 Found 2093 hits with Last Name = 'ma' and Initial = 'q'
Found 2093 hits with Last Name = 'ma' and Initial = 'q' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Xanthine dehydrogenase/oxidase
(Homo sapiens (Human)) | BDBM50320491
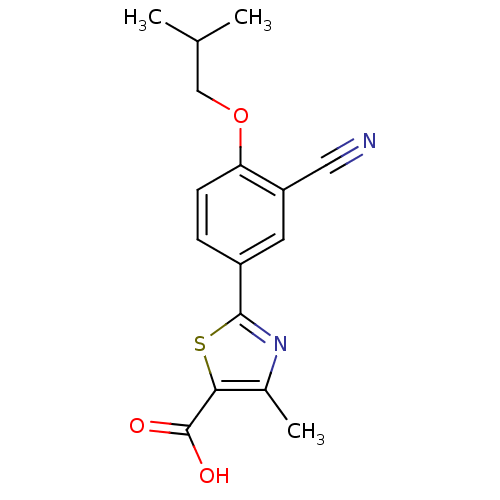
(2-(3-CYANO-4-ISOBUTOXY-PHENYL)-4-METHYL-5-THIAZOLE...)Show InChI InChI=1S/C16H16N2O3S/c1-9(2)8-21-13-5-4-11(6-12(13)7-17)15-18-10(3)14(22-15)16(19)20/h4-6,9H,8H2,1-3H3,(H,19,20) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
| MMDB
PDB
Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.114086
BindingDB Entry DOI: 10.7270/Q27085HM |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM50063581
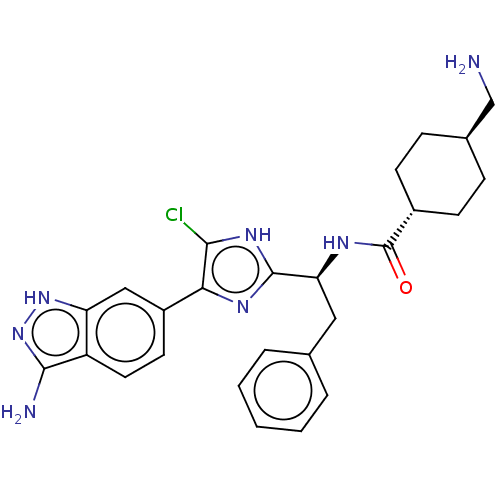
(CHEMBL3398612)Show SMILES NC[C@H]1CC[C@@H](CC1)C(=O)N[C@@H](Cc1ccccc1)c1nc(c(Cl)[nH]1)-c1ccc2c(N)n[nH]c2c1 |r,wU:5.8,wD:2.1,11.11,(25.62,-40.59,;26.39,-39.26,;27.93,-39.26,;28.7,-40.59,;30.24,-40.59,;31.01,-39.26,;30.24,-37.93,;28.7,-37.93,;32.55,-39.26,;33.32,-40.59,;33.32,-37.93,;34.86,-37.93,;35.63,-39.26,;37.17,-39.26,;37.94,-37.93,;39.48,-37.93,;40.25,-39.26,;39.48,-40.59,;37.94,-40.59,;35.63,-36.59,;35.01,-35.19,;36.14,-34.15,;37.48,-34.93,;38.89,-34.3,;37.16,-36.43,;35.98,-32.62,;37.23,-31.72,;37.07,-30.19,;35.66,-29.56,;35.19,-28.09,;36.09,-26.85,;33.65,-28.09,;33.17,-29.56,;34.42,-30.47,;34.58,-32,)| Show InChI InChI=1S/C26H28ClN7O/c27-23-22(18-10-11-19-20(13-18)33-34-24(19)29)31-25(32-23)21(12-15-4-2-1-3-5-15)30-26(35)17-8-6-16(14-28)7-9-17/h1-5,10-11,13,16-17,21H,6-9,12,14,28-29H2,(H,30,35)/b22-18-/t16-,17-,21-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to coagulation factor 11a (unknown origin) assessed as inhibition constant using (p-Glu-Pro-Arg-pNa,HCl) as substrate preincubated f... |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113437
BindingDB Entry DOI: 10.7270/Q22N5622 |
More data for this
Ligand-Target Pair | |
Xanthine dehydrogenase/oxidase
(Homo sapiens (Human)) | BDBM50603070

(CHEMBL5197177)Show SMILES CC(C)Oc1ccc(cc1C#N)-c1ncc2c(n1)[nH][nH]c2=O | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 3.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.114086
BindingDB Entry DOI: 10.7270/Q27085HM |
More data for this
Ligand-Target Pair | |
Xanthine dehydrogenase/oxidase
(Bos taurus (Bovine)) | BDBM50517021
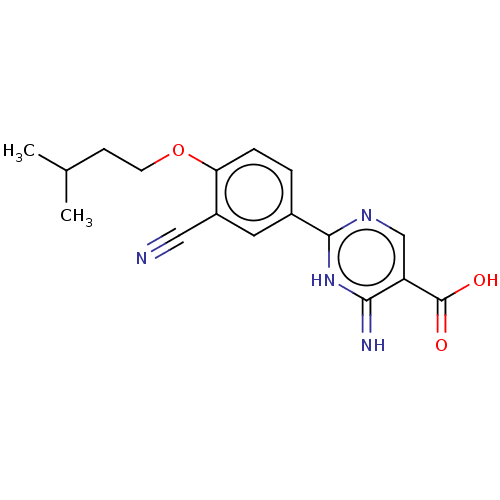
(CHEMBL4445985)Show SMILES CC(C)CCOc1ccc(cc1C#N)-c1ncc(C(O)=O)c(=N)[nH]1 Show InChI InChI=1S/C17H18N4O3/c1-10(2)5-6-24-14-4-3-11(7-12(14)8-18)16-20-9-13(17(22)23)15(19)21-16/h3-4,7,9-10H,5-6H2,1-2H3,(H,22,23)(H2,19,20,21) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 4.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shenyang Pharmaceutical University
Curated by ChEMBL
| Assay Description
Mixed type inhibition of bovine xanthine oxidase using xanthine as substrate preincubated with enzyme for 15 mins followed by substrate addition and ... |
Eur J Med Chem 181: (2019)
Article DOI: 10.1016/j.ejmech.2019.07.061
BindingDB Entry DOI: 10.7270/Q2ST7T69 |
More data for this
Ligand-Target Pair | |
Xanthine dehydrogenase/oxidase
(Homo sapiens (Human)) | BDBM50517021

(CHEMBL4445985)Show SMILES CC(C)CCOc1ccc(cc1C#N)-c1ncc(C(O)=O)c(=N)[nH]1 Show InChI InChI=1S/C17H18N4O3/c1-10(2)5-6-24-14-4-3-11(7-12(14)8-18)16-20-9-13(17(22)23)15(19)21-16/h3-4,7,9-10H,5-6H2,1-2H3,(H,22,23)(H2,19,20,21) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 4.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.114086
BindingDB Entry DOI: 10.7270/Q27085HM |
More data for this
Ligand-Target Pair | |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM50572517
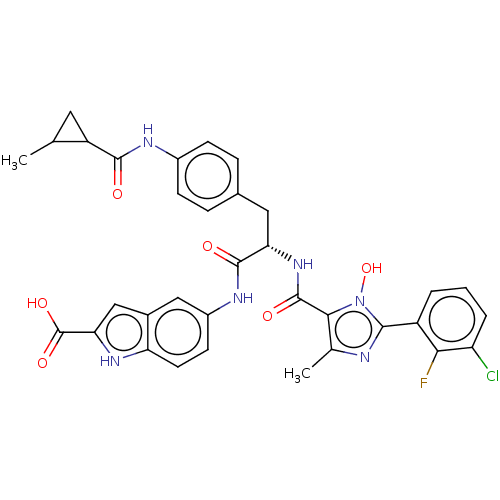
(CHEMBL4865339)Show SMILES CC1CC1C(=O)Nc1ccc(C[C@H](NC(=O)c2c(C)nc(-c3cccc(Cl)c3F)n2O)C(=O)Nc2ccc3[nH]c(cc3c2)C(O)=O)cc1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to coagulation factor 11a (unknown origin) assessed as inhibition constant using (p-Glu-Pro-Arg-pNa,HCl) as substrate preincubated f... |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113437
BindingDB Entry DOI: 10.7270/Q22N5622 |
More data for this
Ligand-Target Pair | |
Xanthine dehydrogenase/oxidase
(Bos taurus (Bovine)) | BDBM50570624

(CHEMBL4869246) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Mixed type inhibition of bovine milk xanthine oxidase assessed as inhibitory constant using xanthine as substrate preincubated for 15 mins followed b... |
Citation and Details
Article DOI: 10.1016/j.bmc.2021.116117
BindingDB Entry DOI: 10.7270/Q261144N |
More data for this
Ligand-Target Pair | |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM50572518
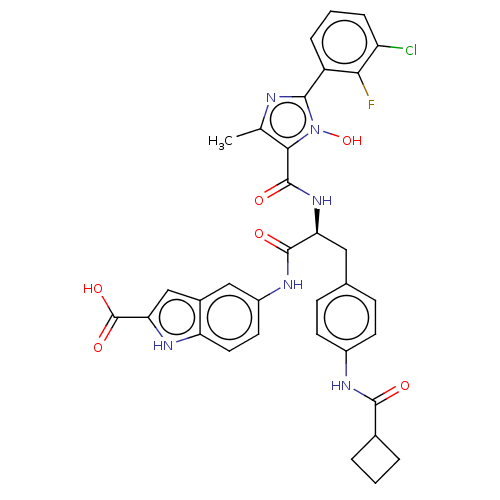
(CHEMBL4849470)Show SMILES Cc1nc(-c2cccc(Cl)c2F)n(O)c1C(=O)N[C@@H](Cc1ccc(NC(=O)C2CCC2)cc1)C(=O)Nc1ccc2[nH]c(cc2c1)C(O)=O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 23 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to coagulation factor 11a (unknown origin) assessed as inhibition constant using (p-Glu-Pro-Arg-pNa,HCl) as substrate preincubated f... |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113437
BindingDB Entry DOI: 10.7270/Q22N5622 |
More data for this
Ligand-Target Pair | |
Xanthine dehydrogenase/oxidase
(Homo sapiens (Human)) | BDBM50603060
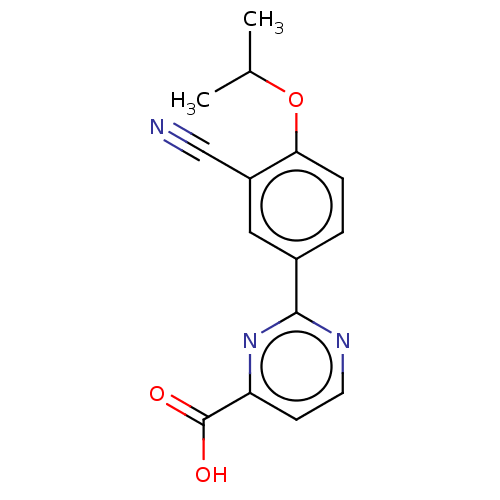
(CHEMBL5186383) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 24 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.114086
BindingDB Entry DOI: 10.7270/Q27085HM |
More data for this
Ligand-Target Pair | |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM50572513

(CHEMBL4862896)Show SMILES CC(C)CC(=O)Nc1ccc(C[C@H](NC(=O)c2c(C)nc(-c3cccc(Cl)c3F)n2O)C(=O)Nc2ccc3[nH]c(cc3c2)C(O)=O)cc1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 26 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to coagulation factor 11a (unknown origin) assessed as inhibition constant using (p-Glu-Pro-Arg-pNa,HCl) as substrate preincubated f... |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113437
BindingDB Entry DOI: 10.7270/Q22N5622 |
More data for this
Ligand-Target Pair | |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM50572515
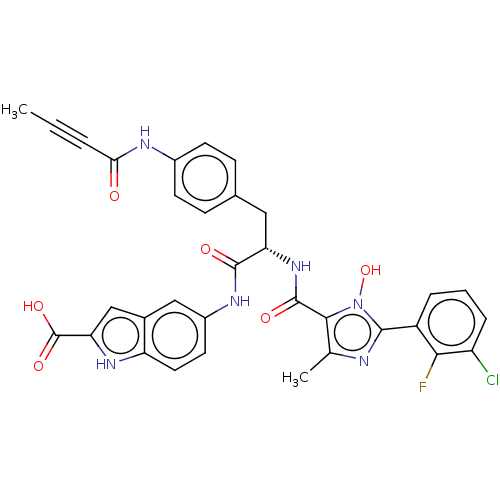
(CHEMBL4863349)Show SMILES CC#CC(=O)Nc1ccc(C[C@H](NC(=O)c2c(C)nc(-c3cccc(Cl)c3F)n2O)C(=O)Nc2ccc3[nH]c(cc3c2)C(O)=O)cc1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 31 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to coagulation factor 11a (unknown origin) assessed as inhibition constant using (p-Glu-Pro-Arg-pNa,HCl) as substrate preincubated f... |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113437
BindingDB Entry DOI: 10.7270/Q22N5622 |
More data for this
Ligand-Target Pair | |
Xanthine dehydrogenase/oxidase
(Homo sapiens (Human)) | BDBM50603046
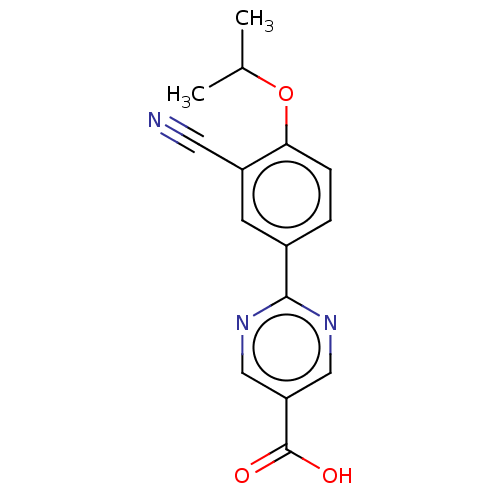
(CHEMBL5189353) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 33 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.114086
BindingDB Entry DOI: 10.7270/Q27085HM |
More data for this
Ligand-Target Pair | |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM50572519

(CHEMBL4849888)Show SMILES Cc1nc(-c2cccc(Cl)c2F)n(O)c1C(=O)N[C@@H](Cc1ccc(NC(=O)C2CCCC2)cc1)C(=O)Nc1ccc2[nH]c(cc2c1)C(O)=O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 34 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to coagulation factor 11a (unknown origin) assessed as inhibition constant using (p-Glu-Pro-Arg-pNa,HCl) as substrate preincubated f... |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113437
BindingDB Entry DOI: 10.7270/Q22N5622 |
More data for this
Ligand-Target Pair | |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM50572514

(CHEMBL4854946)Show SMILES Cc1nc(-c2cccc(Cl)c2F)n(O)c1C(=O)N[C@@H](Cc1ccc(NC(=O)C#C)cc1)C(=O)Nc1ccc2[nH]c(cc2c1)C(O)=O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 53 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to coagulation factor 11a (unknown origin) assessed as inhibition constant using (p-Glu-Pro-Arg-pNa,HCl) as substrate preincubated f... |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113437
BindingDB Entry DOI: 10.7270/Q22N5622 |
More data for this
Ligand-Target Pair | |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM50572520
(CHEMBL4875072)Show SMILES Cc1nc(-c2cccc(Cl)c2F)n(O)c1C(=O)N[C@@H](Cc1ccc(NC(=O)C2CCCCC2)cc1)C(=O)Nc1ccc2[nH]c(cc2c1)C(O)=O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 56 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to coagulation factor 11a (unknown origin) assessed as inhibition constant using (p-Glu-Pro-Arg-pNa,HCl) as substrate preincubated f... |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113437
BindingDB Entry DOI: 10.7270/Q22N5622 |
More data for this
Ligand-Target Pair | |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM50572523

(CHEMBL4872704)Show SMILES Cc1nc(-c2cccc(Cl)c2F)n(O)c1C(=O)N[C@@H](Cc1ccc(NC(=O)c2ncccn2)cc1)C(=O)Nc1ccc2[nH]c(cc2c1)C(O)=O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 81 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to coagulation factor 11a (unknown origin) assessed as inhibition constant using (p-Glu-Pro-Arg-pNa,HCl) as substrate preincubated f... |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113437
BindingDB Entry DOI: 10.7270/Q22N5622 |
More data for this
Ligand-Target Pair | |
Reverse transcriptase/RNaseH
(Human immunodeficiency virus 1) | BDBM50002465
(AZTMPPNP3'-azidothymidine 5'-[alpha,beta-imido]tri...)Show SMILES Cc1cn([C@H]2C[C@@H](N=[N+]=[N-])[C@@H](OP([O-])(=O)OP([O-])(=O)NP([O-])([O-])=O)O2)c(=O)[nH]c1=O Show InChI InChI=1S/C9H15N6O12P3/c1-4-3-15(9(17)11-7(4)16)6-2-5(12-13-10)8(25-6)26-30(23,24)27-29(21,22)14-28(18,19)20/h3,5-6,8H,2H2,1H3,(H,23,24)(H,11,16,17)(H4,14,18,19,20,21,22)/p-4/t5-,6-,8-/m1/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| 87 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California
Curated by ChEMBL
| Assay Description
The compound was tested for the inhibition of human immunodeficiency virus-1 reverse transcriptase(HIV-1 RT). |
J Med Chem 35: 1938-41 (1992)
BindingDB Entry DOI: 10.7270/Q25B0336 |
More data for this
Ligand-Target Pair | |
Xanthine dehydrogenase/oxidase
(Bos taurus (Bovine)) | BDBM50517021

(CHEMBL4445985)Show SMILES CC(C)CCOc1ccc(cc1C#N)-c1ncc(C(O)=O)c(=N)[nH]1 Show InChI InChI=1S/C17H18N4O3/c1-10(2)5-6-24-14-4-3-11(7-12(14)8-18)16-20-9-13(17(22)23)15(19)21-16/h3-4,7,9-10H,5-6H2,1-2H3,(H,22,23)(H2,19,20,21) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 127 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shenyang Pharmaceutical University
Curated by ChEMBL
| Assay Description
Mixed type inhibition of bovine xanthine oxidase using xanthine as substrate preincubated with enzyme for 15 mins followed by substrate addition and ... |
Eur J Med Chem 181: (2019)
Article DOI: 10.1016/j.ejmech.2019.07.061
BindingDB Entry DOI: 10.7270/Q2ST7T69 |
More data for this
Ligand-Target Pair | |
Reverse transcriptase/RNaseH
(Human immunodeficiency virus 1) | BDBM50002470
(AZTTP3'-azido-3'-deoxythymidine 5'-triphosphate)Show SMILES Cc1cn([C@H]2C[C@@H](N=[N+]=[N-])[C@@H](OP([O-])(=O)OP([O-])(=O)OP([O-])([O-])=O)O2)c(=O)[nH]c1=O Show InChI InChI=1S/C9H14N5O13P3/c1-4-3-14(9(16)11-7(4)15)6-2-5(12-13-10)8(24-6)25-29(20,21)27-30(22,23)26-28(17,18)19/h3,5-6,8H,2H2,1H3,(H,20,21)(H,22,23)(H,11,15,16)(H2,17,18,19)/p-4/t5-,6-,8-/m1/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| 130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California
Curated by ChEMBL
| Assay Description
The compound was tested for the inhibition of human immunodeficiency virus-1 reverse transcriptase(HIV-1 RT). |
J Med Chem 35: 1938-41 (1992)
BindingDB Entry DOI: 10.7270/Q25B0336 |
More data for this
Ligand-Target Pair | |
Reverse transcriptase/RNaseH
(Human immunodeficiency virus 1) | BDBM50002470

(AZTTP3'-azido-3'-deoxythymidine 5'-triphosphate)Show SMILES Cc1cn([C@H]2C[C@@H](N=[N+]=[N-])[C@@H](OP([O-])(=O)OP([O-])(=O)OP([O-])([O-])=O)O2)c(=O)[nH]c1=O Show InChI InChI=1S/C9H14N5O13P3/c1-4-3-14(9(16)11-7(4)15)6-2-5(12-13-10)8(24-6)25-29(20,21)27-30(22,23)26-28(17,18)19/h3,5-6,8H,2H2,1H3,(H,20,21)(H,22,23)(H,11,15,16)(H2,17,18,19)/p-4/t5-,6-,8-/m1/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| 130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California
Curated by ChEMBL
| Assay Description
The compound was tested for the inhibition of human immunodeficiency virus-1 reverse transcriptase(HIV-1 RT). |
J Med Chem 35: 1938-41 (1992)
BindingDB Entry DOI: 10.7270/Q25B0336 |
More data for this
Ligand-Target Pair | |
Xanthine dehydrogenase/oxidase
(Bos taurus (Bovine)) | BDBM50564058

(CHEMBL4792232) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 138 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Mixed type inhibition of bovine xanthine oxidase assessed as inhibitory constant using xanthine as substrate preincubated for 25 mins followed by sub... |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112077
BindingDB Entry DOI: 10.7270/Q2280CCG |
More data for this
Ligand-Target Pair | |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM50572522

(CHEMBL4864164)Show SMILES Cc1nc(-c2cccc(Cl)c2F)n(O)c1C(=O)N[C@@H](Cc1ccc(NC(=O)c2ccccn2)cc1)C(=O)Nc1ccc2[nH]c(cc2c1)C(O)=O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 155 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to coagulation factor 11a (unknown origin) assessed as inhibition constant using (p-Glu-Pro-Arg-pNa,HCl) as substrate preincubated f... |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113437
BindingDB Entry DOI: 10.7270/Q22N5622 |
More data for this
Ligand-Target Pair | |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM50572512

(CHEMBL4872406)Show SMILES CC(C)C(=O)Nc1ccc(C[C@H](NC(=O)c2c(C)nc(-c3cccc(Cl)c3F)n2O)C(=O)Nc2ccc3[nH]c(cc3c2)C(O)=O)cc1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 156 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to coagulation factor 11a (unknown origin) assessed as inhibition constant using (p-Glu-Pro-Arg-pNa,HCl) as substrate preincubated f... |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113437
BindingDB Entry DOI: 10.7270/Q22N5622 |
More data for this
Ligand-Target Pair | |
Beta-lactamase TEM
(Escherichia coli) | BDBM50053173

((2S,3S,5R)-3-Methyl-4,4,7-trioxo-3-[1,2,3]triazol-...)Show SMILES C[C@]1(Cn2ccnn2)[C@@H](N2[C@@H](CC2=O)S1(=O)=O)C(O)=O |r| Show InChI InChI=1S/C10H12N4O5S/c1-10(5-13-3-2-11-12-13)8(9(16)17)14-6(15)4-7(14)20(10,18)19/h2-3,7-8H,4-5H2,1H3,(H,16,17)/t7-,8+,10+/m1/s1 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University
Curated by ChEMBL
| Assay Description
Inhibition of bacterial N-terminal His-tagged TEV protease site linked TEM-1 (24 to 286 amino acids) expressed in Escherichia coli Transetta (DE3) pr... |
J Med Chem 62: 7160-7184 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00735
BindingDB Entry DOI: 10.7270/Q22R3W2M |
More data for this
Ligand-Target Pair | |
Plasma kallikrein
(Homo sapiens (Human)) | BDBM50572517

(CHEMBL4865339)Show SMILES CC1CC1C(=O)Nc1ccc(C[C@H](NC(=O)c2c(C)nc(-c3cccc(Cl)c3F)n2O)C(=O)Nc2ccc3[nH]c(cc3c2)C(O)=O)cc1 |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 192 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to plasma kallikrein (unknown origin) assessed as inhibition constant using D-Pro-Phe,Arg,pNa,2HCl as substrate preincubated for 10 ... |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113437
BindingDB Entry DOI: 10.7270/Q22N5622 |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM50569138
(CHEMBL4871070)Show SMILES Cc1noc([C@@H]2[C@H]([C@@H](N[C@@]22C(=O)Nc3cc(Cl)ccc23)C(F)(F)F)c2ccccc2)c1[N+]([O-])=O |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of MDM2 (unknown origin) using peptide as substrate incubated for 30 mins by fluorescence polarization assay |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113359
BindingDB Entry DOI: 10.7270/Q20005VW |
More data for this
Ligand-Target Pair | |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM50572516

(CHEMBL4856139)Show SMILES Cc1nc(-c2cccc(Cl)c2F)n(O)c1C(=O)N[C@@H](Cc1ccc(NC(=O)C2CC2)cc1)C(=O)Nc1ccc2[nH]c(cc2c1)C(O)=O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 259 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to coagulation factor 11a (unknown origin) assessed as inhibition constant using (p-Glu-Pro-Arg-pNa,HCl) as substrate preincubated f... |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113437
BindingDB Entry DOI: 10.7270/Q22N5622 |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM50569140

(CHEMBL4863792)Show SMILES Cc1noc([C@@H]2[C@H]([C@@H](N[C@@]22C(=O)Nc3cc(Br)ccc23)C(F)(F)F)c2ccccc2)c1[N+]([O-])=O |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of MDM2 (unknown origin) using peptide as substrate incubated for 30 mins by fluorescence polarization assay |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113359
BindingDB Entry DOI: 10.7270/Q20005VW |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM31197
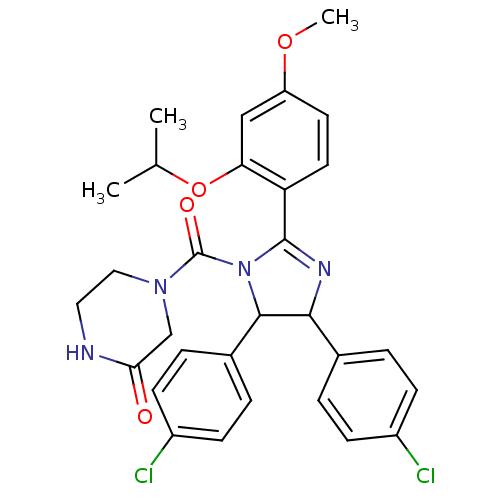
(CHEMBL211045 | Nutlin-3 | med.21724, Compound 186)Show SMILES COc1ccc(C2=NC(C(N2C(=O)N2CCNC(=O)C2)c2ccc(Cl)cc2)c2ccc(Cl)cc2)c(OC(C)C)c1 |t:6| Show InChI InChI=1S/C30H30Cl2N4O4/c1-18(2)40-25-16-23(39-3)12-13-24(25)29-34-27(19-4-8-21(31)9-5-19)28(20-6-10-22(32)11-7-20)36(29)30(38)35-15-14-33-26(37)17-35/h4-13,16,18,27-28H,14-15,17H2,1-3H3,(H,33,37) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of MDM2 (unknown origin) using peptide as substrate incubated for 30 mins by fluorescence polarization assay |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113359
BindingDB Entry DOI: 10.7270/Q20005VW |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM50572507

(CHEMBL4854369)Show SMILES Cc1nc(-c2cccc(Cl)c2F)n(O)c1C(=O)N[C@@H](Cc1ccc(Cl)cc1)C(=O)Nc1ccc2[nH]c(cc2c1)C(O)=O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 356 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to coagulation factor 11a (unknown origin) assessed as inhibition constant using (p-Glu-Pro-Arg-pNa,HCl) as substrate preincubated f... |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113437
BindingDB Entry DOI: 10.7270/Q22N5622 |
More data for this
Ligand-Target Pair | |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM50572521

(CHEMBL4868504)Show SMILES Cc1nc(-c2cccc(Cl)c2F)n(O)c1C(=O)N[C@@H](Cc1ccc(NC(=O)c2ccccc2)cc1)C(=O)Nc1ccc2[nH]c(cc2c1)C(O)=O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 366 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to coagulation factor 11a (unknown origin) assessed as inhibition constant using (p-Glu-Pro-Arg-pNa,HCl) as substrate preincubated f... |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113437
BindingDB Entry DOI: 10.7270/Q22N5622 |
More data for this
Ligand-Target Pair | |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM50572511

(CHEMBL4846470)Show SMILES CC(=O)Nc1ccc(C[C@H](NC(=O)c2c(C)nc(-c3cccc(Cl)c3F)n2O)C(=O)Nc2ccc3[nH]c(cc3c2)C(O)=O)cc1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 392 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to coagulation factor 11a (unknown origin) assessed as inhibition constant using (p-Glu-Pro-Arg-pNa,HCl) as substrate preincubated f... |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113437
BindingDB Entry DOI: 10.7270/Q22N5622 |
More data for this
Ligand-Target Pair | |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM50572506

(CHEMBL4879256)Show SMILES Cc1nc(-c2cccc(Cl)c2F)n(O)c1C(=O)N[C@@H](Cc1ccc(F)cc1)C(=O)Nc1ccc2[nH]c(cc2c1)C(O)=O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 486 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to coagulation factor 11a (unknown origin) assessed as inhibition constant using (p-Glu-Pro-Arg-pNa,HCl) as substrate preincubated f... |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113437
BindingDB Entry DOI: 10.7270/Q22N5622 |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM50569136

(CHEMBL4874111)Show SMILES Cc1noc([C@@H]2[C@H]([C@@H](N[C@@]22C(=O)Nc3ccc(F)cc23)C(F)(F)F)c2ccccc2)c1[N+]([O-])=O |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 490 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of MDM2 (unknown origin) using peptide as substrate incubated for 30 mins by fluorescence polarization assay |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113359
BindingDB Entry DOI: 10.7270/Q20005VW |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM50569137

(CHEMBL4860936)Show SMILES Cc1noc([C@@H]2[C@H]([C@@H](N[C@@]22C(=O)Nc3ccc(Cl)cc23)C(F)(F)F)c2ccccc2)c1[N+]([O-])=O |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 680 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of MDM2 (unknown origin) using peptide as substrate incubated for 30 mins by fluorescence polarization assay |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113359
BindingDB Entry DOI: 10.7270/Q20005VW |
More data for this
Ligand-Target Pair | |
Xanthine dehydrogenase/oxidase
(Homo sapiens (Human)) | BDBM50140241

(Allopurinol | Aloral | Aluline 100 | Aluline 300 |...)Show InChI InChI=1S/C5H4N4O/c10-5-3-1-8-9-4(3)6-2-7-5/h1-2H,(H2,6,7,8,9,10) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.114086
BindingDB Entry DOI: 10.7270/Q27085HM |
More data for this
Ligand-Target Pair | |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM50572508
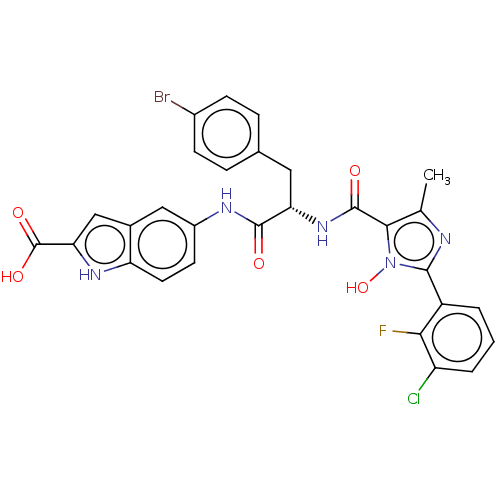
(CHEMBL4865716)Show SMILES Cc1nc(-c2cccc(Cl)c2F)n(O)c1C(=O)N[C@@H](Cc1ccc(Br)cc1)C(=O)Nc1ccc2[nH]c(cc2c1)C(O)=O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 962 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to coagulation factor 11a (unknown origin) assessed as inhibition constant using (p-Glu-Pro-Arg-pNa,HCl) as substrate preincubated f... |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113437
BindingDB Entry DOI: 10.7270/Q22N5622 |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM50569139
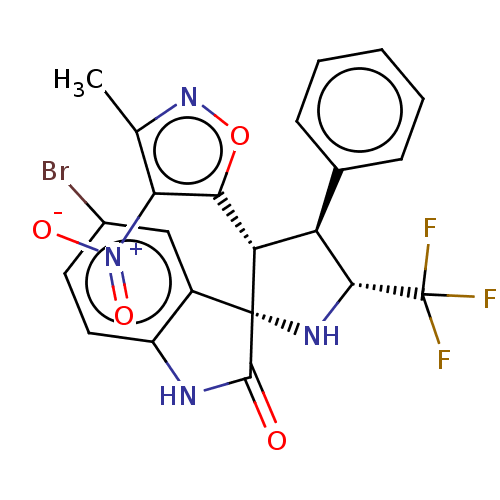
(CHEMBL4863780)Show SMILES Cc1noc([C@@H]2[C@H]([C@@H](N[C@@]22C(=O)Nc3ccc(Br)cc23)C(F)(F)F)c2ccccc2)c1[N+]([O-])=O |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.02E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of MDM2 (unknown origin) using peptide as substrate incubated for 30 mins by fluorescence polarization assay |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113359
BindingDB Entry DOI: 10.7270/Q20005VW |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM50569144

(CHEMBL4861981)Show SMILES Cc1noc([C@@H]2[C@H]([C@@H](N[C@@]22C(=O)Nc3ccccc23)C(F)(F)F)c2ccc(F)cc2)c1[N+]([O-])=O |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.03E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of MDM2 (unknown origin) using peptide as substrate incubated for 30 mins by fluorescence polarization assay |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113359
BindingDB Entry DOI: 10.7270/Q20005VW |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM50569143

(CHEMBL4876458)Show SMILES Cc1noc([C@@H]2[C@H]([C@@H](N[C@@]22C(=O)Nc3ccccc23)C(F)(F)F)c2cccc(F)c2)c1[N+]([O-])=O |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.14E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of MDM2 (unknown origin) using peptide as substrate incubated for 30 mins by fluorescence polarization assay |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113359
BindingDB Entry DOI: 10.7270/Q20005VW |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM50569158

(CHEMBL4856044)Show SMILES Cc1noc([C@@H]2[C@H]([C@@H](N[C@@]22C(=O)Nc3ccccc23)C(F)(F)F)c2ccco2)c1[N+]([O-])=O |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.19E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of MDM2 (unknown origin) using peptide as substrate incubated for 30 mins by fluorescence polarization assay |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113359
BindingDB Entry DOI: 10.7270/Q20005VW |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM50569135
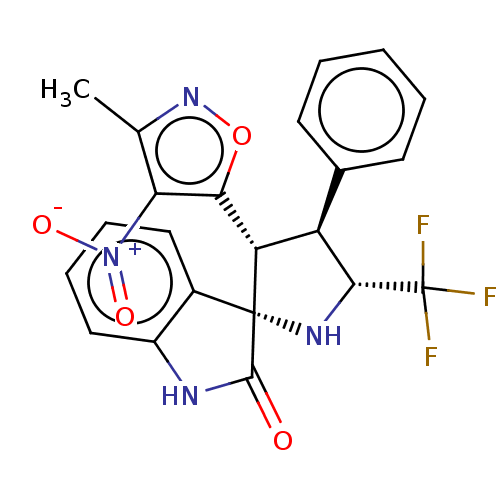
(CHEMBL4874952)Show SMILES Cc1noc([C@@H]2[C@H]([C@@H](N[C@@]22C(=O)Nc3ccccc23)C(F)(F)F)c2ccccc2)c1[N+]([O-])=O |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.28E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of MDM2 (unknown origin) using peptide as substrate incubated for 30 mins by fluorescence polarization assay |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113359
BindingDB Entry DOI: 10.7270/Q20005VW |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM50569159
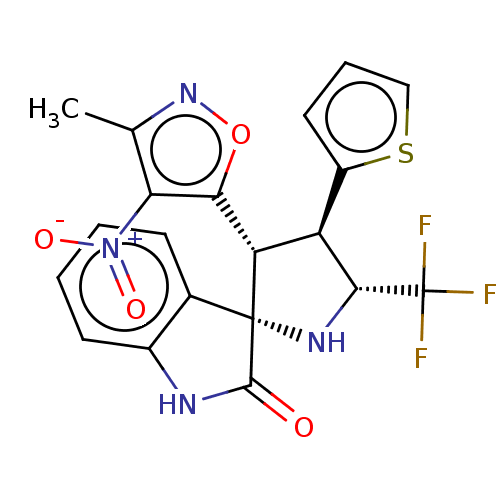
(CHEMBL4870214)Show SMILES Cc1noc([C@@H]2[C@H]([C@@H](N[C@@]22C(=O)Nc3ccccc23)C(F)(F)F)c2cccs2)c1[N+]([O-])=O |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.36E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of MDM2 (unknown origin) using peptide as substrate incubated for 30 mins by fluorescence polarization assay |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113359
BindingDB Entry DOI: 10.7270/Q20005VW |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase Mdm2
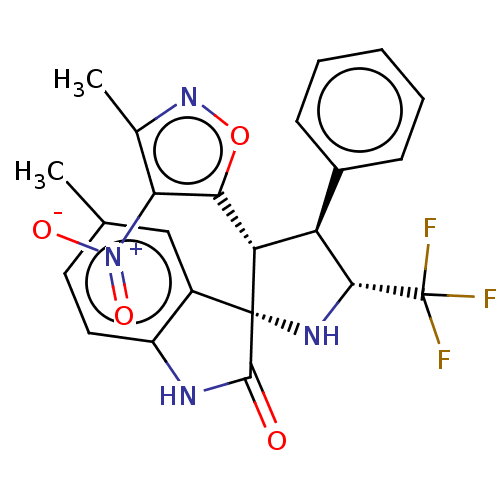
(Homo sapiens (Human)) | BDBM50569142
(CHEMBL4877534)Show SMILES Cc1noc([C@@H]2[C@H]([C@@H](N[C@@]22C(=O)Nc3ccc(C)cc23)C(F)(F)F)c2ccccc2)c1[N+]([O-])=O |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.42E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of MDM2 (unknown origin) using peptide as substrate incubated for 30 mins by fluorescence polarization assay |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113359
BindingDB Entry DOI: 10.7270/Q20005VW |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM50569152
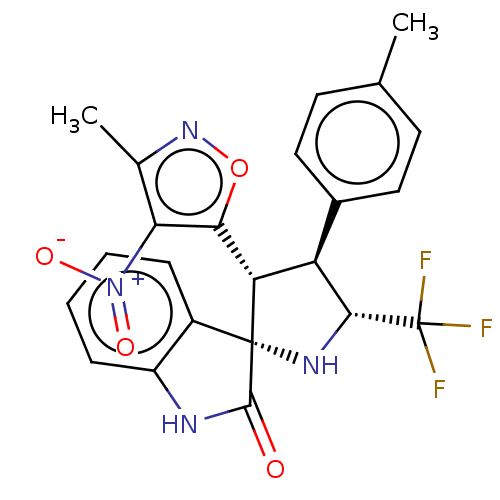
(CHEMBL4859985)Show SMILES Cc1noc([C@@H]2[C@H]([C@@H](N[C@@]22C(=O)Nc3ccccc23)C(F)(F)F)c2ccc(C)cc2)c1[N+]([O-])=O |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.49E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of MDM2 (unknown origin) using peptide as substrate incubated for 30 mins by fluorescence polarization assay |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113359
BindingDB Entry DOI: 10.7270/Q20005VW |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM50569149
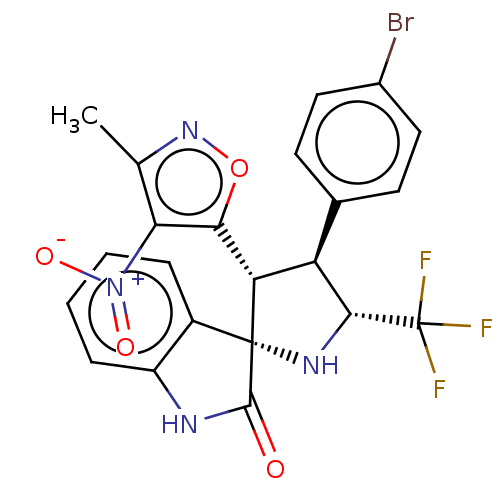
(CHEMBL4877092)Show SMILES Cc1noc([C@@H]2[C@H]([C@@H](N[C@@]22C(=O)Nc3ccccc23)C(F)(F)F)c2ccc(Br)cc2)c1[N+]([O-])=O |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.87E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of MDM2 (unknown origin) using peptide as substrate incubated for 30 mins by fluorescence polarization assay |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113359
BindingDB Entry DOI: 10.7270/Q20005VW |
More data for this
Ligand-Target Pair | |
Beta-lactamase TEM
(Escherichia coli) | BDBM50518879
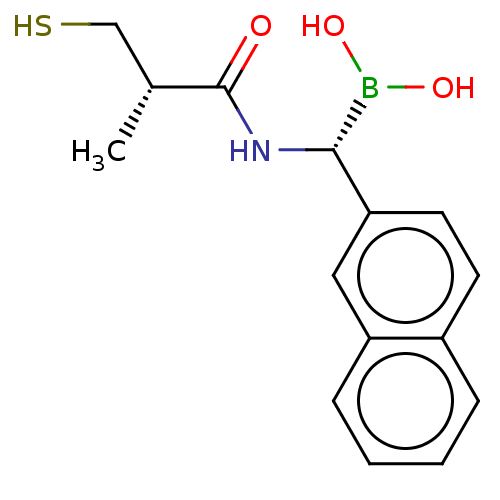
(CHEMBL4450911)Show SMILES C[C@H](CS)C(=O)N[C@H](B(O)O)c1ccc2ccccc2c1 |r| Show InChI InChI=1S/C15H18BNO3S/c1-10(9-21)15(18)17-14(16(19)20)13-7-6-11-4-2-3-5-12(11)8-13/h2-8,10,14,19-21H,9H2,1H3,(H,17,18)/t10-,14+/m1/s1 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.03E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University
Curated by ChEMBL
| Assay Description
Inhibition of bacterial N-terminal His-tagged TEV protease site linked TEM-1 (24 to 286 amino acids) expressed in Escherichia coli Transetta (DE3) pr... |
J Med Chem 62: 7160-7184 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00735
BindingDB Entry DOI: 10.7270/Q22R3W2M |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM50569154
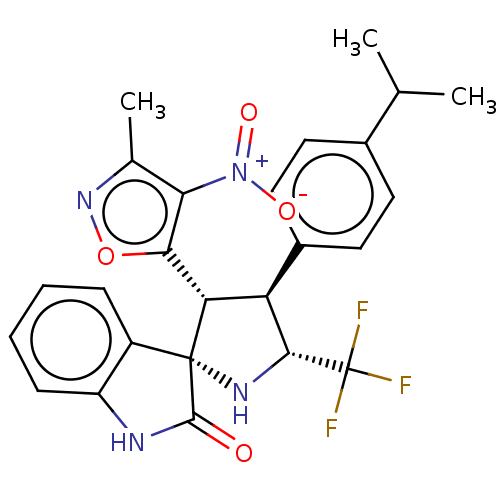
(CHEMBL4860559)Show SMILES CC(C)c1ccc(cc1)[C@H]1[C@@H](N[C@]2([C@@H]1c1onc(C)c1[N+]([O-])=O)C(=O)Nc1ccccc21)C(F)(F)F |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.26E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of MDM2 (unknown origin) using peptide as substrate incubated for 30 mins by fluorescence polarization assay |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113359
BindingDB Entry DOI: 10.7270/Q20005VW |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM50569156

(CHEMBL4878727)Show SMILES Cc1noc([C@@H]2[C@H]([C@@H](N[C@@]22C(=O)Nc3ccccc23)C(F)(F)F)c2ccc(Cl)c(Cl)c2)c1[N+]([O-])=O |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.36E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of MDM2 (unknown origin) using peptide as substrate incubated for 30 mins by fluorescence polarization assay |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113359
BindingDB Entry DOI: 10.7270/Q20005VW |
More data for this
Ligand-Target Pair | |
Reverse transcriptase/RNaseH
(Human immunodeficiency virus 1) | BDBM50002468
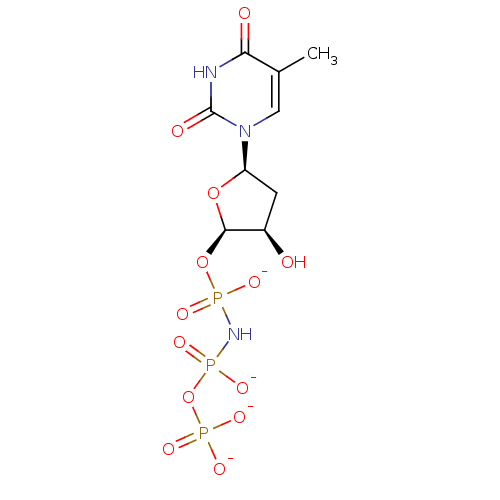
(TMPNPPthymidine 5'-[alpha,beta-imido]triphosphate)Show SMILES Cc1cn([C@H]2C[C@@H](O)[C@@H](OP([O-])(=O)NP([O-])(=O)OP([O-])([O-])=O)O2)c(=O)[nH]c1=O Show InChI InChI=1S/C9H16N3O13P3/c1-4-3-12(9(15)10-7(4)14)6-2-5(13)8(23-6)24-26(16,17)11-27(18,19)25-28(20,21)22/h3,5-6,8,13H,2H2,1H3,(H,10,14,15)(H2,20,21,22)(H3,11,16,17,18,19)/p-4/t5-,6-,8-/m1/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| PubMed
| 2.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California
Curated by ChEMBL
| Assay Description
The compound was tested for the inhibition of human immunodeficiency virus-1 reverse transcriptase(HIV-1 RT). |
J Med Chem 35: 1938-41 (1992)
BindingDB Entry DOI: 10.7270/Q25B0336 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data