

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM8960 ((+/-)-2-[(1-benzylpiperidin-4-yl)methyl]-5,6-dimet...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | 38 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Mixed-type inhibition of human AChE using acetylthiocholine chloride as substrate incubated for 5 mins followed by substrate addition by Lineweaver-B... | Eur J Med Chem 157: 161-176 (2018) Article DOI: 10.1016/j.ejmech.2018.08.005 BindingDB Entry DOI: 10.7270/Q29P34C1 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50469009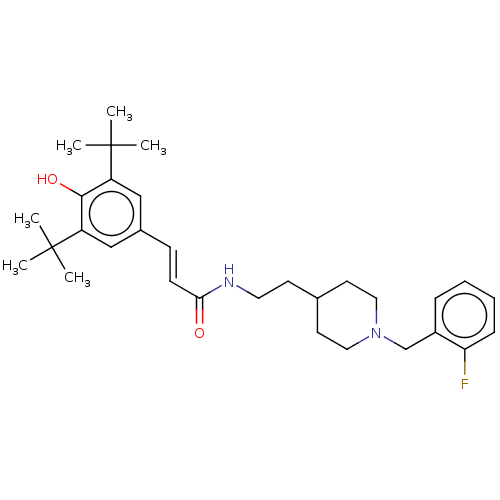 (CHEMBL4292766) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Mixed-type inhibition of human AChE using acetylthiocholine chloride as substrate incubated for 5 mins followed by substrate addition by Lineweaver-B... | Eur J Med Chem 157: 161-176 (2018) Article DOI: 10.1016/j.ejmech.2018.08.005 BindingDB Entry DOI: 10.7270/Q29P34C1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50469008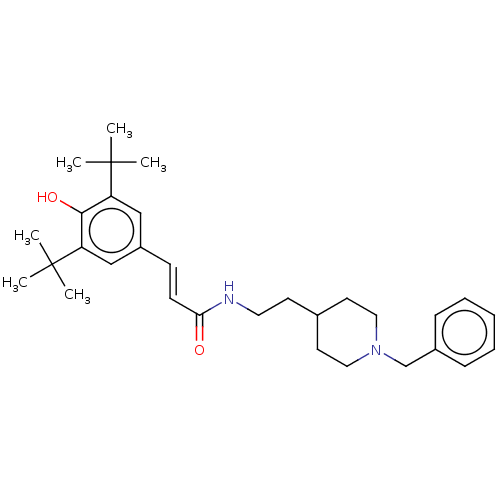 (CHEMBL4283390) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Mixed-type inhibition of human AChE using acetylthiocholine chloride as substrate incubated for 5 mins followed by substrate addition by Lineweaver-B... | Eur J Med Chem 157: 161-176 (2018) Article DOI: 10.1016/j.ejmech.2018.08.005 BindingDB Entry DOI: 10.7270/Q29P34C1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Homo sapiens (Human)) | BDBM50172756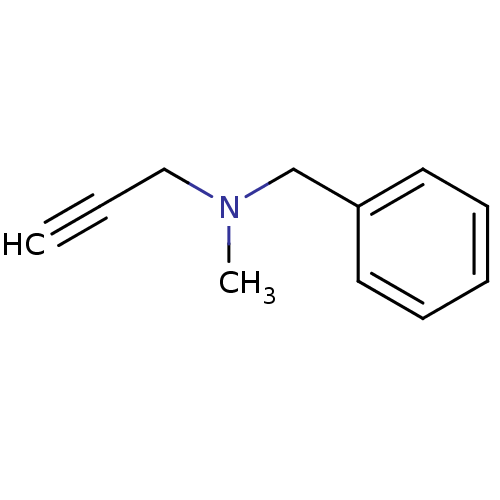 (Benzyl-methyl-prop-2-ynyl-amine | CHEMBL673 | Euto...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 8.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Shenyang Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of MAO-B (unknown origin) | Eur J Med Chem 138: 715-728 (2017) Article DOI: 10.1016/j.ejmech.2017.07.008 BindingDB Entry DOI: 10.7270/Q2S46VKC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A (Homo sapiens (Human)) | BDBM50172756 (Benzyl-methyl-prop-2-ynyl-amine | CHEMBL673 | Euto...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 11.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Shenyang Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of MAO-A (unknown origin) | Eur J Med Chem 138: 715-728 (2017) Article DOI: 10.1016/j.ejmech.2017.07.008 BindingDB Entry DOI: 10.7270/Q2S46VKC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Homo sapiens (Human)) | BDBM50456663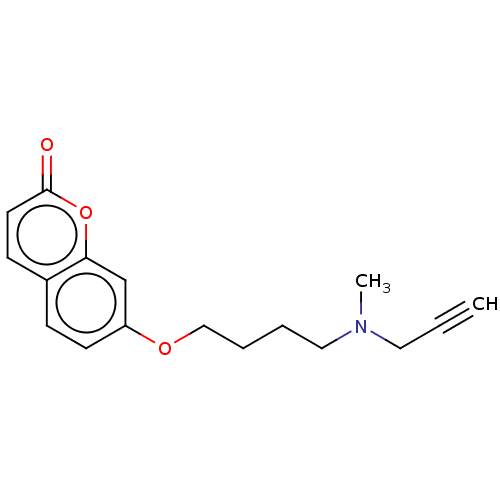 (CHEMBL4215298) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
Shenyang Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of recombinant human MAO-B using p-tyramine as substrate preincubated for 15 mins followed by substrate addition measured after 20 mins by... | Eur J Med Chem 138: 715-728 (2017) Article DOI: 10.1016/j.ejmech.2017.07.008 BindingDB Entry DOI: 10.7270/Q2S46VKC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50204751 (CHEMBL3943450) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of human erythrocytes AChE using acetylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured up ... | Eur J Med Chem 123: 282-297 (2016) Article DOI: 10.1016/j.ejmech.2016.07.052 BindingDB Entry DOI: 10.7270/Q2ZW1NW5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM8961 (1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 41 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of human serum BuChE using s-butyrylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured up to ... | Eur J Med Chem 123: 282-297 (2016) Article DOI: 10.1016/j.ejmech.2016.07.052 BindingDB Entry DOI: 10.7270/Q2ZW1NW5 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50204754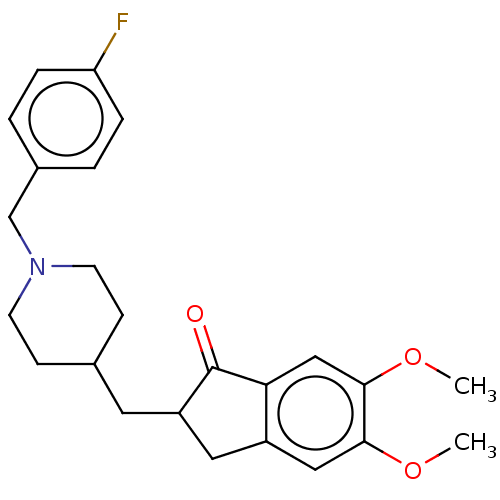 (CHEMBL3985059) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 41 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of human erythrocytes AChE using acetylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured up ... | Eur J Med Chem 123: 282-297 (2016) Article DOI: 10.1016/j.ejmech.2016.07.052 BindingDB Entry DOI: 10.7270/Q2ZW1NW5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50204751 (CHEMBL3943450) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 43 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured up to 3 m... | Eur J Med Chem 123: 282-297 (2016) Article DOI: 10.1016/j.ejmech.2016.07.052 BindingDB Entry DOI: 10.7270/Q2ZW1NW5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM8961 (1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 46 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using s-butyrylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured up to... | Eur J Med Chem 123: 282-297 (2016) Article DOI: 10.1016/j.ejmech.2016.07.052 BindingDB Entry DOI: 10.7270/Q2ZW1NW5 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM8960 ((+/-)-2-[(1-benzylpiperidin-4-yl)methyl]-5,6-dimet...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | n/a | n/a | 48 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of human erythrocyte AChE using acetylthiocholine chloride as substrate incubated for 5 mins followed by substrate addition measured after... | Eur J Med Chem 157: 161-176 (2018) Article DOI: 10.1016/j.ejmech.2018.08.005 BindingDB Entry DOI: 10.7270/Q29P34C1 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM8960 ((+/-)-2-[(1-benzylpiperidin-4-yl)methyl]-5,6-dimet...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | n/a | n/a | 48 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of human erythrocytes AChE using acetylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured up ... | Eur J Med Chem 123: 282-297 (2016) Article DOI: 10.1016/j.ejmech.2016.07.052 BindingDB Entry DOI: 10.7270/Q2ZW1NW5 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM8960 ((+/-)-2-[(1-benzylpiperidin-4-yl)methyl]-5,6-dimet...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine chloride as substrate incubated for 5 mins followed by substrate addition measured after 180 ... | Eur J Med Chem 157: 161-176 (2018) Article DOI: 10.1016/j.ejmech.2018.08.005 BindingDB Entry DOI: 10.7270/Q29P34C1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM8960 ((+/-)-2-[(1-benzylpiperidin-4-yl)methyl]-5,6-dimet...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 51 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured up to 3 m... | Eur J Med Chem 123: 282-297 (2016) Article DOI: 10.1016/j.ejmech.2016.07.052 BindingDB Entry DOI: 10.7270/Q2ZW1NW5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50204831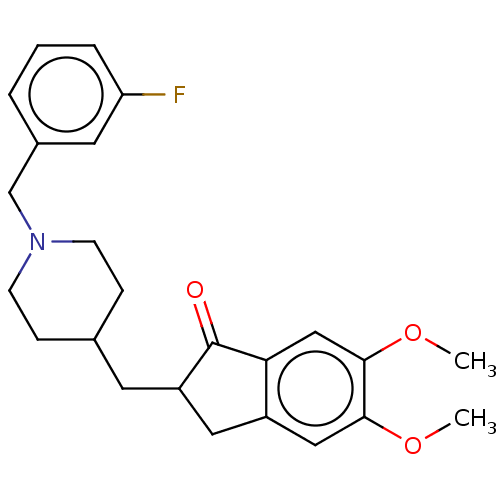 (CHEMBL3927363) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 54 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of human erythrocytes AChE using acetylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured up ... | Eur J Med Chem 123: 282-297 (2016) Article DOI: 10.1016/j.ejmech.2016.07.052 BindingDB Entry DOI: 10.7270/Q2ZW1NW5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50204835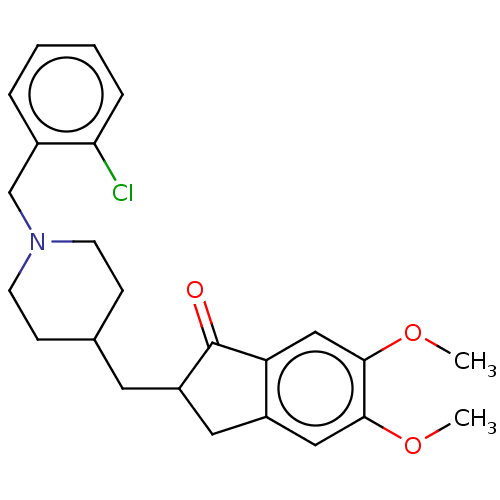 (CHEMBL3933395) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured up to 3 m... | Eur J Med Chem 123: 282-297 (2016) Article DOI: 10.1016/j.ejmech.2016.07.052 BindingDB Entry DOI: 10.7270/Q2ZW1NW5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50204831 (CHEMBL3927363) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 68 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured up to 3 m... | Eur J Med Chem 123: 282-297 (2016) Article DOI: 10.1016/j.ejmech.2016.07.052 BindingDB Entry DOI: 10.7270/Q2ZW1NW5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50204752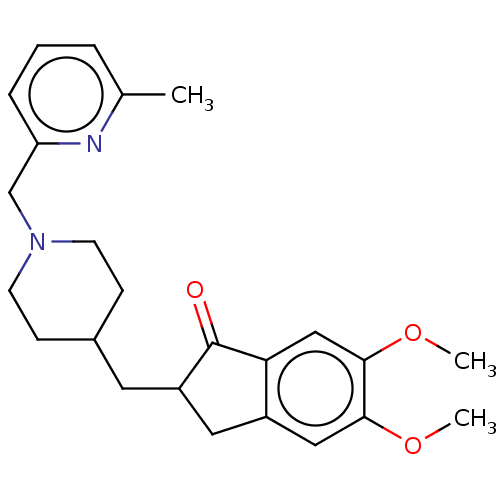 (CHEMBL3919441) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 73 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of human erythrocytes AChE using acetylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured up ... | Eur J Med Chem 123: 282-297 (2016) Article DOI: 10.1016/j.ejmech.2016.07.052 BindingDB Entry DOI: 10.7270/Q2ZW1NW5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50469009 (CHEMBL4292766) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 75 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine chloride as substrate incubated for 5 mins followed by substrate addition measured after 180 ... | Eur J Med Chem 157: 161-176 (2018) Article DOI: 10.1016/j.ejmech.2018.08.005 BindingDB Entry DOI: 10.7270/Q29P34C1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50204752 (CHEMBL3919441) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 85 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured up to 3 m... | Eur J Med Chem 123: 282-297 (2016) Article DOI: 10.1016/j.ejmech.2016.07.052 BindingDB Entry DOI: 10.7270/Q2ZW1NW5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50029944 (5,6-Dimethoxy-2-[1-(3-methyl-benzyl)-piperidin-4-y...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 88 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of human erythrocytes AChE using acetylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured up ... | Eur J Med Chem 123: 282-297 (2016) Article DOI: 10.1016/j.ejmech.2016.07.052 BindingDB Entry DOI: 10.7270/Q2ZW1NW5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50204835 (CHEMBL3933395) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 97 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of human erythrocytes AChE using acetylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured up ... | Eur J Med Chem 123: 282-297 (2016) Article DOI: 10.1016/j.ejmech.2016.07.052 BindingDB Entry DOI: 10.7270/Q2ZW1NW5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50029921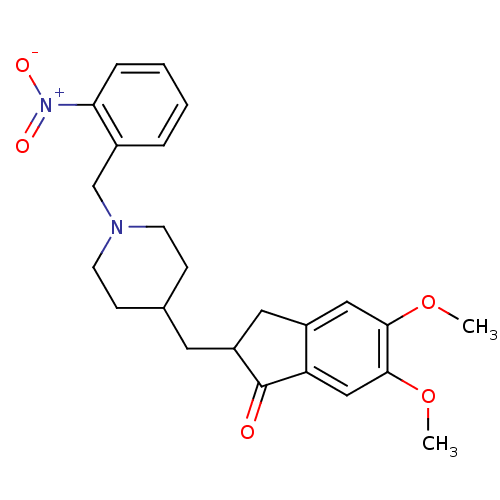 (5,6-Dimethoxy-2-[1-(2-nitro-benzyl)-piperidin-4-yl...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 106 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured up to 3 m... | Eur J Med Chem 123: 282-297 (2016) Article DOI: 10.1016/j.ejmech.2016.07.052 BindingDB Entry DOI: 10.7270/Q2ZW1NW5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50204758 (CHEMBL3891369) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 108 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured up to 3 m... | Eur J Med Chem 123: 282-297 (2016) Article DOI: 10.1016/j.ejmech.2016.07.052 BindingDB Entry DOI: 10.7270/Q2ZW1NW5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50029944 (5,6-Dimethoxy-2-[1-(3-methyl-benzyl)-piperidin-4-y...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 108 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured up to 3 m... | Eur J Med Chem 123: 282-297 (2016) Article DOI: 10.1016/j.ejmech.2016.07.052 BindingDB Entry DOI: 10.7270/Q2ZW1NW5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50204758 (CHEMBL3891369) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 113 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of human erythrocytes AChE using acetylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured up ... | Eur J Med Chem 123: 282-297 (2016) Article DOI: 10.1016/j.ejmech.2016.07.052 BindingDB Entry DOI: 10.7270/Q2ZW1NW5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50204757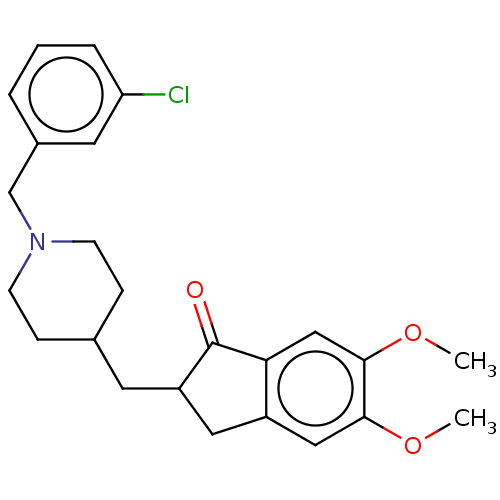 (CHEMBL3960861) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 114 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of human erythrocytes AChE using acetylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured up ... | Eur J Med Chem 123: 282-297 (2016) Article DOI: 10.1016/j.ejmech.2016.07.052 BindingDB Entry DOI: 10.7270/Q2ZW1NW5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50029937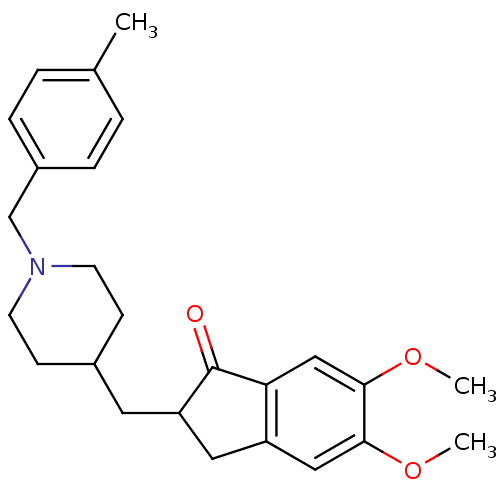 (5,6-Dimethoxy-2-[1-(4-methyl-benzyl)-piperidin-4-y...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 127 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of human erythrocytes AChE using acetylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured up ... | Eur J Med Chem 123: 282-297 (2016) Article DOI: 10.1016/j.ejmech.2016.07.052 BindingDB Entry DOI: 10.7270/Q2ZW1NW5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50204754 (CHEMBL3985059) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured up to 3 m... | Eur J Med Chem 123: 282-297 (2016) Article DOI: 10.1016/j.ejmech.2016.07.052 BindingDB Entry DOI: 10.7270/Q2ZW1NW5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50204755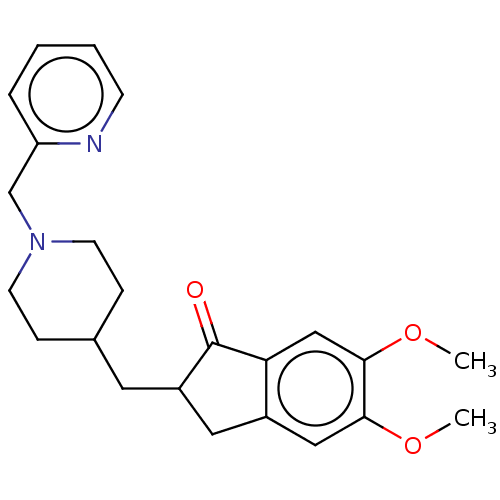 (CHEMBL3910513) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 147 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of human erythrocytes AChE using acetylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured up ... | Eur J Med Chem 123: 282-297 (2016) Article DOI: 10.1016/j.ejmech.2016.07.052 BindingDB Entry DOI: 10.7270/Q2ZW1NW5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50029945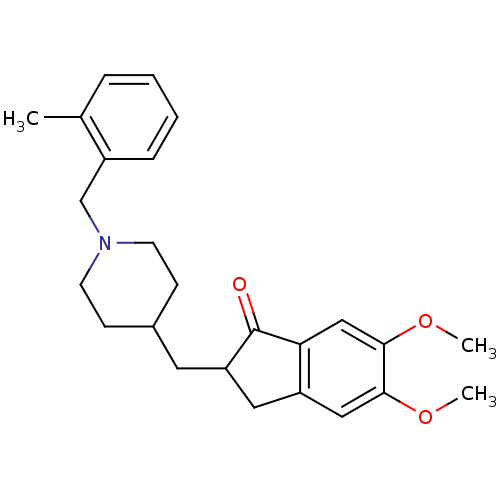 (5,6-Dimethoxy-2-[1-(2-methyl-benzyl)-piperidin-4-y...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 184 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured up to 3 m... | Eur J Med Chem 123: 282-297 (2016) Article DOI: 10.1016/j.ejmech.2016.07.052 BindingDB Entry DOI: 10.7270/Q2ZW1NW5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Homo sapiens (Human)) | BDBM50172756 (Benzyl-methyl-prop-2-ynyl-amine | CHEMBL673 | Euto...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 194 | n/a | n/a | n/a | n/a | n/a | n/a |
Shenyang Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of recombinant human MAO-B using p-tyramine as substrate preincubated for 15 mins followed by substrate addition measured after 20 mins by... | Eur J Med Chem 138: 715-728 (2017) Article DOI: 10.1016/j.ejmech.2017.07.008 BindingDB Entry DOI: 10.7270/Q2S46VKC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50204830 (CHEMBL3928343) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 195 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of human erythrocytes AChE using acetylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured up ... | Eur J Med Chem 123: 282-297 (2016) Article DOI: 10.1016/j.ejmech.2016.07.052 BindingDB Entry DOI: 10.7270/Q2ZW1NW5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50204829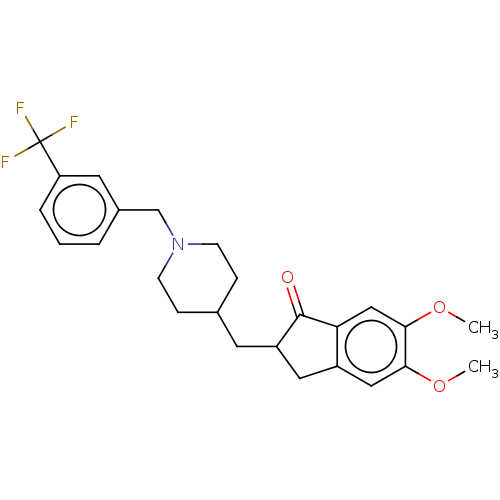 (CHEMBL3961494) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 197 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured up to 3 m... | Eur J Med Chem 123: 282-297 (2016) Article DOI: 10.1016/j.ejmech.2016.07.052 BindingDB Entry DOI: 10.7270/Q2ZW1NW5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Homo sapiens (Human)) | BDBM50172756 (Benzyl-methyl-prop-2-ynyl-amine | CHEMBL673 | Euto...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Shenyang Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of human MAO-B using p-tyramine as substrate preincubated for 15 mins followed by substrate addition measured after 20 mins by fluorescenc... | Bioorg Med Chem 25: 5917-5928 (2017) Article DOI: 10.1016/j.bmc.2017.08.048 BindingDB Entry DOI: 10.7270/Q2TH8Q8J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM8961 (1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 218 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured up to 3 m... | Eur J Med Chem 123: 282-297 (2016) Article DOI: 10.1016/j.ejmech.2016.07.052 BindingDB Entry DOI: 10.7270/Q2ZW1NW5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50029937 (5,6-Dimethoxy-2-[1-(4-methyl-benzyl)-piperidin-4-y...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 221 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured up to 3 m... | Eur J Med Chem 123: 282-297 (2016) Article DOI: 10.1016/j.ejmech.2016.07.052 BindingDB Entry DOI: 10.7270/Q2ZW1NW5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50204757 (CHEMBL3960861) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 237 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured up to 3 m... | Eur J Med Chem 123: 282-297 (2016) Article DOI: 10.1016/j.ejmech.2016.07.052 BindingDB Entry DOI: 10.7270/Q2ZW1NW5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50029945 (5,6-Dimethoxy-2-[1-(2-methyl-benzyl)-piperidin-4-y...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 243 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of human erythrocytes AChE using acetylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured up ... | Eur J Med Chem 123: 282-297 (2016) Article DOI: 10.1016/j.ejmech.2016.07.052 BindingDB Entry DOI: 10.7270/Q2ZW1NW5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50204830 (CHEMBL3928343) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 246 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured up to 3 m... | Eur J Med Chem 123: 282-297 (2016) Article DOI: 10.1016/j.ejmech.2016.07.052 BindingDB Entry DOI: 10.7270/Q2ZW1NW5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50204838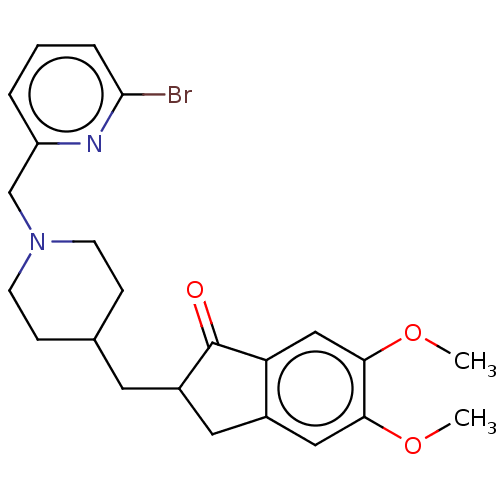 (CHEMBL3973337) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 253 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured up to 3 m... | Eur J Med Chem 123: 282-297 (2016) Article DOI: 10.1016/j.ejmech.2016.07.052 BindingDB Entry DOI: 10.7270/Q2ZW1NW5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50469018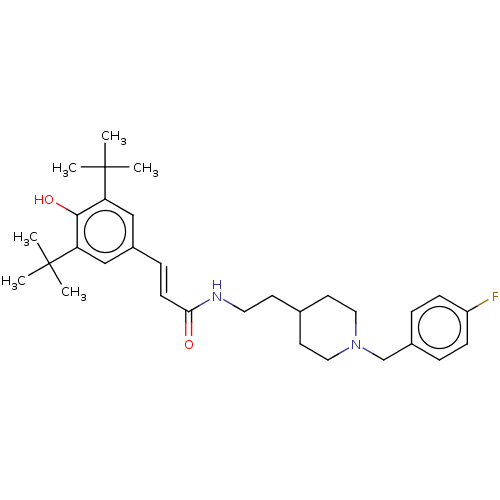 (CHEMBL4284475) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 320 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine chloride as substrate incubated for 5 mins followed by substrate addition measured after 180 ... | Eur J Med Chem 157: 161-176 (2018) Article DOI: 10.1016/j.ejmech.2018.08.005 BindingDB Entry DOI: 10.7270/Q29P34C1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50469020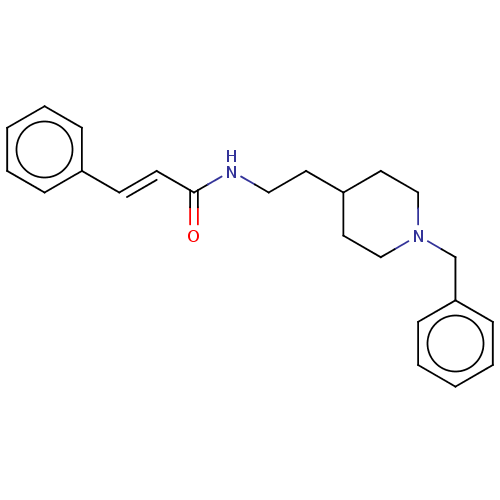 (CHEMBL3819548) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 390 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using butyrylthiocholine iodide as substrate incubated for 5 mins followed by substrate addition measured after 180 ... | Eur J Med Chem 157: 161-176 (2018) Article DOI: 10.1016/j.ejmech.2018.08.005 BindingDB Entry DOI: 10.7270/Q29P34C1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50204761 (CHEMBL3944652) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 409 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured up to 3 m... | Eur J Med Chem 123: 282-297 (2016) Article DOI: 10.1016/j.ejmech.2016.07.052 BindingDB Entry DOI: 10.7270/Q2ZW1NW5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50029932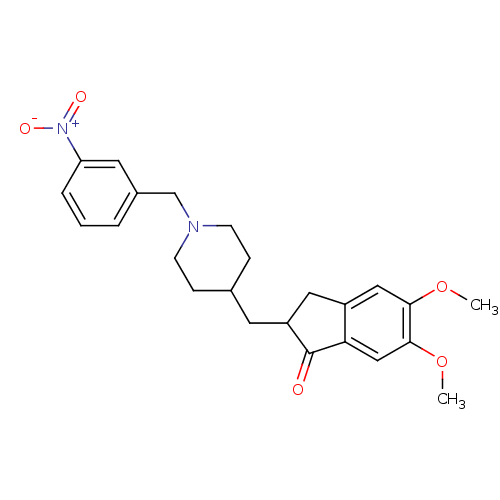 (5,6-Dimethoxy-2-[1-(3-nitro-benzyl)-piperidin-4-yl...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 414 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured up to 3 m... | Eur J Med Chem 123: 282-297 (2016) Article DOI: 10.1016/j.ejmech.2016.07.052 BindingDB Entry DOI: 10.7270/Q2ZW1NW5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50469003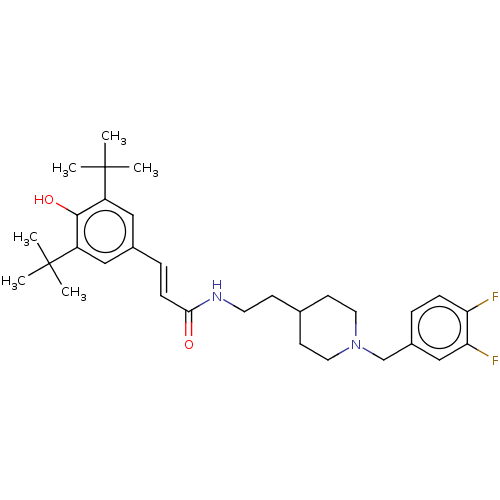 (CHEMBL4281066) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 420 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine chloride as substrate incubated for 5 mins followed by substrate addition measured after 180 ... | Eur J Med Chem 157: 161-176 (2018) Article DOI: 10.1016/j.ejmech.2018.08.005 BindingDB Entry DOI: 10.7270/Q29P34C1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50469020 (CHEMBL3819548) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 450 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of human erythrocyte AChE using acetylthiocholine chloride as substrate incubated for 5 mins followed by substrate addition measured after... | Eur J Med Chem 157: 161-176 (2018) Article DOI: 10.1016/j.ejmech.2018.08.005 BindingDB Entry DOI: 10.7270/Q29P34C1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM8961 (1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 459 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of human erythrocytes AChE using acetylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured up ... | Eur J Med Chem 123: 282-297 (2016) Article DOI: 10.1016/j.ejmech.2016.07.052 BindingDB Entry DOI: 10.7270/Q2ZW1NW5 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50204750 (CHEMBL3889713) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 511 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured up to 3 m... | Eur J Med Chem 123: 282-297 (2016) Article DOI: 10.1016/j.ejmech.2016.07.052 BindingDB Entry DOI: 10.7270/Q2ZW1NW5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 375 total ) | Next | Last >> |