 Found 150 hits with Last Name = 'loiseau' and Initial = 'pm'
Found 150 hits with Last Name = 'loiseau' and Initial = 'pm' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Trypanothione reductase
(Trypanosoma brucei brucei) | BDBM50096500

(CHEMBL86281 | N,N'-Bis-(3-phenyl-propyl)-N,N'-bis-...)Show SMILES C(CCN(CCCNCCCc1ccccc1)CCCc1ccccc1)CN(CCCNCCCc1ccccc1)CCCc1ccccc1 Show InChI InChI=1S/C46H66N4/c1-5-21-43(22-6-1)29-15-33-47-35-19-41-49(39-17-31-45-25-9-3-10-26-45)37-13-14-38-50(40-18-32-46-27-11-4-12-28-46)42-20-36-48-34-16-30-44-23-7-2-8-24-44/h1-12,21-28,47-48H,13-20,29-42H2 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | 600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Trypanothione reductase
(Crithidia fasciculata) | BDBM50240622
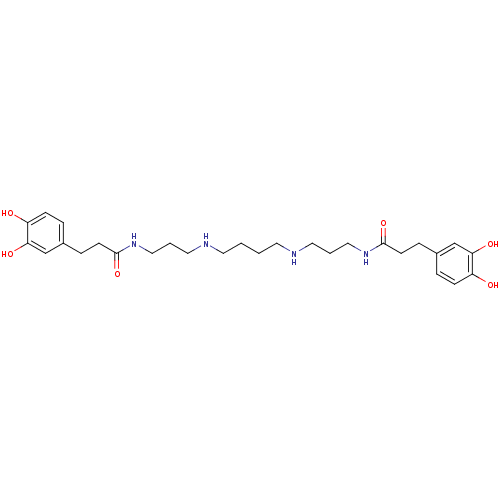
(3-(3,4-Dihydroxy-phenyl)-N-[3-(4-{3-[3-(3,4-dihydr...)Show SMILES Oc1ccc(CCC(=O)NCCCNCCCCNCCCNC(=O)CCc2ccc(O)c(O)c2)cc1O Show InChI InChI=1S/C28H42N4O6/c33-23-9-5-21(19-25(23)35)7-11-27(37)31-17-3-15-29-13-1-2-14-30-16-4-18-32-28(38)12-8-22-6-10-24(34)26(36)20-22/h5-6,9-10,19-20,29-30,33-36H,1-4,7-8,11-18H2,(H,31,37)(H,32,38) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| | 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Trypanothione reductase
(Trypanosoma cruzi) | BDBM50615394
(CHEMBL5266592)Show SMILES CCCNCCCNC(=O)CNC(=O)[C@H](CCCC[C@H](NC(=O)OCC1c2ccccc2-c2ccccc12)C(=O)NCC(=O)NCCCNCCC)NC(=O)OCC1c2ccccc2-c2ccccc12 |r| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Trypanothione reductase
(Trypanosoma brucei brucei) | BDBM50096496
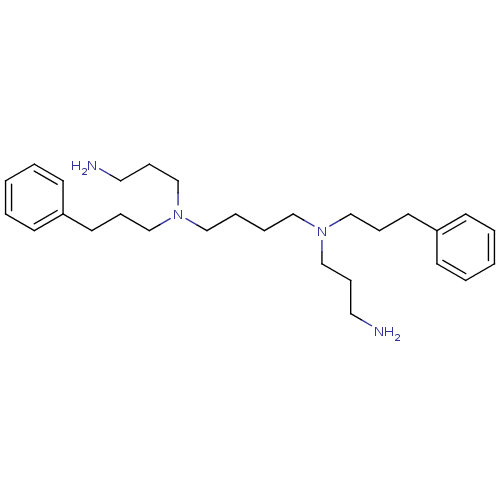
(CHEMBL62048 | N,N'-Bis-(3-amino-propyl)-N,N'-bis-(...)Show InChI InChI=1S/C28H46N4/c29-19-11-25-31(23-9-17-27-13-3-1-4-14-27)21-7-8-22-32(26-12-20-30)24-10-18-28-15-5-2-6-16-28/h1-6,13-16H,7-12,17-26,29-30H2 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Trypanothione reductase
(Trypanosoma cruzi) | BDBM50096493

(CHEMBL82641 | N,N'-Bis-(3-phenyl-propyl)-N-[3-(3-p...)Show SMILES C(CCN(CCCNCCCc1ccccc1)CCCc1ccccc1)CNCCCc1ccccc1 Show InChI InChI=1S/C34H49N3/c1-4-16-32(17-5-1)22-12-26-35-25-10-11-29-37(30-14-24-34-20-8-3-9-21-34)31-15-28-36-27-13-23-33-18-6-2-7-19-33/h1-9,16-21,35-36H,10-15,22-31H2 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | 3.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Trypanothione reductase
(Trypanosoma cruzi) | BDBM50408797
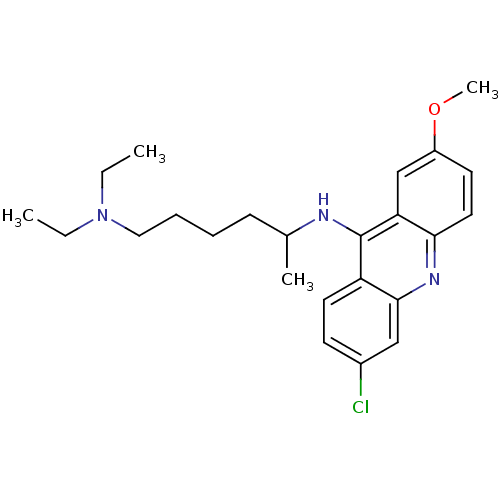
(CHEMBL151228)Show SMILES CCN(CC)CCCCC(C)Nc1c2ccc(Cl)cc2nc2ccc(OC)cc12 Show InChI InChI=1S/C24H32ClN3O/c1-5-28(6-2)14-8-7-9-17(3)26-24-20-12-10-18(25)15-23(20)27-22-13-11-19(29-4)16-21(22)24/h10-13,15-17H,5-9,14H2,1-4H3,(H,26,27) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | 5.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Trypanothione reductase
(Trypanosoma brucei brucei) | BDBM50462235
(CHEMBL4244566)Show InChI InChI=1S/C21H31N3/c22-14-7-8-15-23-16-9-17-24(18-20-10-3-1-4-11-20)19-21-12-5-2-6-13-21/h1-6,10-13,23H,7-9,14-19,22H2 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 7.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Paris-Saclay
Curated by ChEMBL
| Assay Description
Mixed-type inhibition of Trypanosoma brucei trypanothione reductase assessed as enzyme-inhibitor complex using varying levels of TS2 as substrate in ... |
Eur J Med Chem 150: 655-666 (2018)
Article DOI: 10.1016/j.ejmech.2018.02.087
BindingDB Entry DOI: 10.7270/Q22N54ZW |
More data for this
Ligand-Target Pair | |
Trypanothione reductase
(Trypanosoma brucei brucei) | BDBM50096498
(CHEMBL86096 | N,N'-Bis-[3-(3-phenyl-propylamino)-p...)Show InChI InChI=1S/C28H46N4/c1-3-13-27(14-4-1)17-9-21-31-25-11-23-29-19-7-8-20-30-24-12-26-32-22-10-18-28-15-5-2-6-16-28/h1-6,13-16,29-32H,7-12,17-26H2 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | 1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Trypanothione reductase
(Trypanosoma cruzi) | BDBM50615395
(CHEMBL5280891)Show SMILES CCCNCCCNC(=O)CNC(=O)[C@H](CC(Br)C(Br)C[C@H](NC(=O)OCc1ccccc1)C(=O)NCC(=O)NCCCNCCC)NC(=O)OCc1ccccc1 |r| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 1.59E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Trypanothione reductase
(Trypanosoma brucei brucei) | BDBM50009371

(1, 12-DB-3-4-3 | CHEMBL81614 | N,N'-Bis-(3-benzyla...)Show InChI InChI=1S/C24H38N4/c1-3-11-23(12-4-1)21-27-19-9-17-25-15-7-8-16-26-18-10-20-28-22-24-13-5-2-6-14-24/h1-6,11-14,25-28H,7-10,15-22H2 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| | 1.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Trypanothione reductase
(Trypanosoma cruzi) | BDBM50015214
(6-chloro-2-methoxy-9-acridinyl(4-diethylamino-1-me...)Show SMILES CCN(CC)CCCC(C)Nc1c2ccc(Cl)cc2nc2ccc(OC)cc12 Show InChI InChI=1S/C23H30ClN3O/c1-5-27(6-2)13-7-8-16(3)25-23-19-11-9-17(24)14-22(19)26-21-12-10-18(28-4)15-20(21)23/h9-12,14-16H,5-8,13H2,1-4H3,(H,25,26) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| | 1.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Trypanothione reductase
(Trypanosoma brucei brucei) | BDBM50462235

(CHEMBL4244566)Show InChI InChI=1S/C21H31N3/c22-14-7-8-15-23-16-9-17-24(18-20-10-3-1-4-11-20)19-21-12-5-2-6-13-21/h1-6,10-13,23H,7-9,14-19,22H2 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Paris-Saclay
Curated by ChEMBL
| Assay Description
Mixed-type inhibition of Trypanosoma brucei trypanothione reductase assessed as enzyme-inhibitor-substrate complex using varying levels of TS2 as sub... |
Eur J Med Chem 150: 655-666 (2018)
Article DOI: 10.1016/j.ejmech.2018.02.087
BindingDB Entry DOI: 10.7270/Q22N54ZW |
More data for this
Ligand-Target Pair | |
Trypanothione reductase
(Trypanosoma cruzi) | BDBM50615396
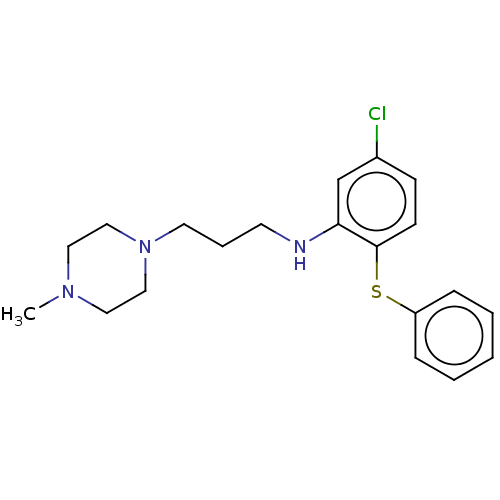
(CHEMBL311566) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 2.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Trypanothione reductase
(Trypanosoma cruzi) | BDBM50096497

(CHEMBL85779 | N-(3-Amino-propyl)-N'-[3-(3-phenyl-p...)Show InChI InChI=1S/C19H36N4/c20-12-7-16-21-13-4-5-14-22-17-8-18-23-15-6-11-19-9-2-1-3-10-19/h1-3,9-10,21-23H,4-8,11-18,20H2 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | 3.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Trypanothione reductase
(Crithidia fasciculata) | BDBM50615384
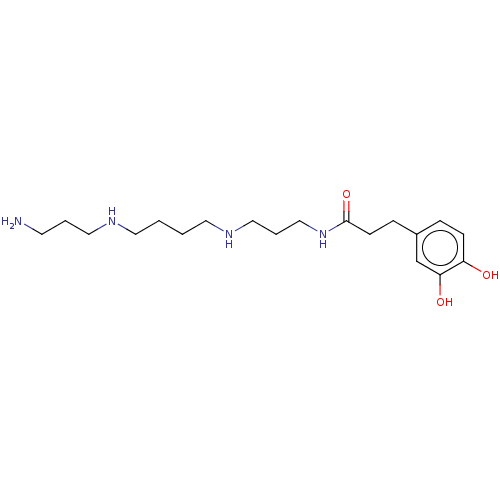
(CHEMBL80264) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 8.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Trypanothione reductase
(Trypanosoma cruzi) | BDBM50615399
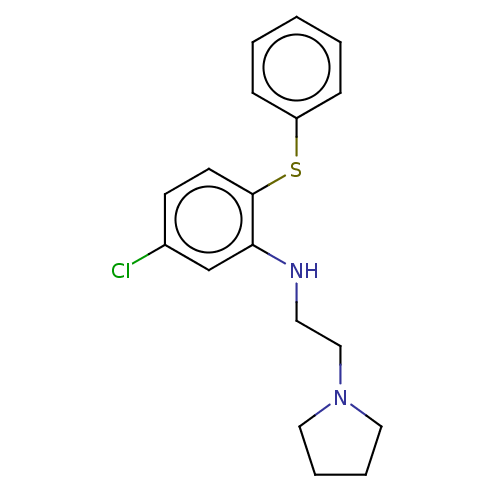
(CHEMBL82315) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 8.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Trypanothione reductase
(Trypanosoma cruzi) | BDBM50615398
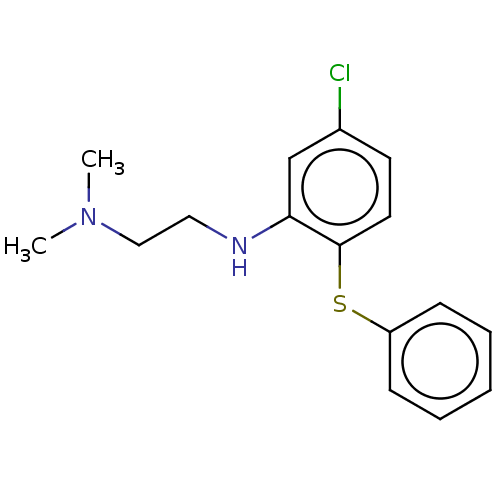
(CHEMBL77450) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 9.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Trypanothione reductase
(Trypanosoma cruzi) | BDBM50615397
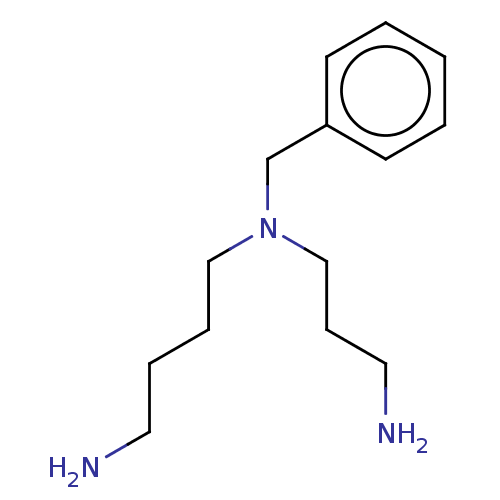
(CHEMBL210576) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Trypanothione reductase
(Trypanosoma cruzi) | BDBM50184788

((4-Amino-butyl)-(3-amino-propyl)-carbamic acid ben...)Show InChI InChI=1S/C15H25N3O2/c16-9-4-5-11-18(12-6-10-17)15(19)20-13-14-7-2-1-3-8-14/h1-3,7-8H,4-6,9-13,16-17H2 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Trypanothione reductase
(Trypanosoma brucei brucei) | BDBM50615382
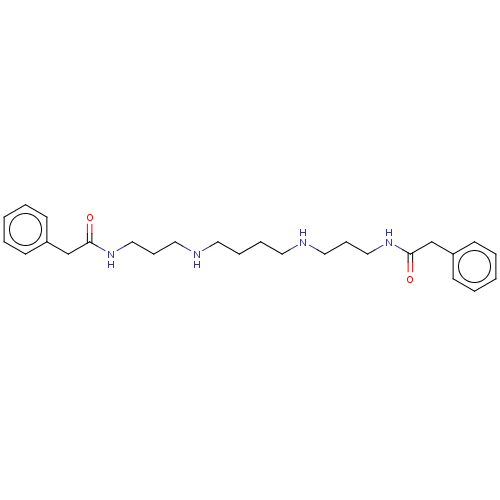
(CHEMBL211029) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Trypanothione reductase
(Trypanosoma cruzi) | BDBM50184783

(CHEMBL298628 | N*1*-(3-Amino-propyl)-N*1*-naphthal...)Show InChI InChI=1S/C18H27N3/c19-10-3-4-12-21(13-5-11-20)15-16-8-9-17-6-1-2-7-18(17)14-16/h1-2,6-9,14H,3-5,10-13,15,19-20H2 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Trypanothione reductase
(Trypanosoma cruzi) | BDBM50615390
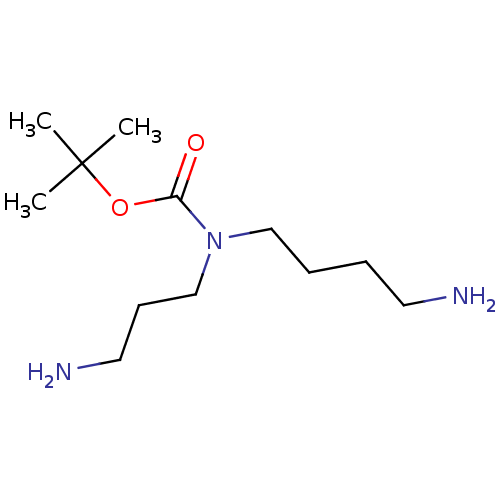
(CHEMBL210734) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | >2.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Trypanothione reductase
(Trypanosoma cruzi) | BDBM50615391
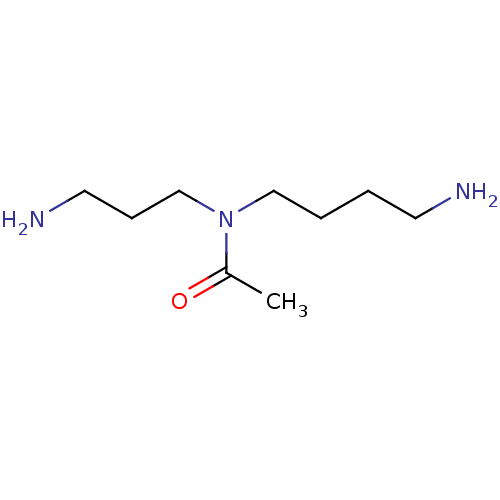
(CHEMBL5277812) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | >2.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Trypanothione reductase
(Trypanosoma cruzi) | BDBM50615392
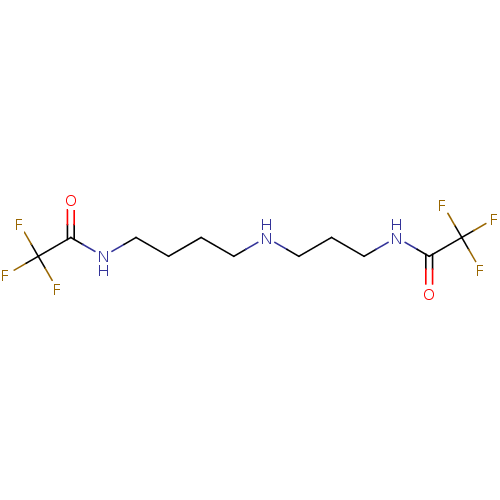
(CHEMBL2009956) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | >2.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Trypanothione reductase
(Trypanosoma cruzi) | BDBM50615393
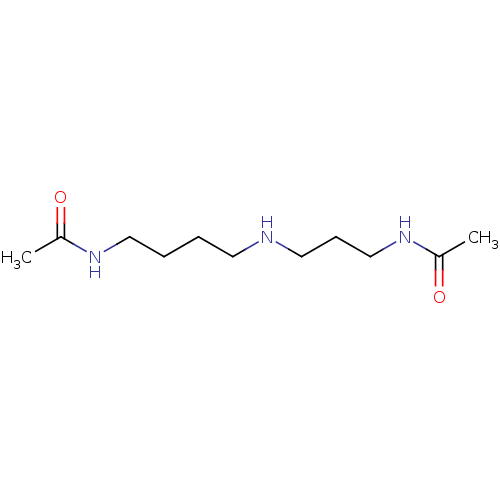
(CHEMBL2008816) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | >2.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50139469
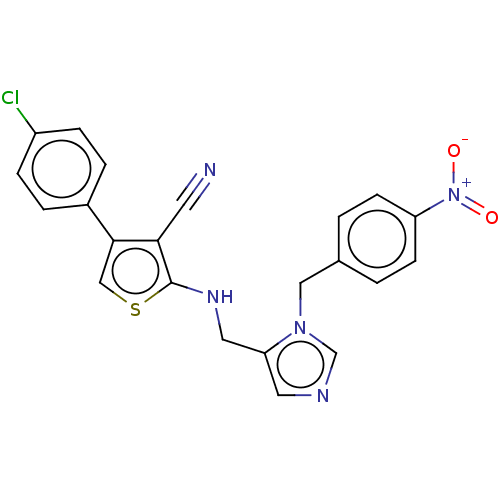
(CHEMBL3763335)Show SMILES [O-][N+](=O)c1ccc(Cn2cncc2CNc2scc(c2C#N)-c2ccc(Cl)cc2)cc1 Show InChI InChI=1S/C22H16ClN5O2S/c23-17-5-3-16(4-6-17)21-13-31-22(20(21)9-24)26-11-19-10-25-14-27(19)12-15-1-7-18(8-2-15)28(29)30/h1-8,10,13-14,26H,11-12H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS UPR2301
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant farnesyltransferase using farnesyl pyrophosphate and Dansyl-GCVLS peptide after 15 mins by fluorescence assay |
Eur J Med Chem 109: 173-86 (2016)
Article DOI: 10.1016/j.ejmech.2015.12.045
BindingDB Entry DOI: 10.7270/Q25B04C6 |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50139637

(CHEMBL3765675)Show SMILES CCOC(=O)c1sc(N(Cc2cncn2Cc2ccc(cc2)C#N)Cc2cncn2Cc2ccc(cc2)C#N)c(C#N)c1-c1ccc(Cl)cc1 Show InChI InChI=1S/C38H29ClN8O2S/c1-2-49-38(48)36-35(30-11-13-31(39)14-12-30)34(17-42)37(50-36)45(22-32-18-43-24-46(32)20-28-7-3-26(15-40)4-8-28)23-33-19-44-25-47(33)21-29-9-5-27(16-41)6-10-29/h3-14,18-19,24-25H,2,20-23H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS UPR2301
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant farnesyltransferase using farnesyl pyrophosphate and Dansyl-GCVLS peptide after 15 mins by fluorescence assay |
Eur J Med Chem 109: 173-86 (2016)
Article DOI: 10.1016/j.ejmech.2015.12.045
BindingDB Entry DOI: 10.7270/Q25B04C6 |
More data for this
Ligand-Target Pair | |
Trypanothione synthetase
(Crithidia fasciculata) | BDBM50615400
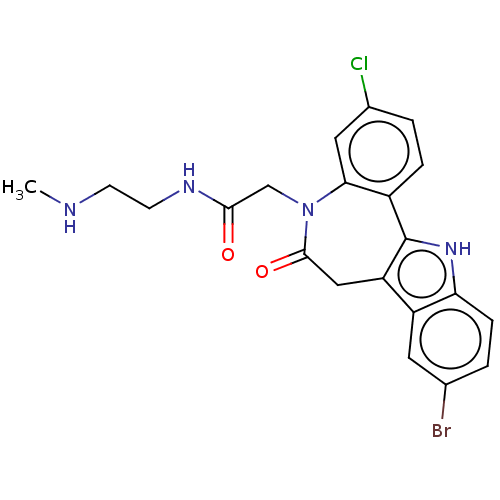
(CHEMBL4092938)Show SMILES CNCCNC(=O)CN1c2cc(Cl)ccc2-c2[nH]c3ccc(Br)cc3c2CC1=O | MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Dual specificity protein kinase CLK1
(Mus musculus) | BDBM50568546

(CHEMBL4853288) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant mouse CLK1 expressed in bacteria using GRSRSRSRSRSR as substrate in presence of [gamma-33P]ATP by radiometric assay |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112956
BindingDB Entry DOI: 10.7270/Q2125XD2 |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50139457
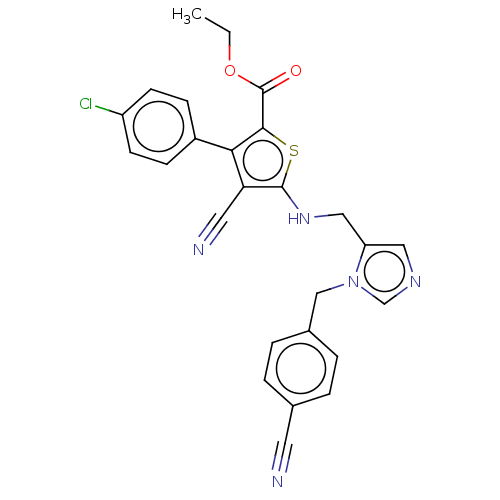
(CHEMBL3765006)Show SMILES CCOC(=O)c1sc(NCc2cncn2Cc2ccc(cc2)C#N)c(C#N)c1-c1ccc(Cl)cc1 Show InChI InChI=1S/C26H20ClN5O2S/c1-2-34-26(33)24-23(19-7-9-20(27)10-8-19)22(12-29)25(35-24)31-14-21-13-30-16-32(21)15-18-5-3-17(11-28)4-6-18/h3-10,13,16,31H,2,14-15H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS UPR2301
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant farnesyltransferase using farnesyl pyrophosphate and Dansyl-GCVLS peptide after 15 mins by fluorescence assay |
Eur J Med Chem 109: 173-86 (2016)
Article DOI: 10.1016/j.ejmech.2015.12.045
BindingDB Entry DOI: 10.7270/Q25B04C6 |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50139466
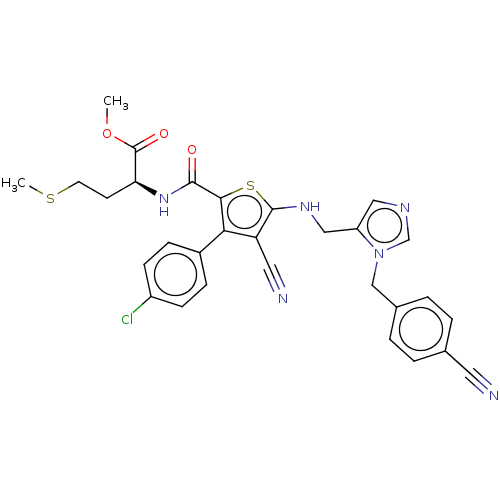
(CHEMBL3765154)Show SMILES COC(=O)[C@H](CCSC)NC(=O)c1sc(NCc2cncn2Cc2ccc(cc2)C#N)c(C#N)c1-c1ccc(Cl)cc1 |r| Show InChI InChI=1S/C30H27ClN6O3S2/c1-40-30(39)25(11-12-41-2)36-28(38)27-26(21-7-9-22(31)10-8-21)24(14-33)29(42-27)35-16-23-15-34-18-37(23)17-20-5-3-19(13-32)4-6-20/h3-10,15,18,25,35H,11-12,16-17H2,1-2H3,(H,36,38)/t25-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS UPR2301
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant farnesyltransferase using farnesyl pyrophosphate and Dansyl-GCVLS peptide after 15 mins by fluorescence assay |
Eur J Med Chem 109: 173-86 (2016)
Article DOI: 10.1016/j.ejmech.2015.12.045
BindingDB Entry DOI: 10.7270/Q25B04C6 |
More data for this
Ligand-Target Pair | |
Casein kinase I isoform epsilon
(Homo sapiens (Human)) | BDBM50568544

(CHEMBL4870529)Show SMILES CN(C)c1nccn2c(c(nc12)-c1ccc(F)cc1)-c1ccnc(N)n1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 160 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human CK1 epsilon expressed in baculovirus infected Sf9 cells using RRKHAAIGSpAYSITA as substrate |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112956
BindingDB Entry DOI: 10.7270/Q2125XD2 |
More data for this
Ligand-Target Pair | |
Dual specificity protein kinase CLK1
(Mus musculus) | BDBM50568545

(CHEMBL4877608)Show SMILES CSc1nccc(n1)-c1c(nc2c(N)nccn12)-c1ccc(F)cc1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 160 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant mouse CLK1 expressed in bacteria using GRSRSRSRSRSR as substrate in presence of [gamma-33P]ATP by radiometric assay |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112956
BindingDB Entry DOI: 10.7270/Q2125XD2 |
More data for this
Ligand-Target Pair | |
Casein kinase I isoform epsilon
(Homo sapiens (Human)) | BDBM50568545

(CHEMBL4877608)Show SMILES CSc1nccc(n1)-c1c(nc2c(N)nccn12)-c1ccc(F)cc1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 180 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human CK1 epsilon expressed in baculovirus infected Sf9 cells using RRKHAAIGSpAYSITA as substrate |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112956
BindingDB Entry DOI: 10.7270/Q2125XD2 |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50139468
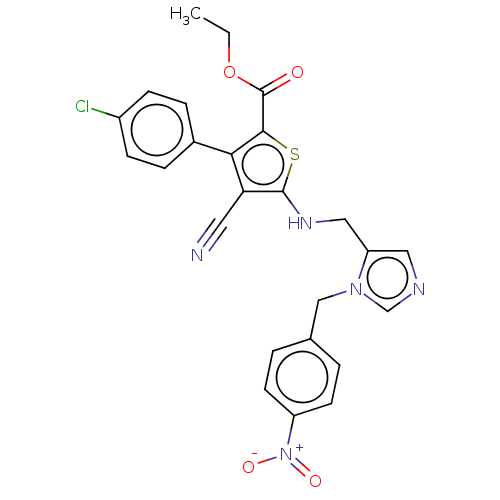
(CHEMBL3763857)Show SMILES CCOC(=O)c1sc(NCc2cncn2Cc2ccc(cc2)[N+]([O-])=O)c(C#N)c1-c1ccc(Cl)cc1 Show InChI InChI=1S/C25H20ClN5O4S/c1-2-35-25(32)23-22(17-5-7-18(26)8-6-17)21(11-27)24(36-23)29-13-20-12-28-15-30(20)14-16-3-9-19(10-4-16)31(33)34/h3-10,12,15,29H,2,13-14H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 190 | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS UPR2301
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant farnesyltransferase using farnesyl pyrophosphate and Dansyl-GCVLS peptide after 15 mins by fluorescence assay |
Eur J Med Chem 109: 173-86 (2016)
Article DOI: 10.1016/j.ejmech.2015.12.045
BindingDB Entry DOI: 10.7270/Q25B04C6 |
More data for this
Ligand-Target Pair | |
Trypanothione reductase
(Trypanosoma cruzi) | BDBM50615386

(CHEMBL5283195)Show SMILES CCCCCCCCCCCCCC(=O)NCCCC(=O)N(CC(=O)Nc1cccc(Sc2ccc(Br)cc2NC(=O)CCN2CCN(C)CC2)c1)C(=O)Nc1ccccc1Sc1ccc(Br)cc1NC(=O)CCN1CCN(C)CC1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Casein kinase I isoform epsilon
(Homo sapiens (Human)) | BDBM50568546

(CHEMBL4853288) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human CK1 epsilon expressed in baculovirus infected Sf9 cells using RRKHAAIGSpAYSITA as substrate |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112956
BindingDB Entry DOI: 10.7270/Q2125XD2 |
More data for this
Ligand-Target Pair | |
Dual specificity protein kinase CLK1
(Mus musculus) | BDBM50568544

(CHEMBL4870529)Show SMILES CN(C)c1nccn2c(c(nc12)-c1ccc(F)cc1)-c1ccnc(N)n1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 250 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant mouse CLK1 expressed in bacteria using GRSRSRSRSRSR as substrate in presence of [gamma-33P]ATP by radiometric assay |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112956
BindingDB Entry DOI: 10.7270/Q2125XD2 |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50139472

(CHEMBL3763746)Show SMILES CCOC(=O)c1sc(N(Cc2cncn2Cc2ccc(cc2)C#N)C(=O)OC(C)(C)C)c(C#N)c1-c1ccc(Cl)cc1 Show InChI InChI=1S/C31H28ClN5O4S/c1-5-40-29(38)27-26(22-10-12-23(32)13-11-22)25(15-34)28(42-27)37(30(39)41-31(2,3)4)18-24-16-35-19-36(24)17-21-8-6-20(14-33)7-9-21/h6-13,16,19H,5,17-18H2,1-4H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 290 | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS UPR2301
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant farnesyltransferase using farnesyl pyrophosphate and Dansyl-GCVLS peptide after 15 mins by fluorescence assay |
Eur J Med Chem 109: 173-86 (2016)
Article DOI: 10.1016/j.ejmech.2015.12.045
BindingDB Entry DOI: 10.7270/Q25B04C6 |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50139477

(CHEMBL3765776)Show SMILES CCOC(=O)c1sc(N(Cc2cn(Cc3ccc(cc3)C#N)cn2)C(=O)OC(C)(C)C)c(C#N)c1-c1ccc(Cl)cc1 Show InChI InChI=1S/C31H28ClN5O4S/c1-5-40-29(38)27-26(22-10-12-23(32)13-11-22)25(15-34)28(42-27)37(30(39)41-31(2,3)4)18-24-17-36(19-35-24)16-21-8-6-20(14-33)7-9-21/h6-13,17,19H,5,16,18H2,1-4H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 330 | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS UPR2301
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant farnesyltransferase using farnesyl pyrophosphate and Dansyl-GCVLS peptide after 15 mins by fluorescence assay |
Eur J Med Chem 109: 173-86 (2016)
Article DOI: 10.1016/j.ejmech.2015.12.045
BindingDB Entry DOI: 10.7270/Q25B04C6 |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50139458
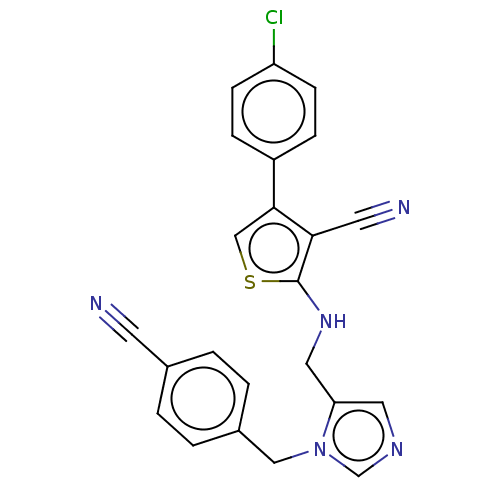
(CHEMBL3764994)Show SMILES Clc1ccc(cc1)-c1csc(NCc2cncn2Cc2ccc(cc2)C#N)c1C#N Show InChI InChI=1S/C23H16ClN5S/c24-19-7-5-18(6-8-19)22-14-30-23(21(22)10-26)28-12-20-11-27-15-29(20)13-17-3-1-16(9-25)2-4-17/h1-8,11,14-15,28H,12-13H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 370 | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS UPR2301
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant farnesyltransferase using farnesyl pyrophosphate and Dansyl-GCVLS peptide after 15 mins by fluorescence assay |
Eur J Med Chem 109: 173-86 (2016)
Article DOI: 10.1016/j.ejmech.2015.12.045
BindingDB Entry DOI: 10.7270/Q25B04C6 |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50139478
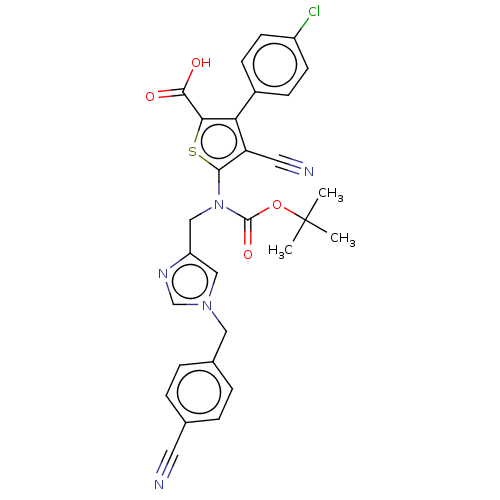
(CHEMBL3764593)Show SMILES CC(C)(C)OC(=O)N(Cc1cn(Cc2ccc(cc2)C#N)cn1)c1sc(C(O)=O)c(c1C#N)-c1ccc(Cl)cc1 Show InChI InChI=1S/C29H24ClN5O4S/c1-29(2,3)39-28(38)35(16-22-15-34(17-33-22)14-19-6-4-18(12-31)5-7-19)26-23(13-32)24(25(40-26)27(36)37)20-8-10-21(30)11-9-20/h4-11,15,17H,14,16H2,1-3H3,(H,36,37) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 380 | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS UPR2301
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant farnesyltransferase using farnesyl pyrophosphate and Dansyl-GCVLS peptide after 15 mins by fluorescence assay |
Eur J Med Chem 109: 173-86 (2016)
Article DOI: 10.1016/j.ejmech.2015.12.045
BindingDB Entry DOI: 10.7270/Q25B04C6 |
More data for this
Ligand-Target Pair | |
Cyclin-T1/Cyclin-dependent kinase 9
(Homo sapiens (Human)) | BDBM50568546

(CHEMBL4853288) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human recombinant CDK9/CyclinT expressed in baculovirus infected Sf9 cells using YSPTSPSYSPTSPSYSPTSPSKKK as substrate in presence of [... |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112956
BindingDB Entry DOI: 10.7270/Q2125XD2 |
More data for this
Ligand-Target Pair | |
Trypanothione reductase
(Trypanosoma cruzi) | BDBM50070270
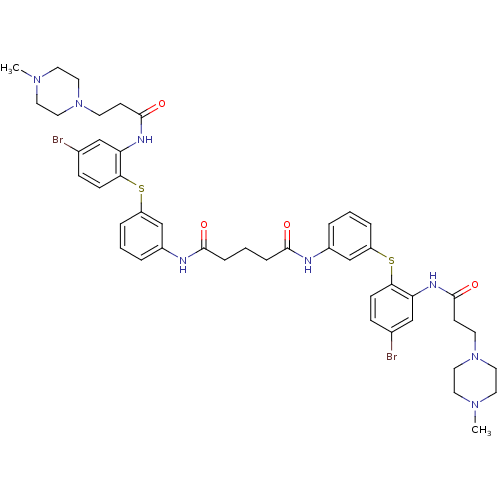
(CHEMBL279374 | Pentanedioic acid bis-[(3-{4-bromo-...)Show SMILES CN1CCN(CCC(=O)Nc2cc(Br)ccc2Sc2cccc(NC(=O)CCCC(=O)Nc3cccc(Sc4ccc(Br)cc4NC(=O)CCN4CCN(C)CC4)c3)c2)CC1 Show InChI InChI=1S/C45H54Br2N8O4S2/c1-52-20-24-54(25-21-52)18-16-44(58)50-38-28-32(46)12-14-40(38)60-36-8-3-6-34(30-36)48-42(56)10-5-11-43(57)49-35-7-4-9-37(31-35)61-41-15-13-33(47)29-39(41)51-45(59)17-19-55-26-22-53(2)23-27-55/h3-4,6-9,12-15,28-31H,5,10-11,16-27H2,1-2H3,(H,48,56)(H,49,57)(H,50,58)(H,51,59) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 550 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Dual specificity protein kinase CLK1
(Mus musculus) | BDBM50568541
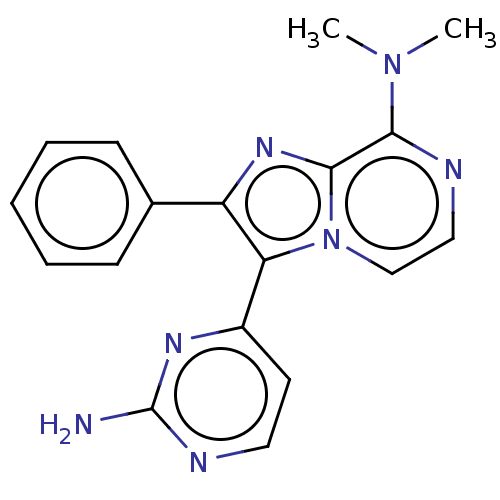
(CHEMBL4860872)Show SMILES CN(C)c1nccn2c(c(nc12)-c1ccccc1)-c1ccnc(N)n1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 590 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant mouse CLK1 expressed in bacteria using GRSRSRSRSRSR as substrate in presence of [gamma-33P]ATP by radiometric assay |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112956
BindingDB Entry DOI: 10.7270/Q2125XD2 |
More data for this
Ligand-Target Pair | |
Trypanothione reductase
(Trypanosoma cruzi) | BDBM50615388
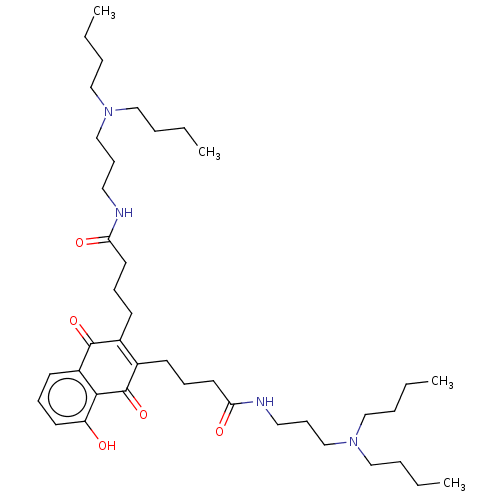
(CHEMBL5280255)Show SMILES CCCCN(CCCC)CCCNC(=O)CCCC1=C(CCCC(=O)NCCCN(CCCC)CCCC)C(=O)c2c(O)cccc2C1=O |c:18| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50139473
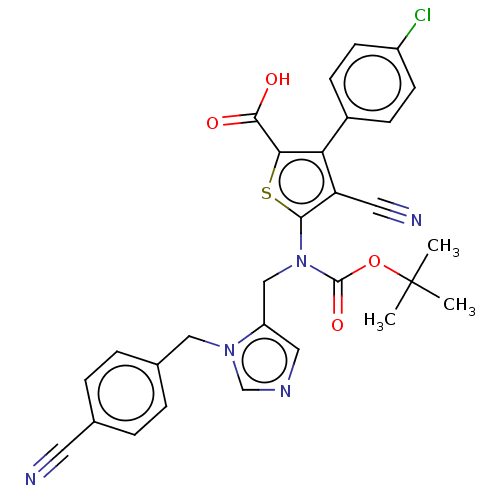
(CHEMBL3763689)Show SMILES CC(C)(C)OC(=O)N(Cc1cncn1Cc1ccc(cc1)C#N)c1sc(C(O)=O)c(c1C#N)-c1ccc(Cl)cc1 Show InChI InChI=1S/C29H24ClN5O4S/c1-29(2,3)39-28(38)35(16-22-14-33-17-34(22)15-19-6-4-18(12-31)5-7-19)26-23(13-32)24(25(40-26)27(36)37)20-8-10-21(30)11-9-20/h4-11,14,17H,15-16H2,1-3H3,(H,36,37) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 640 | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS UPR2301
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant farnesyltransferase using farnesyl pyrophosphate and Dansyl-GCVLS peptide after 15 mins by fluorescence assay |
Eur J Med Chem 109: 173-86 (2016)
Article DOI: 10.1016/j.ejmech.2015.12.045
BindingDB Entry DOI: 10.7270/Q25B04C6 |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50139474
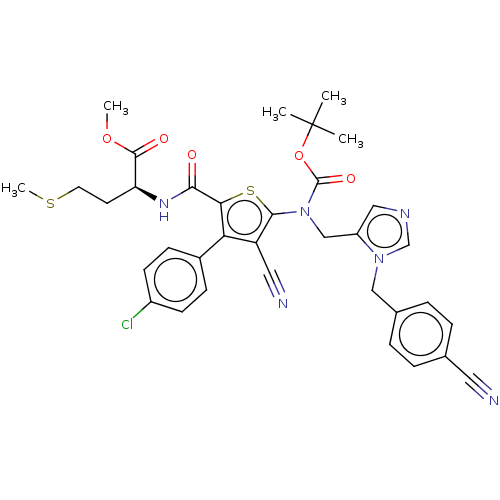
(CHEMBL3765605)Show SMILES COC(=O)[C@H](CCSC)NC(=O)c1sc(N(Cc2cncn2Cc2ccc(cc2)C#N)C(=O)OC(C)(C)C)c(C#N)c1-c1ccc(Cl)cc1 |r| Show InChI InChI=1S/C35H35ClN6O5S2/c1-35(2,3)47-34(45)42(20-26-18-39-21-41(26)19-23-8-6-22(16-37)7-9-23)32-27(17-38)29(24-10-12-25(36)13-11-24)30(49-32)31(43)40-28(14-15-48-5)33(44)46-4/h6-13,18,21,28H,14-15,19-20H2,1-5H3,(H,40,43)/t28-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 680 | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS UPR2301
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant farnesyltransferase using farnesyl pyrophosphate and Dansyl-GCVLS peptide after 15 mins by fluorescence assay |
Eur J Med Chem 109: 173-86 (2016)
Article DOI: 10.1016/j.ejmech.2015.12.045
BindingDB Entry DOI: 10.7270/Q25B04C6 |
More data for this
Ligand-Target Pair | |
Trypanothione reductase
(Trypanosoma brucei brucei) | BDBM50615401
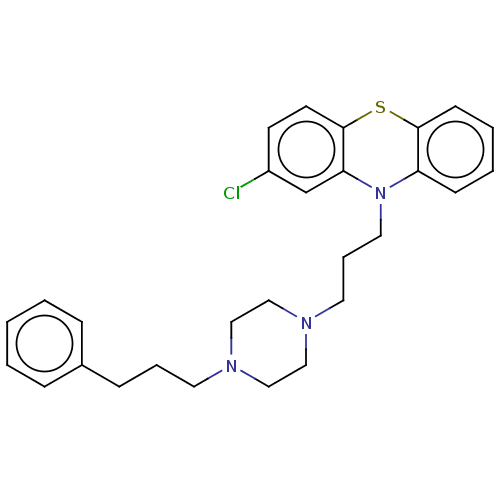
(CHEMBL5081809)Show SMILES Clc1ccc2Sc3ccccc3N(CCCN3CCN(CCCc4ccccc4)CC3)c2c1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 750 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Trypanothione reductase
(Trypanosoma cruzi) | BDBM50615389
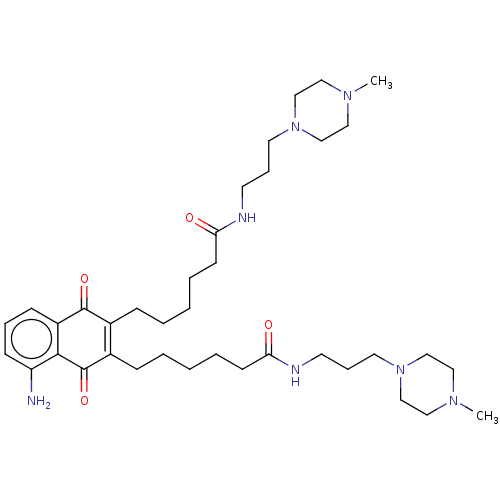
(CHEMBL5283514)Show SMILES CN1CCN(CCCNC(=O)CCCCCC2=C(CCCCCC(=O)NCCCN3CCN(C)CC3)C(=O)c3c(N)cccc3C2=O)CC1 |c:16| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 800 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data