 Found 41 hits with Last Name = 'leung' and Initial = 'cs'
Found 41 hits with Last Name = 'leung' and Initial = 'cs' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Aromatase
(Homo sapiens (Human)) | BDBM50024544
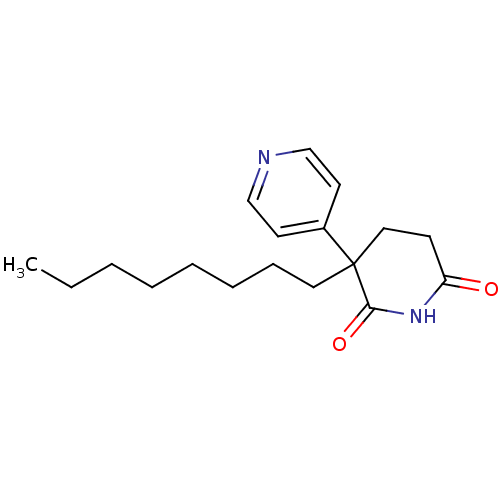
(3-Octyl-4,5-dihydro-3H-[3,4']bipyridinyl-2,6-dione...)Show InChI InChI=1S/C18H26N2O2/c1-2-3-4-5-6-7-11-18(15-9-13-19-14-10-15)12-8-16(21)20-17(18)22/h9-10,13-14H,2-8,11-12H2,1H3,(H,20,21,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of human placental cytochrome P450 19A1 with androstenedione |
J Med Chem 30: 1550-4 (1987)
BindingDB Entry DOI: 10.7270/Q2GM869Q |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50015983
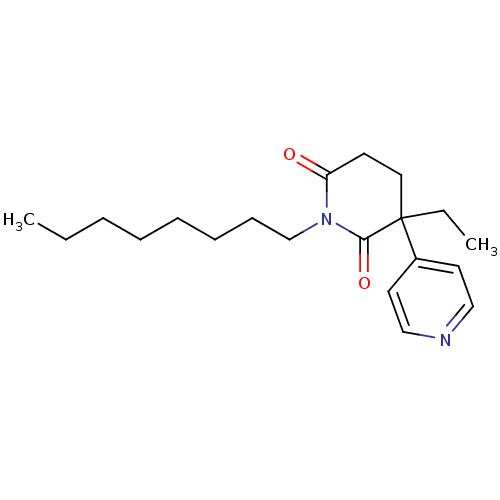
((S)-3-Ethyl-1-octyl-4,5-dihydro-3H-[3,4']bipyridin...)Show InChI InChI=1S/C20H30N2O2/c1-3-5-6-7-8-9-16-22-18(23)10-13-20(4-2,19(22)24)17-11-14-21-15-12-17/h11-12,14-15H,3-10,13,16H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of human placental cytochrome P450 19A1 with testosterone |
J Med Chem 30: 1550-4 (1987)
BindingDB Entry DOI: 10.7270/Q2GM869Q |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50024544

(3-Octyl-4,5-dihydro-3H-[3,4']bipyridinyl-2,6-dione...)Show InChI InChI=1S/C18H26N2O2/c1-2-3-4-5-6-7-11-18(15-9-13-19-14-10-15)12-8-16(21)20-17(18)22/h9-10,13-14H,2-8,11-12H2,1H3,(H,20,21,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of human placental cytochrome P450 19A1 with androstenedione |
J Med Chem 30: 1550-4 (1987)
BindingDB Entry DOI: 10.7270/Q2GM869Q |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50015983

((S)-3-Ethyl-1-octyl-4,5-dihydro-3H-[3,4']bipyridin...)Show InChI InChI=1S/C20H30N2O2/c1-3-5-6-7-8-9-16-22-18(23)10-13-20(4-2,19(22)24)17-11-14-21-15-12-17/h11-12,14-15H,3-10,13,16H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 480 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of human placental cytochrome P450 19A1 with androstenedione |
J Med Chem 30: 1550-4 (1987)
BindingDB Entry DOI: 10.7270/Q2GM869Q |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM9460

(3-(4-aminophenyl)-3-ethyl-piperidine-2,6-dione | 3...)Show InChI InChI=1S/C13H16N2O2/c1-2-13(8-7-11(16)15-12(13)17)9-3-5-10(14)6-4-9/h3-6H,2,7-8,14H2,1H3,(H,15,16,17) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PubMed
| 600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against human placental cytochrome P450 19A1 |
J Med Chem 30: 1550-4 (1987)
BindingDB Entry DOI: 10.7270/Q2GM869Q |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM8885
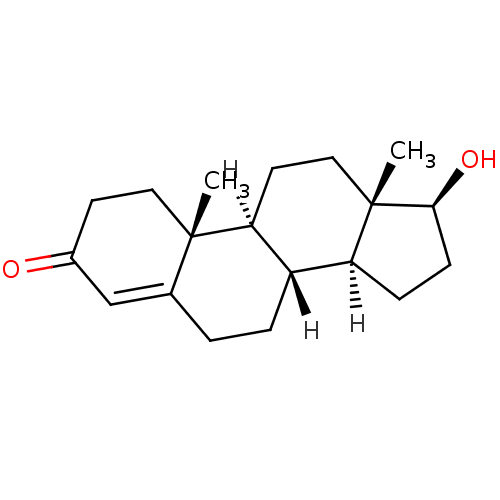
((1S,2R,10R,11S,14S,15S)-14-hydroxy-2,15-dimethylte...)Show SMILES [H][C@@]12CC[C@H](O)[C@@]1(C)CC[C@@]1([H])[C@@]2([H])CCC2=CC(=O)CC[C@]12C |r,t:18| Show InChI InChI=1S/C19H28O2/c1-18-9-7-13(20)11-12(18)3-4-14-15-5-6-17(21)19(15,2)10-8-16(14)18/h11,14-17,21H,3-10H2,1-2H3/t14-,15-,16-,17-,18-,19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| DrugBank
PDB
PubMed
| 600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of human placental aromatase Cytochrome P450 19A1 |
J Med Chem 26: 50-4 (1983)
BindingDB Entry DOI: 10.7270/Q2V125CG |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Aromatase
(Homo sapiens (Human)) | BDBM9460

(3-(4-aminophenyl)-3-ethyl-piperidine-2,6-dione | 3...)Show InChI InChI=1S/C13H16N2O2/c1-2-13(8-7-11(16)15-12(13)17)9-3-5-10(14)6-4-9/h3-6H,2,7-8,14H2,1H3,(H,15,16,17) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PubMed
| 600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory constant (Ki) for Cytochrome P450 19A1 |
J Med Chem 28: 200-4 (1985)
BindingDB Entry DOI: 10.7270/Q2WM1FK8 |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50015985

(3-Ethyl-4,5-dihydro-3H-[3,4']bipyridinyl-2,6-dione...)Show InChI InChI=1S/C12H14N2O2/c1-2-12(9-4-7-13-8-5-9)6-3-10(15)14-11(12)16/h4-5,7-8H,2-3,6H2,1H3,(H,14,15,16) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory constant (Ki) for Cytochrome P450 19A1 |
J Med Chem 28: 200-4 (1985)
BindingDB Entry DOI: 10.7270/Q2WM1FK8 |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50015985

(3-Ethyl-4,5-dihydro-3H-[3,4']bipyridinyl-2,6-dione...)Show InChI InChI=1S/C12H14N2O2/c1-2-12(9-4-7-13-8-5-9)6-3-10(15)14-11(12)16/h4-5,7-8H,2-3,6H2,1H3,(H,14,15,16) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of human placental cytochrome P450 19A1 with androstenedione |
J Med Chem 30: 1550-4 (1987)
BindingDB Entry DOI: 10.7270/Q2GM869Q |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM9460

(3-(4-aminophenyl)-3-ethyl-piperidine-2,6-dione | 3...)Show InChI InChI=1S/C13H16N2O2/c1-2-13(8-7-11(16)15-12(13)17)9-3-5-10(14)6-4-9/h3-6H,2,7-8,14H2,1H3,(H,15,16,17) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PubMed
| 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of human placental cytochrome P450 19A1 with androstenedione |
J Med Chem 30: 1550-4 (1987)
BindingDB Entry DOI: 10.7270/Q2GM869Q |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50015985

(3-Ethyl-4,5-dihydro-3H-[3,4']bipyridinyl-2,6-dione...)Show InChI InChI=1S/C12H14N2O2/c1-2-12(9-4-7-13-8-5-9)6-3-10(15)14-11(12)16/h4-5,7-8H,2-3,6H2,1H3,(H,14,15,16) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 1.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of human placental cytochrome P450 19A1 with androstenedione |
J Med Chem 30: 1550-4 (1987)
BindingDB Entry DOI: 10.7270/Q2GM869Q |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50024544

(3-Octyl-4,5-dihydro-3H-[3,4']bipyridinyl-2,6-dione...)Show InChI InChI=1S/C18H26N2O2/c1-2-3-4-5-6-7-11-18(15-9-13-19-14-10-15)12-8-16(21)20-17(18)22/h9-10,13-14H,2-8,11-12H2,1H3,(H,20,21,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 330 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against human placental cytochrome P450 19A1 |
J Med Chem 30: 1550-4 (1987)
BindingDB Entry DOI: 10.7270/Q2GM869Q |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50024544

(3-Octyl-4,5-dihydro-3H-[3,4']bipyridinyl-2,6-dione...)Show InChI InChI=1S/C18H26N2O2/c1-2-3-4-5-6-7-11-18(15-9-13-19-14-10-15)12-8-16(21)20-17(18)22/h9-10,13-14H,2-8,11-12H2,1H3,(H,20,21,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of human placental cytochrome P450 19A1 with testosterone |
J Med Chem 30: 1550-4 (1987)
BindingDB Entry DOI: 10.7270/Q2GM869Q |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50015983

((S)-3-Ethyl-1-octyl-4,5-dihydro-3H-[3,4']bipyridin...)Show InChI InChI=1S/C20H30N2O2/c1-3-5-6-7-8-9-16-22-18(23)10-13-20(4-2,19(22)24)17-11-14-21-15-12-17/h11-12,14-15H,3-10,13,16H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 800 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of human placental cytochrome P450 19A1 with testosterone |
J Med Chem 30: 1550-4 (1987)
BindingDB Entry DOI: 10.7270/Q2GM869Q |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50024550
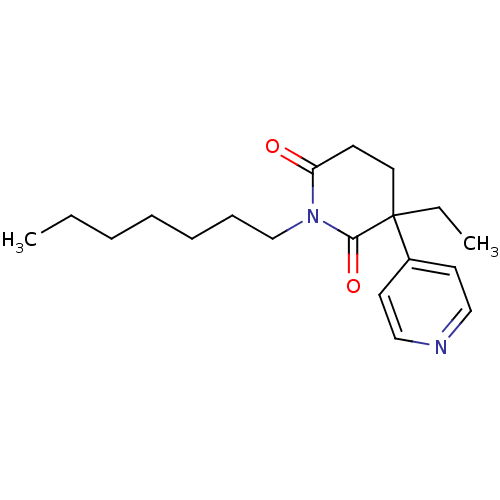
(3-Ethyl-1-heptyl-4,5-dihydro-3H-[3,4']bipyridinyl-...)Show InChI InChI=1S/C19H28N2O2/c1-3-5-6-7-8-15-21-17(22)9-12-19(4-2,18(21)23)16-10-13-20-14-11-16/h10-11,13-14H,3-9,12,15H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against human placental cytochrome P450 19A1 |
J Med Chem 30: 1550-4 (1987)
BindingDB Entry DOI: 10.7270/Q2GM869Q |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50024555
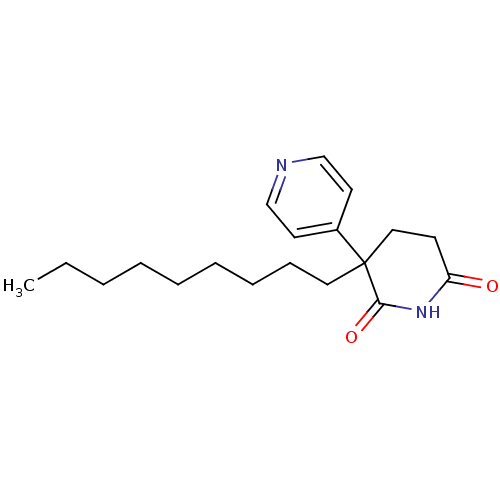
(3-Nonyl-4,5-dihydro-3H-[3,4']bipyridinyl-2,6-dione...)Show InChI InChI=1S/C19H28N2O2/c1-2-3-4-5-6-7-8-12-19(16-10-14-20-15-11-16)13-9-17(22)21-18(19)23/h10-11,14-15H,2-9,12-13H2,1H3,(H,21,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against human placental cytochrome P450 19A1 |
J Med Chem 30: 1550-4 (1987)
BindingDB Entry DOI: 10.7270/Q2GM869Q |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50024557
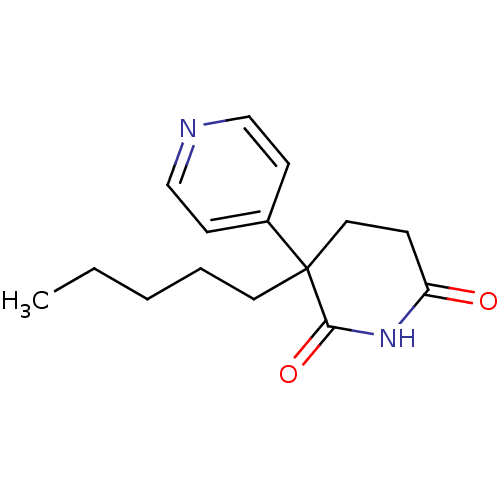
(3-Pentyl-4,5-dihydro-3H-[3,4']bipyridinyl-2,6-dion...)Show InChI InChI=1S/C15H20N2O2/c1-2-3-4-8-15(12-6-10-16-11-7-12)9-5-13(18)17-14(15)19/h6-7,10-11H,2-5,8-9H2,1H3,(H,17,18,19) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against human placental cytochrome P450 19A1 |
J Med Chem 30: 1550-4 (1987)
BindingDB Entry DOI: 10.7270/Q2GM869Q |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50024553

(3-Ethyl-1-nonyl-4,5-dihydro-3H-[3,4']bipyridinyl-2...)Show InChI InChI=1S/C21H32N2O2/c1-3-5-6-7-8-9-10-17-23-19(24)11-14-21(4-2,20(23)25)18-12-15-22-16-13-18/h12-13,15-16H,3-11,14,17H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against human placental cytochrome P450 19A1 |
J Med Chem 30: 1550-4 (1987)
BindingDB Entry DOI: 10.7270/Q2GM869Q |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50024547
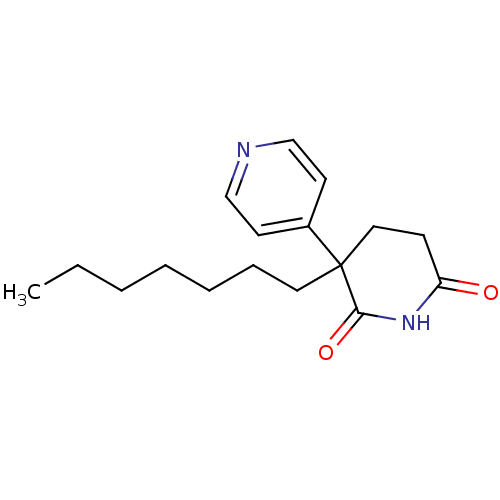
(3-Heptyl-4,5-dihydro-3H-[3,4']bipyridinyl-2,6-dion...)Show InChI InChI=1S/C17H24N2O2/c1-2-3-4-5-6-10-17(14-8-12-18-13-9-14)11-7-15(20)19-16(17)21/h8-9,12-13H,2-7,10-11H2,1H3,(H,19,20,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 3.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against human placental cytochrome P450 19A1 |
J Med Chem 30: 1550-4 (1987)
BindingDB Entry DOI: 10.7270/Q2GM869Q |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50024546
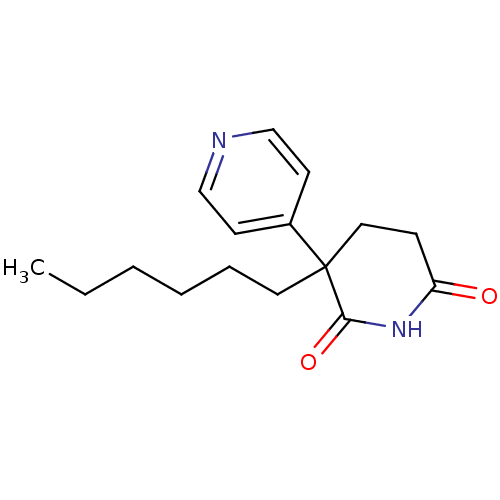
(3-Hexyl-4,5-dihydro-3H-[3,4']bipyridinyl-2,6-dione...)Show InChI InChI=1S/C16H22N2O2/c1-2-3-4-5-9-16(13-7-11-17-12-8-13)10-6-14(19)18-15(16)20/h7-8,11-12H,2-6,9-10H2,1H3,(H,18,19,20) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 3.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against human placental cytochrome P450 19A1 |
J Med Chem 30: 1550-4 (1987)
BindingDB Entry DOI: 10.7270/Q2GM869Q |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50024548
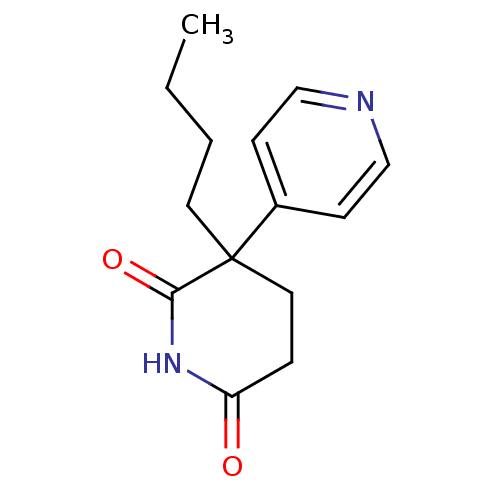
(3-Butyl-4,5-dihydro-3H-[3,4']bipyridinyl-2,6-dione...)Show InChI InChI=1S/C14H18N2O2/c1-2-3-7-14(11-5-9-15-10-6-11)8-4-12(17)16-13(14)18/h5-6,9-10H,2-4,7-8H2,1H3,(H,16,17,18) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against human placental cytochrome P450 19A1 |
J Med Chem 30: 1550-4 (1987)
BindingDB Entry DOI: 10.7270/Q2GM869Q |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50015983

((S)-3-Ethyl-1-octyl-4,5-dihydro-3H-[3,4']bipyridin...)Show InChI InChI=1S/C20H30N2O2/c1-3-5-6-7-8-9-16-22-18(23)10-13-20(4-2,19(22)24)17-11-14-21-15-12-17/h11-12,14-15H,3-10,13,16H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against human placental cytochrome P450 19A1 |
J Med Chem 30: 1550-4 (1987)
BindingDB Entry DOI: 10.7270/Q2GM869Q |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50024556
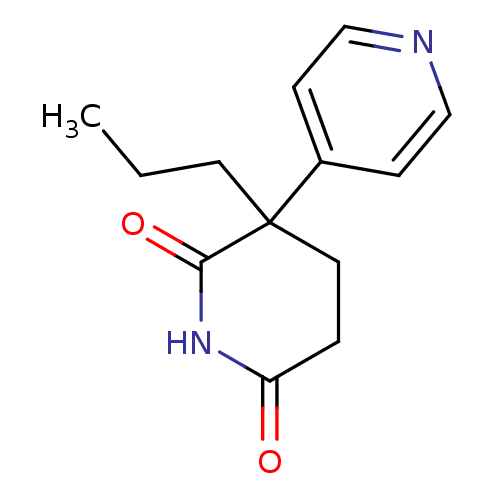
(3-Propyl-4,5-dihydro-3H-[3,4']bipyridinyl-2,6-dion...)Show InChI InChI=1S/C13H16N2O2/c1-2-6-13(10-4-8-14-9-5-10)7-3-11(16)15-12(13)17/h4-5,8-9H,2-3,6-7H2,1H3,(H,15,16,17) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 6.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against human placental cytochrome P450 19A1 |
J Med Chem 30: 1550-4 (1987)
BindingDB Entry DOI: 10.7270/Q2GM869Q |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50024559
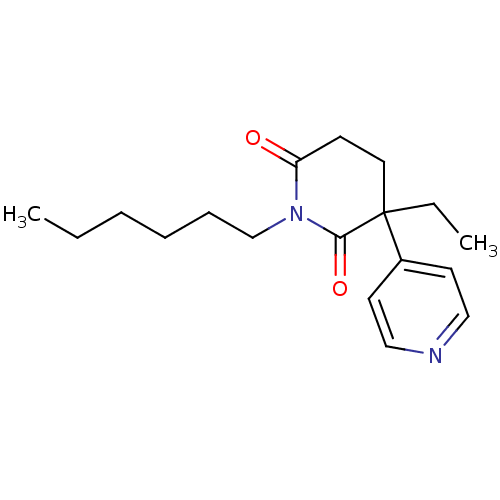
(3-Ethyl-1-hexyl-4,5-dihydro-3H-[3,4']bipyridinyl-2...)Show InChI InChI=1S/C18H26N2O2/c1-3-5-6-7-14-20-16(21)8-11-18(4-2,17(20)22)15-9-12-19-13-10-15/h9-10,12-13H,3-8,11,14H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 6.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against human placental cytochrome P450 19A1 |
J Med Chem 30: 1550-4 (1987)
BindingDB Entry DOI: 10.7270/Q2GM869Q |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM9460

(3-(4-aminophenyl)-3-ethyl-piperidine-2,6-dione | 3...)Show InChI InChI=1S/C13H16N2O2/c1-2-13(8-7-11(16)15-12(13)17)9-3-5-10(14)6-4-9/h3-6H,2,7-8,14H2,1H3,(H,15,16,17) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PubMed
| n/a | n/a | 8.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against human placental cytochrome P450 19A1 |
J Med Chem 30: 1550-4 (1987)
BindingDB Entry DOI: 10.7270/Q2GM869Q |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50024549
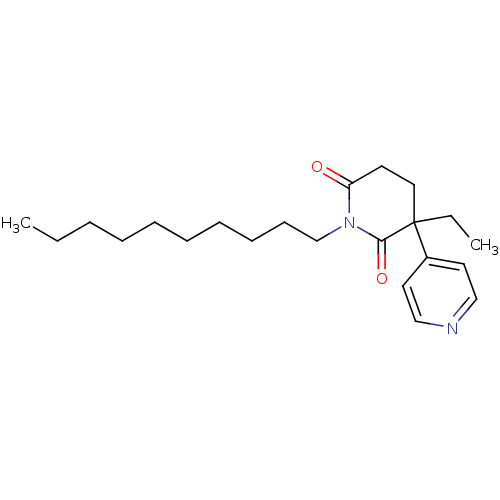
(1-Decyl-3-ethyl-4,5-dihydro-3H-[3,4']bipyridinyl-2...)Show InChI InChI=1S/C22H34N2O2/c1-3-5-6-7-8-9-10-11-18-24-20(25)12-15-22(4-2,21(24)26)19-13-16-23-17-14-19/h13-14,16-17H,3-12,15,18H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against human placental cytochrome P450 19A1 |
J Med Chem 30: 1550-4 (1987)
BindingDB Entry DOI: 10.7270/Q2GM869Q |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50015985

(3-Ethyl-4,5-dihydro-3H-[3,4']bipyridinyl-2,6-dione...)Show InChI InChI=1S/C12H14N2O2/c1-2-12(9-4-7-13-8-5-9)6-3-10(15)14-11(12)16/h4-5,7-8H,2-3,6H2,1H3,(H,14,15,16) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of human placental cytochrome P450 19A1 with androstenedione |
J Med Chem 30: 1550-4 (1987)
BindingDB Entry DOI: 10.7270/Q2GM869Q |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM9460

(3-(4-aminophenyl)-3-ethyl-piperidine-2,6-dione | 3...)Show InChI InChI=1S/C13H16N2O2/c1-2-13(8-7-11(16)15-12(13)17)9-3-5-10(14)6-4-9/h3-6H,2,7-8,14H2,1H3,(H,15,16,17) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PubMed
| n/a | n/a | 1.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of human placental cytochrome P450 19A1 with androstenedione |
J Med Chem 30: 1550-4 (1987)
BindingDB Entry DOI: 10.7270/Q2GM869Q |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50024543
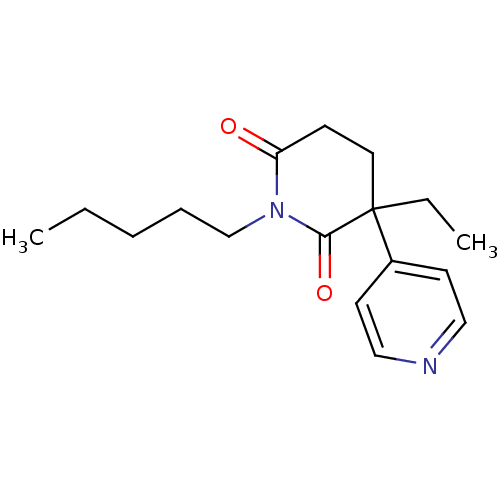
(3-Ethyl-1-pentyl-4,5-dihydro-3H-[3,4']bipyridinyl-...)Show InChI InChI=1S/C17H24N2O2/c1-3-5-6-13-19-15(20)7-10-17(4-2,16(19)21)14-8-11-18-12-9-14/h8-9,11-12H,3-7,10,13H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against human placental cytochrome P450 19A1 |
J Med Chem 30: 1550-4 (1987)
BindingDB Entry DOI: 10.7270/Q2GM869Q |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50024558
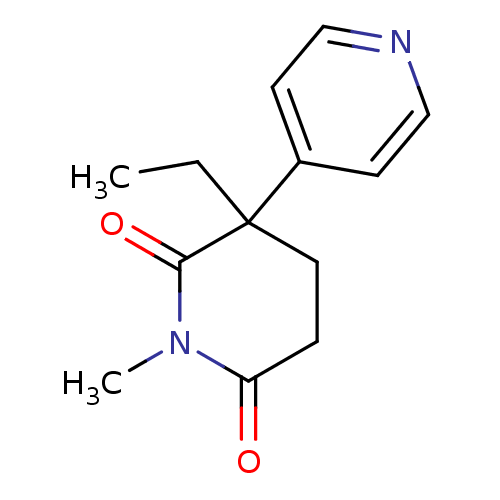
(3-Ethyl-1-methyl-4,5-dihydro-3H-[3,4']bipyridinyl-...)Show InChI InChI=1S/C13H16N2O2/c1-3-13(10-5-8-14-9-6-10)7-4-11(16)15(2)12(13)17/h5-6,8-9H,3-4,7H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against human placental cytochrome P450 19A1 |
J Med Chem 30: 1550-4 (1987)
BindingDB Entry DOI: 10.7270/Q2GM869Q |
More data for this
Ligand-Target Pair | |
Cholesterol side-chain cleavage enzyme, mitochondrial
(Bos taurus (Bovine)) | BDBM9460

(3-(4-aminophenyl)-3-ethyl-piperidine-2,6-dione | 3...)Show InChI InChI=1S/C13H16N2O2/c1-2-13(8-7-11(16)15-12(13)17)9-3-5-10(14)6-4-9/h3-6H,2,7-8,14H2,1H3,(H,15,16,17) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of bovine desmolase, cytochrome P450 11A1 |
J Med Chem 30: 1550-4 (1987)
BindingDB Entry DOI: 10.7270/Q2GM869Q |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50024545
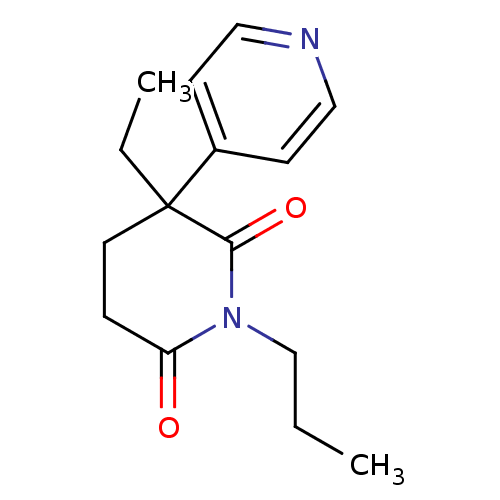
(3-Ethyl-1-propyl-4,5-dihydro-3H-[3,4']bipyridinyl-...)Show InChI InChI=1S/C15H20N2O2/c1-3-11-17-13(18)5-8-15(4-2,14(17)19)12-6-9-16-10-7-12/h6-7,9-10H,3-5,8,11H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 3.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against human placental cytochrome P450 19A1 |
J Med Chem 30: 1550-4 (1987)
BindingDB Entry DOI: 10.7270/Q2GM869Q |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50024554
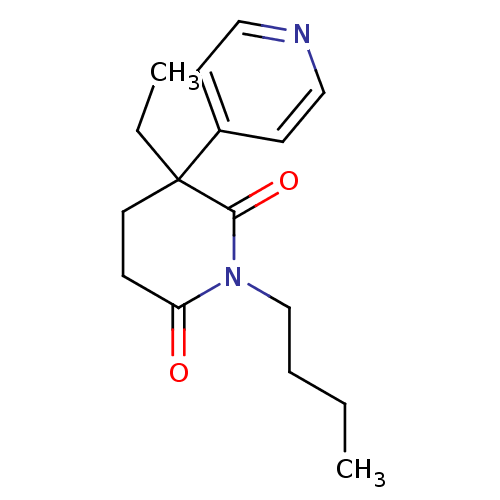
(1-Butyl-3-ethyl-4,5-dihydro-3H-[3,4']bipyridinyl-2...)Show InChI InChI=1S/C16H22N2O2/c1-3-5-12-18-14(19)6-9-16(4-2,15(18)20)13-7-10-17-11-8-13/h7-8,10-11H,3-6,9,12H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against human placental cytochrome P450 19A1 |
J Med Chem 30: 1550-4 (1987)
BindingDB Entry DOI: 10.7270/Q2GM869Q |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50015985

(3-Ethyl-4,5-dihydro-3H-[3,4']bipyridinyl-2,6-dione...)Show InChI InChI=1S/C12H14N2O2/c1-2-12(9-4-7-13-8-5-9)6-3-10(15)14-11(12)16/h4-5,7-8H,2-3,6H2,1H3,(H,14,15,16) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 4.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of human placental cytochrome P450 19A1 with testosterone |
J Med Chem 30: 1550-4 (1987)
BindingDB Entry DOI: 10.7270/Q2GM869Q |
More data for this
Ligand-Target Pair | |
Cholesterol side-chain cleavage enzyme, mitochondrial
(Bos taurus (Bovine)) | BDBM50015983

((S)-3-Ethyl-1-octyl-4,5-dihydro-3H-[3,4']bipyridin...)Show InChI InChI=1S/C20H30N2O2/c1-3-5-6-7-8-9-16-22-18(23)10-13-20(4-2,19(22)24)17-11-14-21-15-12-17/h11-12,14-15H,3-10,13,16H2,1-2H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.10E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of bovine desmolase, cytochrome P450 11A1 |
J Med Chem 30: 1550-4 (1987)
BindingDB Entry DOI: 10.7270/Q2GM869Q |
More data for this
Ligand-Target Pair | |
Cholesterol side-chain cleavage enzyme, mitochondrial
(Bos taurus (Bovine)) | BDBM50024553

(3-Ethyl-1-nonyl-4,5-dihydro-3H-[3,4']bipyridinyl-2...)Show InChI InChI=1S/C21H32N2O2/c1-3-5-6-7-8-9-10-17-23-19(24)11-14-21(4-2,20(23)25)18-12-15-22-16-13-18/h12-13,15-16H,3-11,14,17H2,1-2H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.75E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of bovine desmolase, cytochrome P450 11A1 |
J Med Chem 30: 1550-4 (1987)
BindingDB Entry DOI: 10.7270/Q2GM869Q |
More data for this
Ligand-Target Pair | |
Cholesterol side-chain cleavage enzyme, mitochondrial
(Bos taurus (Bovine)) | BDBM50024543

(3-Ethyl-1-pentyl-4,5-dihydro-3H-[3,4']bipyridinyl-...)Show InChI InChI=1S/C17H24N2O2/c1-3-5-6-13-19-15(20)7-10-17(4-2,16(19)21)14-8-11-18-12-9-14/h8-9,11-12H,3-7,10,13H2,1-2H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2.09E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of bovine desmolase, cytochrome P450 11A1 |
J Med Chem 30: 1550-4 (1987)
BindingDB Entry DOI: 10.7270/Q2GM869Q |
More data for this
Ligand-Target Pair | |
Cholesterol side-chain cleavage enzyme, mitochondrial
(Bos taurus (Bovine)) | BDBM50024550

(3-Ethyl-1-heptyl-4,5-dihydro-3H-[3,4']bipyridinyl-...)Show InChI InChI=1S/C19H28N2O2/c1-3-5-6-7-8-15-21-17(22)9-12-19(4-2,18(21)23)16-10-13-20-14-11-16/h10-11,13-14H,3-9,12,15H2,1-2H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2.10E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of bovine desmolase, cytochrome P450 11A1 |
J Med Chem 30: 1550-4 (1987)
BindingDB Entry DOI: 10.7270/Q2GM869Q |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50024551
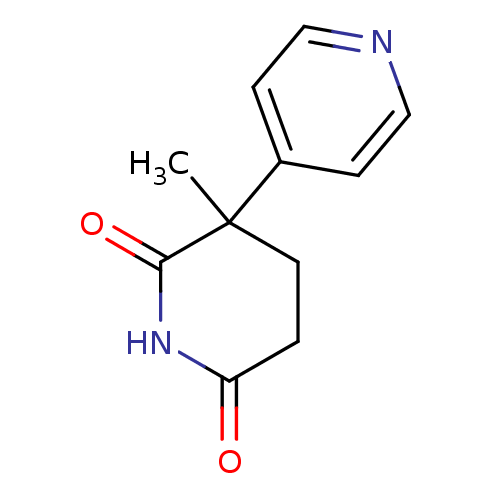
(3-Methyl-4,5-dihydro-3H-[3,4']bipyridinyl-2,6-dion...)Show InChI InChI=1S/C11H12N2O2/c1-11(8-3-6-12-7-4-8)5-2-9(14)13-10(11)15/h3-4,6-7H,2,5H2,1H3,(H,13,14,15) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2.45E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against human placental cytochrome P450 19A1 |
J Med Chem 30: 1550-4 (1987)
BindingDB Entry DOI: 10.7270/Q2GM869Q |
More data for this
Ligand-Target Pair | |
Cholesterol side-chain cleavage enzyme, mitochondrial
(Bos taurus (Bovine)) | BDBM50024549

(1-Decyl-3-ethyl-4,5-dihydro-3H-[3,4']bipyridinyl-2...)Show InChI InChI=1S/C22H34N2O2/c1-3-5-6-7-8-9-10-11-18-24-20(25)12-15-22(4-2,21(24)26)19-13-16-23-17-14-19/h13-14,16-17H,3-12,15,18H2,1-2H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 3.40E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of bovine desmolase, cytochrome P450 11A1 |
J Med Chem 30: 1550-4 (1987)
BindingDB Entry DOI: 10.7270/Q2GM869Q |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50024552
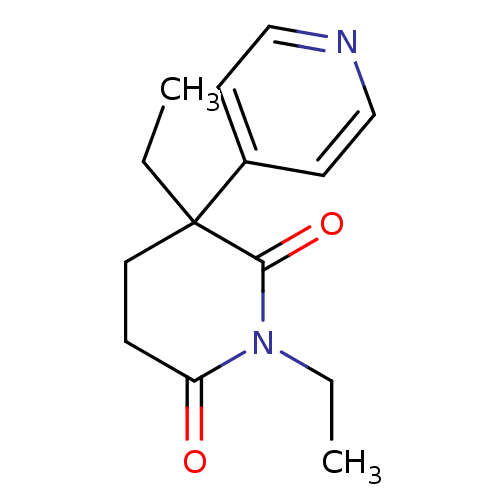
(1,3-Diethyl-4,5-dihydro-3H-[3,4']bipyridinyl-2,6-d...)Show InChI InChI=1S/C14H18N2O2/c1-3-14(11-6-9-15-10-7-11)8-5-12(17)16(4-2)13(14)18/h6-7,9-10H,3-5,8H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 3.85E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against human placental cytochrome P450 19A1 |
J Med Chem 30: 1550-4 (1987)
BindingDB Entry DOI: 10.7270/Q2GM869Q |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data