 Found 983 hits with Last Name = 'luo' and Initial = 'hb'
Found 983 hits with Last Name = 'luo' and Initial = 'hb' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
High affinity cGMP-specific 3',5'-cyclic phosphodiesterase 9A
(Homo sapiens (Human)) | BDBM50581177
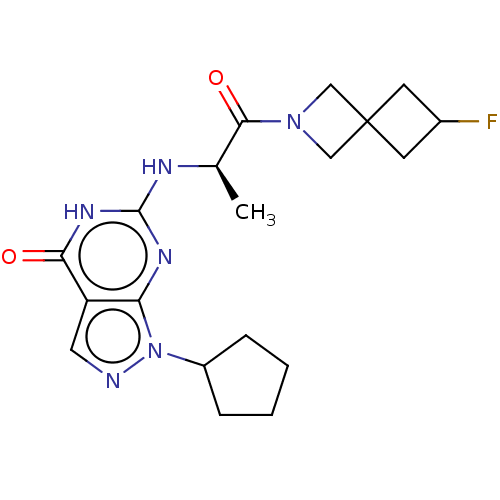
(CHEMBL5075032)Show SMILES C[C@@H](Nc1nc2n(ncc2c(=O)[nH]1)C1CCCC1)C(=O)N1CC2(CC(F)C2)C1 |r| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to PDE9A2 (unknown origin) |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00862
BindingDB Entry DOI: 10.7270/Q2J96B8P |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM50443370
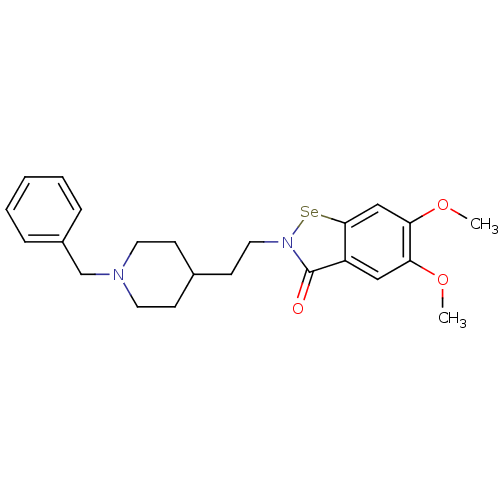
(CHEMBL3086276)Show SMILES COc1cc2[se]n(CCC3CCN(Cc4ccccc4)CC3)c(=O)c2cc1OC Show InChI InChI=1S/C23H28N2O3Se/c1-27-20-14-19-22(15-21(20)28-2)29-25(23(19)26)13-10-17-8-11-24(12-9-17)16-18-6-4-3-5-7-18/h3-7,14-15,17H,8-13,16H2,1-2H3 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University
Curated by ChEMBL
| Assay Description
Competitive inhibition of electric eel AChE using acetylthiocholine chloride as substrate preincubated for 15 mins followed by substrate addition by ... |
J Med Chem 56: 9089-99 (2013)
Article DOI: 10.1021/jm401047q
BindingDB Entry DOI: 10.7270/Q21R6RZF |
More data for this
Ligand-Target Pair | |
Lactoylglutathione lyase
(Homo sapiens (Human)) | BDBM50067040
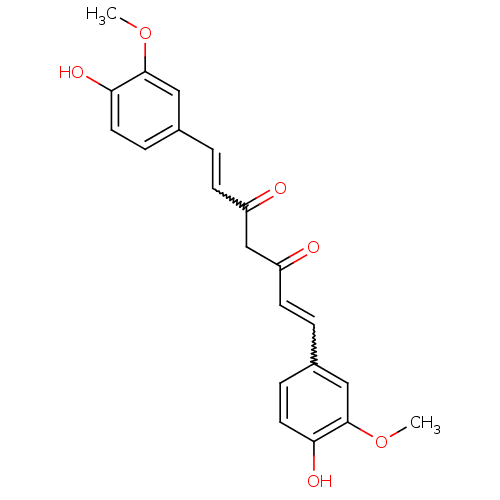
(((E,E)-1,7-bis(4-hydroxy-3-methoxyphenyl)-1,6-hept...)Show SMILES COc1cc(C=CC(=O)CC(=O)C=Cc2ccc(O)c(OC)c2)ccc1O |w:12.11,5.4| Show InChI InChI=1S/C21H20O6/c1-26-20-11-14(5-9-18(20)24)3-7-16(22)13-17(23)8-4-15-6-10-19(25)21(12-15)27-2/h3-12,24-25H,13H2,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 1.03E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-Sen University
Curated by ChEMBL
| Assay Description
Inhibition of human glyoxalase 1 |
Bioorg Med Chem Lett 21: 4243-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.05.095
BindingDB Entry DOI: 10.7270/Q23R0T76 |
More data for this
Ligand-Target Pair | |
Lactoylglutathione lyase
(Homo sapiens (Human)) | BDBM50059989
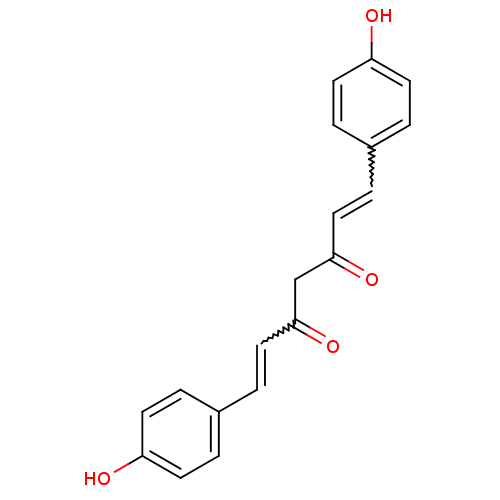
((1E,4Z,6E)-5-Hydroxy-1,7-bis-(4-hydroxy-phenyl)-he...)Show SMILES Oc1ccc(C=CC(=O)CC(=O)C=Cc2ccc(O)cc2)cc1 |w:12.11,5.4| Show InChI InChI=1S/C19H16O4/c20-16-7-1-14(2-8-16)5-11-18(22)13-19(23)12-6-15-3-9-17(21)10-4-15/h1-12,20-21H,13H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.82E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-Sen University
Curated by ChEMBL
| Assay Description
Inhibition of human glyoxalase 1 |
Bioorg Med Chem Lett 21: 4243-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.05.095
BindingDB Entry DOI: 10.7270/Q23R0T76 |
More data for this
Ligand-Target Pair | |
Lactoylglutathione lyase
(Homo sapiens (Human)) | BDBM17638

(2-{1-[(4-chlorophenyl)carbonyl]-5-methoxy-2-methyl...)Show SMILES COc1ccc2n(C(=O)c3ccc(Cl)cc3)c(C)c(CC(O)=O)c2c1 Show InChI InChI=1S/C19H16ClNO4/c1-11-15(10-18(22)23)16-9-14(25-2)7-8-17(16)21(11)19(24)12-3-5-13(20)6-4-12/h3-9H,10H2,1-2H3,(H,22,23) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 2.44E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-Sen University
Curated by ChEMBL
| Assay Description
Inhibition of human glyoxalase 1 |
Bioorg Med Chem Lett 21: 4243-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.05.095
BindingDB Entry DOI: 10.7270/Q23R0T76 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Lactoylglutathione lyase
(Homo sapiens (Human)) | BDBM50295287
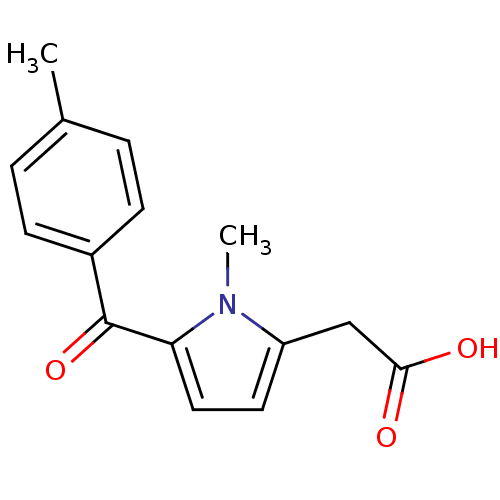
(2-(1-methyl-5-(4-methylbenzoyl)-1H-pyrrol-2-yl)ace...)Show InChI InChI=1S/C15H15NO3/c1-10-3-5-11(6-4-10)15(19)13-8-7-12(16(13)2)9-14(17)18/h3-8H,9H2,1-2H3,(H,17,18) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
| Article
PubMed
| 8.78E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-Sen University
Curated by ChEMBL
| Assay Description
Inhibition of human glyoxalase 1 |
Bioorg Med Chem Lett 21: 4243-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.05.095
BindingDB Entry DOI: 10.7270/Q23R0T76 |
More data for this
Ligand-Target Pair | |
Lactoylglutathione lyase
(Homo sapiens (Human)) | BDBM50027952
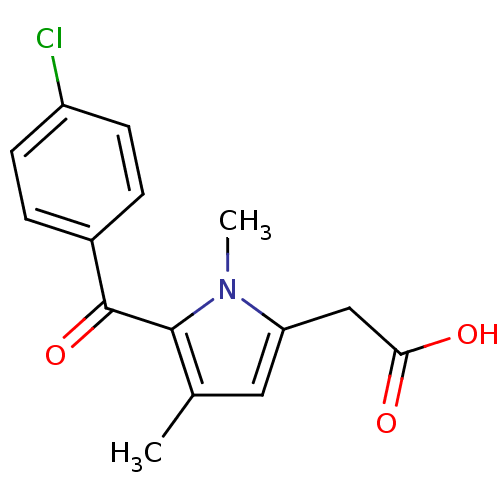
(5-(4-chlorobenzoyl)-1,4-dimethyl-1H-pyrrole-2-acet...)Show InChI InChI=1S/C15H14ClNO3/c1-9-7-12(8-13(18)19)17(2)14(9)15(20)10-3-5-11(16)6-4-10/h3-7H,8H2,1-2H3,(H,18,19) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 3.35E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-Sen University
Curated by ChEMBL
| Assay Description
Inhibition of human glyoxalase 1 |
Bioorg Med Chem Lett 21: 4243-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.05.095
BindingDB Entry DOI: 10.7270/Q23R0T76 |
More data for this
Ligand-Target Pair | |
Lactoylglutathione lyase
(Homo sapiens (Human)) | BDBM54705

(CHEMBL1297 | Fenoprofen | UNM-0000306101 | calcium...)Show InChI InChI=1S/C15H14O3/c1-11(15(16)17)12-6-5-9-14(10-12)18-13-7-3-2-4-8-13/h2-11H,1H3,(H,16,17) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 3.83E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-Sen University
Curated by ChEMBL
| Assay Description
Inhibition of human glyoxalase 1 |
Bioorg Med Chem Lett 21: 4243-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.05.095
BindingDB Entry DOI: 10.7270/Q23R0T76 |
More data for this
Ligand-Target Pair | |
Lactoylglutathione lyase
(Homo sapiens (Human)) | BDBM50022271
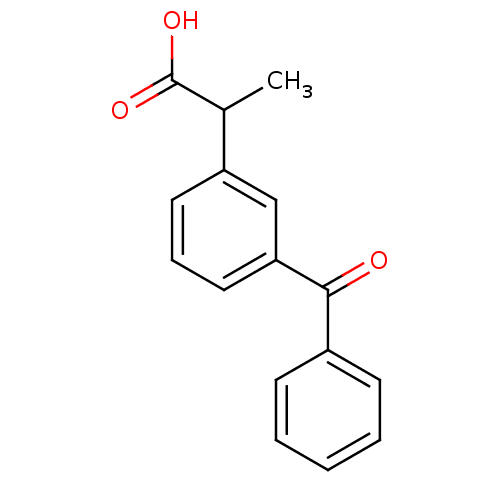
(2-(3-Benzoylphenyl)propionic acid | 2-(3-benzoylph...)Show InChI InChI=1S/C16H14O3/c1-11(16(18)19)13-8-5-9-14(10-13)15(17)12-6-3-2-4-7-12/h2-11H,1H3,(H,18,19) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 8.43E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-Sen University
Curated by ChEMBL
| Assay Description
Inhibition of human glyoxalase 1 |
Bioorg Med Chem Lett 21: 4243-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.05.095
BindingDB Entry DOI: 10.7270/Q23R0T76 |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM50408487
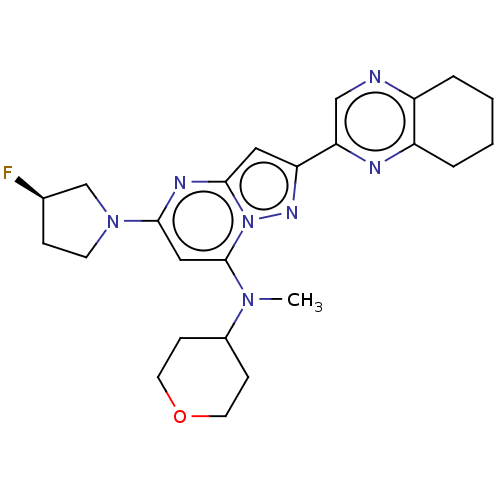
(CHEMBL5281567)Show InChI InChI=1S/C18H21N3OS/c1-20(2)10-5-11-21-14-6-3-4-7-16(14)23-17-9-8-13(18(19)22)12-15(17)21/h3-4,6-9,12H,5,10-11H2,1-2H3,(H2,19,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity at CCK2 receptor in immature rat stomach assessed as pentagastrin-induced acid secretion |
Citation and Details
|
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM50408486
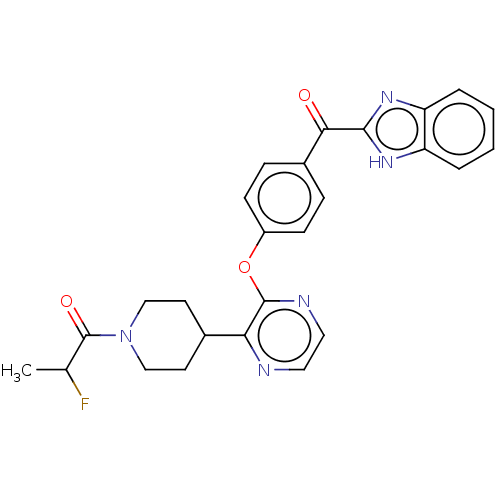
(CHEMBL5268340)Show InChI InChI=1S/C18H21ClN2S/c1-3-20(4-2)11-12-21-15-7-5-6-8-17(15)22-18-10-9-14(19)13-16(18)21/h5-10,13H,3-4,11-12H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 0.130 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity at CCK2 receptor in immature rat stomach assessed as pentagastrin-induced acid secretion |
Citation and Details
|
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM50497974
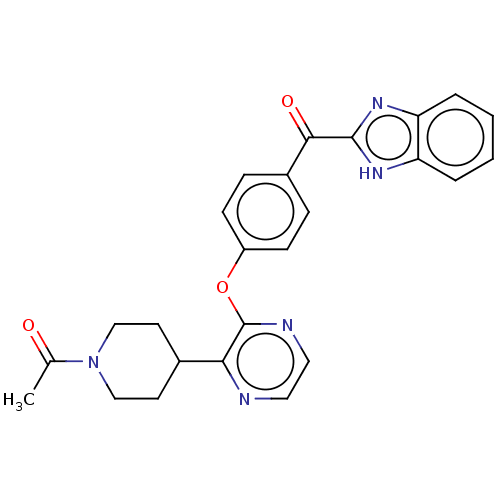
(CHEMBL3319209)Show SMILES CC(=O)N1CCC(CC1)c1nccnc1Oc1ccc(cc1)C(=O)c1nc2ccccc2[nH]1 Show InChI InChI=1S/C25H23N5O3/c1-16(31)30-14-10-17(11-15-30)22-25(27-13-12-26-22)33-19-8-6-18(7-9-19)23(32)24-28-20-4-2-3-5-21(20)29-24/h2-9,12-13,17H,10-11,14-15H2,1H3,(H,28,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| | n/a | n/a | 0.180 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity at CCK2 receptor in immature rat stomach assessed as pentagastrin-induced acid secretion |
Citation and Details
|
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM50513787
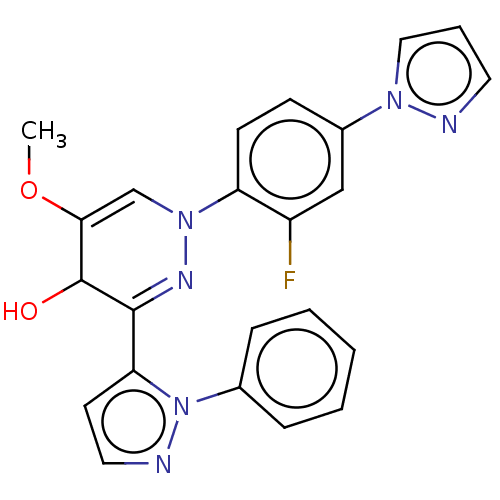
(CHEMBL4445635)Show SMILES COC1=CN(N=C(C1O)c1ccnn1-c1ccccc1)c1ccc(cc1F)-n1cccn1 |c:5,t:2| Show InChI InChI=1S/C23H19FN6O2/c1-32-21-15-29(19-9-8-17(14-18(19)24)28-13-5-11-25-28)27-22(23(21)31)20-10-12-26-30(20)16-6-3-2-4-7-16/h2-15,23,31H,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University
Curated by ChEMBL
| Assay Description
Inhibition of human PDE10A2 catalytic domain (446 to 789 residues) expressed in Escherichia coli BL21 cells using [3H]-cGMP as substrate after 15 min... |
J Med Chem 62: 3707-3721 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00224
BindingDB Entry DOI: 10.7270/Q22B92CV |
More data for this
Ligand-Target Pair | |
High affinity cAMP-specific and IBMX-insensitive 3',5'-cyclic phosphodiesterase 8B
(Homo sapiens (Human)) | BDBM50594973
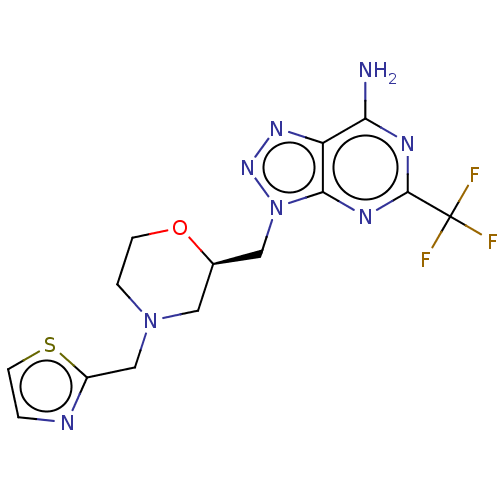
(CHEMBL5169811)Show SMILES Nc1nc(nc2n(C[C@H]3CN(Cc4nccs4)CCO3)nnc12)C(F)(F)F |r| | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity at AT1 receptor assessed inhibition of angiotensin-2-induced contraction of rabbit thoracic aortic rings |
Citation and Details
|
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50459162
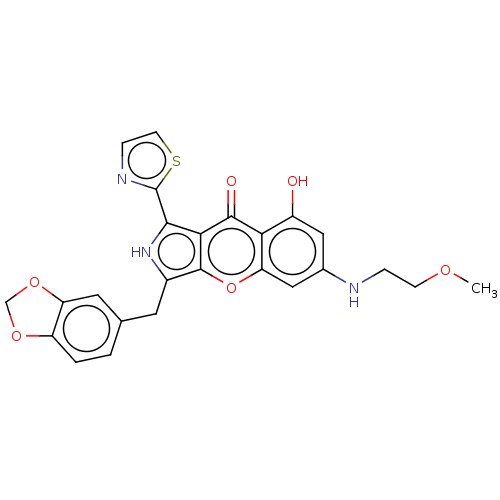
(CHEMBL4218772)Show SMILES COCCNc1cc(O)c2c(c1)oc1c(Cc3ccc4OCOc4c3)[nH]c(-c3nccs3)c1c2=O Show InChI InChI=1S/C25H21N3O6S/c1-31-6-4-26-14-10-16(29)20-19(11-14)34-24-15(8-13-2-3-17-18(9-13)33-12-32-17)28-22(21(24)23(20)30)25-27-5-7-35-25/h2-3,5,7,9-11,26,28-29H,4,6,8,12H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.320 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-Sen University
Curated by ChEMBL
| Assay Description
Inhibition of human PDE5A1 catalytic domain (535 to 860 residues) expressed in Escherichia coli BL21 using 3H-cGMP as substrate after 15 mins by liqu... |
J Med Chem 61: 8468-8473 (2018)
Article DOI: 10.1021/acs.jmedchem.8b01209
BindingDB Entry DOI: 10.7270/Q26D5WMB |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM31592
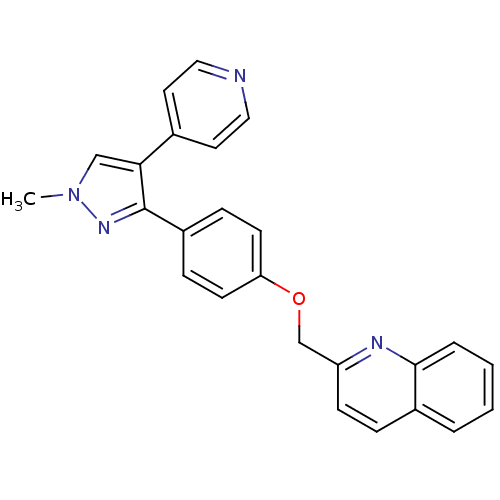
(PF-2545920 | US9138494, MP-10 | substituted pyraz...)Show SMILES Cn1cc(c(n1)-c1ccc(OCc2ccc3ccccc3n2)cc1)-c1ccncc1 Show InChI InChI=1S/C25H20N4O/c1-29-16-23(18-12-14-26-15-13-18)25(28-29)20-7-10-22(11-8-20)30-17-21-9-6-19-4-2-3-5-24(19)27-21/h2-16H,17H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 0.370 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University
Curated by ChEMBL
| Assay Description
Inhibition of human PDE10A2 catalytic domain (446 to 789 residues) expressed in Escherichia coli BL21 cells using [3H]-cGMP as substrate after 15 min... |
J Med Chem 62: 3707-3721 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00224
BindingDB Entry DOI: 10.7270/Q22B92CV |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Rattus norvegicus (rat)) | BDBM50398017
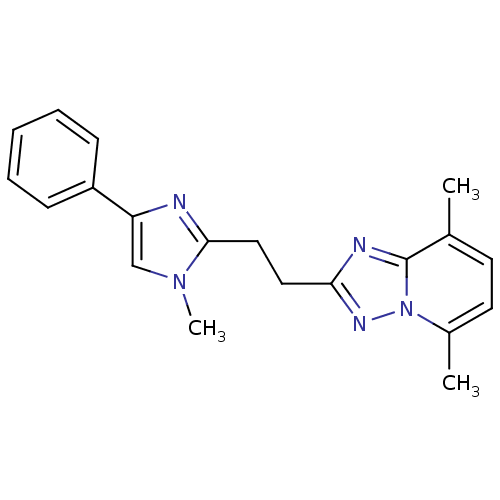
(CHEMBL2180411 | US9018217, 5,8-Dimethyl-2-[2-(1-me...)Show InChI InChI=1S/C20H21N5/c1-14-9-10-15(2)25-20(14)22-18(23-25)11-12-19-21-17(13-24(19)3)16-7-5-4-6-8-16/h4-10,13H,11-12H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 0.390 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of P-glycoprotein expressed in MDCK-MDR1 cells by calcein AM assay |
Citation and Details
|
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50459175

(CHEMBL4204687)Show SMILES CN1CCN(CCNc2cc(O)c3c(c2)oc2c(Cc4ccc5OCOc5c4)[nH]c(-c4nccs4)c2c3=O)CC1 Show InChI InChI=1S/C29H29N5O5S/c1-33-7-9-34(10-8-33)6-4-30-18-14-20(35)24-23(15-18)39-28-19(12-17-2-3-21-22(13-17)38-16-37-21)32-26(25(28)27(24)36)29-31-5-11-40-29/h2-3,5,11,13-15,30,32,35H,4,6-10,12,16H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.390 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-Sen University
Curated by ChEMBL
| Assay Description
Inhibition of human PDE5A1 catalytic domain (535 to 860 residues) expressed in Escherichia coli BL21 using 3H-cGMP as substrate after 15 mins by liqu... |
J Med Chem 61: 8468-8473 (2018)
Article DOI: 10.1021/acs.jmedchem.8b01209
BindingDB Entry DOI: 10.7270/Q26D5WMB |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4D
(Homo sapiens (Human)) | BDBM14774

(3-(cyclopropylmethoxy)-N-(3,5-dichloropyridin-4-yl...)Show SMILES FC(F)Oc1ccc(cc1OCC1CC1)C(=O)Nc1c(Cl)cncc1Cl Show InChI InChI=1S/C17H14Cl2F2N2O3/c18-11-6-22-7-12(19)15(11)23-16(24)10-3-4-13(26-17(20)21)14(5-10)25-8-9-1-2-9/h3-7,9,17H,1-2,8H2,(H,22,23,24) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c02058
BindingDB Entry DOI: 10.7270/Q2DF6W9W |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
cAMP-specific 3',5'-cyclic phosphodiesterase 4D
(Homo sapiens (Human)) | BDBM14774

(3-(cyclopropylmethoxy)-N-(3,5-dichloropyridin-4-yl...)Show SMILES FC(F)Oc1ccc(cc1OCC1CC1)C(=O)Nc1c(Cl)cncc1Cl Show InChI InChI=1S/C17H14Cl2F2N2O3/c18-11-6-22-7-12(19)15(11)23-16(24)10-3-4-13(26-17(20)21)14(5-10)25-8-9-1-2-9/h3-7,9,17H,1-2,8H2,(H,22,23,24) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Guangzhou University of Chinese Medicine
Curated by ChEMBL
| Assay Description
Inhibition of PDE4D2 (unknown origin) |
J Med Chem 63: 3370-3380 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00060
BindingDB Entry DOI: 10.7270/Q20Z76QT |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM50513788

(CHEMBL4558162)Show SMILES Cc1ccc2n(c(CCN3C(=O)c4cccc5cccc(C3=O)c45)nc2c1)-c1ccncc1 Show InChI InChI=1S/C27H20N4O2/c1-17-8-9-23-22(16-17)29-24(31(23)19-10-13-28-14-11-19)12-15-30-26(32)20-6-2-4-18-5-3-7-21(25(18)20)27(30)33/h2-11,13-14,16H,12,15H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 0.440 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University
Curated by ChEMBL
| Assay Description
Inhibition of human PDE10A2 catalytic domain (446 to 789 residues) expressed in Escherichia coli BL21 cells using [3H]-cGMP as substrate after 15 min... |
J Med Chem 62: 3707-3721 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00224
BindingDB Entry DOI: 10.7270/Q22B92CV |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM50419436
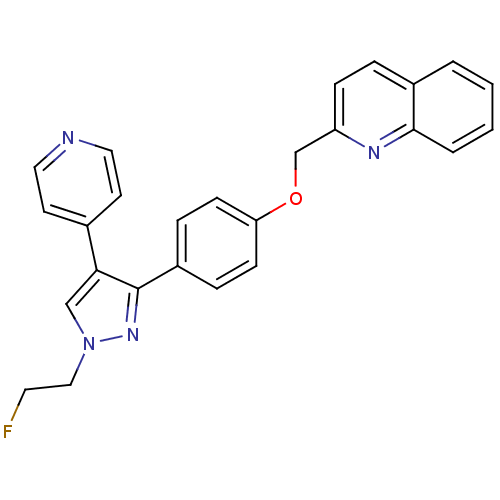
(CHEMBL1915747 | US9138494, JNJ-41510417)Show SMILES FCCn1cc(c(n1)-c1ccc(OCc2ccc3ccccc3n2)cc1)-c1ccncc1 Show InChI InChI=1S/C26H21FN4O/c27-13-16-31-17-24(19-11-14-28-15-12-19)26(30-31)21-6-9-23(10-7-21)32-18-22-8-5-20-3-1-2-4-25(20)29-22/h1-12,14-15,17H,13,16,18H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity at CCK2 receptor in immature rat stomach assessed as pentagastrin-induced acid secretion |
Citation and Details
|
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50459172
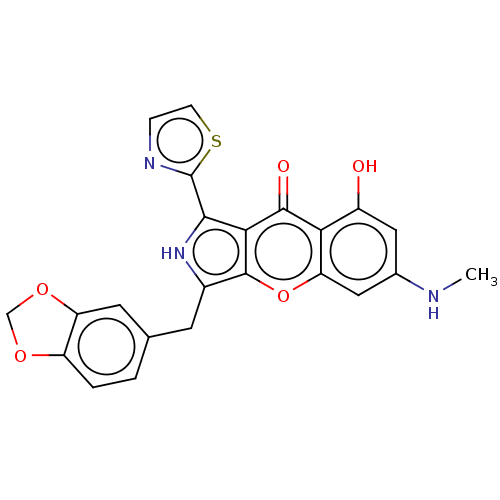
(CHEMBL4217826)Show SMILES CNc1cc(O)c2c(c1)oc1c(Cc3ccc4OCOc4c3)[nH]c(-c3nccs3)c1c2=O Show InChI InChI=1S/C23H17N3O5S/c1-24-12-8-14(27)18-17(9-12)31-22-13(6-11-2-3-15-16(7-11)30-10-29-15)26-20(19(22)21(18)28)23-25-4-5-32-23/h2-5,7-9,24,26-27H,6,10H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.520 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-Sen University
Curated by ChEMBL
| Assay Description
Inhibition of human PDE5A1 catalytic domain (535 to 860 residues) expressed in Escherichia coli BL21 using 3H-cGMP as substrate after 15 mins by liqu... |
J Med Chem 61: 8468-8473 (2018)
Article DOI: 10.1021/acs.jmedchem.8b01209
BindingDB Entry DOI: 10.7270/Q26D5WMB |
More data for this
Ligand-Target Pair | |
High affinity cGMP-specific 3',5'-cyclic phosphodiesterase 9A
(Homo sapiens (Human)) | BDBM50034641
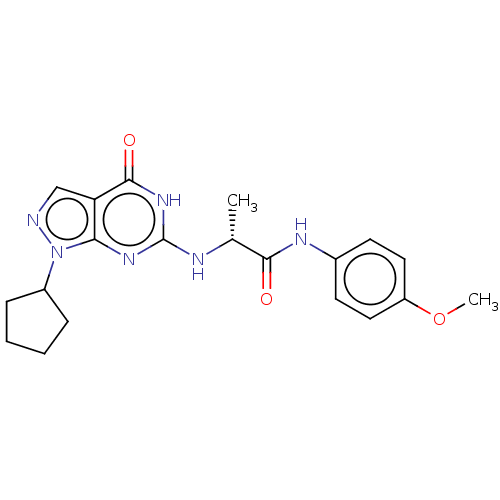
(CHEMBL3360415)Show SMILES COc1ccc(NC(=O)[C@@H](C)Nc2nc3n(ncc3c(=O)[nH]2)C2CCCC2)cc1 |r| Show InChI InChI=1S/C20H24N6O3/c1-12(18(27)23-13-7-9-15(29-2)10-8-13)22-20-24-17-16(19(28)25-20)11-21-26(17)14-5-3-4-6-14/h7-12,14H,3-6H2,1-2H3,(H,23,27)(H2,22,24,25,28)/t12-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University
Curated by ChEMBL
| Assay Description
Inhibition of PDE9A (unknown origin) |
Bioorg Med Chem Lett 30: (2020)
Article DOI: 10.1016/j.bmcl.2020.127254
BindingDB Entry DOI: 10.7270/Q2S1862H |
More data for this
Ligand-Target Pair | |
High affinity cGMP-specific 3',5'-cyclic phosphodiesterase 9A
(Homo sapiens (Human)) | BDBM50034641

(CHEMBL3360415)Show SMILES COc1ccc(NC(=O)[C@@H](C)Nc2nc3n(ncc3c(=O)[nH]2)C2CCCC2)cc1 |r| Show InChI InChI=1S/C20H24N6O3/c1-12(18(27)23-13-7-9-15(29-2)10-8-13)22-20-24-17-16(19(28)25-20)11-21-26(17)14-5-3-4-6-14/h7-12,14H,3-6H2,1-2H3,(H,23,27)(H2,22,24,25,28)/t12-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-Sen University
Curated by ChEMBL
| Assay Description
Inhibition of PDE9A2 catalytic domain (unknown origin) using [3H]-cGMP/[3H]-cAMP as substrate after 15 mins by liquid scintillation counting analysis |
J Med Chem 57: 10304-13 (2014)
Article DOI: 10.1021/jm500836h
BindingDB Entry DOI: 10.7270/Q28P624W |
More data for this
Ligand-Target Pair | |
High affinity cAMP-specific and IBMX-insensitive 3',5'-cyclic phosphodiesterase 8A
(Homo sapiens (Human)) | BDBM50594973

(CHEMBL5169811)Show SMILES Nc1nc(nc2n(C[C@H]3CN(Cc4nccs4)CCO3)nnc12)C(F)(F)F |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity at human EP1 receptor expressed in CHO cells receptor by NFTA reporter gene assay |
Citation and Details
|
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM50513799
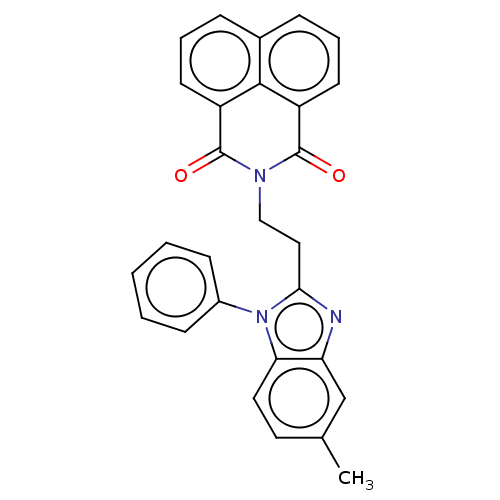
(CHEMBL4527952)Show SMILES Cc1ccc2n(c(CCN3C(=O)c4cccc5cccc(C3=O)c45)nc2c1)-c1ccccc1 Show InChI InChI=1S/C28H21N3O2/c1-18-13-14-24-23(17-18)29-25(31(24)20-9-3-2-4-10-20)15-16-30-27(32)21-11-5-7-19-8-6-12-22(26(19)21)28(30)33/h2-14,17H,15-16H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.760 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University
Curated by ChEMBL
| Assay Description
Inhibition of human PDE10A2 catalytic domain (446 to 789 residues) expressed in Escherichia coli BL21 cells using [3H]-cGMP as substrate after 15 min... |
J Med Chem 62: 3707-3721 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00224
BindingDB Entry DOI: 10.7270/Q22B92CV |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM290889

(1-[2-fluoro-4- (tetrahydro-2H- pyran-4-yl)phenyl]-...)Show SMILES COc1cn(nc(-c2ccnn2-c2ccccc2)c1=O)-c1ccc(cc1F)C1CCOCC1 Show InChI InChI=1S/C25H23FN4O3/c1-32-23-16-29(21-8-7-18(15-20(21)26)17-10-13-33-14-11-17)28-24(25(23)31)22-9-12-27-30(22)19-5-3-2-4-6-19/h2-9,12,15-17H,10-11,13-14H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 0.770 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity at CCK2 receptor in immature rat stomach assessed as pentagastrin-induced acid secretion |
Citation and Details
|
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50459173
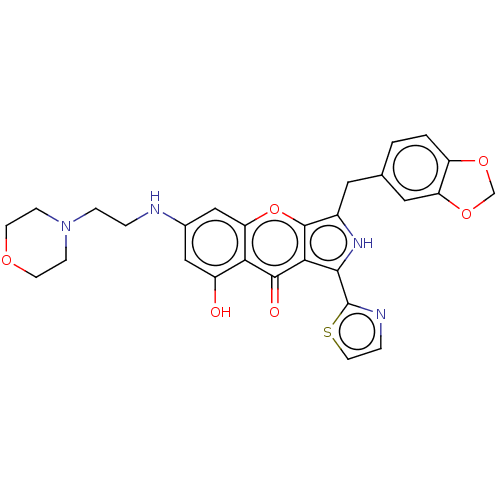
(CHEMBL4209550)Show SMILES Oc1cc(NCCN2CCOCC2)cc2oc3c(Cc4ccc5OCOc5c4)[nH]c(-c4nccs4)c3c(=O)c12 Show InChI InChI=1S/C28H26N4O6S/c33-19-13-17(29-3-5-32-6-8-35-9-7-32)14-22-23(19)26(34)24-25(28-30-4-10-39-28)31-18(27(24)38-22)11-16-1-2-20-21(12-16)37-15-36-20/h1-2,4,10,12-14,29,31,33H,3,5-9,11,15H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.790 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-Sen University
Curated by ChEMBL
| Assay Description
Inhibition of human PDE5A1 catalytic domain (535 to 860 residues) expressed in Escherichia coli BL21 using 3H-cGMP as substrate after 15 mins by liqu... |
J Med Chem 61: 8468-8473 (2018)
Article DOI: 10.1021/acs.jmedchem.8b01209
BindingDB Entry DOI: 10.7270/Q26D5WMB |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM50530262
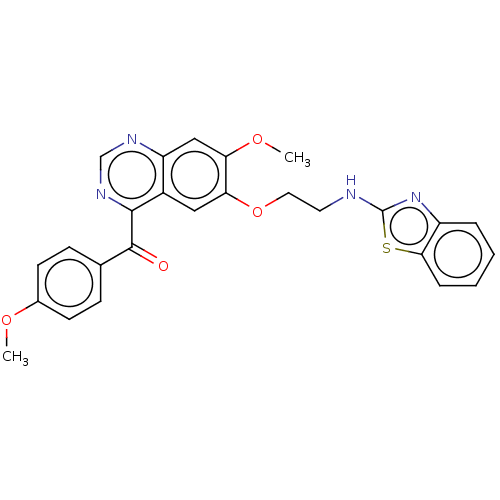
(CHEMBL4456564)Show SMILES COc1ccc(cc1)C(=O)c1ncnc2cc(OC)c(OCCNc3nc4ccccc4s3)cc12 Show InChI InChI=1S/C26H22N4O4S/c1-32-17-9-7-16(8-10-17)25(31)24-18-13-22(21(33-2)14-20(18)28-15-29-24)34-12-11-27-26-30-19-5-3-4-6-23(19)35-26/h3-10,13-15H,11-12H2,1-2H3,(H,27,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.870 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-Sen University
Curated by ChEMBL
| Assay Description
Inhibition of PDE10 (unknown origin) |
J Med Chem 62: 2099-2111 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01763
BindingDB Entry DOI: 10.7270/Q2222Z7D |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM50530262

(CHEMBL4456564)Show SMILES COc1ccc(cc1)C(=O)c1ncnc2cc(OC)c(OCCNc3nc4ccccc4s3)cc12 Show InChI InChI=1S/C26H22N4O4S/c1-32-17-9-7-16(8-10-17)25(31)24-18-13-22(21(33-2)14-20(18)28-15-29-24)34-12-11-27-26-30-19-5-3-4-6-23(19)35-26/h3-10,13-15H,11-12H2,1-2H3,(H,27,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.870 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-Sen University
Curated by ChEMBL
| Assay Description
Inhibition of PDE10 (unknown origin) |
J Med Chem 62: 2099-2111 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01763
BindingDB Entry DOI: 10.7270/Q2222Z7D |
More data for this
Ligand-Target Pair | |
Dual specificity calcium/calmodulin-dependent 3',5'-cyclic nucleotide phosphodiesterase 1C
(Homo sapiens (Human)) | BDBM50540037
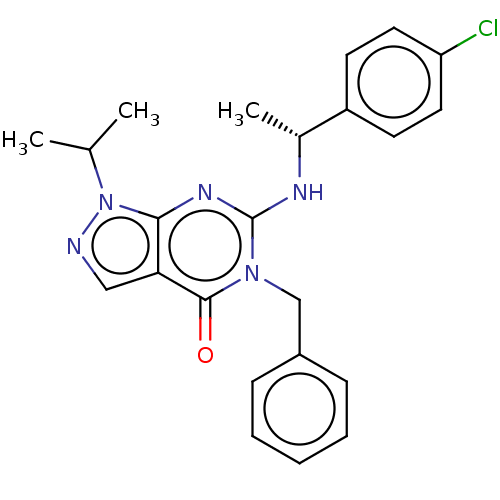
(CHEMBL4639341)Show SMILES CC(C)n1ncc2c1nc(N[C@H](C)c1ccc(Cl)cc1)n(Cc1ccccc1)c2=O |r| Show InChI InChI=1S/C23H24ClN5O/c1-15(2)29-21-20(13-25-29)22(30)28(14-17-7-5-4-6-8-17)23(27-21)26-16(3)18-9-11-19(24)12-10-18/h4-13,15-16H,14H2,1-3H3,(H,26,27)/t16-/m1/s1 | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University
Curated by ChEMBL
| Assay Description
Inhibition of PDE1C2 (147 to 531 residues) (unknown origin) using [3H]-cGMP substrate incubated for 15 mins by liquid scintillation counting method |
J Med Chem 63: 7867-7879 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00711
BindingDB Entry DOI: 10.7270/Q20V8HBZ |
More data for this
Ligand-Target Pair | |
cGMP-dependent 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM107767
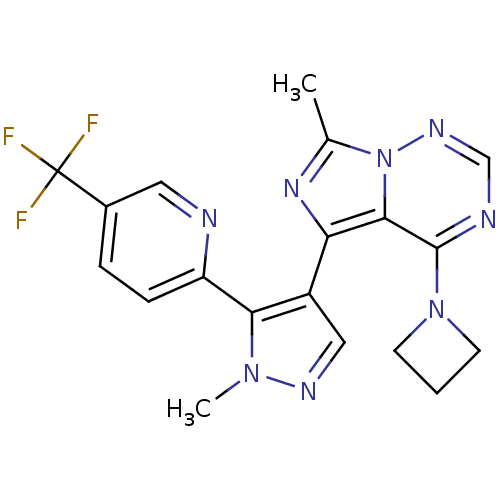
(US11419874, PF-05180999 | US8598155, 2)Show SMILES Cc1nc(-c2cnn(C)c2-c2ccc(cn2)C(F)(F)F)c2c(ncnn12)N1CCC1 Show InChI InChI=1S/C19H17F3N8/c1-11-27-15(17-18(29-6-3-7-29)24-10-26-30(11)17)13-9-25-28(2)16(13)14-5-4-12(8-23-14)19(20,21)22/h4-5,8-10H,3,6-7H2,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity at AT1 receptor assessed inhibition of angiotensin-2-induced contraction of rabbit thoracic aortic rings |
Citation and Details
|
More data for this
Ligand-Target Pair | |
cGMP-dependent 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM566472
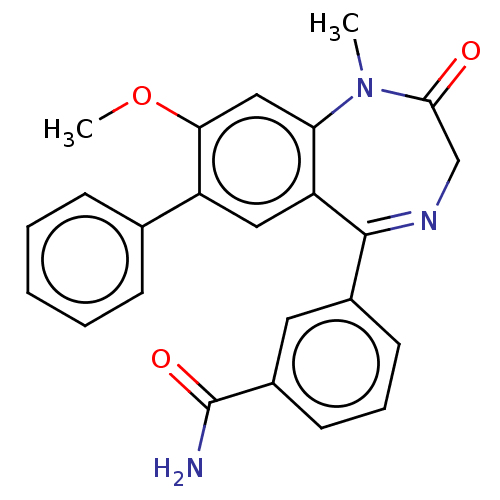
(3-(8-methoxy-1- methyl-2-oxo-7- phenyl-2,3-dihydro...)Show SMILES COc1cc2N(C)C(=O)CN=C(c3cccc(c3)C(N)=O)c2cc1-c1ccccc1 |t:10| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity at AT1 receptor assessed inhibition of angiotensin-2-induced contraction of rabbit thoracic aortic rings |
Citation and Details
|
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM50513795

(CHEMBL4459590)Show SMILES Cc1cnc2n(c(CCN3C(=O)c4cccc5cccc(C3=O)c45)nc2c1)-c1ccccc1 Show InChI InChI=1S/C27H20N4O2/c1-17-15-22-25(28-16-17)31(19-9-3-2-4-10-19)23(29-22)13-14-30-26(32)20-11-5-7-18-8-6-12-21(24(18)20)27(30)33/h2-12,15-16H,13-14H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University
Curated by ChEMBL
| Assay Description
Inhibition of human PDE10A2 catalytic domain (446 to 789 residues) expressed in Escherichia coli BL21 cells using [3H]-cGMP as substrate after 15 min... |
J Med Chem 62: 3707-3721 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00224
BindingDB Entry DOI: 10.7270/Q22B92CV |
More data for this
Ligand-Target Pair | |
High affinity cGMP-specific 3',5'-cyclic phosphodiesterase 9A
(Homo sapiens (Human)) | BDBM50034645
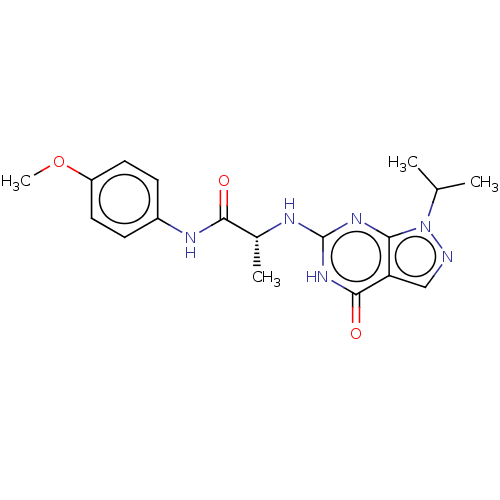
(CHEMBL3360419)Show SMILES COc1ccc(NC(=O)[C@@H](C)Nc2nc3n(ncc3c(=O)[nH]2)C(C)C)cc1 |r| Show InChI InChI=1S/C18H22N6O3/c1-10(2)24-15-14(9-19-24)17(26)23-18(22-15)20-11(3)16(25)21-12-5-7-13(27-4)8-6-12/h5-11H,1-4H3,(H,21,25)(H2,20,22,23,26)/t11-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-Sen University
Curated by ChEMBL
| Assay Description
Inhibition of PDE9A2 catalytic domain (unknown origin) using [3H]-cGMP/[3H]-cAMP as substrate after 15 mins by liquid scintillation counting analysis |
J Med Chem 57: 10304-13 (2014)
Article DOI: 10.1021/jm500836h
BindingDB Entry DOI: 10.7270/Q28P624W |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50459164
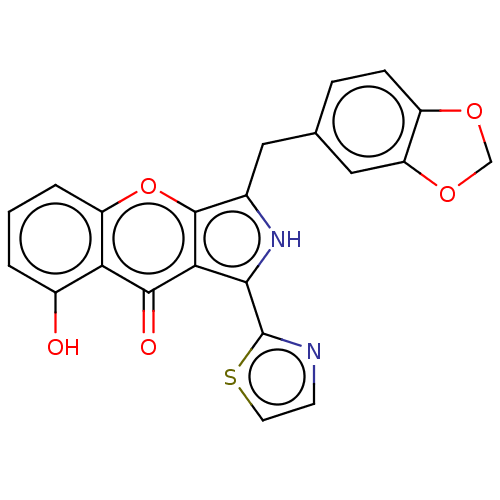
(CHEMBL4215845)Show SMILES Oc1cccc2oc3c(Cc4ccc5OCOc5c4)[nH]c(-c4nccs4)c3c(=O)c12 Show InChI InChI=1S/C22H14N2O5S/c25-13-2-1-3-15-17(13)20(26)18-19(22-23-6-7-30-22)24-12(21(18)29-15)8-11-4-5-14-16(9-11)28-10-27-14/h1-7,9,24-25H,8,10H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-Sen University
Curated by ChEMBL
| Assay Description
Inhibition of human PDE5A1 catalytic domain (535 to 860 residues) expressed in Escherichia coli BL21 using 3H-cGMP as substrate after 15 mins by liqu... |
J Med Chem 61: 8468-8473 (2018)
Article DOI: 10.1021/acs.jmedchem.8b01209
BindingDB Entry DOI: 10.7270/Q26D5WMB |
More data for this
Ligand-Target Pair | |
High affinity cGMP-specific 3',5'-cyclic phosphodiesterase 9A
(Homo sapiens (Human)) | BDBM50034648
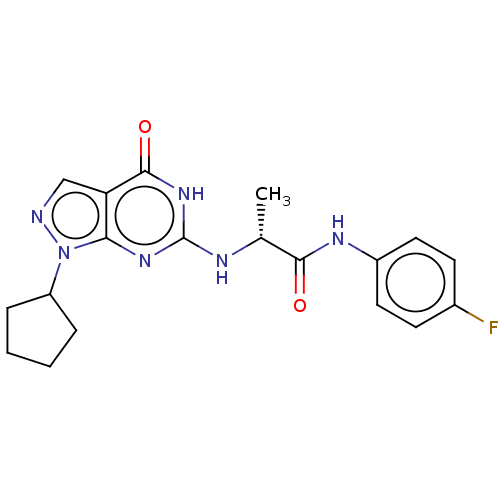
(CHEMBL3360421)Show SMILES C[C@@H](Nc1nc2n(ncc2c(=O)[nH]1)C1CCCC1)C(=O)Nc1ccc(F)cc1 |r| Show InChI InChI=1S/C19H21FN6O2/c1-11(17(27)23-13-8-6-12(20)7-9-13)22-19-24-16-15(18(28)25-19)10-21-26(16)14-4-2-3-5-14/h6-11,14H,2-5H2,1H3,(H,23,27)(H2,22,24,25,28)/t11-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-Sen University
Curated by ChEMBL
| Assay Description
Inhibition of PDE9A2 catalytic domain (unknown origin) using [3H]-cGMP/[3H]-cAMP as substrate after 15 mins by liquid scintillation counting analysis |
J Med Chem 57: 10304-13 (2014)
Article DOI: 10.1021/jm500836h
BindingDB Entry DOI: 10.7270/Q28P624W |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM14777

((2R,8R)-2-(2H-1,3-benzodioxol-5-yl)-6-methyl-3,6,1...)Show SMILES [H][C@]12Cc3c([nH]c4ccccc34)[C@H](N1C(=O)CN(C)C2=O)c1ccc2OCOc2c1 |r| Show InChI InChI=1S/C22H19N3O4/c1-24-10-19(26)25-16(22(24)27)9-14-13-4-2-3-5-15(13)23-20(14)21(25)12-6-7-17-18(8-12)29-11-28-17/h2-8,16,21,23H,9-11H2,1H3/t16-,21-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University
Curated by ChEMBL
| Assay Description
Inhibition of PDE5A (unknown origin) |
J Med Chem 61: 5467-5483 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01370
BindingDB Entry DOI: 10.7270/Q22B91MK |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cone cGMP-specific 3',5'-cyclic phosphodiesterase subunit alpha'
(Homo sapiens (Human)) | BDBM50459162

(CHEMBL4218772)Show SMILES COCCNc1cc(O)c2c(c1)oc1c(Cc3ccc4OCOc4c3)[nH]c(-c3nccs3)c1c2=O Show InChI InChI=1S/C25H21N3O6S/c1-31-6-4-26-14-10-16(29)20-19(11-14)34-24-15(8-13-2-3-17-18(9-13)33-12-32-17)28-22(21(24)23(20)30)25-27-5-7-35-25/h2-3,5,7,9-11,26,28-29H,4,6,8,12H2,1H3 | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-Sen University
Curated by ChEMBL
| Assay Description
Inhibition of PDE6C catalytic domain (1 to 858 residues) (unknown origin) expressed in Escherichia coli BL21 using 3H-cGMP as substrate after 15 mins... |
J Med Chem 61: 8468-8473 (2018)
Article DOI: 10.1021/acs.jmedchem.8b01209
BindingDB Entry DOI: 10.7270/Q26D5WMB |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50459166
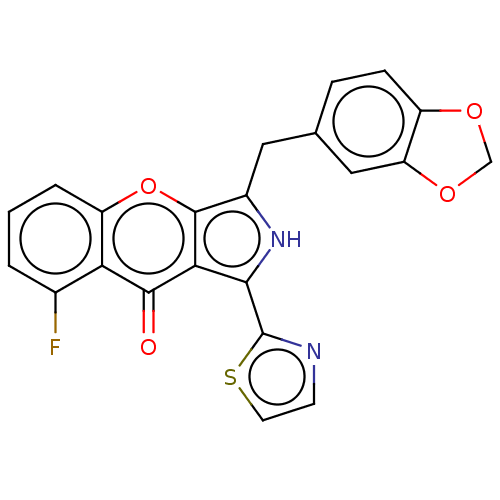
(CHEMBL4206819)Show SMILES Fc1cccc2oc3c(Cc4ccc5OCOc5c4)[nH]c(-c4nccs4)c3c(=O)c12 Show InChI InChI=1S/C22H13FN2O4S/c23-12-2-1-3-15-17(12)20(26)18-19(22-24-6-7-30-22)25-13(21(18)29-15)8-11-4-5-14-16(9-11)28-10-27-14/h1-7,9,25H,8,10H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-Sen University
Curated by ChEMBL
| Assay Description
Inhibition of human PDE5A1 catalytic domain (535 to 860 residues) expressed in Escherichia coli BL21 using 3H-cGMP as substrate after 15 mins by liqu... |
J Med Chem 61: 8468-8473 (2018)
Article DOI: 10.1021/acs.jmedchem.8b01209
BindingDB Entry DOI: 10.7270/Q26D5WMB |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
cGMP-dependent 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50444038
(CHEMBL3092563 | US9669035, B-5)Show SMILES Cc1nc2ccc(CN3CCOCC3)cc2n2c(nnc12)-c1c(F)cccc1Cl |(32.38,-11.31,;31.04,-12.08,;29.71,-11.31,;28.38,-12.08,;27.05,-11.3,;25.72,-12.08,;25.72,-13.62,;24.39,-14.39,;23.06,-13.63,;23.06,-12.09,;21.73,-11.32,;20.39,-12.09,;20.39,-13.63,;21.73,-14.4,;27.05,-14.38,;28.38,-13.62,;29.71,-14.39,;30.03,-15.9,;31.56,-16.06,;32.19,-14.65,;31.04,-13.62,;28.92,-16.96,;27.44,-16.51,;27.08,-15.02,;26.32,-17.57,;26.68,-19.07,;28.16,-19.51,;29.28,-18.45,;30.75,-18.88,)| Show InChI InChI=1S/C21H19ClFN5O/c1-13-20-25-26-21(19-15(22)3-2-4-16(19)23)28(20)18-11-14(5-6-17(18)24-13)12-27-7-9-29-10-8-27/h2-6,11H,7-10,12H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity at AT1 receptor assessed inhibition of angiotensin-2-induced contraction of rabbit thoracic aortic rings |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Dual specificity calcium/calmodulin-dependent 3',5'-cyclic nucleotide phosphodiesterase 1C
(Homo sapiens (Human)) | BDBM50540040

(CHEMBL4640793)Show SMILES C[C@@H](Nc1nc2n(ncc2c(=O)n1Cc1ccccc1)C(C)(C)C)c1ccc(Cl)cc1 |r| Show InChI InChI=1S/C24H26ClN5O/c1-16(18-10-12-19(25)13-11-18)27-23-28-21-20(14-26-30(21)24(2,3)4)22(31)29(23)15-17-8-6-5-7-9-17/h5-14,16H,15H2,1-4H3,(H,27,28)/t16-/m1/s1 | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University
Curated by ChEMBL
| Assay Description
Inhibition of PDE1C2 (147 to 531 residues) (unknown origin) using [3H]-cGMP substrate incubated for 15 mins by liquid scintillation counting method |
J Med Chem 63: 7867-7879 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00711
BindingDB Entry DOI: 10.7270/Q20V8HBZ |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50459169
(CHEMBL4214877)Show SMILES Fc1cc(F)c2oc3c(Cc4ccc5OCOc5c4)[nH]c(-c4nccs4)c3c(=O)c2c1 Show InChI InChI=1S/C22H12F2N2O4S/c23-11-7-12-19(27)17-18(22-25-3-4-31-22)26-14(21(17)30-20(12)13(24)8-11)5-10-1-2-15-16(6-10)29-9-28-15/h1-4,6-8,26H,5,9H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-Sen University
Curated by ChEMBL
| Assay Description
Inhibition of human PDE5A1 catalytic domain (535 to 860 residues) expressed in Escherichia coli BL21 using 3H-cGMP as substrate after 15 mins by liqu... |
J Med Chem 61: 8468-8473 (2018)
Article DOI: 10.1021/acs.jmedchem.8b01209
BindingDB Entry DOI: 10.7270/Q26D5WMB |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50459170

(CHEMBL4209964)Show SMILES Brc1ccc2c(c1)oc1c(Cc3ccc4OCOc4c3)[nH]c(-c3nccs3)c1c2=O Show InChI InChI=1S/C22H13BrN2O4S/c23-12-2-3-13-16(9-12)29-21-14(7-11-1-4-15-17(8-11)28-10-27-15)25-19(18(21)20(13)26)22-24-5-6-30-22/h1-6,8-9,25H,7,10H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-Sen University
Curated by ChEMBL
| Assay Description
Inhibition of human PDE5A1 catalytic domain (535 to 860 residues) expressed in Escherichia coli BL21 using 3H-cGMP as substrate after 15 mins by liqu... |
J Med Chem 61: 8468-8473 (2018)
Article DOI: 10.1021/acs.jmedchem.8b01209
BindingDB Entry DOI: 10.7270/Q26D5WMB |
More data for this
Ligand-Target Pair | |
cGMP-dependent 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50569589
(CHEMBL4863742)Show SMILES Cc1nc2ccc(cc2n2c(nnc12)-c1ccccc1)C(=O)NCc1ccccc1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of PDE2A (unknown origin) |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128016
BindingDB Entry DOI: 10.7270/Q27948FJ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM50530258

(CHEMBL4437555)Show SMILES COc1cc2ncnc(N3CCOCC3)c2cc1OCCNc1nc2ccccc2s1 Show InChI InChI=1S/C22H23N5O3S/c1-28-18-13-17-15(21(25-14-24-17)27-7-10-29-11-8-27)12-19(18)30-9-6-23-22-26-16-4-2-3-5-20(16)31-22/h2-5,12-14H,6-11H2,1H3,(H,23,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-Sen University
Curated by ChEMBL
| Assay Description
Inhibition of PDE10 (unknown origin) |
J Med Chem 62: 2099-2111 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01763
BindingDB Entry DOI: 10.7270/Q2222Z7D |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM50530258

(CHEMBL4437555)Show SMILES COc1cc2ncnc(N3CCOCC3)c2cc1OCCNc1nc2ccccc2s1 Show InChI InChI=1S/C22H23N5O3S/c1-28-18-13-17-15(21(25-14-24-17)27-7-10-29-11-8-27)12-19(18)30-9-6-23-22-26-16-4-2-3-5-20(16)31-22/h2-5,12-14H,6-11H2,1H3,(H,23,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-Sen University
Curated by ChEMBL
| Assay Description
Inhibition of PDE10 (unknown origin) |
J Med Chem 62: 2099-2111 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01763
BindingDB Entry DOI: 10.7270/Q2222Z7D |
More data for this
Ligand-Target Pair | |
Platelet-derived growth factor receptor alpha/beta
(Mus musculus (mouse)) | BDBM50408479
(CHEMBL5180503)Show InChI InChI=1S/C18H22N2S/c1-18(2,19(3)4)13-20-14-9-5-7-11-16(14)21-17-12-8-6-10-15(17)20/h5-12H,13H2,1-4H3 | MMDB
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity at human P2X7 receptor transfected in THP1 cells assessed as inhibition of benzoyl-ATP-induced changes in plasma membrane pore fo... |
Citation and Details
|
More data for this
Ligand-Target Pair | |
High affinity cGMP-specific 3',5'-cyclic phosphodiesterase 9A
(Homo sapiens (Human)) | BDBM50543967

(CHEMBL4638644)Show SMILES C[C@@H](Nc1nc2n(ncc2c(=O)[nH]1)C1CCCC1)C(=O)Nc1ccc2[nH]ncc2c1 |r| Show InChI InChI=1S/C20H22N8O2/c1-11(18(29)24-13-6-7-16-12(8-13)9-21-27-16)23-20-25-17-15(19(30)26-20)10-22-28(17)14-4-2-3-5-14/h6-11,14H,2-5H2,1H3,(H,21,27)(H,24,29)(H2,23,25,26,30)/t11-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University
Curated by ChEMBL
| Assay Description
Inhibition of PDE9A (unknown origin) |
Bioorg Med Chem Lett 30: (2020)
Article DOI: 10.1016/j.bmcl.2020.127254
BindingDB Entry DOI: 10.7270/Q2S1862H |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data