

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Gastrin/cholecystokinin type B receptor (Homo sapiens (Human)) | BDBM50040674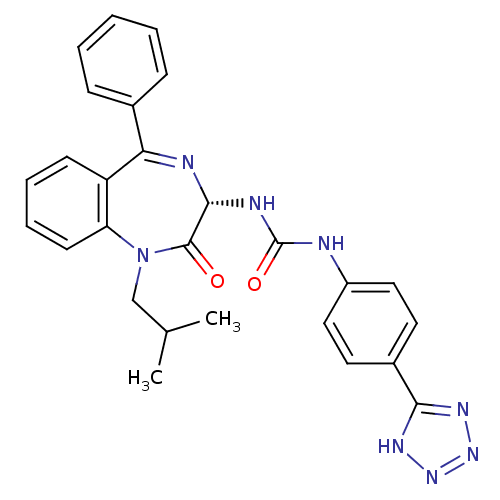 (1-((S)-1-Isobutyl-2-oxo-5-phenyl-2,3-dihydro-1H-be...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.142 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description The compound was tested for binding activity against Cholecystokinin type B receptor from rat pancreatic tissue using [125]BH CCK-8 as radioligand | J Med Chem 37: 722-4 (1994) BindingDB Entry DOI: 10.7270/Q2CN72ZM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (Homo sapiens (Human)) | BDBM50043556 (CHEMBL137516 | N,N-Diethyl-2-{3-[3-(3-methoxy-phen...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.220 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of binding of [125I]-CCK-8 to the cholecystokinin type B receptor | J Med Chem 36: 4276-92 (1994) BindingDB Entry DOI: 10.7270/Q22J6CH9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (Homo sapiens (Human)) | BDBM50043546 (1-(3-Methoxy-phenyl)-3-[2-oxo-1-(2-oxo-2-pyrrolidi...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.240 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of binding of [125I]-CCK-8 to the cholecystokinin type B receptor | J Med Chem 36: 4276-92 (1994) BindingDB Entry DOI: 10.7270/Q22J6CH9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (RAT) | BDBM50456295 (CHEMBL2111920) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.260 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of binding of [125I]CCK-8 to cholecystokinin type A receptor in rat pancreatic tissue | J Med Chem 36: 4276-92 (1994) BindingDB Entry DOI: 10.7270/Q22J6CH9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (Homo sapiens (Human)) | BDBM50040678 (1-((S)-1-Methyl-2-oxo-5-phenyl-2,3-dihydro-1H-benz...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.266 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description The compound was tested for binding activity against Cholecystokinin type B receptor from rat pancreatic tissue using [125]BH CCK-8 as radioligand | J Med Chem 37: 722-4 (1994) BindingDB Entry DOI: 10.7270/Q2CN72ZM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (Homo sapiens (Human)) | BDBM50043493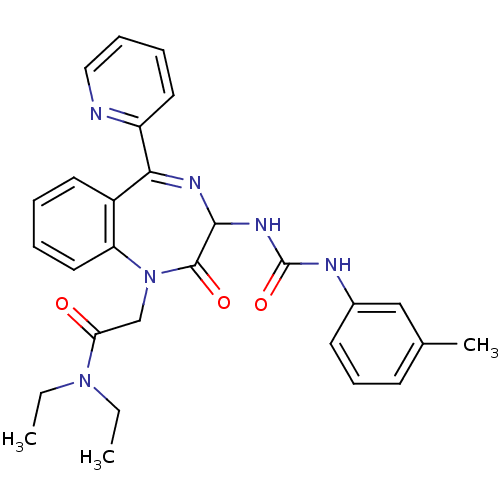 (CHEMBL313813 | N,N-Diethyl-2-[2-oxo-5-pyridin-2-yl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.560 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of binding of [125I]-CCK-8 to the cholecystokinin type B receptor | J Med Chem 36: 4276-92 (1994) BindingDB Entry DOI: 10.7270/Q22J6CH9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (Homo sapiens (Human)) | BDBM50043575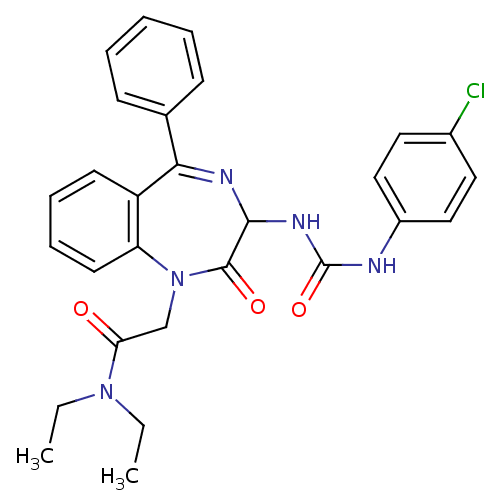 (2-{3-[3-(4-Chloro-phenyl)-ureido]-2-oxo-5-phenyl-2...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of binding of [125I]-CCK-8 to the cholecystokinin type B receptor | J Med Chem 36: 4276-92 (1994) BindingDB Entry DOI: 10.7270/Q22J6CH9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (Homo sapiens (Human)) | BDBM50043548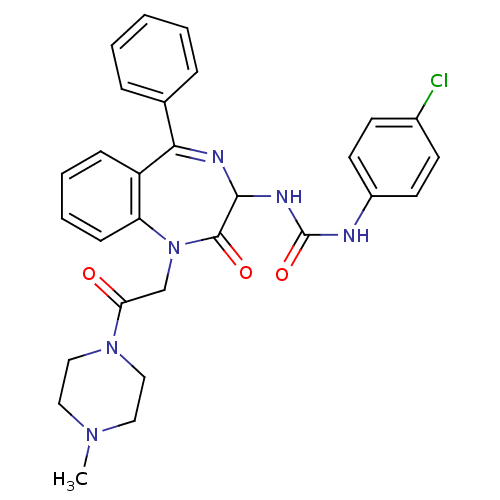 (1-(4-Chloro-phenyl)-3-{1-[2-(4-methyl-piperazin-1-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of binding of [125I]-CCK-8 to the cholecystokinin type B receptor | J Med Chem 36: 4276-92 (1994) BindingDB Entry DOI: 10.7270/Q22J6CH9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (Homo sapiens (Human)) | BDBM50020269 ((RS)-{3-[3-(4-Chloro-phenyl)-ureido]-2-oxo-5-pheny...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of binding of [125I]-CCK-8 to the cholecystokinin type B receptor | J Med Chem 36: 4276-92 (1994) BindingDB Entry DOI: 10.7270/Q22J6CH9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (Homo sapiens (Human)) | BDBM50043541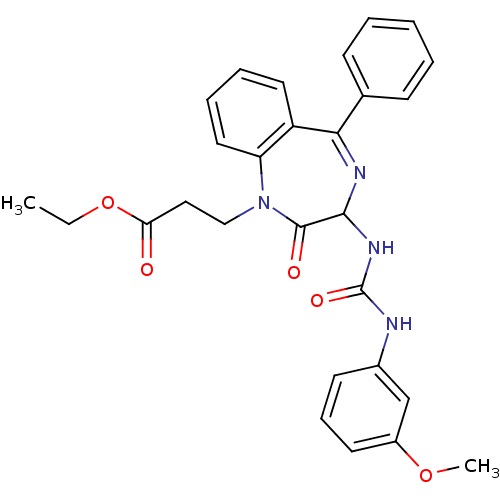 (3-{3-[3-(3-Methoxy-phenyl)-ureido]-2-oxo-5-phenyl-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of binding of [125I]-CCK-8 to the cholecystokinin type B receptor | J Med Chem 36: 4276-92 (1994) BindingDB Entry DOI: 10.7270/Q22J6CH9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (Homo sapiens (Human)) | BDBM50040679 (1-((S)-1-Methyl-2-oxo-5-phenyl-2,3-dihydro-1H-benz...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.02 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description The compound was tested for binding activity against Cholecystokinin type B receptor from rat pancreatic tissue using [125]BH CCK-8 as radioligand | J Med Chem 37: 722-4 (1994) BindingDB Entry DOI: 10.7270/Q2CN72ZM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (Homo sapiens (Human)) | BDBM50020271 ((RS)-[3-[3-(4-Chloro-phenyl)-ureido]-5-(2-fluoro-p...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of binding of [125I]-CCK-8 to the cholecystokinin type B receptor | J Med Chem 36: 4276-92 (1994) BindingDB Entry DOI: 10.7270/Q22J6CH9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (RAT) | BDBM50456293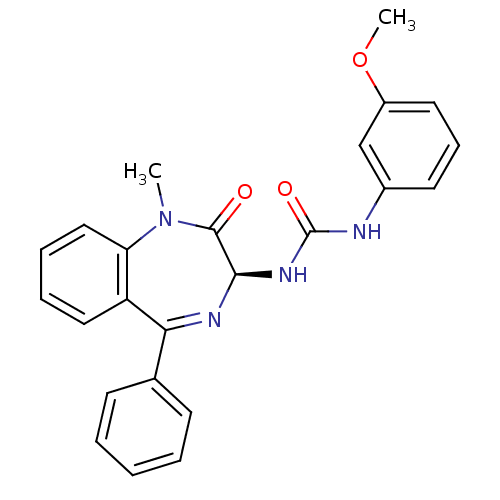 (CHEMBL2111922) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of binding of [125I]CCK-8 to cholecystokinin type A receptor in rat pancreatic tissue | J Med Chem 36: 4276-92 (1994) BindingDB Entry DOI: 10.7270/Q22J6CH9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (Homo sapiens (Human)) | BDBM50456298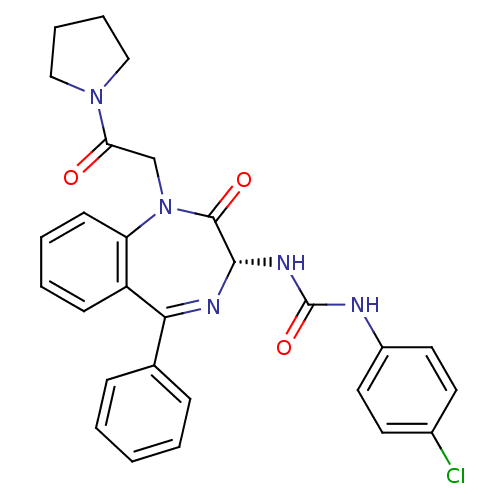 (CHEMBL2092976) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of binding of [125I]-CCK-8 to the cholecystokinin type B receptor | J Med Chem 36: 4276-92 (1994) BindingDB Entry DOI: 10.7270/Q22J6CH9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (Homo sapiens (Human)) | BDBM50020270 ((RS)-1-(4-Chloro-phenyl)-3-[2-oxo-1-(2-oxo-2-pyrro...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of binding of [125I]-CCK-8 to the cholecystokinin type B receptor | J Med Chem 36: 4276-92 (1994) BindingDB Entry DOI: 10.7270/Q22J6CH9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C theta type (Homo sapiens (Human)) | BDBM50028420 (CHEMBL3355137) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Bioresearch Center Curated by ChEMBL | Assay Description Inhibition of human recombinant PKCtheta expressed in Sf21 cells using Bio-cdc peptide substrate and ATP after 60 mins by time-resolved fluorescence ... | J Med Chem 58: 333-46 (2015) Article DOI: 10.1021/jm5013006 BindingDB Entry DOI: 10.7270/Q2B859P2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C theta type (Homo sapiens (Human)) | BDBM50028417 (CHEMBL3355134) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Bioresearch Center Curated by ChEMBL | Assay Description Inhibition of human recombinant PKCtheta expressed in Sf21 cells using Bio-cdc peptide substrate and ATP after 60 mins by time-resolved fluorescence ... | J Med Chem 58: 333-46 (2015) Article DOI: 10.1021/jm5013006 BindingDB Entry DOI: 10.7270/Q2B859P2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C theta type (Homo sapiens (Human)) | BDBM50028422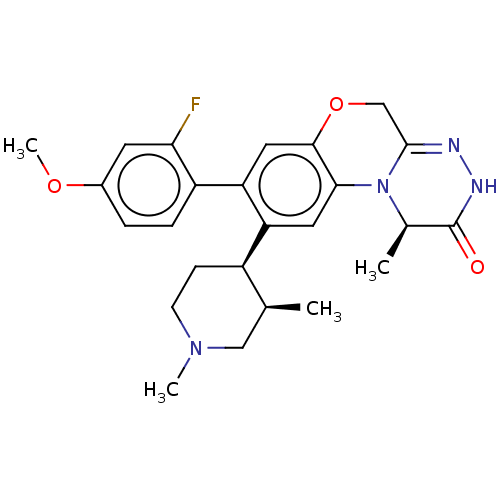 (CHEMBL3355138) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Bioresearch Center Curated by ChEMBL | Assay Description Inhibition of human recombinant PKCtheta expressed in Sf21 cells using Bio-cdc peptide substrate and ATP after 60 mins by time-resolved fluorescence ... | J Med Chem 58: 333-46 (2015) Article DOI: 10.1021/jm5013006 BindingDB Entry DOI: 10.7270/Q2B859P2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (Homo sapiens (Human)) | BDBM50456285 (CHEMBL2448131) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of binding of [125I]-CCK-8 to the cholecystokinin type B receptor | J Med Chem 36: 4276-92 (1994) BindingDB Entry DOI: 10.7270/Q22J6CH9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (Homo sapiens (Human)) | BDBM50043507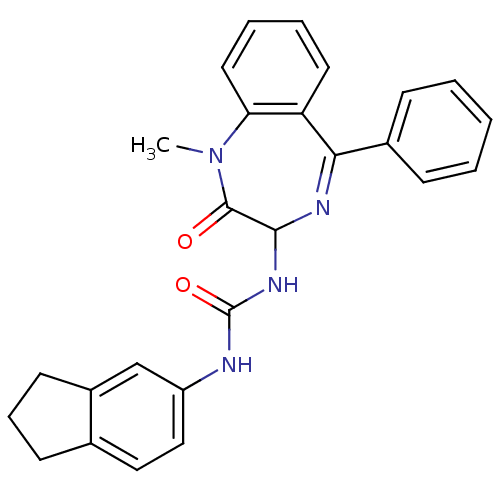 (1-Indan-5-yl-3-(1-methyl-2-oxo-5-phenyl-2,3-dihydr...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of binding of [125I]-CCK-8 to the cholecystokinin type B receptor | J Med Chem 36: 4276-92 (1994) BindingDB Entry DOI: 10.7270/Q22J6CH9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (RAT) | BDBM50043497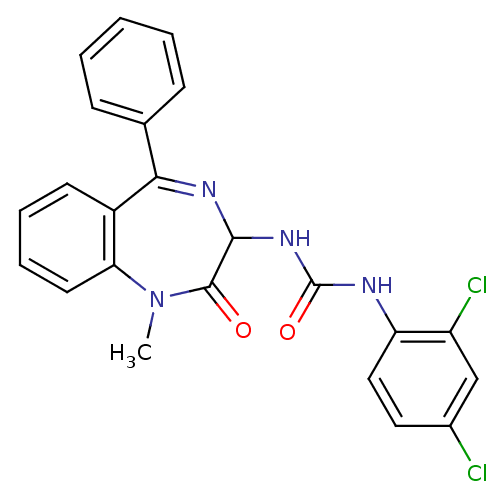 (1-(2,4-Dichloro-phenyl)-3-(1-methyl-2-oxo-5-phenyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of binding of [125I]CCK-8 to cholecystokinin type A receptor in rat pancreatic tissue | J Med Chem 36: 4276-92 (1994) BindingDB Entry DOI: 10.7270/Q22J6CH9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C theta type (Homo sapiens (Human)) | BDBM50028433 (CHEMBL3355125) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Bioresearch Center Curated by ChEMBL | Assay Description Inhibition of human recombinant PKCtheta expressed in Sf21 cells using Bio-cdc peptide substrate and ATP after 60 mins by time-resolved fluorescence ... | J Med Chem 58: 333-46 (2015) Article DOI: 10.1021/jm5013006 BindingDB Entry DOI: 10.7270/Q2B859P2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C theta type (Homo sapiens (Human)) | BDBM50028423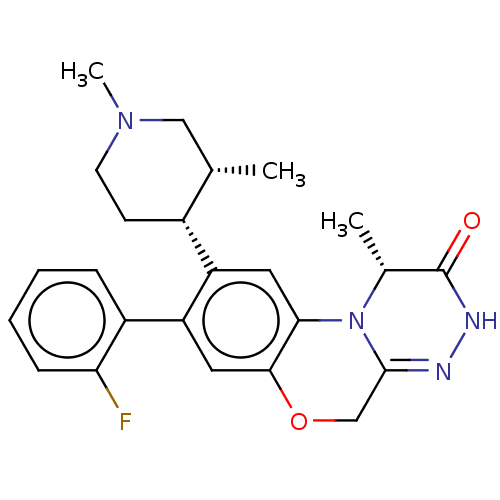 (CHEMBL3355127) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Bioresearch Center Curated by ChEMBL | Assay Description Inhibition of human recombinant PKCtheta expressed in Sf21 cells using Bio-cdc peptide substrate and ATP after 60 mins by time-resolved fluorescence ... | J Med Chem 58: 333-46 (2015) Article DOI: 10.1021/jm5013006 BindingDB Entry DOI: 10.7270/Q2B859P2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C theta type (Homo sapiens (Human)) | BDBM50028439 (CHEMBL3355119) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Bioresearch Center Curated by ChEMBL | Assay Description Inhibition of human recombinant PKCtheta expressed in Sf21 cells using Bio-cdc peptide substrate and ATP after 60 mins by time-resolved fluorescence ... | J Med Chem 58: 333-46 (2015) Article DOI: 10.1021/jm5013006 BindingDB Entry DOI: 10.7270/Q2B859P2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-activating factor acetylhydrolase (Homo sapiens (Human)) | BDBM50429094 (CHEMBL2335783) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
ActivX Biosciences, Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant N-terminal hexahistidine-tagged Lp-PLA2 expressed in Escherichia coli using 2- thio-PAF as substrate incubated for 20... | Bioorg Med Chem Lett 23: 1553-6 (2013) Article DOI: 10.1016/j.bmcl.2012.11.048 BindingDB Entry DOI: 10.7270/Q2571DCV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (Homo sapiens (Human)) | BDBM50043537 (1-(3-Methoxy-phenyl)-3-(1-methyl-2-oxo-5-phenyl-2,...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of binding of [125I]-CCK-8 to the cholecystokinin type B receptor | J Med Chem 36: 4276-92 (1994) BindingDB Entry DOI: 10.7270/Q22J6CH9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (RAT) | BDBM50043563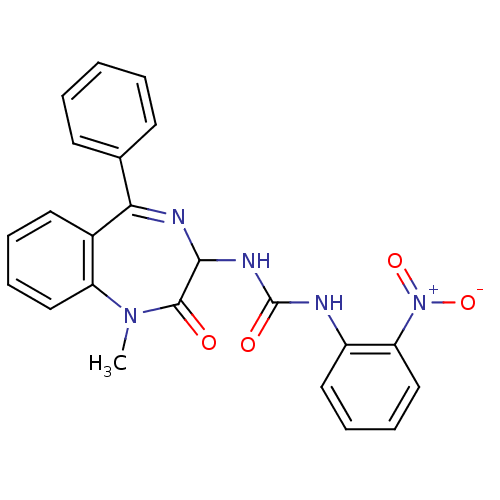 (1-(1-Methyl-2-oxo-5-phenyl-2,3-dihydro-1H-benzo[e]...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of binding of [125I]CCK-8 to cholecystokinin type A receptor in rat pancreatic tissue | J Med Chem 36: 4276-92 (1994) BindingDB Entry DOI: 10.7270/Q22J6CH9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-activating factor acetylhydrolase (Homo sapiens (Human)) | BDBM50429098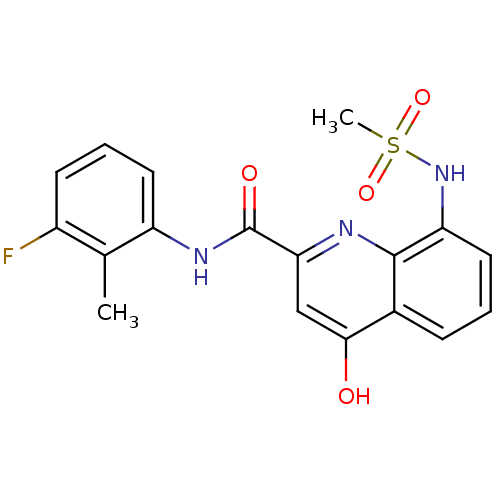 (CHEMBL2335784) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
ActivX Biosciences, Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant N-terminal hexahistidine-tagged Lp-PLA2 expressed in Escherichia coli using 2- thio-PAF as substrate incubated for 20... | Bioorg Med Chem Lett 23: 1553-6 (2013) Article DOI: 10.1016/j.bmcl.2012.11.048 BindingDB Entry DOI: 10.7270/Q2571DCV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (Homo sapiens (Human)) | BDBM50043481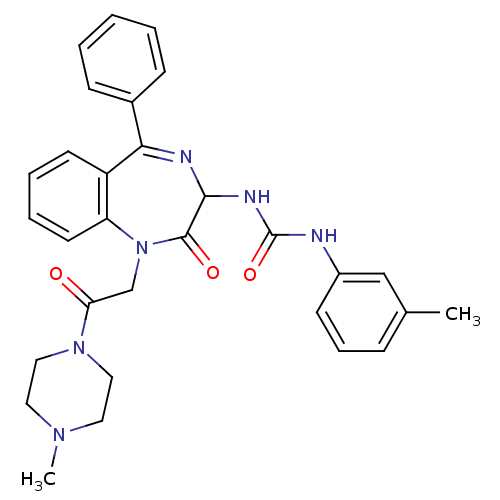 (1-{1-[2-(4-Methyl-piperazin-1-yl)-2-oxo-ethyl]-2-o...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of binding of [125I]-CCK-8 to the cholecystokinin type B receptor | J Med Chem 36: 4276-92 (1994) BindingDB Entry DOI: 10.7270/Q22J6CH9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (Homo sapiens (Human)) | BDBM50456296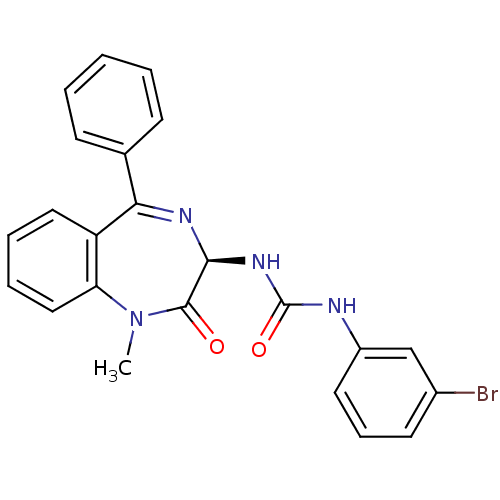 (CHEMBL2111923) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of binding of [125I]-CCK-8 to the cholecystokinin type B receptor | J Med Chem 36: 4276-92 (1994) BindingDB Entry DOI: 10.7270/Q22J6CH9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (Homo sapiens (Human)) | BDBM50043488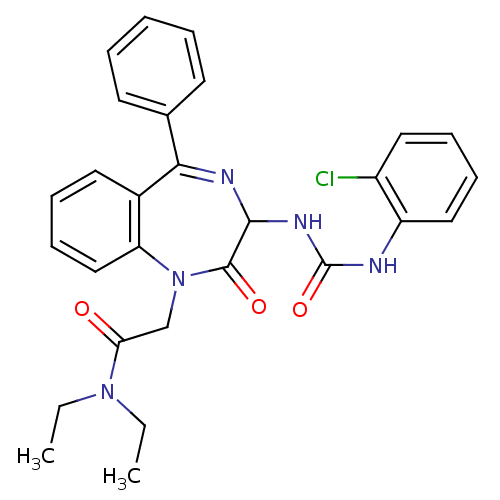 (2-{3-[3-(2-Chloro-phenyl)-ureido]-2-oxo-5-phenyl-2...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 3.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of binding of [125I]-CCK-8 to the cholecystokinin type B receptor | J Med Chem 36: 4276-92 (1994) BindingDB Entry DOI: 10.7270/Q22J6CH9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (Homo sapiens (Human)) | BDBM50043505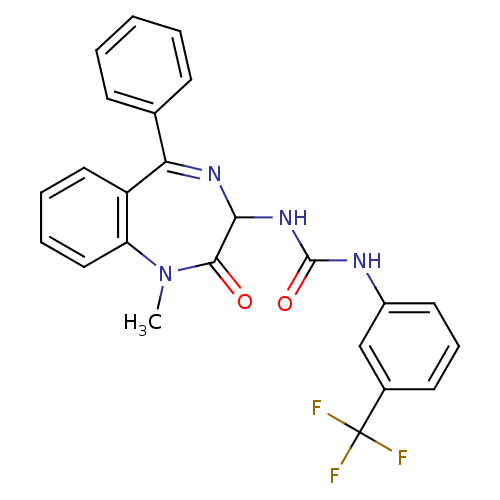 (1-(1-Methyl-2-oxo-5-phenyl-2,3-dihydro-1H-benzo[e]...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 3.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of binding of [125I]-CCK-8 to the cholecystokinin type B receptor | J Med Chem 36: 4276-92 (1994) BindingDB Entry DOI: 10.7270/Q22J6CH9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-activating factor acetylhydrolase (Homo sapiens (Human)) | BDBM50429070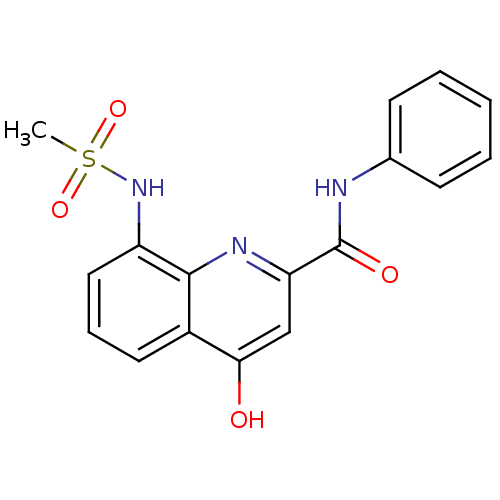 (CHEMBL2335742) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
ActivX Biosciences, Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant N-terminal hexahistidine-tagged Lp-PLA2 expressed in Escherichia coli using 2- thio-PAF as substrate incubated for 20... | Bioorg Med Chem Lett 23: 1553-6 (2013) Article DOI: 10.1016/j.bmcl.2012.11.048 BindingDB Entry DOI: 10.7270/Q2571DCV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C theta type (Homo sapiens (Human)) | BDBM50028443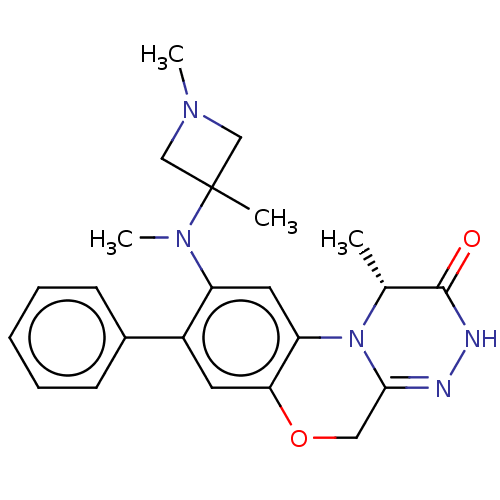 (CHEMBL3355117) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Bioresearch Center Curated by ChEMBL | Assay Description Inhibition of human recombinant PKCtheta expressed in Sf21 cells using Bio-cdc peptide substrate and ATP after 60 mins by time-resolved fluorescence ... | J Med Chem 58: 333-46 (2015) Article DOI: 10.1021/jm5013006 BindingDB Entry DOI: 10.7270/Q2B859P2 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Platelet-activating factor acetylhydrolase (Homo sapiens (Human)) | BDBM50429101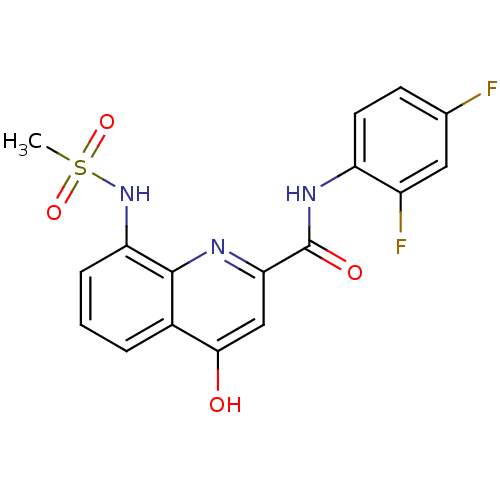 (CHEMBL2335780) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
ActivX Biosciences, Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant N-terminal hexahistidine-tagged Lp-PLA2 expressed in Escherichia coli using 2- thio-PAF as substrate incubated for 20... | Bioorg Med Chem Lett 23: 1553-6 (2013) Article DOI: 10.1016/j.bmcl.2012.11.048 BindingDB Entry DOI: 10.7270/Q2571DCV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C theta type (Homo sapiens (Human)) | BDBM50028442 (CHEMBL3355118) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Bioresearch Center Curated by ChEMBL | Assay Description Inhibition of human recombinant PKCtheta expressed in Sf21 cells using Bio-cdc peptide substrate and ATP after 60 mins by time-resolved fluorescence ... | J Med Chem 58: 333-46 (2015) Article DOI: 10.1021/jm5013006 BindingDB Entry DOI: 10.7270/Q2B859P2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C theta type (Homo sapiens (Human)) | BDBM50028435 (CHEMBL3355123) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Bioresearch Center Curated by ChEMBL | Assay Description Inhibition of human recombinant PKCtheta expressed in Sf21 cells using Bio-cdc peptide substrate and ATP after 60 mins by time-resolved fluorescence ... | J Med Chem 58: 333-46 (2015) Article DOI: 10.1021/jm5013006 BindingDB Entry DOI: 10.7270/Q2B859P2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (RAT) | BDBM50043513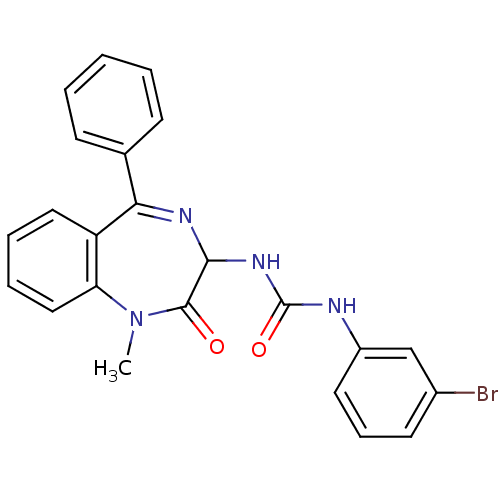 (1-(3-Bromo-phenyl)-3-(1-methyl-2-oxo-5-phenyl-2,3-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 4.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of binding of [125I]CCK-8 to cholecystokinin type A receptor in rat pancreatic tissue | J Med Chem 36: 4276-92 (1994) BindingDB Entry DOI: 10.7270/Q22J6CH9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (Homo sapiens (Human)) | BDBM50043568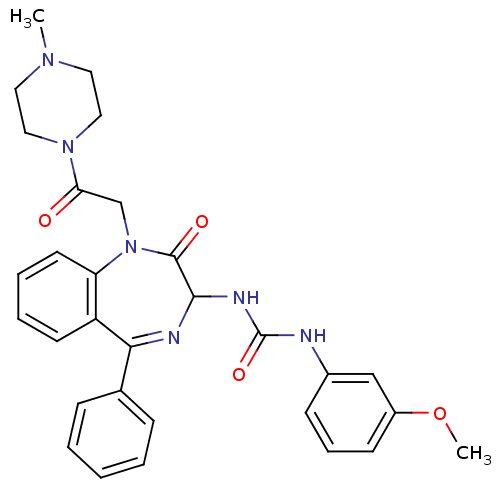 (1-(3-Methoxy-phenyl)-3-{1-[2-(4-methyl-piperazin-1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of binding of [125I]-CCK-8 to the cholecystokinin type B receptor | J Med Chem 36: 4276-92 (1994) BindingDB Entry DOI: 10.7270/Q22J6CH9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (RAT) | BDBM50061220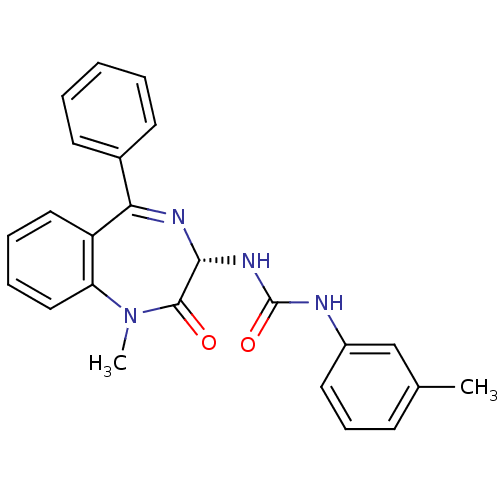 (1-((S)-1-Methyl-2-oxo-5-phenyl-2,3-dihydro-1H-benz...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 4.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of binding of [125I]CCK-8 to cholecystokinin type A receptor in rat pancreatic tissue | J Med Chem 36: 4276-92 (1994) BindingDB Entry DOI: 10.7270/Q22J6CH9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (Homo sapiens (Human)) | BDBM50043572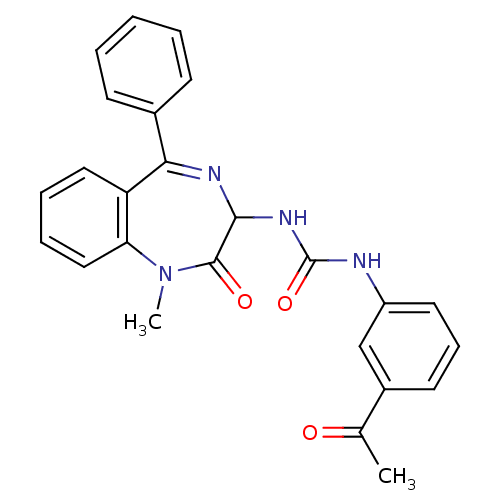 (1-(3-Acetyl-phenyl)-3-(1-methyl-2-oxo-5-phenyl-2,3...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 4.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of binding of [125I]-CCK-8 to the cholecystokinin type B receptor | J Med Chem 36: 4276-92 (1994) BindingDB Entry DOI: 10.7270/Q22J6CH9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (Homo sapiens (Human)) | BDBM50043567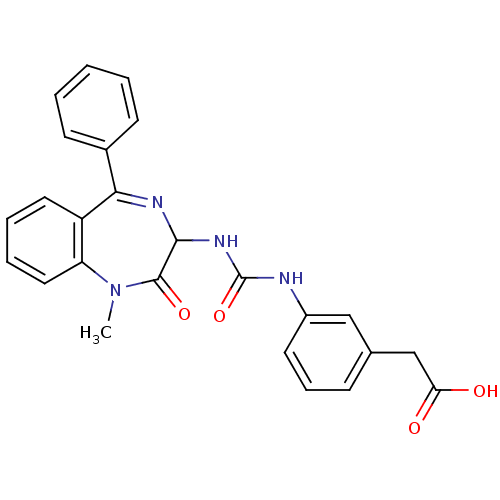 (CHEMBL137567 | {3-[3-(1-Methyl-2-oxo-5-phenyl-2,3-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of binding of [125I]-CCK-8 to the cholecystokinin type B receptor | J Med Chem 36: 4276-92 (1994) BindingDB Entry DOI: 10.7270/Q22J6CH9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (Homo sapiens (Human)) | BDBM50043513 (1-(3-Bromo-phenyl)-3-(1-methyl-2-oxo-5-phenyl-2,3-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of binding of [125I]-CCK-8 to the cholecystokinin type B receptor | J Med Chem 36: 4276-92 (1994) BindingDB Entry DOI: 10.7270/Q22J6CH9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C theta type (Homo sapiens (Human)) | BDBM50028419 (CHEMBL3355136) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Bioresearch Center Curated by ChEMBL | Assay Description Inhibition of human recombinant PKCtheta expressed in Sf21 cells using Bio-cdc peptide substrate and ATP after 60 mins by time-resolved fluorescence ... | J Med Chem 58: 333-46 (2015) Article DOI: 10.1021/jm5013006 BindingDB Entry DOI: 10.7270/Q2B859P2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C theta type (Homo sapiens (Human)) | BDBM50028432 (CHEMBL3355126) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Bioresearch Center Curated by ChEMBL | Assay Description Inhibition of human recombinant PKCtheta expressed in Sf21 cells using Bio-cdc peptide substrate and ATP after 60 mins by time-resolved fluorescence ... | J Med Chem 58: 333-46 (2015) Article DOI: 10.1021/jm5013006 BindingDB Entry DOI: 10.7270/Q2B859P2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (Homo sapiens (Human)) | BDBM50043503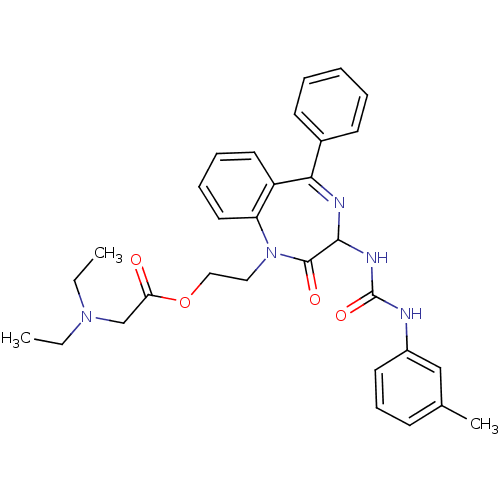 (CHEMBL135218 | Diethylamino-acetic acid 2-[2-oxo-5...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 5.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of binding of [125I]-CCK-8 to the cholecystokinin type B receptor | J Med Chem 36: 4276-92 (1994) BindingDB Entry DOI: 10.7270/Q22J6CH9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (Homo sapiens (Human)) | BDBM50043518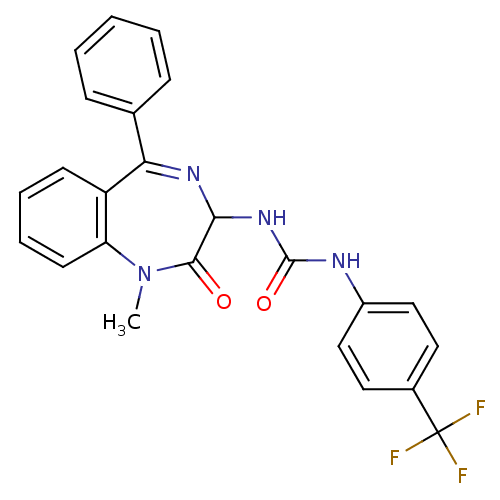 (1-(1-Methyl-2-oxo-5-phenyl-2,3-dihydro-1H-benzo[e]...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 5.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of binding of [125I]-CCK-8 to the cholecystokinin type B receptor | J Med Chem 36: 4276-92 (1994) BindingDB Entry DOI: 10.7270/Q22J6CH9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (Homo sapiens (Human)) | BDBM50043521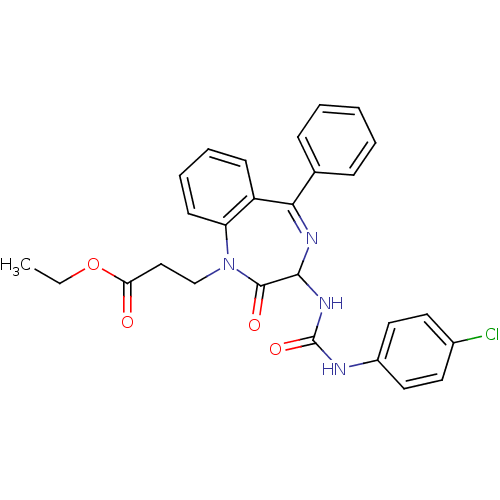 (3-{3-[3-(4-Chloro-phenyl)-ureido]-2-oxo-5-phenyl-2...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 5.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of binding of [125I]-CCK-8 to the cholecystokinin type B receptor | J Med Chem 36: 4276-92 (1994) BindingDB Entry DOI: 10.7270/Q22J6CH9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (Homo sapiens (Human)) | BDBM50043577 (1-[3-(1-Hydroxy-1-methyl-ethyl)-benzyl]-3-(1-methy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 5.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of binding of [125I]-CCK-8 to the cholecystokinin type B receptor | J Med Chem 36: 4276-92 (1994) BindingDB Entry DOI: 10.7270/Q22J6CH9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (Homo sapiens (Human)) | BDBM50043559 (1-[1-(3-Chloro-2-hydroxy-propyl)-2-oxo-5-phenyl-2,...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of binding of [125I]-CCK-8 to the cholecystokinin type B receptor | J Med Chem 36: 4276-92 (1994) BindingDB Entry DOI: 10.7270/Q22J6CH9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 1298 total ) | Next | Last >> |