

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM471715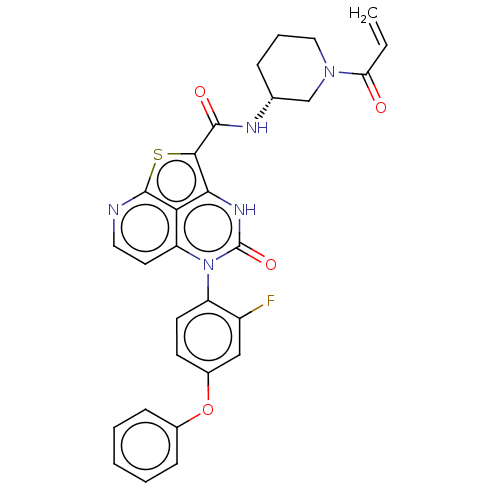 ((R)-N-(1-Acryloylpiperidin-3-yl)-5-(2-fluoro-4-phe...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 9.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Covalent inhibition of recombinant human GST-tagged BTK (2 to 659 end residues) expressed in baculovirus expression system assessed as inhibition con... | Citation and Details Article DOI: 10.1021/acsmedchemlett.1c00044 BindingDB Entry DOI: 10.7270/Q25D8WM5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM467364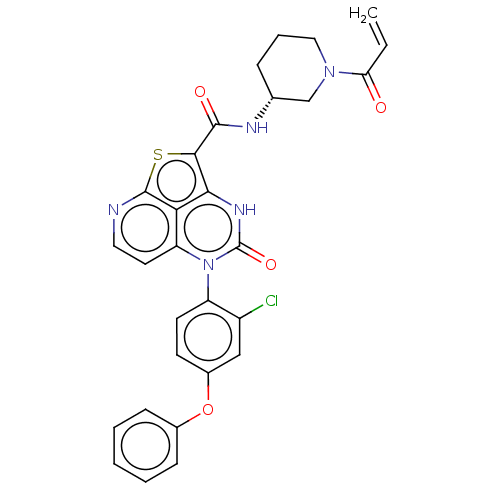 ((R)-N-(1-Acryloylpiperidin-3-yl)-5-(2-chloro-4-phe...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Covalent inhibition of recombinant human GST-tagged BTK (2 to 659 end residues) expressed in baculovirus expression system assessed as inhibition con... | Citation and Details Article DOI: 10.1021/acsmedchemlett.1c00044 BindingDB Entry DOI: 10.7270/Q25D8WM5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM50569791 (CHEMBL4846828) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Covalent inhibition of recombinant human GST-tagged BTK (2 to 659 end residues) expressed in baculovirus expression system assessed as inhibition con... | Citation and Details Article DOI: 10.1021/acsmedchemlett.1c00044 BindingDB Entry DOI: 10.7270/Q25D8WM5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM485273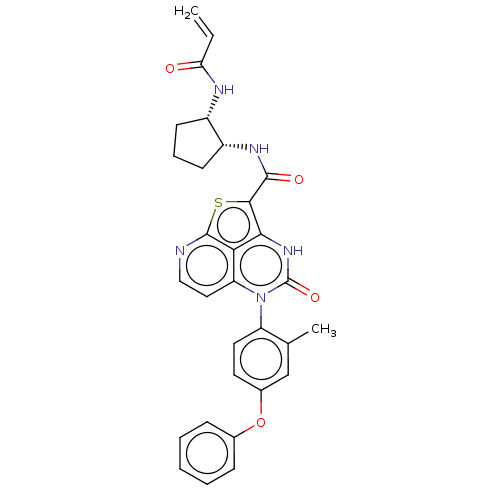 (N-((1R,2S)-2-Acrylamidocyclopentyl)-5-(*S)-(2-meth...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Covalent inhibition of recombinant human GST-tagged BTK (2 to 659 end residues) expressed in baculovirus expression system assessed as inhibition con... | Citation and Details Article DOI: 10.1021/acsmedchemlett.1c00044 BindingDB Entry DOI: 10.7270/Q25D8WM5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM467718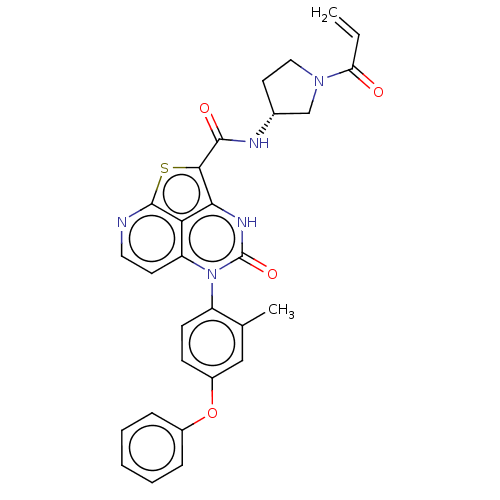 ((R)-N-(1-Acryloylpyrrolidin-3-yl)-5-(2-methyl-4-(2...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 29 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Covalent inhibition of recombinant human GST-tagged BTK (2 to 659 end residues) expressed in baculovirus expression system assessed as inhibition con... | Citation and Details Article DOI: 10.1021/acsmedchemlett.1c00044 BindingDB Entry DOI: 10.7270/Q25D8WM5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM50569790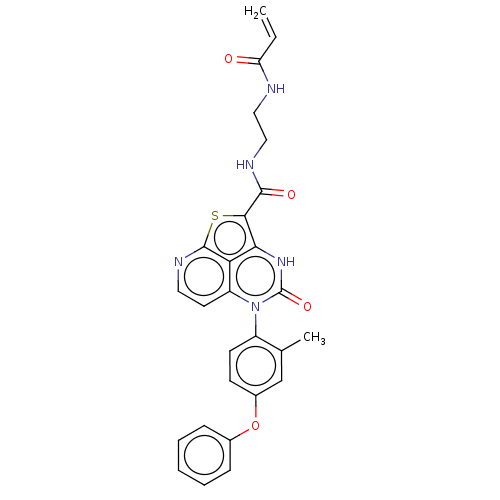 (CHEMBL4852459) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 52 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Covalent inhibition of recombinant human GST-tagged BTK (2 to 659 end residues) expressed in baculovirus expression system assessed as inhibition con... | Citation and Details Article DOI: 10.1021/acsmedchemlett.1c00044 BindingDB Entry DOI: 10.7270/Q25D8WM5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM467457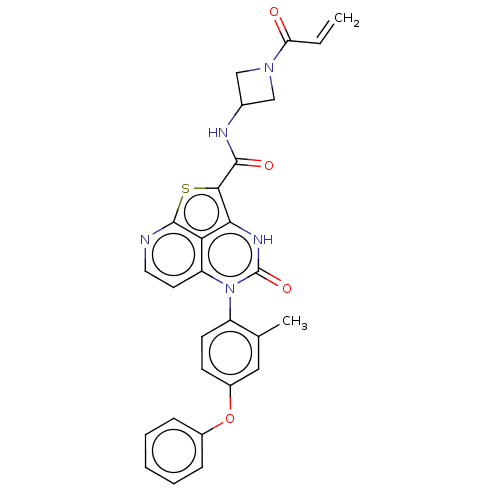 (N-(1-Acryloylazetidin-3-yl)-5-(2-methyl-4-phenoxyp...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 141 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Covalent inhibition of recombinant human GST-tagged BTK (2 to 659 end residues) expressed in baculovirus expression system assessed as inhibition con... | Citation and Details Article DOI: 10.1021/acsmedchemlett.1c00044 BindingDB Entry DOI: 10.7270/Q25D8WM5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM467435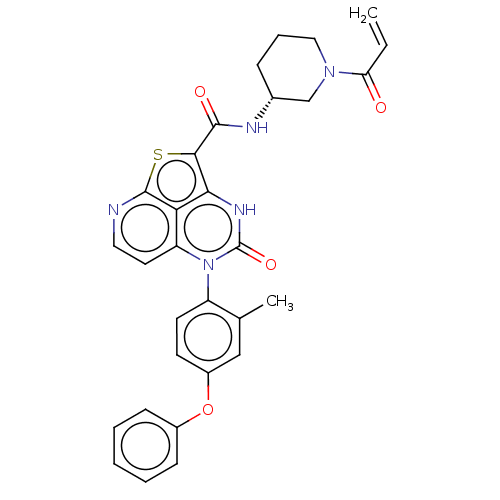 ((R)-N-(1-Acryloylpiperidin-3-yl)-5-(*R)-(2-methyl-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Covalent inhibition of recombinant human GST-tagged BTK (2 to 659 end residues) expressed in baculovirus expression system assessed as inhibition con... | Citation and Details Article DOI: 10.1021/acsmedchemlett.1c00044 BindingDB Entry DOI: 10.7270/Q25D8WM5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM467435 ((R)-N-(1-Acryloylpiperidin-3-yl)-5-(*R)-(2-methyl-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Covalent inhibition of recombinant human GST-tagged BTK (2 to 659 end residues) expressed in baculovirus expression system assessed as inhibition con... | Citation and Details Article DOI: 10.1021/acsmedchemlett.1c00044 BindingDB Entry DOI: 10.7270/Q25D8WM5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM467857 ((S)-N-(1-Acryloylpyrrolidin-3-yl)-5-(*S)-(2-methyl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 217 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Covalent inhibition of recombinant human GST-tagged BTK (2 to 659 end residues) expressed in baculovirus expression system assessed as inhibition con... | Citation and Details Article DOI: 10.1021/acsmedchemlett.1c00044 BindingDB Entry DOI: 10.7270/Q25D8WM5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM467759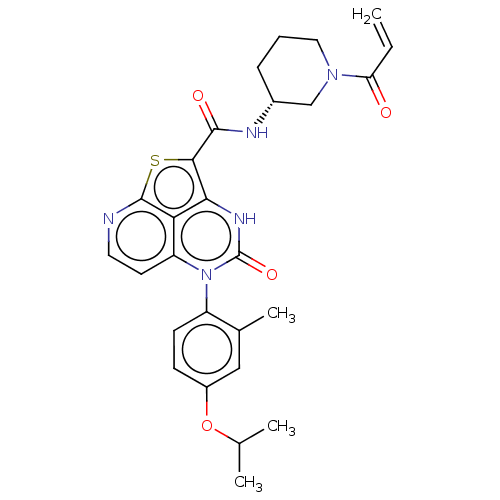 ((R)-N-(1-Acryloylpiperidin-3-yl)-5-(*S)-(4-isoprop...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 337 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Covalent inhibition of recombinant human GST-tagged BTK (2 to 659 end residues) expressed in baculovirus expression system assessed as inhibition con... | Citation and Details Article DOI: 10.1021/acsmedchemlett.1c00044 BindingDB Entry DOI: 10.7270/Q25D8WM5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM467564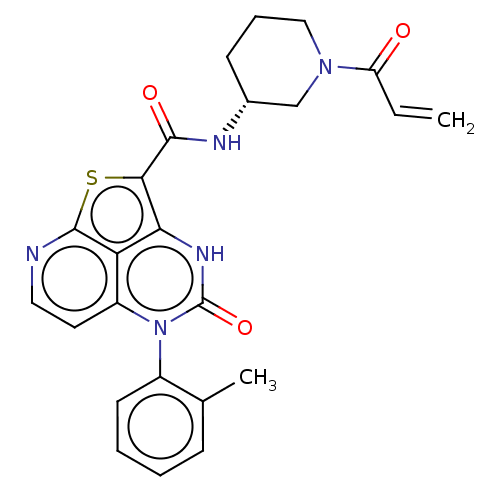 ((R)-N-(1-Acryloylpiperidin-3-yl)-4-oxo-5-(o-tolyl)...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.58E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Covalent inhibition of recombinant human GST-tagged BTK (2 to 659 end residues) expressed in baculovirus expression system assessed as inhibition con... | Citation and Details Article DOI: 10.1021/acsmedchemlett.1c00044 BindingDB Entry DOI: 10.7270/Q25D8WM5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM467625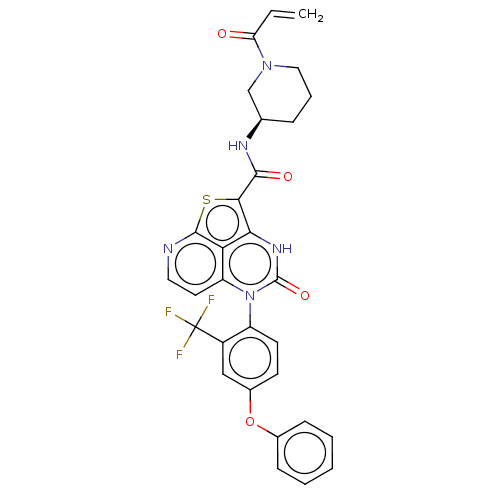 ((R)-N-(1-Acryloylpiperidin-3-yl)-4-oxo-5-(4-phenox...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.85E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Covalent inhibition of recombinant human GST-tagged BTK (2 to 659 end residues) expressed in baculovirus expression system assessed as inhibition con... | Citation and Details Article DOI: 10.1021/acsmedchemlett.1c00044 BindingDB Entry DOI: 10.7270/Q25D8WM5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM467979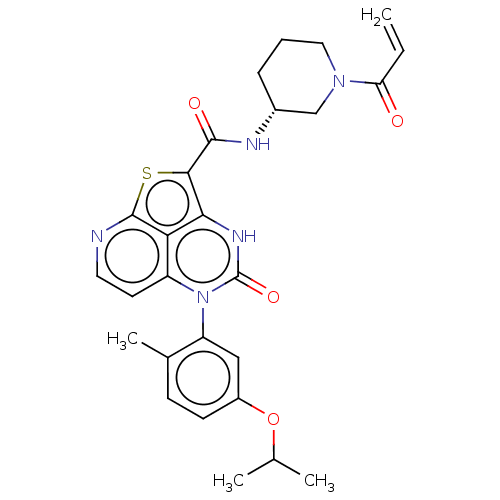 ((R)-N-(1-Acryloylpiperidin-3-yl)-5-(5-isopropoxy-2...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3.29E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Covalent inhibition of recombinant human GST-tagged BTK (2 to 659 end residues) expressed in baculovirus expression system assessed as inhibition con... | Citation and Details Article DOI: 10.1021/acsmedchemlett.1c00044 BindingDB Entry DOI: 10.7270/Q25D8WM5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM467742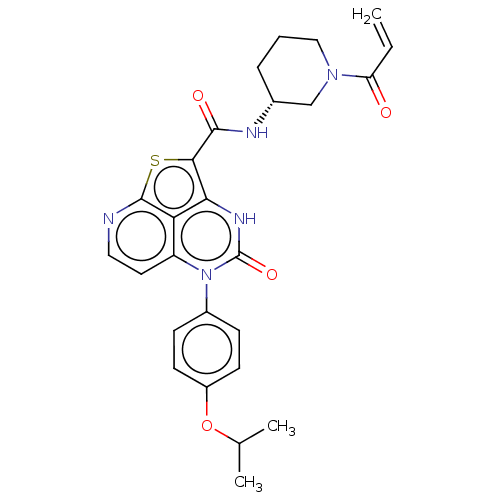 ((R)-N-(1-Acryloylpiperidin-3-yl)-5-(4-isopropoxyph...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 4.22E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Covalent inhibition of recombinant human GST-tagged BTK (2 to 659 end residues) expressed in baculovirus expression system assessed as inhibition con... | Citation and Details Article DOI: 10.1021/acsmedchemlett.1c00044 BindingDB Entry DOI: 10.7270/Q25D8WM5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM467604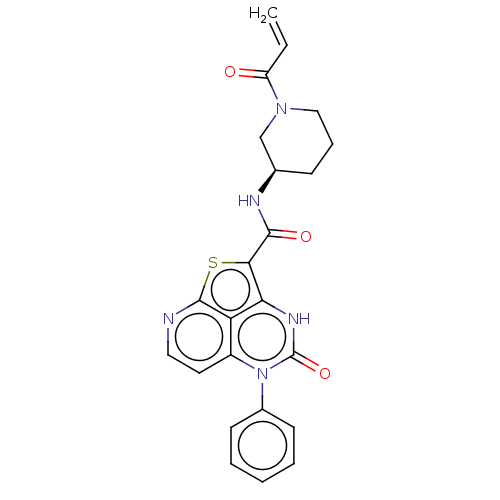 ((R)-N-(1-Acryloylpiperidin-3-yl)-4-oxo-5-phenyl-4,...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 6.42E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Covalent inhibition of recombinant human GST-tagged BTK (2 to 659 end residues) expressed in baculovirus expression system assessed as inhibition con... | Citation and Details Article DOI: 10.1021/acsmedchemlett.1c00044 BindingDB Entry DOI: 10.7270/Q25D8WM5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM50543677 (CHEMBL4635823) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Phenex Pharmaceuticals AG Curated by ChEMBL | Assay Description Binding affinity to recombinant human RORgammat transfected in human HEK293T cells incubated for 24 hrs by dual-glo luciferase reporter gene assay | Bioorg Med Chem Lett 30: (2020) Article DOI: 10.1016/j.bmcl.2020.127174 BindingDB Entry DOI: 10.7270/Q2862M1T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK1 (Homo sapiens (Human)) | BDBM387821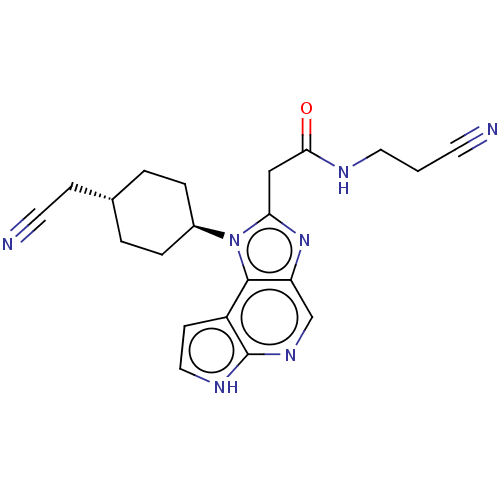 (N-(2-Cyanoethyl)-2-(1-((1r,4r)-4-(cyanomethyl)cycl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research and Development Curated by ChEMBL | Assay Description Inhibition of JAK1-JH1/JH2 domain (574 to 1154 residues) (unknown origin) pre-incubated before NH2-KGGEEEEYFELVKK-CO2 substrate addition and measured... | J Med Chem 63: 2915-2929 (2020) Article DOI: 10.1021/acs.jmedchem.9b01439 BindingDB Entry DOI: 10.7270/Q2H135FN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK1 (Homo sapiens (Human)) | BDBM50527405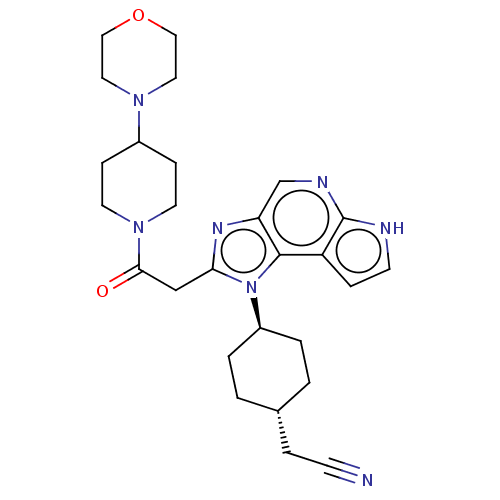 (CHEMBL4456630 | US10981911, Example 26) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research and Development Curated by ChEMBL | Assay Description Inhibition of JAK1-JH1/JH2 domain (574 to 1154 residues) (unknown origin) pre-incubated before NH2-KGGEEEEYFELVKK-CO2 substrate addition and measured... | J Med Chem 63: 2915-2929 (2020) Article DOI: 10.1021/acs.jmedchem.9b01439 BindingDB Entry DOI: 10.7270/Q2H135FN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK2 (Homo sapiens (Human)) | BDBM387815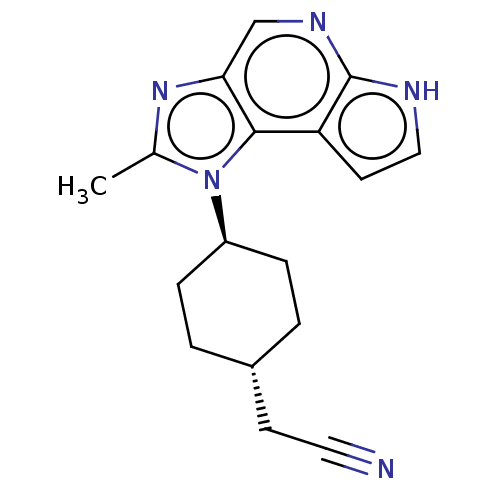 (US10294226, Compound A | US10487083, Example A | U...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | <0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research and Development Curated by ChEMBL | Assay Description Inhibition of JAK2-JH1/JH2 domain (532 to 1132 residues) (unknown origin) pre-incubated before NH2-KGGEEEEYFELVKK-CO2 substrate addition and measured... | J Med Chem 63: 2915-2929 (2020) Article DOI: 10.1021/acs.jmedchem.9b01439 BindingDB Entry DOI: 10.7270/Q2H135FN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK1 (Homo sapiens (Human)) | BDBM387815 (US10294226, Compound A | US10487083, Example A | U...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | <0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research and Development Curated by ChEMBL | Assay Description Inhibition of JAK1-JH1/JH2 domain (574 to 1154 residues) (unknown origin) pre-incubated before NH2-KGGEEEEYFELVKK-CO2 substrate addition and measured... | J Med Chem 63: 2915-2929 (2020) Article DOI: 10.1021/acs.jmedchem.9b01439 BindingDB Entry DOI: 10.7270/Q2H135FN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK1 (Homo sapiens (Human)) | BDBM387824 (N-(2-Cyano-2-methylpropyl)-2-(1-((1r,4r)-4-(cyanom...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.220 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research and Development Curated by ChEMBL | Assay Description Inhibition of JAK1-JH1/JH2 domain (574 to 1154 residues) (unknown origin) pre-incubated before NH2-KGGEEEEYFELVKK-CO2 substrate addition and measured... | J Med Chem 63: 2915-2929 (2020) Article DOI: 10.1021/acs.jmedchem.9b01439 BindingDB Entry DOI: 10.7270/Q2H135FN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK1 (Homo sapiens (Human)) | BDBM50527410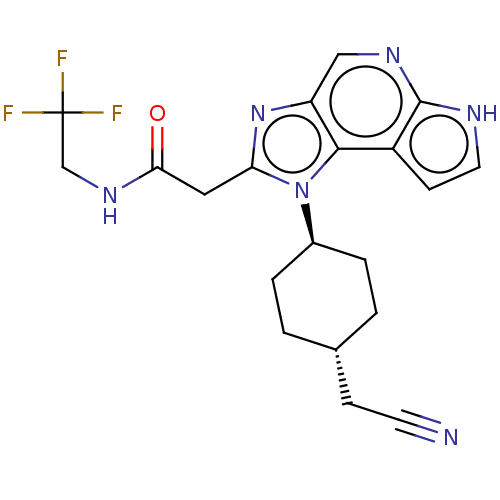 (CHEMBL4439418 | US10981911, Example 57) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research and Development Curated by ChEMBL | Assay Description Inhibition of JAK1-JH1/JH2 domain (574 to 1154 residues) (unknown origin) pre-incubated before NH2-KGGEEEEYFELVKK-CO2 substrate addition and measured... | J Med Chem 63: 2915-2929 (2020) Article DOI: 10.1021/acs.jmedchem.9b01439 BindingDB Entry DOI: 10.7270/Q2H135FN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK1 (Homo sapiens (Human)) | BDBM50527404 (CHEMBL4514898 | US10981911, Example 62) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research and Development Curated by ChEMBL | Assay Description Inhibition of JAK1-JH1/JH2 domain (574 to 1154 residues) (unknown origin) pre-incubated before NH2-KGGEEEEYFELVKK-CO2 substrate addition and measured... | J Med Chem 63: 2915-2929 (2020) Article DOI: 10.1021/acs.jmedchem.9b01439 BindingDB Entry DOI: 10.7270/Q2H135FN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK1 (Homo sapiens (Human)) | BDBM387818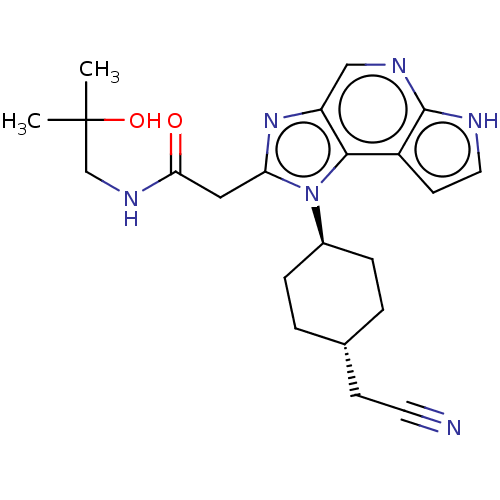 (2-(1-((1r,4r)-4-(Cyanomethyl)cyclohexyl)-1,6-dihyd...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.370 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research and Development Curated by ChEMBL | Assay Description Inhibition of JAK1-JH1/JH2 domain (574 to 1154 residues) (unknown origin) pre-incubated before NH2-KGGEEEEYFELVKK-CO2 substrate addition and measured... | J Med Chem 63: 2915-2929 (2020) Article DOI: 10.1021/acs.jmedchem.9b01439 BindingDB Entry DOI: 10.7270/Q2H135FN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK1 (Homo sapiens (Human)) | BDBM50527406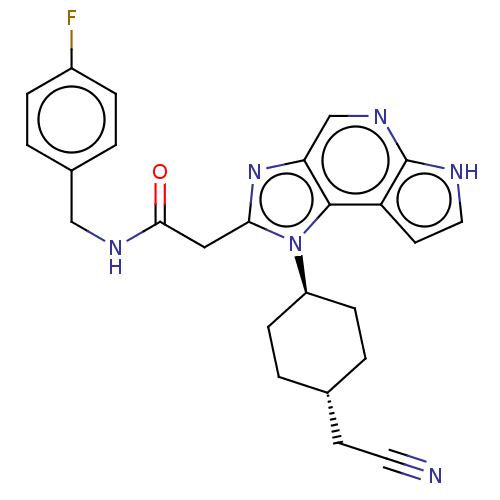 (CHEMBL4588333 | US10981911, Example 45) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.490 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research and Development Curated by ChEMBL | Assay Description Inhibition of JAK1-JH1/JH2 domain (574 to 1154 residues) (unknown origin) pre-incubated before NH2-KGGEEEEYFELVKK-CO2 substrate addition and measured... | J Med Chem 63: 2915-2929 (2020) Article DOI: 10.1021/acs.jmedchem.9b01439 BindingDB Entry DOI: 10.7270/Q2H135FN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK1 (Homo sapiens (Human)) | BDBM50527409 (CHEMBL4447497 | US10981911, Example 58) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.540 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research and Development Curated by ChEMBL | Assay Description Inhibition of JAK1-JH1/JH2 domain (574 to 1154 residues) (unknown origin) pre-incubated before NH2-KGGEEEEYFELVKK-CO2 substrate addition and measured... | J Med Chem 63: 2915-2929 (2020) Article DOI: 10.1021/acs.jmedchem.9b01439 BindingDB Entry DOI: 10.7270/Q2H135FN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK1 (Homo sapiens (Human)) | BDBM50527408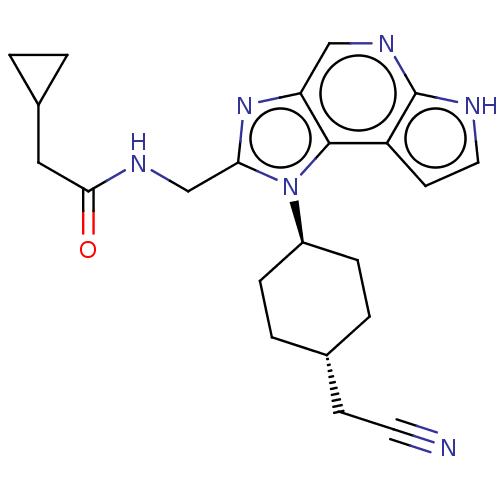 (CHEMBL4437638 | US10981911, Example 94) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.920 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research and Development Curated by ChEMBL | Assay Description Inhibition of JAK1-JH1/JH2 domain (574 to 1154 residues) (unknown origin) pre-incubated before NH2-KGGEEEEYFELVKK-CO2 substrate addition and measured... | J Med Chem 63: 2915-2929 (2020) Article DOI: 10.1021/acs.jmedchem.9b01439 BindingDB Entry DOI: 10.7270/Q2H135FN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK1 (Homo sapiens (Human)) | BDBM50527407 (CHEMBL4582390 | US10981911, Example 33) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research and Development Curated by ChEMBL | Assay Description Inhibition of JAK1-JH1/JH2 domain (574 to 1154 residues) (unknown origin) pre-incubated before NH2-KGGEEEEYFELVKK-CO2 substrate addition and measured... | J Med Chem 63: 2915-2929 (2020) Article DOI: 10.1021/acs.jmedchem.9b01439 BindingDB Entry DOI: 10.7270/Q2H135FN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK1 (Homo sapiens (Human)) | BDBM50193995 (3-((3R,4R)-4-methyl-3-(methyl(7H-pyrrolo[2,3-d]pyr...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research and Development Curated by ChEMBL | Assay Description Inhibition of JAK1-JH1/JH2 domain (574 to 1154 residues) (unknown origin) pre-incubated before NH2-KGGEEEEYFELVKK-CO2 substrate addition and measured... | J Med Chem 63: 2915-2929 (2020) Article DOI: 10.1021/acs.jmedchem.9b01439 BindingDB Entry DOI: 10.7270/Q2H135FN | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM50543664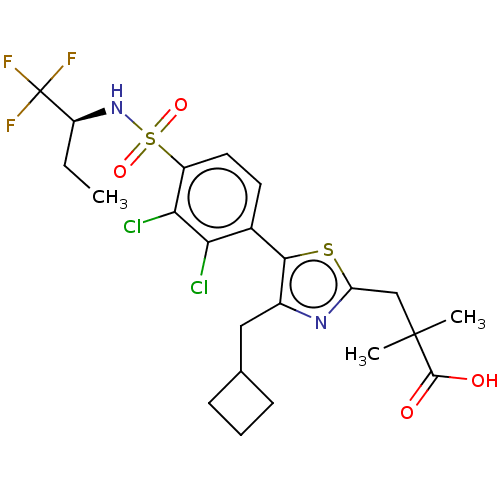 (CHEMBL4647899) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Phenex Pharmaceuticals AG Curated by ChEMBL | Assay Description Binding affinity to recombinant human RORgammat transfected in human HEK293T cells incubated for 24 hrs by dual-glo luciferase reporter gene assay | Bioorg Med Chem Lett 30: (2020) Article DOI: 10.1016/j.bmcl.2020.127174 BindingDB Entry DOI: 10.7270/Q2862M1T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM392076 (US10301272, Example 7/9) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Phenex Pharmaceuticals AG Curated by ChEMBL | Assay Description Binding affinity to recombinant human RORgammat transfected in human HEK293T cells incubated for 24 hrs by dual-glo luciferase reporter gene assay | Bioorg Med Chem Lett 30: (2020) Article DOI: 10.1016/j.bmcl.2020.127174 BindingDB Entry DOI: 10.7270/Q2862M1T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK2 (Homo sapiens (Human)) | BDBM50527404 (CHEMBL4514898 | US10981911, Example 62) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research and Development Curated by ChEMBL | Assay Description Inhibition of JAK2-JH1/JH2 domain (532 to 1132 residues) (unknown origin) pre-incubated before NH2-KGGEEEEYFELVKK-CO2 substrate addition and measured... | J Med Chem 63: 2915-2929 (2020) Article DOI: 10.1021/acs.jmedchem.9b01439 BindingDB Entry DOI: 10.7270/Q2H135FN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM339271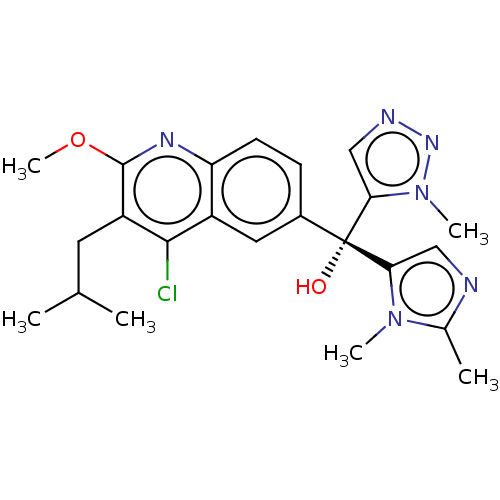 (US10201546, Example 134b) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Pharmaceutica NV US Patent | Assay Description For the RORγt construct used in the ThermoFluorŪ assay, numbering for the nucleotide sequences was based on the reference sequence for human ROR... | US Patent US10201546 (2019) BindingDB Entry DOI: 10.7270/Q2F47R8C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-V/beta-5 (Homo sapiens (Human)) | BDBM50161784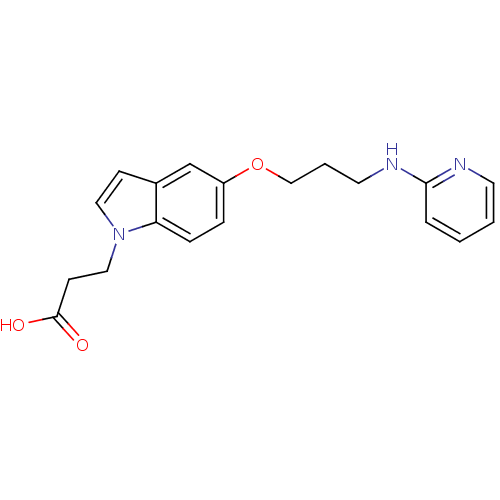 (3-{5-[3-(Pyridin-2-ylamino)-propoxy]-indol-1-yl}-p...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research & Development Curated by ChEMBL | Assay Description In vitro inhibition of human alphaV-beta5 integrin binding in ELISA | J Med Chem 48: 926-34 (2005) Article DOI: 10.1021/jm049725u BindingDB Entry DOI: 10.7270/Q2W37VTG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM392006 (US10301272, Example 6/4) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Phenex Pharmaceuticals AG Curated by ChEMBL | Assay Description Binding affinity to recombinant human RORgammat transfected in human HEK293T cells incubated for 24 hrs by dual-glo luciferase reporter gene assay | Bioorg Med Chem Lett 30: (2020) Article DOI: 10.1016/j.bmcl.2020.127174 BindingDB Entry DOI: 10.7270/Q2862M1T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| JAK3/JAK1 (Homo sapiens (Human)) | BDBM50527406 (CHEMBL4588333 | US10981911, Example 45) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research and Development Curated by ChEMBL | Assay Description Inhibition of JAK1/JAK3 in human PBMC assessed as reduction in IL2-induced STAT5 phosphorylation pre-incubated for 30 mins before IL2 stimulation for... | J Med Chem 63: 2915-2929 (2020) Article DOI: 10.1021/acs.jmedchem.9b01439 BindingDB Entry DOI: 10.7270/Q2H135FN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK2 (Homo sapiens (Human)) | BDBM50527409 (CHEMBL4447497 | US10981911, Example 58) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research and Development Curated by ChEMBL | Assay Description Inhibition of JAK2-JH1/JH2 domain (532 to 1132 residues) (unknown origin) pre-incubated before NH2-KGGEEEEYFELVKK-CO2 substrate addition and measured... | J Med Chem 63: 2915-2929 (2020) Article DOI: 10.1021/acs.jmedchem.9b01439 BindingDB Entry DOI: 10.7270/Q2H135FN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM339272 (US10201546, Example 134c) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Pharmaceutica NV US Patent | Assay Description For the RORγt construct used in the ThermoFluorŪ assay, numbering for the nucleotide sequences was based on the reference sequence for human ROR... | US Patent US10201546 (2019) BindingDB Entry DOI: 10.7270/Q2F47R8C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM285771 (US10080744, Example 3/38) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Phenex Pharmaceuticals AG Curated by ChEMBL | Assay Description Binding affinity to recombinant human RORgammat transfected in human HEK293T cells incubated for 24 hrs by dual-glo luciferase reporter gene assay | Bioorg Med Chem Lett 30: (2020) Article DOI: 10.1016/j.bmcl.2020.127174 BindingDB Entry DOI: 10.7270/Q2862M1T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM285759 (US10080744, Example 3/26) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Phenex Pharmaceuticals AG Curated by ChEMBL | Assay Description Binding affinity to recombinant human RORgammat transfected in human HEK293T cells incubated for 24 hrs by dual-glo luciferase reporter gene assay | Bioorg Med Chem Lett 30: (2020) Article DOI: 10.1016/j.bmcl.2020.127174 BindingDB Entry DOI: 10.7270/Q2862M1T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM50543668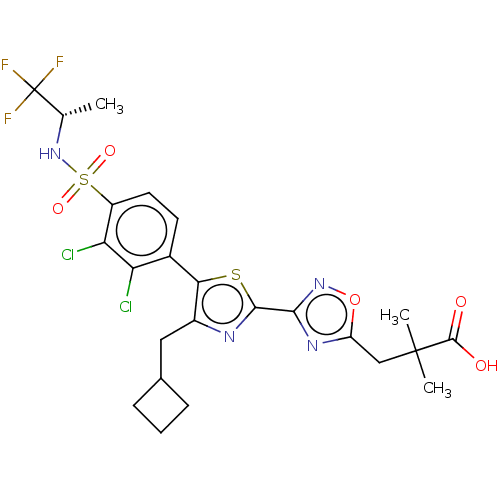 (CHEMBL4632527) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Phenex Pharmaceuticals AG Curated by ChEMBL | Assay Description Binding affinity to recombinant human RORgammat transfected in human HEK293T cells incubated for 24 hrs by dual-glo luciferase reporter gene assay | Bioorg Med Chem Lett 30: (2020) Article DOI: 10.1016/j.bmcl.2020.127174 BindingDB Entry DOI: 10.7270/Q2862M1T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM50543663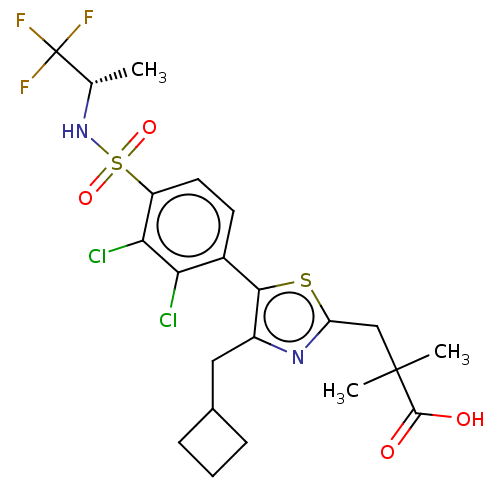 (CHEMBL4647734) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Phenex Pharmaceuticals AG Curated by ChEMBL | Assay Description Binding affinity to recombinant human RORgammat transfected in human HEK293T cells incubated for 24 hrs by dual-glo luciferase reporter gene assay | Bioorg Med Chem Lett 30: (2020) Article DOI: 10.1016/j.bmcl.2020.127174 BindingDB Entry DOI: 10.7270/Q2862M1T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM339171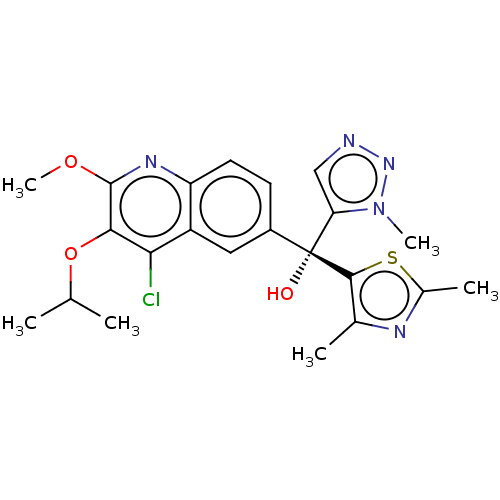 (US10201546, Example 85c) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Pharmaceutica NV US Patent | Assay Description For the RORγt construct used in the ThermoFluorŪ assay, numbering for the nucleotide sequences was based on the reference sequence for human ROR... | US Patent US10201546 (2019) BindingDB Entry DOI: 10.7270/Q2F47R8C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK2 (Homo sapiens (Human)) | BDBM50193995 (3-((3R,4R)-4-methyl-3-(methyl(7H-pyrrolo[2,3-d]pyr...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research and Development Curated by ChEMBL | Assay Description Inhibition of JAK2-JH1/JH2 domain (532 to 1132 residues) (unknown origin) pre-incubated before NH2-KGGEEEEYFELVKK-CO2 substrate addition and measured... | J Med Chem 63: 2915-2929 (2020) Article DOI: 10.1021/acs.jmedchem.9b01439 BindingDB Entry DOI: 10.7270/Q2H135FN | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Tyrosine-protein kinase JAK2 (Homo sapiens (Human)) | BDBM387824 (N-(2-Cyano-2-methylpropyl)-2-(1-((1r,4r)-4-(cyanom...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research and Development Curated by ChEMBL | Assay Description Inhibition of JAK2-JH1/JH2 domain (532 to 1132 residues) (unknown origin) pre-incubated before NH2-KGGEEEEYFELVKK-CO2 substrate addition and measured... | J Med Chem 63: 2915-2929 (2020) Article DOI: 10.1021/acs.jmedchem.9b01439 BindingDB Entry DOI: 10.7270/Q2H135FN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK2 (Homo sapiens (Human)) | BDBM50527410 (CHEMBL4439418 | US10981911, Example 57) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research and Development Curated by ChEMBL | Assay Description Inhibition of JAK2-JH1/JH2 domain (532 to 1132 residues) (unknown origin) pre-incubated before NH2-KGGEEEEYFELVKK-CO2 substrate addition and measured... | J Med Chem 63: 2915-2929 (2020) Article DOI: 10.1021/acs.jmedchem.9b01439 BindingDB Entry DOI: 10.7270/Q2H135FN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM285735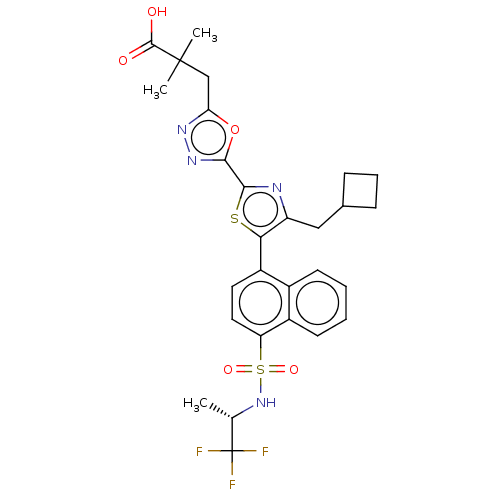 (US10080744, Example 3/3) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Phenex Pharmaceuticals AG Curated by ChEMBL | Assay Description Binding affinity to recombinant human RORgammat transfected in human HEK293T cells incubated for 24 hrs by dual-glo luciferase reporter gene assay | Bioorg Med Chem Lett 30: (2020) Article DOI: 10.1016/j.bmcl.2020.127174 BindingDB Entry DOI: 10.7270/Q2862M1T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK2 (Homo sapiens (Human)) | BDBM50527405 (CHEMBL4456630 | US10981911, Example 26) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research and Development Curated by ChEMBL | Assay Description Inhibition of JAK2-JH1/JH2 domain (532 to 1132 residues) (unknown origin) pre-incubated before NH2-KGGEEEEYFELVKK-CO2 substrate addition and measured... | J Med Chem 63: 2915-2929 (2020) Article DOI: 10.1021/acs.jmedchem.9b01439 BindingDB Entry DOI: 10.7270/Q2H135FN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM339269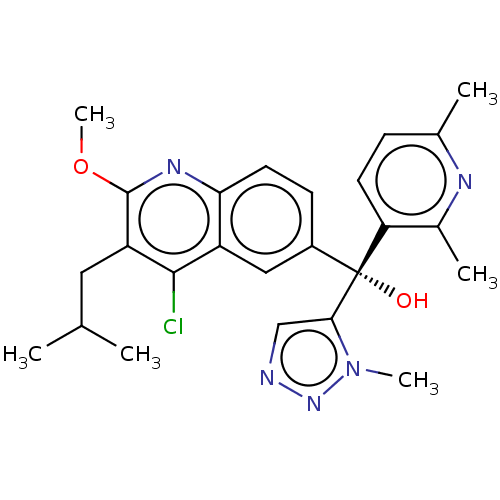 (US10201546, Example 133c) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 6.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Pharmaceutica NV US Patent | Assay Description For the RORγt construct used in the ThermoFluorŪ assay, numbering for the nucleotide sequences was based on the reference sequence for human ROR... | US Patent US10201546 (2019) BindingDB Entry DOI: 10.7270/Q2F47R8C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 1393 total ) | Next | Last >> |