

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50056445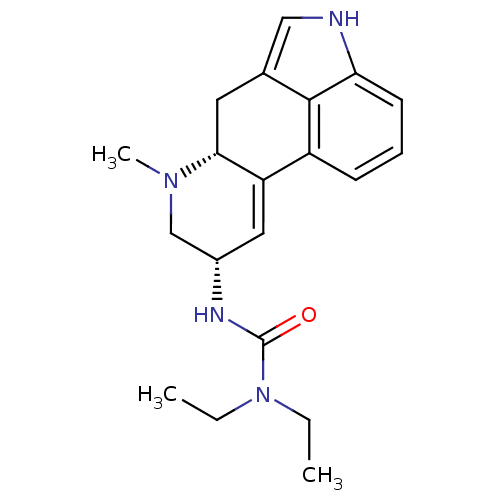 (1,1-diethyl-3-[(8alpha)-6-methyl-9,10-didehydroerg...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia & Upjohn Curated by ChEMBL | Assay Description Binding affinity towards Serotonin 5-hydroxytryptamine 1A receptor by displacement of [3H]-(+)-8-OH-DPAT. | J Med Chem 40: 639-46 (1997) Article DOI: 10.1021/jm960360q BindingDB Entry DOI: 10.7270/Q29P30R9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50056445 (1,1-diethyl-3-[(8alpha)-6-methyl-9,10-didehydroerg...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | DrugBank Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia & Upjohn Curated by ChEMBL | Assay Description Binding affinity towards Dopamine receptor D2 by displacement of [3H]-U-86,170. | J Med Chem 40: 639-46 (1997) Article DOI: 10.1021/jm960360q BindingDB Entry DOI: 10.7270/Q29P30R9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50056445 (1,1-diethyl-3-[(8alpha)-6-methyl-9,10-didehydroerg...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia & Upjohn Curated by ChEMBL | Assay Description Binding affinity towards Dopamine receptor D3 by displacement of [3H]-(+)-7-OH-DPAT. | J Med Chem 40: 639-46 (1997) Article DOI: 10.1021/jm960360q BindingDB Entry DOI: 10.7270/Q29P30R9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50056446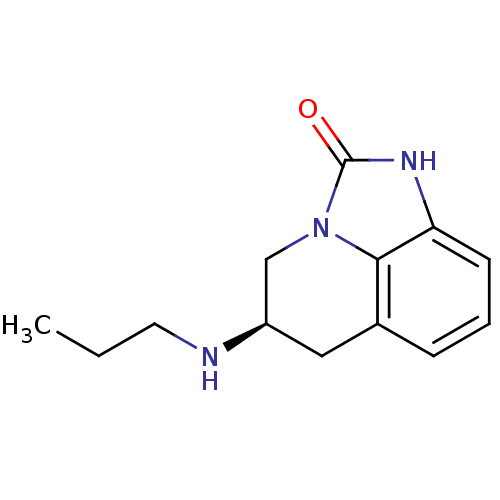 ((R)-5-Propylamino-5,6-dihydro-1H,4H-imidazo[4,5,1-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia & Upjohn Curated by ChEMBL | Assay Description Binding affinity towards Dopamine receptor D2 by displacement of [3H]-U-86,170. | J Med Chem 40: 639-46 (1997) Article DOI: 10.1021/jm960360q BindingDB Entry DOI: 10.7270/Q29P30R9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50017543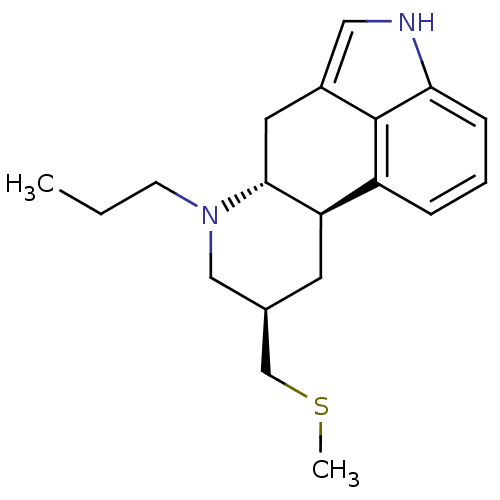 ((6aR,9R,10aR)-9-Methylsulfanylmethyl-7-propyl-4,6,...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | DrugBank Article PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia & Upjohn Curated by ChEMBL | Assay Description Binding affinity towards Dopamine receptor D2 by displacement of [3H]-U-86,170. | J Med Chem 40: 639-46 (1997) Article DOI: 10.1021/jm960360q BindingDB Entry DOI: 10.7270/Q29P30R9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50017543 ((6aR,9R,10aR)-9-Methylsulfanylmethyl-7-propyl-4,6,...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia & Upjohn Curated by ChEMBL | Assay Description Binding affinity towards Dopamine receptor D3 by displacement of [3H]-(+)-7-OH-DPAT. | J Med Chem 40: 639-46 (1997) Article DOI: 10.1021/jm960360q BindingDB Entry DOI: 10.7270/Q29P30R9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50017543 ((6aR,9R,10aR)-9-Methylsulfanylmethyl-7-propyl-4,6,...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia & Upjohn Curated by ChEMBL | Assay Description Binding affinity towards Serotonin 5-hydroxytryptamine 1A receptor by displacement of [3H]-(+)-8-OH-DPAT. | J Med Chem 40: 639-46 (1997) Article DOI: 10.1021/jm960360q BindingDB Entry DOI: 10.7270/Q29P30R9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50020680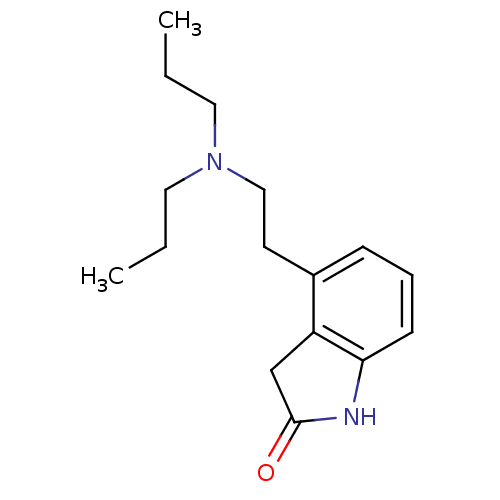 (4-[2-(dipropylamino)ethyl]-1,3-dihydro-2H-indol-2-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | DrugBank Article PubMed | 7.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia & Upjohn Curated by ChEMBL | Assay Description Binding affinity towards Dopamine receptor D2 by displacement of [3H]-U-86,170. | J Med Chem 40: 639-46 (1997) Article DOI: 10.1021/jm960360q BindingDB Entry DOI: 10.7270/Q29P30R9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50056443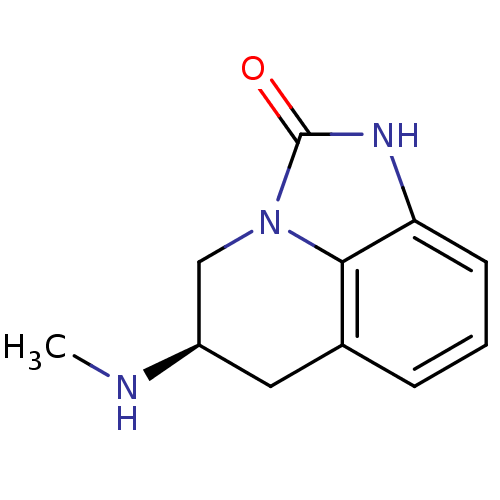 ((R)-5-Methylamino-5,6-dihydro-1H,4H-imidazo[4,5,1-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia & Upjohn Curated by ChEMBL | Assay Description Binding affinity towards Dopamine receptor D2 by displacement of [3H]-U-86,170. | J Med Chem 40: 639-46 (1997) Article DOI: 10.1021/jm960360q BindingDB Entry DOI: 10.7270/Q29P30R9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM81993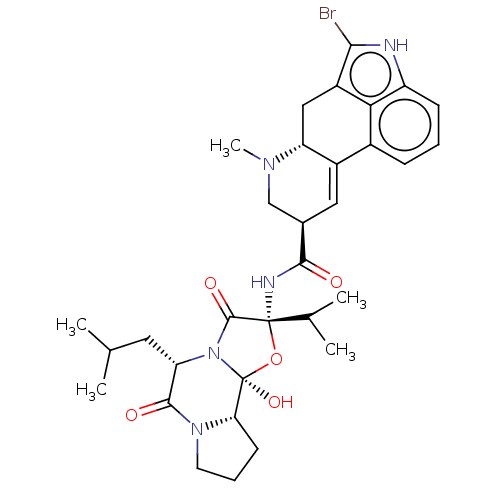 (BROMOCRIPTINE | Bromocriptine+ (GTP+) | Bromocript...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid PDB UniChem Similars | DrugBank PDB Article PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia & Upjohn Curated by ChEMBL | Assay Description Binding affinity towards Dopamine receptor D2 by displacement of [3H]-U-86,170. | J Med Chem 40: 639-46 (1997) Article DOI: 10.1021/jm960360q BindingDB Entry DOI: 10.7270/Q29P30R9 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50056444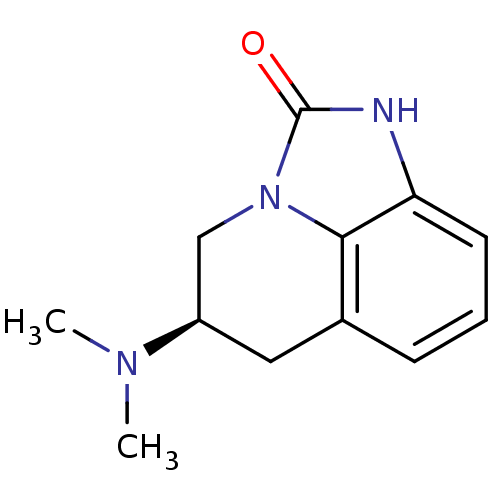 ((R)-5-Dimethylamino-5,6-dihydro-1H,4H-imidazo[4,5,...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia & Upjohn Curated by ChEMBL | Assay Description Binding affinity towards Dopamine receptor D2 by displacement of [3H]-U-86,170. | J Med Chem 40: 639-46 (1997) Article DOI: 10.1021/jm960360q BindingDB Entry DOI: 10.7270/Q29P30R9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50020680 (4-[2-(dipropylamino)ethyl]-1,3-dihydro-2H-indol-2-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia & Upjohn Curated by ChEMBL | Assay Description Binding affinity towards Dopamine receptor D3 by displacement of [3H]-(+)-7-OH-DPAT. | J Med Chem 40: 639-46 (1997) Article DOI: 10.1021/jm960360q BindingDB Entry DOI: 10.7270/Q29P30R9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM81993 (BROMOCRIPTINE | Bromocriptine+ (GTP+) | Bromocript...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid PDB UniChem Similars | Article PubMed | 24 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia & Upjohn Curated by ChEMBL | Assay Description Binding affinity towards Serotonin 5-hydroxytryptamine 1A receptor by displacement of [3H]-(+)-8-OH-DPAT. | J Med Chem 40: 639-46 (1997) Article DOI: 10.1021/jm960360q BindingDB Entry DOI: 10.7270/Q29P30R9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50056446 ((R)-5-Propylamino-5,6-dihydro-1H,4H-imidazo[4,5,1-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 36 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia & Upjohn Curated by ChEMBL | Assay Description Binding affinity towards Dopamine receptor D3 by displacement of [3H]-(+)-7-OH-DPAT. | J Med Chem 40: 639-46 (1997) Article DOI: 10.1021/jm960360q BindingDB Entry DOI: 10.7270/Q29P30R9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50056446 ((R)-5-Propylamino-5,6-dihydro-1H,4H-imidazo[4,5,1-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 58 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia & Upjohn Curated by ChEMBL | Assay Description Binding affinity towards Serotonin 5-hydroxytryptamine 1A receptor by displacement of [3H]-(+)-8-OH-DPAT. | J Med Chem 40: 639-46 (1997) Article DOI: 10.1021/jm960360q BindingDB Entry DOI: 10.7270/Q29P30R9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50056443 ((R)-5-Methylamino-5,6-dihydro-1H,4H-imidazo[4,5,1-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 73 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia & Upjohn Curated by ChEMBL | Assay Description Binding affinity towards Serotonin 5-hydroxytryptamine 1A receptor by displacement of [3H]-(+)-8-OH-DPAT. | J Med Chem 40: 639-46 (1997) Article DOI: 10.1021/jm960360q BindingDB Entry DOI: 10.7270/Q29P30R9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM81993 (BROMOCRIPTINE | Bromocriptine+ (GTP+) | Bromocript...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid PDB UniChem Similars | Article PubMed | 87 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia & Upjohn Curated by ChEMBL | Assay Description Binding affinity towards Dopamine receptor D3 by displacement of [3H]-(+)-7-OH-DPAT. | J Med Chem 40: 639-46 (1997) Article DOI: 10.1021/jm960360q BindingDB Entry DOI: 10.7270/Q29P30R9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50056444 ((R)-5-Dimethylamino-5,6-dihydro-1H,4H-imidazo[4,5,...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 92 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia & Upjohn Curated by ChEMBL | Assay Description Binding affinity towards Serotonin 5-hydroxytryptamine 1A receptor by displacement of [3H]-(+)-8-OH-DPAT. | J Med Chem 40: 639-46 (1997) Article DOI: 10.1021/jm960360q BindingDB Entry DOI: 10.7270/Q29P30R9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50056442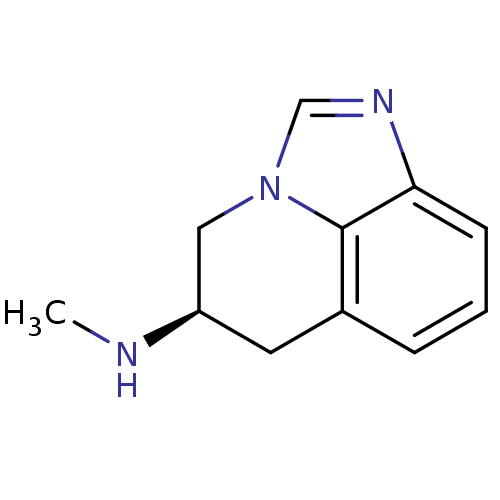 ((R)-5,6-Dihydro-4H-imidazo[4,5,1-ij]quinolin-5-yl-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 355 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia & Upjohn Curated by ChEMBL | Assay Description Binding affinity towards Dopamine receptor D2 by displacement of [3H]-U-86,170. | J Med Chem 40: 639-46 (1997) Article DOI: 10.1021/jm960360q BindingDB Entry DOI: 10.7270/Q29P30R9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50056447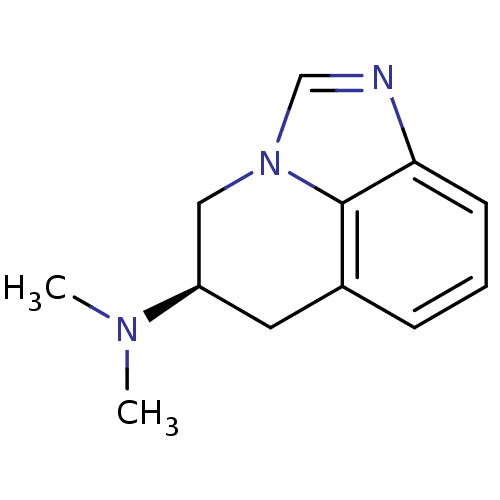 ((R)-5,6-Dihydro-4H-imidazo[4,5,1-ij]quinolin-5-yl-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.28E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia & Upjohn Curated by ChEMBL | Assay Description Binding affinity towards Dopamine receptor D2 by displacement of [3H]-U-86,170. | J Med Chem 40: 639-46 (1997) Article DOI: 10.1021/jm960360q BindingDB Entry DOI: 10.7270/Q29P30R9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50020680 (4-[2-(dipropylamino)ethyl]-1,3-dihydro-2H-indol-2-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 1.71E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia & Upjohn Curated by ChEMBL | Assay Description Binding affinity towards Serotonin 5-hydroxytryptamine 1A receptor by displacement of [3H]-(+)-8-OH-DPAT. | J Med Chem 40: 639-46 (1997) Article DOI: 10.1021/jm960360q BindingDB Entry DOI: 10.7270/Q29P30R9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50056444 ((R)-5-Dimethylamino-5,6-dihydro-1H,4H-imidazo[4,5,...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.93E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia & Upjohn Curated by ChEMBL | Assay Description Binding affinity towards Dopamine receptor D3 by displacement of [3H]-(+)-7-OH-DPAT. | J Med Chem 40: 639-46 (1997) Article DOI: 10.1021/jm960360q BindingDB Entry DOI: 10.7270/Q29P30R9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50056443 ((R)-5-Methylamino-5,6-dihydro-1H,4H-imidazo[4,5,1-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 2.33E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia & Upjohn Curated by ChEMBL | Assay Description Binding affinity towards Dopamine receptor D3 by displacement of [3H]-(+)-7-OH-DPAT. | J Med Chem 40: 639-46 (1997) Article DOI: 10.1021/jm960360q BindingDB Entry DOI: 10.7270/Q29P30R9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50056442 ((R)-5,6-Dihydro-4H-imidazo[4,5,1-ij]quinolin-5-yl-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 2.46E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia & Upjohn Curated by ChEMBL | Assay Description Binding affinity towards Dopamine receptor D3 by displacement of [3H]-(+)-7-OH-DPAT. | J Med Chem 40: 639-46 (1997) Article DOI: 10.1021/jm960360q BindingDB Entry DOI: 10.7270/Q29P30R9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50056447 ((R)-5,6-Dihydro-4H-imidazo[4,5,1-ij]quinolin-5-yl-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.95E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia & Upjohn Curated by ChEMBL | Assay Description Binding affinity towards Dopamine receptor D3 by displacement of [3H]-(+)-7-OH-DPAT. | J Med Chem 40: 639-46 (1997) Article DOI: 10.1021/jm960360q BindingDB Entry DOI: 10.7270/Q29P30R9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50056442 ((R)-5,6-Dihydro-4H-imidazo[4,5,1-ij]quinolin-5-yl-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | >3.26E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia & Upjohn Curated by ChEMBL | Assay Description Binding affinity towards Serotonin 5-hydroxytryptamine 1A receptor by displacement of [3H]-(+)-8-OH-DPAT. | J Med Chem 40: 639-46 (1997) Article DOI: 10.1021/jm960360q BindingDB Entry DOI: 10.7270/Q29P30R9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50056447 ((R)-5,6-Dihydro-4H-imidazo[4,5,1-ij]quinolin-5-yl-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >3.52E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia & Upjohn Curated by ChEMBL | Assay Description Binding affinity towards Serotonin 5-hydroxytryptamine 1A receptor by displacement of [3H]-(+)-8-OH-DPAT. | J Med Chem 40: 639-46 (1997) Article DOI: 10.1021/jm960360q BindingDB Entry DOI: 10.7270/Q29P30R9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Rattus norvegicus) | BDBM50014102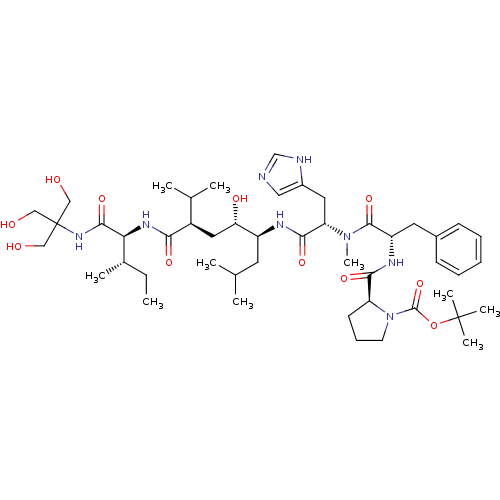 (Boc-Pro-Phe-N(Me) His-Leu[CHOHCH2]Val-Ile-THAM Ami...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.260 | n/a | n/a | n/a | n/a | n/a | n/a |
Upjohn Company Curated by ChEMBL | Assay Description Tested invitro for inhibitory activity against plasma renin in rat models | J Med Chem 33: 2276-83 (1990) BindingDB Entry DOI: 10.7270/Q28C9V7S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Rattus norvegicus) | BDBM50014100 (2-(1-{[1-{2-Hydroxy-1-isobutyl-5-methyl-4-[2-methy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.260 | n/a | n/a | n/a | n/a | n/a | n/a |
Upjohn Company Curated by ChEMBL | Assay Description Tested invitro for inhibitory activity against plasma renin in rat models | J Med Chem 33: 2276-83 (1990) BindingDB Entry DOI: 10.7270/Q28C9V7S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Rattus norvegicus) | BDBM50014106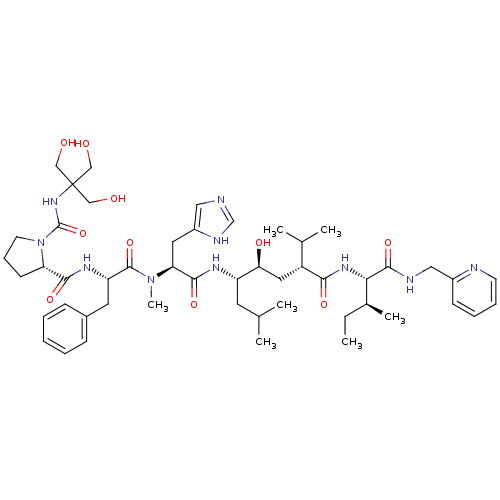 (CHEMBL429882 | N-[[[2-Hydroxy-1,1-bis(hydroxymethy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.330 | n/a | n/a | n/a | n/a | n/a | n/a |
Upjohn Company Curated by ChEMBL | Assay Description Tested invitro for inhibitory activity against plasma renin in rat models | J Med Chem 33: 2276-83 (1990) BindingDB Entry DOI: 10.7270/Q28C9V7S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Rattus norvegicus) | BDBM50014105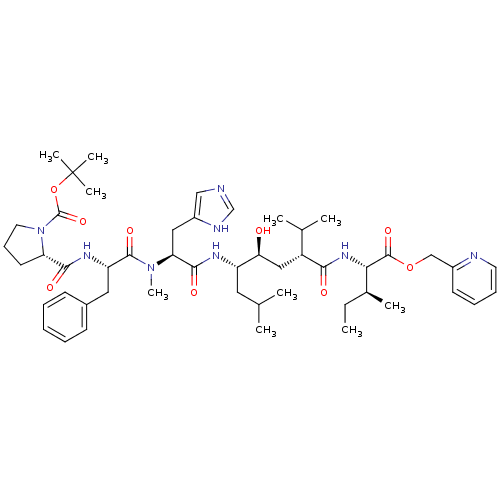 (2-(1-{[1-{2-Hydroxy-1-isobutyl-5-methyl-4-[2-methy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.330 | n/a | n/a | n/a | n/a | n/a | n/a |
Upjohn Company Curated by ChEMBL | Assay Description Tested invitro for inhibitory activity against plasma renin in rat models | J Med Chem 33: 2276-83 (1990) BindingDB Entry DOI: 10.7270/Q28C9V7S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Rattus norvegicus) | BDBM50014099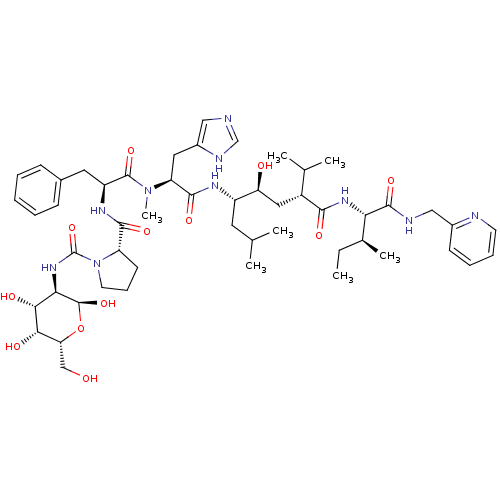 (CHEMBL414795 | N-[(D-Glucosylamino)carbonyl]-Pro-P...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.490 | n/a | n/a | n/a | n/a | n/a | n/a |
Upjohn Company Curated by ChEMBL | Assay Description Tested invitro for inhibitory activity against plasma renin in rat models | J Med Chem 33: 2276-83 (1990) BindingDB Entry DOI: 10.7270/Q28C9V7S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Rattus norvegicus) | BDBM50014104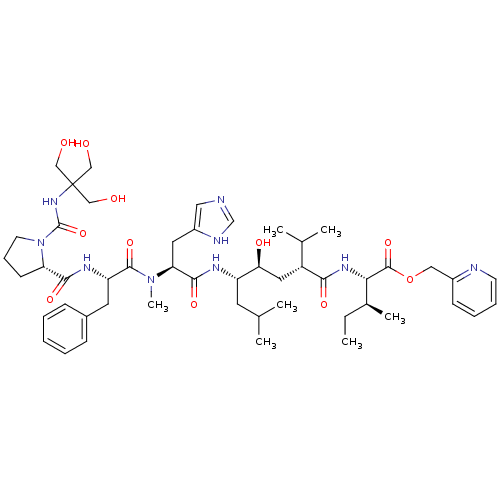 (CHEMBL410067 | N-[[[2-Hydroxy-1,1-bis(hydroxymethy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.580 | n/a | n/a | n/a | n/a | n/a | n/a |
Upjohn Company Curated by ChEMBL | Assay Description Tested invitro for inhibitory activity against plasma renin in rat models | J Med Chem 33: 2276-83 (1990) BindingDB Entry DOI: 10.7270/Q28C9V7S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Rattus norvegicus) | BDBM50014101 (CHEMBL265833 | N-[4,5-Dihydro-4,4-bis(hydroxymethy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Upjohn Company Curated by ChEMBL | Assay Description Tested invitro for inhibitory activity against plasma renin in rat models | J Med Chem 33: 2276-83 (1990) BindingDB Entry DOI: 10.7270/Q28C9V7S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Rattus norvegicus) | BDBM50014103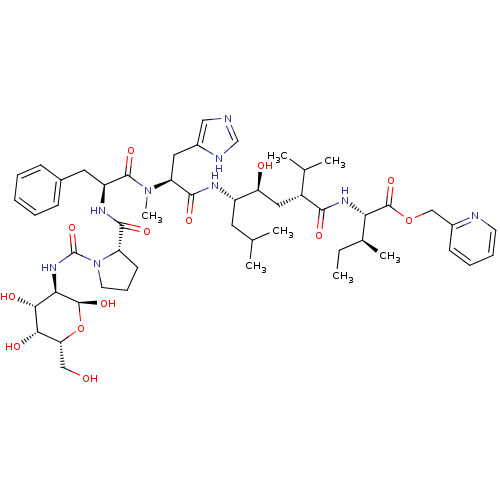 (CHEMBL263257 | N-[(D-Glucosylamino)carbonyl]-Pro-P...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Upjohn Company Curated by ChEMBL | Assay Description Tested invitro for inhibitory activity against plasma renin in rat models | J Med Chem 33: 2276-83 (1990) BindingDB Entry DOI: 10.7270/Q28C9V7S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||