 Found 461 hits with Last Name = 'mackerell' and Initial = 'ad'
Found 461 hits with Last Name = 'mackerell' and Initial = 'ad' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Mu-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50000787

((1S,5R,13R,17S)-4-(cyclopropylmethyl)-10,17-dihydr...)Show SMILES Oc1ccc2C[C@H]3N(CC4CC4)CC[C@@]45[C@@H](Oc1c24)C(=O)CC[C@@]35O |r| Show InChI InChI=1S/C20H23NO4/c22-13-4-3-12-9-15-20(24)6-5-14(23)18-19(20,16(12)17(13)25-18)7-8-21(15)10-11-1-2-11/h3-4,11,15,18,22,24H,1-2,5-10H2/t15-,18+,19+,20-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.460 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Maryland
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from mu opioid receptor in rat brain homogenate after 1 hr by liquid scintillation counting |
Bioorg Med Chem 20: 4556-63 (2012)
Article DOI: 10.1016/j.bmc.2012.05.006
BindingDB Entry DOI: 10.7270/Q2DJ5GPH |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50000787

((1S,5R,13R,17S)-4-(cyclopropylmethyl)-10,17-dihydr...)Show SMILES Oc1ccc2C[C@H]3N(CC4CC4)CC[C@@]45[C@@H](Oc1c24)C(=O)CC[C@@]35O |r| Show InChI InChI=1S/C20H23NO4/c22-13-4-3-12-9-15-20(24)6-5-14(23)18-19(20,16(12)17(13)25-18)7-8-21(15)10-11-1-2-11/h3-4,11,15,18,22,24H,1-2,5-10H2/t15-,18+,19+,20-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.07 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Maryland
Curated by ChEMBL
| Assay Description
Displacement of [3H]U69593 from kappa opioid receptor in rat brain homogenate after 1 hr by liquid scintillation counting |
Bioorg Med Chem 20: 4556-63 (2012)
Article DOI: 10.1016/j.bmc.2012.05.006
BindingDB Entry DOI: 10.7270/Q2DJ5GPH |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50000787

((1S,5R,13R,17S)-4-(cyclopropylmethyl)-10,17-dihydr...)Show SMILES Oc1ccc2C[C@H]3N(CC4CC4)CC[C@@]45[C@@H](Oc1c24)C(=O)CC[C@@]35O |r| Show InChI InChI=1S/C20H23NO4/c22-13-4-3-12-9-15-20(24)6-5-14(23)18-19(20,16(12)17(13)25-18)7-8-21(15)10-11-1-2-11/h3-4,11,15,18,22,24H,1-2,5-10H2/t15-,18+,19+,20-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Maryland
Curated by ChEMBL
| Assay Description
Displacement of [3H]DPDPE from delta opioid receptor in rat brain homogenate after 1 hr by liquid scintillation counting |
Bioorg Med Chem 20: 4556-63 (2012)
Article DOI: 10.1016/j.bmc.2012.05.006
BindingDB Entry DOI: 10.7270/Q2DJ5GPH |
More data for this
Ligand-Target Pair | |
Induced myeloid leukemia cell differentiation protein Mcl-1
(Homo sapiens (Human)) | BDBM50158355
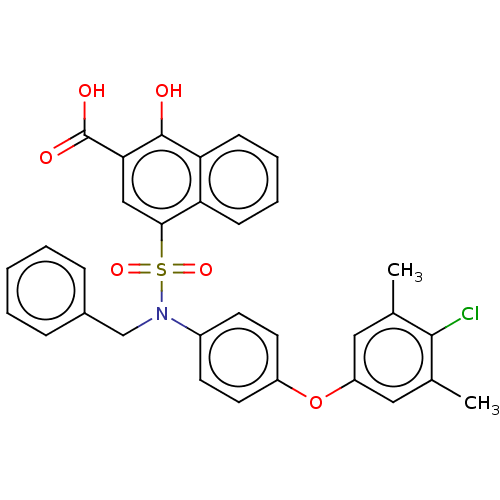
(CHEMBL3781822 | US10858316, Compound 3bl)Show SMILES Cc1cc(Oc2ccc(cc2)N(Cc2ccccc2)S(=O)(=O)c2cc(C(O)=O)c(O)c3ccccc23)cc(C)c1Cl Show InChI InChI=1S/C32H26ClNO6S/c1-20-16-25(17-21(2)30(20)33)40-24-14-12-23(13-15-24)34(19-22-8-4-3-5-9-22)41(38,39)29-18-28(32(36)37)31(35)27-11-7-6-10-26(27)29/h3-18,35H,19H2,1-2H3,(H,36,37) | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 31 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Maryland
Curated by ChEMBL
| Assay Description
Inhibition of His6-MBP tagged recombinant human Mcl-1 residues 172 to 327 expressed in Escherichia coli assessed as inhibition of interaction with Ba... |
Eur J Med Chem 113: 273-92 (2016)
Article DOI: 10.1016/j.ejmech.2016.02.006
BindingDB Entry DOI: 10.7270/Q2HX1FJH |
More data for this
Ligand-Target Pair | |
Induced myeloid leukemia cell differentiation protein Mcl-1
(Homo sapiens (Human)) | BDBM50158354
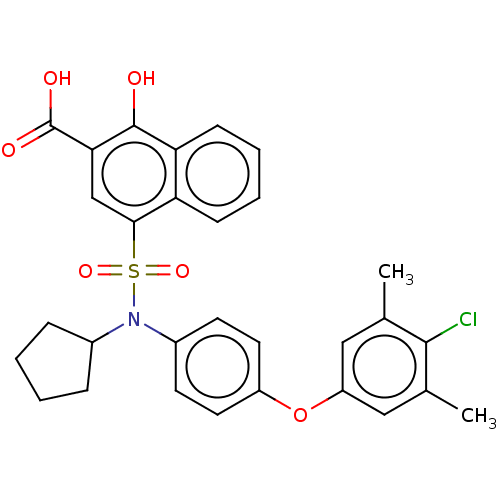
(CHEMBL3780397)Show SMILES Cc1cc(Oc2ccc(cc2)N(C2CCCC2)S(=O)(=O)c2cc(C(O)=O)c(O)c3ccccc23)cc(C)c1Cl Show InChI InChI=1S/C30H28ClNO6S/c1-18-15-23(16-19(2)28(18)31)38-22-13-11-21(12-14-22)32(20-7-3-4-8-20)39(36,37)27-17-26(30(34)35)29(33)25-10-6-5-9-24(25)27/h5-6,9-17,20,33H,3-4,7-8H2,1-2H3,(H,34,35) | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 33 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Maryland
Curated by ChEMBL
| Assay Description
Inhibition of His6-MBP tagged recombinant human Mcl-1 residues 172 to 327 expressed in Escherichia coli assessed as inhibition of interaction with Ba... |
Eur J Med Chem 113: 273-92 (2016)
Article DOI: 10.1016/j.ejmech.2016.02.006
BindingDB Entry DOI: 10.7270/Q2HX1FJH |
More data for this
Ligand-Target Pair | |
Induced myeloid leukemia cell differentiation protein Mcl-1
(Homo sapiens (Human)) | BDBM50589742
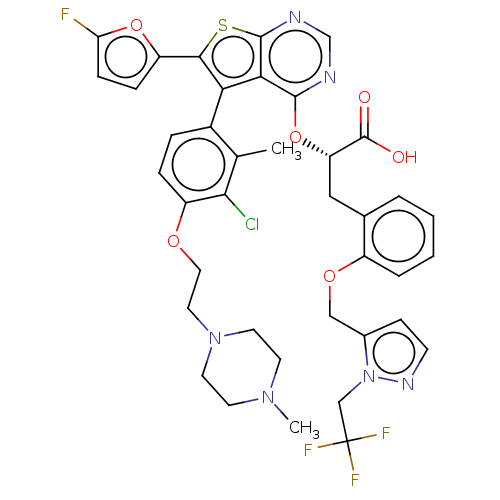
(CHEMBL5173872)Show SMILES CN1CCN(CCOc2ccc(-c3c(sc4ncnc(O[C@@H](Cc5ccccc5OCc5ccnn5CC(F)(F)F)C(O)=O)c34)-c3ccc(F)o3)c(C)c2Cl)CC1 |r,wU:21.21,(.89,-8.59,;.6,-7.39,;1.71,-6.33,;1.35,-4.83,;-.13,-4.4,;-.49,-2.9,;.63,-1.84,;.27,-.34,;1.38,.72,;1.02,2.22,;2.13,3.28,;3.61,2.84,;4.64,3.99,;6.17,3.83,;6.8,5.23,;5.66,6.26,;5.66,7.8,;4.32,8.57,;2.99,7.8,;2.99,6.26,;1.65,5.5,;.32,6.27,;-1.02,5.5,;-2.35,6.27,;-2.35,7.81,;-3.67,8.59,;-5.01,7.82,;-5.01,6.28,;-3.69,5.51,;-3.69,3.97,;-5.04,3.2,;-5.04,1.66,;-3.8,.78,;-4.28,-.69,;-5.82,-.69,;-6.29,.78,;-7.75,1.27,;-8.9,.25,;-9.83,-.56,;-8.66,-.95,;-10.07,.64,;.32,7.81,;1.39,8.43,;-.75,8.43,;4.32,5.49,;6.95,2.49,;6.42,1.07,;7.64,.13,;8.91,1,;10.07,.59,;8.47,2.49,;3.98,1.35,;5.16,1.01,;2.86,.29,;3.15,-.91,;-1.24,-5.46,;-.88,-6.96,)| | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 39 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1039/d2md00095d
BindingDB Entry DOI: 10.7270/Q2XK8KHN |
More data for this
Ligand-Target Pair | |
Bcl-2-like protein 1
(Homo sapiens (Human)) | BDBM21447

(4-(4-{[2-(4-chlorophenyl)phenyl]methyl}piperazin-1...)Show SMILES CN(C)CC[C@H](CSc1ccccc1)Nc1ccc(cc1[N+]([O-])=O)S(=O)(=O)NC(=O)c1ccc(cc1)N1CCN(Cc2ccccc2-c2ccc(Cl)cc2)CC1 |r| Show InChI InChI=1S/C42H45ClN6O5S2/c1-46(2)23-22-35(30-55-37-9-4-3-5-10-37)44-40-21-20-38(28-41(40)49(51)52)56(53,54)45-42(50)32-14-18-36(19-15-32)48-26-24-47(25-27-48)29-33-8-6-7-11-39(33)31-12-16-34(43)17-13-31/h3-21,28,35,44H,22-27,29-30H2,1-2H3,(H,45,50)/t35-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| <40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1039/d2md00095d
BindingDB Entry DOI: 10.7270/Q2XK8KHN |
More data for this
Ligand-Target Pair | |
Apoptosis regulator Bcl-2
(Homo sapiens (Human)) | BDBM21447

(4-(4-{[2-(4-chlorophenyl)phenyl]methyl}piperazin-1...)Show SMILES CN(C)CC[C@H](CSc1ccccc1)Nc1ccc(cc1[N+]([O-])=O)S(=O)(=O)NC(=O)c1ccc(cc1)N1CCN(Cc2ccccc2-c2ccc(Cl)cc2)CC1 |r| Show InChI InChI=1S/C42H45ClN6O5S2/c1-46(2)23-22-35(30-55-37-9-4-3-5-10-37)44-40-21-20-38(28-41(40)49(51)52)56(53,54)45-42(50)32-14-18-36(19-15-32)48-26-24-47(25-27-48)29-33-8-6-7-11-39(33)31-12-16-34(43)17-13-31/h3-21,28,35,44H,22-27,29-30H2,1-2H3,(H,45,50)/t35-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| <40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1039/d2md00095d
BindingDB Entry DOI: 10.7270/Q2XK8KHN |
More data for this
Ligand-Target Pair | |
Induced myeloid leukemia cell differentiation protein Mcl-1
(Homo sapiens (Human)) | BDBM50158326
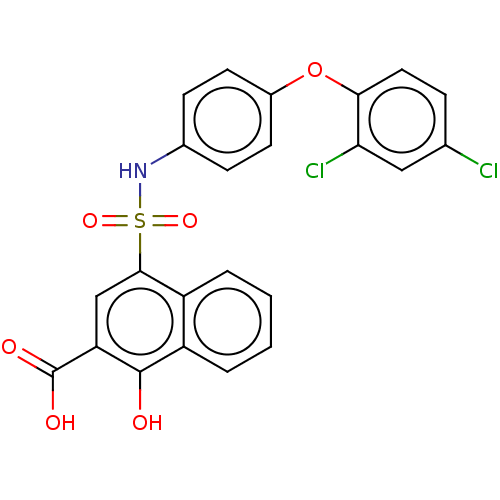
(CHEMBL3781669 | US10858316, Compound 3bg)Show SMILES OC(=O)c1cc(c2ccccc2c1O)S(=O)(=O)Nc1ccc(Oc2ccc(Cl)cc2Cl)cc1 Show InChI InChI=1S/C23H15Cl2NO6S/c24-13-5-10-20(19(25)11-13)32-15-8-6-14(7-9-15)26-33(30,31)21-12-18(23(28)29)22(27)17-4-2-1-3-16(17)21/h1-12,26-27H,(H,28,29) | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 79 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Maryland
Curated by ChEMBL
| Assay Description
Inhibition of His6-MBP tagged recombinant human Mcl-1 residues 172 to 327 expressed in Escherichia coli assessed as inhibition of interaction with Ba... |
Eur J Med Chem 113: 273-92 (2016)
Article DOI: 10.1016/j.ejmech.2016.02.006
BindingDB Entry DOI: 10.7270/Q2HX1FJH |
More data for this
Ligand-Target Pair | |
Induced myeloid leukemia cell differentiation protein Mcl-1
(Homo sapiens (Human)) | BDBM50158353
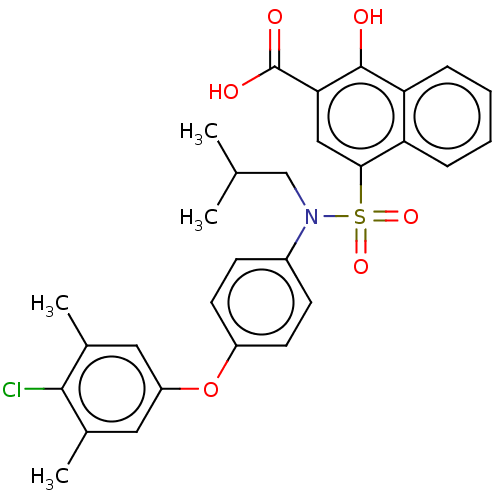
(CHEMBL3780803 | US10858316, Compound 3bj)Show SMILES CC(C)CN(c1ccc(Oc2cc(C)c(Cl)c(C)c2)cc1)S(=O)(=O)c1cc(C(O)=O)c(O)c2ccccc12 Show InChI InChI=1S/C29H28ClNO6S/c1-17(2)16-31(20-9-11-21(12-10-20)37-22-13-18(3)27(30)19(4)14-22)38(35,36)26-15-25(29(33)34)28(32)24-8-6-5-7-23(24)26/h5-15,17,32H,16H2,1-4H3,(H,33,34) | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Maryland
Curated by ChEMBL
| Assay Description
Inhibition of His6-MBP tagged recombinant human Mcl-1 residues 172 to 327 expressed in Escherichia coli assessed as inhibition of interaction with Ba... |
Eur J Med Chem 113: 273-92 (2016)
Article DOI: 10.1016/j.ejmech.2016.02.006
BindingDB Entry DOI: 10.7270/Q2HX1FJH |
More data for this
Ligand-Target Pair | |
Induced myeloid leukemia cell differentiation protein Mcl-1
(Homo sapiens (Human)) | BDBM50158322
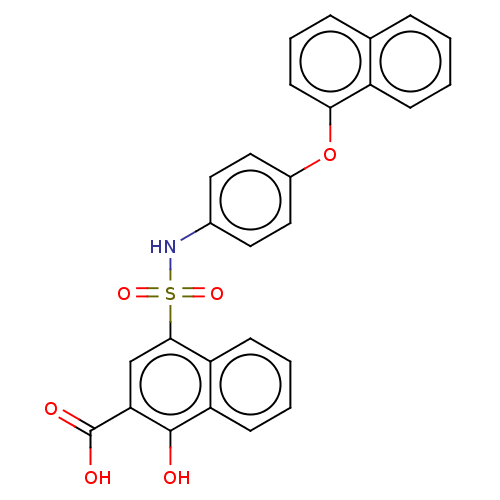
(CHEMBL3780225 | US10858316, Compound 3bd)Show SMILES OC(=O)c1cc(c2ccccc2c1O)S(=O)(=O)Nc1ccc(Oc2cccc3ccccc23)cc1 Show InChI InChI=1S/C27H19NO6S/c29-26-22-10-4-3-9-21(22)25(16-23(26)27(30)31)35(32,33)28-18-12-14-19(15-13-18)34-24-11-5-7-17-6-1-2-8-20(17)24/h1-16,28-29H,(H,30,31) | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 82 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Maryland
Curated by ChEMBL
| Assay Description
Inhibition of His6-MBP tagged recombinant human Mcl-1 residues 172 to 327 expressed in Escherichia coli assessed as inhibition of interaction with Ba... |
Eur J Med Chem 113: 273-92 (2016)
Article DOI: 10.1016/j.ejmech.2016.02.006
BindingDB Entry DOI: 10.7270/Q2HX1FJH |
More data for this
Ligand-Target Pair | |
Induced myeloid leukemia cell differentiation protein Mcl-1
(Homo sapiens (Human)) | BDBM50158324
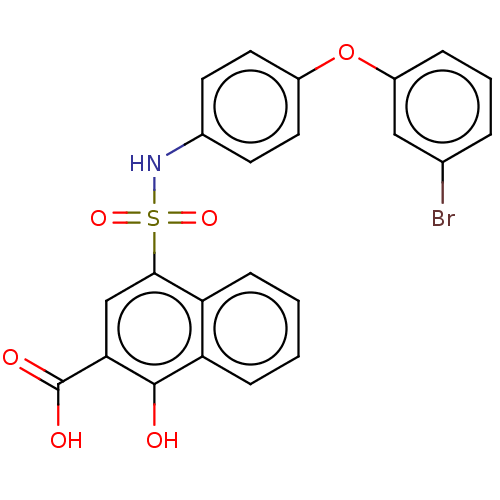
(CHEMBL3780311 | US10858316, Compound 3be)Show SMILES OC(=O)c1cc(c2ccccc2c1O)S(=O)(=O)Nc1ccc(Oc2cccc(Br)c2)cc1 Show InChI InChI=1S/C23H16BrNO6S/c24-14-4-3-5-17(12-14)31-16-10-8-15(9-11-16)25-32(29,30)21-13-20(23(27)28)22(26)19-7-2-1-6-18(19)21/h1-13,25-26H,(H,27,28) | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 114 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Maryland
Curated by ChEMBL
| Assay Description
Inhibition of His6-MBP tagged recombinant human Mcl-1 residues 172 to 327 expressed in Escherichia coli assessed as inhibition of interaction with Ba... |
Eur J Med Chem 113: 273-92 (2016)
Article DOI: 10.1016/j.ejmech.2016.02.006
BindingDB Entry DOI: 10.7270/Q2HX1FJH |
More data for this
Ligand-Target Pair | |
Induced myeloid leukemia cell differentiation protein Mcl-1
(Homo sapiens (Human)) | BDBM50158333

(CHEMBL3780473 | US10858316, Compound 3bi)Show SMILES Cc1cc(Oc2ccc(NS(=O)(=O)c3cc(C(O)=O)c(O)c4ccccc34)cc2)cc(C)c1Cl Show InChI InChI=1S/C25H20ClNO6S/c1-14-11-18(12-15(2)23(14)26)33-17-9-7-16(8-10-17)27-34(31,32)22-13-21(25(29)30)24(28)20-6-4-3-5-19(20)22/h3-13,27-28H,1-2H3,(H,29,30) | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 117 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Maryland
Curated by ChEMBL
| Assay Description
Inhibition of His6-MBP tagged recombinant human Mcl-1 residues 172 to 327 expressed in Escherichia coli assessed as inhibition of interaction with Ba... |
Eur J Med Chem 113: 273-92 (2016)
Article DOI: 10.1016/j.ejmech.2016.02.006
BindingDB Entry DOI: 10.7270/Q2HX1FJH |
More data for this
Ligand-Target Pair | |
Induced myeloid leukemia cell differentiation protein Mcl-1
(Homo sapiens (Human)) | BDBM50158332
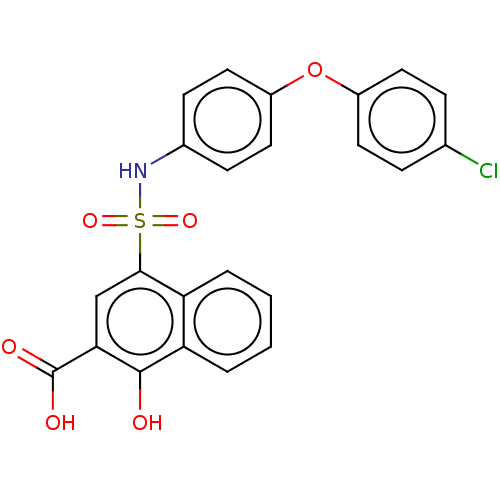
(CHEMBL3780719 | US10858316, Compound 3bh)Show SMILES OC(=O)c1cc(c2ccccc2c1O)S(=O)(=O)Nc1ccc(Oc2ccc(Cl)cc2)cc1 Show InChI InChI=1S/C23H16ClNO6S/c24-14-5-9-16(10-6-14)31-17-11-7-15(8-12-17)25-32(29,30)21-13-20(23(27)28)22(26)19-4-2-1-3-18(19)21/h1-13,25-26H,(H,27,28) | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 173 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Maryland
Curated by ChEMBL
| Assay Description
Inhibition of His6-MBP tagged recombinant human Mcl-1 residues 172 to 327 expressed in Escherichia coli assessed as inhibition of interaction with Ba... |
Eur J Med Chem 113: 273-92 (2016)
Article DOI: 10.1016/j.ejmech.2016.02.006
BindingDB Entry DOI: 10.7270/Q2HX1FJH |
More data for this
Ligand-Target Pair | |
Induced myeloid leukemia cell differentiation protein Mcl-1
(Homo sapiens (Human)) | BDBM50424732

(CHEMBL2314209)Show SMILES Cc1cc(OCCCc2c([nH]c3cc(Cl)ccc23)C(O)=O)cc(C)c1Cl Show InChI InChI=1S/C20H19Cl2NO3/c1-11-8-14(9-12(2)18(11)22)26-7-3-4-16-15-6-5-13(21)10-17(15)23-19(16)20(24)25/h5-6,8-10,23H,3-4,7H2,1-2H3,(H,24,25) | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 247 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1039/d2md00095d
BindingDB Entry DOI: 10.7270/Q2XK8KHN |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Ileal sodium/bile acid cotransporter
(Homo sapiens (Human)) | BDBM50322476

((2S)-5-(4-tert-butylphenylamino)-2-((4R)-4-((3R,7R...)Show SMILES C[C@H](CCC(=O)N[C@@H](CCC(=O)Nc1ccc(cc1)C(C)(C)C)C(O)=O)[C@H]1CC[C@H]2[C@@H]3[C@H](O)C[C@H]4C[C@H](O)CC[C@]4(C)[C@H]3CC[C@]12C |r| Show InChI InChI=1S/C39H60N2O6/c1-23(7-15-34(45)41-31(36(46)47)14-16-33(44)40-26-10-8-24(9-11-26)37(2,3)4)28-12-13-29-35-30(18-20-39(28,29)6)38(5)19-17-27(42)21-25(38)22-32(35)43/h8-11,23,25,27-32,35,42-43H,7,12-22H2,1-6H3,(H,40,44)(H,41,45)(H,46,47)/t23-,25-,27-,28-,29+,30+,31+,32-,35+,38+,39-/m1/s1 | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 275 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Maryland
Curated by ChEMBL
| Assay Description
Inhibition of human ASBT expressed in MDCK cells assessed as inhibition of [3H]taurocholic acid uptake after 10 mins by liquid scintillation counting |
J Med Chem 53: 4749-60 (2010)
Article DOI: 10.1021/jm1003683
BindingDB Entry DOI: 10.7270/Q2H70G0G |
More data for this
Ligand-Target Pair | |
Induced myeloid leukemia cell differentiation protein Mcl-1
(Homo sapiens (Human)) | BDBM50158325
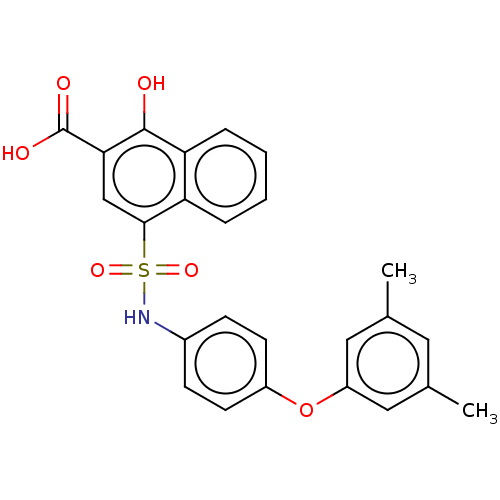
(CHEMBL3781894 | US10858316, Compound 3bf)Show SMILES Cc1cc(C)cc(Oc2ccc(NS(=O)(=O)c3cc(C(O)=O)c(O)c4ccccc34)cc2)c1 Show InChI InChI=1S/C25H21NO6S/c1-15-11-16(2)13-19(12-15)32-18-9-7-17(8-10-18)26-33(30,31)23-14-22(25(28)29)24(27)21-6-4-3-5-20(21)23/h3-14,26-27H,1-2H3,(H,28,29) | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 284 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Maryland
Curated by ChEMBL
| Assay Description
Inhibition of His6-MBP tagged recombinant human Mcl-1 residues 172 to 327 expressed in Escherichia coli assessed as inhibition of interaction with Ba... |
Eur J Med Chem 113: 273-92 (2016)
Article DOI: 10.1016/j.ejmech.2016.02.006
BindingDB Entry DOI: 10.7270/Q2HX1FJH |
More data for this
Ligand-Target Pair | |
Induced myeloid leukemia cell differentiation protein Mcl-1
(Homo sapiens (Human)) | BDBM50158186
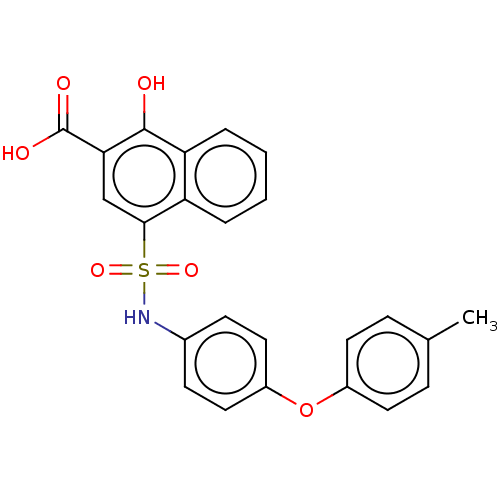
(CHEMBL3781396 | US10858316, Compound 3bc)Show SMILES Cc1ccc(Oc2ccc(NS(=O)(=O)c3cc(C(O)=O)c(O)c4ccccc34)cc2)cc1 Show InChI InChI=1S/C24H19NO6S/c1-15-6-10-17(11-7-15)31-18-12-8-16(9-13-18)25-32(29,30)22-14-21(24(27)28)23(26)20-5-3-2-4-19(20)22/h2-14,25-26H,1H3,(H,27,28) | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 335 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Maryland
Curated by ChEMBL
| Assay Description
Inhibition of His6-MBP tagged recombinant human Mcl-1 residues 172 to 327 expressed in Escherichia coli assessed as inhibition of interaction with Ba... |
Eur J Med Chem 113: 273-92 (2016)
Article DOI: 10.1016/j.ejmech.2016.02.006
BindingDB Entry DOI: 10.7270/Q2HX1FJH |
More data for this
Ligand-Target Pair | |
Apoptosis regulator Bcl-2
(Homo sapiens (Human)) | BDBM50589726
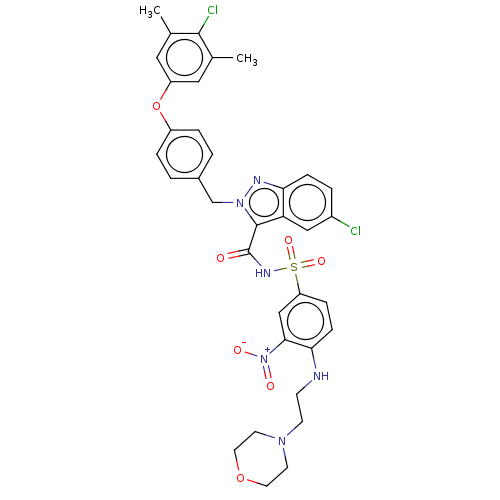
(CHEMBL5206019)Show SMILES Cc1cc(Oc2ccc(Cn3nc4ccc(Cl)cc4c3C(=O)NS(=O)(=O)c3ccc(NCCN4CCOCC4)c(c3)[N+]([O-])=O)cc2)cc(C)c1Cl | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 420 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1039/d2md00095d
BindingDB Entry DOI: 10.7270/Q2XK8KHN |
More data for this
Ligand-Target Pair | |
Induced myeloid leukemia cell differentiation protein Mcl-1
(Homo sapiens (Human)) | BDBM50158168

(CHEMBL3780113 | US10858316, Compound 3o)Show SMILES OC(=O)c1cc(c2ccccc2c1O)S(=O)(=O)Nc1ccc(Br)cc1Br Show InChI InChI=1S/C17H11Br2NO5S/c18-9-5-6-14(13(19)7-9)20-26(24,25)15-8-12(17(22)23)16(21)11-4-2-1-3-10(11)15/h1-8,20-21H,(H,22,23) | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 420 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Maryland
Curated by ChEMBL
| Assay Description
Inhibition of His6-MBP tagged recombinant human Mcl-1 residues 172 to 327 expressed in Escherichia coli assessed as inhibition of interaction with Ba... |
Eur J Med Chem 113: 273-92 (2016)
Article DOI: 10.1016/j.ejmech.2016.02.006
BindingDB Entry DOI: 10.7270/Q2HX1FJH |
More data for this
Ligand-Target Pair | |
Apoptosis regulator Bcl-2
(Homo sapiens (Human)) | BDBM50589731
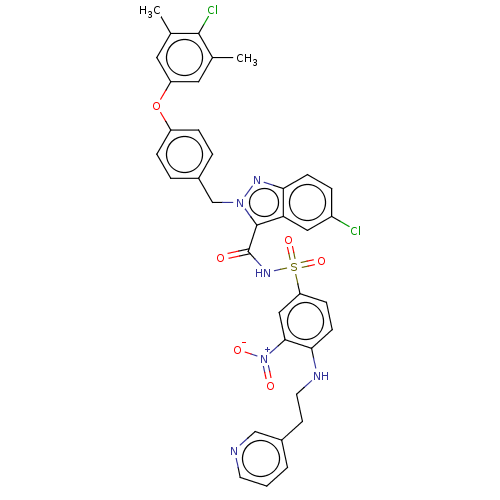
(CHEMBL5182327 | US11760752, Compound 38)Show SMILES Cc1cc(Oc2ccc(Cn3nc4ccc(Cl)cc4c3C(=O)NS(=O)(=O)c3ccc(NCCc4cccnc4)c(c3)[N+]([O-])=O)cc2)cc(C)c1Cl | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 460 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1039/d2md00095d
BindingDB Entry DOI: 10.7270/Q2XK8KHN |
More data for this
Ligand-Target Pair | |
Apoptosis regulator Bcl-2
(Homo sapiens (Human)) | BDBM50589739
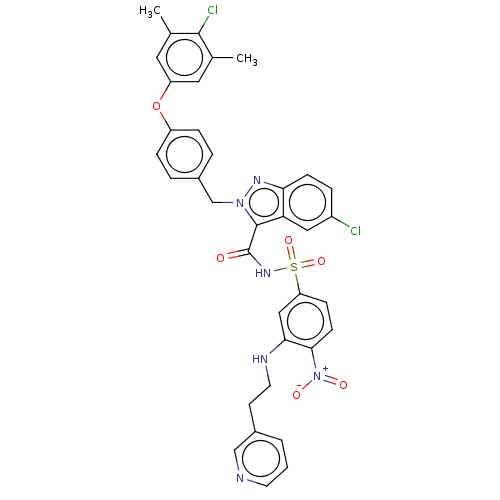
(CHEMBL5197460)Show SMILES Cc1cc(Oc2ccc(Cn3nc4ccc(Cl)cc4c3C(=O)NS(=O)(=O)c3ccc(c(NCCc4cccnc4)c3)[N+]([O-])=O)cc2)cc(C)c1Cl | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 460 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1039/d2md00095d
BindingDB Entry DOI: 10.7270/Q2XK8KHN |
More data for this
Ligand-Target Pair | |
Induced myeloid leukemia cell differentiation protein Mcl-1
(Homo sapiens (Human)) | BDBM50158184
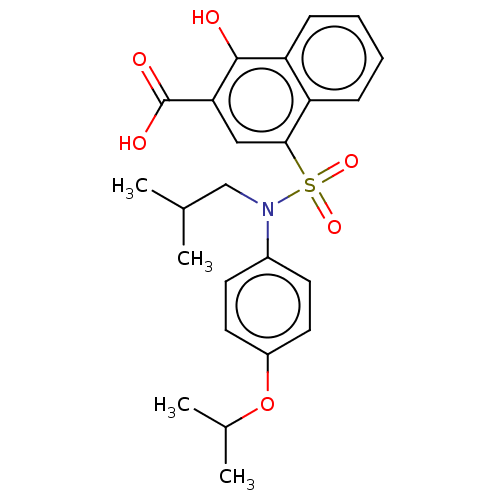
(CHEMBL3780576 | US10858316, Compound 3ba)Show SMILES CC(C)CN(c1ccc(OC(C)C)cc1)S(=O)(=O)c1cc(C(O)=O)c(O)c2ccccc12 Show InChI InChI=1S/C24H27NO6S/c1-15(2)14-25(17-9-11-18(12-10-17)31-16(3)4)32(29,30)22-13-21(24(27)28)23(26)20-8-6-5-7-19(20)22/h5-13,15-16,26H,14H2,1-4H3,(H,27,28) | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 487 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Maryland
Curated by ChEMBL
| Assay Description
Inhibition of His6-MBP tagged recombinant human Mcl-1 residues 172 to 327 expressed in Escherichia coli assessed as inhibition of interaction with Ba... |
Eur J Med Chem 113: 273-92 (2016)
Article DOI: 10.1016/j.ejmech.2016.02.006
BindingDB Entry DOI: 10.7270/Q2HX1FJH |
More data for this
Ligand-Target Pair | |
Apoptosis regulator Bcl-2
(Homo sapiens (Human)) | BDBM50589724
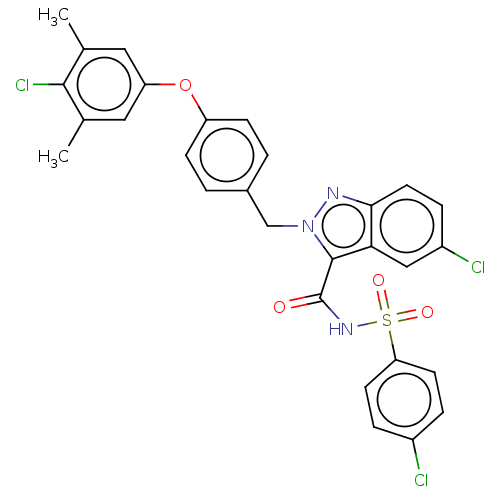
(CHEMBL5188517 | US11760752, Compound 31)Show SMILES Cc1cc(Oc2ccc(Cn3nc4ccc(Cl)cc4c3C(=O)NS(=O)(=O)c3ccc(Cl)cc3)cc2)cc(C)c1Cl | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 510 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1039/d2md00095d
BindingDB Entry DOI: 10.7270/Q2XK8KHN |
More data for this
Ligand-Target Pair | |
Apoptosis regulator Bcl-2
(Homo sapiens (Human)) | BDBM50589725
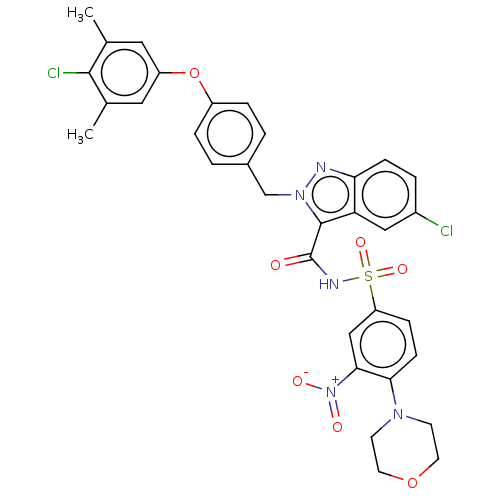
(CHEMBL5209122)Show SMILES Cc1cc(Oc2ccc(Cn3nc4ccc(Cl)cc4c3C(=O)NS(=O)(=O)c3ccc(N4CCOCC4)c(c3)[N+]([O-])=O)cc2)cc(C)c1Cl | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 530 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1039/d2md00095d
BindingDB Entry DOI: 10.7270/Q2XK8KHN |
More data for this
Ligand-Target Pair | |
Induced myeloid leukemia cell differentiation protein Mcl-1
(Homo sapiens (Human)) | BDBM50158356
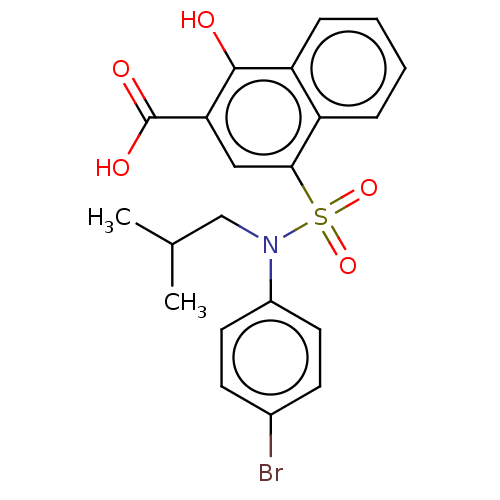
(CHEMBL3781019 | US10858316, Compound 3ca)Show SMILES CC(C)CN(c1ccc(Br)cc1)S(=O)(=O)c1cc(C(O)=O)c(O)c2ccccc12 Show InChI InChI=1S/C21H20BrNO5S/c1-13(2)12-23(15-9-7-14(22)8-10-15)29(27,28)19-11-18(21(25)26)20(24)17-6-4-3-5-16(17)19/h3-11,13,24H,12H2,1-2H3,(H,25,26) | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 566 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Maryland
Curated by ChEMBL
| Assay Description
Inhibition of His6-MBP tagged recombinant human Mcl-1 residues 172 to 327 expressed in Escherichia coli assessed as inhibition of interaction with Ba... |
Eur J Med Chem 113: 273-92 (2016)
Article DOI: 10.1016/j.ejmech.2016.02.006
BindingDB Entry DOI: 10.7270/Q2HX1FJH |
More data for this
Ligand-Target Pair | |
Apoptosis regulator Bcl-2
(Homo sapiens (Human)) | BDBM50589727
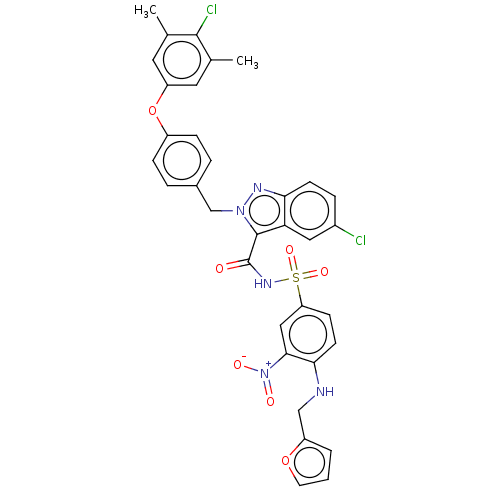
(CHEMBL5172723)Show SMILES Cc1cc(Oc2ccc(Cn3nc4ccc(Cl)cc4c3C(=O)NS(=O)(=O)c3ccc(NCc4ccco4)c(c3)[N+]([O-])=O)cc2)cc(C)c1Cl | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 570 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1039/d2md00095d
BindingDB Entry DOI: 10.7270/Q2XK8KHN |
More data for this
Ligand-Target Pair | |
Ileal sodium/bile acid cotransporter
(Homo sapiens (Human)) | BDBM50322507

((2S)-5-(3-tert-butylphenylamino)-2-((4R)-4-((3R,7R...)Show SMILES C[C@H](CCC(=O)N[C@@H](CCC(=O)Nc1cccc(c1)C(C)(C)C)C(O)=O)[C@H]1CC[C@H]2[C@@H]3[C@H](O)C[C@H]4C[C@H](O)CC[C@]4(C)[C@H]3CC[C@]12C |r| Show InChI InChI=1S/C39H60N2O6/c1-23(10-14-34(45)41-31(36(46)47)13-15-33(44)40-26-9-7-8-24(20-26)37(2,3)4)28-11-12-29-35-30(17-19-39(28,29)6)38(5)18-16-27(42)21-25(38)22-32(35)43/h7-9,20,23,25,27-32,35,42-43H,10-19,21-22H2,1-6H3,(H,40,44)(H,41,45)(H,46,47)/t23-,25-,27-,28-,29+,30+,31+,32-,35+,38+,39-/m1/s1 | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 587 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Maryland
Curated by ChEMBL
| Assay Description
Inhibition of human ASBT expressed in MDCK cells assessed as inhibition of [3H]taurocholic acid uptake after 10 mins by liquid scintillation counting |
J Med Chem 53: 4749-60 (2010)
Article DOI: 10.1021/jm1003683
BindingDB Entry DOI: 10.7270/Q2H70G0G |
More data for this
Ligand-Target Pair | |
Apoptosis regulator Bcl-2
(Homo sapiens (Human)) | BDBM50589729
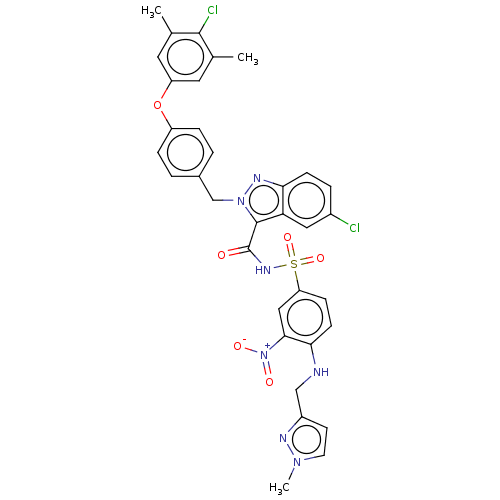
(CHEMBL5176177)Show SMILES Cc1cc(Oc2ccc(Cn3nc4ccc(Cl)cc4c3C(=O)NS(=O)(=O)c3ccc(NCc4ccn(C)n4)c(c3)[N+]([O-])=O)cc2)cc(C)c1Cl | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1039/d2md00095d
BindingDB Entry DOI: 10.7270/Q2XK8KHN |
More data for this
Ligand-Target Pair | |
Apoptosis regulator Bcl-2
(Homo sapiens (Human)) | BDBM50589728
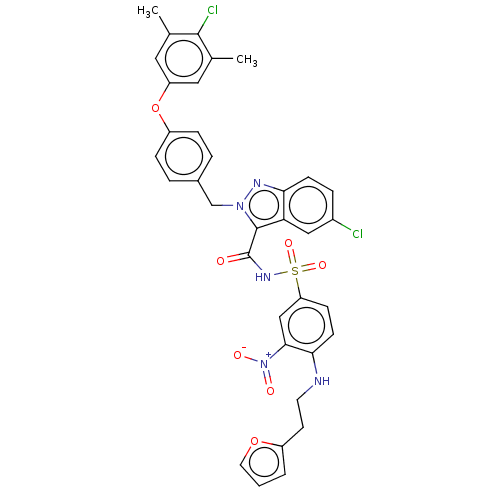
(CHEMBL5181405)Show SMILES Cc1cc(Oc2ccc(Cn3nc4ccc(Cl)cc4c3C(=O)NS(=O)(=O)c3ccc(NCCc4ccco4)c(c3)[N+]([O-])=O)cc2)cc(C)c1Cl | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 620 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1039/d2md00095d
BindingDB Entry DOI: 10.7270/Q2XK8KHN |
More data for this
Ligand-Target Pair | |
Apoptosis regulator Bcl-2
(Homo sapiens (Human)) | BDBM50589733

(CHEMBL5183183 | US11760752, Compound 40)Show SMILES Cc1cc(Oc2ccc(Cn3nc4ccc(Cl)cc4c3C(=O)NS(=O)(=O)c3ccc(NCCc4ccncc4)c(c3)[N+]([O-])=O)cc2)cc(C)c1Cl | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 660 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1039/d2md00095d
BindingDB Entry DOI: 10.7270/Q2XK8KHN |
More data for this
Ligand-Target Pair | |
Apoptosis regulator Bcl-2
(Homo sapiens (Human)) | BDBM50589740
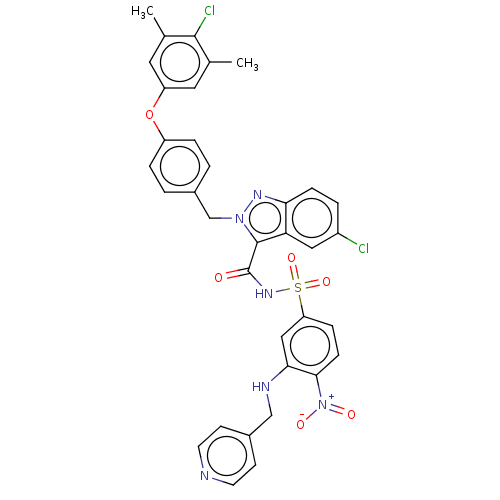
(CHEMBL5192657)Show SMILES Cc1cc(Oc2ccc(Cn3nc4ccc(Cl)cc4c3C(=O)NS(=O)(=O)c3ccc(c(NCc4ccncc4)c3)[N+]([O-])=O)cc2)cc(C)c1Cl | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 710 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1039/d2md00095d
BindingDB Entry DOI: 10.7270/Q2XK8KHN |
More data for this
Ligand-Target Pair | |
Apoptosis regulator Bcl-2
(Homo sapiens (Human)) | BDBM50589737
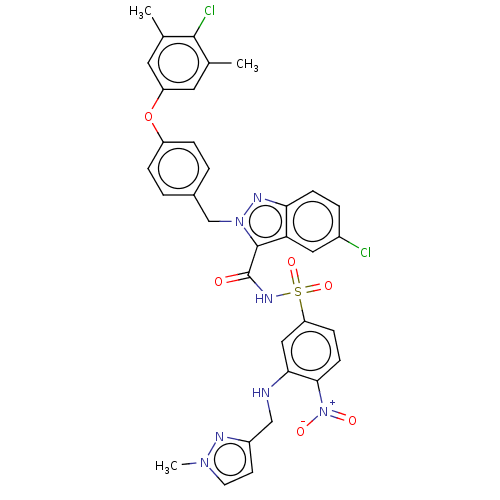
(CHEMBL5192774)Show SMILES Cc1cc(Oc2ccc(Cn3nc4ccc(Cl)cc4c3C(=O)NS(=O)(=O)c3ccc(c(NCc4ccn(C)n4)c3)[N+]([O-])=O)cc2)cc(C)c1Cl | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 710 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1039/d2md00095d
BindingDB Entry DOI: 10.7270/Q2XK8KHN |
More data for this
Ligand-Target Pair | |
Apoptosis regulator Bcl-2
(Homo sapiens (Human)) | BDBM50589741
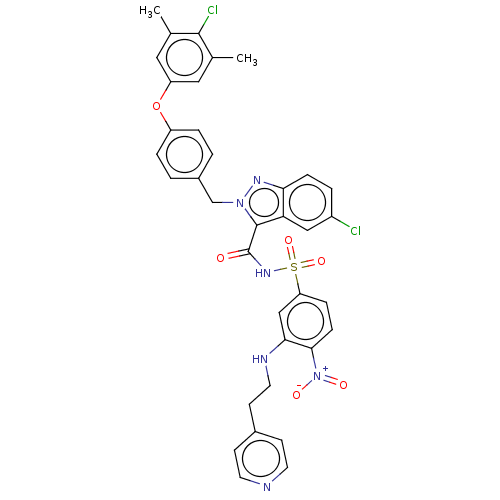
(CHEMBL5182219)Show SMILES Cc1cc(Oc2ccc(Cn3nc4ccc(Cl)cc4c3C(=O)NS(=O)(=O)c3ccc(c(NCCc4ccncc4)c3)[N+]([O-])=O)cc2)cc(C)c1Cl | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 770 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1039/d2md00095d
BindingDB Entry DOI: 10.7270/Q2XK8KHN |
More data for this
Ligand-Target Pair | |
Apoptosis regulator Bcl-2
(Homo sapiens (Human)) | BDBM50589736
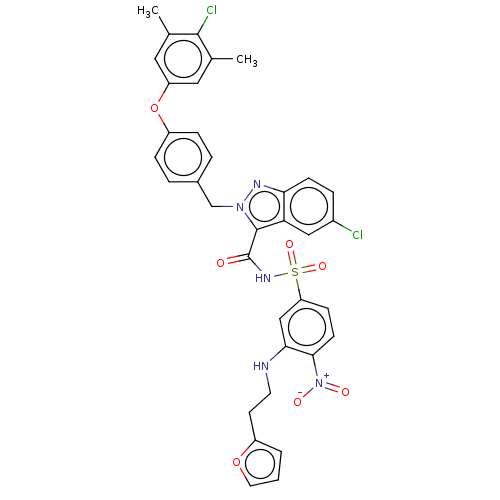
(CHEMBL5209295)Show SMILES Cc1cc(Oc2ccc(Cn3nc4ccc(Cl)cc4c3C(=O)NS(=O)(=O)c3ccc(c(NCCc4ccco4)c3)[N+]([O-])=O)cc2)cc(C)c1Cl | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 840 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1039/d2md00095d
BindingDB Entry DOI: 10.7270/Q2XK8KHN |
More data for this
Ligand-Target Pair | |
Ileal sodium/bile acid cotransporter
(Homo sapiens (Human)) | BDBM50322501

((2S)-2-((4R)-4-((3R,7R,8R,9S,10S,13R,14S,17R)-3,7-...)Show SMILES C[C@H](CCC(=O)N[C@@H](CCC(=O)Nc1cccc(C)c1)C(O)=O)[C@H]1CC[C@H]2[C@@H]3[C@H](O)C[C@H]4C[C@H](O)CC[C@]4(C)[C@H]3CC[C@]12C |r| Show InChI InChI=1S/C36H54N2O6/c1-21-6-5-7-24(18-21)37-31(41)13-11-29(34(43)44)38-32(42)12-8-22(2)26-9-10-27-33-28(15-17-36(26,27)4)35(3)16-14-25(39)19-23(35)20-30(33)40/h5-7,18,22-23,25-30,33,39-40H,8-17,19-20H2,1-4H3,(H,37,41)(H,38,42)(H,43,44)/t22-,23-,25-,26-,27+,28+,29+,30-,33+,35+,36-/m1/s1 | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 889 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Maryland
Curated by ChEMBL
| Assay Description
Inhibition of human ASBT expressed in MDCK cells assessed as inhibition of [3H]taurocholic acid uptake after 10 mins by liquid scintillation counting |
J Med Chem 53: 4749-60 (2010)
Article DOI: 10.1021/jm1003683
BindingDB Entry DOI: 10.7270/Q2H70G0G |
More data for this
Ligand-Target Pair | |
Apoptosis regulator Bcl-2
(Homo sapiens (Human)) | BDBM50589734

(CHEMBL5203579)Show SMILES Cc1cc(Oc2ccc(Cn3nc4ccc(Cl)cc4c3C(=O)NS(=O)(=O)c3ccc(c(c3)N3CCOCC3)[N+]([O-])=O)cc2)cc(C)c1Cl | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1039/d2md00095d
BindingDB Entry DOI: 10.7270/Q2XK8KHN |
More data for this
Ligand-Target Pair | |
Apoptosis regulator Bcl-2
(Homo sapiens (Human)) | BDBM50589735
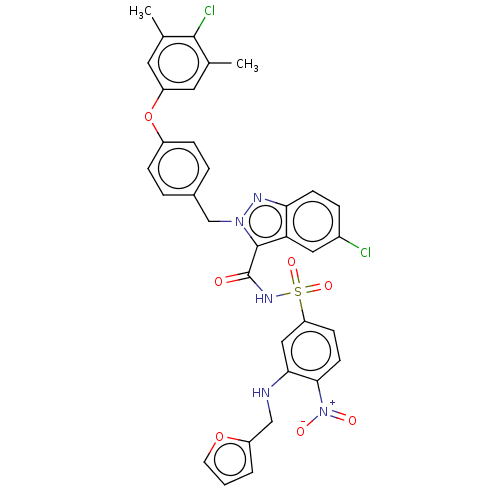
(CHEMBL5191785)Show SMILES Cc1cc(Oc2ccc(Cn3nc4ccc(Cl)cc4c3C(=O)NS(=O)(=O)c3ccc(c(NCc4ccco4)c3)[N+]([O-])=O)cc2)cc(C)c1Cl | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 960 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1039/d2md00095d
BindingDB Entry DOI: 10.7270/Q2XK8KHN |
More data for this
Ligand-Target Pair | |
Induced myeloid leukemia cell differentiation protein Mcl-1
(Homo sapiens (Human)) | BDBM50589738
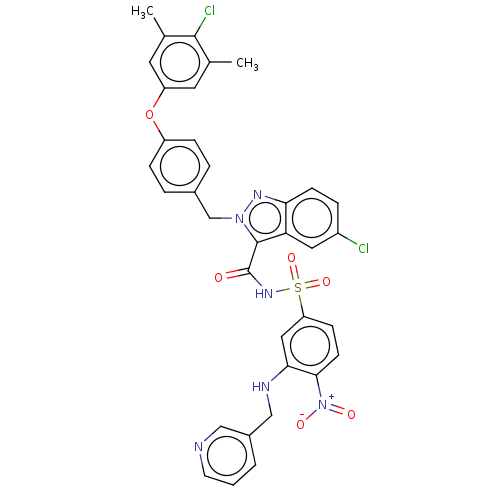
(CHEMBL5180236)Show SMILES Cc1cc(Oc2ccc(Cn3nc4ccc(Cl)cc4c3C(=O)NS(=O)(=O)c3ccc(c(NCc4cccnc4)c3)[N+]([O-])=O)cc2)cc(C)c1Cl | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1039/d2md00095d
BindingDB Entry DOI: 10.7270/Q2XK8KHN |
More data for this
Ligand-Target Pair | |
Induced myeloid leukemia cell differentiation protein Mcl-1
(Homo sapiens (Human)) | BDBM50589730
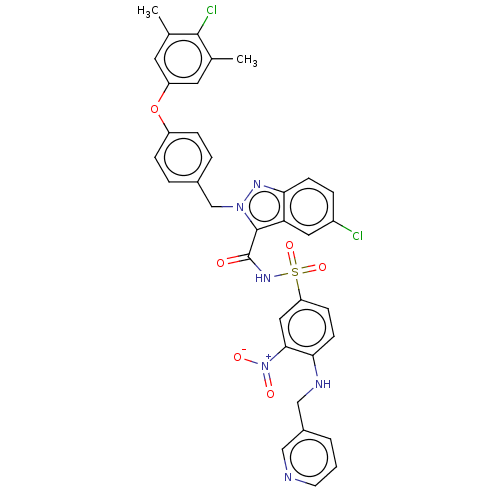
(CHEMBL5178609)Show SMILES Cc1cc(Oc2ccc(Cn3nc4ccc(Cl)cc4c3C(=O)NS(=O)(=O)c3ccc(NCc4cccnc4)c(c3)[N+]([O-])=O)cc2)cc(C)c1Cl | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.12E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1039/d2md00095d
BindingDB Entry DOI: 10.7270/Q2XK8KHN |
More data for this
Ligand-Target Pair | |
Ileal sodium/bile acid cotransporter
(Homo sapiens (Human)) | BDBM50322480
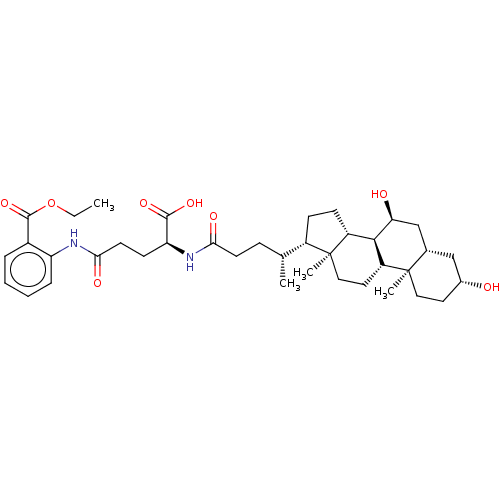
((2S)-2-((4R)-4-((3R,7R,8R,9S,10S,13R,14S,17R)-3,7-...)Show SMILES CCOC(=O)c1ccccc1NC(=O)CCC(NC(=O)CCC(C)C1CCC2C3C(O)CC4CC(O)CCC4(C)C3CCC12C)C(O)=O Show InChI InChI=1S/C38H56N2O8/c1-5-48-36(47)25-8-6-7-9-29(25)39-33(44)15-13-30(35(45)46)40-32(43)14-10-22(2)26-11-12-27-34-28(17-19-38(26,27)4)37(3)18-16-24(41)20-23(37)21-31(34)42/h6-9,22-24,26-28,30-31,34,41-42H,5,10-21H2,1-4H3,(H,39,44)(H,40,43)(H,45,46)/t22-,23-,24-,26-,27+,28+,30+,31-,34+,37+,38-/m1/s1 | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.15E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Maryland
Curated by ChEMBL
| Assay Description
Inhibition of human ASBT expressed in MDCK cells assessed as inhibition of [3H]taurocholic acid uptake after 10 mins by liquid scintillation counting |
J Med Chem 53: 4749-60 (2010)
Article DOI: 10.1021/jm1003683
BindingDB Entry DOI: 10.7270/Q2H70G0G |
More data for this
Ligand-Target Pair | |
Induced myeloid leukemia cell differentiation protein Mcl-1
(Homo sapiens (Human)) | BDBM50158185
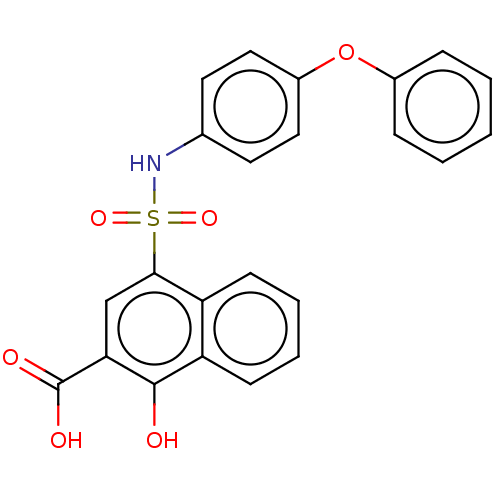
(CHEMBL3781946 | US10858316, Compound 3bb)Show SMILES OC(=O)c1cc(c2ccccc2c1O)S(=O)(=O)Nc1ccc(Oc2ccccc2)cc1 Show InChI InChI=1S/C23H17NO6S/c25-22-19-9-5-4-8-18(19)21(14-20(22)23(26)27)31(28,29)24-15-10-12-17(13-11-15)30-16-6-2-1-3-7-16/h1-14,24-25H,(H,26,27) | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.15E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Maryland
Curated by ChEMBL
| Assay Description
Inhibition of His6-MBP tagged recombinant human Mcl-1 residues 172 to 327 expressed in Escherichia coli assessed as inhibition of interaction with Ba... |
Eur J Med Chem 113: 273-92 (2016)
Article DOI: 10.1016/j.ejmech.2016.02.006
BindingDB Entry DOI: 10.7270/Q2HX1FJH |
More data for this
Ligand-Target Pair | |
Apoptosis regulator Bcl-2
(Homo sapiens (Human)) | BDBM50589730

(CHEMBL5178609)Show SMILES Cc1cc(Oc2ccc(Cn3nc4ccc(Cl)cc4c3C(=O)NS(=O)(=O)c3ccc(NCc4cccnc4)c(c3)[N+]([O-])=O)cc2)cc(C)c1Cl | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.18E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1039/d2md00095d
BindingDB Entry DOI: 10.7270/Q2XK8KHN |
More data for this
Ligand-Target Pair | |
Induced myeloid leukemia cell differentiation protein Mcl-1
(Homo sapiens (Human)) | BDBM50589724

(CHEMBL5188517 | US11760752, Compound 31)Show SMILES Cc1cc(Oc2ccc(Cn3nc4ccc(Cl)cc4c3C(=O)NS(=O)(=O)c3ccc(Cl)cc3)cc2)cc(C)c1Cl | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1039/d2md00095d
BindingDB Entry DOI: 10.7270/Q2XK8KHN |
More data for this
Ligand-Target Pair | |
Apoptosis regulator Bcl-2
(Homo sapiens (Human)) | BDBM50424732

(CHEMBL2314209)Show SMILES Cc1cc(OCCCc2c([nH]c3cc(Cl)ccc23)C(O)=O)cc(C)c1Cl Show InChI InChI=1S/C20H19Cl2NO3/c1-11-8-14(9-12(2)18(11)22)26-7-3-4-16-15-6-5-13(21)10-17(15)23-19(16)20(24)25/h5-6,8-10,23H,3-4,7H2,1-2H3,(H,24,25) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1039/d2md00095d
BindingDB Entry DOI: 10.7270/Q2XK8KHN |
More data for this
Ligand-Target Pair | |
Apoptosis regulator Bcl-2
(Homo sapiens (Human)) | BDBM50589738

(CHEMBL5180236)Show SMILES Cc1cc(Oc2ccc(Cn3nc4ccc(Cl)cc4c3C(=O)NS(=O)(=O)c3ccc(c(NCc4cccnc4)c3)[N+]([O-])=O)cc2)cc(C)c1Cl | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.22E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1039/d2md00095d
BindingDB Entry DOI: 10.7270/Q2XK8KHN |
More data for this
Ligand-Target Pair | |
Ileal sodium/bile acid cotransporter
(Homo sapiens (Human)) | BDBM50322498

((2S)-2-((4R)-4-((3R,7R,8R,9S,10S,13R,14S,17R)-3,7-...)Show SMILES C[C@H](CCC(=O)N[C@@H](CCC(=O)Nc1cccc(F)c1)C(O)=O)[C@H]1CC[C@H]2[C@@H]3[C@H](O)C[C@H]4C[C@H](O)CC[C@]4(C)[C@H]3CC[C@]12C |r| Show InChI InChI=1S/C35H51FN2O6/c1-20(7-11-31(42)38-28(33(43)44)10-12-30(41)37-23-6-4-5-22(36)19-23)25-8-9-26-32-27(14-16-35(25,26)3)34(2)15-13-24(39)17-21(34)18-29(32)40/h4-6,19-21,24-29,32,39-40H,7-18H2,1-3H3,(H,37,41)(H,38,42)(H,43,44)/t20-,21-,24-,25-,26+,27+,28+,29-,32+,34+,35-/m1/s1 | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.25E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Maryland
Curated by ChEMBL
| Assay Description
Inhibition of human ASBT expressed in MDCK cells assessed as inhibition of [3H]taurocholic acid uptake after 10 mins by liquid scintillation counting |
J Med Chem 53: 4749-60 (2010)
Article DOI: 10.1021/jm1003683
BindingDB Entry DOI: 10.7270/Q2H70G0G |
More data for this
Ligand-Target Pair | |
Induced myeloid leukemia cell differentiation protein Mcl-1
(Homo sapiens (Human)) | BDBM50589728

(CHEMBL5181405)Show SMILES Cc1cc(Oc2ccc(Cn3nc4ccc(Cl)cc4c3C(=O)NS(=O)(=O)c3ccc(NCCc4ccco4)c(c3)[N+]([O-])=O)cc2)cc(C)c1Cl | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.28E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1039/d2md00095d
BindingDB Entry DOI: 10.7270/Q2XK8KHN |
More data for this
Ligand-Target Pair | |
Induced myeloid leukemia cell differentiation protein Mcl-1
(Homo sapiens (Human)) | BDBM50589727

(CHEMBL5172723)Show SMILES Cc1cc(Oc2ccc(Cn3nc4ccc(Cl)cc4c3C(=O)NS(=O)(=O)c3ccc(NCc4ccco4)c(c3)[N+]([O-])=O)cc2)cc(C)c1Cl | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1039/d2md00095d
BindingDB Entry DOI: 10.7270/Q2XK8KHN |
More data for this
Ligand-Target Pair | |
Induced myeloid leukemia cell differentiation protein Mcl-1
(Homo sapiens (Human)) | BDBM50589725

(CHEMBL5209122)Show SMILES Cc1cc(Oc2ccc(Cn3nc4ccc(Cl)cc4c3C(=O)NS(=O)(=O)c3ccc(N4CCOCC4)c(c3)[N+]([O-])=O)cc2)cc(C)c1Cl | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.32E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1039/d2md00095d
BindingDB Entry DOI: 10.7270/Q2XK8KHN |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data