

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50140562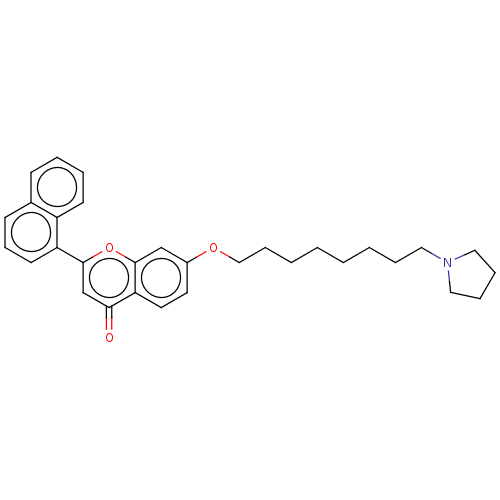 (CHEMBL3753044) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 206 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Henan University Curated by ChEMBL | Assay Description Mixed-type inhibition of electric eel AChE using ATC as substrate assessed as hydrolysis of ATC preincubated with protein for 15 mins followed by sub... | Bioorg Med Chem 24: 672-80 (2016) Article DOI: 10.1016/j.bmc.2015.12.031 BindingDB Entry DOI: 10.7270/Q2ZP4801 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50443692 (CHEMBL3093837) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 386 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Henan University Curated by ChEMBL | Assay Description Mixed-type inhibition of electric eel AChE using acetylthiocholine chloride as substrate incubated for 15 mins prior to substrate addition by Linewea... | Bioorg Med Chem 21: 7275-82 (2013) Article DOI: 10.1016/j.bmc.2013.09.061 BindingDB Entry DOI: 10.7270/Q25B03ZD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B-cell CLL/lymphoma 9 protein (Homo sapiens) | BDBM50571672 (CHEMBL4865480) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of full length C-terminal 6-His-tagged beta-catenin (1 to 781 residues) (unknown origin) expressed in Escherichia coli BL21 (DE3)-N-termin... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00046 BindingDB Entry DOI: 10.7270/Q28G8QHC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B-cell CLL/lymphoma 9 protein (Homo sapiens) | BDBM50571674 (CHEMBL4867597) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of full length C-terminal 6-His-tagged beta-catenin (1 to 781 residues) (unknown origin) expressed in Escherichia coli BL21 (DE3)-N-termin... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00046 BindingDB Entry DOI: 10.7270/Q28G8QHC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B-cell CLL/lymphoma 9 protein (Homo sapiens) | BDBM50571673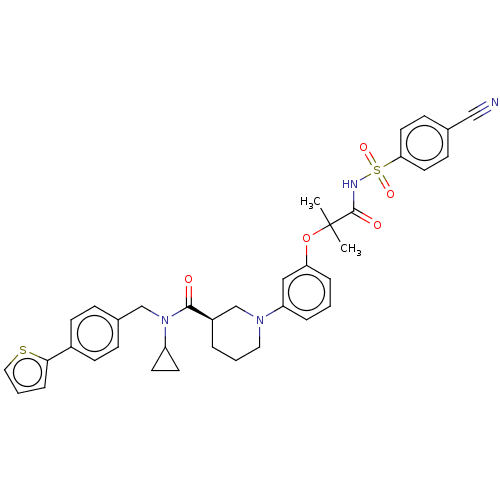 (CHEMBL4871518) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of full length C-terminal 6-His-tagged beta-catenin (1 to 781 residues) (unknown origin) expressed in Escherichia coli BL21 (DE3)-N-termin... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00046 BindingDB Entry DOI: 10.7270/Q28G8QHC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B-cell CLL/lymphoma 9 protein (Homo sapiens) | BDBM50571670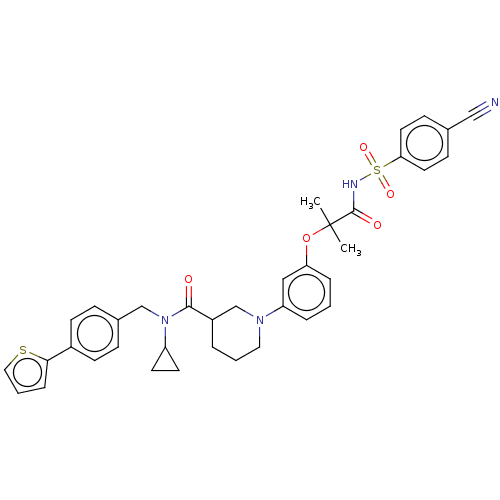 (CHEMBL4863831) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of full length C-terminal 6-His-tagged beta-catenin (1 to 781 residues) (unknown origin) expressed in Escherichia coli BL21 (DE3)-N-termin... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00046 BindingDB Entry DOI: 10.7270/Q28G8QHC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B-cell CLL/lymphoma 9 protein (Homo sapiens) | BDBM50571666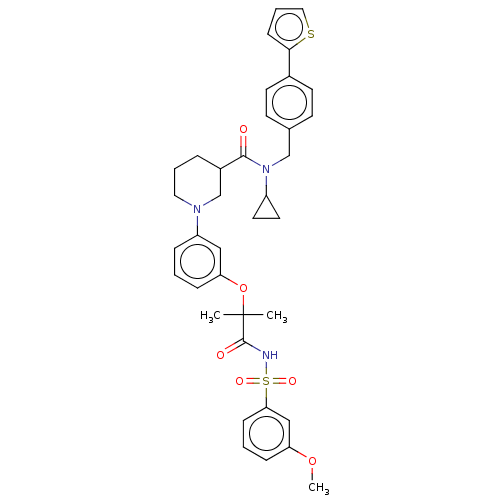 (CHEMBL4876302) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 4.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of full length C-terminal 6-His-tagged beta-catenin (1 to 781 residues) (unknown origin) expressed in Escherichia coli BL21 (DE3)-N-termin... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00046 BindingDB Entry DOI: 10.7270/Q28G8QHC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B-cell CLL/lymphoma 9 protein (Homo sapiens) | BDBM50571671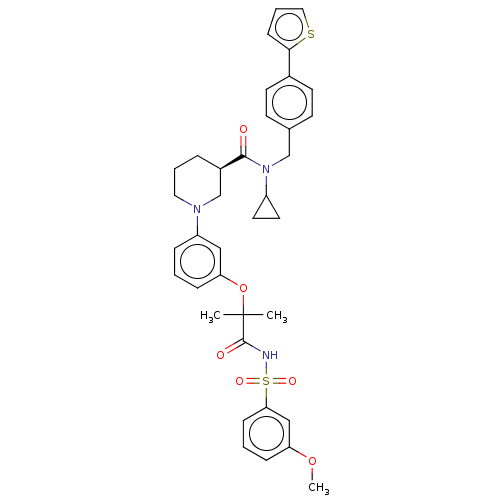 (CHEMBL4873369) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 4.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of full length C-terminal 6-His-tagged beta-catenin (1 to 781 residues) (unknown origin) expressed in Escherichia coli BL21 (DE3)-N-termin... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00046 BindingDB Entry DOI: 10.7270/Q28G8QHC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B-cell CLL/lymphoma 9 protein (Homo sapiens) | BDBM50571667 (CHEMBL4859967) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 4.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of full length C-terminal 6-His-tagged beta-catenin (1 to 781 residues) (unknown origin) expressed in Escherichia coli BL21 (DE3)-N-termin... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00046 BindingDB Entry DOI: 10.7270/Q28G8QHC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B-cell CLL/lymphoma 9 protein (Homo sapiens) | BDBM50571669 (CHEMBL4870345) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 5.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of full length C-terminal 6-His-tagged beta-catenin (1 to 781 residues) (unknown origin) expressed in Escherichia coli BL21 (DE3)-N-termin... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00046 BindingDB Entry DOI: 10.7270/Q28G8QHC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B-cell CLL/lymphoma 9 protein (Homo sapiens) | BDBM50571668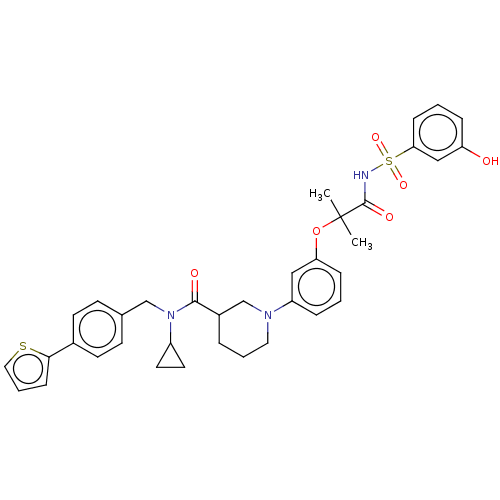 (CHEMBL4855648) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 5.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of full length C-terminal 6-His-tagged beta-catenin (1 to 781 residues) (unknown origin) expressed in Escherichia coli BL21 (DE3)-N-termin... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00046 BindingDB Entry DOI: 10.7270/Q28G8QHC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B-cell CLL/lymphoma 9 protein (Homo sapiens) | BDBM50571665 (CHEMBL4853758) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 6.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of full length C-terminal 6-His-tagged beta-catenin (1 to 781 residues) (unknown origin) expressed in Escherichia coli BL21 (DE3)-N-termin... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00046 BindingDB Entry DOI: 10.7270/Q28G8QHC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B-cell CLL/lymphoma 9 protein (Homo sapiens) | BDBM50571664 (CHEMBL4866328) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of full length C-terminal 6-His-tagged beta-catenin (1 to 781 residues) (unknown origin) expressed in Escherichia coli BL21 (DE3)-N-termin... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00046 BindingDB Entry DOI: 10.7270/Q28G8QHC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B-cell CLL/lymphoma 9 protein (Homo sapiens) | BDBM50571645 (CHEMBL4861591) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 1.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of full length C-terminal 6-His-tagged beta-catenin (1 to 781 residues) (unknown origin) expressed in Escherichia coli BL21 (DE3)-N-termin... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00046 BindingDB Entry DOI: 10.7270/Q28G8QHC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B-cell CLL/lymphoma 9 protein (Homo sapiens) | BDBM50571656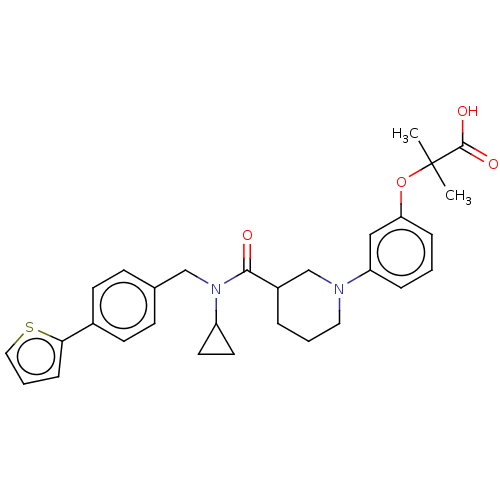 (CHEMBL4864865) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of full length C-terminal 6-His-tagged beta-catenin (1 to 781 residues) (unknown origin) expressed in Escherichia coli BL21 (DE3)-N-termin... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00046 BindingDB Entry DOI: 10.7270/Q28G8QHC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B-cell CLL/lymphoma 9 protein (Homo sapiens) | BDBM50571663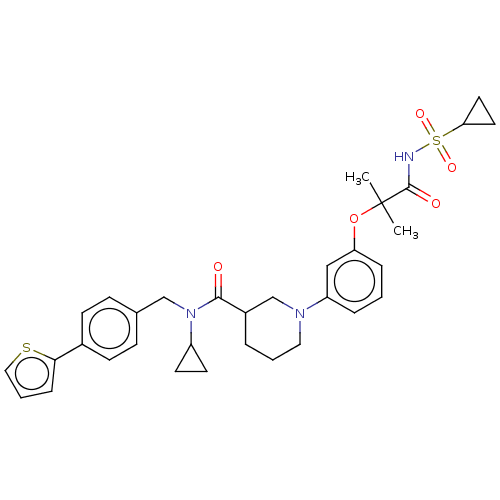 (CHEMBL4855866) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of full length C-terminal 6-His-tagged beta-catenin (1 to 781 residues) (unknown origin) expressed in Escherichia coli BL21 (DE3)-N-termin... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00046 BindingDB Entry DOI: 10.7270/Q28G8QHC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B-cell CLL/lymphoma 9 protein (Homo sapiens) | BDBM50571662 (CHEMBL4875573) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of full length C-terminal 6-His-tagged beta-catenin (1 to 781 residues) (unknown origin) expressed in Escherichia coli BL21 (DE3)-N-termin... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00046 BindingDB Entry DOI: 10.7270/Q28G8QHC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B-cell CLL/lymphoma 9 protein (Homo sapiens) | BDBM50571657 (CHEMBL4863201) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of full length C-terminal 6-His-tagged beta-catenin (1 to 781 residues) (unknown origin) expressed in Escherichia coli BL21 (DE3)-N-termin... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00046 BindingDB Entry DOI: 10.7270/Q28G8QHC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B-cell CLL/lymphoma 9 protein (Homo sapiens) | BDBM50571651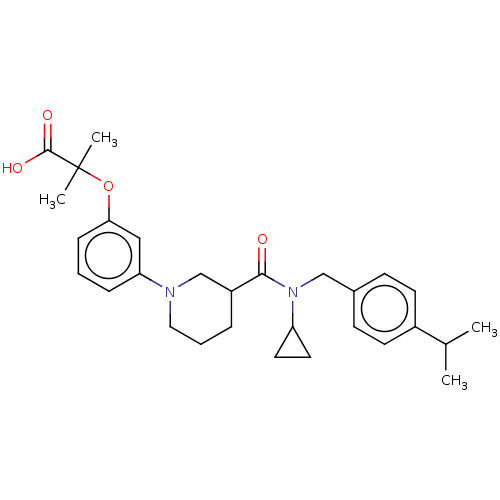 (CHEMBL4855222) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of full length C-terminal 6-His-tagged beta-catenin (1 to 781 residues) (unknown origin) expressed in Escherichia coli BL21 (DE3)-N-termin... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00046 BindingDB Entry DOI: 10.7270/Q28G8QHC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B-cell CLL/lymphoma 9 protein (Homo sapiens) | BDBM50571658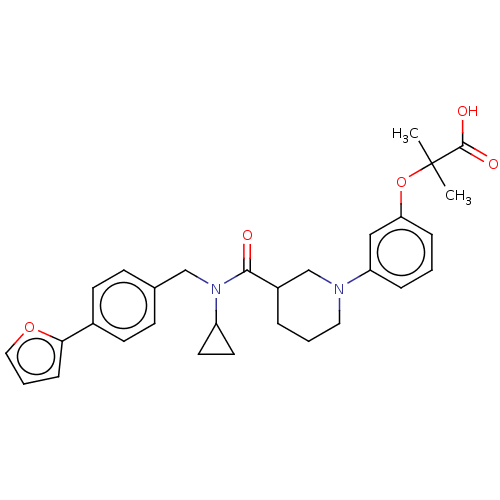 (CHEMBL4860163) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 4.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of full length C-terminal 6-His-tagged beta-catenin (1 to 781 residues) (unknown origin) expressed in Escherichia coli BL21 (DE3)-N-termin... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00046 BindingDB Entry DOI: 10.7270/Q28G8QHC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B-cell CLL/lymphoma 9 protein (Homo sapiens) | BDBM50571650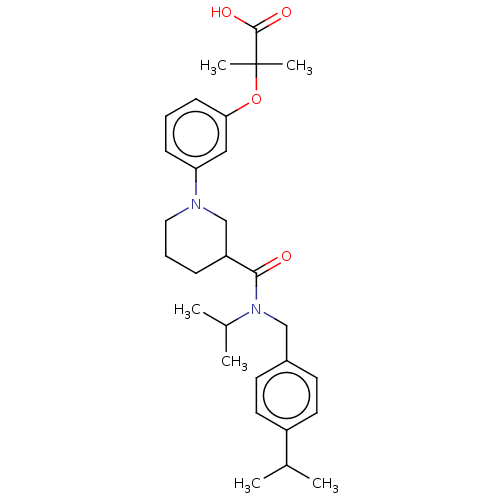 (CHEMBL4868062) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 4.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of full length C-terminal 6-His-tagged beta-catenin (1 to 781 residues) (unknown origin) expressed in Escherichia coli BL21 (DE3)-N-termin... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00046 BindingDB Entry DOI: 10.7270/Q28G8QHC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B-cell CLL/lymphoma 9 protein (Homo sapiens) | BDBM50571649 (CHEMBL4853547) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of full length C-terminal 6-His-tagged beta-catenin (1 to 781 residues) (unknown origin) expressed in Escherichia coli BL21 (DE3)-N-termin... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00046 BindingDB Entry DOI: 10.7270/Q28G8QHC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B-cell CLL/lymphoma 9 protein (Homo sapiens) | BDBM50571648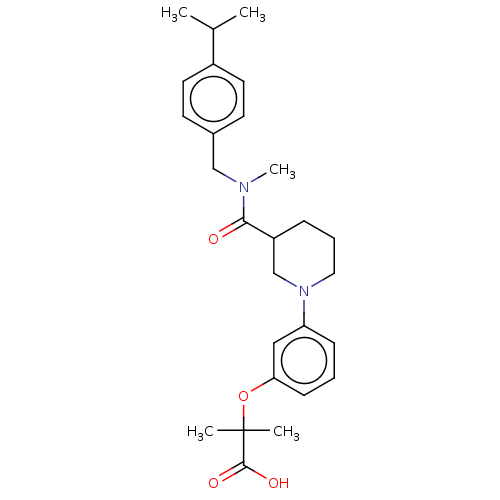 (CHEMBL4856471) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 5.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of full length C-terminal 6-His-tagged beta-catenin (1 to 781 residues) (unknown origin) expressed in Escherichia coli BL21 (DE3)-N-termin... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00046 BindingDB Entry DOI: 10.7270/Q28G8QHC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B-cell CLL/lymphoma 9 protein (Homo sapiens) | BDBM50571654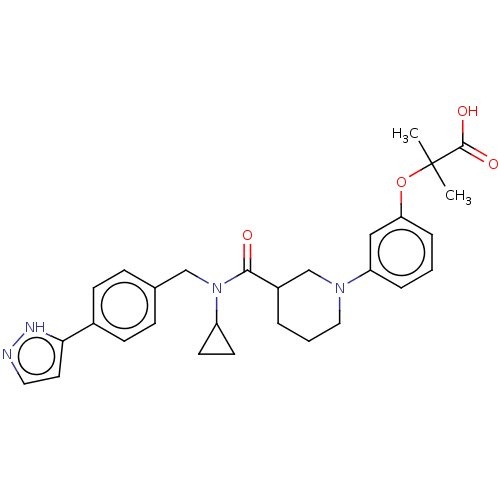 (CHEMBL4869581) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 6.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of full length C-terminal 6-His-tagged beta-catenin (1 to 781 residues) (unknown origin) expressed in Escherichia coli BL21 (DE3)-N-termin... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00046 BindingDB Entry DOI: 10.7270/Q28G8QHC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B-cell CLL/lymphoma 9 protein (Homo sapiens) | BDBM50571646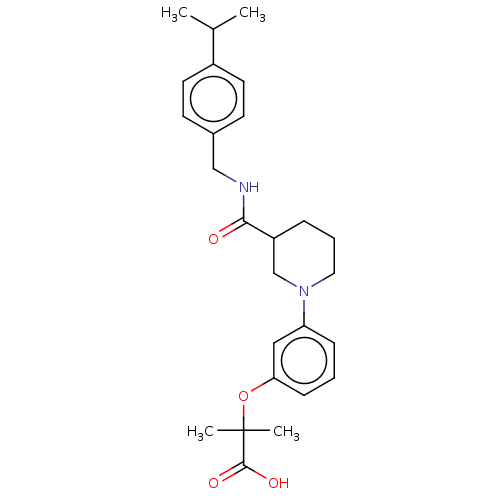 (CHEMBL4859575) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 7.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of full length C-terminal 6-His-tagged beta-catenin (1 to 781 residues) (unknown origin) expressed in Escherichia coli BL21 (DE3)-N-termin... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00046 BindingDB Entry DOI: 10.7270/Q28G8QHC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B-cell CLL/lymphoma 9 protein (Homo sapiens) | BDBM50571647 (CHEMBL4873220) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 8.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of full length C-terminal 6-His-tagged beta-catenin (1 to 781 residues) (unknown origin) expressed in Escherichia coli BL21 (DE3)-N-termin... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00046 BindingDB Entry DOI: 10.7270/Q28G8QHC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B-cell CLL/lymphoma 9 protein (Homo sapiens) | BDBM50571661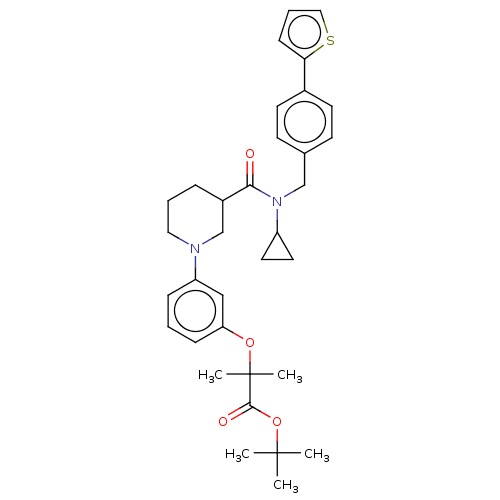 (CHEMBL4861802) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | >9.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of full length C-terminal 6-His-tagged beta-catenin (1 to 781 residues) (unknown origin) expressed in Escherichia coli BL21 (DE3)-N-termin... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00046 BindingDB Entry DOI: 10.7270/Q28G8QHC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B-cell CLL/lymphoma 9 protein (Homo sapiens) | BDBM50571652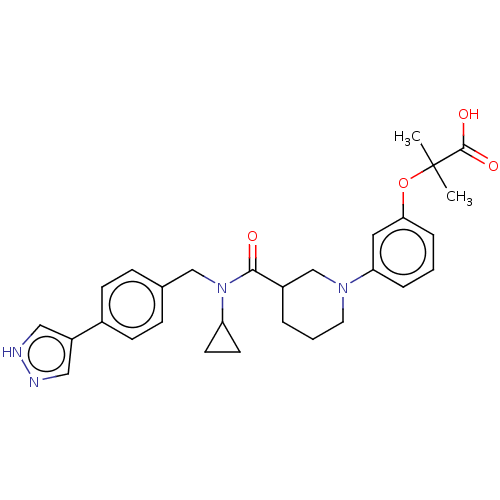 (CHEMBL4857876) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | >9.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of full length C-terminal 6-His-tagged beta-catenin (1 to 781 residues) (unknown origin) expressed in Escherichia coli BL21 (DE3)-N-termin... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00046 BindingDB Entry DOI: 10.7270/Q28G8QHC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B-cell CLL/lymphoma 9 protein (Homo sapiens) | BDBM50571655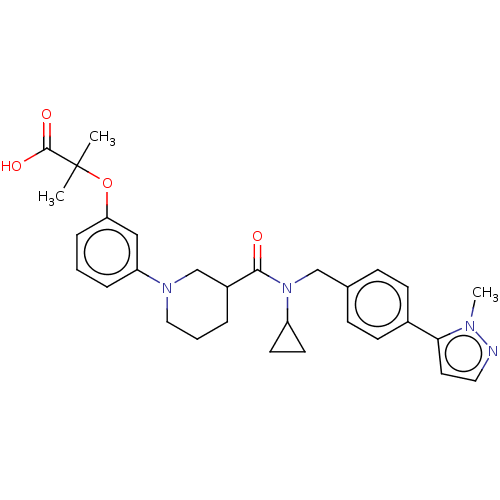 (CHEMBL4868027) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | >9.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of full length C-terminal 6-His-tagged beta-catenin (1 to 781 residues) (unknown origin) expressed in Escherichia coli BL21 (DE3)-N-termin... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00046 BindingDB Entry DOI: 10.7270/Q28G8QHC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B-cell CLL/lymphoma 9 protein (Homo sapiens) | BDBM50571659 (CHEMBL4855718) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | >9.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of full length C-terminal 6-His-tagged beta-catenin (1 to 781 residues) (unknown origin) expressed in Escherichia coli BL21 (DE3)-N-termin... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00046 BindingDB Entry DOI: 10.7270/Q28G8QHC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B-cell CLL/lymphoma 9 protein (Homo sapiens) | BDBM50571660 (CHEMBL4863752) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | >9.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of full length C-terminal 6-His-tagged beta-catenin (1 to 781 residues) (unknown origin) expressed in Escherichia coli BL21 (DE3)-N-termin... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00046 BindingDB Entry DOI: 10.7270/Q28G8QHC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B-cell CLL/lymphoma 9 protein (Homo sapiens) | BDBM50571653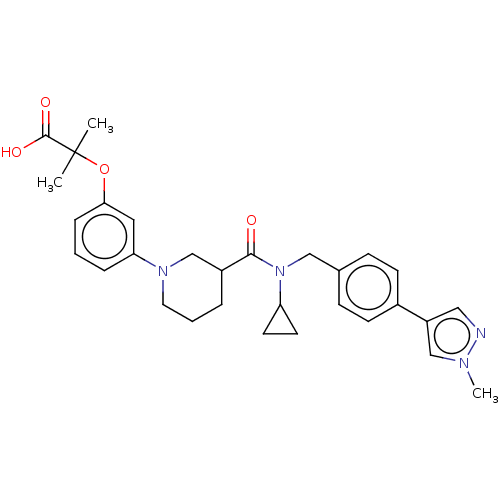 (CHEMBL4859974) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | >9.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of full length C-terminal 6-His-tagged beta-catenin (1 to 781 residues) (unknown origin) expressed in Escherichia coli BL21 (DE3)-N-termin... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00046 BindingDB Entry DOI: 10.7270/Q28G8QHC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM10616 ((3aS)-3a,8-dimethyl-2H,3H,3aH,8H,8aH-furo[2,3-b]in...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institutes of Health | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changes at 412 nm were recorded for 5 min with ... | J Med Chem 49: 2174-85 (2006) Article DOI: 10.1021/jm050578p BindingDB Entry DOI: 10.7270/Q2S75DJ6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM10616 ((3aS)-3a,8-dimethyl-2H,3H,3aH,8H,8aH-furo[2,3-b]in...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Aging Curated by ChEMBL | Assay Description Inhibitory concentration against human plasma Butyrylcholinesterase | J Med Chem 48: 986-94 (2005) Article DOI: 10.1021/jm049309+ BindingDB Entry DOI: 10.7270/Q2TM7BWQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM50333767 (CHEMBL1644288 | N1-(1,2,3,4-Tetrahydroacridin-9-yl...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.59 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University Curated by ChEMBL | Assay Description Inhibition of equine BChE by Ellman's method | Bioorg Med Chem 19: 763-70 (2011) Article DOI: 10.1016/j.bmc.2010.12.022 BindingDB Entry DOI: 10.7270/Q2T43TB0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM50333763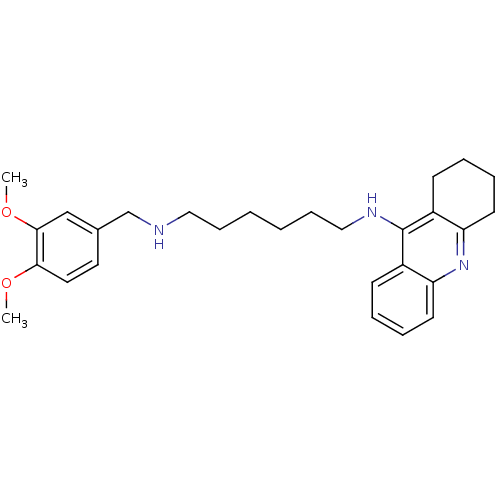 (CHEMBL1644292 | N1-(3,4-Dimethoxybenzyl)-N6-(1,2,3...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.68 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University Curated by ChEMBL | Assay Description Inhibition of equine BChE by Ellman's method | Bioorg Med Chem 19: 763-70 (2011) Article DOI: 10.1016/j.bmc.2010.12.022 BindingDB Entry DOI: 10.7270/Q2T43TB0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM10598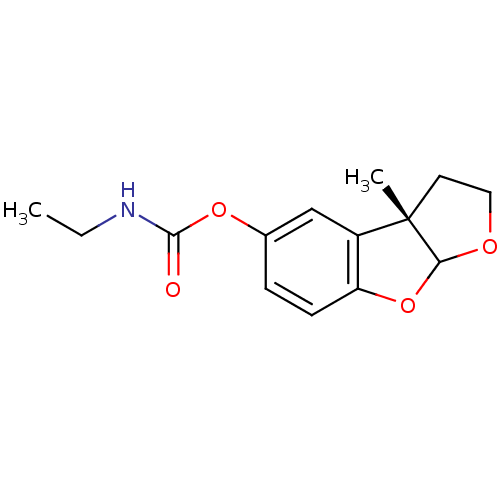 ((2S)-2-methyl-5,7-dioxatricyclo[6.4.0.0^{2,6}]dode...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institutes of Health | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changes at 412 nm were recorded for 5 min with ... | J Med Chem 49: 2174-85 (2006) Article DOI: 10.1021/jm050578p BindingDB Entry DOI: 10.7270/Q2S75DJ6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM50333765 (CHEMBL1644290 | N1-(1,2,3,4-Tetrahydroacridin-9-yl...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.38 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University Curated by ChEMBL | Assay Description Inhibition of equine BChE by Ellman's method | Bioorg Med Chem 19: 763-70 (2011) Article DOI: 10.1016/j.bmc.2010.12.022 BindingDB Entry DOI: 10.7270/Q2T43TB0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50345200 (2-Methoxy-4-((8-(1,2,3,4-tetrahydroacridin-9-ylami...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.41 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE assessed as inhibition of butylthiocholine chloride substrate hydrolysis incubated for 15 mins before substrate addi... | Eur J Med Chem 46: 2609-16 (2011) Article DOI: 10.1016/j.ejmech.2011.03.058 BindingDB Entry DOI: 10.7270/Q2BV7GZB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50345205 (CHEMBL1783241 | N1-(Benzo[d][1,3]dioxol-5-ylmethyl...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.44 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE assessed as inhibition of butylthiocholine chloride substrate hydrolysis incubated for 15 mins before substrate addi... | Eur J Med Chem 46: 2609-16 (2011) Article DOI: 10.1016/j.ejmech.2011.03.058 BindingDB Entry DOI: 10.7270/Q2BV7GZB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM50333766 (CHEMBL1644289 | N1-(1,2,3,4-Tetrahydroacridin-9-yl...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.55 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University Curated by ChEMBL | Assay Description Inhibition of equine BChE by Ellman's method | Bioorg Med Chem 19: 763-70 (2011) Article DOI: 10.1016/j.bmc.2010.12.022 BindingDB Entry DOI: 10.7270/Q2T43TB0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM10610 ((3aS)-1,3a,8-trimethyl-1H,2H,3H,3aH,8H,8aH-pyrrolo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institutes of Health | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changes at 412 nm were recorded for 5 min with ... | J Med Chem 49: 2174-85 (2006) Article DOI: 10.1021/jm050578p BindingDB Entry DOI: 10.7270/Q2S75DJ6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM10614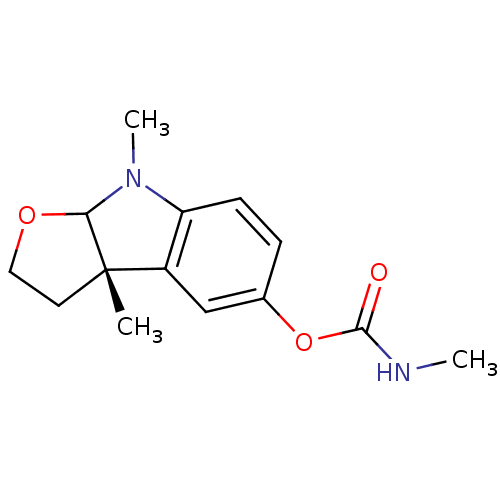 ((3aS)-3a,8-dimethyl-2H,3H,3aH,8H,8aH-furo[2,3-b]in...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institutes of Health | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changes at 412 nm were recorded for 5 min with ... | J Med Chem 49: 2174-85 (2006) Article DOI: 10.1021/jm050578p BindingDB Entry DOI: 10.7270/Q2S75DJ6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM10610 ((3aS)-1,3a,8-trimethyl-1H,2H,3H,3aH,8H,8aH-pyrrolo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Aging Curated by ChEMBL | Assay Description Inhibitory concentration against human plasma Butyrylcholinesterase | J Med Chem 48: 986-94 (2005) Article DOI: 10.1021/jm049309+ BindingDB Entry DOI: 10.7270/Q2TM7BWQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM10614 ((3aS)-3a,8-dimethyl-2H,3H,3aH,8H,8aH-furo[2,3-b]in...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Aging Curated by ChEMBL | Assay Description Inhibitory concentration against human plasma Butyrylcholinesterase | J Med Chem 48: 986-94 (2005) Article DOI: 10.1021/jm049309+ BindingDB Entry DOI: 10.7270/Q2TM7BWQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50289517 (CHEMBL4163312) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Henan University Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using butyrylthiocholine chloride as substrate preincubated for 15 mins followed by substrate addition measured ever... | Eur J Med Chem 144: 128-136 (2018) Article DOI: 10.1016/j.ejmech.2017.12.005 BindingDB Entry DOI: 10.7270/Q24B33WB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50345201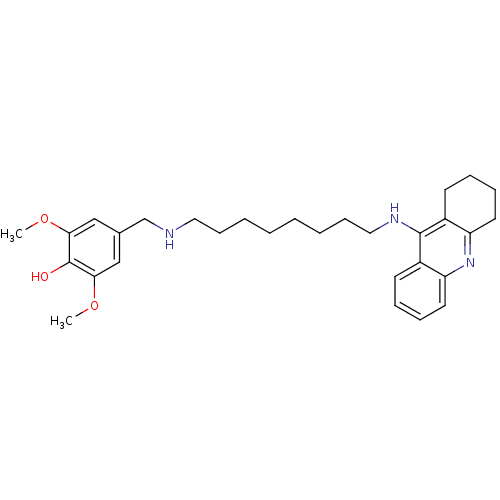 (2,6-Dimethoxy-4-((8-(1,2,3,4-tetrahydroacridin-9-y...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.08 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE assessed as inhibition of butylthiocholine chloride substrate hydrolysis incubated for 15 mins before substrate addi... | Eur J Med Chem 46: 2609-16 (2011) Article DOI: 10.1016/j.ejmech.2011.03.058 BindingDB Entry DOI: 10.7270/Q2BV7GZB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50345200 (2-Methoxy-4-((8-(1,2,3,4-tetrahydroacridin-9-ylami...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.55 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE assessed as inhibition of acetylthiocholine chloride substrate hydrolysis incubated for 15 mins before substrate addi... | Eur J Med Chem 46: 2609-16 (2011) Article DOI: 10.1016/j.ejmech.2011.03.058 BindingDB Entry DOI: 10.7270/Q2BV7GZB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50345201 (2,6-Dimethoxy-4-((8-(1,2,3,4-tetrahydroacridin-9-y...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.61 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE assessed as inhibition of acetylthiocholine chloride substrate hydrolysis incubated for 15 mins before substrate addi... | Eur J Med Chem 46: 2609-16 (2011) Article DOI: 10.1016/j.ejmech.2011.03.058 BindingDB Entry DOI: 10.7270/Q2BV7GZB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50345206 (CHEMBL1783242 | N1-(Pyridin-4-ylmethyl)-N8-(1,2,3,...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.66 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE assessed as inhibition of butylthiocholine chloride substrate hydrolysis incubated for 15 mins before substrate addi... | Eur J Med Chem 46: 2609-16 (2011) Article DOI: 10.1016/j.ejmech.2011.03.058 BindingDB Entry DOI: 10.7270/Q2BV7GZB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 694 total ) | Next | Last >> |