

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50485688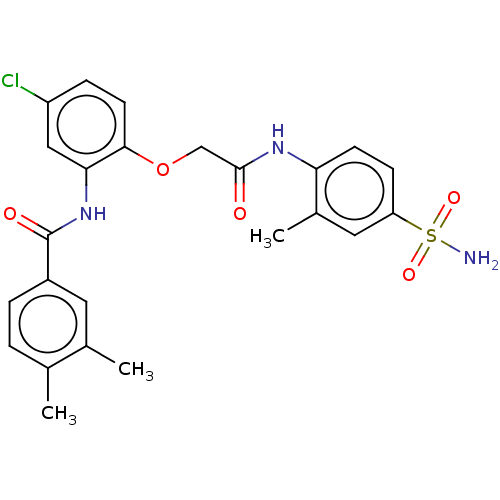 (CHEMBL2151836) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University Curated by ChEMBL | Assay Description Inhibition of wild type HIV1 reverse transcriptase activity using poly (rA)/oligo (dT)15 homopolymer template after 1 hr by ELISA | Eur J Med Chem 58: 504-12 (2012) Article DOI: 10.1016/j.ejmech.2012.03.032 BindingDB Entry DOI: 10.7270/Q2K07747 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50485689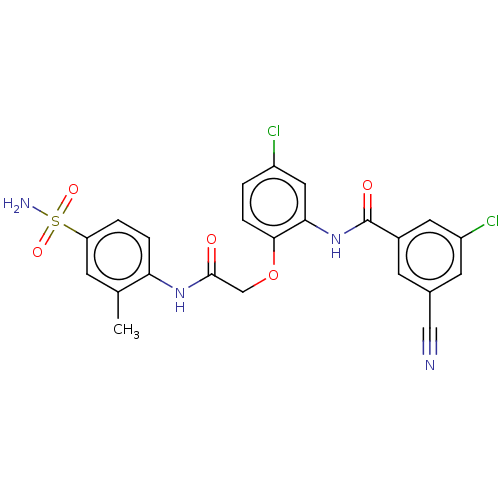 (CHEMBL2151844) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University Curated by ChEMBL | Assay Description Inhibition of wild type HIV1 reverse transcriptase activity using poly (rA)/oligo (dT)15 homopolymer template after 1 hr by ELISA | Eur J Med Chem 58: 504-12 (2012) Article DOI: 10.1016/j.ejmech.2012.03.032 BindingDB Entry DOI: 10.7270/Q2K07747 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50478026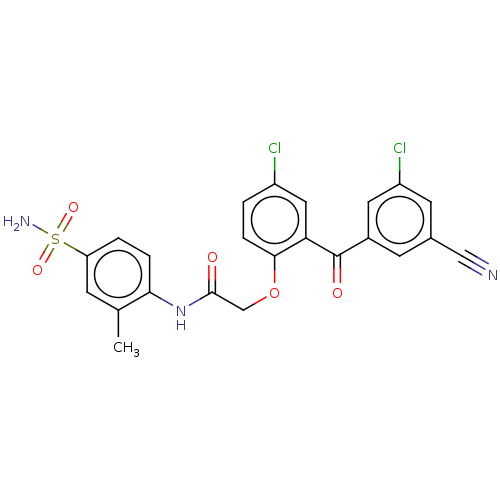 (CHEMBL203420 | GW678248 | GW8248) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University Curated by ChEMBL | Assay Description Inhibition of wild type HIV1 reverse transcriptase activity using poly (rA)/oligo (dT)15 homopolymer template after 1 hr by ELISA | Eur J Med Chem 58: 504-12 (2012) Article DOI: 10.1016/j.ejmech.2012.03.032 BindingDB Entry DOI: 10.7270/Q2K07747 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM2483 ((4S)-6-chloro-4-(2-cyclopropylethynyl)-4-(trifluor...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 48 | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University Curated by ChEMBL | Assay Description Inhibition of recombinant wild type HIV1 RT using poly(rA)/oligo(dT)16 as template after 40 mins by spectrofluorometric analysis | Eur J Med Chem 65: 134-43 (2013) Article DOI: 10.1016/j.ejmech.2013.04.052 BindingDB Entry DOI: 10.7270/Q2736TTS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50485192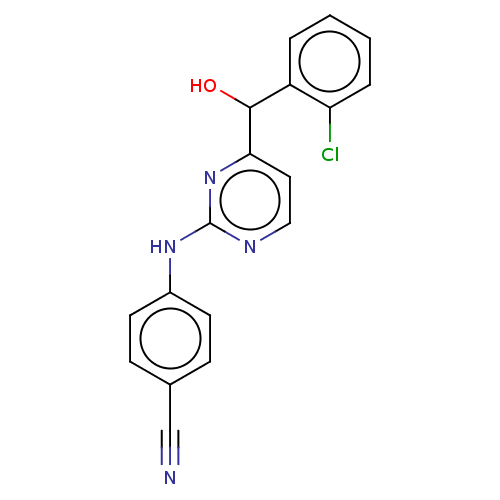 (CHEMBL1817673) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 450 | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University Curated by ChEMBL | Assay Description Inhibition of HIV1 wildtype reverse transcriptase using poly(rA)/oligo(dT)15 as template by colometric streptavidin alkaline phosphate reporter assay | Eur J Med Chem 53: 229-34 (2012) Article DOI: 10.1016/j.ejmech.2012.04.004 BindingDB Entry DOI: 10.7270/Q2KK9FN2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50103642 (4-(6-amino-5-bromo-2-(4-cyanophenylamino)pyrimidin...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 980 | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University Curated by ChEMBL | Assay Description Inhibition of HIV1 wildtype reverse transcriptase using poly(rA)/oligo(dT)15 as template by colometric streptavidin alkaline phosphate reporter assay | Eur J Med Chem 53: 229-34 (2012) Article DOI: 10.1016/j.ejmech.2012.04.004 BindingDB Entry DOI: 10.7270/Q2KK9FN2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50492329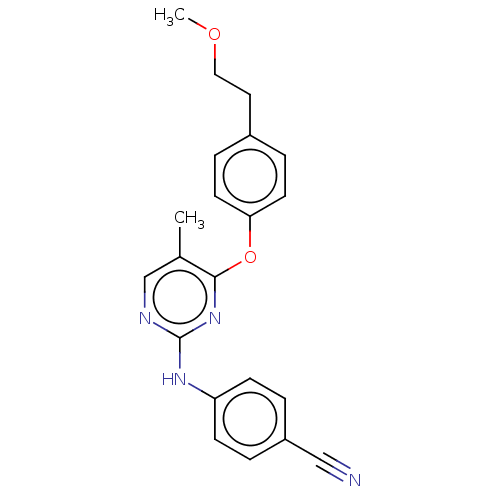 (CHEMBL2402968) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.27E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University Curated by ChEMBL | Assay Description Inhibition of recombinant wild type HIV1 RT using poly(rA)/oligo(dT)16 as template after 40 mins by spectrofluorometric analysis | Eur J Med Chem 65: 134-43 (2013) Article DOI: 10.1016/j.ejmech.2013.04.052 BindingDB Entry DOI: 10.7270/Q2736TTS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50492328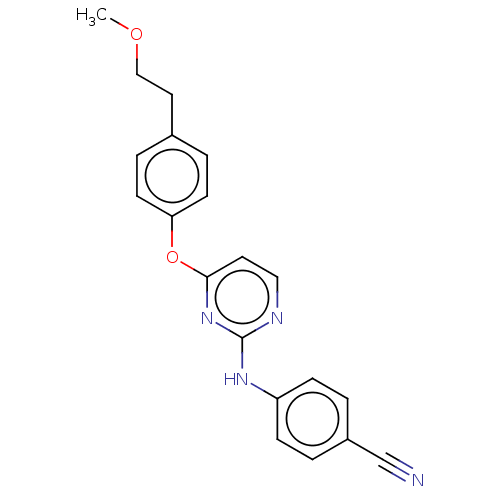 (CHEMBL2402857) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.69E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University Curated by ChEMBL | Assay Description Inhibition of recombinant wild type HIV1 RT using poly(rA)/oligo(dT)16 as template after 40 mins by spectrofluorometric analysis | Eur J Med Chem 65: 134-43 (2013) Article DOI: 10.1016/j.ejmech.2013.04.052 BindingDB Entry DOI: 10.7270/Q2736TTS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM1434 (11-cyclopropyl-5,11-dihydro-4-methyl-6H-dipyrido[3...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 2.74E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University Curated by ChEMBL | Assay Description Inhibition of recombinant wild type HIV1 RT using poly(rA)/oligo(dT)16 as template after 40 mins by spectrofluorometric analysis | Eur J Med Chem 65: 134-43 (2013) Article DOI: 10.1016/j.ejmech.2013.04.052 BindingDB Entry DOI: 10.7270/Q2736TTS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50492334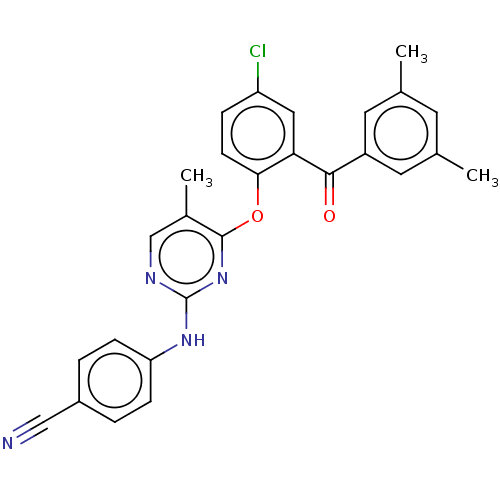 (CHEMBL2402963) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.06E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University Curated by ChEMBL | Assay Description Inhibition of recombinant wild type HIV1 RT using poly(rA)/oligo(dT)16 as template after 40 mins by spectrofluorometric analysis | Eur J Med Chem 65: 134-43 (2013) Article DOI: 10.1016/j.ejmech.2013.04.052 BindingDB Entry DOI: 10.7270/Q2736TTS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50492327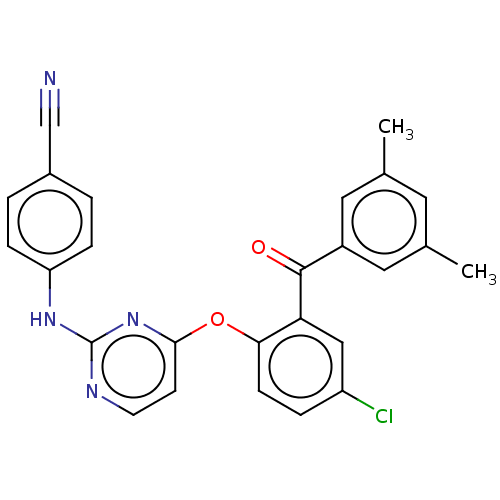 (CHEMBL2402852) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.22E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University Curated by ChEMBL | Assay Description Inhibition of recombinant wild type HIV1 RT using poly(rA)/oligo(dT)16 as template after 40 mins by spectrofluorometric analysis | Eur J Med Chem 65: 134-43 (2013) Article DOI: 10.1016/j.ejmech.2013.04.052 BindingDB Entry DOI: 10.7270/Q2736TTS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50492326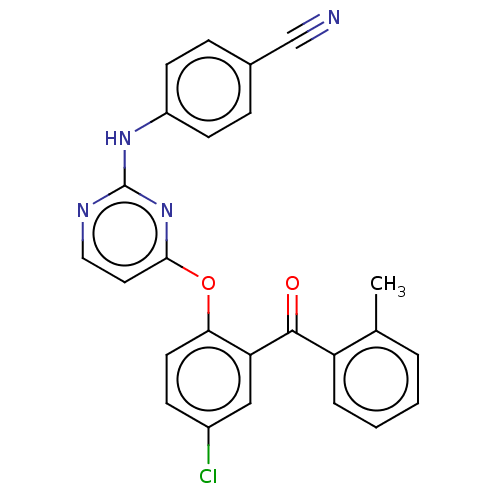 (CHEMBL2402853) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.45E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University Curated by ChEMBL | Assay Description Inhibition of recombinant wild type HIV1 RT using poly(rA)/oligo(dT)16 as template after 40 mins by spectrofluorometric analysis | Eur J Med Chem 65: 134-43 (2013) Article DOI: 10.1016/j.ejmech.2013.04.052 BindingDB Entry DOI: 10.7270/Q2736TTS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50492336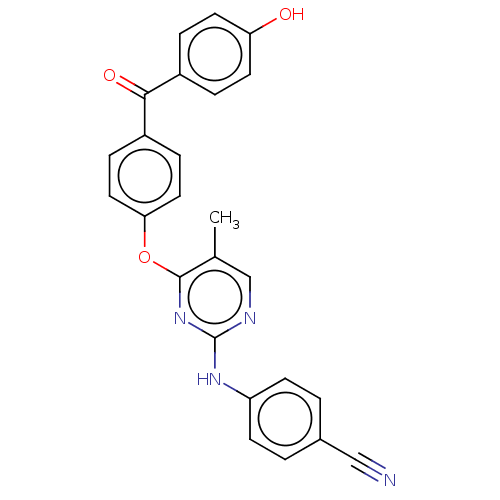 (CHEMBL2402966) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.21E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University Curated by ChEMBL | Assay Description Inhibition of recombinant wild type HIV1 RT using poly(rA)/oligo(dT)16 as template after 40 mins by spectrofluorometric analysis | Eur J Med Chem 65: 134-43 (2013) Article DOI: 10.1016/j.ejmech.2013.04.052 BindingDB Entry DOI: 10.7270/Q2736TTS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50492325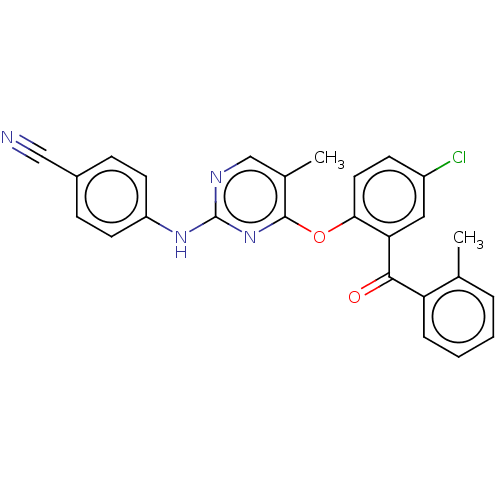 (CHEMBL2402964) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.23E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University Curated by ChEMBL | Assay Description Inhibition of recombinant wild type HIV1 RT using poly(rA)/oligo(dT)16 as template after 40 mins by spectrofluorometric analysis | Eur J Med Chem 65: 134-43 (2013) Article DOI: 10.1016/j.ejmech.2013.04.052 BindingDB Entry DOI: 10.7270/Q2736TTS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50492332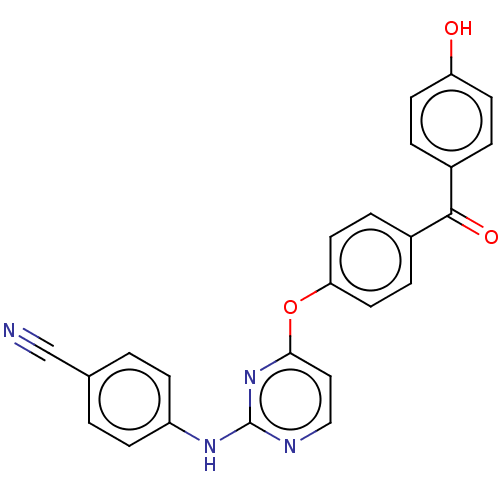 (CHEMBL2402855) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7.13E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University Curated by ChEMBL | Assay Description Inhibition of recombinant wild type HIV1 RT using poly(rA)/oligo(dT)16 as template after 40 mins by spectrofluorometric analysis | Eur J Med Chem 65: 134-43 (2013) Article DOI: 10.1016/j.ejmech.2013.04.052 BindingDB Entry DOI: 10.7270/Q2736TTS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50492335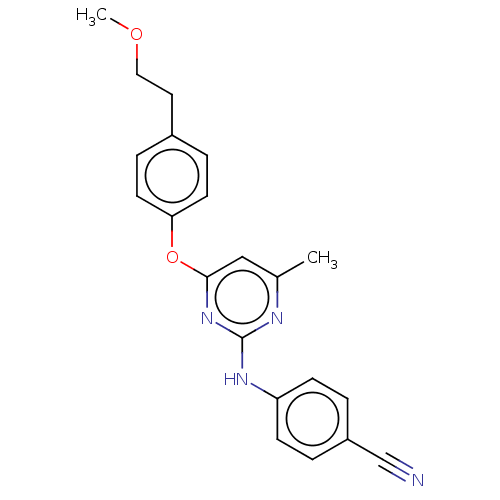 (CHEMBL2402961) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.16E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University Curated by ChEMBL | Assay Description Inhibition of recombinant wild type HIV1 RT using poly(rA)/oligo(dT)16 as template after 40 mins by spectrofluorometric analysis | Eur J Med Chem 65: 134-43 (2013) Article DOI: 10.1016/j.ejmech.2013.04.052 BindingDB Entry DOI: 10.7270/Q2736TTS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50492331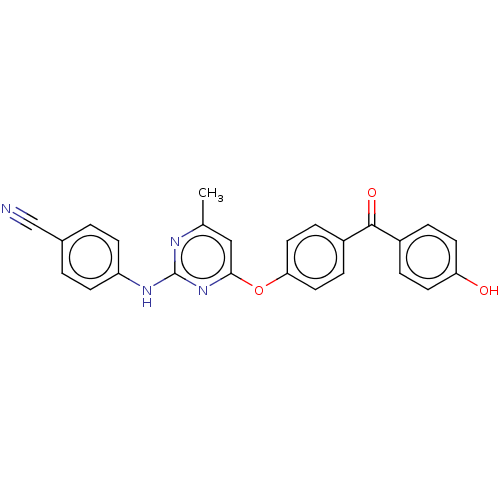 (CHEMBL2402959) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.69E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University Curated by ChEMBL | Assay Description Inhibition of recombinant wild type HIV1 RT using poly(rA)/oligo(dT)16 as template after 40 mins by spectrofluorometric analysis | Eur J Med Chem 65: 134-43 (2013) Article DOI: 10.1016/j.ejmech.2013.04.052 BindingDB Entry DOI: 10.7270/Q2736TTS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50492330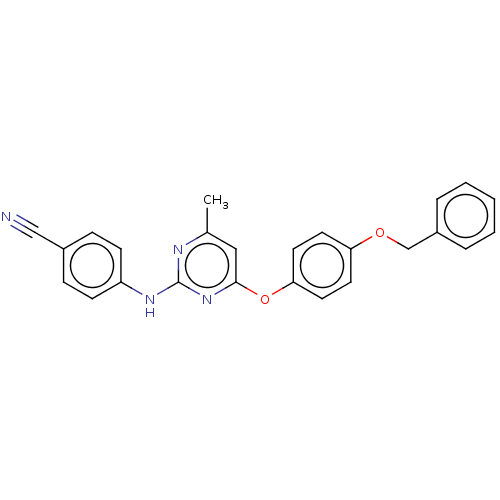 (CHEMBL2402960) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.74E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University Curated by ChEMBL | Assay Description Inhibition of recombinant wild type HIV1 RT using poly(rA)/oligo(dT)16 as template after 40 mins by spectrofluorometric analysis | Eur J Med Chem 65: 134-43 (2013) Article DOI: 10.1016/j.ejmech.2013.04.052 BindingDB Entry DOI: 10.7270/Q2736TTS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50492333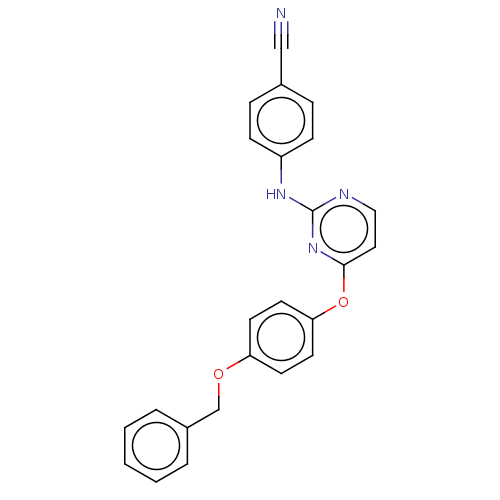 (CHEMBL2402856) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University Curated by ChEMBL | Assay Description Inhibition of recombinant wild type HIV1 RT using poly(rA)/oligo(dT)16 as template after 40 mins by spectrofluorometric analysis | Eur J Med Chem 65: 134-43 (2013) Article DOI: 10.1016/j.ejmech.2013.04.052 BindingDB Entry DOI: 10.7270/Q2736TTS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase protein (Human immunodeficiency virus 1) | BDBM1944 (BHAP deriv. | CHEMBL593 | DELAVIRDINE MESYLATE | D...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
Fudan University Curated by ChEMBL | Assay Description Inhibition of HIV RT K103N/Y181C mutant by cell based assay | Eur J Med Chem 65: 134-43 (2013) Article DOI: 10.1016/j.ejmech.2013.04.052 BindingDB Entry DOI: 10.7270/Q2736TTS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase protein (Human immunodeficiency virus 1) | BDBM1434 (11-cyclopropyl-5,11-dihydro-4-methyl-6H-dipyrido[3...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
Fudan University Curated by ChEMBL | Assay Description Inhibition of HIV RT K103N/Y181C mutant by cell based assay | Eur J Med Chem 65: 134-43 (2013) Article DOI: 10.1016/j.ejmech.2013.04.052 BindingDB Entry DOI: 10.7270/Q2736TTS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase protein (Human immunodeficiency virus 1) | BDBM222178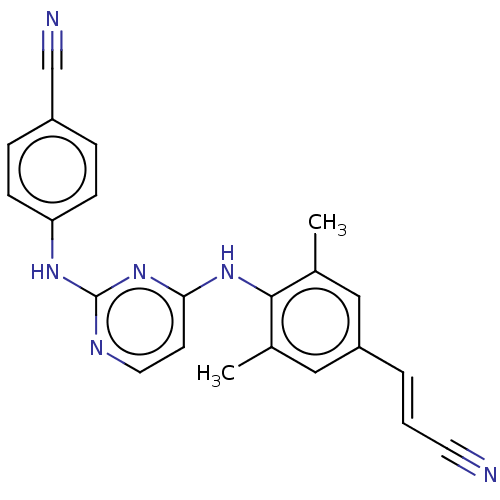 (Rilpivirine) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase DrugBank MCE PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a |
Fudan University Curated by ChEMBL | Assay Description Inhibition of HIV RT K103N/Y181C mutant by cell based assay | Eur J Med Chem 65: 134-43 (2013) Article DOI: 10.1016/j.ejmech.2013.04.052 BindingDB Entry DOI: 10.7270/Q2736TTS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase protein (Human immunodeficiency virus 1) | BDBM50103642 (4-(6-amino-5-bromo-2-(4-cyanophenylamino)pyrimidin...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 5 | n/a | n/a | n/a | n/a |
Fudan University Curated by ChEMBL | Assay Description Inhibition of HIV RT K103N/Y181C mutant by cell based assay | Eur J Med Chem 65: 134-43 (2013) Article DOI: 10.1016/j.ejmech.2013.04.052 BindingDB Entry DOI: 10.7270/Q2736TTS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||