 Found 91 hits with Last Name = 'letts' and Initial = 'lg'
Found 91 hits with Last Name = 'letts' and Initial = 'lg' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50136106

(4-(6-Benzyl-benzo[1,3]dioxol-5-yl)-benzenesulfonam...)Show SMILES NS(=O)(=O)c1ccc(cc1)-c1cc2OCOc2cc1Cc1ccccc1 Show InChI InChI=1S/C20H17NO4S/c21-26(22,23)17-8-6-15(7-9-17)18-12-20-19(24-13-25-20)11-16(18)10-14-4-2-1-3-5-14/h1-9,11-12H,10,13H2,(H2,21,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
NitroMed, Inc
Curated by ChEMBL
| Assay Description
Compound was tested for the inhibition of human Prostaglandin G/H synthase 2 (COX-2) in human whole blood |
J Med Chem 46: 5484-504 (2003)
Article DOI: 10.1021/jm030268b
BindingDB Entry DOI: 10.7270/Q2FX78WK |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM11639

(4-[5-(4-methylphenyl)-3-(trifluoromethyl)-1H-pyraz...)Show SMILES Cc1ccc(cc1)-c1cc(nn1-c1ccc(cc1)S(N)(=O)=O)C(F)(F)F Show InChI InChI=1S/C17H14F3N3O2S/c1-11-2-4-12(5-3-11)15-10-16(17(18,19)20)22-23(15)13-6-8-14(9-7-13)26(21,24)25/h2-10H,1H3,(H2,21,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 54 | n/a | n/a | n/a | n/a | n/a | n/a |
NitroMed, Inc
Curated by ChEMBL
| Assay Description
Compound was tested for the inhibition of recombinant human Prostaglandin G/H synthase 2 (COX-2) |
J Med Chem 46: 5484-504 (2003)
Article DOI: 10.1021/jm030268b
BindingDB Entry DOI: 10.7270/Q2FX78WK |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50136109
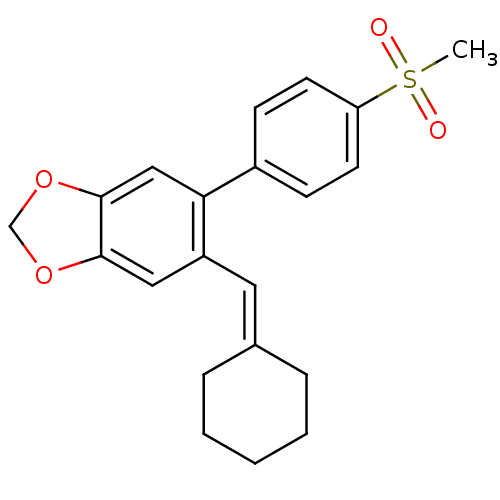
(5-Cyclohexylidenemethyl-6-(4-methanesulfonyl-pheny...)Show SMILES [#6]S(=O)(=O)c1ccc(cc1)-c1cc2-[#8]-[#6]-[#8]-c2cc1\[#6]=[#6]-1\[#6]-[#6]-[#6]-[#6]-[#6]-1 Show InChI InChI=1S/C21H22O4S/c1-26(22,23)18-9-7-16(8-10-18)19-13-21-20(24-14-25-21)12-17(19)11-15-5-3-2-4-6-15/h7-13H,2-6,14H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
NitroMed, Inc
Curated by ChEMBL
| Assay Description
Compound was tested for the inhibition of recombinant human Prostaglandin G/H synthase 2 (COX-2) by enzyme Assay |
J Med Chem 46: 5484-504 (2003)
Article DOI: 10.1021/jm030268b
BindingDB Entry DOI: 10.7270/Q2FX78WK |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50136108
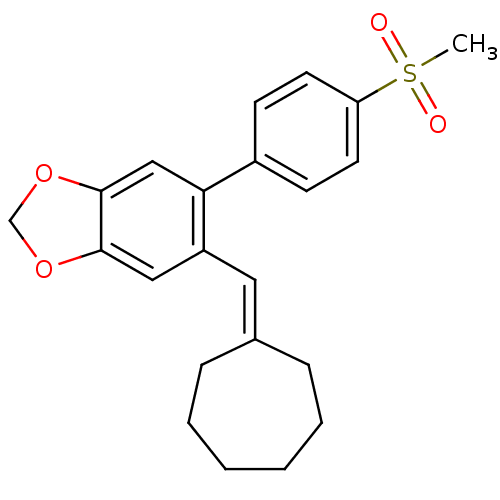
(5-Cycloheptylidenemethyl-6-(4-methanesulfonyl-phen...)Show SMILES [#6]S(=O)(=O)c1ccc(cc1)-c1cc2-[#8]-[#6]-[#8]-c2cc1\[#6]=[#6]-1/[#6]-[#6]-[#6]-[#6]-[#6]-[#6]-1 Show InChI InChI=1S/C22H24O4S/c1-27(23,24)19-10-8-17(9-11-19)20-14-22-21(25-15-26-22)13-18(20)12-16-6-4-2-3-5-7-16/h8-14H,2-7,15H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
NitroMed, Inc
Curated by ChEMBL
| Assay Description
Compound was tested for the inhibition of human Prostaglandin G/H synthase 2 (COX-2) in human whole blood |
J Med Chem 46: 5484-504 (2003)
Article DOI: 10.1021/jm030268b
BindingDB Entry DOI: 10.7270/Q2FX78WK |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50168167
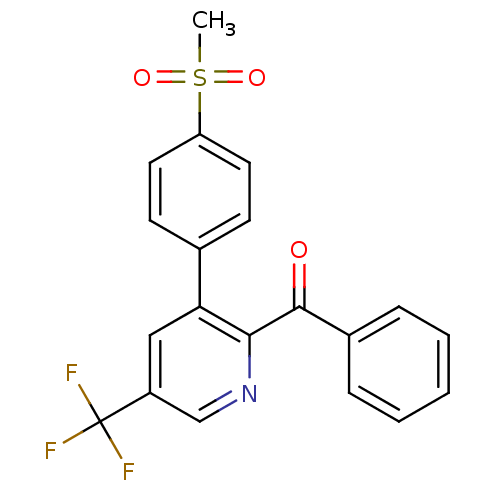
(CHEMBL364966 | [3-(4-Methanesulfonyl-phenyl)-5-tri...)Show SMILES CS(=O)(=O)c1ccc(cc1)-c1cc(cnc1C(=O)c1ccccc1)C(F)(F)F Show InChI InChI=1S/C20H14F3NO3S/c1-28(26,27)16-9-7-13(8-10-16)17-11-15(20(21,22)23)12-24-18(17)19(25)14-5-3-2-4-6-14/h2-12H,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 250 | n/a | n/a | n/a | n/a | n/a | n/a |
NitroMed, Inc.
Curated by ChEMBL
| Assay Description
In vitro inhibitory concentration against Prostaglandin G/H synthase 2 (COX-2) in human whole blood |
J Med Chem 48: 3930-4 (2005)
Article DOI: 10.1021/jm0582064
BindingDB Entry DOI: 10.7270/Q28C9VSZ |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM22369

(4-(4-methanesulfonylphenyl)-3-phenyl-2,5-dihydrofu...)Show SMILES CS(=O)(=O)c1ccc(cc1)C1=C(C(=O)OC1)c1ccccc1 |t:11| Show InChI InChI=1S/C17H14O4S/c1-22(19,20)14-9-7-12(8-10-14)15-11-21-17(18)16(15)13-5-3-2-4-6-13/h2-10H,11H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
NitroMed Inc.
Curated by ChEMBL
| Assay Description
In vitro inhibition of Prostaglandin G/H synthase 2 in human whole blood |
Bioorg Med Chem Lett 14: 6049-52 (2004)
Article DOI: 10.1016/j.bmcl.2004.09.073
BindingDB Entry DOI: 10.7270/Q2Q81CJ0 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM22369

(4-(4-methanesulfonylphenyl)-3-phenyl-2,5-dihydrofu...)Show SMILES CS(=O)(=O)c1ccc(cc1)C1=C(C(=O)OC1)c1ccccc1 |t:11| Show InChI InChI=1S/C17H14O4S/c1-22(19,20)14-9-7-12(8-10-14)15-11-21-17(18)16(15)13-5-3-2-4-6-13/h2-10H,11H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
NitroMed, Inc
Curated by ChEMBL
| Assay Description
Compound was tested for the inhibition of human Prostaglandin G/H synthase 2 (COX-2) in human whole blood |
J Med Chem 46: 5484-504 (2003)
Article DOI: 10.1021/jm030268b
BindingDB Entry DOI: 10.7270/Q2FX78WK |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM22369

(4-(4-methanesulfonylphenyl)-3-phenyl-2,5-dihydrofu...)Show SMILES CS(=O)(=O)c1ccc(cc1)C1=C(C(=O)OC1)c1ccccc1 |t:11| Show InChI InChI=1S/C17H14O4S/c1-22(19,20)14-9-7-12(8-10-14)15-11-21-17(18)16(15)13-5-3-2-4-6-13/h2-10H,11H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
NitroMed Inc.
Curated by ChEMBL
| Assay Description
Compound was evaluated for inhibition concentration of prostaglandin G/H synthase 2 in human blood |
J Med Chem 47: 2180-93 (2004)
Article DOI: 10.1021/jm030276s
BindingDB Entry DOI: 10.7270/Q2P55MZF |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50144866
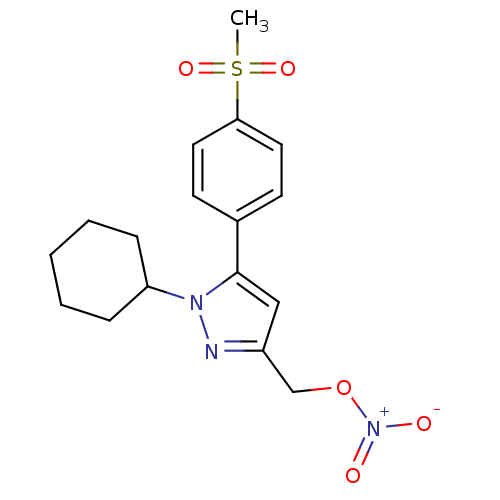
(1-Cyclohexyl-5-(4-methanesulfonyl-phenyl)-3-nitroo...)Show SMILES CS(=O)(=O)c1ccc(cc1)-c1cc(CO[N+]([O-])=O)nn1C1CCCCC1 Show InChI InChI=1S/C17H21N3O5S/c1-26(23,24)16-9-7-13(8-10-16)17-11-14(12-25-20(21)22)18-19(17)15-5-3-2-4-6-15/h7-11,15H,2-6,12H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
NitroMed Inc.
Curated by ChEMBL
| Assay Description
Compound was evaluated for inhibition concentration of prostaglandin G/H synthase 2 in human blood |
J Med Chem 47: 2180-93 (2004)
Article DOI: 10.1021/jm030276s
BindingDB Entry DOI: 10.7270/Q2P55MZF |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50024275
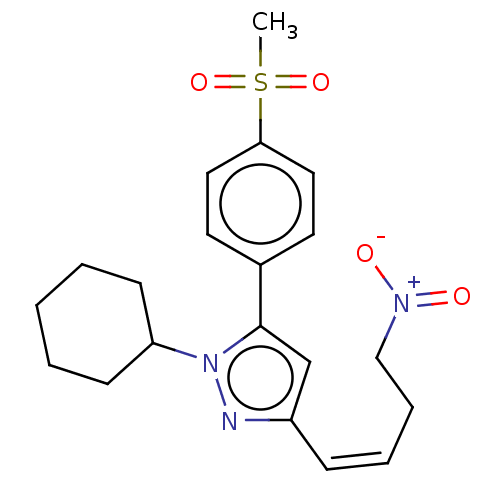
(CHEMBL2111539)Show SMILES CS(=O)(=O)c1ccc(cc1)-c1cc(\C=C/CC[N+]([O-])=O)nn1C1CCCCC1 Show InChI InChI=1S/C20H25N3O4S/c1-28(26,27)19-12-10-16(11-13-19)20-15-17(7-5-6-14-22(24)25)21-23(20)18-8-3-2-4-9-18/h5,7,10-13,15,18H,2-4,6,8-9,14H2,1H3/b7-5- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
NitroMed Inc.
Curated by ChEMBL
| Assay Description
Compound was evaluated for inhibition concentration of prostaglandin G/H synthase 2 in human blood |
J Med Chem 47: 2180-93 (2004)
Article DOI: 10.1021/jm030276s
BindingDB Entry DOI: 10.7270/Q2P55MZF |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Homo sapiens (Human)) | BDBM17638

(2-{1-[(4-chlorophenyl)carbonyl]-5-methoxy-2-methyl...)Show SMILES COc1ccc2n(C(=O)c3ccc(Cl)cc3)c(C)c(CC(O)=O)c2c1 Show InChI InChI=1S/C19H16ClNO4/c1-11-15(10-18(22)23)16-9-14(25-2)7-8-17(16)21(11)19(24)12-3-5-13(20)6-4-12/h3-9H,10H2,1-2H3,(H,22,23) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
NitroMed, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of COX1 in human whole blood assessed as effect on A23187-induced thromboxane B2 production |
J Med Chem 50: 6367-82 (2007)
Article DOI: 10.1021/jm0611861
BindingDB Entry DOI: 10.7270/Q2RJ4K9J |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM22369

(4-(4-methanesulfonylphenyl)-3-phenyl-2,5-dihydrofu...)Show SMILES CS(=O)(=O)c1ccc(cc1)C1=C(C(=O)OC1)c1ccccc1 |t:11| Show InChI InChI=1S/C17H14O4S/c1-22(19,20)14-9-7-12(8-10-14)15-11-21-17(18)16(15)13-5-3-2-4-6-13/h2-10H,11H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
NitroMed, Inc.
Curated by ChEMBL
| Assay Description
In vitro inhibitory concentration against Prostaglandin G/H synthase 2 (COX-2) in human whole blood |
J Med Chem 48: 3930-4 (2005)
Article DOI: 10.1021/jm0582064
BindingDB Entry DOI: 10.7270/Q28C9VSZ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM17638

(2-{1-[(4-chlorophenyl)carbonyl]-5-methoxy-2-methyl...)Show SMILES COc1ccc2n(C(=O)c3ccc(Cl)cc3)c(C)c(CC(O)=O)c2c1 Show InChI InChI=1S/C19H16ClNO4/c1-11-15(10-18(22)23)16-9-14(25-2)7-8-17(16)21(11)19(24)12-3-5-13(20)6-4-12/h3-9H,10H2,1-2H3,(H,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 700 | n/a | n/a | n/a | n/a | n/a | n/a |
NitroMed, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of COX2 in human whole blood assessed as effect on LPS-induced thromboxane B2 production |
J Med Chem 50: 6367-82 (2007)
Article DOI: 10.1021/jm0611861
BindingDB Entry DOI: 10.7270/Q2RJ4K9J |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50226637
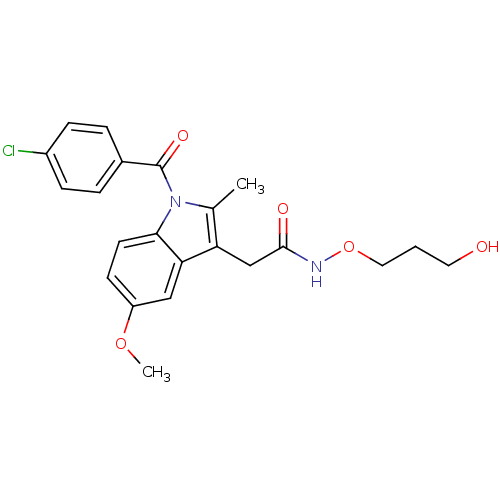
(2-{1-[(4-chlorophenyl)carbonyl]-5-methoxy-2-methyl...)Show SMILES COc1ccc2n(C(=O)c3ccc(Cl)cc3)c(C)c(CC(=O)NOCCCO)c2c1 Show InChI InChI=1S/C22H23ClN2O5/c1-14-18(13-21(27)24-30-11-3-10-26)19-12-17(29-2)8-9-20(19)25(14)22(28)15-4-6-16(23)7-5-15/h4-9,12,26H,3,10-11,13H2,1-2H3,(H,24,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 700 | n/a | n/a | n/a | n/a | n/a | n/a |
NitroMed, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of COX2 in human whole blood assessed as effect on LPS-induced thromboxane B2 production |
J Med Chem 50: 6367-82 (2007)
Article DOI: 10.1021/jm0611861
BindingDB Entry DOI: 10.7270/Q2RJ4K9J |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50226637

(2-{1-[(4-chlorophenyl)carbonyl]-5-methoxy-2-methyl...)Show SMILES COc1ccc2n(C(=O)c3ccc(Cl)cc3)c(C)c(CC(=O)NOCCCO)c2c1 Show InChI InChI=1S/C22H23ClN2O5/c1-14-18(13-21(27)24-30-11-3-10-26)19-12-17(29-2)8-9-20(19)25(14)22(28)15-4-6-16(23)7-5-15/h4-9,12,26H,3,10-11,13H2,1-2H3,(H,24,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 700 | n/a | n/a | n/a | n/a | n/a | n/a |
NitroMed, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of COX2 in human whole blood assessed as effect on LPS-induced thromboxane B2 production |
J Med Chem 50: 6367-82 (2007)
Article DOI: 10.1021/jm0611861
BindingDB Entry DOI: 10.7270/Q2RJ4K9J |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50136107
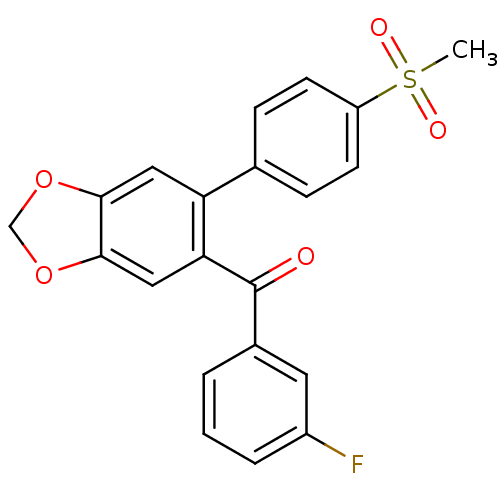
((3-Fluoro-phenyl)-[6-(4-methanesulfonyl-phenyl)-be...)Show SMILES CS(=O)(=O)c1ccc(cc1)-c1cc2OCOc2cc1C(=O)c1cccc(F)c1 Show InChI InChI=1S/C21H15FO5S/c1-28(24,25)16-7-5-13(6-8-16)17-10-19-20(27-12-26-19)11-18(17)21(23)14-3-2-4-15(22)9-14/h2-11H,12H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
NitroMed, Inc
Curated by ChEMBL
| Assay Description
Compound was tested for the inhibition of human Prostaglandin G/H synthase 2 (COX-2) in human whole blood |
J Med Chem 46: 5484-504 (2003)
Article DOI: 10.1021/jm030268b
BindingDB Entry DOI: 10.7270/Q2FX78WK |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM22369

(4-(4-methanesulfonylphenyl)-3-phenyl-2,5-dihydrofu...)Show SMILES CS(=O)(=O)c1ccc(cc1)C1=C(C(=O)OC1)c1ccccc1 |t:11| Show InChI InChI=1S/C17H14O4S/c1-22(19,20)14-9-7-12(8-10-14)15-11-21-17(18)16(15)13-5-3-2-4-6-13/h2-10H,11H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
NitroMed, Inc
Curated by ChEMBL
| Assay Description
Compound was tested for the inhibition of human Prostaglandin G/H synthase 2 (COX-2) in human whole blood |
J Med Chem 46: 5484-504 (2003)
Article DOI: 10.1021/jm030268b
BindingDB Entry DOI: 10.7270/Q2FX78WK |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50226646
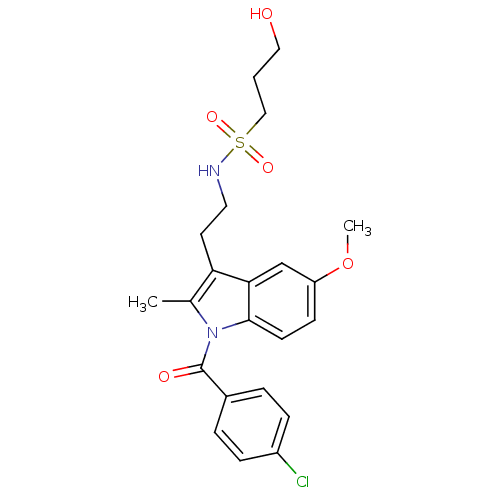
(4-chlorophenyl 3-(2-{[(3-hydroxypropyl)sulfonyl]am...)Show SMILES COc1ccc2n(C(=O)c3ccc(Cl)cc3)c(C)c(CCNS(=O)(=O)CCCO)c2c1 Show InChI InChI=1S/C22H25ClN2O5S/c1-15-19(10-11-24-31(28,29)13-3-12-26)20-14-18(30-2)8-9-21(20)25(15)22(27)16-4-6-17(23)7-5-16/h4-9,14,24,26H,3,10-13H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
NitroMed, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of COX2 in human whole blood assessed as effect on LPS-induced thromboxane B2 production |
J Med Chem 50: 6367-82 (2007)
Article DOI: 10.1021/jm0611861
BindingDB Entry DOI: 10.7270/Q2RJ4K9J |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50226646

(4-chlorophenyl 3-(2-{[(3-hydroxypropyl)sulfonyl]am...)Show SMILES COc1ccc2n(C(=O)c3ccc(Cl)cc3)c(C)c(CCNS(=O)(=O)CCCO)c2c1 Show InChI InChI=1S/C22H25ClN2O5S/c1-15-19(10-11-24-31(28,29)13-3-12-26)20-14-18(30-2)8-9-21(20)25(15)22(27)16-4-6-17(23)7-5-16/h4-9,14,24,26H,3,10-13H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
NitroMed, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of COX2 in human whole blood assessed as effect on LPS-induced thromboxane B2 production |
J Med Chem 50: 6367-82 (2007)
Article DOI: 10.1021/jm0611861
BindingDB Entry DOI: 10.7270/Q2RJ4K9J |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50226643
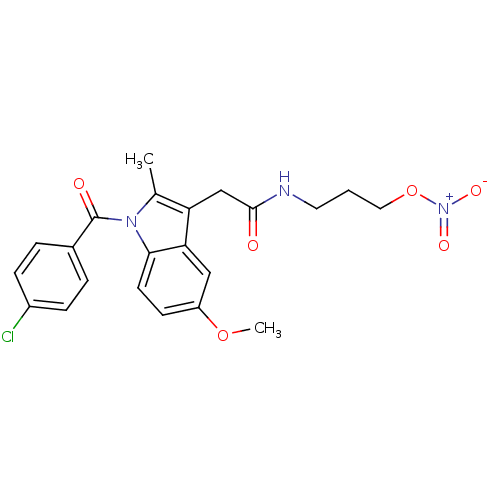
(2-{1-[(4-chlorophenyl)carbonyl]-5-methoxy-2-methyl...)Show SMILES COc1ccc2n(C(=O)c3ccc(Cl)cc3)c(C)c(CC(=O)NCCCO[N+]([O-])=O)c2c1 Show InChI InChI=1S/C22H22ClN3O6/c1-14-18(13-21(27)24-10-3-11-32-26(29)30)19-12-17(31-2)8-9-20(19)25(14)22(28)15-4-6-16(23)7-5-15/h4-9,12H,3,10-11,13H2,1-2H3,(H,24,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
NitroMed, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of COX2 in human whole blood assessed as effect on LPS-induced thromboxane B2 production |
J Med Chem 50: 6367-82 (2007)
Article DOI: 10.1021/jm0611861
BindingDB Entry DOI: 10.7270/Q2RJ4K9J |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50112552
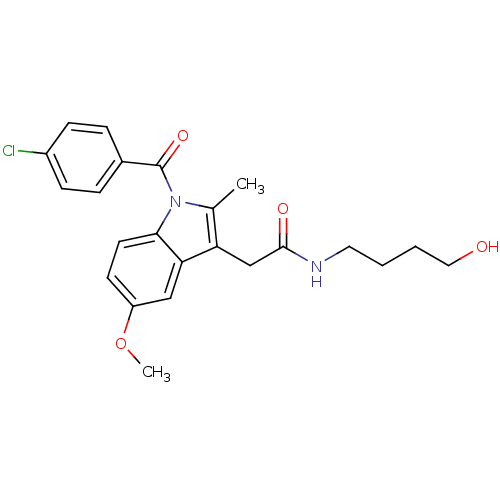
(2-[1-(4-Chloro-benzoyl)-5-methoxy-2-methyl-1H-indo...)Show SMILES COc1ccc2n(C(=O)c3ccc(Cl)cc3)c(C)c(CC(=O)NCCCCO)c2c1 Show InChI InChI=1S/C23H25ClN2O4/c1-15-19(14-22(28)25-11-3-4-12-27)20-13-18(30-2)9-10-21(20)26(15)23(29)16-5-7-17(24)8-6-16/h5-10,13,27H,3-4,11-12,14H2,1-2H3,(H,25,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
NitroMed, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of COX2 in human whole blood assessed as effect on LPS-induced thromboxane B2 production |
J Med Chem 50: 6367-82 (2007)
Article DOI: 10.1021/jm0611861
BindingDB Entry DOI: 10.7270/Q2RJ4K9J |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM11639

(4-[5-(4-methylphenyl)-3-(trifluoromethyl)-1H-pyraz...)Show SMILES Cc1ccc(cc1)-c1cc(nn1-c1ccc(cc1)S(N)(=O)=O)C(F)(F)F Show InChI InChI=1S/C17H14F3N3O2S/c1-11-2-4-12(5-3-11)15-10-16(17(18,19)20)22-23(15)13-6-8-14(9-7-13)26(21,24)25/h2-10H,1H3,(H2,21,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
NitroMed, Inc.
Curated by ChEMBL
| Assay Description
In vitro inhibitory concentration against Prostaglandin G/H synthase 2 (COX-2) in human whole blood |
J Med Chem 48: 3930-4 (2005)
Article DOI: 10.1021/jm0582064
BindingDB Entry DOI: 10.7270/Q28C9VSZ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50156456
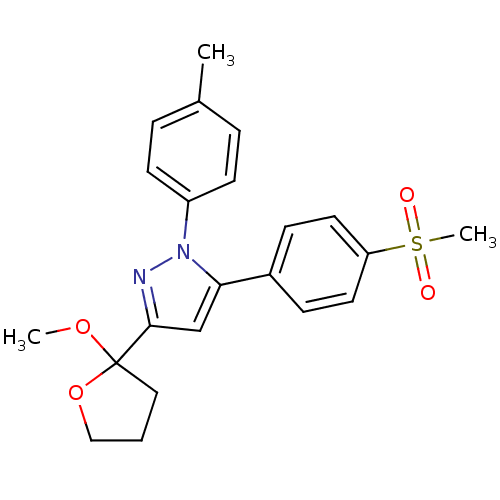
(5-(4-Methanesulfonyl-phenyl)-3-(2-methoxy-tetrahyd...)Show SMILES COC1(CCCO1)c1cc(-c2ccc(cc2)S(C)(=O)=O)n(n1)-c1ccc(C)cc1 Show InChI InChI=1S/C22H24N2O4S/c1-16-5-9-18(10-6-16)24-20(17-7-11-19(12-8-17)29(3,25)26)15-21(23-24)22(27-2)13-4-14-28-22/h5-12,15H,4,13-14H2,1-3H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
NitroMed Inc.
Curated by ChEMBL
| Assay Description
In vitro inhibition of Prostaglandin G/H synthase 2 in human whole blood |
Bioorg Med Chem Lett 14: 6049-52 (2004)
Article DOI: 10.1016/j.bmcl.2004.09.073
BindingDB Entry DOI: 10.7270/Q2Q81CJ0 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM11639

(4-[5-(4-methylphenyl)-3-(trifluoromethyl)-1H-pyraz...)Show SMILES Cc1ccc(cc1)-c1cc(nn1-c1ccc(cc1)S(N)(=O)=O)C(F)(F)F Show InChI InChI=1S/C17H14F3N3O2S/c1-11-2-4-12(5-3-11)15-10-16(17(18,19)20)22-23(15)13-6-8-14(9-7-13)26(21,24)25/h2-10H,1H3,(H2,21,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
NitroMed Inc.
Curated by ChEMBL
| Assay Description
In vitro inhibition of Prostaglandin G/H synthase 2 in human whole blood |
Bioorg Med Chem Lett 14: 6049-52 (2004)
Article DOI: 10.1016/j.bmcl.2004.09.073
BindingDB Entry DOI: 10.7270/Q2Q81CJ0 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM11639

(4-[5-(4-methylphenyl)-3-(trifluoromethyl)-1H-pyraz...)Show SMILES Cc1ccc(cc1)-c1cc(nn1-c1ccc(cc1)S(N)(=O)=O)C(F)(F)F Show InChI InChI=1S/C17H14F3N3O2S/c1-11-2-4-12(5-3-11)15-10-16(17(18,19)20)22-23(15)13-6-8-14(9-7-13)26(21,24)25/h2-10H,1H3,(H2,21,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
NitroMed Inc.
Curated by ChEMBL
| Assay Description
Compound was evaluated for inhibition concentration of prostaglandin G/H synthase 2 in human blood |
J Med Chem 47: 2180-93 (2004)
Article DOI: 10.1021/jm030276s
BindingDB Entry DOI: 10.7270/Q2P55MZF |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM11639

(4-[5-(4-methylphenyl)-3-(trifluoromethyl)-1H-pyraz...)Show SMILES Cc1ccc(cc1)-c1cc(nn1-c1ccc(cc1)S(N)(=O)=O)C(F)(F)F Show InChI InChI=1S/C17H14F3N3O2S/c1-11-2-4-12(5-3-11)15-10-16(17(18,19)20)22-23(15)13-6-8-14(9-7-13)26(21,24)25/h2-10H,1H3,(H2,21,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
NitroMed, Inc
Curated by ChEMBL
| Assay Description
Compound was tested for the inhibition of human Prostaglandin G/H synthase 2 (COX-2) in human whole blood |
J Med Chem 46: 5484-504 (2003)
Article DOI: 10.1021/jm030268b
BindingDB Entry DOI: 10.7270/Q2FX78WK |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50226652
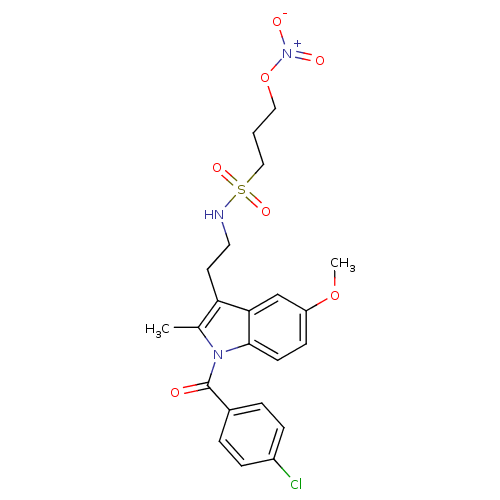
(4-chlorophenyl 5-methoxy-2-methyl-3-[2-({[3-(nitro...)Show SMILES COc1ccc2n(C(=O)c3ccc(Cl)cc3)c(C)c(CCNS(=O)(=O)CCCO[N+]([O-])=O)c2c1 Show InChI InChI=1S/C22H24ClN3O7S/c1-15-19(10-11-24-34(30,31)13-3-12-33-26(28)29)20-14-18(32-2)8-9-21(20)25(15)22(27)16-4-6-17(23)7-5-16/h4-9,14,24H,3,10-13H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
NitroMed, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of COX2 in human whole blood assessed as effect on LPS-induced thromboxane B2 production |
J Med Chem 50: 6367-82 (2007)
Article DOI: 10.1021/jm0611861
BindingDB Entry DOI: 10.7270/Q2RJ4K9J |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50226641
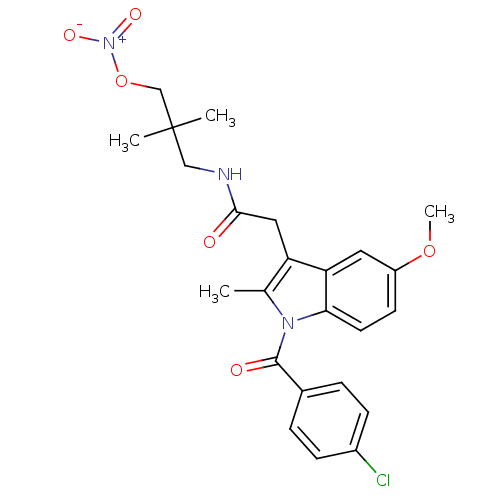
(CHEMBL399198 | N-[2,2-dimethyl-3-(nitrooxy)propyl]...)Show SMILES COc1ccc2n(C(=O)c3ccc(Cl)cc3)c(C)c(CC(=O)NCC(C)(C)CO[N+]([O-])=O)c2c1 Show InChI InChI=1S/C24H26ClN3O6/c1-15-19(12-22(29)26-13-24(2,3)14-34-28(31)32)20-11-18(33-4)9-10-21(20)27(15)23(30)16-5-7-17(25)8-6-16/h5-11H,12-14H2,1-4H3,(H,26,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
NitroMed, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of COX2 in human whole blood assessed as effect on LPS-induced thromboxane B2 production |
J Med Chem 50: 6367-82 (2007)
Article DOI: 10.1021/jm0611861
BindingDB Entry DOI: 10.7270/Q2RJ4K9J |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50226652

(4-chlorophenyl 5-methoxy-2-methyl-3-[2-({[3-(nitro...)Show SMILES COc1ccc2n(C(=O)c3ccc(Cl)cc3)c(C)c(CCNS(=O)(=O)CCCO[N+]([O-])=O)c2c1 Show InChI InChI=1S/C22H24ClN3O7S/c1-15-19(10-11-24-34(30,31)13-3-12-33-26(28)29)20-14-18(32-2)8-9-21(20)25(15)22(27)16-4-6-17(23)7-5-16/h4-9,14,24H,3,10-13H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
NitroMed, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of COX2 in human whole blood assessed as effect on LPS-induced thromboxane B2 production |
J Med Chem 50: 6367-82 (2007)
Article DOI: 10.1021/jm0611861
BindingDB Entry DOI: 10.7270/Q2RJ4K9J |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Homo sapiens (Human)) | BDBM50226656
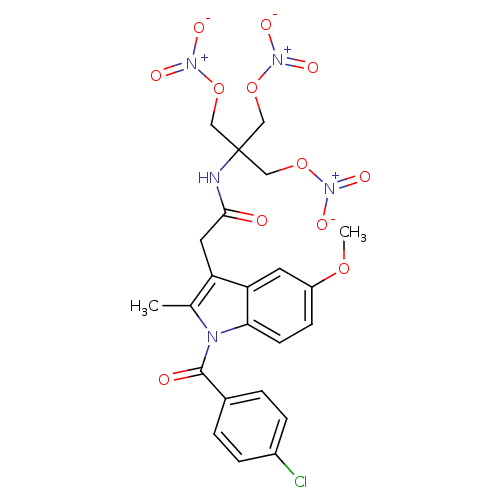
(CHEMBL393680 | N-{1,1-bis[(nitrooxy)methyl]-2-(nit...)Show SMILES COc1ccc2n(C(=O)c3ccc(Cl)cc3)c(C)c(CC(=O)NC(CO[N+]([O-])=O)(CO[N+]([O-])=O)CO[N+]([O-])=O)c2c1 Show InChI InChI=1S/C23H22ClN5O12/c1-14-18(10-21(30)25-23(11-39-27(32)33,12-40-28(34)35)13-41-29(36)37)19-9-17(38-2)7-8-20(19)26(14)22(31)15-3-5-16(24)6-4-15/h3-9H,10-13H2,1-2H3,(H,25,30) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
NitroMed, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of COX1 in human whole blood assessed as effect on A23187-induced thromboxane B2 production |
J Med Chem 50: 6367-82 (2007)
Article DOI: 10.1021/jm0611861
BindingDB Entry DOI: 10.7270/Q2RJ4K9J |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM11639

(4-[5-(4-methylphenyl)-3-(trifluoromethyl)-1H-pyraz...)Show SMILES Cc1ccc(cc1)-c1cc(nn1-c1ccc(cc1)S(N)(=O)=O)C(F)(F)F Show InChI InChI=1S/C17H14F3N3O2S/c1-11-2-4-12(5-3-11)15-10-16(17(18,19)20)22-23(15)13-6-8-14(9-7-13)26(21,24)25/h2-10H,1H3,(H2,21,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
NitroMed, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of COX2 in human whole blood assessed as effect on LPS-induced thromboxane B2 production |
J Med Chem 50: 6367-82 (2007)
Article DOI: 10.1021/jm0611861
BindingDB Entry DOI: 10.7270/Q2RJ4K9J |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Prostaglandin G/H synthase 1
(Homo sapiens (Human)) | BDBM50144867
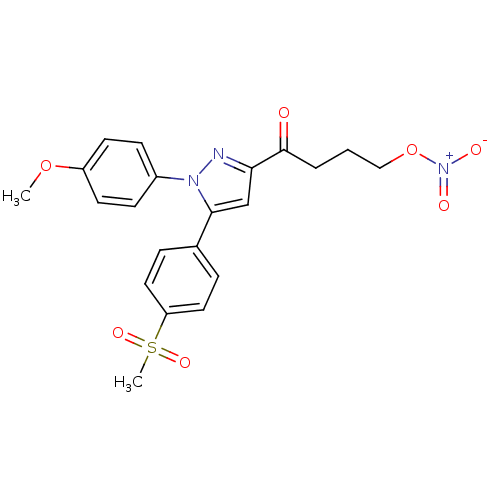
(1-[5-(4-Methanesulfonyl-phenyl)-1-(4-methoxy-pheny...)Show SMILES COc1ccc(cc1)-n1nc(cc1-c1ccc(cc1)S(C)(=O)=O)C(=O)CCCO[N+]([O-])=O Show InChI InChI=1S/C21H21N3O7S/c1-30-17-9-7-16(8-10-17)23-20(15-5-11-18(12-6-15)32(2,28)29)14-19(22-23)21(25)4-3-13-31-24(26)27/h5-12,14H,3-4,13H2,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
NitroMed Inc.
Curated by ChEMBL
| Assay Description
Compound was evaluated for inhibition concentration of prostaglandin G/H synthase 1 in human blood |
J Med Chem 47: 2180-93 (2004)
Article DOI: 10.1021/jm030276s
BindingDB Entry DOI: 10.7270/Q2P55MZF |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50226639
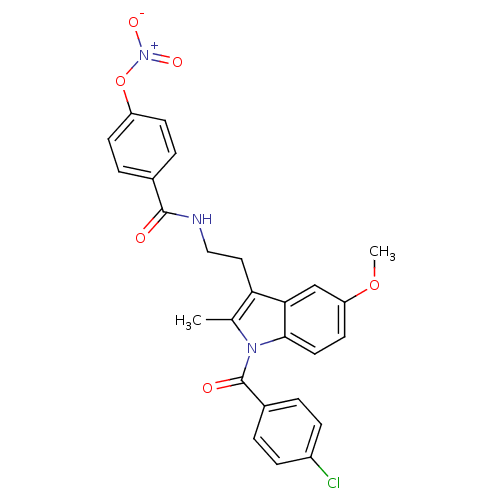
(CHEMBL238240 | N-(2-{1-[(4-chlorophenyl)carbonyl]-...)Show SMILES COc1ccc2n(C(=O)c3ccc(Cl)cc3)c(C)c(CCNC(=O)c3ccc(O[N+]([O-])=O)cc3)c2c1 Show InChI InChI=1S/C26H22ClN3O6/c1-16-22(13-14-28-25(31)17-5-9-20(10-6-17)36-30(33)34)23-15-21(35-2)11-12-24(23)29(16)26(32)18-3-7-19(27)8-4-18/h3-12,15H,13-14H2,1-2H3,(H,28,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
NitroMed, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of COX2 in human whole blood assessed as effect on LPS-induced thromboxane B2 production |
J Med Chem 50: 6367-82 (2007)
Article DOI: 10.1021/jm0611861
BindingDB Entry DOI: 10.7270/Q2RJ4K9J |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Homo sapiens (Human)) | BDBM50226653
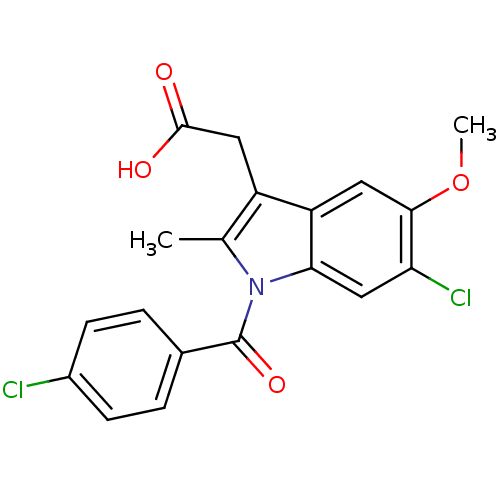
(2-{6-chloro-1-[(4-chlorophenyl)carbonyl]-5-methoxy...)Show SMILES COc1cc2c(CC(O)=O)c(C)n(C(=O)c3ccc(Cl)cc3)c2cc1Cl Show InChI InChI=1S/C19H15Cl2NO4/c1-10-13(8-18(23)24)14-7-17(26-2)15(21)9-16(14)22(10)19(25)11-3-5-12(20)6-4-11/h3-7,9H,8H2,1-2H3,(H,23,24) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
NitroMed, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of COX1 in human whole blood assessed as effect on A23187-induced thromboxane B2 production |
J Med Chem 50: 6367-82 (2007)
Article DOI: 10.1021/jm0611861
BindingDB Entry DOI: 10.7270/Q2RJ4K9J |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50112548
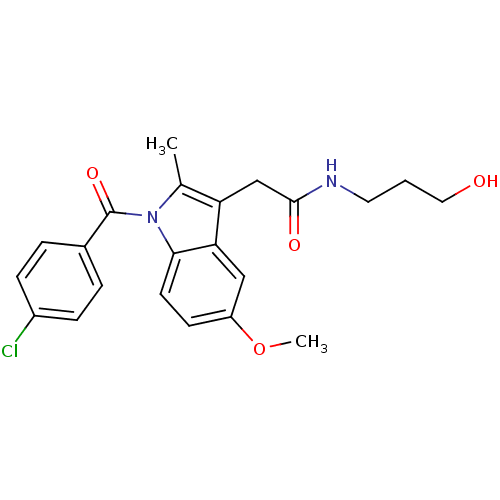
(2-[1-(4-Chloro-benzoyl)-5-methoxy-2-methyl-1H-indo...)Show SMILES COc1ccc2n(C(=O)c3ccc(Cl)cc3)c(C)c(CC(=O)NCCCO)c2c1 Show InChI InChI=1S/C22H23ClN2O4/c1-14-18(13-21(27)24-10-3-11-26)19-12-17(29-2)8-9-20(19)25(14)22(28)15-4-6-16(23)7-5-15/h4-9,12,26H,3,10-11,13H2,1-2H3,(H,24,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
NitroMed, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of COX2 in human whole blood assessed as effect on LPS-induced thromboxane B2 production |
J Med Chem 50: 6367-82 (2007)
Article DOI: 10.1021/jm0611861
BindingDB Entry DOI: 10.7270/Q2RJ4K9J |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Homo sapiens (Human)) | BDBM50226654
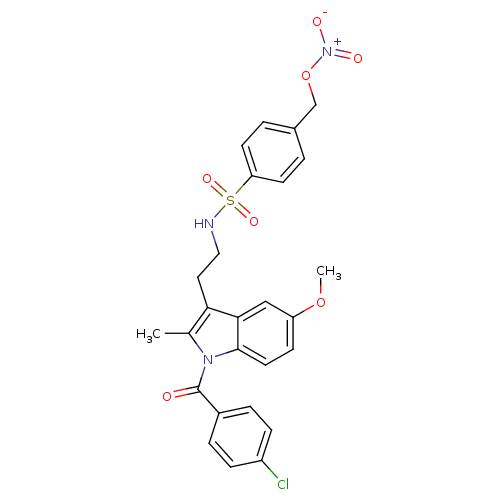
(4-chlorophenyl 5-methoxy-2-methyl-3-{2-[({4-[(nitr...)Show SMILES COc1ccc2n(C(=O)c3ccc(Cl)cc3)c(C)c(CCNS(=O)(=O)c3ccc(CO[N+]([O-])=O)cc3)c2c1 Show InChI InChI=1S/C26H24ClN3O7S/c1-17-23(13-14-28-38(34,35)22-10-3-18(4-11-22)16-37-30(32)33)24-15-21(36-2)9-12-25(24)29(17)26(31)19-5-7-20(27)8-6-19/h3-12,15,28H,13-14,16H2,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
NitroMed, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of COX1 in human whole blood assessed as effect on A23187-induced thromboxane B2 production |
J Med Chem 50: 6367-82 (2007)
Article DOI: 10.1021/jm0611861
BindingDB Entry DOI: 10.7270/Q2RJ4K9J |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50072064

(5-Chloro-3-(4-methanesulfonyl-phenyl)-6''-methyl-[...)Show SMILES Cc1ccc(cn1)-c1ncc(Cl)cc1-c1ccc(cc1)S(C)(=O)=O Show InChI InChI=1S/C18H15ClN2O2S/c1-12-3-4-14(10-20-12)18-17(9-15(19)11-21-18)13-5-7-16(8-6-13)24(2,22)23/h3-11H,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
NitroMed, Inc.
Curated by ChEMBL
| Assay Description
In vitro inhibitory concentration against Prostaglandin G/H synthase 2 (COX-2) in human whole blood |
J Med Chem 48: 3930-4 (2005)
Article DOI: 10.1021/jm0582064
BindingDB Entry DOI: 10.7270/Q28C9VSZ |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Homo sapiens (Human)) | BDBM50226648
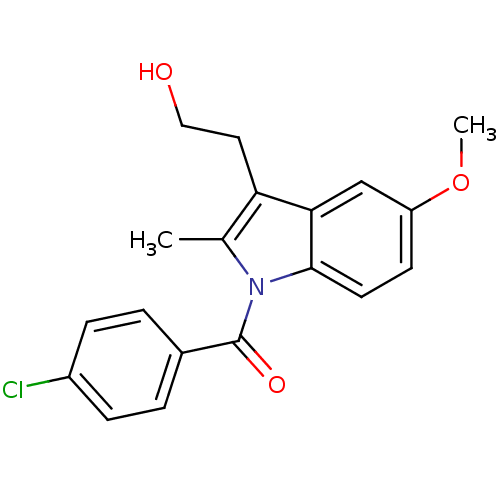
(4-chlorophenyl 3-(2-hydroxyethyl)-5-methoxy-2-meth...)Show InChI InChI=1S/C19H18ClNO3/c1-12-16(9-10-22)17-11-15(24-2)7-8-18(17)21(12)19(23)13-3-5-14(20)6-4-13/h3-8,11,22H,9-10H2,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
NitroMed, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of COX1 in human whole blood assessed as effect on A23187-induced thromboxane B2 production |
J Med Chem 50: 6367-82 (2007)
Article DOI: 10.1021/jm0611861
BindingDB Entry DOI: 10.7270/Q2RJ4K9J |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Homo sapiens (Human)) | BDBM50112552

(2-[1-(4-Chloro-benzoyl)-5-methoxy-2-methyl-1H-indo...)Show SMILES COc1ccc2n(C(=O)c3ccc(Cl)cc3)c(C)c(CC(=O)NCCCCO)c2c1 Show InChI InChI=1S/C23H25ClN2O4/c1-15-19(14-22(28)25-11-3-4-12-27)20-13-18(30-2)9-10-21(20)26(15)23(29)16-5-7-17(24)8-6-16/h5-10,13,27H,3-4,11-12,14H2,1-2H3,(H,25,28) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
NitroMed, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of COX1 in human whole blood assessed as effect on A23187-induced thromboxane B2 production |
J Med Chem 50: 6367-82 (2007)
Article DOI: 10.1021/jm0611861
BindingDB Entry DOI: 10.7270/Q2RJ4K9J |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50144867

(1-[5-(4-Methanesulfonyl-phenyl)-1-(4-methoxy-pheny...)Show SMILES COc1ccc(cc1)-n1nc(cc1-c1ccc(cc1)S(C)(=O)=O)C(=O)CCCO[N+]([O-])=O Show InChI InChI=1S/C21H21N3O7S/c1-30-17-9-7-16(8-10-17)23-20(15-5-11-18(12-6-15)32(2,28)29)14-19(22-23)21(25)4-3-13-31-24(26)27/h5-12,14H,3-4,13H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
NitroMed Inc.
Curated by ChEMBL
| Assay Description
Compound was evaluated for inhibition concentration of prostaglandin G/H synthase 2 in human blood |
J Med Chem 47: 2180-93 (2004)
Article DOI: 10.1021/jm030276s
BindingDB Entry DOI: 10.7270/Q2P55MZF |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Homo sapiens (Human)) | BDBM50226641

(CHEMBL399198 | N-[2,2-dimethyl-3-(nitrooxy)propyl]...)Show SMILES COc1ccc2n(C(=O)c3ccc(Cl)cc3)c(C)c(CC(=O)NCC(C)(C)CO[N+]([O-])=O)c2c1 Show InChI InChI=1S/C24H26ClN3O6/c1-15-19(12-22(29)26-13-24(2,3)14-34-28(31)32)20-11-18(33-4)9-10-21(20)27(15)23(30)16-5-7-17(25)8-6-16/h5-11H,12-14H2,1-4H3,(H,26,29) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
NitroMed, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of COX1 in human whole blood assessed as effect on A23187-induced thromboxane B2 production |
J Med Chem 50: 6367-82 (2007)
Article DOI: 10.1021/jm0611861
BindingDB Entry DOI: 10.7270/Q2RJ4K9J |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Homo sapiens (Human)) | BDBM50012893
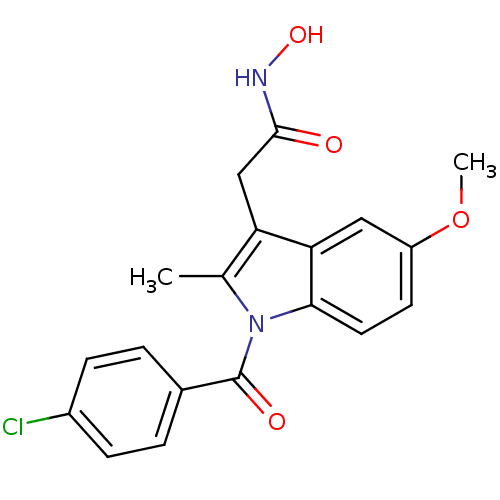
(2-[1-(4-Chloro-benzoyl)-5-methoxy-2-methyl-1H-indo...)Show SMILES COc1ccc2n(C(=O)c3ccc(Cl)cc3)c(C)c(CC(=O)NO)c2c1 Show InChI InChI=1S/C19H17ClN2O4/c1-11-15(10-18(23)21-25)16-9-14(26-2)7-8-17(16)22(11)19(24)12-3-5-13(20)6-4-12/h3-9,25H,10H2,1-2H3,(H,21,23) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
NitroMed, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of COX1 in human whole blood assessed as effect on A23187-induced thromboxane B2 production |
J Med Chem 50: 6367-82 (2007)
Article DOI: 10.1021/jm0611861
BindingDB Entry DOI: 10.7270/Q2RJ4K9J |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Homo sapiens (Human)) | BDBM50226638
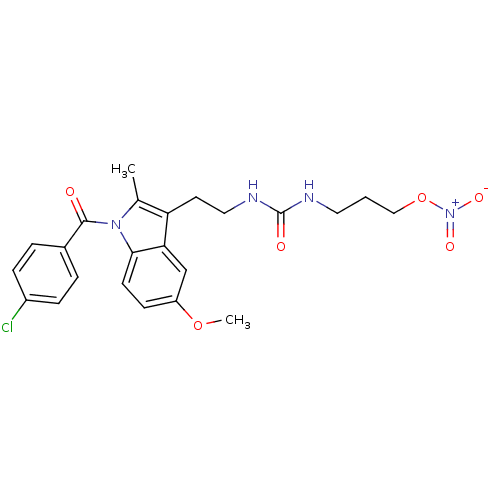
(CHEMBL237022 | N-(2-{1-[(4-chlorophenyl)carbonyl]-...)Show SMILES COc1ccc2n(C(=O)c3ccc(Cl)cc3)c(C)c(CCNC(=O)NCCCO[N+]([O-])=O)c2c1 Show InChI InChI=1S/C23H25ClN4O6/c1-15-19(10-12-26-23(30)25-11-3-13-34-28(31)32)20-14-18(33-2)8-9-21(20)27(15)22(29)16-4-6-17(24)7-5-16/h4-9,14H,3,10-13H2,1-2H3,(H2,25,26,30) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
NitroMed, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of COX1 in human whole blood assessed as effect on A23187-induced thromboxane B2 production |
J Med Chem 50: 6367-82 (2007)
Article DOI: 10.1021/jm0611861
BindingDB Entry DOI: 10.7270/Q2RJ4K9J |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50226655
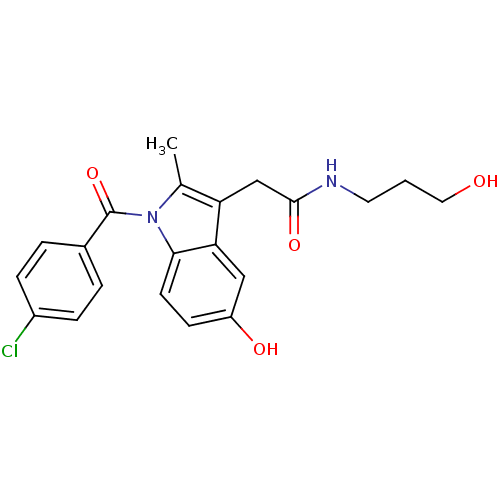
(2-{1-[(4-chlorophenyl)carbonyl]-5-hydroxy-2-methyl...)Show SMILES Cc1c(CC(=O)NCCCO)c2cc(O)ccc2n1C(=O)c1ccc(Cl)cc1 Show InChI InChI=1S/C21H21ClN2O4/c1-13-17(12-20(27)23-9-2-10-25)18-11-16(26)7-8-19(18)24(13)21(28)14-3-5-15(22)6-4-14/h3-8,11,25-26H,2,9-10,12H2,1H3,(H,23,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
NitroMed, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of COX2 in human whole blood assessed as effect on LPS-induced thromboxane B2 production |
J Med Chem 50: 6367-82 (2007)
Article DOI: 10.1021/jm0611861
BindingDB Entry DOI: 10.7270/Q2RJ4K9J |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Homo sapiens (Human)) | BDBM50112548

(2-[1-(4-Chloro-benzoyl)-5-methoxy-2-methyl-1H-indo...)Show SMILES COc1ccc2n(C(=O)c3ccc(Cl)cc3)c(C)c(CC(=O)NCCCO)c2c1 Show InChI InChI=1S/C22H23ClN2O4/c1-14-18(13-21(27)24-10-3-11-26)19-12-17(29-2)8-9-20(19)25(14)22(28)15-4-6-16(23)7-5-15/h4-9,12,26H,3,10-11,13H2,1-2H3,(H,24,27) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
NitroMed, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of COX1 in human whole blood assessed as effect on A23187-induced thromboxane B2 production |
J Med Chem 50: 6367-82 (2007)
Article DOI: 10.1021/jm0611861
BindingDB Entry DOI: 10.7270/Q2RJ4K9J |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50226657
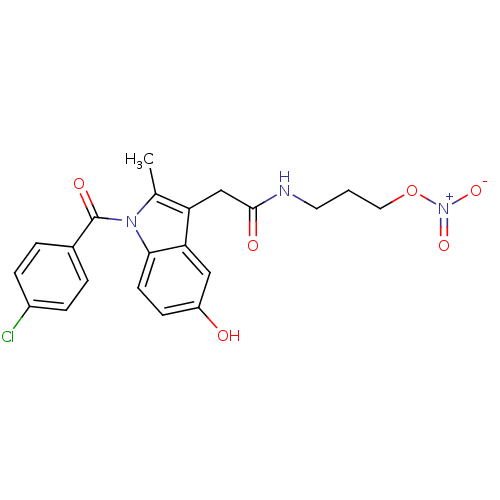
(2-{1-[(4-chlorophenyl)carbonyl]-5-hydroxy-2-methyl...)Show SMILES Cc1c(CC(=O)NCCCO[N+]([O-])=O)c2cc(O)ccc2n1C(=O)c1ccc(Cl)cc1 Show InChI InChI=1S/C21H20ClN3O6/c1-13-17(12-20(27)23-9-2-10-31-25(29)30)18-11-16(26)7-8-19(18)24(13)21(28)14-3-5-15(22)6-4-14/h3-8,11,26H,2,9-10,12H2,1H3,(H,23,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
NitroMed, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of COX2 in human whole blood assessed as effect on LPS-induced thromboxane B2 production |
J Med Chem 50: 6367-82 (2007)
Article DOI: 10.1021/jm0611861
BindingDB Entry DOI: 10.7270/Q2RJ4K9J |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Homo sapiens (Human)) | BDBM50226647
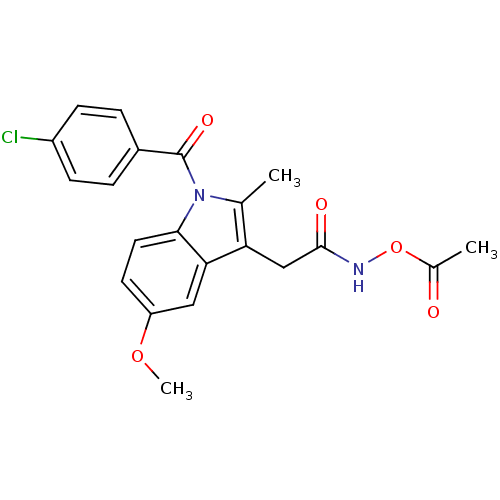
((2-{1-[(4-chlorophenyl)carbonyl]-5-methoxy-2-methy...)Show SMILES COc1ccc2n(C(=O)c3ccc(Cl)cc3)c(C)c(CC(=O)NOC(C)=O)c2c1 Show InChI InChI=1S/C21H19ClN2O5/c1-12-17(11-20(26)23-29-13(2)25)18-10-16(28-3)8-9-19(18)24(12)21(27)14-4-6-15(22)7-5-14/h4-10H,11H2,1-3H3,(H,23,26) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
NitroMed, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of COX1 in human whole blood assessed as effect on A23187-induced thromboxane B2 production |
J Med Chem 50: 6367-82 (2007)
Article DOI: 10.1021/jm0611861
BindingDB Entry DOI: 10.7270/Q2RJ4K9J |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Homo sapiens (Human)) | BDBM50226646

(4-chlorophenyl 3-(2-{[(3-hydroxypropyl)sulfonyl]am...)Show SMILES COc1ccc2n(C(=O)c3ccc(Cl)cc3)c(C)c(CCNS(=O)(=O)CCCO)c2c1 Show InChI InChI=1S/C22H25ClN2O5S/c1-15-19(10-11-24-31(28,29)13-3-12-26)20-14-18(30-2)8-9-21(20)25(15)22(27)16-4-6-17(23)7-5-16/h4-9,14,24,26H,3,10-13H2,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
NitroMed, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of COX1 in human whole blood assessed as effect on A23187-induced thromboxane B2 production |
J Med Chem 50: 6367-82 (2007)
Article DOI: 10.1021/jm0611861
BindingDB Entry DOI: 10.7270/Q2RJ4K9J |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Homo sapiens (Human)) | BDBM50226646

(4-chlorophenyl 3-(2-{[(3-hydroxypropyl)sulfonyl]am...)Show SMILES COc1ccc2n(C(=O)c3ccc(Cl)cc3)c(C)c(CCNS(=O)(=O)CCCO)c2c1 Show InChI InChI=1S/C22H25ClN2O5S/c1-15-19(10-11-24-31(28,29)13-3-12-26)20-14-18(30-2)8-9-21(20)25(15)22(27)16-4-6-17(23)7-5-16/h4-9,14,24,26H,3,10-13H2,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
NitroMed, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of COX1 in human whole blood assessed as effect on A23187-induced thromboxane B2 production |
J Med Chem 50: 6367-82 (2007)
Article DOI: 10.1021/jm0611861
BindingDB Entry DOI: 10.7270/Q2RJ4K9J |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50226653

(2-{6-chloro-1-[(4-chlorophenyl)carbonyl]-5-methoxy...)Show SMILES COc1cc2c(CC(O)=O)c(C)n(C(=O)c3ccc(Cl)cc3)c2cc1Cl Show InChI InChI=1S/C19H15Cl2NO4/c1-10-13(8-18(23)24)14-7-17(26-2)15(21)9-16(14)22(10)19(25)11-3-5-12(20)6-4-11/h3-7,9H,8H2,1-2H3,(H,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
NitroMed, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of COX2 in human whole blood assessed as effect on LPS-induced thromboxane B2 production |
J Med Chem 50: 6367-82 (2007)
Article DOI: 10.1021/jm0611861
BindingDB Entry DOI: 10.7270/Q2RJ4K9J |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data