 Found 99 hits with Last Name = 'liao' and Initial = 'sy'
Found 99 hits with Last Name = 'liao' and Initial = 'sy' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Replicase polyprotein 1ab
(Human SARS coronavirus (SARS-CoV) (Severe acute re...) | BDBM11233

(N-[(benzyloxy)carbonyl]-O-(tert-butyl)-L-threonyl-...)Show SMILES C[C@H](OC(C)(C)C)[C@H](NC(=O)OCc1ccccc1)C(=O)N[C@@H](CC1CCCCC1)C(=O)N[C@@H](C[C@@H]1CCNC1=O)C=O |r| Show InChI InChI=1S/C32H48N4O7/c1-21(43-32(2,3)4)27(36-31(41)42-20-23-13-9-6-10-14-23)30(40)35-26(17-22-11-7-5-8-12-22)29(39)34-25(19-37)18-24-15-16-33-28(24)38/h6,9-10,13-14,19,21-22,24-27H,5,7-8,11-12,15-18,20H2,1-4H3,(H,33,38)(H,34,39)(H,35,40)(H,36,41)/t21-,24-,25-,26-,27-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 53 | -41.5 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
TaiGen Biotechnology Co.
| Assay Description
The effects of compound on enzyme activity were measured by using peptide cleavage assay. Cleavage products were resolved and analyzed with a reverse... |
J Med Chem 49: 4971-80 (2006)
Article DOI: 10.1021/jm0603926
BindingDB Entry DOI: 10.7270/Q24B2ZJT |
More data for this
Ligand-Target Pair | |
Replicase polyprotein 1ab
(Human SARS coronavirus (SARS-CoV) (Severe acute re...) | BDBM11232
(N-[(benzyloxy)carbonyl]-O-(tert-butyl)-L-threonyl-...)Show SMILES CCOC(=O)\C=C\[C@H](C[C@@H]1CCNC1=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@@H](NC(=O)OCc1ccccc1)C(C)OC(C)(C)C |r| Show InChI InChI=1S/C33H50N4O8/c1-8-43-27(38)15-14-25(19-24-16-17-34-29(24)39)35-30(40)26(18-21(2)3)36-31(41)28(22(4)45-33(5,6)7)37-32(42)44-20-23-12-10-9-11-13-23/h9-15,21-22,24-26,28H,8,16-20H2,1-7H3,(H,34,39)(H,35,40)(H,36,41)(H,37,42)/b15-14+/t22?,24-,25+,26-,28-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 58 | -41.3 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
TaiGen Biotechnology Co.
| Assay Description
The effects of compound on enzyme activity were measured by using peptide cleavage assay. Cleavage products were resolved and analyzed with a reverse... |
J Med Chem 49: 4971-80 (2006)
Article DOI: 10.1021/jm0603926
BindingDB Entry DOI: 10.7270/Q24B2ZJT |
More data for this
Ligand-Target Pair | |
Replicase polyprotein 1ab
(Human SARS coronavirus (SARS-CoV) (Severe acute re...) | BDBM11231

(N-[(benzyloxy)carbonyl]-L-valyl-N1-((1S,2E)-4-etho...)Show SMILES CCOC(=O)\C=C\[C@H](C[C@@H]1CCNC1=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@@H](NC(=O)OCc1ccccc1)C(C)C |r| Show InChI InChI=1S/C30H44N4O7/c1-6-40-25(35)13-12-23(17-22-14-15-31-27(22)36)32-28(37)24(16-19(2)3)33-29(38)26(20(4)5)34-30(39)41-18-21-10-8-7-9-11-21/h7-13,19-20,22-24,26H,6,14-18H2,1-5H3,(H,31,36)(H,32,37)(H,33,38)(H,34,39)/b13-12+/t22-,23+,24-,26-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 660 | -35.3 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
TaiGen Biotechnology Co.
| Assay Description
The effects of compound on enzyme activity were measured by using peptide cleavage assay. Cleavage products were resolved and analyzed with a reverse... |
J Med Chem 49: 4971-80 (2006)
Article DOI: 10.1021/jm0603926
BindingDB Entry DOI: 10.7270/Q24B2ZJT |
More data for this
Ligand-Target Pair | |
Replicase polyprotein 1ab
(Human SARS coronavirus (SARS-CoV) (Severe acute re...) | BDBM11230
(AG7088 analogue 2d | CHEMBL277716 | N-[(benzyloxy)...)Show SMILES CCOC(=O)\C=C\[C@H](C[C@@H]1CCNC1=O)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@@H](NC(=O)OCc1ccccc1)C(C)C |r| Show InChI InChI=1S/C33H42N4O7/c1-4-43-28(38)16-15-26(20-25-17-18-34-30(25)39)35-31(40)27(19-23-11-7-5-8-12-23)36-32(41)29(22(2)3)37-33(42)44-21-24-13-9-6-10-14-24/h5-16,22,25-27,29H,4,17-21H2,1-3H3,(H,34,39)(H,35,40)(H,36,41)(H,37,42)/b16-15+/t25-,26+,27-,29-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.26E+3 | -32.2 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
TaiGen Biotechnology Co.
| Assay Description
The effects of compound on enzyme activity were measured by using peptide cleavage assay. Cleavage products were resolved and analyzed with a reverse... |
J Med Chem 49: 4971-80 (2006)
Article DOI: 10.1021/jm0603926
BindingDB Entry DOI: 10.7270/Q24B2ZJT |
More data for this
Ligand-Target Pair | |
Replicase polyprotein 1ab
(Human SARS coronavirus (SARS-CoV) (Severe acute re...) | BDBM11229
(AG7088 analogue 2a | CHEMBL20636 | N-[(5-methyliso...)Show SMILES CCOC(=O)\C=C\[C@H](C[C@@H]1CCNC1=O)NC(=O)[C@H](Cc1ccc(F)cc1)NC(=O)[C@@H](NC(=O)c1cc(C)on1)C(C)C |r| Show InChI InChI=1S/C30H38FN5O7/c1-5-42-25(37)11-10-22(16-20-12-13-32-27(20)38)33-28(39)23(15-19-6-8-21(31)9-7-19)34-30(41)26(17(2)3)35-29(40)24-14-18(4)43-36-24/h6-11,14,17,20,22-23,26H,5,12-13,15-16H2,1-4H3,(H,32,38)(H,33,39)(H,34,41)(H,35,40)/b11-10+/t20-,22+,23-,26-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >1.00E+4 | >-28.5 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
TaiGen Biotechnology Co.
| Assay Description
The effects of compound on enzyme activity were measured by using peptide cleavage assay. Cleavage products were resolved and analyzed with a reverse... |
J Med Chem 49: 4971-80 (2006)
Article DOI: 10.1021/jm0603926
BindingDB Entry DOI: 10.7270/Q24B2ZJT |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50610140
(CHEMBL5266817)Show SMILES [H][C@]12CCN(c3ncnc4[nH]ccc34)[C@@]1([H])CN(CC2)C(=O)CNc1cc(Cl)cc(Cl)c1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50610140

(CHEMBL5266817)Show SMILES [H][C@]12CCN(c3ncnc4[nH]ccc34)[C@@]1([H])CN(CC2)C(=O)CNc1cc(Cl)cc(Cl)c1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50610137
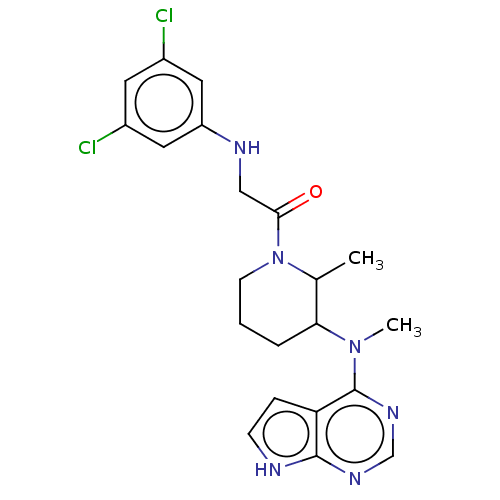
(CHEMBL5269111)Show SMILES CC1C(CCCN1C(=O)CNc1cc(Cl)cc(Cl)c1)N(C)c1ncnc2[nH]ccc12 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50610128
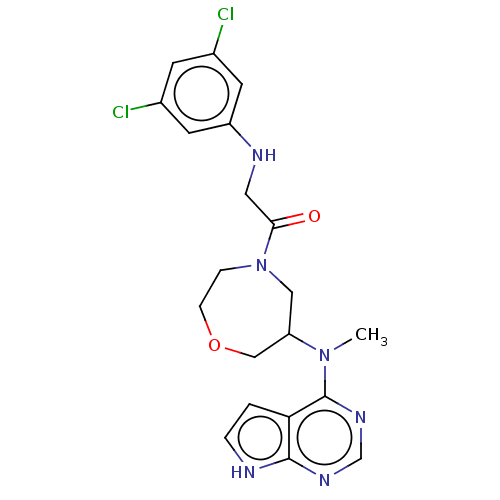
(CHEMBL5284310)Show SMILES CN(C1COCCN(C1)C(=O)CNc1cc(Cl)cc(Cl)c1)c1ncnc2[nH]ccc12 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50610136
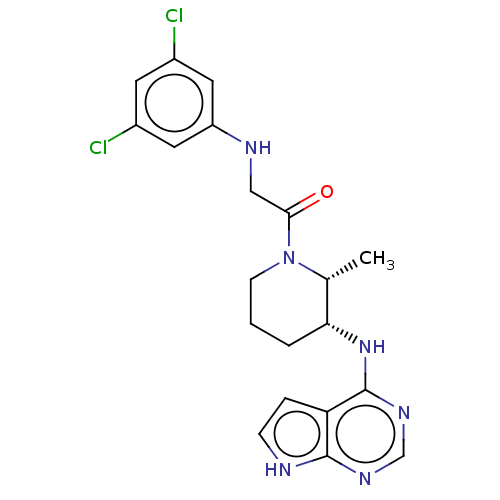
(CHEMBL5274179)Show SMILES C[C@@H]1[C@@H](CCCN1C(=O)CNc1cc(Cl)cc(Cl)c1)Nc1ncnc2[nH]ccc12 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50610139
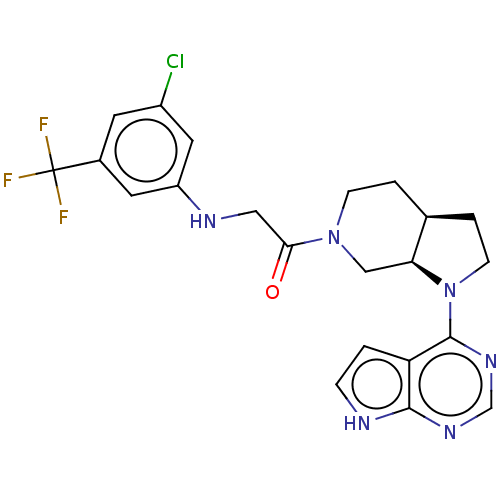
(CHEMBL5287698)Show SMILES [H][C@]12CCN(c3ncnc4[nH]ccc34)[C@@]1([H])CN(CC2)C(=O)CNc1cc(Cl)cc(c1)C(F)(F)F |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50610138

(CHEMBL5268773)Show SMILES [H][C@]12CCN(c3ncnc4[nH]ccc34)[C@@]1([H])CN(CC2)C(=O)CNc1cc(F)cc(Cl)c1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50610134
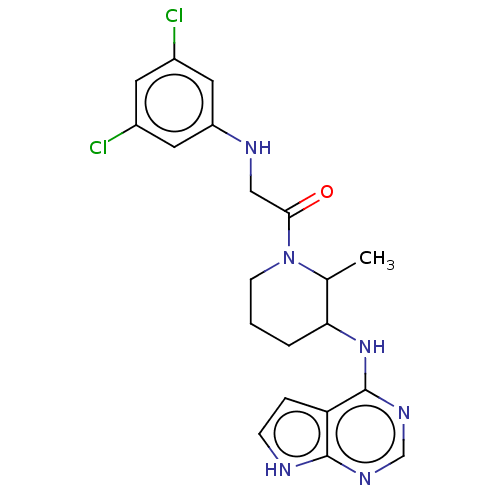
(CHEMBL5280787)Show SMILES CC1C(CCCN1C(=O)CNc1cc(Cl)cc(Cl)c1)Nc1ncnc2[nH]ccc12 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50610129
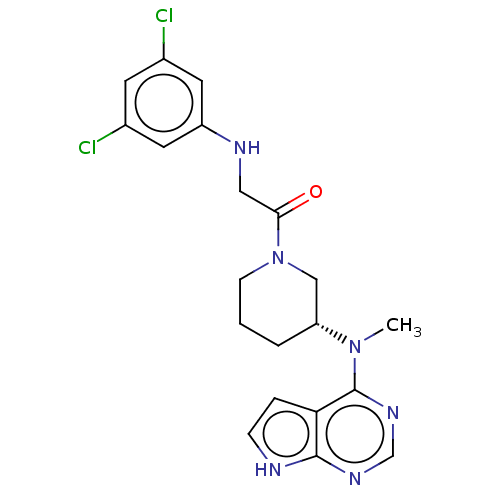
(CHEMBL5276931)Show SMILES CN([C@@H]1CCCN(C1)C(=O)CNc1cc(Cl)cc(Cl)c1)c1ncnc2[nH]ccc12 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PDB
UniChem
| PDB
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM50165323
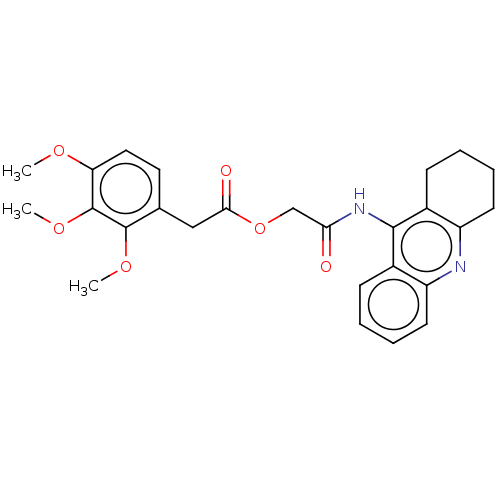
(CHEMBL3799558)Show SMILES COc1ccc(CC(=O)OCC(=O)Nc2c3CCCCc3nc3ccccc23)c(OC)c1OC Show InChI InChI=1S/C26H28N2O6/c1-31-21-13-12-16(25(32-2)26(21)33-3)14-23(30)34-15-22(29)28-24-17-8-4-6-10-19(17)27-20-11-7-5-9-18(20)24/h4,6,8,10,12-13H,5,7,9,11,14-15H2,1-3H3,(H,27,28,29) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Guangzhou Medical University
Curated by ChEMBL
| Assay Description
Inhibition of electric eel AChE using acetylthiocholine chloride as substrate preincubated for 20 mins followed by substrate addition by Ellman's met... |
Eur J Med Chem 116: 200-209 (2016)
Article DOI: 10.1016/j.ejmech.2016.03.077
BindingDB Entry DOI: 10.7270/Q2XK8HGC |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50610127
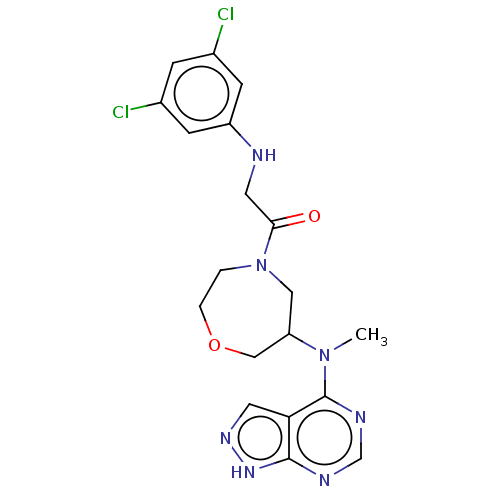
(CHEMBL5269279)Show SMILES CN(C1COCCN(C1)C(=O)CNc1cc(Cl)cc(Cl)c1)c1ncnc2[nH]ncc12 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM50165358

(CHEMBL3799641)Show SMILES O=C(COC(=O)Cc1c[nH]c2ccccc12)Nc1c2CCCCc2nc2ccccc12 Show InChI InChI=1S/C25H23N3O3/c29-23(15-31-24(30)13-16-14-26-20-10-4-1-7-17(16)20)28-25-18-8-2-5-11-21(18)27-22-12-6-3-9-19(22)25/h1-2,4-5,7-8,10-11,14,26H,3,6,9,12-13,15H2,(H,27,28,29) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Guangzhou Medical University
Curated by ChEMBL
| Assay Description
Inhibition of electric eel AChE using acetylthiocholine chloride as substrate preincubated for 20 mins followed by substrate addition by Ellman's met... |
Eur J Med Chem 116: 200-209 (2016)
Article DOI: 10.1016/j.ejmech.2016.03.077
BindingDB Entry DOI: 10.7270/Q2XK8HGC |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM50165334
(CHEMBL3797363)Show SMILES COc1cc(cc(OC)c1OC)C(=O)OCC(=O)Nc1c2CCCCc2nc2ccccc12 Show InChI InChI=1S/C25H26N2O6/c1-30-20-12-15(13-21(31-2)24(20)32-3)25(29)33-14-22(28)27-23-16-8-4-6-10-18(16)26-19-11-7-5-9-17(19)23/h4,6,8,10,12-13H,5,7,9,11,14H2,1-3H3,(H,26,27,28) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Guangzhou Medical University
Curated by ChEMBL
| Assay Description
Inhibition of electric eel AChE using acetylthiocholine chloride as substrate preincubated for 20 mins followed by substrate addition by Ellman's met... |
Eur J Med Chem 116: 200-209 (2016)
Article DOI: 10.1016/j.ejmech.2016.03.077
BindingDB Entry DOI: 10.7270/Q2XK8HGC |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50610126
(CHEMBL5266943)Show SMILES CN(C1CCCCN(C1)C(=O)CNc1cc(Cl)cc(Cl)c1)c1ncnc2[nH]ccc12 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PDB
UniChem
| PDB
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Amyloid-beta precursor protein
(Homo sapiens (Human)) | BDBM8961

(1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...)Show InChI InChI=1S/C13H14N2/c14-13-9-5-1-3-7-11(9)15-12-8-4-2-6-10(12)13/h1,3,5,7H,2,4,6,8H2,(H2,14,15) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Guangzhou Medical University
Curated by ChEMBL
| Assay Description
Inhibition of self-mediated amyloid beta (1 to 42) (unknown origin) aggregation after 24 hrs by thioflavin T fluorescence assay |
Eur J Med Chem 116: 200-209 (2016)
Article DOI: 10.1016/j.ejmech.2016.03.077
BindingDB Entry DOI: 10.7270/Q2XK8HGC |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50588008
(CHEMBL5196798)Show SMILES Clc1cc(Cl)cc(NCC(=O)NC2CCCN(C2)c2ncnc3[nH]ccc23)c1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Cholinesterase
(Equus caballus (Horse)) | BDBM8961

(1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...)Show InChI InChI=1S/C13H14N2/c14-13-9-5-1-3-7-11(9)15-12-8-4-2-6-10(12)13/h1,3,5,7H,2,4,6,8H2,(H2,14,15) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Guangzhou Medical University
Curated by ChEMBL
| Assay Description
Inhibition of equine serum BuChE using butylthiocholine chloride as substrate preincubated for 20 mins followed by substrate addition by Ellman's met... |
Eur J Med Chem 116: 200-209 (2016)
Article DOI: 10.1016/j.ejmech.2016.03.077
BindingDB Entry DOI: 10.7270/Q2XK8HGC |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50610141
(CHEMBL5274934)Show SMILES [H][C@]12CCN(c3ncnc4[nH]ccc34)[C@@]1([H])CN(CC2)C(=O)CNc1ccccc1S(=O)(=O)c1ccccc1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM50165336

(CHEMBL3799703)Show SMILES COc1ccc(C(=O)OCC(=O)Nc2c3CCCCc3nc3ccccc23)c(OC)c1OC Show InChI InChI=1S/C25H26N2O6/c1-30-20-13-12-17(23(31-2)24(20)32-3)25(29)33-14-21(28)27-22-15-8-4-6-10-18(15)26-19-11-7-5-9-16(19)22/h4,6,8,10,12-13H,5,7,9,11,14H2,1-3H3,(H,26,27,28) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Guangzhou Medical University
Curated by ChEMBL
| Assay Description
Inhibition of electric eel AChE using acetylthiocholine chloride as substrate preincubated for 20 mins followed by substrate addition by Ellman's met... |
Eur J Med Chem 116: 200-209 (2016)
Article DOI: 10.1016/j.ejmech.2016.03.077
BindingDB Entry DOI: 10.7270/Q2XK8HGC |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM50165345

(CHEMBL3800317)Show SMILES COc1ccc(\C=C\C(=O)OCC(=O)Nc2c3CCCCc3nc3ccccc23)cc1OC Show InChI InChI=1S/C26H26N2O5/c1-31-22-13-11-17(15-23(22)32-2)12-14-25(30)33-16-24(29)28-26-18-7-3-5-9-20(18)27-21-10-6-4-8-19(21)26/h3,5,7,9,11-15H,4,6,8,10,16H2,1-2H3,(H,27,28,29)/b14-12+ | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Guangzhou Medical University
Curated by ChEMBL
| Assay Description
Inhibition of electric eel AChE using acetylthiocholine chloride as substrate preincubated for 20 mins followed by substrate addition by Ellman's met... |
Eur J Med Chem 116: 200-209 (2016)
Article DOI: 10.1016/j.ejmech.2016.03.077
BindingDB Entry DOI: 10.7270/Q2XK8HGC |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM50165333

(CHEMBL3798989)Show SMILES COc1cc(OC)cc(c1)C(=O)OCC(=O)Nc1c2CCCCc2nc2ccccc12 Show InChI InChI=1S/C24H24N2O5/c1-29-16-11-15(12-17(13-16)30-2)24(28)31-14-22(27)26-23-18-7-3-5-9-20(18)25-21-10-6-4-8-19(21)23/h3,5,7,9,11-13H,4,6,8,10,14H2,1-2H3,(H,25,26,27) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Guangzhou Medical University
Curated by ChEMBL
| Assay Description
Inhibition of electric eel AChE using acetylthiocholine chloride as substrate preincubated for 20 mins followed by substrate addition by Ellman's met... |
Eur J Med Chem 116: 200-209 (2016)
Article DOI: 10.1016/j.ejmech.2016.03.077
BindingDB Entry DOI: 10.7270/Q2XK8HGC |
More data for this
Ligand-Target Pair | |
Amyloid-beta precursor protein
(Homo sapiens (Human)) | BDBM50165344
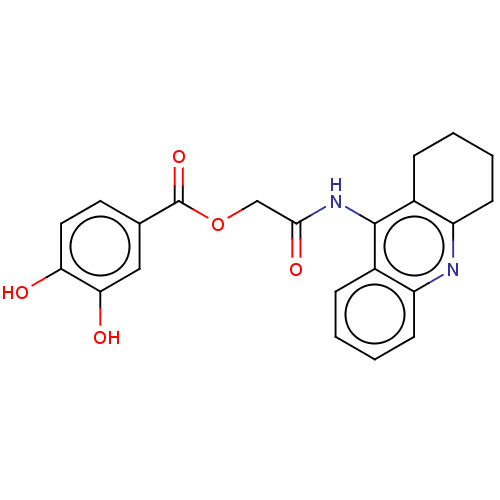
(CHEMBL3800453)Show SMILES Oc1ccc(cc1O)C(=O)OCC(=O)Nc1c2CCCCc2nc2ccccc12 Show InChI InChI=1S/C22H20N2O5/c25-18-10-9-13(11-19(18)26)22(28)29-12-20(27)24-21-14-5-1-3-7-16(14)23-17-8-4-2-6-15(17)21/h1,3,5,7,9-11,25-26H,2,4,6,8,12H2,(H,23,24,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Guangzhou Medical University
Curated by ChEMBL
| Assay Description
Inhibition of self-mediated amyloid beta (1 to 42) (unknown origin) aggregation after 24 hrs by thioflavin T fluorescence assay |
Eur J Med Chem 116: 200-209 (2016)
Article DOI: 10.1016/j.ejmech.2016.03.077
BindingDB Entry DOI: 10.7270/Q2XK8HGC |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM50165346

(CHEMBL3799950)Show SMILES COc1cc(\C=C\C(=O)OCC(=O)Nc2c3CCCCc3nc3ccccc23)cc(OC)c1OC Show InChI InChI=1S/C27H28N2O6/c1-32-22-14-17(15-23(33-2)27(22)34-3)12-13-25(31)35-16-24(30)29-26-18-8-4-6-10-20(18)28-21-11-7-5-9-19(21)26/h4,6,8,10,12-15H,5,7,9,11,16H2,1-3H3,(H,28,29,30)/b13-12+ | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Guangzhou Medical University
Curated by ChEMBL
| Assay Description
Inhibition of electric eel AChE using acetylthiocholine chloride as substrate preincubated for 20 mins followed by substrate addition by Ellman's met... |
Eur J Med Chem 116: 200-209 (2016)
Article DOI: 10.1016/j.ejmech.2016.03.077
BindingDB Entry DOI: 10.7270/Q2XK8HGC |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50610119
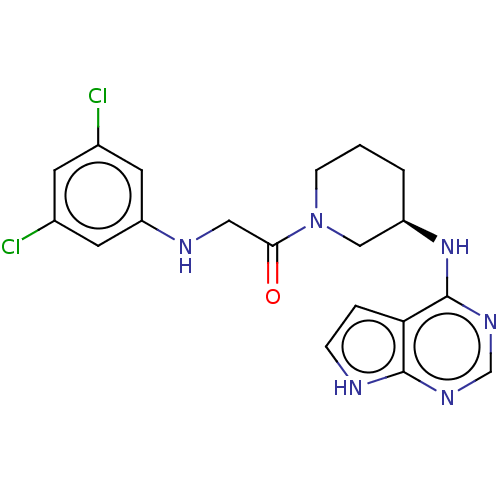
(CHEMBL5285015)Show SMILES Clc1cc(Cl)cc(NCC(=O)N2CCC[C@H](C2)Nc2ncnc3[nH]ccc23)c1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50610125
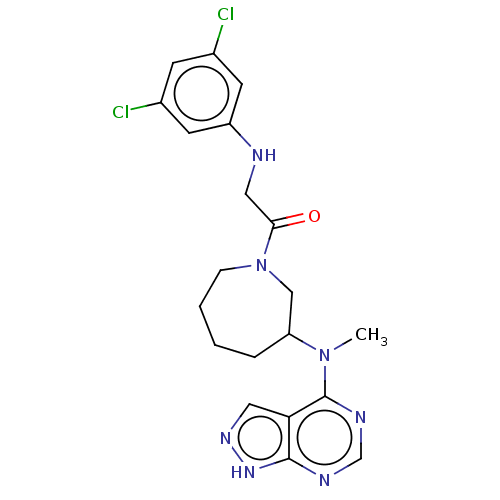
(CHEMBL5276135)Show SMILES CN(C1CCCCN(C1)C(=O)CNc1cc(Cl)cc(Cl)c1)c1ncnc2[nH]ncc12 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Amyloid-beta precursor protein
(Homo sapiens (Human)) | BDBM50165322

(CHEMBL3797706)Show SMILES COc1ccc(CC(=O)OCC(=O)Nc2c3CCCCc3nc3ccccc23)cc1OC Show InChI InChI=1S/C25H26N2O5/c1-30-21-12-11-16(13-22(21)31-2)14-24(29)32-15-23(28)27-25-17-7-3-5-9-19(17)26-20-10-6-4-8-18(20)25/h3,5,7,9,11-13H,4,6,8,10,14-15H2,1-2H3,(H,26,27,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Guangzhou Medical University
Curated by ChEMBL
| Assay Description
Inhibition of self-mediated amyloid beta (1 to 42) (unknown origin) aggregation after 24 hrs by thioflavin T fluorescence assay |
Eur J Med Chem 116: 200-209 (2016)
Article DOI: 10.1016/j.ejmech.2016.03.077
BindingDB Entry DOI: 10.7270/Q2XK8HGC |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM50165321
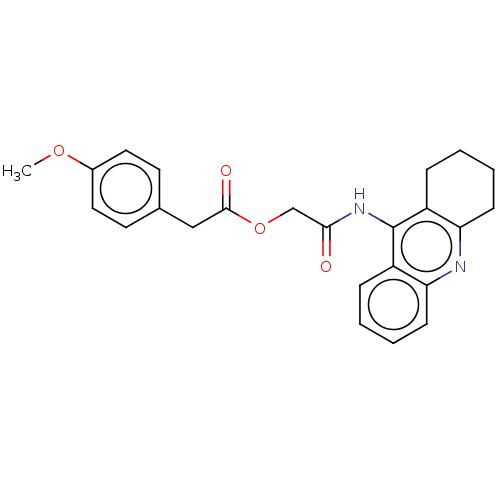
(CHEMBL3797646)Show SMILES COc1ccc(CC(=O)OCC(=O)Nc2c3CCCCc3nc3ccccc23)cc1 Show InChI InChI=1S/C24H24N2O4/c1-29-17-12-10-16(11-13-17)14-23(28)30-15-22(27)26-24-18-6-2-4-8-20(18)25-21-9-5-3-7-19(21)24/h2,4,6,8,10-13H,3,5,7,9,14-15H2,1H3,(H,25,26,27) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Guangzhou Medical University
Curated by ChEMBL
| Assay Description
Inhibition of electric eel AChE using acetylthiocholine chloride as substrate preincubated for 20 mins followed by substrate addition by Ellman's met... |
Eur J Med Chem 116: 200-209 (2016)
Article DOI: 10.1016/j.ejmech.2016.03.077
BindingDB Entry DOI: 10.7270/Q2XK8HGC |
More data for this
Ligand-Target Pair | |
Amyloid-beta precursor protein
(Homo sapiens (Human)) | BDBM50165334

(CHEMBL3797363)Show SMILES COc1cc(cc(OC)c1OC)C(=O)OCC(=O)Nc1c2CCCCc2nc2ccccc12 Show InChI InChI=1S/C25H26N2O6/c1-30-20-12-15(13-21(31-2)24(20)32-3)25(29)33-14-22(28)27-23-16-8-4-6-10-18(16)26-19-11-7-5-9-17(19)23/h4,6,8,10,12-13H,5,7,9,11,14H2,1-3H3,(H,26,27,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
Guangzhou Medical University
Curated by ChEMBL
| Assay Description
Inhibition of self-mediated amyloid beta (1 to 42) (unknown origin) aggregation after 24 hrs by thioflavin T fluorescence assay |
Eur J Med Chem 116: 200-209 (2016)
Article DOI: 10.1016/j.ejmech.2016.03.077
BindingDB Entry DOI: 10.7270/Q2XK8HGC |
More data for this
Ligand-Target Pair | |
Amyloid-beta precursor protein
(Homo sapiens (Human)) | BDBM50165343
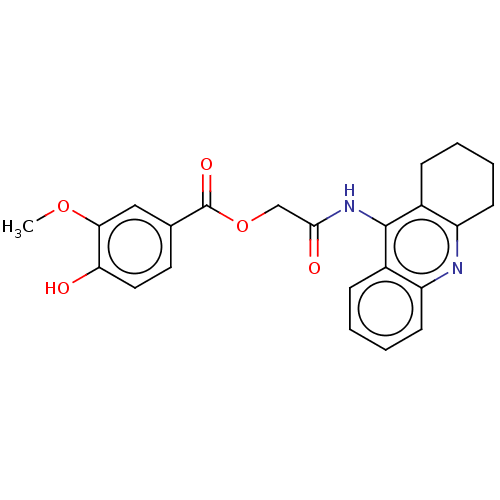
(CHEMBL3797381)Show SMILES COc1cc(ccc1O)C(=O)OCC(=O)Nc1c2CCCCc2nc2ccccc12 Show InChI InChI=1S/C23H22N2O5/c1-29-20-12-14(10-11-19(20)26)23(28)30-13-21(27)25-22-15-6-2-4-8-17(15)24-18-9-5-3-7-16(18)22/h2,4,6,8,10-12,26H,3,5,7,9,13H2,1H3,(H,24,25,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 37 | n/a | n/a | n/a | n/a | n/a | n/a |
Guangzhou Medical University
Curated by ChEMBL
| Assay Description
Inhibition of self-mediated amyloid beta (1 to 42) (unknown origin) aggregation after 24 hrs by thioflavin T fluorescence assay |
Eur J Med Chem 116: 200-209 (2016)
Article DOI: 10.1016/j.ejmech.2016.03.077
BindingDB Entry DOI: 10.7270/Q2XK8HGC |
More data for this
Ligand-Target Pair | |
Amyloid-beta precursor protein
(Homo sapiens (Human)) | BDBM50165358

(CHEMBL3799641)Show SMILES O=C(COC(=O)Cc1c[nH]c2ccccc12)Nc1c2CCCCc2nc2ccccc12 Show InChI InChI=1S/C25H23N3O3/c29-23(15-31-24(30)13-16-14-26-20-10-4-1-7-17(16)20)28-25-18-8-2-5-11-21(18)27-22-12-6-3-9-19(22)25/h1-2,4-5,7-8,10-11,14,26H,3,6,9,12-13,15H2,(H,27,28,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 39 | n/a | n/a | n/a | n/a | n/a | n/a |
Guangzhou Medical University
Curated by ChEMBL
| Assay Description
Inhibition of self-mediated amyloid beta (1 to 42) (unknown origin) aggregation after 24 hrs by thioflavin T fluorescence assay |
Eur J Med Chem 116: 200-209 (2016)
Article DOI: 10.1016/j.ejmech.2016.03.077
BindingDB Entry DOI: 10.7270/Q2XK8HGC |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50610124

(CHEMBL5274214)Show SMILES Clc1cc(Cl)cc(NCC(=O)N2CCCCC(C2)Nc2ncnc3[nH]ncc23)c1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 43 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Amyloid-beta precursor protein
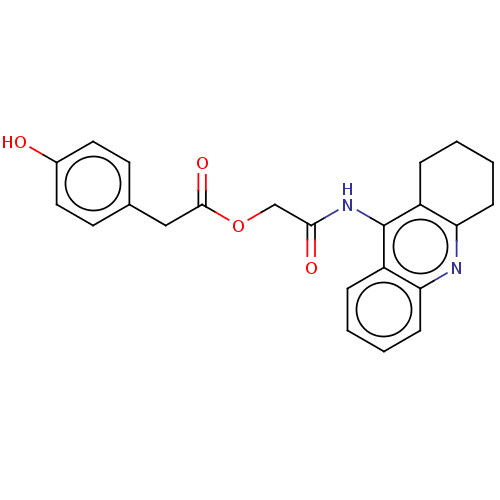
(Homo sapiens (Human)) | BDBM50165324
(CHEMBL3798857)Show SMILES Oc1ccc(CC(=O)OCC(=O)Nc2c3CCCCc3nc3ccccc23)cc1 Show InChI InChI=1S/C23H22N2O4/c26-16-11-9-15(10-12-16)13-22(28)29-14-21(27)25-23-17-5-1-3-7-19(17)24-20-8-4-2-6-18(20)23/h1,3,5,7,9-12,26H,2,4,6,8,13-14H2,(H,24,25,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 45 | n/a | n/a | n/a | n/a | n/a | n/a |
Guangzhou Medical University
Curated by ChEMBL
| Assay Description
Inhibition of self-mediated amyloid beta (1 to 42) (unknown origin) aggregation after 24 hrs by thioflavin T fluorescence assay |
Eur J Med Chem 116: 200-209 (2016)
Article DOI: 10.1016/j.ejmech.2016.03.077
BindingDB Entry DOI: 10.7270/Q2XK8HGC |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50610131
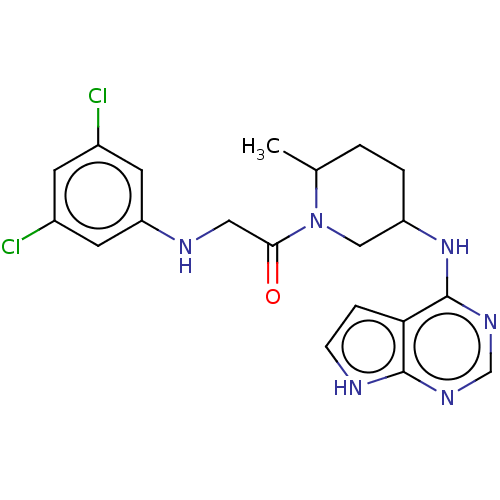
(CHEMBL5289054)Show SMILES CC1CCC(CN1C(=O)CNc1cc(Cl)cc(Cl)c1)Nc1ncnc2[nH]ccc12 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 45 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50610132
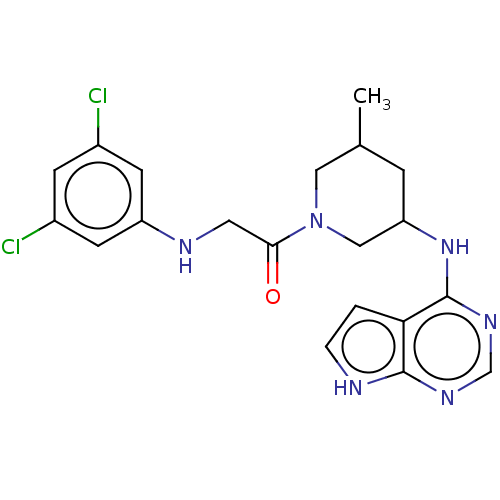
(CHEMBL5290011)Show SMILES CC1CC(CN(C1)C(=O)CNc1cc(Cl)cc(Cl)c1)Nc1ncnc2[nH]ccc12 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 45 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Amyloid-beta precursor protein
(Homo sapiens (Human)) | BDBM50165346

(CHEMBL3799950)Show SMILES COc1cc(\C=C\C(=O)OCC(=O)Nc2c3CCCCc3nc3ccccc23)cc(OC)c1OC Show InChI InChI=1S/C27H28N2O6/c1-32-22-14-17(15-23(33-2)27(22)34-3)12-13-25(31)35-16-24(30)29-26-18-8-4-6-10-20(18)28-21-11-7-5-9-19(21)26/h4,6,8,10,12-15H,5,7,9,11,16H2,1-3H3,(H,28,29,30)/b13-12+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 46 | n/a | n/a | n/a | n/a | n/a | n/a |
Guangzhou Medical University
Curated by ChEMBL
| Assay Description
Inhibition of self-mediated amyloid beta (1 to 42) (unknown origin) aggregation after 24 hrs by thioflavin T fluorescence assay |
Eur J Med Chem 116: 200-209 (2016)
Article DOI: 10.1016/j.ejmech.2016.03.077
BindingDB Entry DOI: 10.7270/Q2XK8HGC |
More data for this
Ligand-Target Pair | |
Amyloid-beta precursor protein
(Homo sapiens (Human)) | BDBM50165347

(CHEMBL3798813)Show SMILES COc1ccc(\C=C\C(=O)OCC(=O)Nc2c3CCCCc3nc3ccccc23)cc1O Show InChI InChI=1S/C25H24N2O5/c1-31-22-12-10-16(14-21(22)28)11-13-24(30)32-15-23(29)27-25-17-6-2-4-8-19(17)26-20-9-5-3-7-18(20)25/h2,4,6,8,10-14,28H,3,5,7,9,15H2,1H3,(H,26,27,29)/b13-11+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 46 | n/a | n/a | n/a | n/a | n/a | n/a |
Guangzhou Medical University
Curated by ChEMBL
| Assay Description
Inhibition of self-mediated amyloid beta (1 to 42) (unknown origin) aggregation after 24 hrs by thioflavin T fluorescence assay |
Eur J Med Chem 116: 200-209 (2016)
Article DOI: 10.1016/j.ejmech.2016.03.077
BindingDB Entry DOI: 10.7270/Q2XK8HGC |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50610142
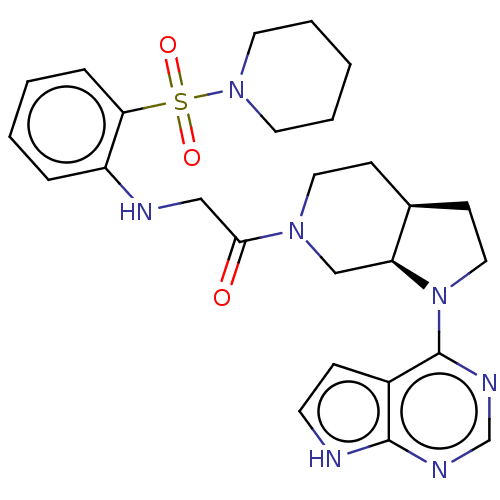
(CHEMBL5286967)Show SMILES [H][C@]12CCN(c3ncnc4[nH]ccc34)[C@@]1([H])CN(CC2)C(=O)CNc1ccccc1S(=O)(=O)N1CCCCC1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 49 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Amyloid-beta precursor protein
(Homo sapiens (Human)) | BDBM50165345

(CHEMBL3800317)Show SMILES COc1ccc(\C=C\C(=O)OCC(=O)Nc2c3CCCCc3nc3ccccc23)cc1OC Show InChI InChI=1S/C26H26N2O5/c1-31-22-13-11-17(15-23(22)32-2)12-14-25(30)33-16-24(29)28-26-18-7-3-5-9-20(18)27-21-10-6-4-8-19(21)26/h3,5,7,9,11-15H,4,6,8,10,16H2,1-2H3,(H,27,28,29)/b14-12+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 49 | n/a | n/a | n/a | n/a | n/a | n/a |
Guangzhou Medical University
Curated by ChEMBL
| Assay Description
Inhibition of self-mediated amyloid beta (1 to 42) (unknown origin) aggregation after 24 hrs by thioflavin T fluorescence assay |
Eur J Med Chem 116: 200-209 (2016)
Article DOI: 10.1016/j.ejmech.2016.03.077
BindingDB Entry DOI: 10.7270/Q2XK8HGC |
More data for this
Ligand-Target Pair | |
Amyloid-beta precursor protein
(Homo sapiens (Human)) | BDBM50165323

(CHEMBL3799558)Show SMILES COc1ccc(CC(=O)OCC(=O)Nc2c3CCCCc3nc3ccccc23)c(OC)c1OC Show InChI InChI=1S/C26H28N2O6/c1-31-21-13-12-16(25(32-2)26(21)33-3)14-23(30)34-15-22(29)28-24-17-8-4-6-10-19(17)27-20-11-7-5-9-18(20)24/h4,6,8,10,12-13H,5,7,9,11,14-15H2,1-3H3,(H,27,28,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 52 | n/a | n/a | n/a | n/a | n/a | n/a |
Guangzhou Medical University
Curated by ChEMBL
| Assay Description
Inhibition of self-mediated amyloid beta (1 to 42) (unknown origin) aggregation after 24 hrs by thioflavin T fluorescence assay |
Eur J Med Chem 116: 200-209 (2016)
Article DOI: 10.1016/j.ejmech.2016.03.077
BindingDB Entry DOI: 10.7270/Q2XK8HGC |
More data for this
Ligand-Target Pair | |
Amyloid-beta precursor protein
(Homo sapiens (Human)) | BDBM50165354

(CHEMBL3799175)Show SMILES Clc1ccc(\C=C\C(=O)OCC(=O)Nc2c3CCCCc3nc3ccccc23)cc1 Show InChI InChI=1S/C24H21ClN2O3/c25-17-12-9-16(10-13-17)11-14-23(29)30-15-22(28)27-24-18-5-1-3-7-20(18)26-21-8-4-2-6-19(21)24/h1,3,5,7,9-14H,2,4,6,8,15H2,(H,26,27,28)/b14-11+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 52 | n/a | n/a | n/a | n/a | n/a | n/a |
Guangzhou Medical University
Curated by ChEMBL
| Assay Description
Inhibition of self-mediated amyloid beta (1 to 42) (unknown origin) aggregation after 24 hrs by thioflavin T fluorescence assay |
Eur J Med Chem 116: 200-209 (2016)
Article DOI: 10.1016/j.ejmech.2016.03.077
BindingDB Entry DOI: 10.7270/Q2XK8HGC |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50610123
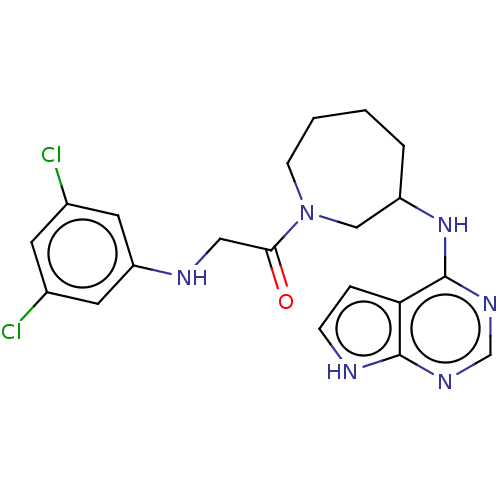
(CHEMBL5276339)Show SMILES Clc1cc(Cl)cc(NCC(=O)N2CCCCC(C2)Nc2ncnc3[nH]ccc23)c1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 53 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Amyloid-beta precursor protein
(Homo sapiens (Human)) | BDBM50165333

(CHEMBL3798989)Show SMILES COc1cc(OC)cc(c1)C(=O)OCC(=O)Nc1c2CCCCc2nc2ccccc12 Show InChI InChI=1S/C24H24N2O5/c1-29-16-11-15(12-17(13-16)30-2)24(28)31-14-22(27)26-23-18-7-3-5-9-20(18)25-21-10-6-4-8-19(21)23/h3,5,7,9,11-13H,4,6,8,10,14H2,1-2H3,(H,25,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 56 | n/a | n/a | n/a | n/a | n/a | n/a |
Guangzhou Medical University
Curated by ChEMBL
| Assay Description
Inhibition of self-mediated amyloid beta (1 to 42) (unknown origin) aggregation after 24 hrs by thioflavin T fluorescence assay |
Eur J Med Chem 116: 200-209 (2016)
Article DOI: 10.1016/j.ejmech.2016.03.077
BindingDB Entry DOI: 10.7270/Q2XK8HGC |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50610135
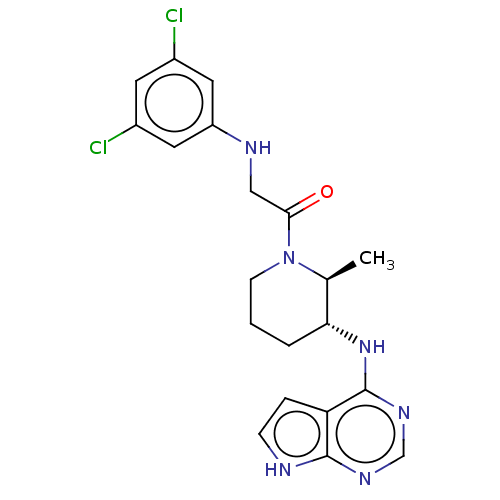
(CHEMBL5267533)Show SMILES C[C@H]1[C@@H](CCCN1C(=O)CNc1cc(Cl)cc(Cl)c1)Nc1ncnc2[nH]ccc12 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 58 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM50165322

(CHEMBL3797706)Show SMILES COc1ccc(CC(=O)OCC(=O)Nc2c3CCCCc3nc3ccccc23)cc1OC Show InChI InChI=1S/C25H26N2O5/c1-30-21-12-11-16(13-22(21)31-2)14-24(29)32-15-23(28)27-25-17-7-3-5-9-19(17)26-20-10-6-4-8-18(20)25/h3,5,7,9,11-13H,4,6,8,10,14-15H2,1-2H3,(H,26,27,28) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 65 | n/a | n/a | n/a | n/a | n/a | n/a |
Guangzhou Medical University
Curated by ChEMBL
| Assay Description
Inhibition of electric eel AChE using acetylthiocholine chloride as substrate preincubated for 20 mins followed by substrate addition by Ellman's met... |
Eur J Med Chem 116: 200-209 (2016)
Article DOI: 10.1016/j.ejmech.2016.03.077
BindingDB Entry DOI: 10.7270/Q2XK8HGC |
More data for this
Ligand-Target Pair | |
Amyloid-beta precursor protein
(Homo sapiens (Human)) | BDBM50165336

(CHEMBL3799703)Show SMILES COc1ccc(C(=O)OCC(=O)Nc2c3CCCCc3nc3ccccc23)c(OC)c1OC Show InChI InChI=1S/C25H26N2O6/c1-30-20-13-12-17(23(31-2)24(20)32-3)25(29)33-14-21(28)27-22-15-8-4-6-10-18(15)26-19-11-7-5-9-16(19)22/h4,6,8,10,12-13H,5,7,9,11,14H2,1-3H3,(H,26,27,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 66 | n/a | n/a | n/a | n/a | n/a | n/a |
Guangzhou Medical University
Curated by ChEMBL
| Assay Description
Inhibition of self-mediated amyloid beta (1 to 42) (unknown origin) aggregation after 24 hrs by thioflavin T fluorescence assay |
Eur J Med Chem 116: 200-209 (2016)
Article DOI: 10.1016/j.ejmech.2016.03.077
BindingDB Entry DOI: 10.7270/Q2XK8HGC |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data