

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50120543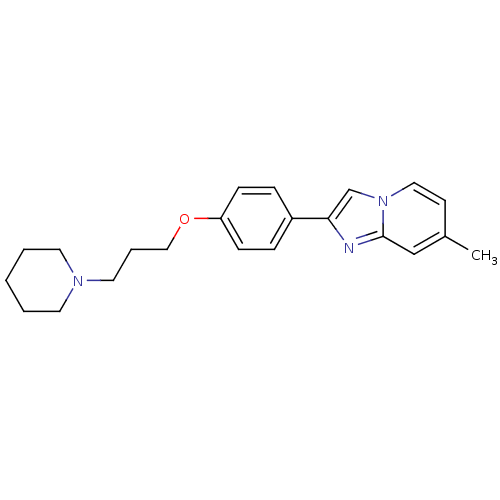 (7-Methyl-2-[4-(3-piperidin-1-yl-propoxy)-phenyl]-i...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Binding affinity for human histamine H3 receptor | Bioorg Med Chem Lett 12: 3309-12 (2002) BindingDB Entry DOI: 10.7270/Q2KH0MPG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50120559 (7-Methyl-2-[2-methyl-4-(3-piperidin-1-yl-propoxy)-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Binding affinity for human histamine H3 receptor | Bioorg Med Chem Lett 12: 3309-12 (2002) BindingDB Entry DOI: 10.7270/Q2KH0MPG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50120537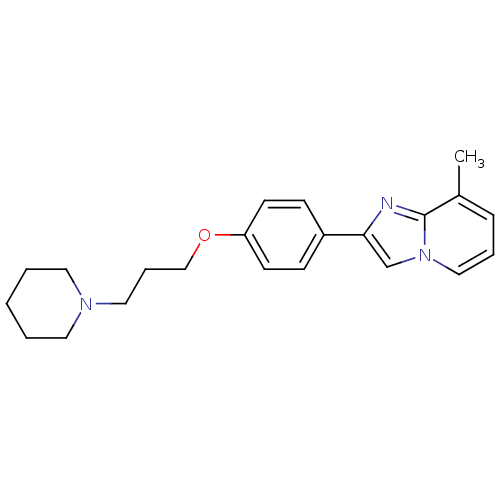 (8-Methyl-2-[4-(3-piperidin-1-yl-propoxy)-phenyl]-i...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Antagonist activity for human histamine H3 receptor was determined | Bioorg Med Chem Lett 12: 3309-12 (2002) BindingDB Entry DOI: 10.7270/Q2KH0MPG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50120537 (8-Methyl-2-[4-(3-piperidin-1-yl-propoxy)-phenyl]-i...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Binding affinity for human histamine H3 receptor | Bioorg Med Chem Lett 12: 3309-12 (2002) BindingDB Entry DOI: 10.7270/Q2KH0MPG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin receptor type 2 (Homo sapiens (Human)) | BDBM50412863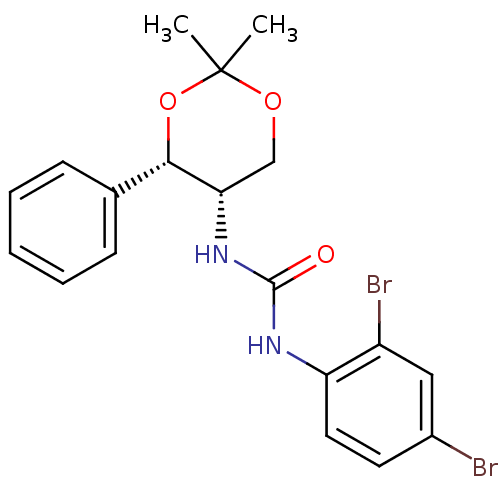 (CHEMBL359632 | JNJ-10397049) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Binding affinity towards human orexin receptor type 2 was determined using [125I]-Orexin A as radio ligand | Bioorg Med Chem Lett 14: 4225-9 (2004) Article DOI: 10.1016/j.bmcl.2004.06.032 BindingDB Entry DOI: 10.7270/Q2K64MV4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50120544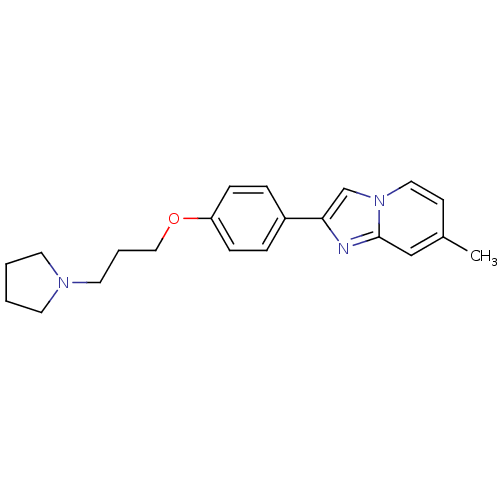 (7-Methyl-2-[4-(3-pyrrolidin-1-yl-propoxy)-phenyl]-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Binding affinity for human histamine H3 receptor | Bioorg Med Chem Lett 12: 3309-12 (2002) BindingDB Entry DOI: 10.7270/Q2KH0MPG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50120562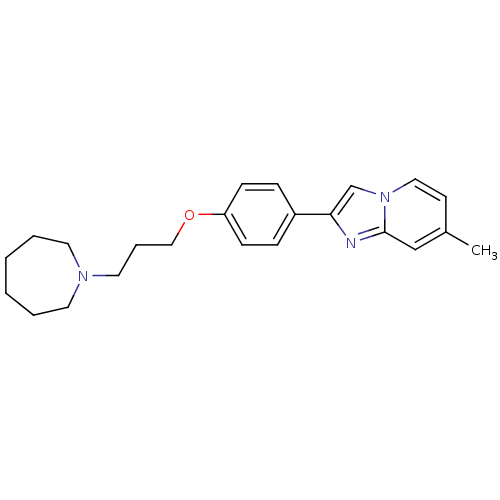 (2-[4-(3-Azepan-1-yl-propoxy)-phenyl]-7-methyl-imid...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Binding affinity for human histamine H3 receptor | Bioorg Med Chem Lett 12: 3309-12 (2002) BindingDB Entry DOI: 10.7270/Q2KH0MPG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50120542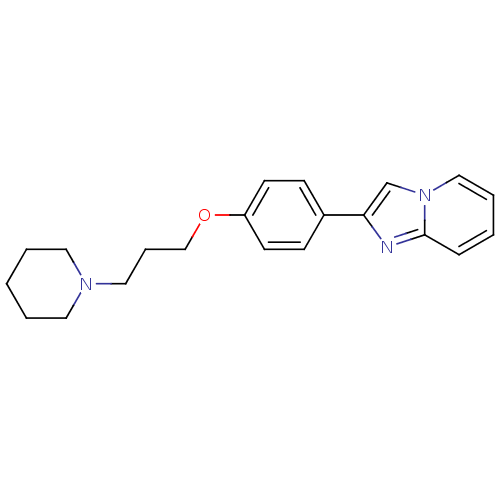 (2-[4-(3-Piperidin-1-yl-propoxy)-phenyl]-imidazo[1,...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Binding affinity for human histamine H3 receptor | Bioorg Med Chem Lett 12: 3309-12 (2002) BindingDB Entry DOI: 10.7270/Q2KH0MPG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin receptor type 2 (Homo sapiens (Human)) | BDBM50412861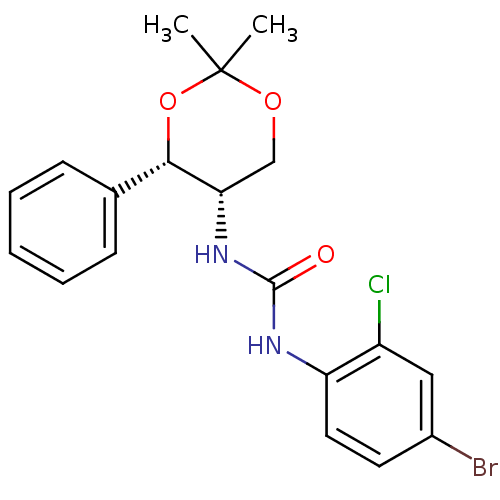 (CHEMBL185088) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Binding affinity towards human orexin receptor type 2 was determined using [125I]-Orexin A as radio ligand | Bioorg Med Chem Lett 14: 4225-9 (2004) Article DOI: 10.1016/j.bmcl.2004.06.032 BindingDB Entry DOI: 10.7270/Q2K64MV4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50120561 (7-Methyl-2-[4-(4-piperidin-1-yl-but-1-ynyl)-phenyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Binding affinity for human histamine H3 receptor | Bioorg Med Chem Lett 12: 3309-12 (2002) BindingDB Entry DOI: 10.7270/Q2KH0MPG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50120551 (2-[2-Fluoro-4-(3-piperidin-1-yl-propoxy)-phenyl]-7...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Binding affinity for human histamine H3 receptor | Bioorg Med Chem Lett 12: 3309-12 (2002) BindingDB Entry DOI: 10.7270/Q2KH0MPG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50120543 (7-Methyl-2-[4-(3-piperidin-1-yl-propoxy)-phenyl]-i...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Compound was tested for its binding affinity for rat histamine H3 receptor | Bioorg Med Chem Lett 12: 3309-12 (2002) BindingDB Entry DOI: 10.7270/Q2KH0MPG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50120549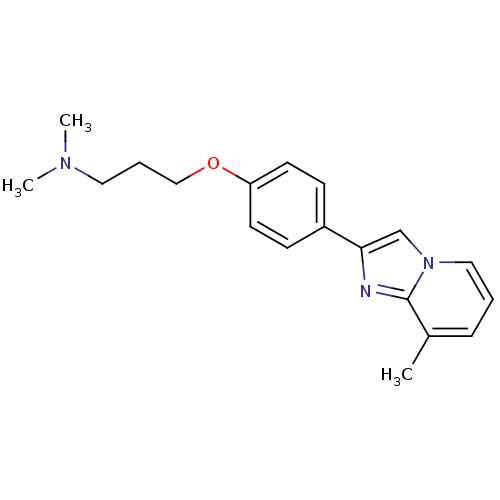 (CHEMBL70944 | Dimethyl-{3-[4-(8-methyl-imidazo[1,2...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Binding affinity for human histamine H3 receptor | Bioorg Med Chem Lett 12: 3309-12 (2002) BindingDB Entry DOI: 10.7270/Q2KH0MPG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50120539 (CHEMBL113236 | [4-(8-Methyl-imidazo[1,2-a]pyridin-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Binding affinity for human histamine H3 receptor | Bioorg Med Chem Lett 12: 3309-12 (2002) BindingDB Entry DOI: 10.7270/Q2KH0MPG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50120565 (7-Methyl-2-[4-(4-piperidin-1-yl-butyl)-phenyl]-imi...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Binding affinity for human histamine H3 receptor | Bioorg Med Chem Lett 12: 3309-12 (2002) BindingDB Entry DOI: 10.7270/Q2KH0MPG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin receptor type 2 (Homo sapiens (Human)) | BDBM50412862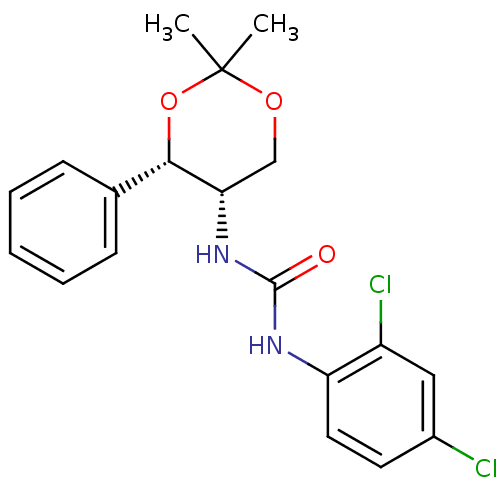 (CHEMBL185136) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Binding affinity towards human orexin receptor type 2 was determined using [125I]-Orexin A as radio ligand | Bioorg Med Chem Lett 14: 4225-9 (2004) Article DOI: 10.1016/j.bmcl.2004.06.032 BindingDB Entry DOI: 10.7270/Q2K64MV4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50120556 (7-Methyl-2-[4-((E)-4-piperidin-1-yl-but-1-enyl)-ph...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | 22 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Binding affinity for human histamine H3 receptor | Bioorg Med Chem Lett 12: 3309-12 (2002) BindingDB Entry DOI: 10.7270/Q2KH0MPG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin receptor type 2 (Homo sapiens (Human)) | BDBM50474855 (CHEMBL364814) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Binding affinity towards human orexin receptor type 2 was determined using [125I]-Orexin A as radio ligand | Bioorg Med Chem Lett 14: 4225-9 (2004) Article DOI: 10.1016/j.bmcl.2004.06.032 BindingDB Entry DOI: 10.7270/Q2K64MV4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50120547 (2-[3-Methyl-4-(3-piperidin-1-yl-propoxy)-phenyl]-i...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 28 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Binding affinity for human histamine H3 receptor | Bioorg Med Chem Lett 12: 3309-12 (2002) BindingDB Entry DOI: 10.7270/Q2KH0MPG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin receptor type 2 (Homo sapiens (Human)) | BDBM50412860 (CHEMBL185093) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Binding affinity towards human orexin receptor type 2 was determined using [125I]-Orexin A as radio ligand | Bioorg Med Chem Lett 14: 4225-9 (2004) Article DOI: 10.1016/j.bmcl.2004.06.032 BindingDB Entry DOI: 10.7270/Q2K64MV4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50120569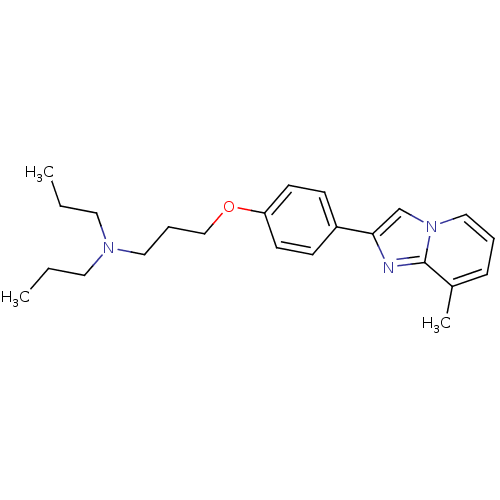 (CHEMBL306911 | {3-[4-(8-Methyl-imidazo[1,2-a]pyrid...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 43 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Binding affinity for human histamine H3 receptor | Bioorg Med Chem Lett 12: 3309-12 (2002) BindingDB Entry DOI: 10.7270/Q2KH0MPG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin receptor type 2 (Homo sapiens (Human)) | BDBM50474849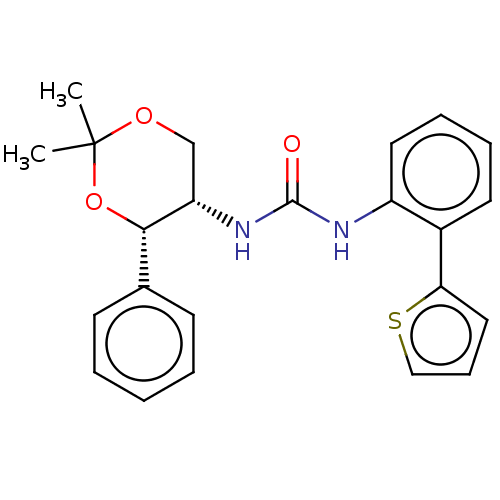 (CHEMBL185424) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Binding affinity towards human orexin receptor type 2 was determined using [125I]-Orexin A as radio ligand | Bioorg Med Chem Lett 14: 4225-9 (2004) Article DOI: 10.1016/j.bmcl.2004.06.032 BindingDB Entry DOI: 10.7270/Q2K64MV4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin receptor type 2 (Homo sapiens (Human)) | BDBM50412857 (CHEMBL361716) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 63 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Binding affinity towards human orexin receptor type 2 was determined using [125I]-Orexin A as radio ligand | Bioorg Med Chem Lett 14: 4225-9 (2004) Article DOI: 10.1016/j.bmcl.2004.06.032 BindingDB Entry DOI: 10.7270/Q2K64MV4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin receptor type 2 (Homo sapiens (Human)) | BDBM50412859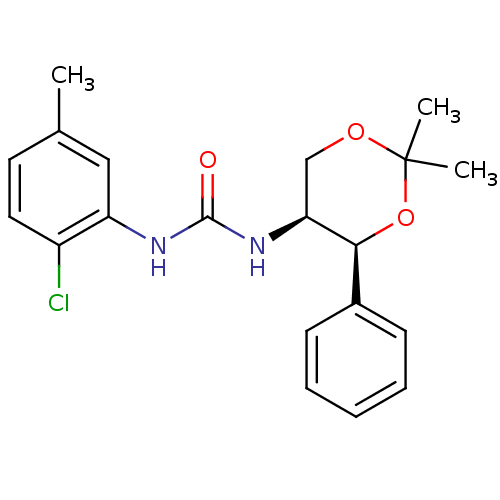 (CHEMBL182473) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 63 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Binding affinity towards human orexin receptor type 2 was determined using [125I]-Orexin A as radio ligand | Bioorg Med Chem Lett 14: 4225-9 (2004) Article DOI: 10.1016/j.bmcl.2004.06.032 BindingDB Entry DOI: 10.7270/Q2K64MV4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50120566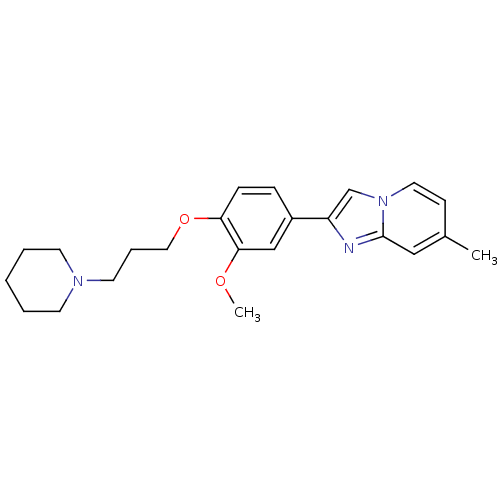 (2-[3-Methoxy-4-(3-piperidin-1-yl-propoxy)-phenyl]-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 69 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Binding affinity for human histamine H3 receptor | Bioorg Med Chem Lett 12: 3309-12 (2002) BindingDB Entry DOI: 10.7270/Q2KH0MPG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin receptor type 2 (Homo sapiens (Human)) | BDBM50474846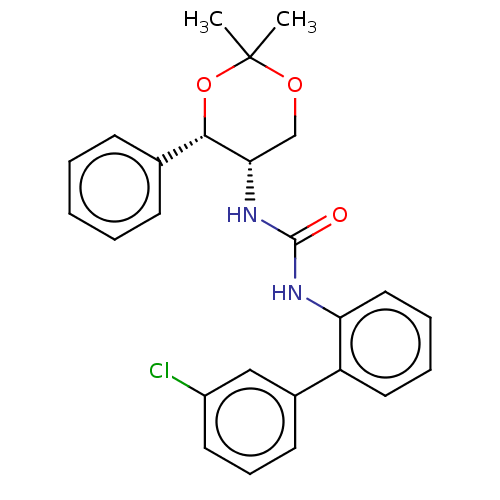 (CHEMBL185131) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 79 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Binding affinity towards human orexin receptor type 2 was determined using [125I]-Orexin A as radio ligand | Bioorg Med Chem Lett 14: 4225-9 (2004) Article DOI: 10.1016/j.bmcl.2004.06.032 BindingDB Entry DOI: 10.7270/Q2K64MV4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin receptor type 2 (Homo sapiens (Human)) | BDBM50412855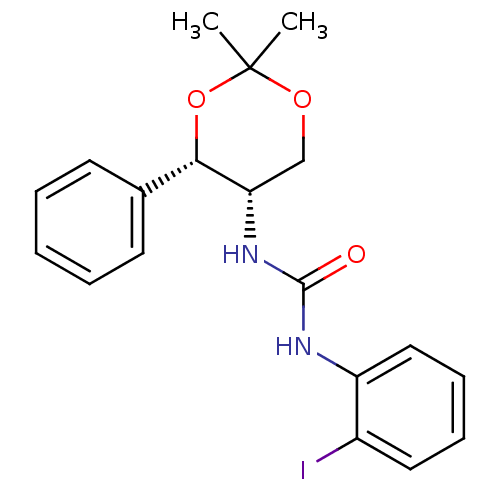 (CHEMBL185382) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 79 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Binding affinity towards human orexin receptor type 2 was determined using [125I]-Orexin A as radio ligand | Bioorg Med Chem Lett 14: 4225-9 (2004) Article DOI: 10.1016/j.bmcl.2004.06.032 BindingDB Entry DOI: 10.7270/Q2K64MV4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin receptor type 2 (Homo sapiens (Human)) | BDBM50412854 (CHEMBL185435) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 79 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Binding affinity towards human orexin receptor type 2 was determined using [125I]-Orexin A as radio ligand | Bioorg Med Chem Lett 14: 4225-9 (2004) Article DOI: 10.1016/j.bmcl.2004.06.032 BindingDB Entry DOI: 10.7270/Q2K64MV4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin receptor type 2 (Homo sapiens (Human)) | BDBM50474848 (CHEMBL184596) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 79 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Binding affinity towards human orexin receptor type 2 was determined using [125I]-Orexin A as radio ligand | Bioorg Med Chem Lett 14: 4225-9 (2004) Article DOI: 10.1016/j.bmcl.2004.06.032 BindingDB Entry DOI: 10.7270/Q2K64MV4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50120570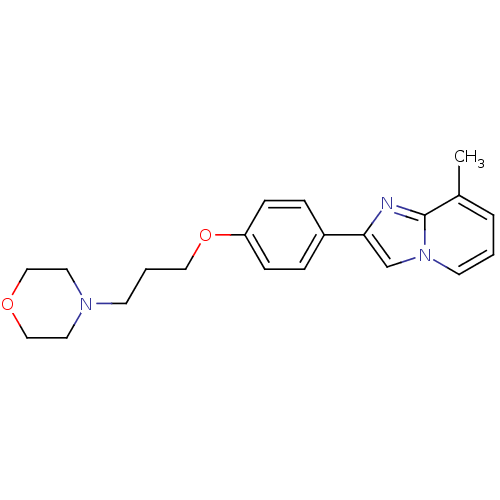 (8-Methyl-2-[4-(3-morpholin-4-yl-propoxy)-phenyl]-i...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Binding affinity for human histamine H3 receptor | Bioorg Med Chem Lett 12: 3309-12 (2002) BindingDB Entry DOI: 10.7270/Q2KH0MPG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin receptor type 2 (Homo sapiens (Human)) | BDBM50474847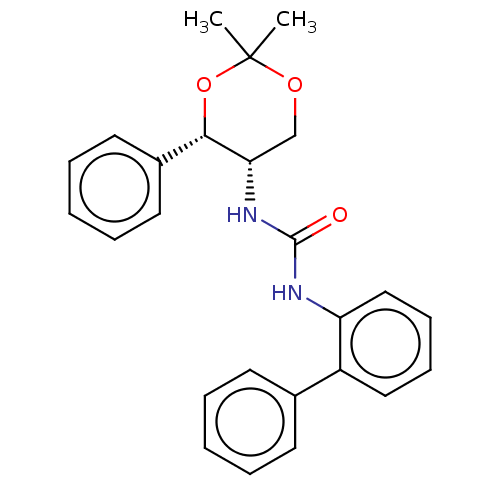 (CHEMBL183421) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Binding affinity towards human orexin receptor type 2 was determined using [125I]-Orexin A as radio ligand | Bioorg Med Chem Lett 14: 4225-9 (2004) Article DOI: 10.1016/j.bmcl.2004.06.032 BindingDB Entry DOI: 10.7270/Q2K64MV4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin receptor type 2 (Homo sapiens (Human)) | BDBM50412849 (CHEMBL363743) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Binding affinity towards human orexin receptor type 2 was determined using [125I]-Orexin A as radio ligand | Bioorg Med Chem Lett 14: 4225-9 (2004) Article DOI: 10.1016/j.bmcl.2004.06.032 BindingDB Entry DOI: 10.7270/Q2K64MV4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin receptor type 2 (Homo sapiens (Human)) | BDBM50412851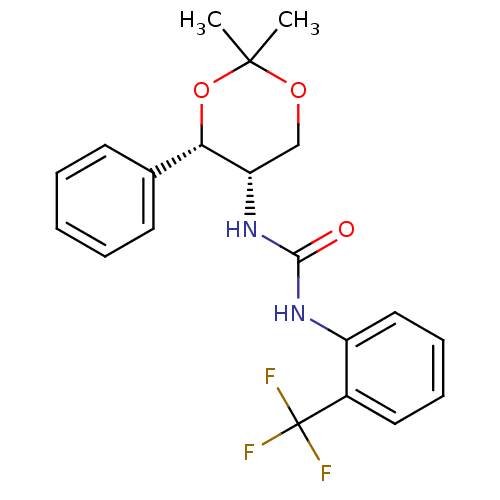 (CHEMBL363951) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Binding affinity towards human orexin receptor type 2 was determined using [125I]-Orexin A as radio ligand | Bioorg Med Chem Lett 14: 4225-9 (2004) Article DOI: 10.1016/j.bmcl.2004.06.032 BindingDB Entry DOI: 10.7270/Q2K64MV4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin/Hypocretin receptor type 1 (Homo sapiens (Human)) | BDBM50474855 (CHEMBL364814) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 126 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Binding affinity towards human orexin receptor type 1 was determined using [125I]-Orexin A as radio ligand | Bioorg Med Chem Lett 14: 4225-9 (2004) Article DOI: 10.1016/j.bmcl.2004.06.032 BindingDB Entry DOI: 10.7270/Q2K64MV4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin receptor type 2 (Homo sapiens (Human)) | BDBM50412858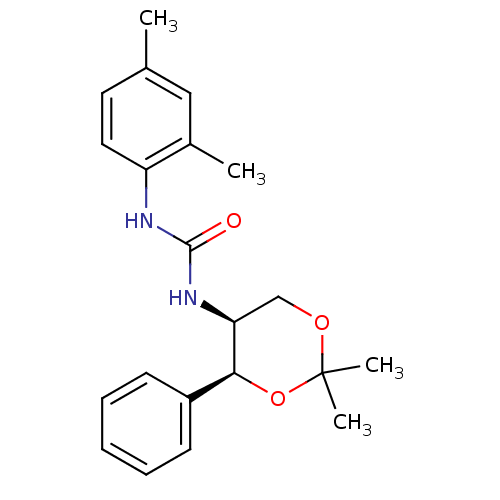 (CHEMBL183576) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 126 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Binding affinity towards human orexin receptor type 2 was determined using [125I]-Orexin A as radio ligand | Bioorg Med Chem Lett 14: 4225-9 (2004) Article DOI: 10.1016/j.bmcl.2004.06.032 BindingDB Entry DOI: 10.7270/Q2K64MV4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin receptor type 2 (Homo sapiens (Human)) | BDBM50474844 (CHEMBL183734) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Binding affinity towards human orexin receptor type 2 was determined using [125I]-Orexin A as radio ligand | Bioorg Med Chem Lett 14: 4225-9 (2004) Article DOI: 10.1016/j.bmcl.2004.06.032 BindingDB Entry DOI: 10.7270/Q2K64MV4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin receptor type 2 (Homo sapiens (Human)) | BDBM50474850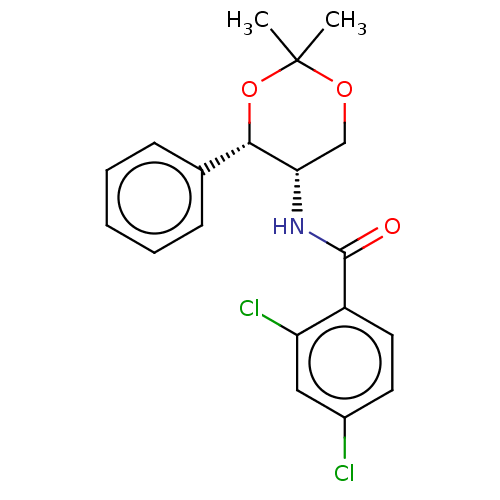 (CHEMBL185324) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Binding affinity towards human orexin receptor type 2 was determined using [125I]-Orexin A as radio ligand | Bioorg Med Chem Lett 14: 4225-9 (2004) Article DOI: 10.1016/j.bmcl.2004.06.032 BindingDB Entry DOI: 10.7270/Q2K64MV4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin receptor type 2 (Homo sapiens (Human)) | BDBM50412864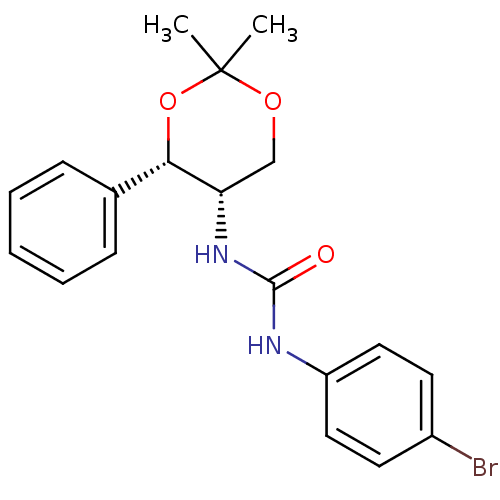 (CHEMBL184936) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 251 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Binding affinity towards human orexin receptor type 2 was determined using [125I]-Orexin A as radio ligand | Bioorg Med Chem Lett 14: 4225-9 (2004) Article DOI: 10.1016/j.bmcl.2004.06.032 BindingDB Entry DOI: 10.7270/Q2K64MV4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50120567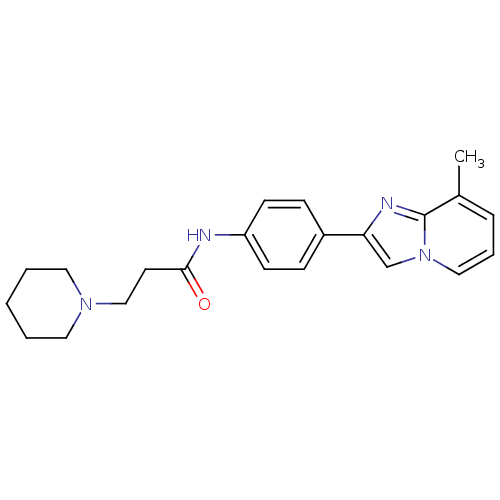 (CHEMBL114231 | N-[4-(8-Methyl-imidazo[1,2-a]pyridi...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Binding affinity for human histamine H3 receptor | Bioorg Med Chem Lett 12: 3309-12 (2002) BindingDB Entry DOI: 10.7270/Q2KH0MPG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin receptor type 2 (Homo sapiens (Human)) | BDBM50474845 (CHEMBL182269) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 316 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Binding affinity towards human orexin receptor type 2 was determined using [125I]-Orexin A as radio ligand | Bioorg Med Chem Lett 14: 4225-9 (2004) Article DOI: 10.1016/j.bmcl.2004.06.032 BindingDB Entry DOI: 10.7270/Q2K64MV4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin receptor type 2 (Homo sapiens (Human)) | BDBM50474854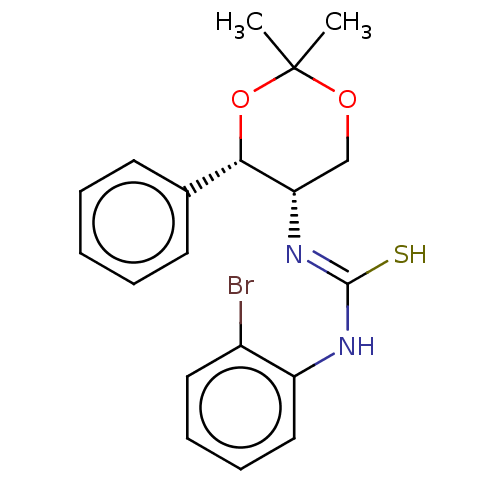 (CHEMBL184303) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 398 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Binding affinity towards human orexin receptor type 2 was determined using [125I]-Orexin A as radio ligand | Bioorg Med Chem Lett 14: 4225-9 (2004) Article DOI: 10.1016/j.bmcl.2004.06.032 BindingDB Entry DOI: 10.7270/Q2K64MV4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin receptor type 2 (Homo sapiens (Human)) | BDBM50412853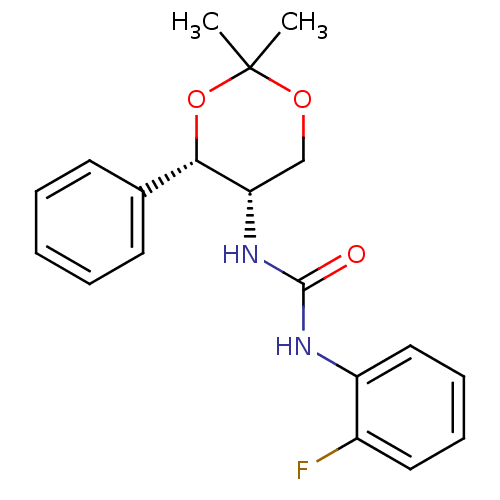 (CHEMBL361748) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 398 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Binding affinity towards human orexin receptor type 2 was determined using [125I]-Orexin A as radio ligand | Bioorg Med Chem Lett 14: 4225-9 (2004) Article DOI: 10.1016/j.bmcl.2004.06.032 BindingDB Entry DOI: 10.7270/Q2K64MV4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50120550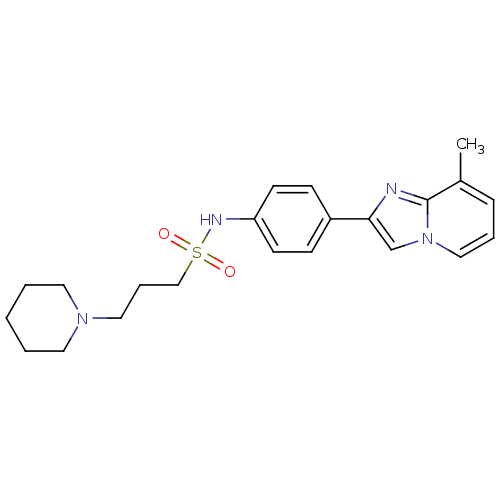 (3-Piperidin-1-yl-propane-1-sulfonic acid [4-(8-met...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | 500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Binding affinity for human histamine H3 receptor | Bioorg Med Chem Lett 12: 3309-12 (2002) BindingDB Entry DOI: 10.7270/Q2KH0MPG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin receptor type 2 (Homo sapiens (Human)) | BDBM50412866 (CHEMBL360377) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 501 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Binding affinity towards human orexin receptor type 2 was determined using [125I]-Orexin A as radio ligand | Bioorg Med Chem Lett 14: 4225-9 (2004) Article DOI: 10.1016/j.bmcl.2004.06.032 BindingDB Entry DOI: 10.7270/Q2K64MV4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin/Hypocretin receptor type 1 (Homo sapiens (Human)) | BDBM50474846 (CHEMBL185131) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 501 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Binding affinity towards human orexin receptor type 1 was determined using [125I]-Orexin A as radio ligand | Bioorg Med Chem Lett 14: 4225-9 (2004) Article DOI: 10.1016/j.bmcl.2004.06.032 BindingDB Entry DOI: 10.7270/Q2K64MV4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin/Hypocretin receptor type 1 (Homo sapiens (Human)) | BDBM50474847 (CHEMBL183421) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 501 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Binding affinity towards human orexin receptor type 1 was determined using [125I]-Orexin A as radio ligand | Bioorg Med Chem Lett 14: 4225-9 (2004) Article DOI: 10.1016/j.bmcl.2004.06.032 BindingDB Entry DOI: 10.7270/Q2K64MV4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50120538 (CHEMBL445619 | Dibutyl-{3-[4-(3,8-dimethyl-imidazo...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 870 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Binding affinity for human histamine H3 receptor | Bioorg Med Chem Lett 12: 3309-12 (2002) BindingDB Entry DOI: 10.7270/Q2KH0MPG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50120560 (2-[4-(3-Imidazol-1-yl-propoxy)-phenyl]-8-methyl-im...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Binding affinity for human histamine H3 receptor | Bioorg Med Chem Lett 12: 3309-12 (2002) BindingDB Entry DOI: 10.7270/Q2KH0MPG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin receptor type 2 (Homo sapiens (Human)) | BDBM50412865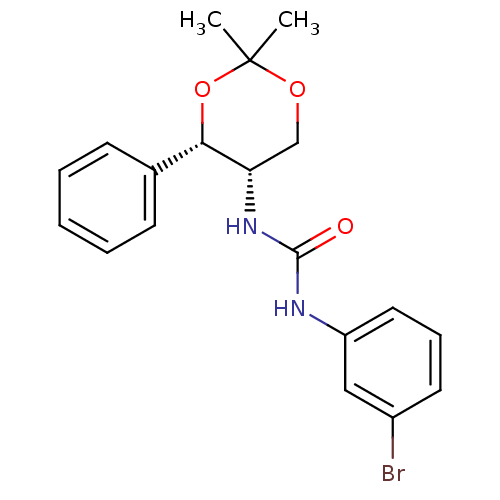 (CHEMBL185080) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Binding affinity towards human orexin receptor type 2 was determined using [125I]-Orexin A as radio ligand | Bioorg Med Chem Lett 14: 4225-9 (2004) Article DOI: 10.1016/j.bmcl.2004.06.032 BindingDB Entry DOI: 10.7270/Q2K64MV4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin/Hypocretin receptor type 1 (Homo sapiens (Human)) | BDBM50474849 (CHEMBL185424) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Binding affinity towards human orexin receptor type 1 was determined using [125I]-Orexin A as radio ligand | Bioorg Med Chem Lett 14: 4225-9 (2004) Article DOI: 10.1016/j.bmcl.2004.06.032 BindingDB Entry DOI: 10.7270/Q2K64MV4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 189 total ) | Next | Last >> |