccc2CCN(Cc2c1)C(N)=N)C(O)=O Show InChI InChI=1S/C25H30N4O5/c1-16(30)28-12-9-21(10-13-28)33-20-5-3-18(4-6-20)23(24(31)32)34-22-7-2-17-8-11-29(25(26)27)15-19(17)14-22/h2-7,14,21,23H,8-13,15H2,1H3,(H3,26,27)(H,31,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Mannheim GmbH
Curated by ChEMBL
| Assay Description
The inhibition constant against human Coagulation factor X |
J Med Chem 41: 4983-94 (1999)
Article DOI: 10.1021/jm9800402
BindingDB Entry DOI: 10.7270/Q2611112 |
More data for this
Ligand-Target Pair | |
Serine protease 1/Trypsin-2
(Homo sapiens (Human)) | BDBM50068494

((2-Carbamimidoyl-1,2,3,4-tetrahydro-isoquinolin-7-...)Show SMILES CCOC(=O)C(Oc1ccc2CCN(Cc2c1)C(N)=N)c1ccc(OC2CCN(CC2)C(C)=N)cc1 Show InChI InChI=1S/C27H35N5O4/c1-3-34-26(33)25(36-24-9-4-19-10-13-32(27(29)30)17-21(19)16-24)20-5-7-22(8-6-20)35-23-11-14-31(15-12-23)18(2)28/h4-9,16,23,25,28H,3,10-15,17H2,1-2H3,(H3,29,30) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Mannheim GmbH
Curated by ChEMBL
| Assay Description
The inhibition constant against human trypsin |
J Med Chem 41: 4983-94 (1999)
Article DOI: 10.1021/jm9800402
BindingDB Entry DOI: 10.7270/Q2611112 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50070622
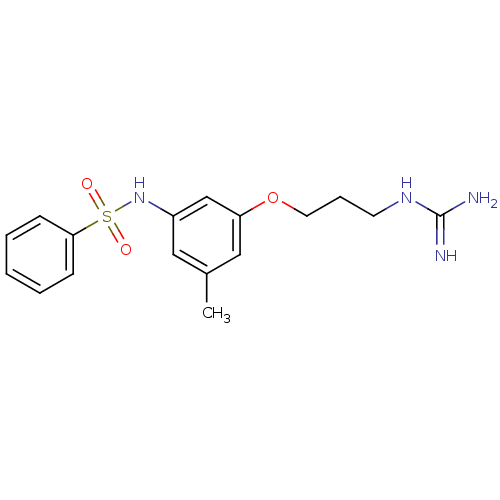
(CHEMBL40704 | N-[3-(3-Guanidino-propoxy)-5-methyl-...)Show InChI InChI=1S/C17H22N4O3S/c1-13-10-14(21-25(22,23)16-6-3-2-4-7-16)12-15(11-13)24-9-5-8-20-17(18)19/h2-4,6-7,10-12,21H,5,8-9H2,1H3,(H4,18,19,20) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 630 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmazeutisch-Chemisches Institut der Universität
Curated by ChEMBL
| Assay Description
Inhibitory activity against thrombin |
Bioorg Med Chem Lett 8: 1613-8 (1999)
BindingDB Entry DOI: 10.7270/Q2VH5N09 |
More data for this
Ligand-Target Pair | |
Serine protease 1
(Bos taurus (bovine)) | BDBM50037996

(1-[N-(naphthalen-2-ylsulfonyl)glycyl-4-carbamimido...)Show SMILES NC(=N)c1ccc(C[C@@H](NC(=O)CNS(=O)(=O)c2ccc3ccccc3c2)C(=O)N2CCCCC2)cc1 Show InChI InChI=1S/C27H31N5O4S/c28-26(29)21-10-8-19(9-11-21)16-24(27(34)32-14-4-1-5-15-32)31-25(33)18-30-37(35,36)23-13-12-20-6-2-3-7-22(20)17-23/h2-3,6-13,17,24,30H,1,4-5,14-16,18H2,(H3,28,29)(H,31,33)/t24-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
| 690 | n/a | n/a | n/a | n/a | n/a | n/a | 7.5 | n/a |
TBA
Curated by ChEMBL
| Assay Description
Tested for the Inhibition of the catalytic activity of bovine trypsin at 25 degree C and pH 7.5. |
Bioorg Med Chem Lett 7: 1283-1288 (1997)
Article DOI: 10.1016/S0960-894X(97)00210-2
BindingDB Entry DOI: 10.7270/Q29W0FZ9 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Prothrombin
(Homo sapiens (Human)) | BDBM50126700
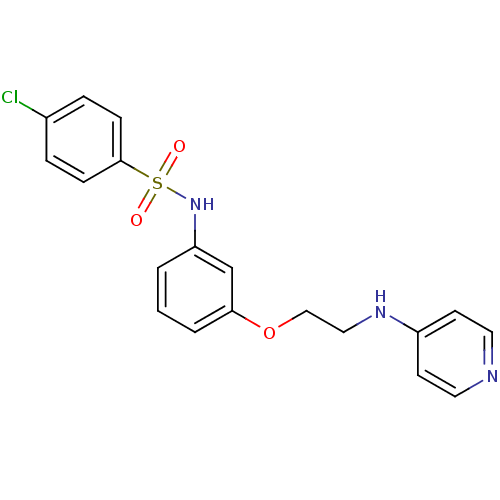
(4-Chloro-N-{3-[2-(pyridin-4-ylamino)-ethoxy]-pheny...)Show SMILES Clc1ccc(cc1)S(=O)(=O)Nc1cccc(OCCNc2ccncc2)c1 Show InChI InChI=1S/C19H18ClN3O3S/c20-15-4-6-19(7-5-15)27(24,25)23-17-2-1-3-18(14-17)26-13-12-22-16-8-10-21-11-9-16/h1-11,14,23H,12-13H2,(H,21,22) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| 700 | n/a | n/a | n/a | n/a | n/a | n/a | 7.5 | n/a |
TBA
Curated by ChEMBL
| Assay Description
Tested for the Inhibition of the catalytic activity of human Thrombin at 25 degree C and pH 7.5. |
Bioorg Med Chem Lett 7: 1283-1288 (1997)
Article DOI: 10.1016/S0960-894X(97)00210-2
BindingDB Entry DOI: 10.7270/Q29W0FZ9 |
More data for this
Ligand-Target Pair | |
Serine protease 1/Trypsin-2
(Homo sapiens (Human)) | BDBM50068486

(CHEMBL147988 | [4-((S)-1-Carbamimidoyl-pyrrolidin-...)Show SMILES NC(=N)N1CC[C@@H](C1)Oc1ccc(cc1)C(Oc1ccc2CCN(Cc2c1)C(N)=N)C(O)=O Show InChI InChI=1S/C23H28N6O4/c24-22(25)28-9-7-14-1-6-18(11-16(14)12-28)33-20(21(30)31)15-2-4-17(5-3-15)32-19-8-10-29(13-19)23(26)27/h1-6,11,19-20H,7-10,12-13H2,(H3,24,25)(H3,26,27)(H,30,31)/t19-,20?/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Mannheim GmbH
Curated by ChEMBL
| Assay Description
The inhibition constant against human trypsin |
J Med Chem 41: 4983-94 (1999)
Article DOI: 10.1021/jm9800402
BindingDB Entry DOI: 10.7270/Q2611112 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50068496

((2-Carbamimidoyl-1,2,3,4-tetrahydro-isoquinolin-7-...)Show SMILES NC(=N)N1CCc2ccc(OC(C(O)=O)c3ccc(O[C@H]4CCNC4)cc3)cc2C1 Show InChI InChI=1S/C22H26N4O4/c23-22(24)26-10-8-14-1-6-18(11-16(14)13-26)30-20(21(27)28)15-2-4-17(5-3-15)29-19-7-9-25-12-19/h1-6,11,19-20,25H,7-10,12-13H2,(H3,23,24)(H,27,28)/t19-,20?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Mannheim GmbH
Curated by ChEMBL
| Assay Description
The inhibition constant against human Coagulation factor X |
J Med Chem 41: 4983-94 (1999)
Article DOI: 10.1021/jm9800402
BindingDB Entry DOI: 10.7270/Q2611112 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50068498
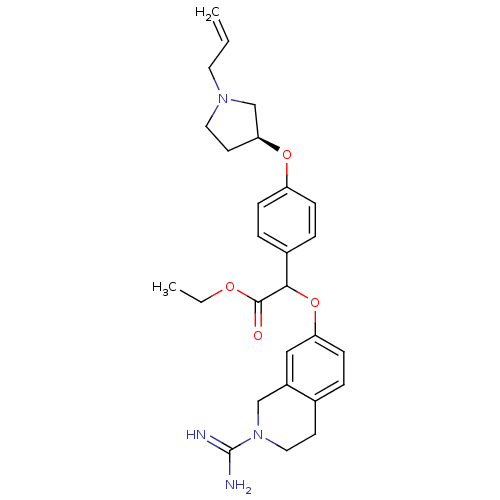
(CHEMBL342568 | [4-((S)-1-Allyl-pyrrolidin-3-yloxy)...)Show SMILES CCOC(=O)C(Oc1ccc2CCN(Cc2c1)C(N)=N)c1ccc(O[C@H]2CCN(CC=C)C2)cc1 Show InChI InChI=1S/C27H34N4O4/c1-3-13-30-14-12-24(18-30)34-22-8-6-20(7-9-22)25(26(32)33-4-2)35-23-10-5-19-11-15-31(27(28)29)17-21(19)16-23/h3,5-10,16,24-25H,1,4,11-15,17-18H2,2H3,(H3,28,29)/t24-,25?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Mannheim GmbH
Curated by ChEMBL
| Assay Description
The inhibition constant against human Coagulation factor X |
J Med Chem 41: 4983-94 (1999)
Article DOI: 10.1021/jm9800402
BindingDB Entry DOI: 10.7270/Q2611112 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50289377
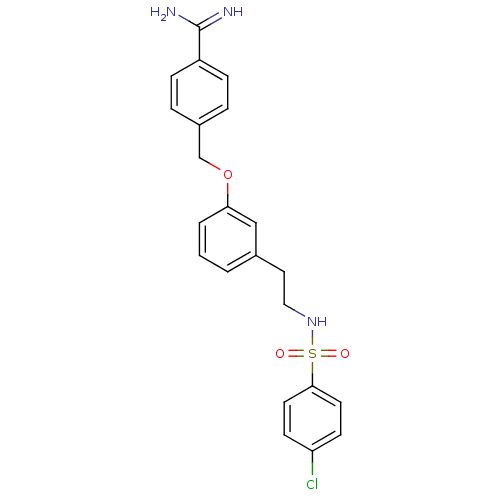
(4-{3-[2-(4-Chloro-benzenesulfonylamino)-ethyl]-phe...)Show SMILES NC(=N)c1ccc(COc2cccc(CCNS(=O)(=O)c3ccc(Cl)cc3)c2)cc1 Show InChI InChI=1S/C22H22ClN3O3S/c23-19-8-10-21(11-9-19)30(27,28)26-13-12-16-2-1-3-20(14-16)29-15-17-4-6-18(7-5-17)22(24)25/h1-11,14,26H,12-13,15H2,(H3,24,25) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
| 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | 7.5 | n/a |
TBA
Curated by ChEMBL
| Assay Description
Tested for the Inhibition of the catalytic activity of human Thrombin at 25 degree C and pH 7.5. |
Bioorg Med Chem Lett 7: 1283-1288 (1997)
Article DOI: 10.1016/S0960-894X(97)00210-2
BindingDB Entry DOI: 10.7270/Q29W0FZ9 |
More data for this
Ligand-Target Pair | |
Serine protease 1
(Bos taurus (bovine)) | BDBM50289377

(4-{3-[2-(4-Chloro-benzenesulfonylamino)-ethyl]-phe...)Show SMILES NC(=N)c1ccc(COc2cccc(CCNS(=O)(=O)c3ccc(Cl)cc3)c2)cc1 Show InChI InChI=1S/C22H22ClN3O3S/c23-19-8-10-21(11-9-19)30(27,28)26-13-12-16-2-1-3-20(14-16)29-15-17-4-6-18(7-5-17)22(24)25/h1-11,14,26H,12-13,15H2,(H3,24,25) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
| 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | 7.5 | n/a |
TBA
Curated by ChEMBL
| Assay Description
Tested for the Inhibition of the catalytic activity of bovine trypsin at 25 degree C and pH 7.5. |
Bioorg Med Chem Lett 7: 1283-1288 (1997)
Article DOI: 10.1016/S0960-894X(97)00210-2
BindingDB Entry DOI: 10.7270/Q29W0FZ9 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50068503
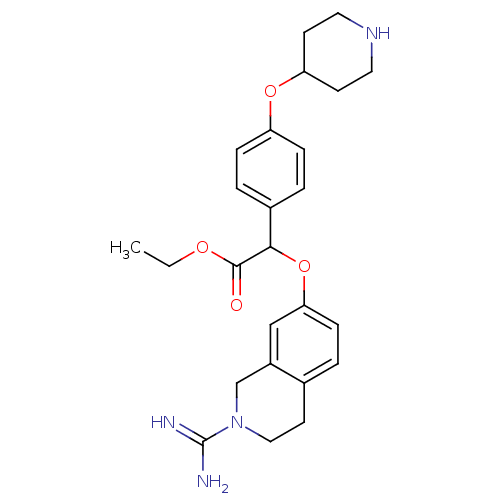
((2-Carbamimidoyl-1,2,3,4-tetrahydro-isoquinolin-7-...)Show SMILES CCOC(=O)C(Oc1ccc2CCN(Cc2c1)C(N)=N)c1ccc(OC2CCNCC2)cc1 Show InChI InChI=1S/C25H32N4O4/c1-2-31-24(30)23(18-4-6-20(7-5-18)32-21-9-12-28-13-10-21)33-22-8-3-17-11-14-29(25(26)27)16-19(17)15-22/h3-8,15,21,23,28H,2,9-14,16H2,1H3,(H3,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Mannheim GmbH
Curated by ChEMBL
| Assay Description
The inhibition constant against human Coagulation factor X |
J Med Chem 41: 4983-94 (1999)
Article DOI: 10.1021/jm9800402
BindingDB Entry DOI: 10.7270/Q2611112 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50070630
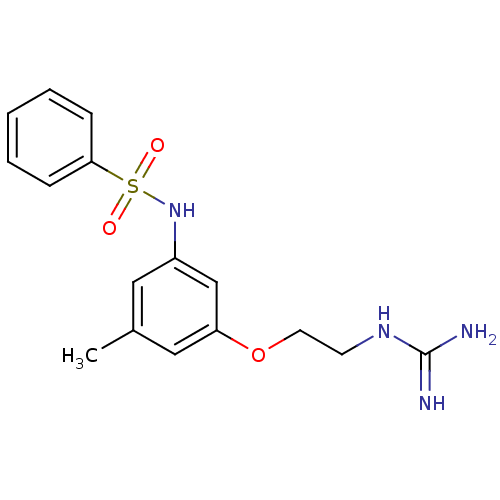
(CHEMBL290226 | N-[3-(2-Guanidino-ethoxy)-5-methyl-...)Show InChI InChI=1S/C16H20N4O3S/c1-12-9-13(11-14(10-12)23-8-7-19-16(17)18)20-24(21,22)15-5-3-2-4-6-15/h2-6,9-11,20H,7-8H2,1H3,(H4,17,18,19) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmazeutisch-Chemisches Institut der Universität
Curated by ChEMBL
| Assay Description
Inhibitory activity against thrombin |
Bioorg Med Chem Lett 8: 1613-8 (1999)
BindingDB Entry DOI: 10.7270/Q2VH5N09 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50068492
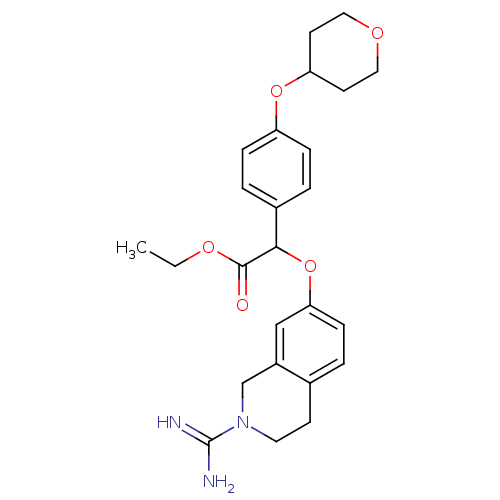
((2-Carbamimidoyl-1,2,3,4-tetrahydro-isoquinolin-7-...)Show SMILES CCOC(=O)C(Oc1ccc2CCN(Cc2c1)C(N)=N)c1ccc(OC2CCOCC2)cc1 Show InChI InChI=1S/C25H31N3O5/c1-2-31-24(29)23(18-4-6-20(7-5-18)32-21-10-13-30-14-11-21)33-22-8-3-17-9-12-28(25(26)27)16-19(17)15-22/h3-8,15,21,23H,2,9-14,16H2,1H3,(H3,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Mannheim GmbH
Curated by ChEMBL
| Assay Description
The inhibition constant against human Coagulation factor X |
J Med Chem 41: 4983-94 (1999)
Article DOI: 10.1021/jm9800402
BindingDB Entry DOI: 10.7270/Q2611112 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50068504
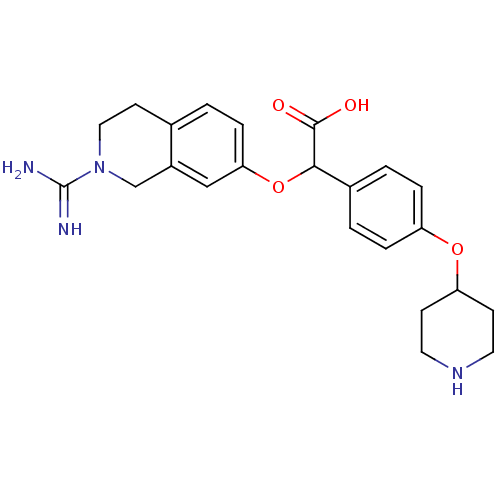
((2-Carbamimidoyl-1,2,3,4-tetrahydro-isoquinolin-7-...)Show SMILES NC(=N)N1CCc2ccc(OC(C(O)=O)c3ccc(OC4CCNCC4)cc3)cc2C1 Show InChI InChI=1S/C23H28N4O4/c24-23(25)27-12-9-15-1-6-20(13-17(15)14-27)31-21(22(28)29)16-2-4-18(5-3-16)30-19-7-10-26-11-8-19/h1-6,13,19,21,26H,7-12,14H2,(H3,24,25)(H,28,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Mannheim GmbH
Curated by ChEMBL
| Assay Description
The inhibition constant against human Coagulation factor X |
J Med Chem 41: 4983-94 (1999)
Article DOI: 10.1021/jm9800402
BindingDB Entry DOI: 10.7270/Q2611112 |
More data for this
Ligand-Target Pair | |
Serine protease 1/Trypsin-2
(Homo sapiens (Human)) | BDBM50068500

(CHEMBL344309 | [4-((S)-1-Carbamimidoyl-pyrrolidin-...)Show SMILES CCOC(=O)C(Oc1ccc2CCN(Cc2c1)C(N)=N)c1ccc(O[C@H]2CCN(C2)C(N)=N)cc1 Show InChI InChI=1S/C25H32N6O4/c1-2-33-23(32)22(35-20-8-3-16-9-11-30(24(26)27)14-18(16)13-20)17-4-6-19(7-5-17)34-21-10-12-31(15-21)25(28)29/h3-8,13,21-22H,2,9-12,14-15H2,1H3,(H3,26,27)(H3,28,29)/t21-,22?/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Mannheim GmbH
Curated by ChEMBL
| Assay Description
The inhibition constant against human trypsin |
J Med Chem 41: 4983-94 (1999)
Article DOI: 10.1021/jm9800402
BindingDB Entry DOI: 10.7270/Q2611112 |
More data for this
Ligand-Target Pair | |
Serine protease 1/Trypsin-2
(Homo sapiens (Human)) | BDBM50068488

((S)-3-(7-Carbamimidoyl-naphthalen-2-yl)-2-{4-[(S)-...)Show SMILES CC(=N)N1CC[C@@H](C1)Oc1ccc(cc1)[C@H](Cc1ccc2ccc(cc2c1)C(N)=N)C(O)=O Show InChI InChI=1S/C26H28N4O3/c1-16(27)30-11-10-23(15-30)33-22-8-6-19(7-9-22)24(26(31)32)13-17-2-3-18-4-5-20(25(28)29)14-21(18)12-17/h2-9,12,14,23-24,27H,10-11,13,15H2,1H3,(H3,28,29)(H,31,32)/t23-,24-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Mannheim GmbH
Curated by ChEMBL
| Assay Description
The inhibition constant against human trypsin |
J Med Chem 41: 4983-94 (1999)
Article DOI: 10.1021/jm9800402
BindingDB Entry DOI: 10.7270/Q2611112 |
More data for this
Ligand-Target Pair | |
Serine protease 1/Trypsin-2
(Homo sapiens (Human)) | BDBM50068506

((2-Carbamimidoyl-1,2,3,4-tetrahydro-isoquinolin-7-...)Show SMILES CC(=N)N1CCC(CC1)Oc1ccc(cc1)C(Oc1ccc2CCN(Cc2c1)C(N)=N)C(O)=O Show InChI InChI=1S/C25H31N5O4/c1-16(26)29-12-9-21(10-13-29)33-20-5-3-18(4-6-20)23(24(31)32)34-22-7-2-17-8-11-30(25(27)28)15-19(17)14-22/h2-7,14,21,23,26H,8-13,15H2,1H3,(H3,27,28)(H,31,32) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Mannheim GmbH
Curated by ChEMBL
| Assay Description
The inhibition constant against human trypsin |
J Med Chem 41: 4983-94 (1999)
Article DOI: 10.1021/jm9800402
BindingDB Entry DOI: 10.7270/Q2611112 |
More data for this
Ligand-Target Pair | |
Serine protease 1/Trypsin-2
(Homo sapiens (Human)) | BDBM50068502

((2-Carbamimidoyl-1,2,3,4-tetrahydro-isoquinolin-7-...)Show SMILES CCOC(=O)C(Oc1ccc2CCN(Cc2c1)C(N)=N)c1ccc(O[C@H]2CCN(C2)C(C)=N)cc1 Show InChI InChI=1S/C26H33N5O4/c1-3-33-25(32)24(35-22-9-4-18-10-12-31(26(28)29)15-20(18)14-22)19-5-7-21(8-6-19)34-23-11-13-30(16-23)17(2)27/h4-9,14,23-24,27H,3,10-13,15-16H2,1-2H3,(H3,28,29)/t23-,24?/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Mannheim GmbH
Curated by ChEMBL
| Assay Description
The inhibition constant against human trypsin |
J Med Chem 41: 4983-94 (1999)
Article DOI: 10.1021/jm9800402
BindingDB Entry DOI: 10.7270/Q2611112 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50289373
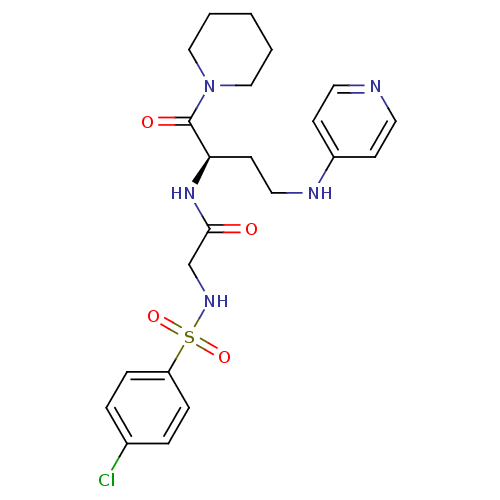
(2-(4-Chloro-benzenesulfonylamino)-N-[(R)-1-(piperi...)Show SMILES Clc1ccc(cc1)S(=O)(=O)NCC(=O)N[C@H](CCNc1ccncc1)C(=O)N1CCCCC1 Show InChI InChI=1S/C22H28ClN5O4S/c23-17-4-6-19(7-5-17)33(31,32)26-16-21(29)27-20(22(30)28-14-2-1-3-15-28)10-13-25-18-8-11-24-12-9-18/h4-9,11-12,20,26H,1-3,10,13-16H2,(H,24,25)(H,27,29)/t20-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | 7.5 | n/a |
TBA
Curated by ChEMBL
| Assay Description
Tested for the Inhibition of the catalytic activity of human Thrombin at 25 degree C and pH 7.5. |
Bioorg Med Chem Lett 7: 1283-1288 (1997)
Article DOI: 10.1016/S0960-894X(97)00210-2
BindingDB Entry DOI: 10.7270/Q29W0FZ9 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50289382
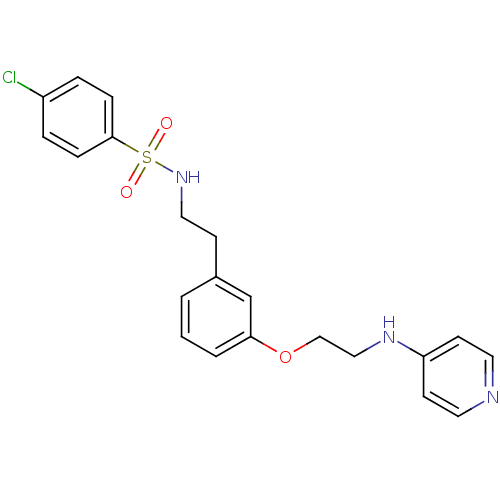
(4-Chloro-N-(2-{3-[2-(pyridin-4-ylamino)-ethoxy]-ph...)Show SMILES Clc1ccc(cc1)S(=O)(=O)NCCc1cccc(OCCNc2ccncc2)c1 Show InChI InChI=1S/C21H22ClN3O3S/c22-18-4-6-21(7-5-18)29(26,27)25-13-8-17-2-1-3-20(16-17)28-15-14-24-19-9-11-23-12-10-19/h1-7,9-12,16,25H,8,13-15H2,(H,23,24) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
| 2.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | 7.5 | n/a |
TBA
Curated by ChEMBL
| Assay Description
Tested for the Inhibition of the catalytic activity of human Thrombin at 25 degree C and pH 7.5. |
Bioorg Med Chem Lett 7: 1283-1288 (1997)
Article DOI: 10.1016/S0960-894X(97)00210-2
BindingDB Entry DOI: 10.7270/Q29W0FZ9 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50068489
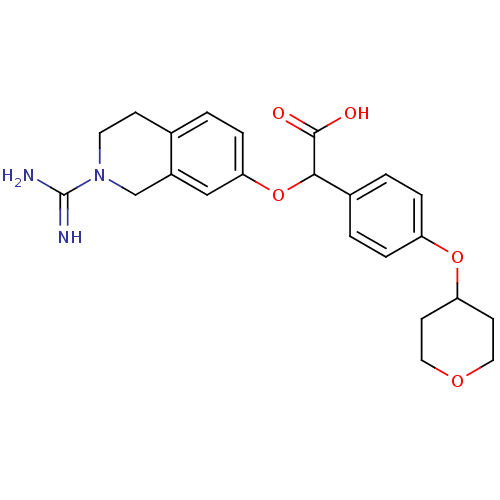
((2-Carbamimidoyl-1,2,3,4-tetrahydro-isoquinolin-7-...)Show SMILES NC(=N)N1CCc2ccc(OC(C(O)=O)c3ccc(OC4CCOCC4)cc3)cc2C1 Show InChI InChI=1S/C23H27N3O5/c24-23(25)26-10-7-15-1-6-20(13-17(15)14-26)31-21(22(27)28)16-2-4-18(5-3-16)30-19-8-11-29-12-9-19/h1-6,13,19,21H,7-12,14H2,(H3,24,25)(H,27,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Mannheim GmbH
Curated by ChEMBL
| Assay Description
The inhibition constant against human Coagulation factor X |
J Med Chem 41: 4983-94 (1999)
Article DOI: 10.1021/jm9800402
BindingDB Entry DOI: 10.7270/Q2611112 |
More data for this
Ligand-Target Pair | |
Serine protease 1/Trypsin-2
(Homo sapiens (Human)) | BDBM50068503

((2-Carbamimidoyl-1,2,3,4-tetrahydro-isoquinolin-7-...)Show SMILES CCOC(=O)C(Oc1ccc2CCN(Cc2c1)C(N)=N)c1ccc(OC2CCNCC2)cc1 Show InChI InChI=1S/C25H32N4O4/c1-2-31-24(30)23(18-4-6-20(7-5-18)32-21-9-12-28-13-10-21)33-22-8-3-17-11-14-29(25(26)27)16-19(17)15-22/h3-8,15,21,23,28H,2,9-14,16H2,1H3,(H3,26,27) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Mannheim GmbH
Curated by ChEMBL
| Assay Description
The inhibition constant against human trypsin |
J Med Chem 41: 4983-94 (1999)
Article DOI: 10.1021/jm9800402
BindingDB Entry DOI: 10.7270/Q2611112 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data