 Found 37 hits with Last Name = 'lewandowicz' and Initial = 'a'
Found 37 hits with Last Name = 'lewandowicz' and Initial = 'a' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Purine nucleoside phosphorylase
(Homo sapiens (Human)) | BDBM50135920
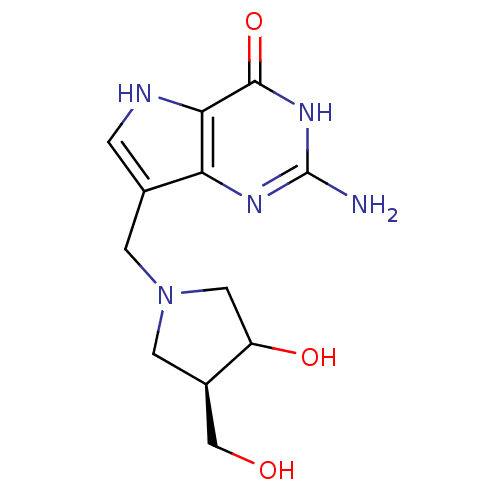
(2-Amino-7-(3-hydroxy-4-hydroxymethyl-pyrrolidin-1-...)Show SMILES Nc1nc2c(CN3CC(O)[C@@H](CO)C3)c[nH]c2c(=O)[nH]1 Show InChI InChI=1S/C12H17N5O3/c13-12-15-9-6(1-14-10(9)11(20)16-12)2-17-3-7(5-18)8(19)4-17/h1,7-8,14,18-19H,2-5H2,(H3,13,15,16,20)/t7-,8?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.00680 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Industrial Research Limited
Curated by ChEMBL
| Assay Description
Dissociation constant against Human Purine Nucleoside Phosphorylase was reported |
J Med Chem 46: 5271-6 (2003)
Article DOI: 10.1021/jm030305z
BindingDB Entry DOI: 10.7270/Q23N2449 |
More data for this
Ligand-Target Pair | |
Purine nucleoside phosphorylase
(Homo sapiens (Human)) | BDBM22109
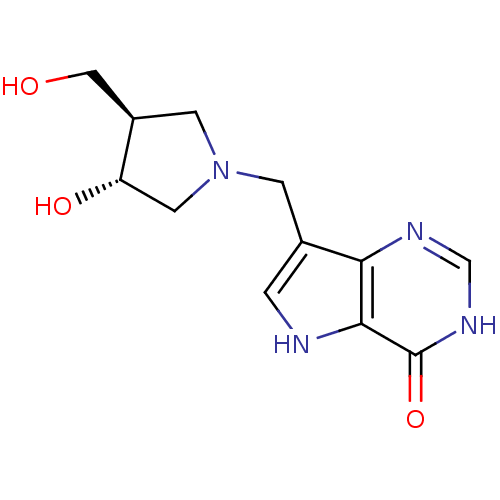
(7-{[(3R,4R)-3-hydroxy-4-(hydroxymethyl)pyrrolidin-...)Show SMILES OC[C@H]1CN(Cc2c[nH]c3c2nc[nH]c3=O)C[C@@H]1O |r| Show InChI InChI=1S/C12H16N4O3/c17-5-8-3-16(4-9(8)18)2-7-1-13-11-10(7)14-6-15-12(11)19/h1,6,8-9,13,17-18H,2-5H2,(H,14,15,19)/t8-,9+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| MMDB
PDB
Article
PubMed
| 0.0160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Industrial Research Limited
Curated by ChEMBL
| Assay Description
Dissociation constant against Human Purine Nucleoside Phosphorylase was reported |
J Med Chem 46: 5271-6 (2003)
Article DOI: 10.1021/jm030305z
BindingDB Entry DOI: 10.7270/Q23N2449 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Purine nucleoside phosphorylase
(Homo sapiens (Human)) | BDBM50293086
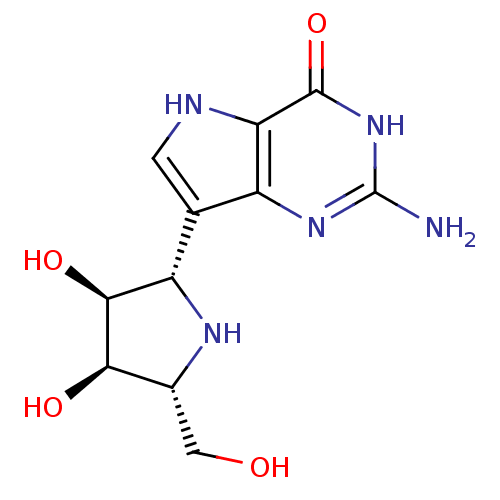
((1S)-1,4-dideoxy-1,4-imino-1-(9-deazaguanin-9-yl)-...)Show SMILES Nc1nc2c(c[nH]c2c(=O)[nH]1)[C@@H]1N[C@H](CO)[C@@H](O)[C@H]1O |r| Show InChI InChI=1S/C11H15N5O4/c12-11-15-5-3(1-13-7(5)10(20)16-11)6-9(19)8(18)4(2-17)14-6/h1,4,6,8-9,13-14,17-19H,2H2,(H3,12,15,16,20)/t4-,6+,8-,9+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
Article
PubMed
| 0.0420 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Industrial Research Limited
Curated by ChEMBL
| Assay Description
Dissociation constant against Human Purine Nucleoside Phosphorylase was reported |
J Med Chem 46: 5271-6 (2003)
Article DOI: 10.1021/jm030305z
BindingDB Entry DOI: 10.7270/Q23N2449 |
More data for this
Ligand-Target Pair | |
Purine nucleoside phosphorylase
(Homo sapiens (Human)) | BDBM50293086

((1S)-1,4-dideoxy-1,4-imino-1-(9-deazaguanin-9-yl)-...)Show SMILES Nc1nc2c(c[nH]c2c(=O)[nH]1)[C@@H]1N[C@H](CO)[C@@H](O)[C@H]1O |r| Show InChI InChI=1S/C11H15N5O4/c12-11-15-5-3(1-13-7(5)10(20)16-11)6-9(19)8(18)4(2-17)14-6/h1,4,6,8-9,13-14,17-19H,2H2,(H3,12,15,16,20)/t4-,6+,8-,9+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
Article
PubMed
| 0.0420 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Industrial Research Limited
Curated by ChEMBL
| Assay Description
Equilibrium dissociation constant determined against human purine nucleoside phosphorylase (PNP) after slow-onset inhibition |
J Med Chem 46: 3412-23 (2003)
Article DOI: 10.1021/jm030145r
BindingDB Entry DOI: 10.7270/Q2X92C29 |
More data for this
Ligand-Target Pair | |
Purine nucleoside phosphorylase
(Homo sapiens (Human)) | BDBM50195587

(1,4-DIDEOXY-4-AZA-1-(S)-(9-DEAZAHYPOXANTHIN-9-YL)-...)Show SMILES OC[C@H]1N[C@H]([C@H](O)[C@@H]1O)c1c[nH]c2c1nc[nH]c2=O |r| Show InChI InChI=1S/C11H14N4O4/c16-2-5-9(17)10(18)7(15-5)4-1-12-8-6(4)13-3-14-11(8)19/h1,3,5,7,9-10,12,15-18H,2H2,(H,13,14,19)/t5-,7+,9-,10+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| 0.0560 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Industrial Research Limited
Curated by ChEMBL
| Assay Description
Dissociation constant against Human Purine Nucleoside Phosphorylase was reported |
J Med Chem 46: 5271-6 (2003)
Article DOI: 10.1021/jm030305z
BindingDB Entry DOI: 10.7270/Q23N2449 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Purine nucleoside phosphorylase
(Homo sapiens (Human)) | BDBM50195587

(1,4-DIDEOXY-4-AZA-1-(S)-(9-DEAZAHYPOXANTHIN-9-YL)-...)Show SMILES OC[C@H]1N[C@H]([C@H](O)[C@@H]1O)c1c[nH]c2c1nc[nH]c2=O |r| Show InChI InChI=1S/C11H14N4O4/c16-2-5-9(17)10(18)7(15-5)4-1-12-8-6(4)13-3-14-11(8)19/h1,3,5,7,9-10,12,15-18H,2H2,(H,13,14,19)/t5-,7+,9-,10+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| 0.0560 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Industrial Research Limited
Curated by ChEMBL
| Assay Description
Equilibrium dissociation constant determined against human purine nucleoside phosphorylase (PNP) after slow-onset inhibition |
J Med Chem 46: 3412-23 (2003)
Article DOI: 10.1021/jm030145r
BindingDB Entry DOI: 10.7270/Q2X92C29 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Purine nucleoside phosphorylase
(Homo sapiens (Human)) | BDBM50135920

(2-Amino-7-(3-hydroxy-4-hydroxymethyl-pyrrolidin-1-...)Show SMILES Nc1nc2c(CN3CC(O)[C@@H](CO)C3)c[nH]c2c(=O)[nH]1 Show InChI InChI=1S/C12H17N5O3/c13-12-15-9-6(1-14-10(9)11(20)16-12)2-17-3-7(5-18)8(19)4-17/h1,7-8,14,18-19H,2-5H2,(H3,13,15,16,20)/t7-,8?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.163 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Industrial Research Limited
Curated by ChEMBL
| Assay Description
Binding affinity towards Human Purine Nucleoside Phosphorylase was reported |
J Med Chem 46: 5271-6 (2003)
Article DOI: 10.1021/jm030305z
BindingDB Entry DOI: 10.7270/Q23N2449 |
More data for this
Ligand-Target Pair | |
Purine nucleoside phosphorylase
(Homo sapiens (Human)) | BDBM50422435
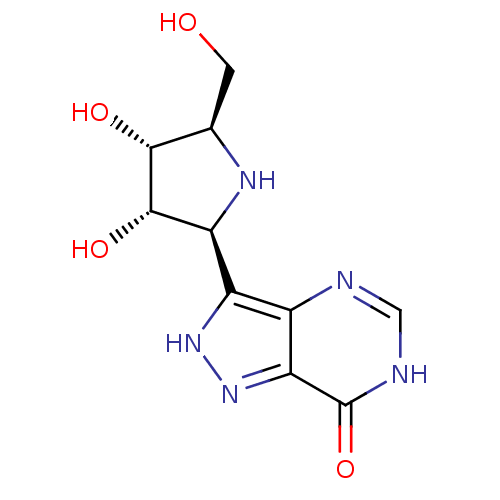
(CHEMBL2311112)Show SMILES OC[C@H]1N[C@H]([C@H](O)[C@@H]1O)c1[nH]nc2c1nc[nH]c2=O Show InChI InChI=1S/C10H13N5O4/c16-1-3-8(17)9(18)6(13-3)5-4-7(15-14-5)10(19)12-2-11-4/h2-3,6,8-9,13,16-18H,1H2,(H,14,15)(H,11,12,19)/t3-,6+,8-,9+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Industrial Research Limited
Curated by ChEMBL
| Assay Description
Equilibrium dissociation constant determined against human purine nucleoside phosphorylase (PNP) after slow-onset inhibition |
J Med Chem 46: 3412-23 (2003)
Article DOI: 10.1021/jm030145r
BindingDB Entry DOI: 10.7270/Q2X92C29 |
More data for this
Ligand-Target Pair | |
Purine nucleoside phosphorylase
(Homo sapiens (Human)) | BDBM50422435

(CHEMBL2311112)Show SMILES OC[C@H]1N[C@H]([C@H](O)[C@@H]1O)c1[nH]nc2c1nc[nH]c2=O Show InChI InChI=1S/C10H13N5O4/c16-1-3-8(17)9(18)6(13-3)5-4-7(15-14-5)10(19)12-2-11-4/h2-3,6,8-9,13,16-18H,1H2,(H,14,15)(H,11,12,19)/t3-,6+,8-,9+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Industrial Research Limited
Curated by ChEMBL
| Assay Description
Dissociation constant against Human Purine Nucleoside Phosphorylase was reported |
J Med Chem 46: 5271-6 (2003)
Article DOI: 10.1021/jm030305z
BindingDB Entry DOI: 10.7270/Q23N2449 |
More data for this
Ligand-Target Pair | |
Purine nucleoside phosphorylase
(Homo sapiens (Human)) | BDBM50370253
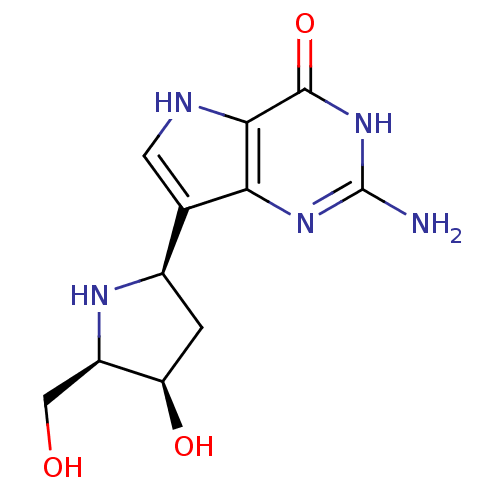
(CHEMBL114781)Show SMILES Nc1nc2c(c[nH]c2c(=O)[nH]1)[C@H]1C[C@@H](O)[C@@H](CO)N1 Show InChI InChI=1S/C11H15N5O3/c12-11-15-8-4(2-13-9(8)10(19)16-11)5-1-7(18)6(3-17)14-5/h2,5-7,13-14,17-18H,1,3H2,(H3,12,15,16,19)/t5-,6-,7-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Industrial Research Limited
Curated by ChEMBL
| Assay Description
Inhibition of human purine nucleoside phosphorylase; Initial rate. |
J Med Chem 46: 3412-23 (2003)
Article DOI: 10.1021/jm030145r
BindingDB Entry DOI: 10.7270/Q2X92C29 |
More data for this
Ligand-Target Pair | |
Purine nucleoside phosphorylase
(Homo sapiens (Human)) | BDBM50370255
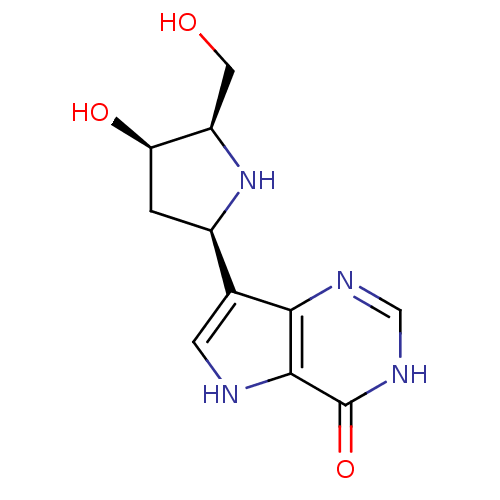
(CHEMBL115146)Show SMILES OC[C@H]1N[C@H](C[C@H]1O)c1c[nH]c2c1nc[nH]c2=O Show InChI InChI=1S/C11H14N4O3/c16-3-7-8(17)1-6(15-7)5-2-12-10-9(5)13-4-14-11(10)18/h2,4,6-8,12,15-17H,1,3H2,(H,13,14,18)/t6-,7-,8-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Industrial Research Limited
Curated by ChEMBL
| Assay Description
Inhibition of human purine nucleoside phosphorylase; Initial rate. |
J Med Chem 46: 3412-23 (2003)
Article DOI: 10.1021/jm030145r
BindingDB Entry DOI: 10.7270/Q2X92C29 |
More data for this
Ligand-Target Pair | |
Purine nucleoside phosphorylase
(Homo sapiens (Human)) | BDBM50370251
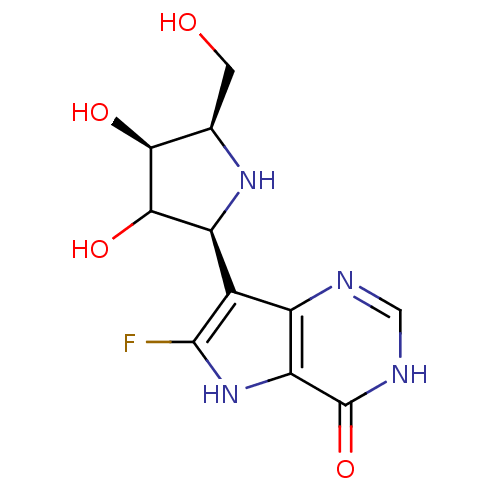
(CHEMBL542455)Show SMILES OC[C@H]1N[C@H](C(O)[C@H]1O)c1c(F)[nH]c2c1nc[nH]c2=O |r| Show InChI InChI=1S/C11H13FN4O4/c12-10-4(5-7(16-10)11(20)14-2-13-5)6-9(19)8(18)3(1-17)15-6/h2-3,6,8-9,15-19H,1H2,(H,13,14,20)/t3-,6+,8+,9?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.390 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Industrial Research Limited
Curated by ChEMBL
| Assay Description
Equilibrium dissociation constant determined against human purine nucleoside phosphorylase (PNP) after slow-onset inhibition |
J Med Chem 46: 3412-23 (2003)
Article DOI: 10.1021/jm030145r
BindingDB Entry DOI: 10.7270/Q2X92C29 |
More data for this
Ligand-Target Pair | |
Purine nucleoside phosphorylase
(Homo sapiens (Human)) | BDBM50293086

((1S)-1,4-dideoxy-1,4-imino-1-(9-deazaguanin-9-yl)-...)Show SMILES Nc1nc2c(c[nH]c2c(=O)[nH]1)[C@@H]1N[C@H](CO)[C@@H](O)[C@H]1O |r| Show InChI InChI=1S/C11H15N5O4/c12-11-15-5-3(1-13-7(5)10(20)16-11)6-9(19)8(18)4(2-17)14-6/h1,4,6,8-9,13-14,17-19H,2H2,(H3,12,15,16,20)/t4-,6+,8-,9+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
Article
PubMed
| 0.540 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Industrial Research Limited
Curated by ChEMBL
| Assay Description
Inhibition of human purine nucleoside phosphorylase; Initial rate. |
J Med Chem 46: 3412-23 (2003)
Article DOI: 10.1021/jm030145r
BindingDB Entry DOI: 10.7270/Q2X92C29 |
More data for this
Ligand-Target Pair | |
Purine nucleoside phosphorylase
(Homo sapiens (Human)) | BDBM50293086

((1S)-1,4-dideoxy-1,4-imino-1-(9-deazaguanin-9-yl)-...)Show SMILES Nc1nc2c(c[nH]c2c(=O)[nH]1)[C@@H]1N[C@H](CO)[C@@H](O)[C@H]1O |r| Show InChI InChI=1S/C11H15N5O4/c12-11-15-5-3(1-13-7(5)10(20)16-11)6-9(19)8(18)4(2-17)14-6/h1,4,6,8-9,13-14,17-19H,2H2,(H3,12,15,16,20)/t4-,6+,8-,9+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
Article
PubMed
| 0.540 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Industrial Research Limited
Curated by ChEMBL
| Assay Description
Binding affinity towards Human Purine Nucleoside Phosphorylase was reported |
J Med Chem 46: 5271-6 (2003)
Article DOI: 10.1021/jm030305z
BindingDB Entry DOI: 10.7270/Q23N2449 |
More data for this
Ligand-Target Pair | |
Purine nucleoside phosphorylase
(Homo sapiens (Human)) | BDBM22109

(7-{[(3R,4R)-3-hydroxy-4-(hydroxymethyl)pyrrolidin-...)Show SMILES OC[C@H]1CN(Cc2c[nH]c3c2nc[nH]c3=O)C[C@@H]1O |r| Show InChI InChI=1S/C12H16N4O3/c17-5-8-3-16(4-9(8)18)2-7-1-13-11-10(7)14-6-15-12(11)19/h1,6,8-9,13,17-18H,2-5H2,(H,14,15,19)/t8-,9+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| MMDB
PDB
Article
PubMed
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Industrial Research Limited
Curated by ChEMBL
| Assay Description
Binding affinity towards Human Purine Nucleoside Phosphorylase was reported |
J Med Chem 46: 5271-6 (2003)
Article DOI: 10.1021/jm030305z
BindingDB Entry DOI: 10.7270/Q23N2449 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Purine nucleoside phosphorylase
(Homo sapiens (Human)) | BDBM50370256
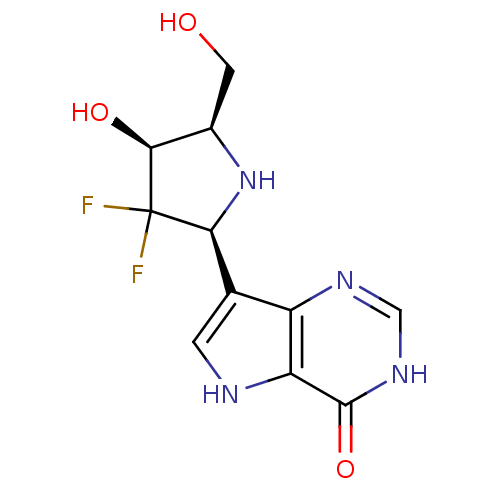
(CHEMBL541578)Show SMILES OC[C@H]1N[C@@H](c2c[nH]c3c2nc[nH]c3=O)C(F)(F)[C@H]1O |r| Show InChI InChI=1S/C11H12F2N4O3/c12-11(13)8(17-5(2-18)9(11)19)4-1-14-7-6(4)15-3-16-10(7)20/h1,3,5,8-9,14,17-19H,2H2,(H,15,16,20)/t5-,8+,9+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Industrial Research Limited
Curated by ChEMBL
| Assay Description
Inhibition of human purine nucleoside phosphorylase; Initial rate. |
J Med Chem 46: 3412-23 (2003)
Article DOI: 10.1021/jm030145r
BindingDB Entry DOI: 10.7270/Q2X92C29 |
More data for this
Ligand-Target Pair | |
Purine nucleoside phosphorylase
(Homo sapiens (Human)) | BDBM50422435

(CHEMBL2311112)Show SMILES OC[C@H]1N[C@H]([C@H](O)[C@@H]1O)c1[nH]nc2c1nc[nH]c2=O Show InChI InChI=1S/C10H13N5O4/c16-1-3-8(17)9(18)6(13-3)5-4-7(15-14-5)10(19)12-2-11-4/h2-3,6,8-9,13,16-18H,1H2,(H,14,15)(H,11,12,19)/t3-,6+,8-,9+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Industrial Research Limited
Curated by ChEMBL
| Assay Description
Binding affinity towards Human Purine Nucleoside Phosphorylase was reported |
J Med Chem 46: 5271-6 (2003)
Article DOI: 10.1021/jm030305z
BindingDB Entry DOI: 10.7270/Q23N2449 |
More data for this
Ligand-Target Pair | |
Purine nucleoside phosphorylase
(Homo sapiens (Human)) | BDBM50422435

(CHEMBL2311112)Show SMILES OC[C@H]1N[C@H]([C@H](O)[C@@H]1O)c1[nH]nc2c1nc[nH]c2=O Show InChI InChI=1S/C10H13N5O4/c16-1-3-8(17)9(18)6(13-3)5-4-7(15-14-5)10(19)12-2-11-4/h2-3,6,8-9,13,16-18H,1H2,(H,14,15)(H,11,12,19)/t3-,6+,8-,9+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Industrial Research Limited
Curated by ChEMBL
| Assay Description
Inhibition of human purine nucleoside phosphorylase; Initial rate. |
J Med Chem 46: 3412-23 (2003)
Article DOI: 10.1021/jm030145r
BindingDB Entry DOI: 10.7270/Q2X92C29 |
More data for this
Ligand-Target Pair | |
Purine nucleoside phosphorylase
(Homo sapiens (Human)) | BDBM50135919

(3-(3-Hydroxy-4-hydroxymethyl-pyrrolidin-1-ylmethyl...)Show InChI InChI=1S/C11H15N5O3/c17-4-6-1-16(3-8(6)18)2-7-9-10(15-14-7)11(19)13-5-12-9/h5-6,8,17-18H,1-4H2,(H,14,15)(H,12,13,19)/t6-,8?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Industrial Research Limited
Curated by ChEMBL
| Assay Description
Binding affinity towards Human Purine Nucleoside Phosphorylase was reported |
J Med Chem 46: 5271-6 (2003)
Article DOI: 10.1021/jm030305z
BindingDB Entry DOI: 10.7270/Q23N2449 |
More data for this
Ligand-Target Pair | |
Purine nucleoside phosphorylase
(Homo sapiens (Human)) | BDBM50370254

(CHEMBL543401)Show SMILES OC[C@@H]1[C@H](O)C(O)CN1Cc1c[nH]c2c1nc[nH]c2=O |r| Show InChI InChI=1S/C12H16N4O4/c17-4-7-11(19)8(18)3-16(7)2-6-1-13-10-9(6)14-5-15-12(10)20/h1,5,7-8,11,13,17-19H,2-4H2,(H,14,15,20)/t7-,8?,11+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.84 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Industrial Research Limited
Curated by ChEMBL
| Assay Description
Inhibition of human purine nucleoside phosphorylase; Initial rate. |
J Med Chem 46: 3412-23 (2003)
Article DOI: 10.1021/jm030145r
BindingDB Entry DOI: 10.7270/Q2X92C29 |
More data for this
Ligand-Target Pair | |
Purine nucleoside phosphorylase
(Homo sapiens (Human)) | BDBM50195587

(1,4-DIDEOXY-4-AZA-1-(S)-(9-DEAZAHYPOXANTHIN-9-YL)-...)Show SMILES OC[C@H]1N[C@H]([C@H](O)[C@@H]1O)c1c[nH]c2c1nc[nH]c2=O |r| Show InChI InChI=1S/C11H14N4O4/c16-2-5-9(17)10(18)7(15-5)4-1-12-8-6(4)13-3-14-11(8)19/h1,3,5,7,9-10,12,15-18H,2H2,(H,13,14,19)/t5-,7+,9-,10+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| 3.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Industrial Research Limited
Curated by ChEMBL
| Assay Description
Inhibition of human purine nucleoside phosphorylase; Initial rate. |
J Med Chem 46: 3412-23 (2003)
Article DOI: 10.1021/jm030145r
BindingDB Entry DOI: 10.7270/Q2X92C29 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Purine nucleoside phosphorylase
(Homo sapiens (Human)) | BDBM50195587

(1,4-DIDEOXY-4-AZA-1-(S)-(9-DEAZAHYPOXANTHIN-9-YL)-...)Show SMILES OC[C@H]1N[C@H]([C@H](O)[C@@H]1O)c1c[nH]c2c1nc[nH]c2=O |r| Show InChI InChI=1S/C11H14N4O4/c16-2-5-9(17)10(18)7(15-5)4-1-12-8-6(4)13-3-14-11(8)19/h1,3,5,7,9-10,12,15-18H,2H2,(H,13,14,19)/t5-,7+,9-,10+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| 3.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Industrial Research Limited
Curated by ChEMBL
| Assay Description
Binding affinity towards Human Purine Nucleoside Phosphorylase was reported |
J Med Chem 46: 5271-6 (2003)
Article DOI: 10.1021/jm030305z
BindingDB Entry DOI: 10.7270/Q23N2449 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Purine nucleoside phosphorylase
(Homo sapiens (Human)) | BDBM50294725

(7-((2S,3R,4R,5R)-3,4-dihydroxy-5-(hydroxymethyl)py...)Show SMILES OC[C@H]1N[C@H]([C@@H](O)[C@@H]1O)c1c[nH]c2c1nc[nH]c2=O |r| Show InChI InChI=1S/C11H14N4O4/c16-2-5-9(17)10(18)7(15-5)4-1-12-8-6(4)13-3-14-11(8)19/h1,3,5,7,9-10,12,15-18H,2H2,(H,13,14,19)/t5-,7+,9-,10-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Industrial Research Limited
Curated by ChEMBL
| Assay Description
Inhibition of human purine nucleoside phosphorylase; Initial rate. |
J Med Chem 46: 3412-23 (2003)
Article DOI: 10.1021/jm030145r
BindingDB Entry DOI: 10.7270/Q2X92C29 |
More data for this
Ligand-Target Pair | |
Purine nucleoside phosphorylase
(Homo sapiens (Human)) | BDBM50370243

(CHEMBL543164)Show SMILES Cn1cc([C@@H]2N[C@H](CO)[C@H](O)C2O)c2nc[nH]c(=O)c12 |r| Show InChI InChI=1S/C12H16N4O4/c1-16-2-5(7-9(16)12(20)14-4-13-7)8-11(19)10(18)6(3-17)15-8/h2,4,6,8,10-11,15,17-19H,3H2,1H3,(H,13,14,20)/t6-,8+,10+,11?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Industrial Research Limited
Curated by ChEMBL
| Assay Description
Inhibition of human purine nucleoside phosphorylase; Initial rate. |
J Med Chem 46: 3412-23 (2003)
Article DOI: 10.1021/jm030145r
BindingDB Entry DOI: 10.7270/Q2X92C29 |
More data for this
Ligand-Target Pair | |
Purine nucleoside phosphorylase
(Homo sapiens (Human)) | BDBM50370245
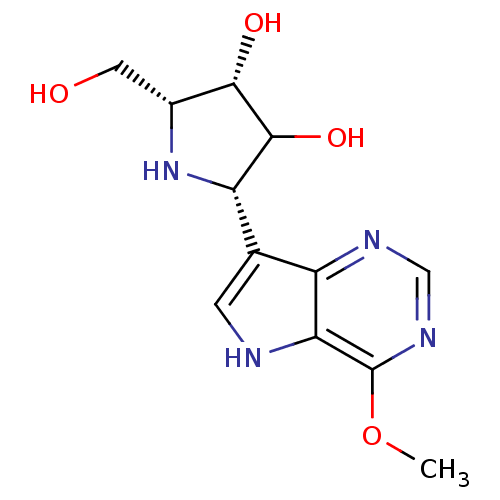
(CHEMBL544117)Show SMILES COc1ncnc2c(c[nH]c12)[C@@H]1N[C@H](CO)[C@H](O)C1O |r| Show InChI InChI=1S/C12H16N4O4/c1-20-12-9-7(14-4-15-12)5(2-13-9)8-11(19)10(18)6(3-17)16-8/h2,4,6,8,10-11,13,16-19H,3H2,1H3/t6-,8+,10+,11?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Industrial Research Limited
Curated by ChEMBL
| Assay Description
Inhibition of human purine nucleoside phosphorylase; Initial rate. |
J Med Chem 46: 3412-23 (2003)
Article DOI: 10.1021/jm030145r
BindingDB Entry DOI: 10.7270/Q2X92C29 |
More data for this
Ligand-Target Pair | |
Purine nucleoside phosphorylase
(Homo sapiens (Human)) | BDBM50370251

(CHEMBL542455)Show SMILES OC[C@H]1N[C@H](C(O)[C@H]1O)c1c(F)[nH]c2c1nc[nH]c2=O |r| Show InChI InChI=1S/C11H13FN4O4/c12-10-4(5-7(16-10)11(20)14-2-13-5)6-9(19)8(18)3(1-17)15-6/h2-3,6,8-9,15-19H,1H2,(H,13,14,20)/t3-,6+,8+,9?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Industrial Research Limited
Curated by ChEMBL
| Assay Description
Inhibition of human purine nucleoside phosphorylase; Initial rate. |
J Med Chem 46: 3412-23 (2003)
Article DOI: 10.1021/jm030145r
BindingDB Entry DOI: 10.7270/Q2X92C29 |
More data for this
Ligand-Target Pair | |
Purine nucleoside phosphorylase
(Homo sapiens (Human)) | BDBM50370246

(CHEMBL325862)Show SMILES COC1[C@@H](O)[C@@H](CO)N[C@H]1c1c[nH]c2c1nc[nH]c2=O Show InChI InChI=1S/C12H16N4O4/c1-20-11-8(16-6(3-17)10(11)18)5-2-13-9-7(5)14-4-15-12(9)19/h2,4,6,8,10-11,13,16-18H,3H2,1H3,(H,14,15,19)/t6-,8+,10+,11?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Industrial Research Limited
Curated by ChEMBL
| Assay Description
Inhibition of human purine nucleoside phosphorylase; Initial rate. |
J Med Chem 46: 3412-23 (2003)
Article DOI: 10.1021/jm030145r
BindingDB Entry DOI: 10.7270/Q2X92C29 |
More data for this
Ligand-Target Pair | |
Purine nucleoside phosphorylase
(Homo sapiens (Human)) | BDBM50370250
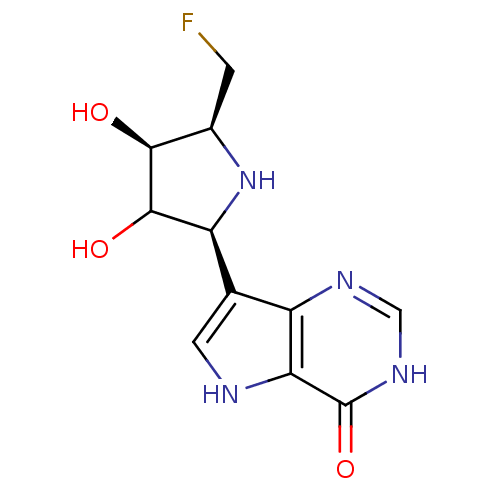
(CHEMBL117383)Show SMILES O[C@H]1[C@@H](CF)N[C@H](C1O)c1c[nH]c2c1nc[nH]c2=O Show InChI InChI=1S/C11H13FN4O3/c12-1-5-9(17)10(18)7(16-5)4-2-13-8-6(4)14-3-15-11(8)19/h2-3,5,7,9-10,13,16-18H,1H2,(H,14,15,19)/t5-,7+,9+,10?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Industrial Research Limited
Curated by ChEMBL
| Assay Description
Equilibrium dissociation constant determined against human purine nucleoside phosphorylase (PNP) after slow-onset inhibition |
J Med Chem 46: 3412-23 (2003)
Article DOI: 10.1021/jm030145r
BindingDB Entry DOI: 10.7270/Q2X92C29 |
More data for this
Ligand-Target Pair | |
Purine nucleoside phosphorylase
(Homo sapiens (Human)) | BDBM50370247
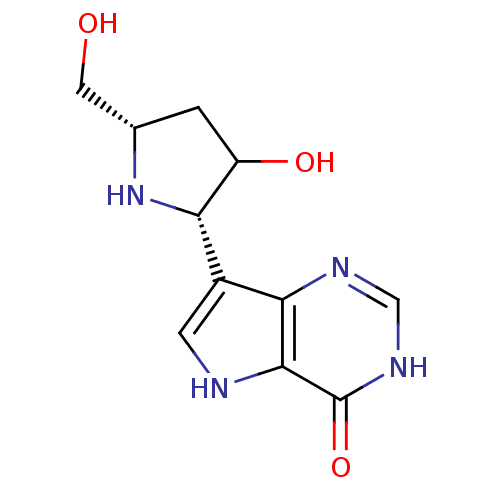
(CHEMBL538773)Show SMILES OC[C@@H]1CC(O)[C@@H](N1)c1c[nH]c2c1nc[nH]c2=O |r| Show InChI InChI=1S/C11H14N4O3/c16-3-5-1-7(17)8(15-5)6-2-12-10-9(6)13-4-14-11(10)18/h2,4-5,7-8,12,15-17H,1,3H2,(H,13,14,18)/t5-,7?,8-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 9.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Industrial Research Limited
Curated by ChEMBL
| Assay Description
Inhibition of human purine nucleoside phosphorylase; Initial rate. |
J Med Chem 46: 3412-23 (2003)
Article DOI: 10.1021/jm030145r
BindingDB Entry DOI: 10.7270/Q2X92C29 |
More data for this
Ligand-Target Pair | |
Purine nucleoside phosphorylase
(Homo sapiens (Human)) | BDBM50370237
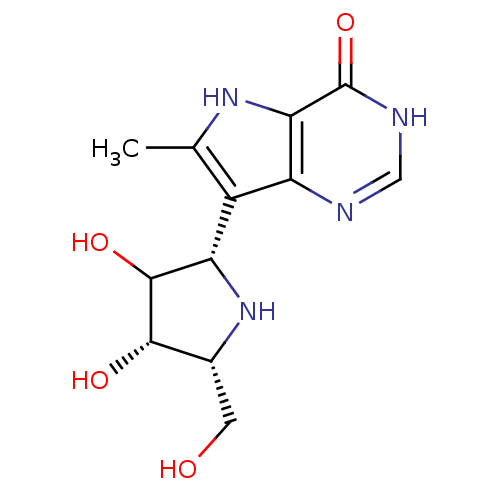
(CHEMBL114003)Show SMILES Cc1[nH]c2c(nc[nH]c2=O)c1[C@@H]1N[C@H](CO)[C@H](O)C1O Show InChI InChI=1S/C12H16N4O4/c1-4-6(7-9(15-4)12(20)14-3-13-7)8-11(19)10(18)5(2-17)16-8/h3,5,8,10-11,15-19H,2H2,1H3,(H,13,14,20)/t5-,8+,10+,11?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 10.1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Industrial Research Limited
Curated by ChEMBL
| Assay Description
Inhibition of human purine nucleoside phosphorylase; Initial rate. |
J Med Chem 46: 3412-23 (2003)
Article DOI: 10.1021/jm030145r
BindingDB Entry DOI: 10.7270/Q2X92C29 |
More data for this
Ligand-Target Pair | |
Purine nucleoside phosphorylase
(Homo sapiens (Human)) | BDBM50370244
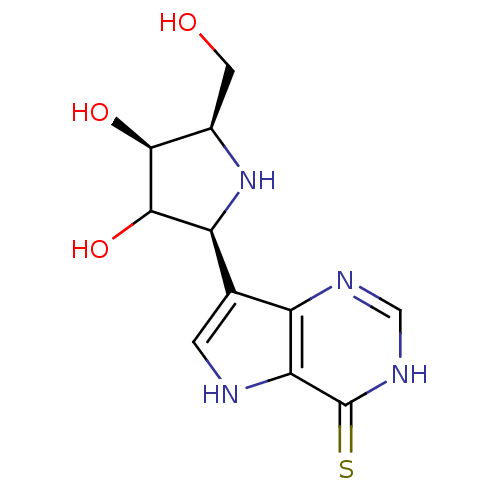
(CHEMBL555985)Show SMILES OC[C@H]1N[C@H](C(O)[C@H]1O)c1c[nH]c2c1nc[nH]c2=S |r| Show InChI InChI=1S/C11H14N4O3S/c16-2-5-9(17)10(18)7(15-5)4-1-12-8-6(4)13-3-14-11(8)19/h1,3,5,7,9-10,12,15-18H,2H2,(H,13,14,19)/t5-,7+,9+,10?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 24.7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Industrial Research Limited
Curated by ChEMBL
| Assay Description
Inhibition of human purine nucleoside phosphorylase; Initial rate. |
J Med Chem 46: 3412-23 (2003)
Article DOI: 10.1021/jm030145r
BindingDB Entry DOI: 10.7270/Q2X92C29 |
More data for this
Ligand-Target Pair | |
Purine nucleoside phosphorylase
(Homo sapiens (Human)) | BDBM50370252
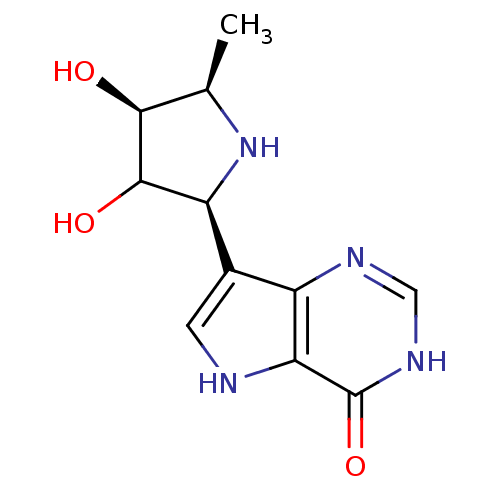
(CHEMBL117065)Show SMILES C[C@H]1N[C@H](C(O)[C@H]1O)c1c[nH]c2c1nc[nH]c2=O Show InChI InChI=1S/C11H14N4O3/c1-4-9(16)10(17)7(15-4)5-2-12-8-6(5)13-3-14-11(8)18/h2-4,7,9-10,12,15-17H,1H3,(H,13,14,18)/t4-,7+,9+,10?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Industrial Research Limited
Curated by ChEMBL
| Assay Description
Inhibition of human purine nucleoside phosphorylase; Initial rate. |
J Med Chem 46: 3412-23 (2003)
Article DOI: 10.1021/jm030145r
BindingDB Entry DOI: 10.7270/Q2X92C29 |
More data for this
Ligand-Target Pair | |
Purine nucleoside phosphorylase
(Homo sapiens (Human)) | BDBM50370250

(CHEMBL117383)Show SMILES O[C@H]1[C@@H](CF)N[C@H](C1O)c1c[nH]c2c1nc[nH]c2=O Show InChI InChI=1S/C11H13FN4O3/c12-1-5-9(17)10(18)7(16-5)4-2-13-8-6(4)14-3-15-11(8)19/h2-3,5,7,9-10,13,16-18H,1H2,(H,14,15,19)/t5-,7+,9+,10?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 81 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Industrial Research Limited
Curated by ChEMBL
| Assay Description
Inhibition of human purine nucleoside phosphorylase; Initial rate. |
J Med Chem 46: 3412-23 (2003)
Article DOI: 10.1021/jm030145r
BindingDB Entry DOI: 10.7270/Q2X92C29 |
More data for this
Ligand-Target Pair | |
Purine nucleoside phosphorylase
(Homo sapiens (Human)) | BDBM50370241

(CHEMBL542704)Show SMILES OC[C@H]1N[C@@H](Cc2c[nH]c3c2nc[nH]c3=O)C(O)[C@H]1O |r| Show InChI InChI=1S/C12H16N4O4/c17-3-7-11(19)10(18)6(16-7)1-5-2-13-9-8(5)14-4-15-12(9)20/h2,4,6-7,10-11,13,16-19H,1,3H2,(H,14,15,20)/t6-,7+,10?,11-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 250 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Industrial Research Limited
Curated by ChEMBL
| Assay Description
Inhibition of human purine nucleoside phosphorylase; Initial rate. |
J Med Chem 46: 3412-23 (2003)
Article DOI: 10.1021/jm030145r
BindingDB Entry DOI: 10.7270/Q2X92C29 |
More data for this
Ligand-Target Pair | |
Purine nucleoside phosphorylase
(Homo sapiens (Human)) | BDBM50370249
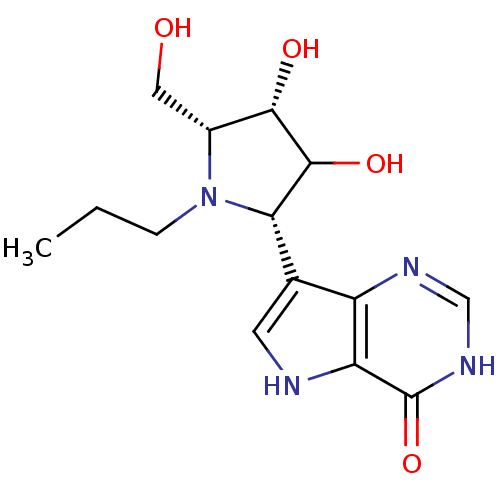
(CHEMBL542702)Show SMILES CCCN1[C@H](CO)[C@H](O)C(O)[C@@H]1c1c[nH]c2c1nc[nH]c2=O |r| Show InChI InChI=1S/C14H20N4O4/c1-2-3-18-8(5-19)12(20)13(21)11(18)7-4-15-10-9(7)16-6-17-14(10)22/h4,6,8,11-13,15,19-21H,2-3,5H2,1H3,(H,16,17,22)/t8-,11+,12+,13?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 415 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Industrial Research Limited
Curated by ChEMBL
| Assay Description
Inhibition of human purine nucleoside phosphorylase; Initial rate. |
J Med Chem 46: 3412-23 (2003)
Article DOI: 10.1021/jm030145r
BindingDB Entry DOI: 10.7270/Q2X92C29 |
More data for this
Ligand-Target Pair | |
Purine nucleoside phosphorylase
(Homo sapiens (Human)) | BDBM50370248

(CHEMBL544582)Show SMILES OC[C@@H]1[C@H](O)C(O)CN1c1c[nH]c2c1nc[nH]c2=O |r| Show InChI InChI=1S/C11H14N4O4/c16-3-6-10(18)7(17)2-15(6)5-1-12-9-8(5)13-4-14-11(9)19/h1,4,6-7,10,12,16-18H,2-3H2,(H,13,14,19)/t6-,7?,10+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 1.27E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Industrial Research Limited
Curated by ChEMBL
| Assay Description
Inhibition of human purine nucleoside phosphorylase; Initial rate. |
J Med Chem 46: 3412-23 (2003)
Article DOI: 10.1021/jm030145r
BindingDB Entry DOI: 10.7270/Q2X92C29 |
More data for this
Ligand-Target Pair | |
Purine nucleoside phosphorylase
(Homo sapiens (Human)) | BDBM50370239

(CHEMBL1743674)Show SMILES Nc1ncnc2c(C[C@@H]3N[C@H](CO)[C@H](O)C3O)c[nH]c12 |r| Show InChI InChI=1S/C12H17N5O3/c13-12-9-8(15-4-16-12)5(2-14-9)1-6-10(19)11(20)7(3-18)17-6/h2,4,6-7,10-11,14,17-20H,1,3H2,(H2,13,15,16)/t6-,7+,10?,11-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Industrial Research Limited
Curated by ChEMBL
| Assay Description
Inhibition of human purine nucleoside phosphorylase; Initial rate. |
J Med Chem 46: 3412-23 (2003)
Article DOI: 10.1021/jm030145r
BindingDB Entry DOI: 10.7270/Q2X92C29 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data