

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Transient receptor potential cation channel subfamily V member 1/2/4 (Rattus norvegicus-Rattus norvegicus (rat)) | BDBM50222640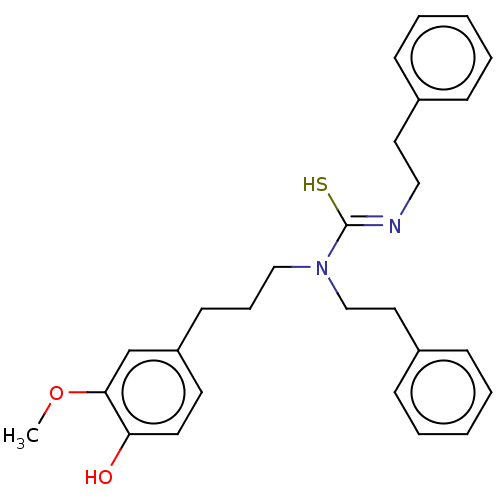 (CHEMBL415543) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.320 | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description Antagonistic activity against vanilloid receptor as concentration to reduce the response to 0.5 mM capsaicin by 50% from neonatal rat culture spinal ... | Bioorg Med Chem Lett 13: 601-4 (2003) BindingDB Entry DOI: 10.7270/Q2XP7747 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1/2/4 (Rattus norvegicus-Rattus norvegicus (rat)) | BDBM50222640 (CHEMBL415543) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.320 | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description Antagonistic activity against vanilloid receptor as concentration to reduce the response to 0.5 mM capsaicin by 50% from neonatal rat culture spinal ... | Bioorg Med Chem Lett 13: 601-4 (2003) BindingDB Entry DOI: 10.7270/Q2XP7747 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1/2/4 (Rattus norvegicus-Rattus norvegicus (rat)) | BDBM50222695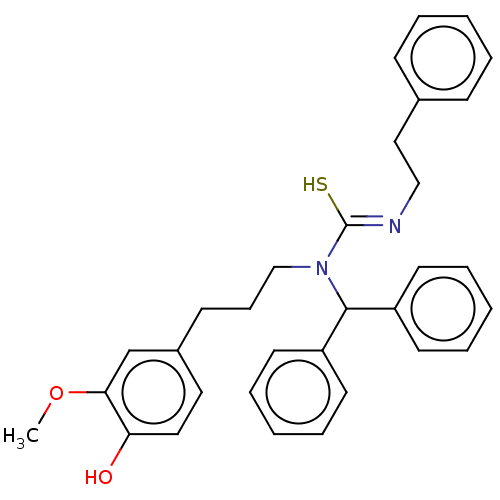 (CHEMBL155433) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.460 | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description Antagonistic activity against vanilloid receptor as concentration to reduce the response to 0.5 mM capsaicin by 50% from neonatal rat culture spinal ... | Bioorg Med Chem Lett 13: 601-4 (2003) BindingDB Entry DOI: 10.7270/Q2XP7747 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1/2/4 (Rattus norvegicus-Rattus norvegicus (rat)) | BDBM50222637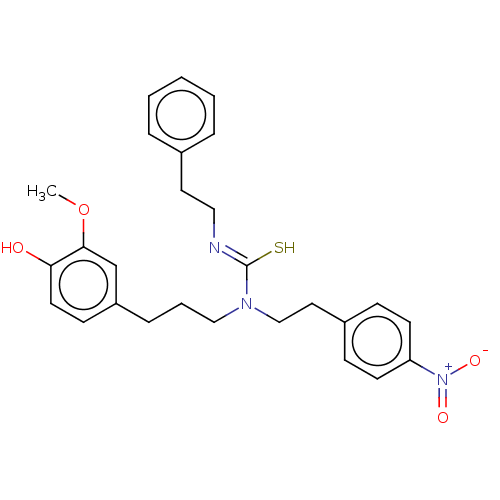 (CHEMBL154743) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description Antagonistic activity against vanilloid receptor as concentration to reduce the response to 0.5 mM capsaicin by 50% from neonatal rat culture spinal ... | Bioorg Med Chem Lett 13: 601-4 (2003) BindingDB Entry DOI: 10.7270/Q2XP7747 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1/2/4 (Rattus norvegicus-Rattus norvegicus (rat)) | BDBM50222696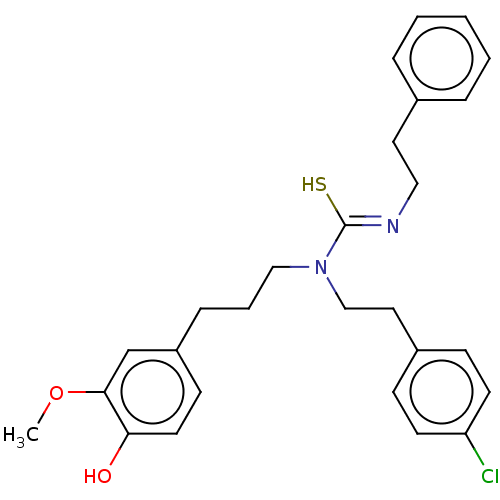 (CHEMBL156041) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.550 | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description Antagonistic activity against vanilloid receptor as concentration to reduce the response to 0.5 mM capsaicin by 50% from neonatal rat culture spinal ... | Bioorg Med Chem Lett 13: 601-4 (2003) BindingDB Entry DOI: 10.7270/Q2XP7747 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1/2/4 (Rattus norvegicus-Rattus norvegicus (rat)) | BDBM50222635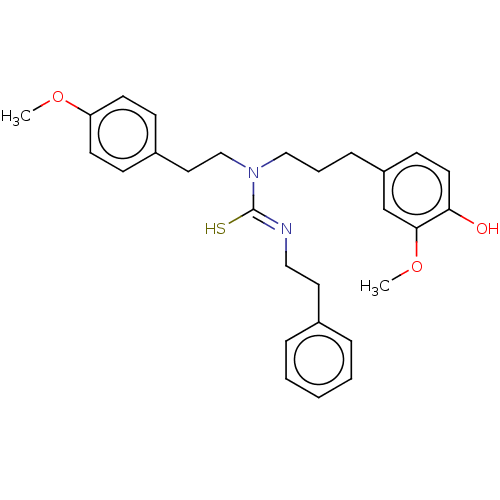 (CHEMBL267205) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.720 | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description Antagonistic activity against vanilloid receptor as concentration to reduce the response to 0.5 mM capsaicin by 50% from neonatal rat culture spinal ... | Bioorg Med Chem Lett 13: 601-4 (2003) BindingDB Entry DOI: 10.7270/Q2XP7747 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1/2/4 (Rattus norvegicus-Rattus norvegicus (rat)) | BDBM50222649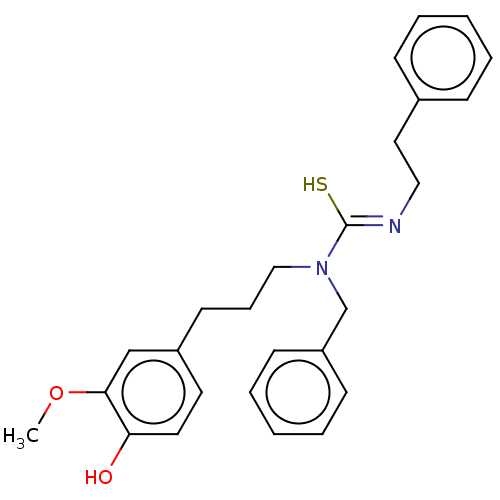 (CHEMBL155730) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.950 | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description Antagonistic activity against vanilloid receptor as concentration to reduce the response to 0.5 mM capsaicin by 50% from neonatal rat culture spinal ... | Bioorg Med Chem Lett 13: 601-4 (2003) BindingDB Entry DOI: 10.7270/Q2XP7747 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1/2/4 (Rattus norvegicus-Rattus norvegicus (rat)) | BDBM50222647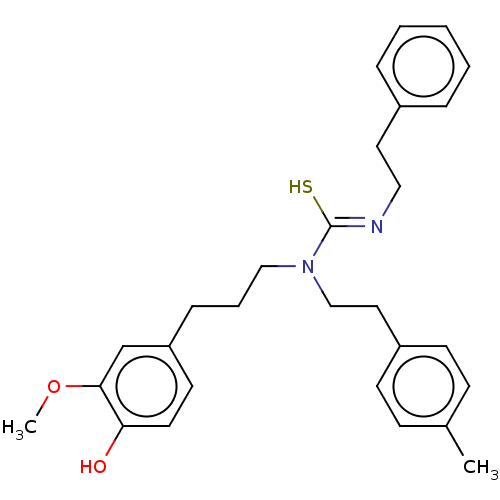 (CHEMBL154990) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.950 | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description Antagonistic activity against vanilloid receptor as concentration to reduce the response to 0.5 mM capsaicin by 50% from neonatal rat culture spinal ... | Bioorg Med Chem Lett 13: 601-4 (2003) BindingDB Entry DOI: 10.7270/Q2XP7747 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1/2/4 (Rattus norvegicus-Rattus norvegicus (rat)) | BDBM50222641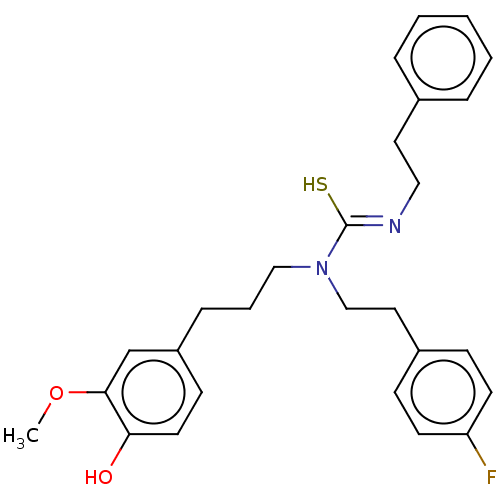 (CHEMBL157962) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description Antagonistic activity against vanilloid receptor as concentration to reduce the response to 0.5 mM capsaicin by 50% from neonatal rat culture spinal ... | Bioorg Med Chem Lett 13: 601-4 (2003) BindingDB Entry DOI: 10.7270/Q2XP7747 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1/2/4 (Rattus norvegicus-Rattus norvegicus (rat)) | BDBM50222638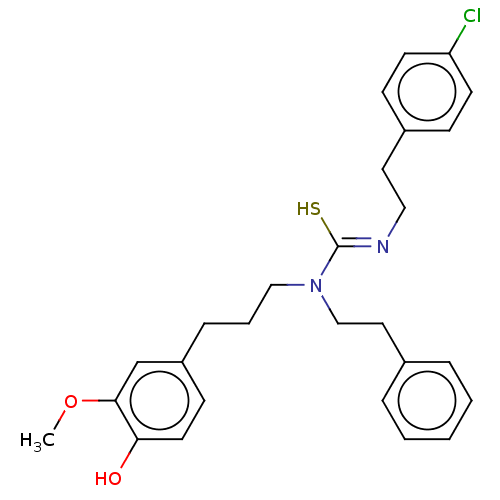 (CHEMBL422702) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description Antagonistic activity against vanilloid receptor as concentration to reduce the response to 0.5 mM capsaicin by 50% from neonatal rat culture spinal ... | Bioorg Med Chem Lett 13: 601-4 (2003) BindingDB Entry DOI: 10.7270/Q2XP7747 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1/2/4 (Rattus norvegicus-Rattus norvegicus (rat)) | BDBM50222644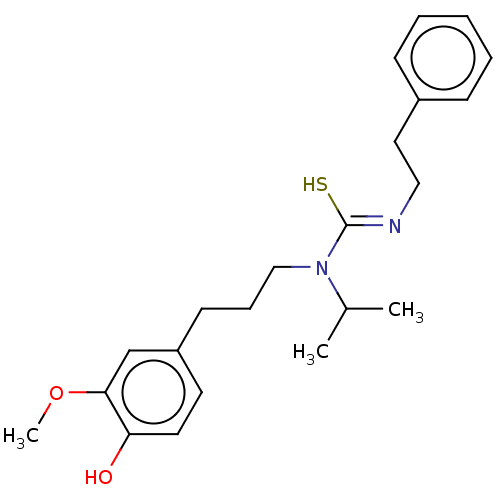 (CHEMBL434295) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description Antagonistic activity against vanilloid receptor as concentration to reduce the response to 0.5 mM capsaicin by 50% from neonatal rat culture spinal ... | Bioorg Med Chem Lett 13: 601-4 (2003) BindingDB Entry DOI: 10.7270/Q2XP7747 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1/2/4 (Rattus norvegicus-Rattus norvegicus (rat)) | BDBM50222648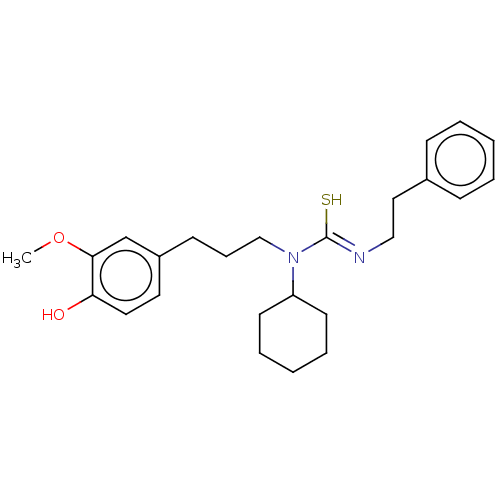 (CHEMBL156693) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description Antagonistic activity against vanilloid receptor as concentration to reduce the response to 0.5 mM capsaicin by 50% from neonatal rat culture spinal ... | Bioorg Med Chem Lett 13: 601-4 (2003) BindingDB Entry DOI: 10.7270/Q2XP7747 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1/2/4 (Rattus norvegicus-Rattus norvegicus (rat)) | BDBM50222639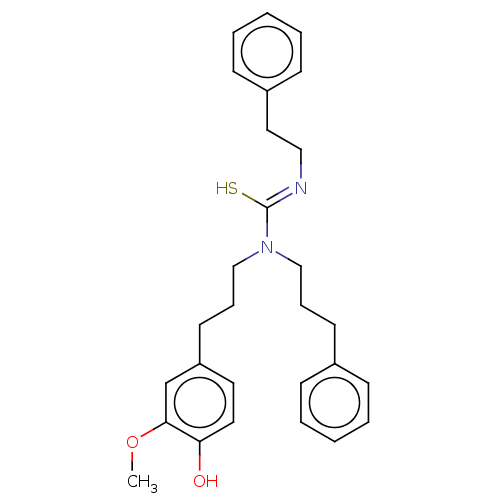 (CHEMBL356682) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description Antagonistic activity against vanilloid receptor as concentration to reduce the response to 0.5 mM capsaicin by 50% from neonatal rat culture spinal ... | Bioorg Med Chem Lett 13: 601-4 (2003) BindingDB Entry DOI: 10.7270/Q2XP7747 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Mus musculus (Mouse)) | BDBM50474740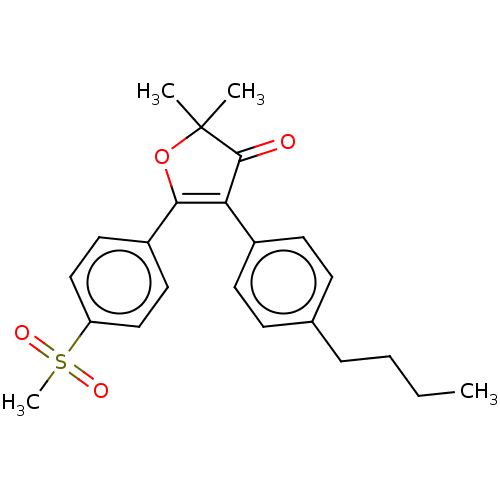 (CHEMBL355074) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.02 | n/a | n/a | n/a | n/a | n/a | n/a |
AmorePacific R&D Center Curated by ChEMBL | Assay Description In vitro inhibitory activity against prostaglandin G/H synthase 2 using mouse peritoneal macrophage method | J Med Chem 47: 792-804 (2004) Article DOI: 10.1021/jm020545z BindingDB Entry DOI: 10.7270/Q2QZ2DPG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1/2/4 (Rattus norvegicus-Rattus norvegicus (rat)) | BDBM50222646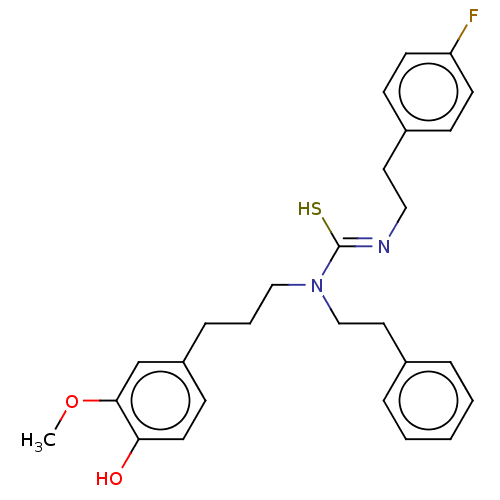 (CHEMBL157917) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 5.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description Antagonistic activity against vanilloid receptor as concentration to reduce the response to 0.5 mM capsaicin by 50% from neonatal rat culture spinal ... | Bioorg Med Chem Lett 13: 601-4 (2003) BindingDB Entry DOI: 10.7270/Q2XP7747 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1/2/4 (Rattus norvegicus-Rattus norvegicus (rat)) | BDBM50222694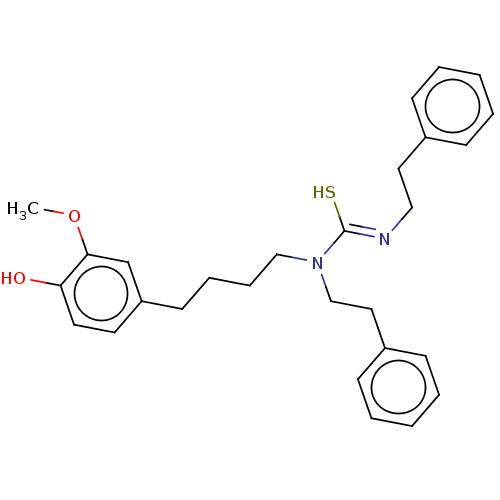 (CHEMBL158189) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 6.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description Antagonistic activity against vanilloid receptor as concentration to reduce the response to 0.5 mM capsaicin by 50% from neonatal rat culture spinal ... | Bioorg Med Chem Lett 13: 601-4 (2003) BindingDB Entry DOI: 10.7270/Q2XP7747 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Mus musculus (Mouse)) | BDBM50474747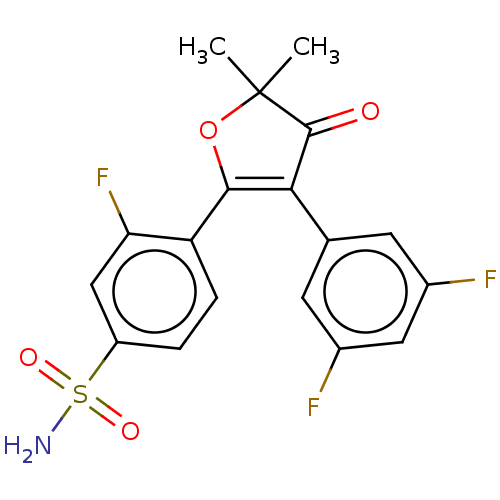 (CHEMBL167357) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7.55 | n/a | n/a | n/a | n/a | n/a | n/a |
AmorePacific R&D Center Curated by ChEMBL | Assay Description In vitro inhibitory activity against prostaglandin G/H synthase 2 using mouse peritoneal macrophage method | J Med Chem 47: 792-804 (2004) Article DOI: 10.1021/jm020545z BindingDB Entry DOI: 10.7270/Q2QZ2DPG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Mus musculus (Mouse)) | BDBM50474743 (CHEMBL353935) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7.91 | n/a | n/a | n/a | n/a | n/a | n/a |
AmorePacific R&D Center Curated by ChEMBL | Assay Description In vitro inhibitory activity against prostaglandin G/H synthase 2 using mouse peritoneal macrophage method | J Med Chem 47: 792-804 (2004) Article DOI: 10.1021/jm020545z BindingDB Entry DOI: 10.7270/Q2QZ2DPG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Mus musculus (Mouse)) | BDBM50474760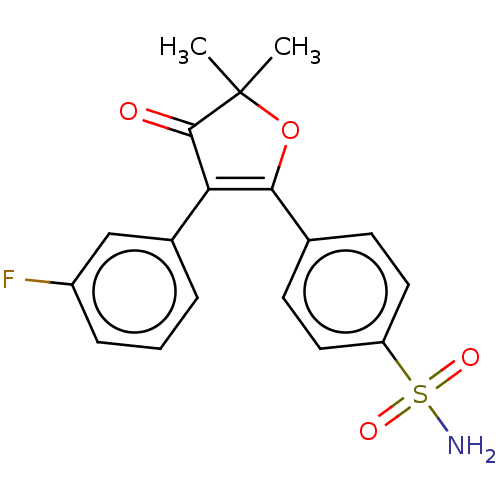 (CG-100649 | CG100649 | Polmacoxib) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 8.30 | n/a | n/a | n/a | n/a | n/a | n/a |
AmorePacific R&D Center Curated by ChEMBL | Assay Description In vitro inhibitory activity against prostaglandin G/H synthase 2 using mouse peritoneal macrophage method | J Med Chem 47: 792-804 (2004) Article DOI: 10.1021/jm020545z BindingDB Entry DOI: 10.7270/Q2QZ2DPG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1/2/4 (Rattus norvegicus-Rattus norvegicus (rat)) | BDBM50222691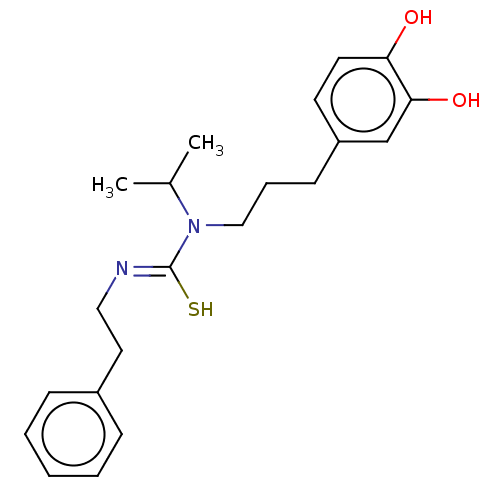 (CHEMBL156927) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | n/a | n/a | 9.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description Antagonistic activity against vanilloid receptor as concentration to reduce the response to 0.5 mM capsaicin by 50% from neonatal rat culture spinal ... | Bioorg Med Chem Lett 13: 601-4 (2003) BindingDB Entry DOI: 10.7270/Q2XP7747 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1/2/4 (Rattus norvegicus-Rattus norvegicus (rat)) | BDBM50222690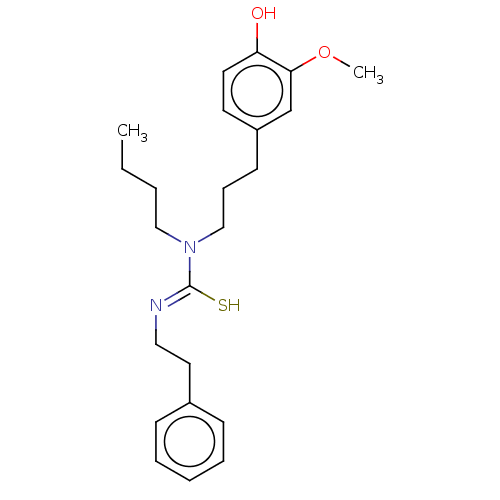 (CHEMBL345157) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description Antagonistic activity against vanilloid receptor as concentration to reduce the response to 0.5 mM capsaicin by 50% from neonatal rat culture spinal ... | Bioorg Med Chem Lett 13: 601-4 (2003) BindingDB Entry DOI: 10.7270/Q2XP7747 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1/2/4 (Rattus norvegicus-Rattus norvegicus (rat)) | BDBM50222645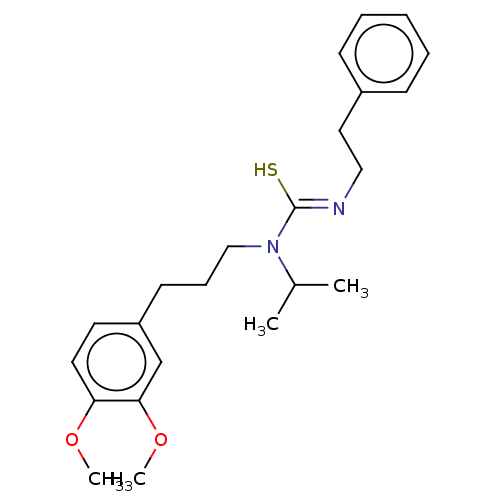 (CHEMBL157918) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description Antagonistic activity against vanilloid receptor as concentration to reduce the response to 0.5 mM capsaicin by 50% from neonatal rat culture spinal ... | Bioorg Med Chem Lett 13: 601-4 (2003) BindingDB Entry DOI: 10.7270/Q2XP7747 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1/2/4 (Rattus norvegicus-Rattus norvegicus (rat)) | BDBM50222693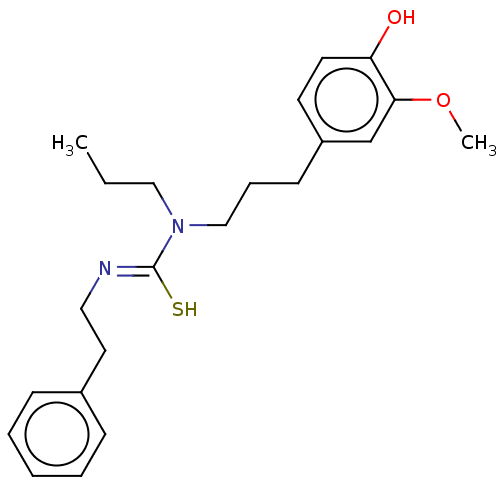 (CHEMBL157579) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description Antagonistic activity against vanilloid receptor as concentration to reduce the response to 0.5 mM capsaicin by 50% from neonatal rat culture spinal ... | Bioorg Med Chem Lett 13: 601-4 (2003) BindingDB Entry DOI: 10.7270/Q2XP7747 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Mus musculus (Mouse)) | BDBM50474737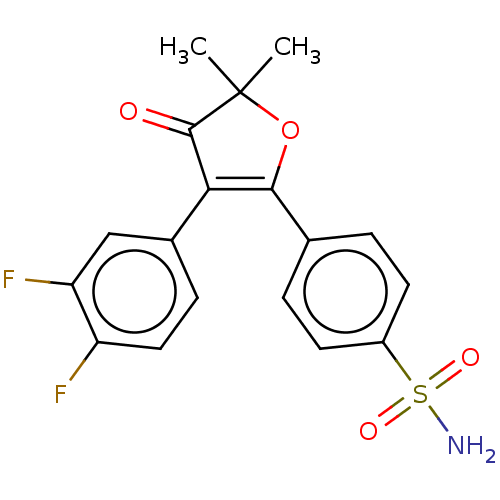 (CHEMBL167011) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 18.5 | n/a | n/a | n/a | n/a | n/a | n/a |
AmorePacific R&D Center Curated by ChEMBL | Assay Description In vitro inhibitory activity against prostaglandin G/H synthase 2 using mouse peritoneal macrophage method | J Med Chem 47: 792-804 (2004) Article DOI: 10.1021/jm020545z BindingDB Entry DOI: 10.7270/Q2QZ2DPG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Mus musculus (Mouse)) | BDBM50474742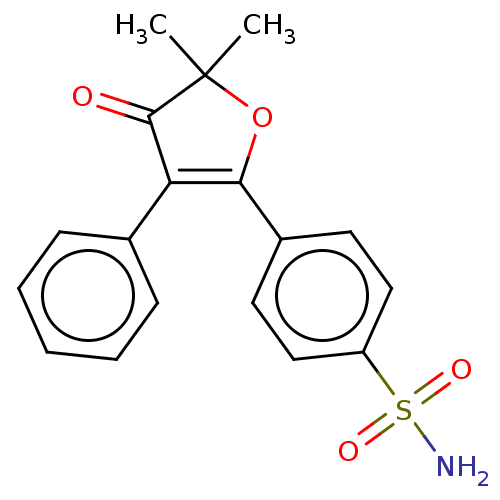 (CHEMBL166295) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 23.3 | n/a | n/a | n/a | n/a | n/a | n/a |
AmorePacific R&D Center Curated by ChEMBL | Assay Description In vitro inhibitory activity against prostaglandin G/H synthase 2 using mouse peritoneal macrophage method | J Med Chem 47: 792-804 (2004) Article DOI: 10.1021/jm020545z BindingDB Entry DOI: 10.7270/Q2QZ2DPG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Mus musculus (Mouse)) | BDBM11639 (4-[5-(4-methylphenyl)-3-(trifluoromethyl)-1H-pyraz...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB PubMed | n/a | n/a | 26.2 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical& Health Research Institute Curated by ChEMBL | Assay Description Inhibitory activity against Prostaglandin G/H synthase 2 from freshly harvested mouse peritoneal macrophages | Bioorg Med Chem Lett 14: 2195-8 (2004) BindingDB Entry DOI: 10.7270/Q2K939PT | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Prostaglandin G/H synthase 2 (Mus musculus (Mouse)) | BDBM50218963 (CHEMBL297626) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 26.5 | n/a | n/a | n/a | n/a | n/a | n/a |
AmorePacific R&D Center Curated by ChEMBL | Assay Description Inhibitory concentration against prostaglandin G/H synthase 2 in mouse peritoneal macrophages | Bioorg Med Chem Lett 14: 1757-60 (2004) BindingDB Entry DOI: 10.7270/Q2ZK5JVW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Mus musculus (Mouse)) | BDBM50218963 (CHEMBL297626) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 26.5 | n/a | n/a | n/a | n/a | n/a | n/a |
AmorePacific R&D Center Curated by ChEMBL | Assay Description In vitro inhibitory activity against prostaglandin G/H synthase 2 using mouse peritoneal macrophage method | J Med Chem 47: 792-804 (2004) Article DOI: 10.1021/jm020545z BindingDB Entry DOI: 10.7270/Q2QZ2DPG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Mus musculus (Mouse)) | BDBM50218963 (CHEMBL297626) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 26.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pacific Corporation R&D Center Curated by ChEMBL | Assay Description Ability to inhibit Prostaglandin G/H synthase 2 by using freshly harvested mouse peritoneal macrophages | Bioorg Med Chem Lett 11: 165-8 (2001) BindingDB Entry DOI: 10.7270/Q2Z03BB2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Mus musculus (Mouse)) | BDBM50474770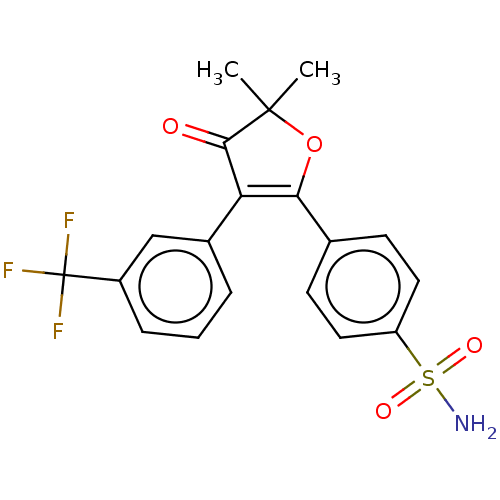 (CHEMBL353965) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 26.7 | n/a | n/a | n/a | n/a | n/a | n/a |
AmorePacific R&D Center Curated by ChEMBL | Assay Description In vitro inhibitory activity against prostaglandin G/H synthase 2 using mouse peritoneal macrophage method | J Med Chem 47: 792-804 (2004) Article DOI: 10.1021/jm020545z BindingDB Entry DOI: 10.7270/Q2QZ2DPG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1/2/4 (Rattus norvegicus-Rattus norvegicus (rat)) | BDBM50222643 (CHEMBL157688) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >30 | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description Antagonistic activity against vanilloid receptor as concentration to reduce the response to 0.5 mM capsaicin by 50% from neonatal rat culture spinal ... | Bioorg Med Chem Lett 13: 601-4 (2003) BindingDB Entry DOI: 10.7270/Q2XP7747 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1/2/4 (Rattus norvegicus-Rattus norvegicus (rat)) | BDBM50222634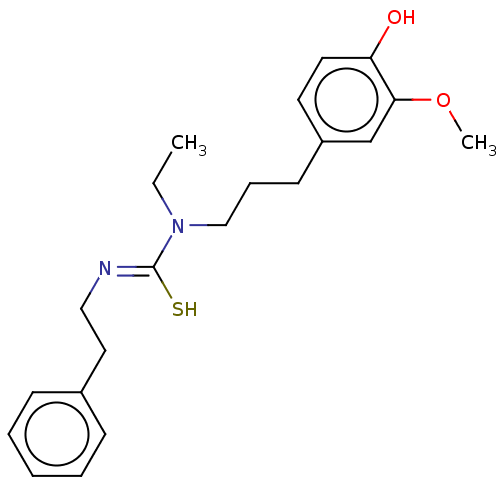 (CHEMBL157473) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >30 | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description Antagonistic activity against vanilloid receptor as concentration to reduce the response to 0.5 mM capsaicin by 50% from neonatal rat culture spinal ... | Bioorg Med Chem Lett 13: 601-4 (2003) BindingDB Entry DOI: 10.7270/Q2XP7747 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1/2/4 (Rattus norvegicus-Rattus norvegicus (rat)) | BDBM50222692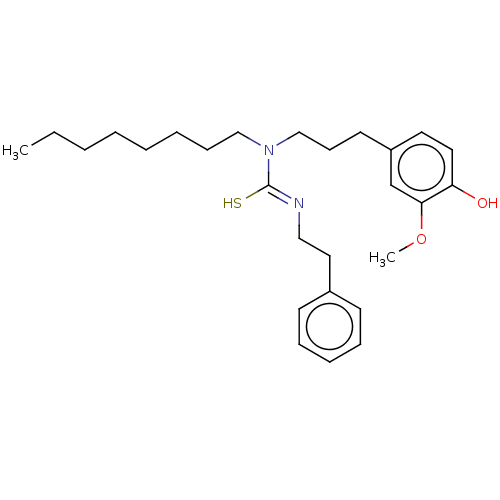 (CHEMBL348634) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | >30 | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description Antagonistic activity against vanilloid receptor as concentration to reduce the response to 0.5 mM capsaicin by 50% from neonatal rat culture spinal ... | Bioorg Med Chem Lett 13: 601-4 (2003) BindingDB Entry DOI: 10.7270/Q2XP7747 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Mus musculus (Mouse)) | BDBM50474765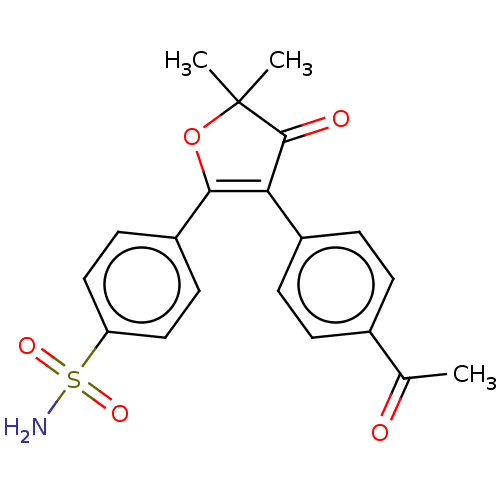 (CHEMBL353959) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 33.7 | n/a | n/a | n/a | n/a | n/a | n/a |
AmorePacific R&D Center Curated by ChEMBL | Assay Description In vitro inhibitory activity against prostaglandin G/H synthase 2 using mouse peritoneal macrophage method | J Med Chem 47: 792-804 (2004) Article DOI: 10.1021/jm020545z BindingDB Entry DOI: 10.7270/Q2QZ2DPG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Mus musculus (Mouse)) | BDBM50474746 (CHEMBL423083) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 37.1 | n/a | n/a | n/a | n/a | n/a | n/a |
AmorePacific R&D Center Curated by ChEMBL | Assay Description In vitro inhibitory activity against prostaglandin G/H synthase 2 using mouse peritoneal macrophage method | J Med Chem 47: 792-804 (2004) Article DOI: 10.1021/jm020545z BindingDB Entry DOI: 10.7270/Q2QZ2DPG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Mus musculus (Mouse)) | BDBM50474771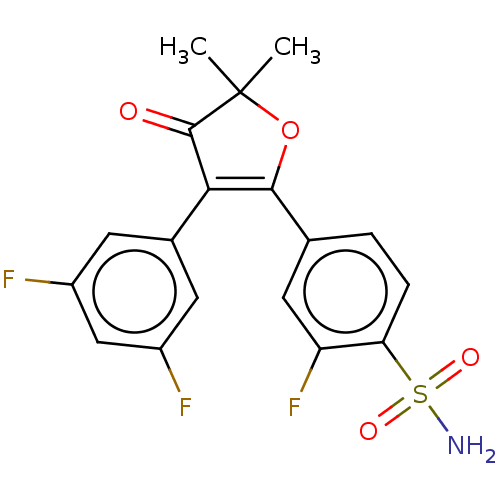 (CHEMBL352602) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 50.3 | n/a | n/a | n/a | n/a | n/a | n/a |
AmorePacific R&D Center Curated by ChEMBL | Assay Description In vitro inhibitory activity against prostaglandin G/H synthase 2 using mouse peritoneal macrophage method | J Med Chem 47: 792-804 (2004) Article DOI: 10.1021/jm020545z BindingDB Entry DOI: 10.7270/Q2QZ2DPG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Mus musculus (Mouse)) | BDBM50474766 (CHEMBL166720) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 50.7 | n/a | n/a | n/a | n/a | n/a | n/a |
AmorePacific R&D Center Curated by ChEMBL | Assay Description In vitro inhibitory activity against prostaglandin G/H synthase 2 using mouse peritoneal macrophage method | J Med Chem 47: 792-804 (2004) Article DOI: 10.1021/jm020545z BindingDB Entry DOI: 10.7270/Q2QZ2DPG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Mus musculus (Mouse)) | BDBM50218974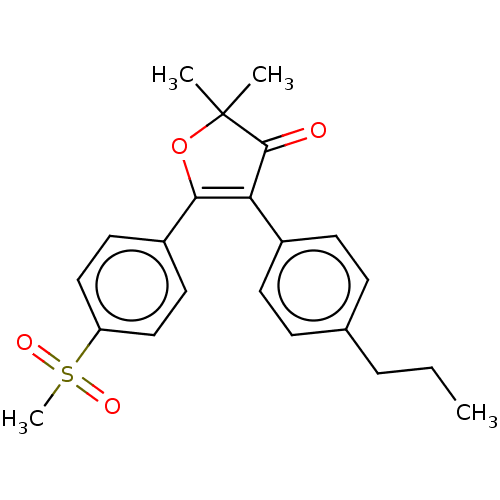 (CHEMBL296206) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 52 | n/a | n/a | n/a | n/a | n/a | n/a |
Pacific Corporation R&D Center Curated by ChEMBL | Assay Description Ability to inhibit Prostaglandin G/H synthase 2 by using freshly harvested mouse peritoneal macrophages | Bioorg Med Chem Lett 11: 165-8 (2001) BindingDB Entry DOI: 10.7270/Q2Z03BB2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Mus musculus (Mouse)) | BDBM11639 (4-[5-(4-methylphenyl)-3-(trifluoromethyl)-1H-pyraz...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB PubMed | n/a | n/a | 52.4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pacific Corporation R&D Center Curated by ChEMBL | Assay Description Ability to inhibit Prostaglandin G/H synthase 2 by using freshly harvested mouse peritoneal macrophages | Bioorg Med Chem Lett 11: 165-8 (2001) BindingDB Entry DOI: 10.7270/Q2Z03BB2 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Prostaglandin G/H synthase 2 (Mus musculus (Mouse)) | BDBM11639 (4-[5-(4-methylphenyl)-3-(trifluoromethyl)-1H-pyraz...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 52.4 | n/a | n/a | n/a | n/a | n/a | n/a |
AmorePacific R&D Center Curated by ChEMBL | Assay Description In vitro inhibitory activity against prostaglandin G/H synthase 2 using mouse peritoneal macrophage method | J Med Chem 47: 792-804 (2004) Article DOI: 10.1021/jm020545z BindingDB Entry DOI: 10.7270/Q2QZ2DPG | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Prostaglandin G/H synthase 2 (Mus musculus (Mouse)) | BDBM50218964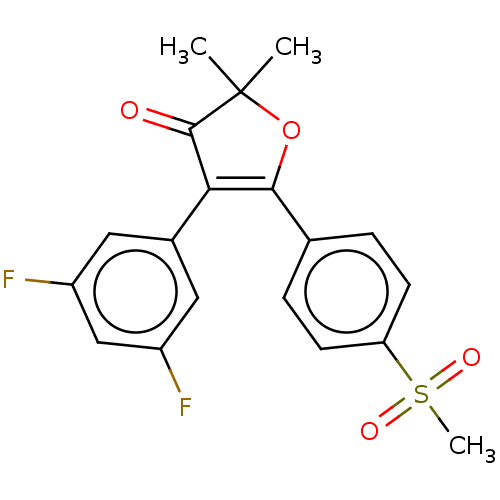 (CHEMBL44223) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 52.9 | n/a | n/a | n/a | n/a | n/a | n/a |
AmorePacific R&D Center Curated by ChEMBL | Assay Description Inhibitory concentration against prostaglandin G/H synthase 2 in mouse peritoneal macrophages | Bioorg Med Chem Lett 14: 1757-60 (2004) BindingDB Entry DOI: 10.7270/Q2ZK5JVW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Mus musculus (Mouse)) | BDBM50218973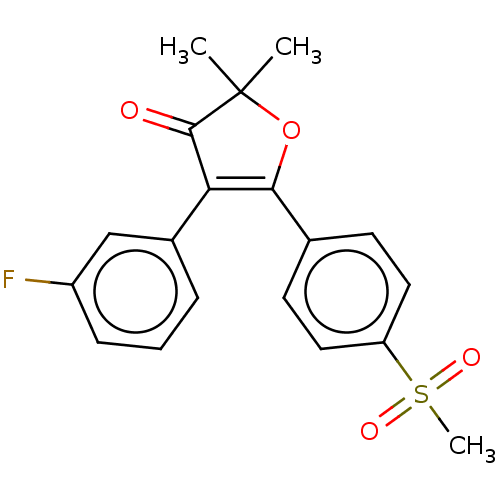 (CHEMBL44001) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 55.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pacific Corporation R&D Center Curated by ChEMBL | Assay Description Ability to inhibit Prostaglandin G/H synthase 2 by using freshly harvested mouse peritoneal macrophages | Bioorg Med Chem Lett 11: 165-8 (2001) BindingDB Entry DOI: 10.7270/Q2Z03BB2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Mus musculus (Mouse)) | BDBM50218973 (CHEMBL44001) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 55.5 | n/a | n/a | n/a | n/a | n/a | n/a |
AmorePacific R&D Center Curated by ChEMBL | Assay Description In vitro inhibitory activity against prostaglandin G/H synthase 2 using mouse peritoneal macrophage method | J Med Chem 47: 792-804 (2004) Article DOI: 10.1021/jm020545z BindingDB Entry DOI: 10.7270/Q2QZ2DPG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Mus musculus (Mouse)) | BDBM50474741 (CHEMBL355729) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 68.4 | n/a | n/a | n/a | n/a | n/a | n/a |
AmorePacific R&D Center Curated by ChEMBL | Assay Description In vitro inhibitory activity against prostaglandin G/H synthase 2 using mouse peritoneal macrophage method | J Med Chem 47: 792-804 (2004) Article DOI: 10.1021/jm020545z BindingDB Entry DOI: 10.7270/Q2QZ2DPG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Mus musculus (Mouse)) | BDBM50474762 (CHEMBL166378) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 70.5 | n/a | n/a | n/a | n/a | n/a | n/a |
AmorePacific R&D Center Curated by ChEMBL | Assay Description In vitro inhibitory activity against prostaglandin G/H synthase 2 using mouse peritoneal macrophage method | J Med Chem 47: 792-804 (2004) Article DOI: 10.1021/jm020545z BindingDB Entry DOI: 10.7270/Q2QZ2DPG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Mus musculus) | BDBM50474774 (CHEMBL352430) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 71.2 | n/a | n/a | n/a | n/a | n/a | n/a |
AmorePacific R&D Center Curated by ChEMBL | Assay Description In vitro inhibitory activity against prostaglandin G/H synthase 1 using mouse peritoneal macrophage method | J Med Chem 47: 792-804 (2004) Article DOI: 10.1021/jm020545z BindingDB Entry DOI: 10.7270/Q2QZ2DPG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Mus musculus (Mouse)) | BDBM50474774 (CHEMBL352430) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 71.2 | n/a | n/a | n/a | n/a | n/a | n/a |
AmorePacific R&D Center Curated by ChEMBL | Assay Description In vitro inhibitory activity against prostaglandin G/H synthase 2 using mouse peritoneal macrophage method | J Med Chem 47: 792-804 (2004) Article DOI: 10.1021/jm020545z BindingDB Entry DOI: 10.7270/Q2QZ2DPG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Mus musculus (Mouse)) | BDBM50218970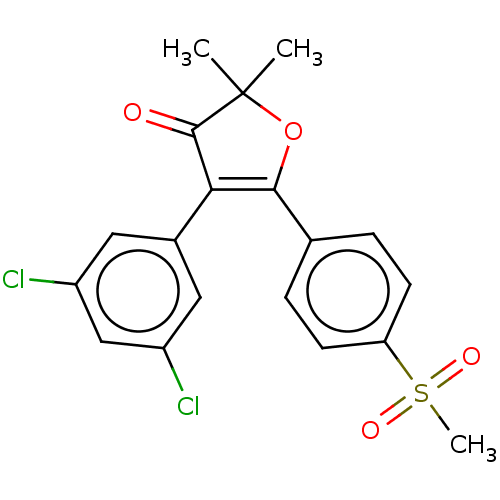 (CHEMBL43893) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 72.9 | n/a | n/a | n/a | n/a | n/a | n/a |
Pacific Corporation R&D Center Curated by ChEMBL | Assay Description Ability to inhibit Prostaglandin G/H synthase 2 by using freshly harvested mouse peritoneal macrophages | Bioorg Med Chem Lett 11: 165-8 (2001) BindingDB Entry DOI: 10.7270/Q2Z03BB2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Mus musculus (Mouse)) | BDBM50218970 (CHEMBL43893) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 72.9 | n/a | n/a | n/a | n/a | n/a | n/a |
AmorePacific R&D Center Curated by ChEMBL | Assay Description In vitro inhibitory activity against prostaglandin G/H synthase 2 using mouse peritoneal macrophage method | J Med Chem 47: 792-804 (2004) Article DOI: 10.1021/jm020545z BindingDB Entry DOI: 10.7270/Q2QZ2DPG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Mus musculus (Mouse)) | BDBM50474752 (CHEMBL352329) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 75.7 | n/a | n/a | n/a | n/a | n/a | n/a |
AmorePacific R&D Center Curated by ChEMBL | Assay Description In vitro inhibitory activity against prostaglandin G/H synthase 2 using mouse peritoneal macrophage method | J Med Chem 47: 792-804 (2004) Article DOI: 10.1021/jm020545z BindingDB Entry DOI: 10.7270/Q2QZ2DPG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 214 total ) | Next | Last >> |