

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cathepsin B (Homo sapiens (Human)) | BDBM50069984 ((R)-1-((S)-2-((S)-2-(benzyloxycarbonyl)-4-methylpe...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PubMed | 6.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ProScript, Inc. Curated by ChEMBL | Assay Description Inhibitory activity against Cathepsin B | Bioorg Med Chem Lett 8: 333-8 (1999) BindingDB Entry DOI: 10.7270/Q2RV0MVQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsinogen B (Homo sapiens (Human)) | BDBM50069989 ((R)-3-methyl-1-((S)-3-phenyl-2-(pyrazine-2-carboxa...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | 320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ProScript, Inc. Curated by ChEMBL | Assay Description Inhibitory activity against human Chymotrypsinogen | Bioorg Med Chem Lett 8: 333-8 (1999) BindingDB Entry DOI: 10.7270/Q2RV0MVQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin G (Homo sapiens (Human)) | BDBM50069989 ((R)-3-methyl-1-((S)-3-phenyl-2-(pyrazine-2-carboxa...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | 630 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ProScript, Inc. Curated by ChEMBL | Assay Description Inhibitory activity against human cathepsin G | Bioorg Med Chem Lett 8: 333-8 (1999) BindingDB Entry DOI: 10.7270/Q2RV0MVQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50069989 ((R)-3-methyl-1-((S)-3-phenyl-2-(pyrazine-2-carboxa...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | 2.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ProScript, Inc. Curated by ChEMBL | Assay Description Inhibitory activity against human leukocyte elastase | Bioorg Med Chem Lett 8: 333-8 (1999) BindingDB Entry DOI: 10.7270/Q2RV0MVQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50069989 ((R)-3-methyl-1-((S)-3-phenyl-2-(pyrazine-2-carboxa...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | 1.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ProScript, Inc. Curated by ChEMBL | Assay Description Inhibitory activity against thrombin | Bioorg Med Chem Lett 8: 333-8 (1999) BindingDB Entry DOI: 10.7270/Q2RV0MVQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prokineticin-2 (Homo sapiens (Human)) | BDBM121129 (A457 | US8722896, (+/-)-1-Benzyl-N-(9-chloro-3,4- ...) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 40 | n/a | 7.80 | n/a | n/a | 7.5 | 37 |
Central South University; Changsha Medical University | Assay Description Briefly, Chinese hamster ovary cells stably expressing the photoprotein aequorin were transiently transfected with WT or respective mutant PKR2-expre... | J Biol Chem 289: 15518-26 (2014) Article DOI: 10.1074/jbc.M114.556381 BindingDB Entry DOI: 10.7270/Q2H9943S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prokineticin receptor 2 (Homo sapiens (Human)) | BDBM121129 (A457 | US8722896, (+/-)-1-Benzyl-N-(9-chloro-3,4- ...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 102 | n/a | n/a | n/a | n/a | 7.5 | 37 |
Central South University; Changsha Medical University | Assay Description Briefly, Chinese hamster ovary cells stably expressing the photoprotein aequorin were transiently transfected with WT or respective mutant PKR2-expre... | J Biol Chem 289: 15518-26 (2014) Article DOI: 10.1074/jbc.M114.556381 BindingDB Entry DOI: 10.7270/Q2H9943S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| N(G),N(G)-dimethylarginine dimethylaminohydrolase 1 (Homo sapiens (Human)) | BDBM155523 (US9011882, Table 1, Compound 18) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 3.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The Board of Trustees of the Leland Stanford Junior University US Patent | Assay Description The L-citrulline assay was based upon an original test-tube method developed by Prescott and Jones in 1969 (Prescott, L. M. & Jones, M. E. Modified m... | US Patent US9011882 (2015) BindingDB Entry DOI: 10.7270/Q2W957XP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| N(G),N(G)-dimethylarginine dimethylaminohydrolase 1 (Homo sapiens (Human)) | BDBM50389868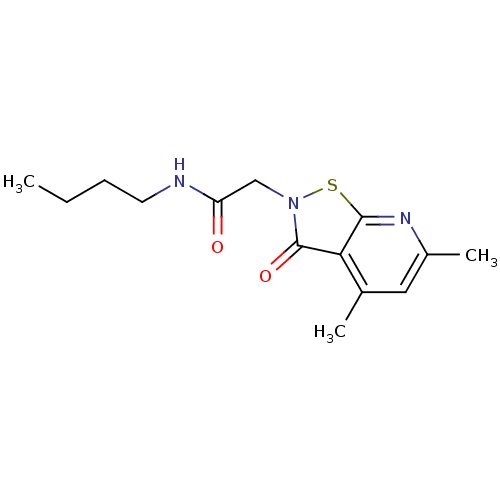 (CHEMBL2070857 | US9011882, Table 1, Compound 16) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 5.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The Board of Trustees of the Leland Stanford Junior University US Patent | Assay Description The L-citrulline assay was based upon an original test-tube method developed by Prescott and Jones in 1969 (Prescott, L. M. & Jones, M. E. Modified m... | US Patent US9011882 (2015) BindingDB Entry DOI: 10.7270/Q2W957XP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| N(G),N(G)-dimethylarginine dimethylaminohydrolase 1 (Homo sapiens (Human)) | BDBM155517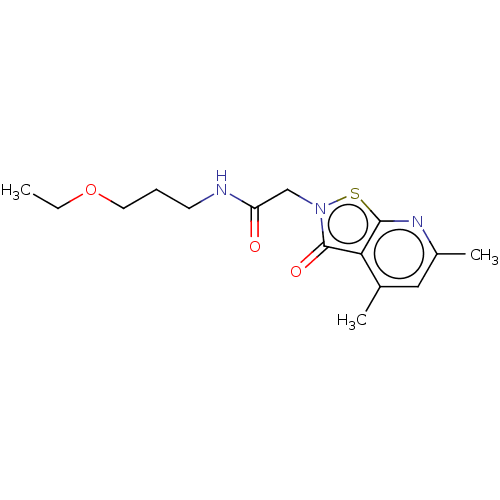 (US9011882, Table 1, Compound 10) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 7.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The Board of Trustees of the Leland Stanford Junior University US Patent | Assay Description The L-citrulline assay was based upon an original test-tube method developed by Prescott and Jones in 1969 (Prescott, L. M. & Jones, M. E. Modified m... | US Patent US9011882 (2015) BindingDB Entry DOI: 10.7270/Q2W957XP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| N(G),N(G)-dimethylarginine dimethylaminohydrolase 1 (Homo sapiens (Human)) | BDBM155515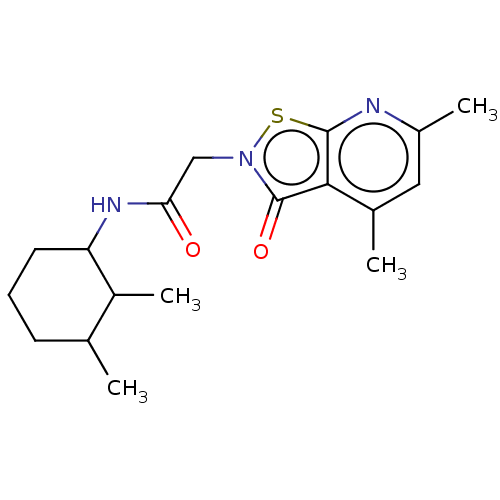 (US9011882, Table 1, Compound 8) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 8.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The Board of Trustees of the Leland Stanford Junior University US Patent | Assay Description The L-citrulline assay was based upon an original test-tube method developed by Prescott and Jones in 1969 (Prescott, L. M. & Jones, M. E. Modified m... | US Patent US9011882 (2015) BindingDB Entry DOI: 10.7270/Q2W957XP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| N(G),N(G)-dimethylarginine dimethylaminohydrolase 1 (Homo sapiens (Human)) | BDBM34513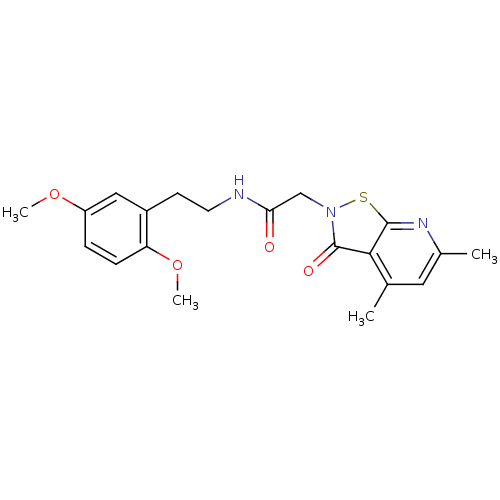 (MLS000118887 | N-[2-(2,5-dimethoxyphenyl)ethyl]-2-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 9.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The Board of Trustees of the Leland Stanford Junior University US Patent | Assay Description The L-citrulline assay was based upon an original test-tube method developed by Prescott and Jones in 1969 (Prescott, L. M. & Jones, M. E. Modified m... | US Patent US9011882 (2015) BindingDB Entry DOI: 10.7270/Q2W957XP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| N(G),N(G)-dimethylarginine dimethylaminohydrolase 1 (Homo sapiens (Human)) | BDBM155510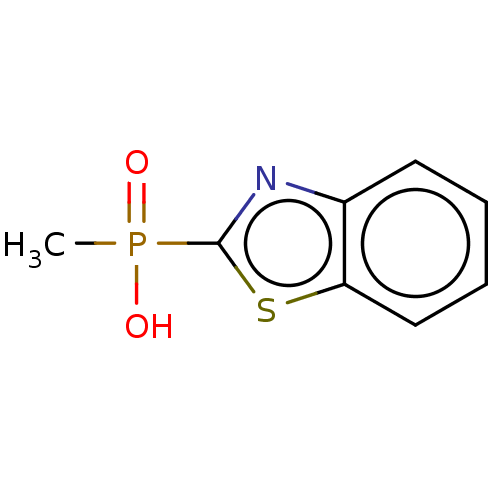 (US9011882, Table 1, Compound 3) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | US Patent | n/a | n/a | 9.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The Board of Trustees of the Leland Stanford Junior University US Patent | Assay Description The L-citrulline assay was based upon an original test-tube method developed by Prescott and Jones in 1969 (Prescott, L. M. & Jones, M. E. Modified m... | US Patent US9011882 (2015) BindingDB Entry DOI: 10.7270/Q2W957XP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| N(G),N(G)-dimethylarginine dimethylaminohydrolase 1 (Homo sapiens (Human)) | BDBM155521 (US9011882, Table 1, Compound 14) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.05E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
The Board of Trustees of the Leland Stanford Junior University US Patent | Assay Description The L-citrulline assay was based upon an original test-tube method developed by Prescott and Jones in 1969 (Prescott, L. M. & Jones, M. E. Modified m... | US Patent US9011882 (2015) BindingDB Entry DOI: 10.7270/Q2W957XP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| N(G),N(G)-dimethylarginine dimethylaminohydrolase 1 (Homo sapiens (Human)) | BDBM155519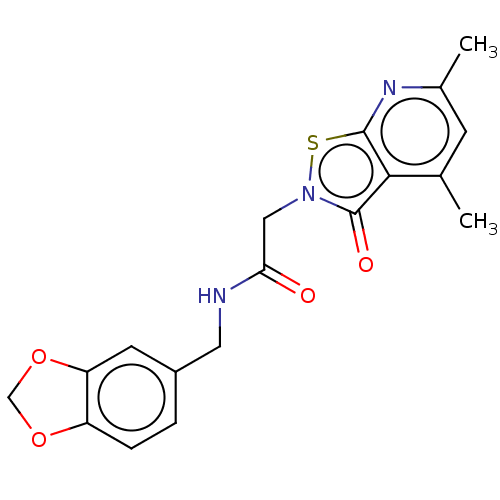 (US9011882, Table 1, Compound 12) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
The Board of Trustees of the Leland Stanford Junior University US Patent | Assay Description The L-citrulline assay was based upon an original test-tube method developed by Prescott and Jones in 1969 (Prescott, L. M. & Jones, M. E. Modified m... | US Patent US9011882 (2015) BindingDB Entry DOI: 10.7270/Q2W957XP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| N(G),N(G)-dimethylarginine dimethylaminohydrolase 1 (Homo sapiens (Human)) | BDBM155522 (US9011882, Table 1, Compound 17) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.18E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
The Board of Trustees of the Leland Stanford Junior University US Patent | Assay Description The L-citrulline assay was based upon an original test-tube method developed by Prescott and Jones in 1969 (Prescott, L. M. & Jones, M. E. Modified m... | US Patent US9011882 (2015) BindingDB Entry DOI: 10.7270/Q2W957XP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| N(G),N(G)-dimethylarginine dimethylaminohydrolase 1 (Homo sapiens (Human)) | BDBM155516 (US9011882, Table 1, Compound 9) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.28E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
The Board of Trustees of the Leland Stanford Junior University US Patent | Assay Description The L-citrulline assay was based upon an original test-tube method developed by Prescott and Jones in 1969 (Prescott, L. M. & Jones, M. E. Modified m... | US Patent US9011882 (2015) BindingDB Entry DOI: 10.7270/Q2W957XP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| N(G),N(G)-dimethylarginine dimethylaminohydrolase 1 (Homo sapiens (Human)) | BDBM155508 (US9011882, Table 1, Compound 1) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.31E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
The Board of Trustees of the Leland Stanford Junior University US Patent | Assay Description The L-citrulline assay was based upon an original test-tube method developed by Prescott and Jones in 1969 (Prescott, L. M. & Jones, M. E. Modified m... | US Patent US9011882 (2015) BindingDB Entry DOI: 10.7270/Q2W957XP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| N(G),N(G)-dimethylarginine dimethylaminohydrolase 1 (Homo sapiens (Human)) | BDBM46658 (1,2-benzothiazol-3-one | MLS-0254244.0001 | US9011...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Patents | US Patent | n/a | n/a | 1.32E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
The Board of Trustees of the Leland Stanford Junior University US Patent | Assay Description The L-citrulline assay was based upon an original test-tube method developed by Prescott and Jones in 1969 (Prescott, L. M. & Jones, M. E. Modified m... | US Patent US9011882 (2015) BindingDB Entry DOI: 10.7270/Q2W957XP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| N(G),N(G)-dimethylarginine dimethylaminohydrolase 1 (Homo sapiens (Human)) | BDBM155520 (US9011882, Table 1, Compound 13) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.34E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
The Board of Trustees of the Leland Stanford Junior University US Patent | Assay Description The L-citrulline assay was based upon an original test-tube method developed by Prescott and Jones in 1969 (Prescott, L. M. & Jones, M. E. Modified m... | US Patent US9011882 (2015) BindingDB Entry DOI: 10.7270/Q2W957XP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| N(G),N(G)-dimethylarginine dimethylaminohydrolase 1 (Homo sapiens (Human)) | BDBM155514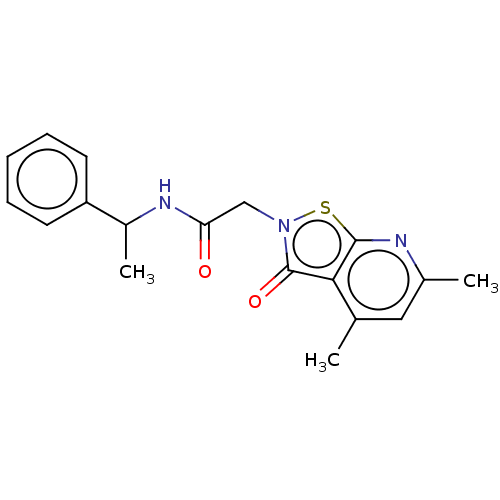 (US9011882, Table 1, Compound 7) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.37E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
The Board of Trustees of the Leland Stanford Junior University US Patent | Assay Description The L-citrulline assay was based upon an original test-tube method developed by Prescott and Jones in 1969 (Prescott, L. M. & Jones, M. E. Modified m... | US Patent US9011882 (2015) BindingDB Entry DOI: 10.7270/Q2W957XP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| N(G),N(G)-dimethylarginine dimethylaminohydrolase 1 (Homo sapiens (Human)) | BDBM155518 (US9011882, Table 1, Compound 11) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.41E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
The Board of Trustees of the Leland Stanford Junior University US Patent | Assay Description The L-citrulline assay was based upon an original test-tube method developed by Prescott and Jones in 1969 (Prescott, L. M. & Jones, M. E. Modified m... | US Patent US9011882 (2015) BindingDB Entry DOI: 10.7270/Q2W957XP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| N(G),N(G)-dimethylarginine dimethylaminohydrolase 1 (Homo sapiens (Human)) | BDBM155512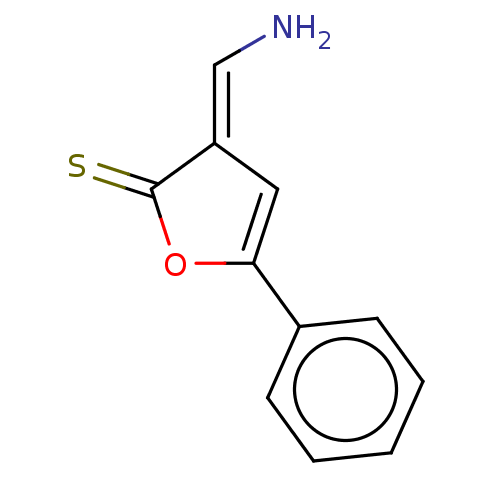 (US9011882, Table 1, Compound 5) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.77E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
The Board of Trustees of the Leland Stanford Junior University US Patent | Assay Description The L-citrulline assay was based upon an original test-tube method developed by Prescott and Jones in 1969 (Prescott, L. M. & Jones, M. E. Modified m... | US Patent US9011882 (2015) BindingDB Entry DOI: 10.7270/Q2W957XP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| N(G),N(G)-dimethylarginine dimethylaminohydrolase 1 (Homo sapiens (Human)) | BDBM155511 (US9011882, Table 1, Compound 4) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | US Patent | n/a | n/a | 2.12E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
The Board of Trustees of the Leland Stanford Junior University US Patent | Assay Description The L-citrulline assay was based upon an original test-tube method developed by Prescott and Jones in 1969 (Prescott, L. M. & Jones, M. E. Modified m... | US Patent US9011882 (2015) BindingDB Entry DOI: 10.7270/Q2W957XP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| N(G),N(G)-dimethylarginine dimethylaminohydrolase 1 (Homo sapiens (Human)) | BDBM155509 (US9011882, Table 1, Compound 2) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 2.33E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
The Board of Trustees of the Leland Stanford Junior University US Patent | Assay Description The L-citrulline assay was based upon an original test-tube method developed by Prescott and Jones in 1969 (Prescott, L. M. & Jones, M. E. Modified m... | US Patent US9011882 (2015) BindingDB Entry DOI: 10.7270/Q2W957XP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| N(G),N(G)-dimethylarginine dimethylaminohydrolase 1 (Homo sapiens (Human)) | BDBM155524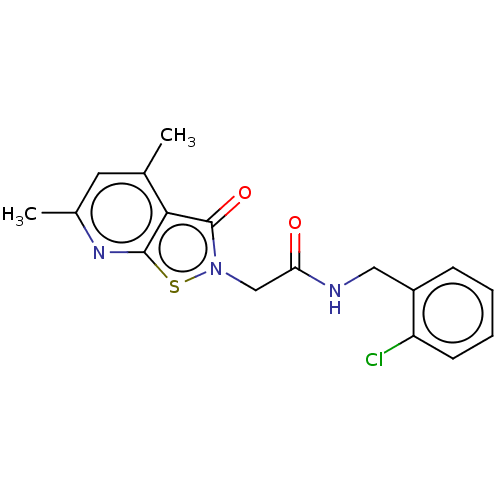 (US9011882, Table 1, Compound 19) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 3.99E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
The Board of Trustees of the Leland Stanford Junior University US Patent | Assay Description The L-citrulline assay was based upon an original test-tube method developed by Prescott and Jones in 1969 (Prescott, L. M. & Jones, M. E. Modified m... | US Patent US9011882 (2015) BindingDB Entry DOI: 10.7270/Q2W957XP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| N(G),N(G)-dimethylarginine dimethylaminohydrolase 1 (Homo sapiens (Human)) | BDBM155513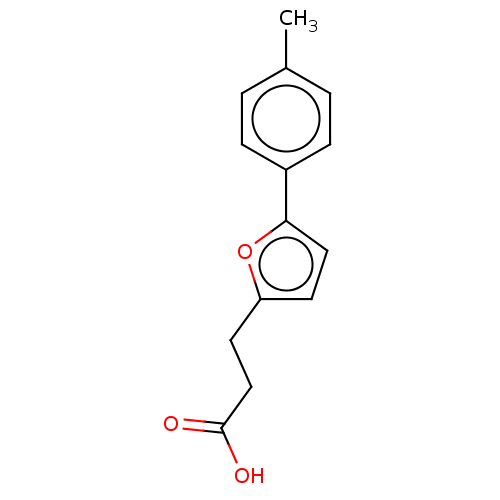 (US9011882, Table 1, Compound 6) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 4.58E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
The Board of Trustees of the Leland Stanford Junior University US Patent | Assay Description The L-citrulline assay was based upon an original test-tube method developed by Prescott and Jones in 1969 (Prescott, L. M. & Jones, M. E. Modified m... | US Patent US9011882 (2015) BindingDB Entry DOI: 10.7270/Q2W957XP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||