

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Proto-oncogene tyrosine-protein kinase ROS (Homo sapiens (Human)) | BDBM50018830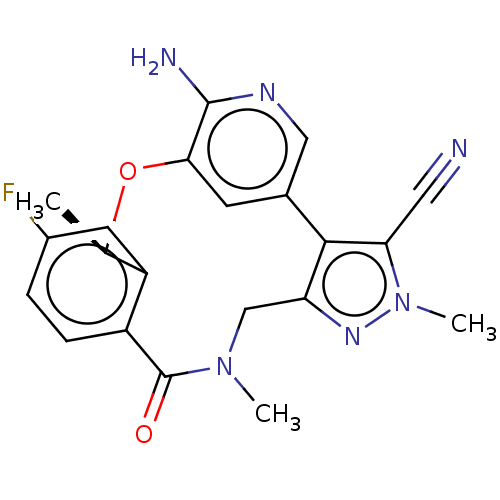 (CHEMBL3286830 | US10543199, Compound PF-06463922 |...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | <0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of ROS1 (unknown origin) by off-chip mobility shift assay | J Med Chem 57: 4720-44 (2014) Article DOI: 10.1021/jm500261q BindingDB Entry DOI: 10.7270/Q2K35W68 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase ROS (Homo sapiens (Human)) | BDBM50448785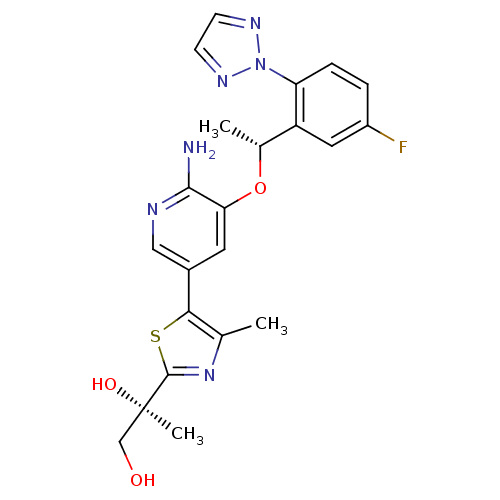 (CHEMBL3128069) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of ROS1 (unknown origin) by Pfizer mobility shift assay | J Med Chem 57: 1170-87 (2014) Article DOI: 10.1021/jm401805h BindingDB Entry DOI: 10.7270/Q29C6ZX5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor (Homo sapiens (Human)) | BDBM50018836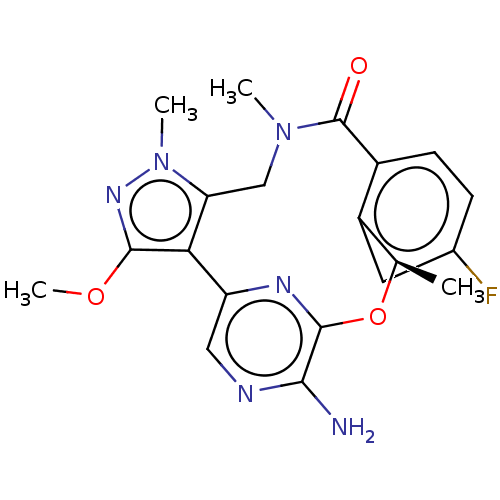 (CHEMBL3286826) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | <0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant ALK L1196M mutant kinase domain (amino acids 1093 to 1141) expressed in baculovirus system using 5'FAM-KKSRGDYMTMQIG-... | J Med Chem 57: 4720-44 (2014) Article DOI: 10.1021/jm500261q BindingDB Entry DOI: 10.7270/Q2K35W68 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM50356880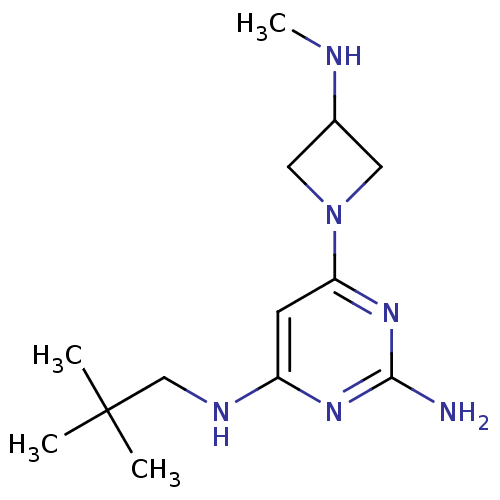 (CHEMBL1915536) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Antagonist activity at full length human H4R expressed in HEK293 cells assessed as reversal of forskolin-induced cAMP production by CRE-beta-lactamas... | Bioorg Med Chem Lett 21: 6596-602 (2011) Article DOI: 10.1016/j.bmcl.2011.07.125 BindingDB Entry DOI: 10.7270/Q20C4W6C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM357210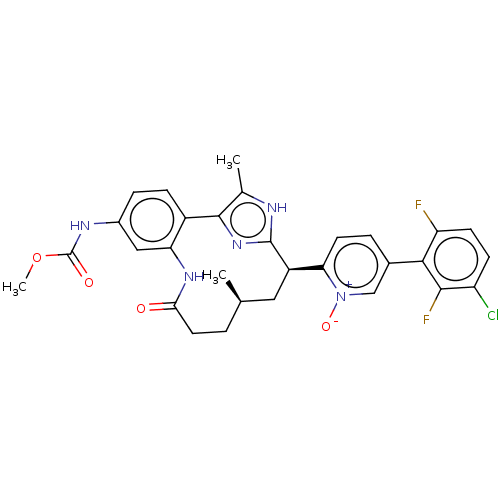 (US10214512, Example 151-a) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The effectiveness of a compound of the present invention as an inhibitor of Coagulation Factor XIa can be determined using a relevant purified serine... | US Patent US10214512 (2019) BindingDB Entry DOI: 10.7270/Q2668GGN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAF proto-oncogene serine/threonine-protein kinase (Homo sapiens (Human)) | BDBM50580080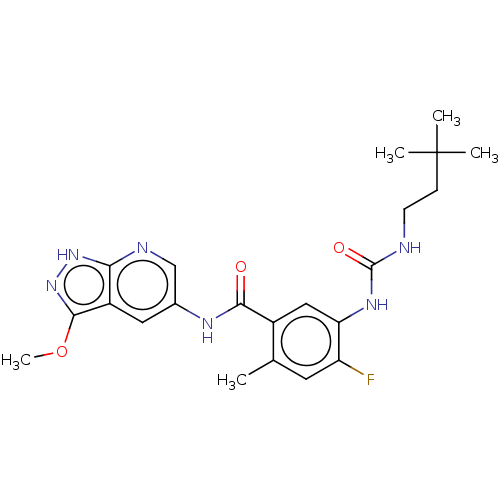 (CHEMBL5090624) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | <0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of CRAF Y340D/Y341D mutant (unknown origin) using inactive MAP2K1 as substrate preincubated for 30 mins measured after 90 mins by DELFIA a... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c02085 BindingDB Entry DOI: 10.7270/Q2GT5S13 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAF proto-oncogene serine/threonine-protein kinase (Homo sapiens (Human)) | BDBM50580082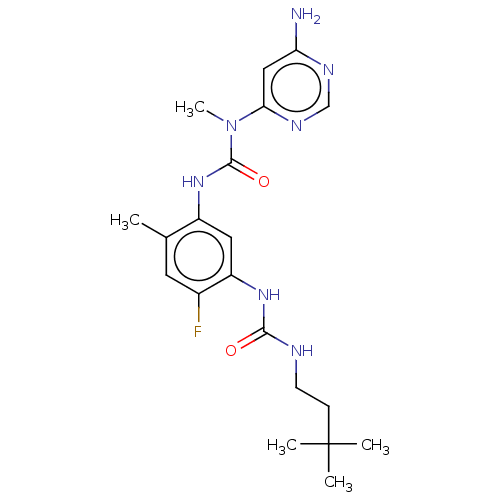 (CHEMBL5079215) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | <0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of CRAF Y340D/Y341D mutant (unknown origin) using inactive MAP2K1 as substrate preincubated for 30 mins measured after 90 mins by DELFIA a... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c02085 BindingDB Entry DOI: 10.7270/Q2GT5S13 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAF proto-oncogene serine/threonine-protein kinase (Homo sapiens (Human)) | BDBM50580083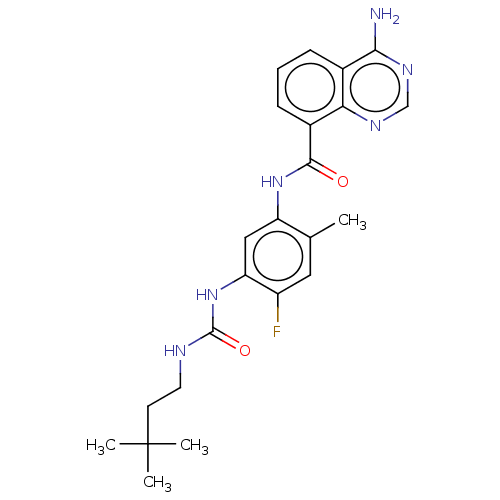 (CHEMBL5094268) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | <0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of CRAF Y340D/Y341D mutant (unknown origin) using inactive MAP2K1 as substrate preincubated for 30 mins measured after 90 mins by DELFIA a... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c02085 BindingDB Entry DOI: 10.7270/Q2GT5S13 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAF proto-oncogene serine/threonine-protein kinase (Homo sapiens (Human)) | BDBM50557775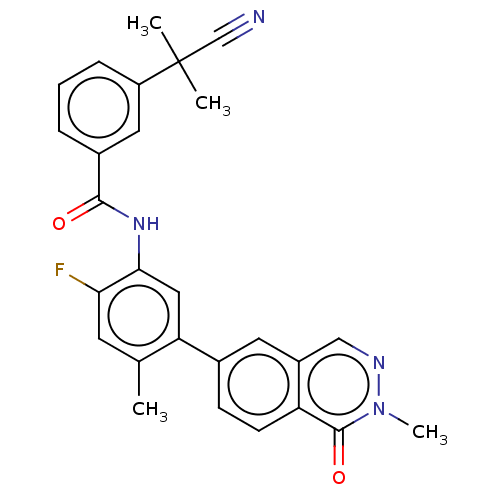 (CHEMBL4758903) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | <0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of CRAF Y340D/Y341D mutant (unknown origin) assessed as using inactive phosphorylated MAP2K1 substrate preincubated for 30 mins measured a... | Citation and Details Article DOI: 10.1021/acsmedchemlett.1c00063 BindingDB Entry DOI: 10.7270/Q2DZ0D0Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAF proto-oncogene serine/threonine-protein kinase (Homo sapiens (Human)) | BDBM50557772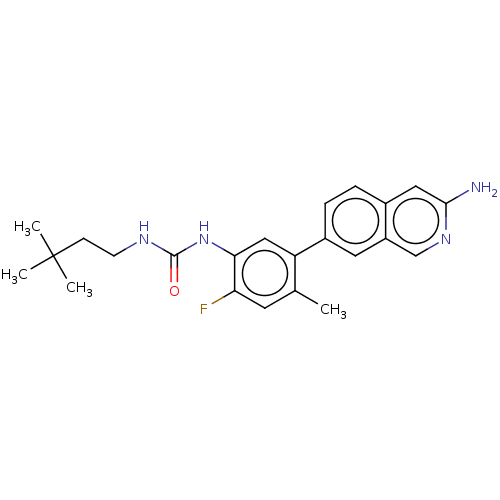 (CHEMBL4775998) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | <0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of CRAF Y340D/Y341D mutant (unknown origin) assessed as using inactive phosphorylated MAP2K1 substrate preincubated for 30 mins measured a... | Citation and Details Article DOI: 10.1021/acsmedchemlett.1c00063 BindingDB Entry DOI: 10.7270/Q2DZ0D0Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAF proto-oncogene serine/threonine-protein kinase (Homo sapiens (Human)) | BDBM50557773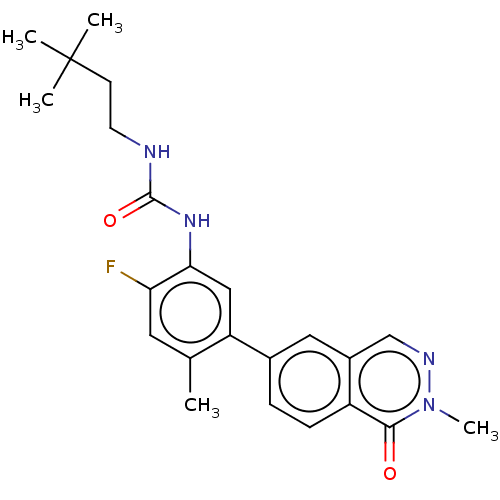 (CHEMBL4778772) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | <0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of CRAF Y340D/Y341D mutant (unknown origin) assessed as using inactive phosphorylated MAP2K1 substrate preincubated for 30 mins measured a... | Citation and Details Article DOI: 10.1021/acsmedchemlett.1c00063 BindingDB Entry DOI: 10.7270/Q2DZ0D0Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAF proto-oncogene serine/threonine-protein kinase (Homo sapiens (Human)) | BDBM50580084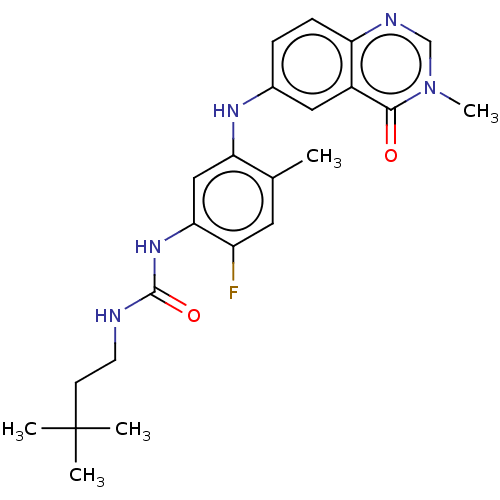 (CHEMBL5075174) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | <0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of CRAF Y340D/Y341D mutant (unknown origin) using inactive MAP2K1 as substrate preincubated for 30 mins measured after 90 mins by DELFIA a... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c02085 BindingDB Entry DOI: 10.7270/Q2GT5S13 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAF proto-oncogene serine/threonine-protein kinase (Homo sapiens (Human)) | BDBM50580081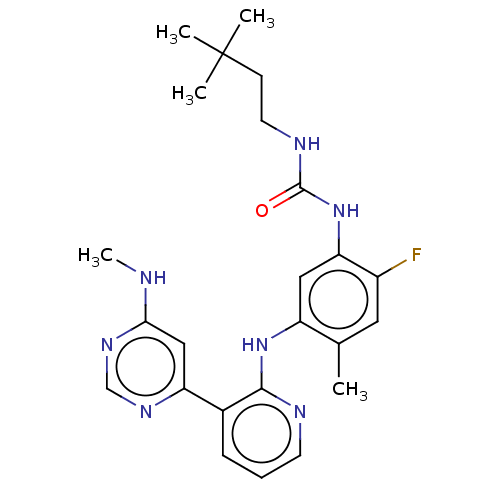 (CHEMBL5094514) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of CRAF Y340D/Y341D mutant (unknown origin) using inactive MAP2K1 as substrate preincubated for 30 mins measured after 90 mins by DELFIA a... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c02085 BindingDB Entry DOI: 10.7270/Q2GT5S13 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAF proto-oncogene serine/threonine-protein kinase (Homo sapiens (Human)) | BDBM50557774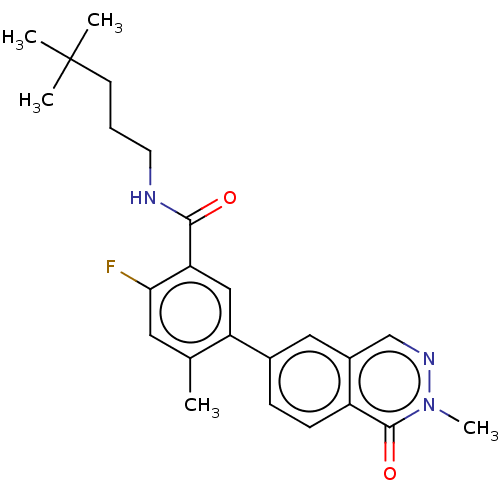 (CHEMBL4776565) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of CRAF Y340D/Y341D mutant (unknown origin) assessed as using inactive phosphorylated MAP2K1 substrate preincubated for 30 mins measured a... | Citation and Details Article DOI: 10.1021/acsmedchemlett.1c00063 BindingDB Entry DOI: 10.7270/Q2DZ0D0Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAF proto-oncogene serine/threonine-protein kinase (Homo sapiens (Human)) | BDBM50096279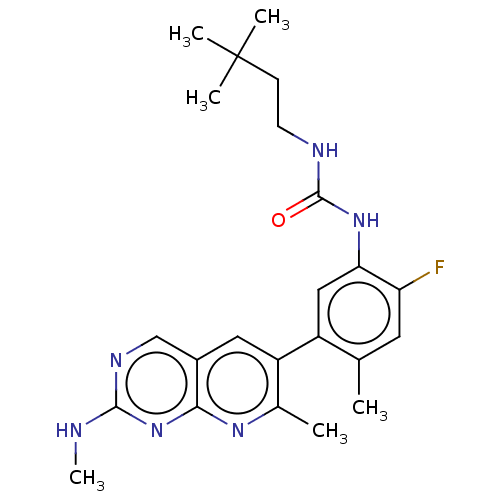 (CHEMBL3577124) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | Article PubMed | 0.0610 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of CRAF Y340D/Y341D mutant (unknown origin) assessed as using inactive phosphorylated MAP2K1 substrate preincubated for 30 mins measured a... | Citation and Details Article DOI: 10.1021/acsmedchemlett.1c00063 BindingDB Entry DOI: 10.7270/Q2DZ0D0Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAF proto-oncogene serine/threonine-protein kinase (Homo sapiens (Human)) | BDBM50557770 (CHEMBL4780060) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | 0.0610 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of CRAF Y340D/Y341D mutant (unknown origin) using inactive MAP2K1 as substrate preincubated for 30 mins measured after 90 mins by DELFIA a... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c02085 BindingDB Entry DOI: 10.7270/Q2GT5S13 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAF proto-oncogene serine/threonine-protein kinase (Homo sapiens (Human)) | BDBM50096279 (CHEMBL3577124) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | Article PubMed | 0.0610 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of CRAF Y340D/Y341D mutant (unknown origin) using inactive MAP2K1 as substrate preincubated for 30 mins measured after 90 mins by DELFIA a... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c02085 BindingDB Entry DOI: 10.7270/Q2GT5S13 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAF proto-oncogene serine/threonine-protein kinase (Homo sapiens (Human)) | BDBM50557770 (CHEMBL4780060) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | 0.0610 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of CRAF Y340D/Y341D mutant (unknown origin) assessed as using inactive phosphorylated MAP2K1 substrate preincubated for 30 mins measured a... | Citation and Details Article DOI: 10.1021/acsmedchemlett.1c00063 BindingDB Entry DOI: 10.7270/Q2DZ0D0Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAF proto-oncogene serine/threonine-protein kinase (Homo sapiens (Human)) | BDBM50557776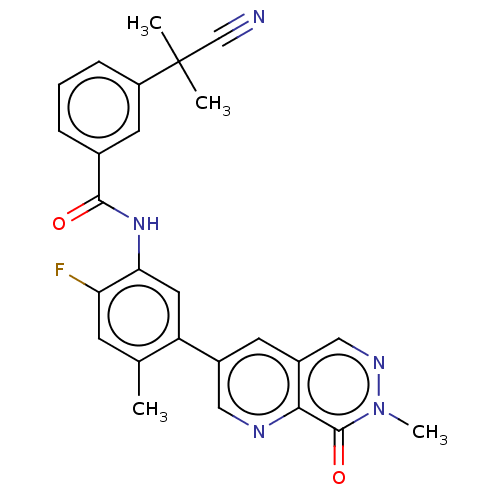 (CHEMBL4778419) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | Article PubMed | 0.0620 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of CRAF Y340D/Y341D mutant (unknown origin) assessed as using inactive phosphorylated MAP2K1 substrate preincubated for 30 mins measured a... | Citation and Details Article DOI: 10.1021/acsmedchemlett.1c00063 BindingDB Entry DOI: 10.7270/Q2DZ0D0Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor (Homo sapiens (Human)) | BDBM50018837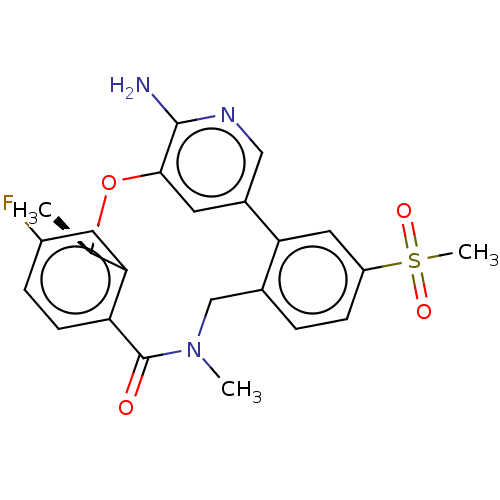 (CHEMBL3286827) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | <0.0700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of wild type human recombinant ALK kinase domain (amino acids 1093 to 1141) expressed in baculovirus system using 5'FAM-KKSRGDYMTMQIG-CONH... | J Med Chem 57: 4720-44 (2014) Article DOI: 10.1021/jm500261q BindingDB Entry DOI: 10.7270/Q2K35W68 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor (Homo sapiens (Human)) | BDBM50018830 (CHEMBL3286830 | US10543199, Compound PF-06463922 |...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | <0.0700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of wild type human recombinant ALK kinase domain (amino acids 1093 to 1141) expressed in baculovirus system using 5'FAM-KKSRGDYMTMQIG-CONH... | J Med Chem 57: 4720-44 (2014) Article DOI: 10.1021/jm500261q BindingDB Entry DOI: 10.7270/Q2K35W68 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor (Homo sapiens (Human)) | BDBM50018836 (CHEMBL3286826) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | <0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of wild type human recombinant ALK kinase domain (amino acids 1093 to 1141) expressed in baculovirus system using 5'FAM-KKSRGDYMTMQIG-CONH... | J Med Chem 57: 4720-44 (2014) Article DOI: 10.1021/jm500261q BindingDB Entry DOI: 10.7270/Q2K35W68 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM357161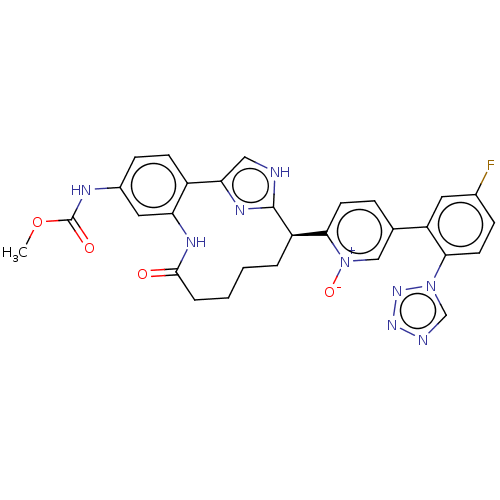 (US10214512, Example 117) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The effectiveness of a compound of the present invention as an inhibitor of Coagulation Factor XIa can be determined using a relevant purified serine... | US Patent US10214512 (2019) BindingDB Entry DOI: 10.7270/Q2668GGN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase B-raf (Homo sapiens (Human)) | BDBM50580081 (CHEMBL5094514) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | <0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of BRAF (unknown origin) (416 to 766) inactive MAP2K1 as substrate preincubated for 30 mins measured after 90 mins by DELFIA assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c02085 BindingDB Entry DOI: 10.7270/Q2GT5S13 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase B-raf (Homo sapiens (Human)) | BDBM50580082 (CHEMBL5079215) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | <0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of BRAF (unknown origin) (416 to 766) inactive MAP2K1 as substrate preincubated for 30 mins measured after 90 mins by DELFIA assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c02085 BindingDB Entry DOI: 10.7270/Q2GT5S13 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase B-raf (Homo sapiens (Human)) | BDBM50580083 (CHEMBL5094268) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | <0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of BRAF (unknown origin) (416 to 766) inactive MAP2K1 as substrate preincubated for 30 mins measured after 90 mins by DELFIA assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c02085 BindingDB Entry DOI: 10.7270/Q2GT5S13 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase B-raf (Homo sapiens (Human)) | BDBM50580084 (CHEMBL5075174) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | <0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of BRAF (unknown origin) (416 to 766) inactive MAP2K1 as substrate preincubated for 30 mins measured after 90 mins by DELFIA assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c02085 BindingDB Entry DOI: 10.7270/Q2GT5S13 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase B-raf (Homo sapiens (Human)) | BDBM50557770 (CHEMBL4780060) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | <0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of BRAF (unknown origin) (416 to 766) inactive MAP2K1 as substrate preincubated for 30 mins measured after 90 mins by DELFIA assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c02085 BindingDB Entry DOI: 10.7270/Q2GT5S13 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Serine/threonine-protein kinase B-raf (Homo sapiens (Human)) | BDBM50557770 (CHEMBL4780060) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | <0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of BRAF (unknown origin) (416 to 766) assessed as using inactive phosphorylated MAP2K1 substrate preincubated for 30 mins measured after 9... | Citation and Details Article DOI: 10.1021/acsmedchemlett.1c00063 BindingDB Entry DOI: 10.7270/Q2DZ0D0Z | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Serine/threonine-protein kinase B-raf (Homo sapiens (Human)) | BDBM50557772 (CHEMBL4775998) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | <0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of BRAF (unknown origin) (416 to 766) assessed as using inactive phosphorylated MAP2K1 substrate preincubated for 30 mins measured after 9... | Citation and Details Article DOI: 10.1021/acsmedchemlett.1c00063 BindingDB Entry DOI: 10.7270/Q2DZ0D0Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase B-raf (Homo sapiens (Human)) | BDBM50557773 (CHEMBL4778772) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | <0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of BRAF (unknown origin) (416 to 766) assessed as using inactive phosphorylated MAP2K1 substrate preincubated for 30 mins measured after 9... | Citation and Details Article DOI: 10.1021/acsmedchemlett.1c00063 BindingDB Entry DOI: 10.7270/Q2DZ0D0Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase B-raf (Homo sapiens (Human)) | BDBM50557775 (CHEMBL4758903) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | <0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of BRAF (unknown origin) (416 to 766) assessed as using inactive phosphorylated MAP2K1 substrate preincubated for 30 mins measured after 9... | Citation and Details Article DOI: 10.1021/acsmedchemlett.1c00063 BindingDB Entry DOI: 10.7270/Q2DZ0D0Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAF proto-oncogene serine/threonine-protein kinase (Homo sapiens (Human)) | BDBM50557771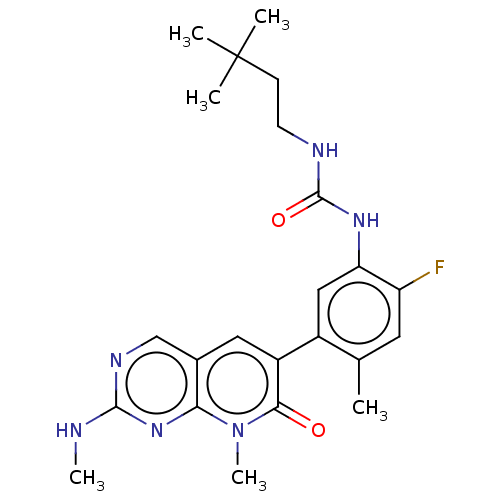 (CHEMBL4740241) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0810 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of CRAF Y340D/Y341D mutant (unknown origin) assessed as using inactive phosphorylated MAP2K1 substrate preincubated for 30 mins measured a... | Citation and Details Article DOI: 10.1021/acsmedchemlett.1c00063 BindingDB Entry DOI: 10.7270/Q2DZ0D0Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM357017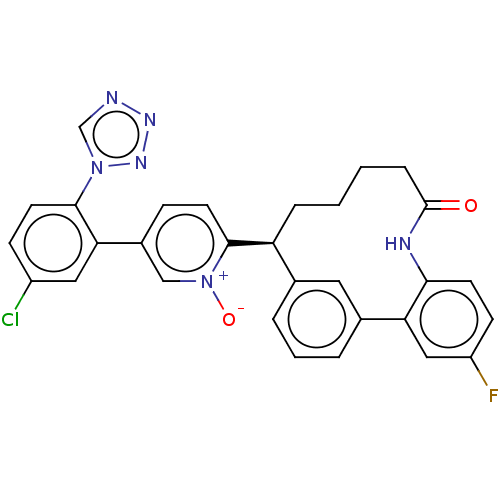 ((S) 5-(5-chloro-2-(1H-tetrazol-1-yl)phenyl)-2-(25-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The effectiveness of a compound of the present invention as an inhibitor of Coagulation Factor XIa can be determined using a relevant purified serine... | US Patent US10214512 (2019) BindingDB Entry DOI: 10.7270/Q2668GGN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM357214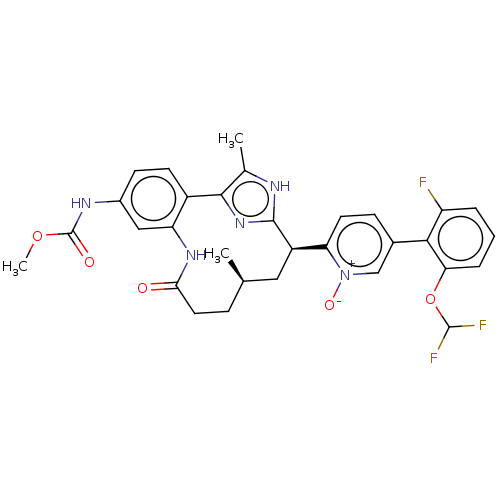 (US10214512, Example 152-a) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The effectiveness of a compound of the present invention as an inhibitor of Coagulation Factor XIa can be determined using a relevant purified serine... | US Patent US10214512 (2019) BindingDB Entry DOI: 10.7270/Q2668GGN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50599186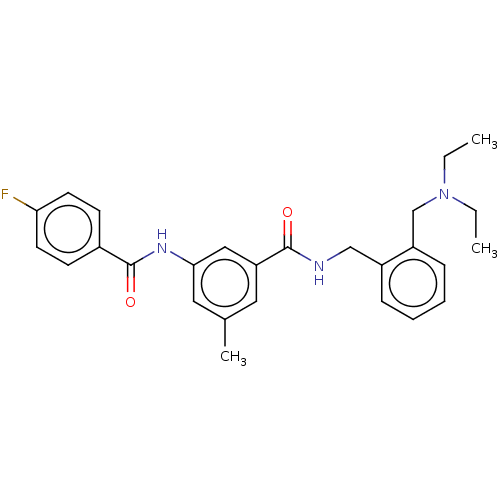 (CHEMBL5201089) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0940 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.2c00944 BindingDB Entry DOI: 10.7270/Q2HX1HP4 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor (Homo sapiens (Human)) | BDBM50018828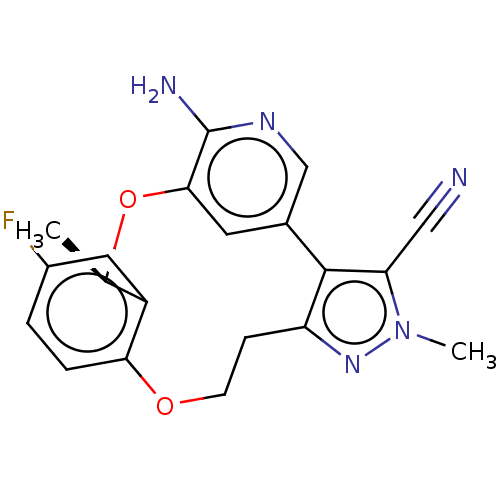 (CHEMBL3286818) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | <0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of wild type human recombinant ALK kinase domain (amino acids 1093 to 1141) expressed in baculovirus system using 5'FAM-KKSRGDYMTMQIG-CONH... | J Med Chem 57: 4720-44 (2014) Article DOI: 10.1021/jm500261q BindingDB Entry DOI: 10.7270/Q2K35W68 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor (Homo sapiens (Human)) | BDBM50018829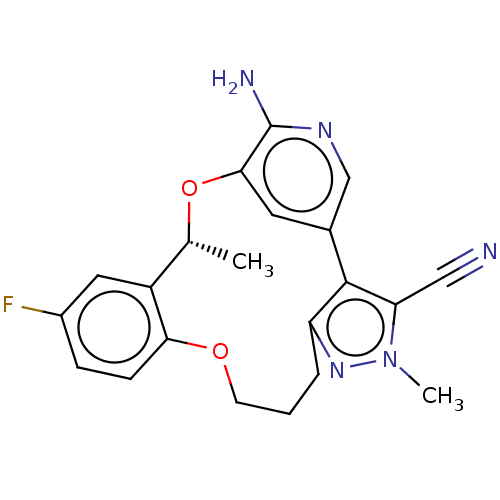 (CHEMBL3286819) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | <0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of wild type human recombinant ALK kinase domain (amino acids 1093 to 1141) expressed in baculovirus system using 5'FAM-KKSRGDYMTMQIG-CONH... | J Med Chem 57: 4720-44 (2014) Article DOI: 10.1021/jm500261q BindingDB Entry DOI: 10.7270/Q2K35W68 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor (Homo sapiens (Human)) | BDBM50018838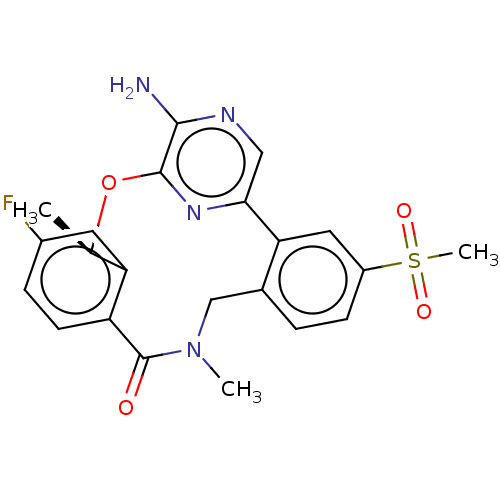 (CHEMBL3286828) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | <0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of wild type human recombinant ALK kinase domain (amino acids 1093 to 1141) expressed in baculovirus system using 5'FAM-KKSRGDYMTMQIG-CONH... | J Med Chem 57: 4720-44 (2014) Article DOI: 10.1021/jm500261q BindingDB Entry DOI: 10.7270/Q2K35W68 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor (Homo sapiens (Human)) | BDBM50018825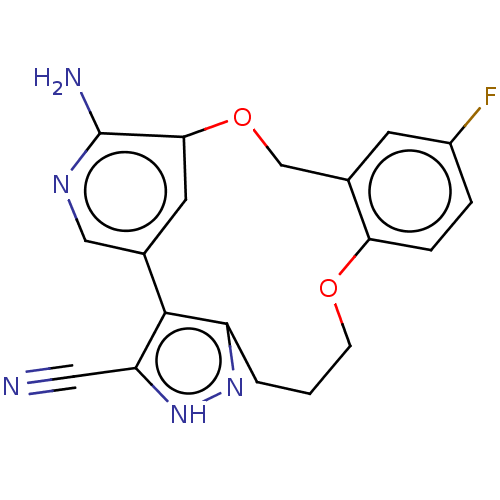 (CHEMBL3286815) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | <0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant ALK L1196M mutant kinase domain (amino acids 1093 to 1141) expressed in baculovirus system using 5'FAM-KKSRGDYMTMQIG-... | J Med Chem 57: 4720-44 (2014) Article DOI: 10.1021/jm500261q BindingDB Entry DOI: 10.7270/Q2K35W68 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor (Homo sapiens (Human)) | BDBM50018833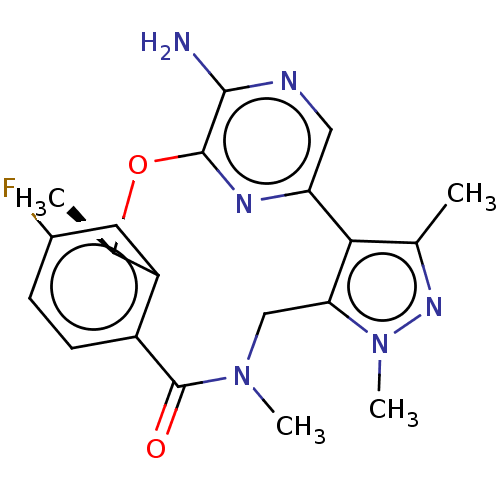 (CHEMBL3286823) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant ALK L1196M mutant kinase domain (amino acids 1093 to 1141) expressed in baculovirus system using 5'FAM-KKSRGDYMTMQIG-... | J Med Chem 57: 4720-44 (2014) Article DOI: 10.1021/jm500261q BindingDB Entry DOI: 10.7270/Q2K35W68 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH2 (Homo sapiens (Human)) | BDBM50246967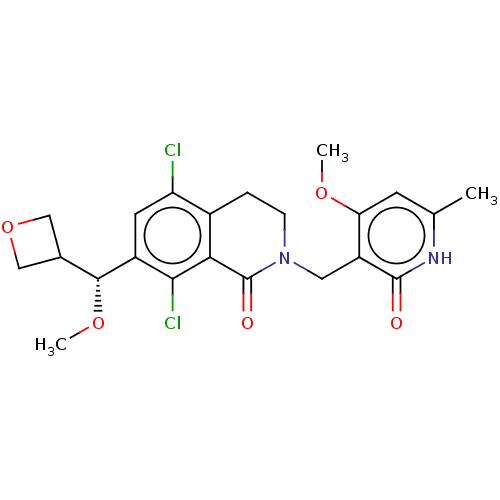 (CHEMBL4080228 | US10570121, Example 81) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | MCE PC cid PC sid PDB UniChem | PDB Article PubMed | <0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
WuXi AppTec Curated by ChEMBL | Assay Description Binding affinity to EZH2 (unknown origin) | J Med Chem 61: 650-665 (2018) Article DOI: 10.1021/acs.jmedchem.7b01375 BindingDB Entry DOI: 10.7270/Q2X069G8 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| ALK tyrosine kinase receptor (Homo sapiens (Human)) | BDBM50018839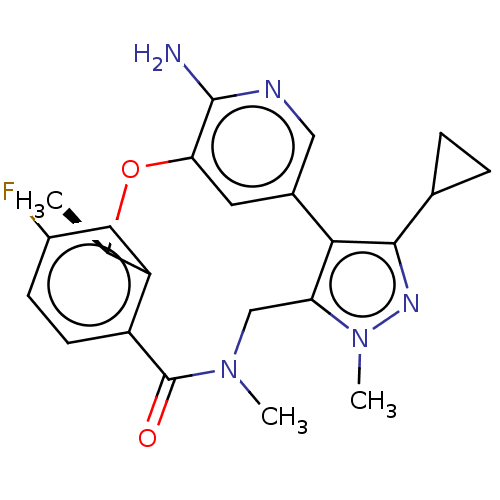 (CHEMBL3286829) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | <0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant ALK L1196M mutant kinase domain (amino acids 1093 to 1141) expressed in baculovirus system using 5'FAM-KKSRGDYMTMQIG-... | J Med Chem 57: 4720-44 (2014) Article DOI: 10.1021/jm500261q BindingDB Entry DOI: 10.7270/Q2K35W68 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| ALK tyrosine kinase receptor (Homo sapiens (Human)) | BDBM50448785 (CHEMBL3128069) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | <0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of wild type human recombinant ALK kinase domain (amino acids 1093 to 1141) expressed in baculovirus system using 5'FAM-KKSRGDYMTMQIG-CONH... | J Med Chem 57: 4720-44 (2014) Article DOI: 10.1021/jm500261q BindingDB Entry DOI: 10.7270/Q2K35W68 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM357026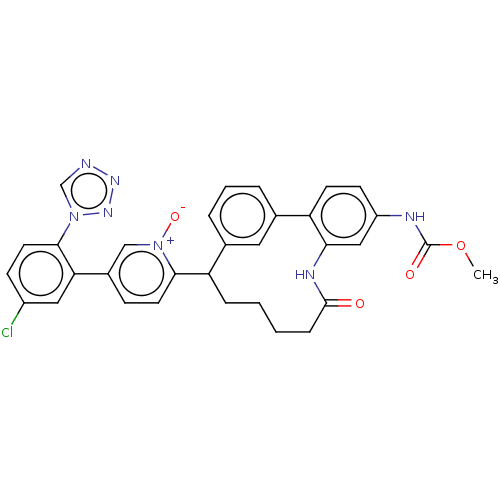 (5-(5-Chloro-2-(1H-tetrazol-1-yl)phenyl)-2-(24-((me...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The effectiveness of a compound of the present invention as an inhibitor of Coagulation Factor XIa can be determined using a relevant purified serine... | US Patent US10214512 (2019) BindingDB Entry DOI: 10.7270/Q2668GGN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM357188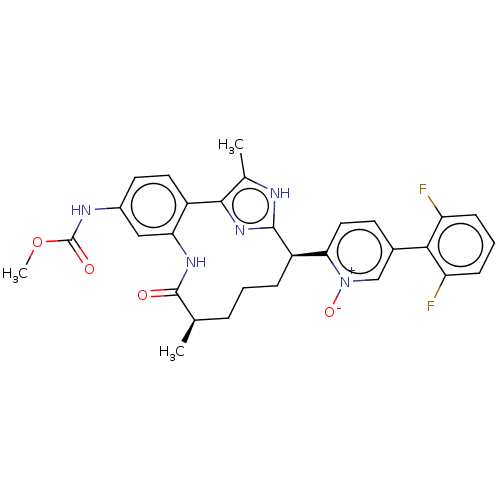 (US10214512, Example 143-b) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The effectiveness of a compound of the present invention as an inhibitor of Coagulation Factor XIa can be determined using a relevant purified serine... | US Patent US10214512 (2019) BindingDB Entry DOI: 10.7270/Q2668GGN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor (Homo sapiens (Human)) | BDBM50018835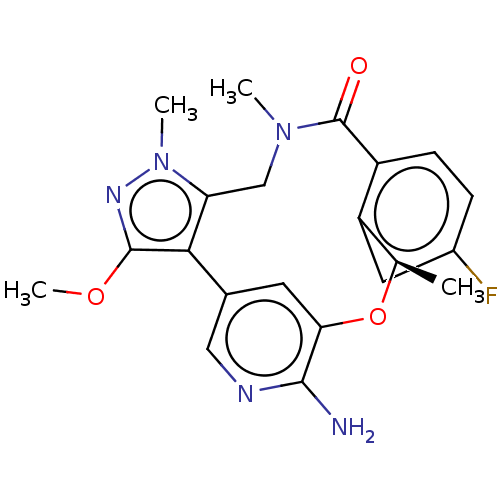 (CHEMBL3286825) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant ALK L1196M mutant kinase domain (amino acids 1093 to 1141) expressed in baculovirus system using 5'FAM-KKSRGDYMTMQIG-... | J Med Chem 57: 4720-44 (2014) Article DOI: 10.1021/jm500261q BindingDB Entry DOI: 10.7270/Q2K35W68 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase B-raf (Homo sapiens (Human)) | BDBM50557771 (CHEMBL4740241) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.102 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of BRAF (unknown origin) (416 to 766) assessed as using inactive phosphorylated MAP2K1 substrate preincubated for 30 mins measured after 9... | Citation and Details Article DOI: 10.1021/acsmedchemlett.1c00063 BindingDB Entry DOI: 10.7270/Q2DZ0D0Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM357058 (US10214512, Example 38) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The effectiveness of a compound of the present invention as an inhibitor of Coagulation Factor XIa can be determined using a relevant purified serine... | US Patent US10214512 (2019) BindingDB Entry DOI: 10.7270/Q2668GGN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM357166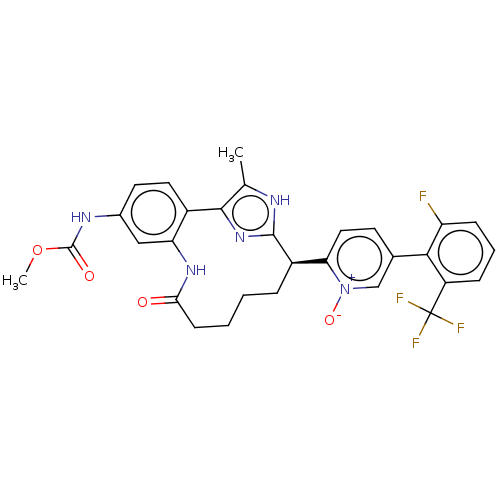 (US10214512, Example 122 | US10214512, Example 125) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The effectiveness of a compound of the present invention as an inhibitor of Coagulation Factor XIa can be determined using a relevant purified serine... | US Patent US10214512 (2019) BindingDB Entry DOI: 10.7270/Q2668GGN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 10838 total ) | Next | Last >> |