 Found 47 hits with Last Name = 'lin' and Initial = 'lc'
Found 47 hits with Last Name = 'lin' and Initial = 'lc' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
1-aminocyclopropane-1-carboxylate synthase 5
(Arabidopsis thaliana) | BDBM50487185
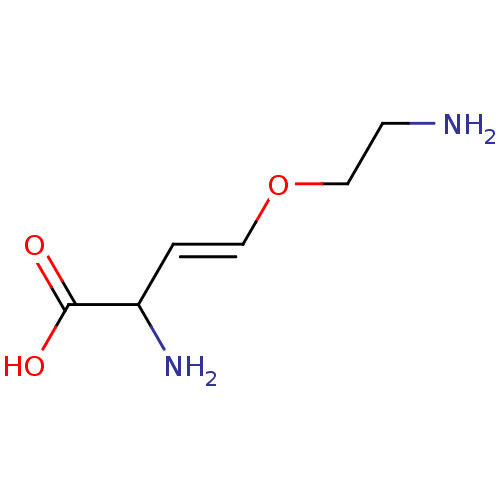
(AMINOETHOXYVINYLGLYCINE)Show InChI InChI=1S/C6H12N2O3/c7-2-4-11-3-1-5(8)6(9)10/h1,3,5H,2,4,7-8H2,(H,9,10)/b3-1+ | MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Defense Medical Center
Curated by ChEMBL
| Assay Description
Competitive inhibition of Arabidopsis thaliana recombinant ACS5 expressed in Escherichia coli (BL21) assessed as oxidation of ACC to ethylene by Line... |
J Biol Chem 285: 33445-56 (2010)
Article DOI: 10.1074/jbc.M110.132498
BindingDB Entry DOI: 10.7270/Q2VQ35J0 |
More data for this
Ligand-Target Pair | |
1-aminocyclopropane-1-carboxylate synthase 5
(Arabidopsis thaliana) | BDBM50487187
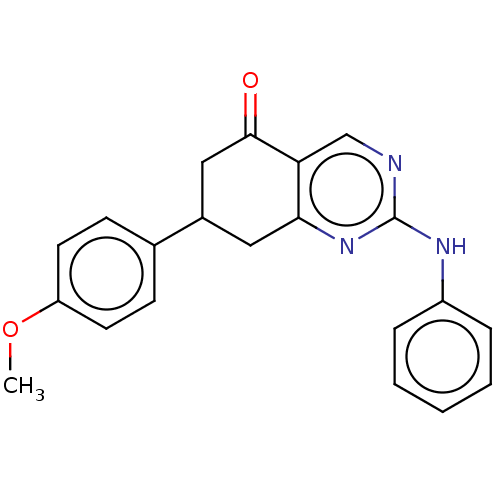
(CHEMBL2251967)Show InChI InChI=1S/C21H19N3O2/c1-26-17-9-7-14(8-10-17)15-11-19-18(20(25)12-15)13-22-21(24-19)23-16-5-3-2-4-6-16/h2-10,13,15H,11-12H2,1H3,(H,22,23,24) | MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| 23 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Defense Medical Center
Curated by ChEMBL
| Assay Description
Competitive inhibition of Arabidopsis thaliana recombinant ACS5 expressed in Escherichia coli (BL21) assessed as oxidation of ACC to ethylene by Line... |
J Biol Chem 285: 33445-56 (2010)
Article DOI: 10.1074/jbc.M110.132498
BindingDB Entry DOI: 10.7270/Q2VQ35J0 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Homo sapiens (Human)) | BDBM17638

(2-{1-[(4-chlorophenyl)carbonyl]-5-methoxy-2-methyl...)Show SMILES COc1ccc2n(C(=O)c3ccc(Cl)cc3)c(C)c(CC(O)=O)c2c1 Show InChI InChI=1S/C19H16ClNO4/c1-11-15(10-18(22)23)16-9-14(25-2)7-8-17(16)21(11)19(24)12-3-5-13(20)6-4-12/h3-9H,10H2,1-2H3,(H,22,23) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Research Institute of Chinese Medicine
Curated by ChEMBL
| Assay Description
Inhibition of ovine COX1 |
Bioorg Med Chem Lett 16: 4440-3 (2006)
Article DOI: 10.1016/j.bmcl.2006.06.036
BindingDB Entry DOI: 10.7270/Q2ZS2W33 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Homo sapiens (Human)) | BDBM17638

(2-{1-[(4-chlorophenyl)carbonyl]-5-methoxy-2-methyl...)Show SMILES COc1ccc2n(C(=O)c3ccc(Cl)cc3)c(C)c(CC(O)=O)c2c1 Show InChI InChI=1S/C19H16ClNO4/c1-11-15(10-18(22)23)16-9-14(25-2)7-8-17(16)21(11)19(24)12-3-5-13(20)6-4-12/h3-9H,10H2,1-2H3,(H,22,23) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
National Research Institute of Chinese Medicine
Curated by ChEMBL
| Assay Description
Inhibition of COX1 assessed as TBX2 production in human whole blood |
Bioorg Med Chem Lett 16: 4440-3 (2006)
Article DOI: 10.1016/j.bmcl.2006.06.036
BindingDB Entry DOI: 10.7270/Q2ZS2W33 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM17638

(2-{1-[(4-chlorophenyl)carbonyl]-5-methoxy-2-methyl...)Show SMILES COc1ccc2n(C(=O)c3ccc(Cl)cc3)c(C)c(CC(O)=O)c2c1 Show InChI InChI=1S/C19H16ClNO4/c1-11-15(10-18(22)23)16-9-14(25-2)7-8-17(16)21(11)19(24)12-3-5-13(20)6-4-12/h3-9H,10H2,1-2H3,(H,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 36 | n/a | n/a | n/a | n/a | n/a | n/a |
National Research Institute of Chinese Medicine
Curated by ChEMBL
| Assay Description
Inhibition of COX2 assessed as LPS-stimulated PGE2 production in human whole blood leukocyte |
Bioorg Med Chem Lett 16: 4440-3 (2006)
Article DOI: 10.1016/j.bmcl.2006.06.036
BindingDB Entry DOI: 10.7270/Q2ZS2W33 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM11639

(4-[5-(4-methylphenyl)-3-(trifluoromethyl)-1H-pyraz...)Show SMILES Cc1ccc(cc1)-c1cc(nn1-c1ccc(cc1)S(N)(=O)=O)C(F)(F)F Show InChI InChI=1S/C17H14F3N3O2S/c1-11-2-4-12(5-3-11)15-10-16(17(18,19)20)22-23(15)13-6-8-14(9-7-13)26(21,24)25/h2-10H,1H3,(H2,21,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 330 | n/a | n/a | n/a | n/a | n/a | n/a |
National Research Institute of Chinese Medicine
Curated by ChEMBL
| Assay Description
Inhibition of COX2 assessed as LPS-stimulated PGE2 production in human whole blood leukocyte |
Bioorg Med Chem Lett 16: 4440-3 (2006)
Article DOI: 10.1016/j.bmcl.2006.06.036
BindingDB Entry DOI: 10.7270/Q2ZS2W33 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Prostaglandin G/H synthase 2
(Ovis aries (Sheep)) | BDBM17638

(2-{1-[(4-chlorophenyl)carbonyl]-5-methoxy-2-methyl...)Show SMILES COc1ccc2n(C(=O)c3ccc(Cl)cc3)c(C)c(CC(O)=O)c2c1 Show InChI InChI=1S/C19H16ClNO4/c1-11-15(10-18(22)23)16-9-14(25-2)7-8-17(16)21(11)19(24)12-3-5-13(20)6-4-12/h3-9H,10H2,1-2H3,(H,22,23) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 340 | n/a | n/a | n/a | n/a | n/a | n/a |
National Research Institute of Chinese Medicine
Curated by ChEMBL
| Assay Description
Inhibition of ovine COX2 |
Bioorg Med Chem Lett 16: 4440-3 (2006)
Article DOI: 10.1016/j.bmcl.2006.06.036
BindingDB Entry DOI: 10.7270/Q2ZS2W33 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Prostaglandin G/H synthase 1
(Homo sapiens (Human)) | BDBM23926

((E)-resveratrol | 5-[(E)-2-(4-hydroxyphenyl)etheny...)Show InChI InChI=1S/C14H12O3/c15-12-5-3-10(4-6-12)1-2-11-7-13(16)9-14(17)8-11/h1-9,15-17H/b2-1+ | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 380 | n/a | n/a | n/a | n/a | n/a | n/a |
National Research Institute of Chinese Medicine
Curated by ChEMBL
| Assay Description
Inhibition of ovine COX1 |
Bioorg Med Chem Lett 16: 4440-3 (2006)
Article DOI: 10.1016/j.bmcl.2006.06.036
BindingDB Entry DOI: 10.7270/Q2ZS2W33 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Ovis aries (Sheep)) | BDBM11639

(4-[5-(4-methylphenyl)-3-(trifluoromethyl)-1H-pyraz...)Show SMILES Cc1ccc(cc1)-c1cc(nn1-c1ccc(cc1)S(N)(=O)=O)C(F)(F)F Show InChI InChI=1S/C17H14F3N3O2S/c1-11-2-4-12(5-3-11)15-10-16(17(18,19)20)22-23(15)13-6-8-14(9-7-13)26(21,24)25/h2-10H,1H3,(H2,21,24,25) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 440 | n/a | n/a | n/a | n/a | n/a | n/a |
National Research Institute of Chinese Medicine
Curated by ChEMBL
| Assay Description
Inhibition of ovine COX2 |
Bioorg Med Chem Lett 16: 4440-3 (2006)
Article DOI: 10.1016/j.bmcl.2006.06.036
BindingDB Entry DOI: 10.7270/Q2ZS2W33 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
1-aminocyclopropane-1-carboxylate synthase 5
(Arabidopsis thaliana) | BDBM50487187

(CHEMBL2251967)Show InChI InChI=1S/C21H19N3O2/c1-26-17-9-7-14(8-10-17)15-11-19-18(20(25)12-15)13-22-21(24-19)23-16-5-3-2-4-6-16/h2-10,13,15H,11-12H2,1H3,(H,22,23,24) | MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
National Defense Medical Center
Curated by ChEMBL
| Assay Description
Inhibition of Arabidopsis thaliana recombinant ACS5 expressed in Escherichia coli (BL21) assessed as oxidation of ACC to ethylene preincubated for 30... |
J Biol Chem 285: 33445-56 (2010)
Article DOI: 10.1074/jbc.M110.132498
BindingDB Entry DOI: 10.7270/Q2VQ35J0 |
More data for this
Ligand-Target Pair | |
1-aminocyclopropane-1-carboxylate synthase 5
(Arabidopsis thaliana) | BDBM50487185

(AMINOETHOXYVINYLGLYCINE)Show InChI InChI=1S/C6H12N2O3/c7-2-4-11-3-1-5(8)6(9)10/h1,3,5H,2,4,7-8H2,(H,9,10)/b3-1+ | MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 700 | n/a | n/a | n/a | n/a | n/a | n/a |
National Defense Medical Center
Curated by ChEMBL
| Assay Description
Inhibition of Arabidopsis thaliana recombinant ACS5 expressed in Escherichia coli (BL21) assessed as oxidation of ACC to ethylene preincubated for 30... |
J Biol Chem 285: 33445-56 (2010)
Article DOI: 10.1074/jbc.M110.132498
BindingDB Entry DOI: 10.7270/Q2VQ35J0 |
More data for this
Ligand-Target Pair | |
1-aminocyclopropane-1-carboxylate synthase 5
(Arabidopsis thaliana) | BDBM50487184
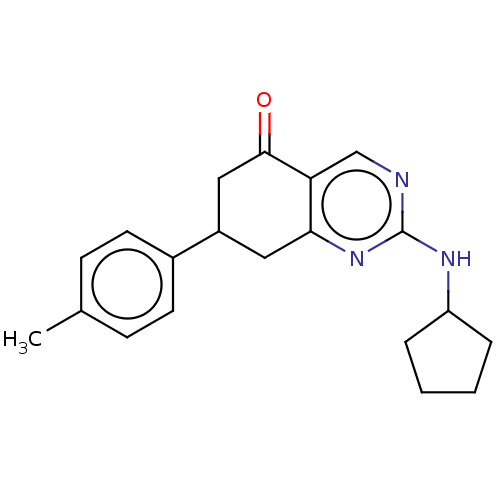
(CHEMBL2251970)Show InChI InChI=1S/C20H23N3O/c1-13-6-8-14(9-7-13)15-10-18-17(19(24)11-15)12-21-20(23-18)22-16-4-2-3-5-16/h6-9,12,15-16H,2-5,10-11H2,1H3,(H,21,22,23) | MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
National Defense Medical Center
Curated by ChEMBL
| Assay Description
Inhibition of Arabidopsis thaliana recombinant ACS5 expressed in Escherichia coli (BL21) assessed as oxidation of ACC to ethylene preincubated for 30... |
J Biol Chem 285: 33445-56 (2010)
Article DOI: 10.1074/jbc.M110.132498
BindingDB Entry DOI: 10.7270/Q2VQ35J0 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50140278
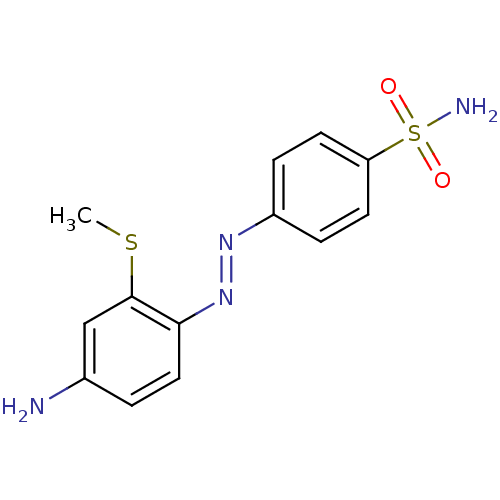
(4-((E)-4-Amino-2-methylsulfanyl-phenylazo)-benzene...)Show InChI InChI=1S/C13H14N4O2S2/c1-20-13-8-9(14)2-7-12(13)17-16-10-3-5-11(6-4-10)21(15,18)19/h2-8H,14H2,1H3,(H2,15,18,19)/b17-16+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.04E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
National Research Institute of Chinese Medicine
Curated by ChEMBL
| Assay Description
Inhibition of COX2 assessed as LPS-stimulated PGE2 production in human whole blood leukocyte |
Bioorg Med Chem Lett 16: 4440-3 (2006)
Article DOI: 10.1016/j.bmcl.2006.06.036
BindingDB Entry DOI: 10.7270/Q2ZS2W33 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Homo sapiens (Human)) | BDBM23926

((E)-resveratrol | 5-[(E)-2-(4-hydroxyphenyl)etheny...)Show InChI InChI=1S/C14H12O3/c15-12-5-3-10(4-6-12)1-2-11-7-13(16)9-14(17)8-11/h1-9,15-17H/b2-1+ | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.84E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
National Research Institute of Chinese Medicine
Curated by ChEMBL
| Assay Description
Inhibition of COX1 assessed as TBX2 production in human whole blood |
Bioorg Med Chem Lett 16: 4440-3 (2006)
Article DOI: 10.1016/j.bmcl.2006.06.036
BindingDB Entry DOI: 10.7270/Q2ZS2W33 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Ovis aries (Sheep)) | BDBM23926

((E)-resveratrol | 5-[(E)-2-(4-hydroxyphenyl)etheny...)Show InChI InChI=1S/C14H12O3/c15-12-5-3-10(4-6-12)1-2-11-7-13(16)9-14(17)8-11/h1-9,15-17H/b2-1+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.49E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
National Research Institute of Chinese Medicine
Curated by ChEMBL
| Assay Description
Inhibition of ovine COX2 |
Bioorg Med Chem Lett 16: 4440-3 (2006)
Article DOI: 10.1016/j.bmcl.2006.06.036
BindingDB Entry DOI: 10.7270/Q2ZS2W33 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50190336
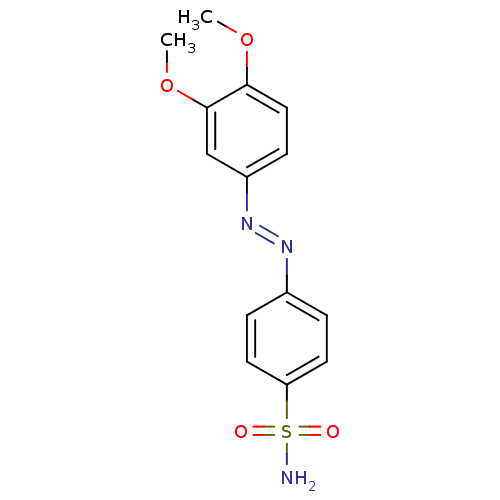
(4-((3,4-dimethoxyphenyl)diazenyl)benzenesulfonamid...)Show InChI InChI=1S/C14H15N3O4S/c1-20-13-8-5-11(9-14(13)21-2)17-16-10-3-6-12(7-4-10)22(15,18)19/h3-9H,1-2H3,(H2,15,18,19)/b17-16+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.28E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
National Research Institute of Chinese Medicine
Curated by ChEMBL
| Assay Description
Inhibition of COX2 assessed as LPS-stimulated PGE2 production in human whole blood leukocyte |
Bioorg Med Chem Lett 16: 4440-3 (2006)
Article DOI: 10.1016/j.bmcl.2006.06.036
BindingDB Entry DOI: 10.7270/Q2ZS2W33 |
More data for this
Ligand-Target Pair | |
1-aminocyclopropane-1-carboxylate synthase 5
(Arabidopsis thaliana) | BDBM50487186
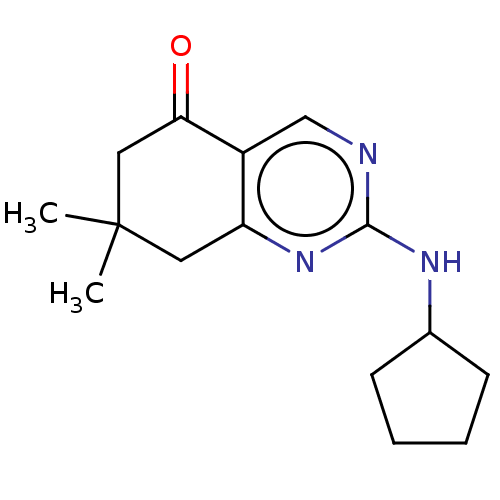
(CHEMBL2251971)Show InChI InChI=1S/C15H21N3O/c1-15(2)7-12-11(13(19)8-15)9-16-14(18-12)17-10-5-3-4-6-10/h9-10H,3-8H2,1-2H3,(H,16,17,18) | MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
National Defense Medical Center
Curated by ChEMBL
| Assay Description
Inhibition of Arabidopsis thaliana recombinant ACS5 expressed in Escherichia coli (BL21) assessed as oxidation of ACC to ethylene preincubated for 30... |
J Biol Chem 285: 33445-56 (2010)
Article DOI: 10.1074/jbc.M110.132498
BindingDB Entry DOI: 10.7270/Q2VQ35J0 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50190337
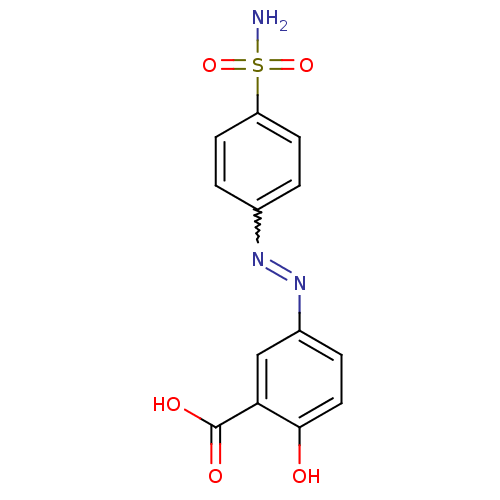
(5-{[4-(aminosulfonyl)phenyl]diazenyl}-2-hydroxyben...)Show SMILES NS(=O)(=O)c1ccc(cc1)N=Nc1ccc(O)c(c1)C(O)=O |w:10.10| Show InChI InChI=1S/C13H11N3O5S/c14-22(20,21)10-4-1-8(2-5-10)15-16-9-3-6-12(17)11(7-9)13(18)19/h1-7,17H,(H,18,19)(H2,14,20,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 8.89E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
National Research Institute of Chinese Medicine
Curated by ChEMBL
| Assay Description
Inhibition of COX2 assessed as LPS-stimulated PGE2 production in human whole blood leukocyte |
Bioorg Med Chem Lett 16: 4440-3 (2006)
Article DOI: 10.1016/j.bmcl.2006.06.036
BindingDB Entry DOI: 10.7270/Q2ZS2W33 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50190333
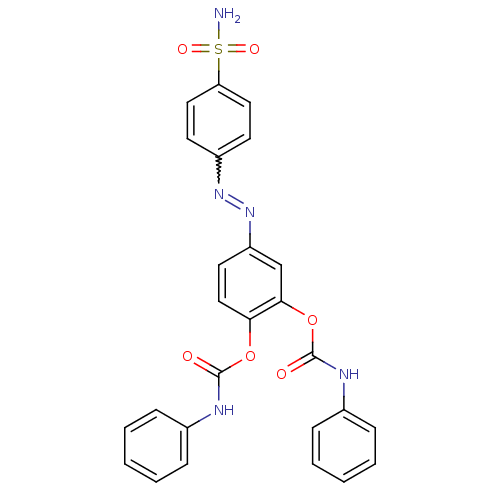
(2-[(phenylcarbamoyl)oxy]-4-[(E)-2-(4-sulfamoylphen...)Show SMILES NS(=O)(=O)c1ccc(cc1)N=Nc1ccc(OC(=O)Nc2ccccc2)c(OC(=O)Nc2ccccc2)c1 |w:10.10| Show InChI InChI=1S/C26H21N5O6S/c27-38(34,35)22-14-11-20(12-15-22)30-31-21-13-16-23(36-25(32)28-18-7-3-1-4-8-18)24(17-21)37-26(33)29-19-9-5-2-6-10-19/h1-17H,(H,28,32)(H,29,33)(H2,27,34,35) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.11E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Research Institute of Chinese Medicine
Curated by ChEMBL
| Assay Description
Inhibition of COX2 assessed as LPS-stimulated PGE2 production in human whole blood leukocyte |
Bioorg Med Chem Lett 16: 4440-3 (2006)
Article DOI: 10.1016/j.bmcl.2006.06.036
BindingDB Entry DOI: 10.7270/Q2ZS2W33 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50190335
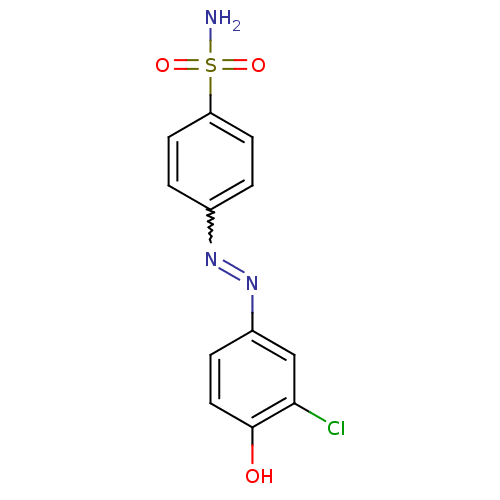
(4-((3-chloro-4-hydroxyphenyl)diazenyl)benzenesulfo...)Show SMILES NS(=O)(=O)c1ccc(cc1)N=Nc1ccc(O)c(Cl)c1 |w:10.10| Show InChI InChI=1S/C12H10ClN3O3S/c13-11-7-9(3-6-12(11)17)16-15-8-1-4-10(5-2-8)20(14,18)19/h1-7,17H,(H2,14,18,19) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.13E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Research Institute of Chinese Medicine
Curated by ChEMBL
| Assay Description
Inhibition of COX2 assessed as LPS-stimulated PGE2 production in human whole blood leukocyte |
Bioorg Med Chem Lett 16: 4440-3 (2006)
Article DOI: 10.1016/j.bmcl.2006.06.036
BindingDB Entry DOI: 10.7270/Q2ZS2W33 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50190338
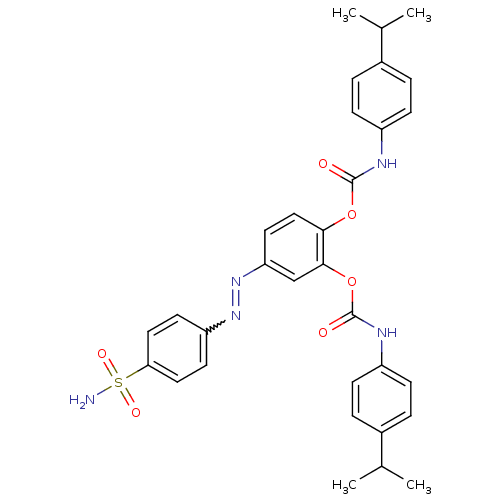
(2-({[4-(propan-2-yl)phenyl]carbamoyl}oxy)-4-[(E)-2...)Show SMILES CC(C)c1ccc(NC(=O)Oc2ccc(cc2OC(=O)Nc2ccc(cc2)C(C)C)N=Nc2ccc(cc2)S(N)(=O)=O)cc1 |w:31.33| Show InChI InChI=1S/C32H33N5O6S/c1-20(2)22-5-9-24(10-6-22)34-31(38)42-29-18-15-27(37-36-26-13-16-28(17-14-26)44(33,40)41)19-30(29)43-32(39)35-25-11-7-23(8-12-25)21(3)4/h5-21H,1-4H3,(H,34,38)(H,35,39)(H2,33,40,41) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.24E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Research Institute of Chinese Medicine
Curated by ChEMBL
| Assay Description
Inhibition of COX2 assessed as LPS-stimulated PGE2 production in human whole blood leukocyte |
Bioorg Med Chem Lett 16: 4440-3 (2006)
Article DOI: 10.1016/j.bmcl.2006.06.036
BindingDB Entry DOI: 10.7270/Q2ZS2W33 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50190334
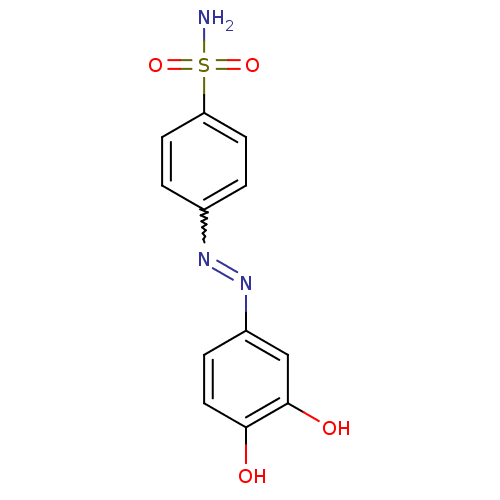
(4-((3,4-dihydroxyphenyl)diazenyl)benzenesulfonamid...)Show SMILES NS(=O)(=O)c1ccc(cc1)N=Nc1ccc(O)c(O)c1 |w:10.10| Show InChI InChI=1S/C12H11N3O4S/c13-20(18,19)10-4-1-8(2-5-10)14-15-9-3-6-11(16)12(17)7-9/h1-7,16-17H,(H2,13,18,19) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.24E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Research Institute of Chinese Medicine
Curated by ChEMBL
| Assay Description
Inhibition of COX2 assessed as LPS-stimulated PGE2 production in human whole blood leukocyte |
Bioorg Med Chem Lett 16: 4440-3 (2006)
Article DOI: 10.1016/j.bmcl.2006.06.036
BindingDB Entry DOI: 10.7270/Q2ZS2W33 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50190339
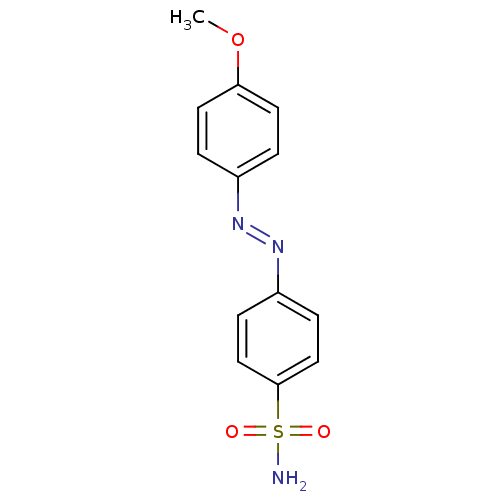
(4-((4-methoxyphenyl)diazenyl)benzenesulfonamide | ...)Show InChI InChI=1S/C13H13N3O3S/c1-19-12-6-2-10(3-7-12)15-16-11-4-8-13(9-5-11)20(14,17)18/h2-9H,1H3,(H2,14,17,18)/b16-15+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.46E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Research Institute of Chinese Medicine
Curated by ChEMBL
| Assay Description
Inhibition of COX2 assessed as LPS-stimulated PGE2 production in human whole blood leukocyte |
Bioorg Med Chem Lett 16: 4440-3 (2006)
Article DOI: 10.1016/j.bmcl.2006.06.036
BindingDB Entry DOI: 10.7270/Q2ZS2W33 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50190332
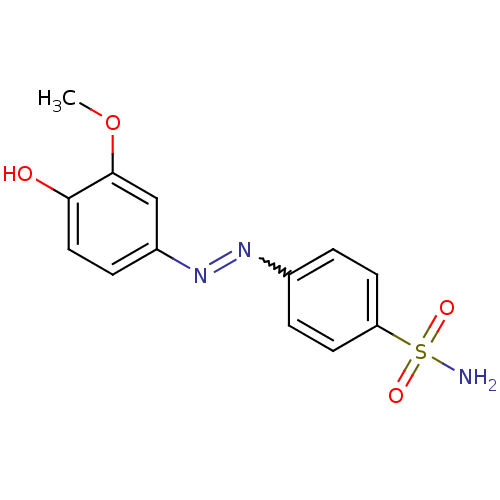
(4-((4-hydroxy-3-methoxyphenyl)diazenyl)benzenesulf...)Show SMILES COc1cc(ccc1O)N=Nc1ccc(cc1)S(N)(=O)=O |w:10.11| Show InChI InChI=1S/C13H13N3O4S/c1-20-13-8-10(4-7-12(13)17)16-15-9-2-5-11(6-3-9)21(14,18)19/h2-8,17H,1H3,(H2,14,18,19) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.69E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Research Institute of Chinese Medicine
Curated by ChEMBL
| Assay Description
Inhibition of COX2 assessed as LPS-stimulated PGE2 production in human whole blood leukocyte |
Bioorg Med Chem Lett 16: 4440-3 (2006)
Article DOI: 10.1016/j.bmcl.2006.06.036
BindingDB Entry DOI: 10.7270/Q2ZS2W33 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Ovis aries (Sheep)) | BDBM50140278

(4-((E)-4-Amino-2-methylsulfanyl-phenylazo)-benzene...)Show InChI InChI=1S/C13H14N4O2S2/c1-20-13-8-9(14)2-7-12(13)17-16-10-3-5-11(6-4-10)21(15,18)19/h2-8H,14H2,1H3,(H2,15,18,19)/b17-16+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Research Institute of Chinese Medicine
Curated by ChEMBL
| Assay Description
Inhibition of ovine COX2 |
Bioorg Med Chem Lett 16: 4440-3 (2006)
Article DOI: 10.1016/j.bmcl.2006.06.036
BindingDB Entry DOI: 10.7270/Q2ZS2W33 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Homo sapiens (Human)) | BDBM11639

(4-[5-(4-methylphenyl)-3-(trifluoromethyl)-1H-pyraz...)Show SMILES Cc1ccc(cc1)-c1cc(nn1-c1ccc(cc1)S(N)(=O)=O)C(F)(F)F Show InChI InChI=1S/C17H14F3N3O2S/c1-11-2-4-12(5-3-11)15-10-16(17(18,19)20)22-23(15)13-6-8-14(9-7-13)26(21,24)25/h2-10H,1H3,(H2,21,24,25) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Research Institute of Chinese Medicine
Curated by ChEMBL
| Assay Description
Inhibition of COX1 assessed as TBX2 production in human whole blood |
Bioorg Med Chem Lett 16: 4440-3 (2006)
Article DOI: 10.1016/j.bmcl.2006.06.036
BindingDB Entry DOI: 10.7270/Q2ZS2W33 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Homo sapiens (Human)) | BDBM50140278

(4-((E)-4-Amino-2-methylsulfanyl-phenylazo)-benzene...)Show InChI InChI=1S/C13H14N4O2S2/c1-20-13-8-9(14)2-7-12(13)17-16-10-3-5-11(6-4-10)21(15,18)19/h2-8H,14H2,1H3,(H2,15,18,19)/b17-16+ | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.33E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Research Institute of Chinese Medicine
Curated by ChEMBL
| Assay Description
Inhibition of COX1 assessed as TBX2 production in human whole blood |
Bioorg Med Chem Lett 16: 4440-3 (2006)
Article DOI: 10.1016/j.bmcl.2006.06.036
BindingDB Entry DOI: 10.7270/Q2ZS2W33 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Ovis aries (Sheep)) | BDBM50190337

(5-{[4-(aminosulfonyl)phenyl]diazenyl}-2-hydroxyben...)Show SMILES NS(=O)(=O)c1ccc(cc1)N=Nc1ccc(O)c(c1)C(O)=O |w:10.10| Show InChI InChI=1S/C13H11N3O5S/c14-22(20,21)10-4-1-8(2-5-10)15-16-9-3-6-12(17)11(7-9)13(18)19/h1-7,17H,(H,18,19)(H2,14,20,21) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.36E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Research Institute of Chinese Medicine
Curated by ChEMBL
| Assay Description
Inhibition of ovine COX2 |
Bioorg Med Chem Lett 16: 4440-3 (2006)
Article DOI: 10.1016/j.bmcl.2006.06.036
BindingDB Entry DOI: 10.7270/Q2ZS2W33 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Homo sapiens (Human)) | BDBM50190338

(2-({[4-(propan-2-yl)phenyl]carbamoyl}oxy)-4-[(E)-2...)Show SMILES CC(C)c1ccc(NC(=O)Oc2ccc(cc2OC(=O)Nc2ccc(cc2)C(C)C)N=Nc2ccc(cc2)S(N)(=O)=O)cc1 |w:31.33| Show InChI InChI=1S/C32H33N5O6S/c1-20(2)22-5-9-24(10-6-22)34-31(38)42-29-18-15-27(37-36-26-13-16-28(17-14-26)44(33,40)41)19-30(29)43-32(39)35-25-11-7-23(8-12-25)21(3)4/h5-21H,1-4H3,(H,34,38)(H,35,39)(H2,33,40,41) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.63E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Research Institute of Chinese Medicine
Curated by ChEMBL
| Assay Description
Inhibition of COX1 assessed as TBX2 production in human whole blood |
Bioorg Med Chem Lett 16: 4440-3 (2006)
Article DOI: 10.1016/j.bmcl.2006.06.036
BindingDB Entry DOI: 10.7270/Q2ZS2W33 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Homo sapiens (Human)) | BDBM11639

(4-[5-(4-methylphenyl)-3-(trifluoromethyl)-1H-pyraz...)Show SMILES Cc1ccc(cc1)-c1cc(nn1-c1ccc(cc1)S(N)(=O)=O)C(F)(F)F Show InChI InChI=1S/C17H14F3N3O2S/c1-11-2-4-12(5-3-11)15-10-16(17(18,19)20)22-23(15)13-6-8-14(9-7-13)26(21,24)25/h2-10H,1H3,(H2,21,24,25) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.66E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Research Institute of Chinese Medicine
Curated by ChEMBL
| Assay Description
Inhibition of ovine COX1 |
Bioorg Med Chem Lett 16: 4440-3 (2006)
Article DOI: 10.1016/j.bmcl.2006.06.036
BindingDB Entry DOI: 10.7270/Q2ZS2W33 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Homo sapiens (Human)) | BDBM50190337

(5-{[4-(aminosulfonyl)phenyl]diazenyl}-2-hydroxyben...)Show SMILES NS(=O)(=O)c1ccc(cc1)N=Nc1ccc(O)c(c1)C(O)=O |w:10.10| Show InChI InChI=1S/C13H11N3O5S/c14-22(20,21)10-4-1-8(2-5-10)15-16-9-3-6-12(17)11(7-9)13(18)19/h1-7,17H,(H,18,19)(H2,14,20,21) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.93E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Research Institute of Chinese Medicine
Curated by ChEMBL
| Assay Description
Inhibition of ovine COX1 |
Bioorg Med Chem Lett 16: 4440-3 (2006)
Article DOI: 10.1016/j.bmcl.2006.06.036
BindingDB Entry DOI: 10.7270/Q2ZS2W33 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Homo sapiens (Human)) | BDBM50190337

(5-{[4-(aminosulfonyl)phenyl]diazenyl}-2-hydroxyben...)Show SMILES NS(=O)(=O)c1ccc(cc1)N=Nc1ccc(O)c(c1)C(O)=O |w:10.10| Show InChI InChI=1S/C13H11N3O5S/c14-22(20,21)10-4-1-8(2-5-10)15-16-9-3-6-12(17)11(7-9)13(18)19/h1-7,17H,(H,18,19)(H2,14,20,21) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.16E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Research Institute of Chinese Medicine
Curated by ChEMBL
| Assay Description
Inhibition of COX1 assessed as TBX2 production in human whole blood |
Bioorg Med Chem Lett 16: 4440-3 (2006)
Article DOI: 10.1016/j.bmcl.2006.06.036
BindingDB Entry DOI: 10.7270/Q2ZS2W33 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM23926

((E)-resveratrol | 5-[(E)-2-(4-hydroxyphenyl)etheny...)Show InChI InChI=1S/C14H12O3/c15-12-5-3-10(4-6-12)1-2-11-7-13(16)9-14(17)8-11/h1-9,15-17H/b2-1+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.32E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Research Institute of Chinese Medicine
Curated by ChEMBL
| Assay Description
Inhibition of COX2 assessed as LPS-stimulated PGE2 production in human whole blood leukocyte |
Bioorg Med Chem Lett 16: 4440-3 (2006)
Article DOI: 10.1016/j.bmcl.2006.06.036
BindingDB Entry DOI: 10.7270/Q2ZS2W33 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Homo sapiens (Human)) | BDBM50190335

(4-((3-chloro-4-hydroxyphenyl)diazenyl)benzenesulfo...)Show SMILES NS(=O)(=O)c1ccc(cc1)N=Nc1ccc(O)c(Cl)c1 |w:10.10| Show InChI InChI=1S/C12H10ClN3O3S/c13-11-7-9(3-6-12(11)17)16-15-8-1-4-10(5-2-8)20(14,18)19/h1-7,17H,(H2,14,18,19) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.33E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Research Institute of Chinese Medicine
Curated by ChEMBL
| Assay Description
Inhibition of COX1 assessed as TBX2 production in human whole blood |
Bioorg Med Chem Lett 16: 4440-3 (2006)
Article DOI: 10.1016/j.bmcl.2006.06.036
BindingDB Entry DOI: 10.7270/Q2ZS2W33 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Homo sapiens (Human)) | BDBM50140278

(4-((E)-4-Amino-2-methylsulfanyl-phenylazo)-benzene...)Show InChI InChI=1S/C13H14N4O2S2/c1-20-13-8-9(14)2-7-12(13)17-16-10-3-5-11(6-4-10)21(15,18)19/h2-8H,14H2,1H3,(H2,15,18,19)/b17-16+ | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.41E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Research Institute of Chinese Medicine
Curated by ChEMBL
| Assay Description
Inhibition of ovine COX1 |
Bioorg Med Chem Lett 16: 4440-3 (2006)
Article DOI: 10.1016/j.bmcl.2006.06.036
BindingDB Entry DOI: 10.7270/Q2ZS2W33 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Homo sapiens (Human)) | BDBM50190333

(2-[(phenylcarbamoyl)oxy]-4-[(E)-2-(4-sulfamoylphen...)Show SMILES NS(=O)(=O)c1ccc(cc1)N=Nc1ccc(OC(=O)Nc2ccccc2)c(OC(=O)Nc2ccccc2)c1 |w:10.10| Show InChI InChI=1S/C26H21N5O6S/c27-38(34,35)22-14-11-20(12-15-22)30-31-21-13-16-23(36-25(32)28-18-7-3-1-4-8-18)24(17-21)37-26(33)29-19-9-5-2-6-10-19/h1-17H,(H,28,32)(H,29,33)(H2,27,34,35) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.51E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Research Institute of Chinese Medicine
Curated by ChEMBL
| Assay Description
Inhibition of COX1 assessed as TBX2 production in human whole blood |
Bioorg Med Chem Lett 16: 4440-3 (2006)
Article DOI: 10.1016/j.bmcl.2006.06.036
BindingDB Entry DOI: 10.7270/Q2ZS2W33 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Ovis aries (Sheep)) | BDBM50190335

(4-((3-chloro-4-hydroxyphenyl)diazenyl)benzenesulfo...)Show SMILES NS(=O)(=O)c1ccc(cc1)N=Nc1ccc(O)c(Cl)c1 |w:10.10| Show InChI InChI=1S/C12H10ClN3O3S/c13-11-7-9(3-6-12(11)17)16-15-8-1-4-10(5-2-8)20(14,18)19/h1-7,17H,(H2,14,18,19) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.97E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Research Institute of Chinese Medicine
Curated by ChEMBL
| Assay Description
Inhibition of ovine COX2 |
Bioorg Med Chem Lett 16: 4440-3 (2006)
Article DOI: 10.1016/j.bmcl.2006.06.036
BindingDB Entry DOI: 10.7270/Q2ZS2W33 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Homo sapiens (Human)) | BDBM50190335

(4-((3-chloro-4-hydroxyphenyl)diazenyl)benzenesulfo...)Show SMILES NS(=O)(=O)c1ccc(cc1)N=Nc1ccc(O)c(Cl)c1 |w:10.10| Show InChI InChI=1S/C12H10ClN3O3S/c13-11-7-9(3-6-12(11)17)16-15-8-1-4-10(5-2-8)20(14,18)19/h1-7,17H,(H2,14,18,19) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.05E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Research Institute of Chinese Medicine
Curated by ChEMBL
| Assay Description
Inhibition of ovine COX1 |
Bioorg Med Chem Lett 16: 4440-3 (2006)
Article DOI: 10.1016/j.bmcl.2006.06.036
BindingDB Entry DOI: 10.7270/Q2ZS2W33 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Homo sapiens (Human)) | BDBM50190334

(4-((3,4-dihydroxyphenyl)diazenyl)benzenesulfonamid...)Show SMILES NS(=O)(=O)c1ccc(cc1)N=Nc1ccc(O)c(O)c1 |w:10.10| Show InChI InChI=1S/C12H11N3O4S/c13-20(18,19)10-4-1-8(2-5-10)14-15-9-3-6-11(16)12(17)7-9/h1-7,16-17H,(H2,13,18,19) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Research Institute of Chinese Medicine
Curated by ChEMBL
| Assay Description
Inhibition of COX1 assessed as TBX2 production in human whole blood |
Bioorg Med Chem Lett 16: 4440-3 (2006)
Article DOI: 10.1016/j.bmcl.2006.06.036
BindingDB Entry DOI: 10.7270/Q2ZS2W33 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Homo sapiens (Human)) | BDBM50190336

(4-((3,4-dimethoxyphenyl)diazenyl)benzenesulfonamid...)Show InChI InChI=1S/C14H15N3O4S/c1-20-13-8-5-11(9-14(13)21-2)17-16-10-3-6-12(7-4-10)22(15,18)19/h3-9H,1-2H3,(H2,15,18,19)/b17-16+ | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.17E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Research Institute of Chinese Medicine
Curated by ChEMBL
| Assay Description
Inhibition of COX1 assessed as TBX2 production in human whole blood |
Bioorg Med Chem Lett 16: 4440-3 (2006)
Article DOI: 10.1016/j.bmcl.2006.06.036
BindingDB Entry DOI: 10.7270/Q2ZS2W33 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Homo sapiens (Human)) | BDBM50190340
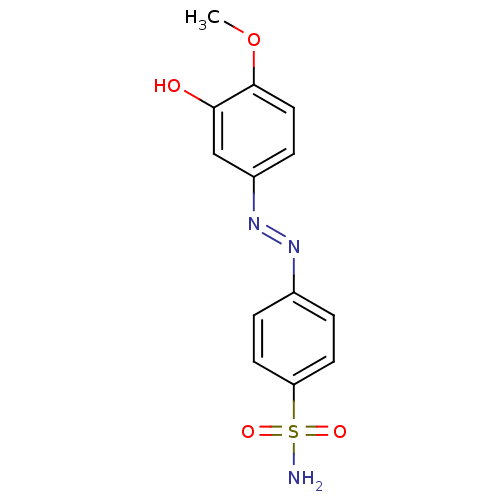
(4-((3-hydroxy-4-methoxyphenyl)diazenyl)benzenesulf...)Show InChI InChI=1S/C13H13N3O4S/c1-20-13-7-4-10(8-12(13)17)16-15-9-2-5-11(6-3-9)21(14,18)19/h2-8,17H,1H3,(H2,14,18,19)/b16-15+ | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.64E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Research Institute of Chinese Medicine
Curated by ChEMBL
| Assay Description
Inhibition of COX1 assessed as TBX2 production in human whole blood |
Bioorg Med Chem Lett 16: 4440-3 (2006)
Article DOI: 10.1016/j.bmcl.2006.06.036
BindingDB Entry DOI: 10.7270/Q2ZS2W33 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Ovis aries (Sheep)) | BDBM50190336

(4-((3,4-dimethoxyphenyl)diazenyl)benzenesulfonamid...)Show InChI InChI=1S/C14H15N3O4S/c1-20-13-8-5-11(9-14(13)21-2)17-16-10-3-6-12(7-4-10)22(15,18)19/h3-9H,1-2H3,(H2,15,18,19)/b17-16+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.45E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Research Institute of Chinese Medicine
Curated by ChEMBL
| Assay Description
Inhibition of ovine COX2 |
Bioorg Med Chem Lett 16: 4440-3 (2006)
Article DOI: 10.1016/j.bmcl.2006.06.036
BindingDB Entry DOI: 10.7270/Q2ZS2W33 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Homo sapiens (Human)) | BDBM33269
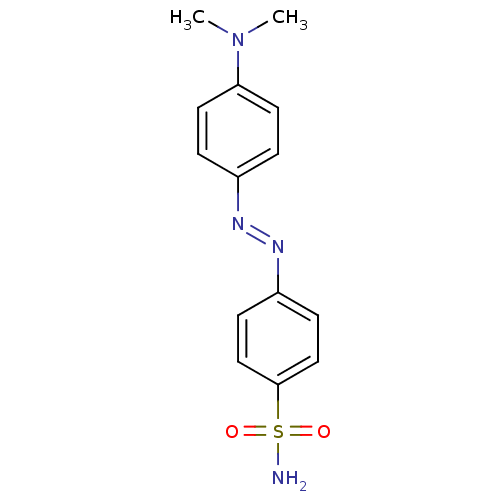
(CHEMBL277836 | azo-sulfonamide, 1d | cid_75522 | p...)Show InChI InChI=1S/C14H16N4O2S/c1-18(2)13-7-3-11(4-8-13)16-17-12-5-9-14(10-6-12)21(15,19)20/h3-10H,1-2H3,(H2,15,19,20)/b17-16+ | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
National Research Institute of Chinese Medicine
Curated by ChEMBL
| Assay Description
Inhibition of COX1 assessed as TBX2 production in human whole blood |
Bioorg Med Chem Lett 16: 4440-3 (2006)
Article DOI: 10.1016/j.bmcl.2006.06.036
BindingDB Entry DOI: 10.7270/Q2ZS2W33 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Homo sapiens (Human)) | BDBM50190339

(4-((4-methoxyphenyl)diazenyl)benzenesulfonamide | ...)Show InChI InChI=1S/C13H13N3O3S/c1-19-12-6-2-10(3-7-12)15-16-11-4-8-13(9-5-11)20(14,17)18/h2-9H,1H3,(H2,14,17,18)/b16-15+ | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
National Research Institute of Chinese Medicine
Curated by ChEMBL
| Assay Description
Inhibition of COX1 assessed as TBX2 production in human whole blood |
Bioorg Med Chem Lett 16: 4440-3 (2006)
Article DOI: 10.1016/j.bmcl.2006.06.036
BindingDB Entry DOI: 10.7270/Q2ZS2W33 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Homo sapiens (Human)) | BDBM50190332

(4-((4-hydroxy-3-methoxyphenyl)diazenyl)benzenesulf...)Show SMILES COc1cc(ccc1O)N=Nc1ccc(cc1)S(N)(=O)=O |w:10.11| Show InChI InChI=1S/C13H13N3O4S/c1-20-13-8-10(4-7-12(13)17)16-15-9-2-5-11(6-3-9)21(14,18)19/h2-8,17H,1H3,(H2,14,18,19) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
National Research Institute of Chinese Medicine
Curated by ChEMBL
| Assay Description
Inhibition of COX1 assessed as TBX2 production in human whole blood |
Bioorg Med Chem Lett 16: 4440-3 (2006)
Article DOI: 10.1016/j.bmcl.2006.06.036
BindingDB Entry DOI: 10.7270/Q2ZS2W33 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Homo sapiens (Human)) | BDBM33266

(CHEMBL379206 | azo-sulfonamide, 1a | p-Phenyldiaze...)Show SMILES NS(=O)(=O)c1ccc(cc1)N=Nc1ccc(O)cc1 |w:10.10| Show InChI InChI=1S/C12H11N3O3S/c13-19(17,18)12-7-3-10(4-8-12)15-14-9-1-5-11(16)6-2-9/h1-8,16H,(H2,13,17,18) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
National Research Institute of Chinese Medicine
Curated by ChEMBL
| Assay Description
Inhibition of COX1 assessed as TBX2 production in human whole blood |
Bioorg Med Chem Lett 16: 4440-3 (2006)
Article DOI: 10.1016/j.bmcl.2006.06.036
BindingDB Entry DOI: 10.7270/Q2ZS2W33 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Homo sapiens (Human)) | BDBM50190336

(4-((3,4-dimethoxyphenyl)diazenyl)benzenesulfonamid...)Show InChI InChI=1S/C14H15N3O4S/c1-20-13-8-5-11(9-14(13)21-2)17-16-10-3-6-12(7-4-10)22(15,18)19/h3-9H,1-2H3,(H2,15,18,19)/b17-16+ | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
National Research Institute of Chinese Medicine
Curated by ChEMBL
| Assay Description
Inhibition of ovine COX1 |
Bioorg Med Chem Lett 16: 4440-3 (2006)
Article DOI: 10.1016/j.bmcl.2006.06.036
BindingDB Entry DOI: 10.7270/Q2ZS2W33 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data