 Found 1032 hits with Last Name = 'meng' and Initial = 'x'
Found 1032 hits with Last Name = 'meng' and Initial = 'x' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM50605126

(CHEMBL5187340)Show SMILES COc1cc(ccc1NC(=O)[C@@H]1N[C@@H](CC(C)(C)C)[C@@](CNCC2CCNCC2)([C@H]1c1cccc(Cl)c1F)c1ccc(Cl)cc1F)C(O)=O |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.00200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00095
BindingDB Entry DOI: 10.7270/Q2V69PP3 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(MOUSE) | BDBM50408679

(CHEMBL5287792)Show SMILES COc1cc2nc(nc(N)c2cc1OC)N(C)CCCCCCN(C)C(=O)c1ccc(CN(C)C)o1 Show InChI InChI=1S/C26H38N6O4/c1-30(2)17-18-11-12-21(36-18)25(33)31(3)13-9-7-8-10-14-32(4)26-28-20-16-23(35-6)22(34-5)15-19(20)24(27)29-26/h11-12,15-16H,7-10,13-14,17H2,1-6H3,(H2,27,28,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | 0.00260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity at cloned muscarinic M2 receptor-Gqi5 chimeric protein expressed in CHO cells assessed as acetylcholine-induced change in cytosol... |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(MOUSE) | BDBM50408509

(CHEMBL5277326)Show InChI InChI=1S/C20H24N2OS/c1-4-18(23)15-10-11-20-17(14-15)22(13-7-12-21(2)3)16-8-5-6-9-19(16)24-20/h5-6,8-11,14H,4,7,12-13H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| | 0.00340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity at AT1 receptor in rat aortic rings |
Citation and Details
|
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM50125967

(CHEMBL3627846)Show SMILES Cc1ccc2c(c1)nc(CCN1C(=O)c3ccccc3C1=O)n(-c1ccc3n(C)ncc3c1)c2=O Show InChI InChI=1S/C27H21N5O3/c1-16-7-9-21-22(13-16)29-24(11-12-31-25(33)19-5-3-4-6-20(19)26(31)34)32(27(21)35)18-8-10-23-17(14-18)15-28-30(23)2/h3-10,13-15H,11-12H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 0.0250 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of PDE10A (unknown origin) |
Bioorg Med Chem Lett 25: 4893-8 (2015)
Article DOI: 10.1016/j.bmcl.2015.05.080
BindingDB Entry DOI: 10.7270/Q2RJ4M9Z |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM135600
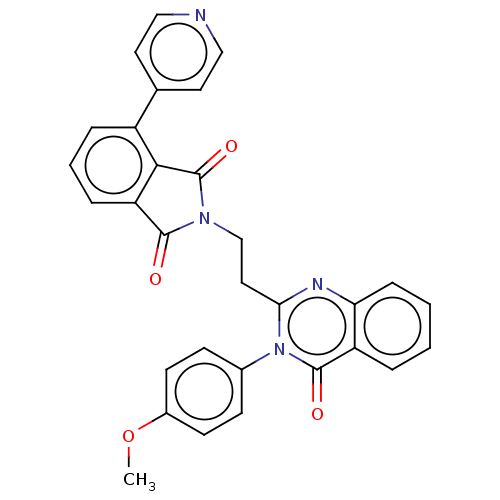
(US8846000, 1-16)Show SMILES COc1ccc(cc1)-n1c(CCN2C(=O)c3cccc(c3C2=O)-c2ccncc2)nc2ccccc2c1=O Show InChI InChI=1S/C30H22N4O4/c1-38-21-11-9-20(10-12-21)34-26(32-25-8-3-2-5-23(25)29(34)36)15-18-33-28(35)24-7-4-6-22(27(24)30(33)37)19-13-16-31-17-14-19/h2-14,16-17H,15,18H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0720 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of PDE10A (unknown origin) |
Bioorg Med Chem Lett 25: 4893-8 (2015)
Article DOI: 10.1016/j.bmcl.2015.05.080
BindingDB Entry DOI: 10.7270/Q2RJ4M9Z |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM135614

(US8846000, D-7)Show SMILES COc1ccc(cc1)-n1c(CCN2C(=O)c3cccc(OC(C)C)c3C2=O)nc2ccccc2c1=O Show InChI InChI=1S/C28H25N3O5/c1-17(2)36-23-10-6-8-21-25(23)28(34)30(26(21)32)16-15-24-29-22-9-5-4-7-20(22)27(33)31(24)18-11-13-19(35-3)14-12-18/h4-14,17H,15-16H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 0.150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of PDE10A (unknown origin) |
Bioorg Med Chem Lett 25: 4893-8 (2015)
Article DOI: 10.1016/j.bmcl.2015.05.080
BindingDB Entry DOI: 10.7270/Q2RJ4M9Z |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Mu-type opioid receptor
(MOUSE) | BDBM50408675
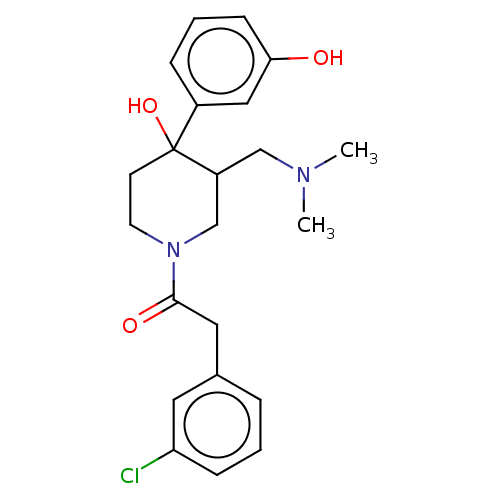
(CHEMBL5270915)Show SMILES COc1cc2nc(nc(N)c2cc1OC)N(C)CCCCCCN(C)C(=O)c1ccc(CCl)o1 Show InChI InChI=1S/C24H32ClN5O4/c1-29(23(31)19-10-9-16(15-25)34-19)11-7-5-6-8-12-30(2)24-27-18-14-21(33-4)20(32-3)13-17(18)22(26)28-24/h9-10,13-14H,5-8,11-12,15H2,1-4H3,(H2,26,27,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity at cloned muscarinic M1 receptor expressed in CHO cells assessed as acetylcholine-induced change in cytosolic calcium concentrati... |
Citation and Details
|
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM135595
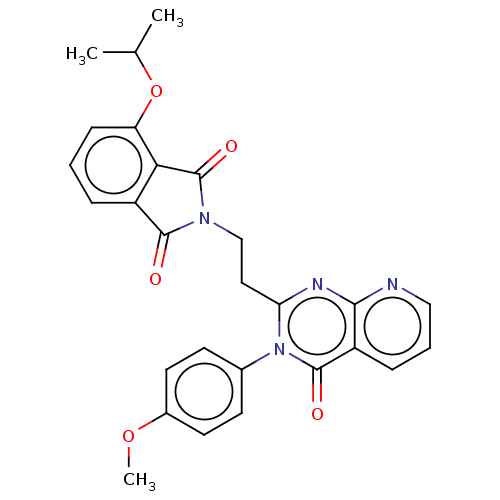
(US8846000, 1-11)Show SMILES COc1ccc(cc1)-n1c(CCN2C(=O)c3cccc(OC(C)C)c3C2=O)nc2ncccc2c1=O Show InChI InChI=1S/C27H24N4O5/c1-16(2)36-21-8-4-6-19-23(21)27(34)30(25(19)32)15-13-22-29-24-20(7-5-14-28-24)26(33)31(22)17-9-11-18(35-3)12-10-17/h4-12,14,16H,13,15H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.370 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of PDE10A (unknown origin) |
Bioorg Med Chem Lett 25: 4893-8 (2015)
Article DOI: 10.1016/j.bmcl.2015.05.080
BindingDB Entry DOI: 10.7270/Q2RJ4M9Z |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM50605132

(CHEMBL5171665)Show SMILES COc1cc(ccc1NC(=O)[C@@H]1N[C@@H](CC(C)(C)C)[C@@](CNS(=O)(=O)c2ccccc2C(O)=O)([C@H]1c1cccc(Cl)c1F)c1ccc(Cl)cc1F)C(O)=O |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00095
BindingDB Entry DOI: 10.7270/Q2V69PP3 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(MOUSE) | BDBM50408677

(CHEMBL5274117)Show SMILES COc1cc2nc(nc(N)c2cc1OC)N(C)CCCCCCN(C)C(=O)c1ccccc1CNCCCCCCN Show InChI InChI=1S/C32H49N7O3/c1-38(31(40)25-16-10-9-15-24(25)23-35-18-12-6-5-11-17-33)19-13-7-8-14-20-39(2)32-36-27-22-29(42-4)28(41-3)21-26(27)30(34)37-32/h9-10,15-16,21-22,35H,5-8,11-14,17-20,23,33H2,1-4H3,(H2,34,36,37) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity at cloned muscarinic M2 receptor-Gqi5 chimeric protein expressed in CHO cells assessed as acetylcholine-induced change in cytosol... |
Citation and Details
|
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM50605125
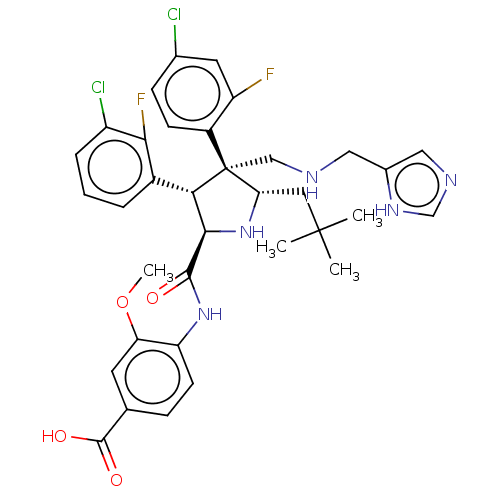
(CHEMBL5197279)Show SMILES COc1cc(ccc1NC(=O)[C@@H]1N[C@@H](CC(C)(C)C)[C@@](CNCc2cnc[nH]2)([C@H]1c1cccc(Cl)c1F)c1ccc(Cl)cc1F)C(O)=O |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00095
BindingDB Entry DOI: 10.7270/Q2V69PP3 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM21130
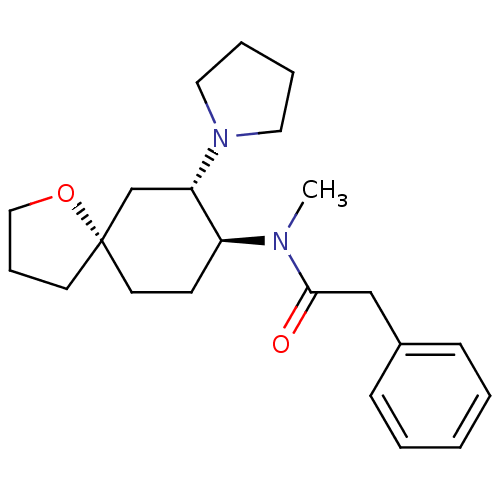
(N-methyl-2-phenyl-N-[(5R,7S,8S)-7-(pyrrolidin-1-yl...)Show SMILES CN([C@H]1CC[C@@]2(CCCO2)C[C@@H]1N1CCCC1)C(=O)Cc1ccccc1 Show InChI InChI=1S/C22H32N2O2/c1-23(21(25)16-18-8-3-2-4-9-18)19-10-12-22(11-7-15-26-22)17-20(19)24-13-5-6-14-24/h2-4,8-9,19-20H,5-7,10-17H2,1H3/t19-,20-,22-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
Similars
| | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity at oxytocin receptor in Wistar rat uterus assessed as inhibition of oxytocin-induced uterotonic activity treated 1 min prior to o... |
Citation and Details
|
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM50126108
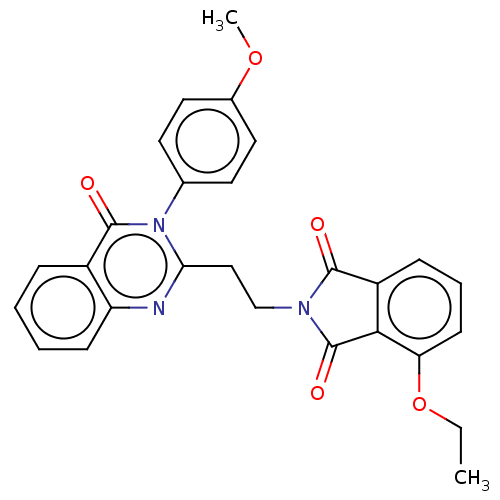
(CHEMBL3627840)Show SMILES CCOc1cccc2C(=O)N(CCc3nc4ccccc4c(=O)n3-c3ccc(OC)cc3)C(=O)c12 Show InChI InChI=1S/C27H23N3O5/c1-3-35-22-10-6-8-20-24(22)27(33)29(25(20)31)16-15-23-28-21-9-5-4-7-19(21)26(32)30(23)17-11-13-18(34-2)14-12-17/h4-14H,3,15-16H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of PDE10A (unknown origin) |
Bioorg Med Chem Lett 25: 4893-8 (2015)
Article DOI: 10.1016/j.bmcl.2015.05.080
BindingDB Entry DOI: 10.7270/Q2RJ4M9Z |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM50605123
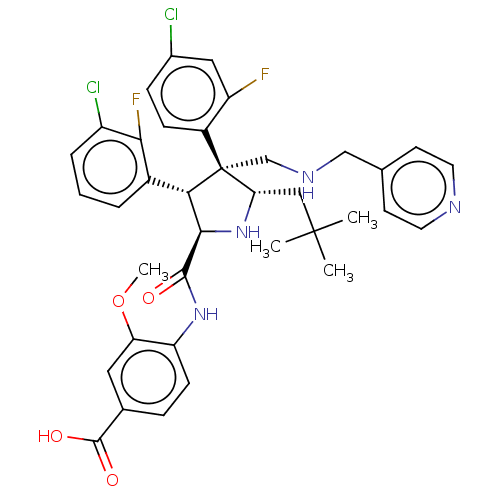
(CHEMBL5181559)Show SMILES COc1cc(ccc1NC(=O)[C@@H]1N[C@@H](CC(C)(C)C)[C@@](CNCc2ccncc2)([C@H]1c1cccc(Cl)c1F)c1ccc(Cl)cc1F)C(O)=O |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00095
BindingDB Entry DOI: 10.7270/Q2V69PP3 |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM50605114
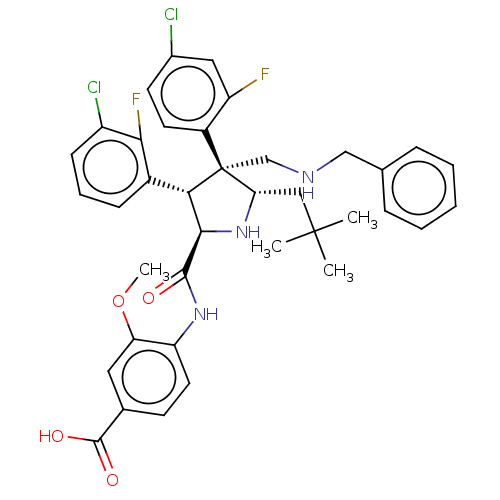
(CHEMBL5173513)Show SMILES COc1cc(ccc1NC(=O)[C@@H]1N[C@@H](CC(C)(C)C)[C@@](CNCc2ccccc2)([C@H]1c1cccc(Cl)c1F)c1ccc(Cl)cc1F)C(O)=O |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00095
BindingDB Entry DOI: 10.7270/Q2V69PP3 |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM50605147
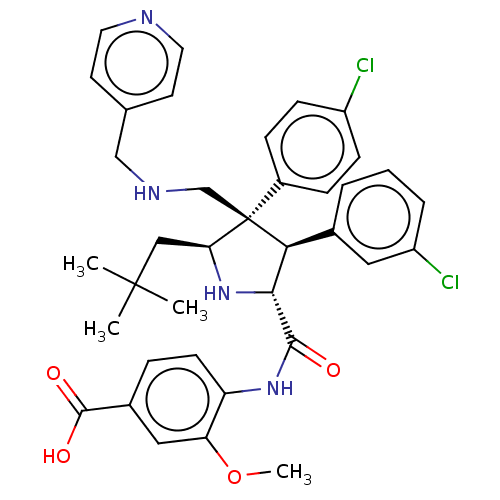
(CHEMBL5169689)Show SMILES COc1cc(ccc1NC(=O)[C@@H]1N[C@@H](CC(C)(C)C)[C@@](CNCc2ccncc2)([C@H]1c1cccc(Cl)c1)c1ccc(Cl)cc1)C(O)=O |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00095
BindingDB Entry DOI: 10.7270/Q2V69PP3 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(MOUSE) | BDBM50408673

(CHEMBL5275873)Show SMILES COc1cc2nc(nc(N)c2cc1OC)N(C)CCSSCCN(C)C(=O)c1ccc(CCl)o1 Show InChI InChI=1S/C22H28ClN5O4S2/c1-27(21(29)17-6-5-14(13-23)32-17)7-9-33-34-10-8-28(2)22-25-16-12-19(31-4)18(30-3)11-15(16)20(24)26-22/h5-6,11-12H,7-10,13H2,1-4H3,(H2,24,25,26) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity at cloned muscarinic M1 receptor expressed in CHO cells assessed as acetylcholine-induced change in cytosolic calcium concentrati... |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(MOUSE) | BDBM50408676
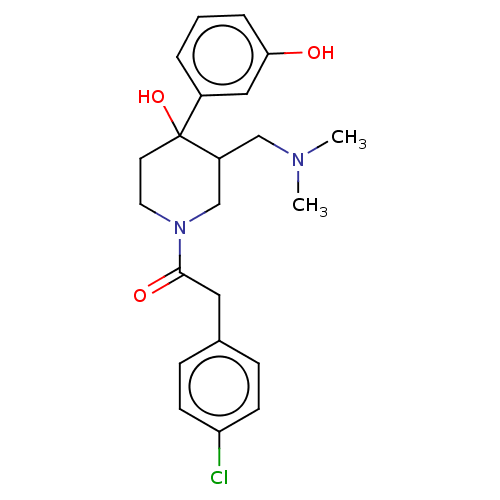
(CHEMBL5272258)Show SMILES CNCCCCCCN(C)Cc1ccc(cc1)C(=O)N(C)CCCCCCN(C)c1nc(N)c2cc(OC)c(OC)cc2n1 Show InChI InChI=1S/C34H53N7O3/c1-36-19-11-7-8-12-20-39(2)25-26-15-17-27(18-16-26)33(42)40(3)21-13-9-10-14-22-41(4)34-37-29-24-31(44-6)30(43-5)23-28(29)32(35)38-34/h15-18,23-24,36H,7-14,19-22,25H2,1-6H3,(H2,35,37,38) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity at cloned muscarinic M2 receptor-Gqi5 chimeric protein expressed in CHO cells assessed as acetylcholine-induced change in cytosol... |
Citation and Details
|
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM50605117
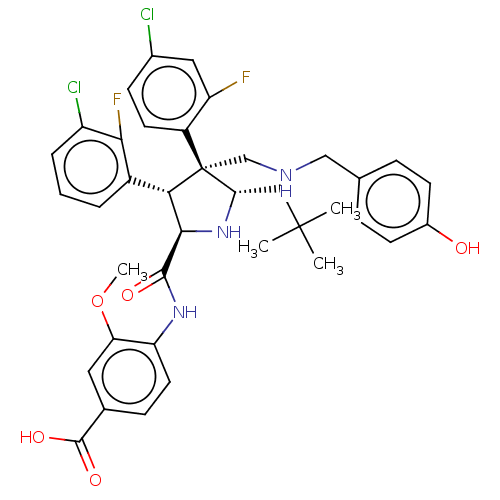
(CHEMBL5205792)Show SMILES COc1cc(ccc1NC(=O)[C@@H]1N[C@@H](CC(C)(C)C)[C@@](CNCc2ccc(O)cc2)([C@H]1c1cccc(Cl)c1F)c1ccc(Cl)cc1F)C(O)=O |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00095
BindingDB Entry DOI: 10.7270/Q2V69PP3 |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM50605116
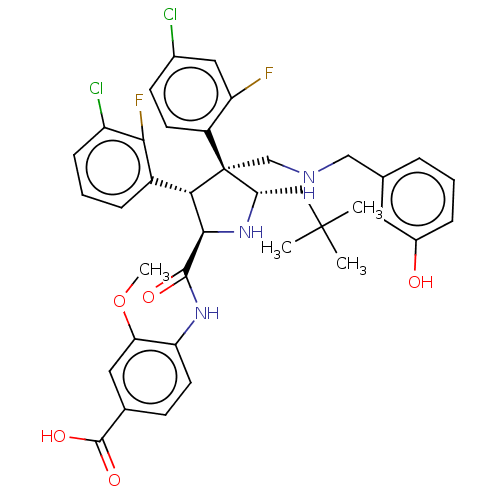
(CHEMBL5181296)Show SMILES COc1cc(ccc1NC(=O)[C@@H]1N[C@@H](CC(C)(C)C)[C@@](CNCc2cccc(O)c2)([C@H]1c1cccc(Cl)c1F)c1ccc(Cl)cc1F)C(O)=O |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00095
BindingDB Entry DOI: 10.7270/Q2V69PP3 |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM50605102
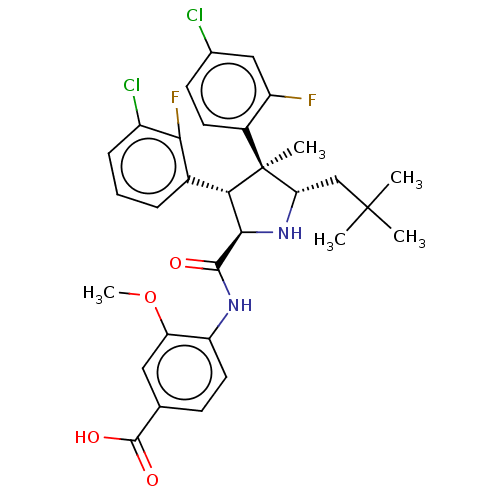
(CHEMBL5196857)Show SMILES COc1cc(ccc1NC(=O)[C@@H]1N[C@@H](CC(C)(C)C)[C@@](C)([C@H]1c1cccc(Cl)c1F)c1ccc(Cl)cc1F)C(O)=O |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00095
BindingDB Entry DOI: 10.7270/Q2V69PP3 |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM50605124
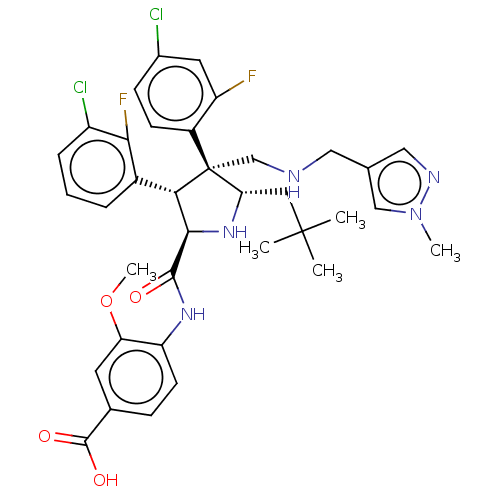
(CHEMBL5196069)Show SMILES COc1cc(ccc1NC(=O)[C@@H]1N[C@@H](CC(C)(C)C)[C@@](CNCc2cnn(C)c2)([C@H]1c1cccc(Cl)c1F)c1ccc(Cl)cc1F)C(O)=O |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00095
BindingDB Entry DOI: 10.7270/Q2V69PP3 |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM50605134
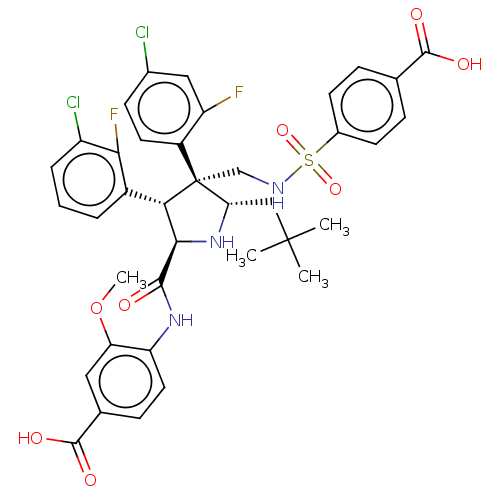
(CHEMBL5207803)Show SMILES COc1cc(ccc1NC(=O)[C@@H]1N[C@@H](CC(C)(C)C)[C@@](CNS(=O)(=O)c2ccc(cc2)C(O)=O)([C@H]1c1cccc(Cl)c1F)c1ccc(Cl)cc1F)C(O)=O |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00095
BindingDB Entry DOI: 10.7270/Q2V69PP3 |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM50126110
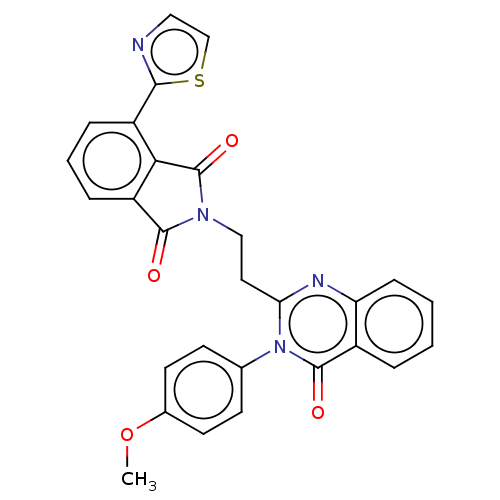
(CHEMBL3627843)Show SMILES COc1ccc(cc1)-n1c(CCN2C(=O)c3cccc(-c4nccs4)c3C2=O)nc2ccccc2c1=O Show InChI InChI=1S/C28H20N4O4S/c1-36-18-11-9-17(10-12-18)32-23(30-22-8-3-2-5-19(22)27(32)34)13-15-31-26(33)21-7-4-6-20(24(21)28(31)35)25-29-14-16-37-25/h2-12,14,16H,13,15H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of PDE10A (unknown origin) |
Bioorg Med Chem Lett 25: 4893-8 (2015)
Article DOI: 10.1016/j.bmcl.2015.05.080
BindingDB Entry DOI: 10.7270/Q2RJ4M9Z |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(MOUSE) | BDBM50408816

(CHEMBL5279890)Show SMILES N[C@H](CCCNC(N)=N)C(=O)N[C@@H](CCCNC(N)=N)C(=O)NC1(CCCCCCC1)C(=O)N[C@@H](CO)C(=O)N1Cc2ccccc2C[C@@H]1C(=O)N1[C@H]2CCCC[C@H]2C[C@H]1C(O)=O Show InChI InChI=1S/C43H68N12O8/c44-29(15-10-20-49-41(45)46)35(57)51-30(16-11-21-50-42(47)48)36(58)53-43(18-8-2-1-3-9-19-43)40(63)52-31(25-56)37(59)54-24-28-14-5-4-12-26(28)22-33(54)38(60)55-32-17-7-6-13-27(32)23-34(55)39(61)62/h4-5,12,14,27,29-34,56H,1-3,6-11,13,15-25,44H2,(H,51,57)(H,52,63)(H,53,58)(H,61,62)(H4,45,46,49)(H4,47,48,50)/t27-,29+,30-,31-,32-,33+,34-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity at histamine H1 receptor in guinea pig ileum assessed as histamine-induced contractions |
Citation and Details
|
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM50605146
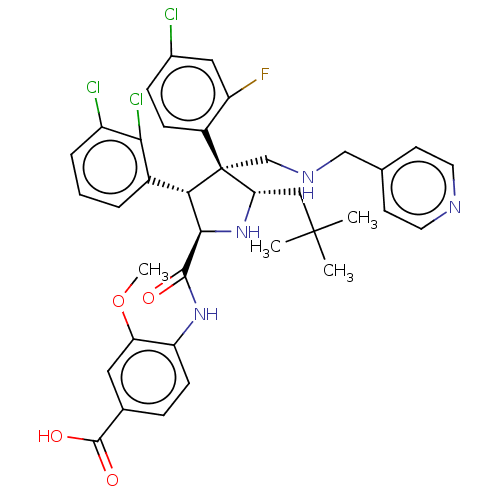
(CHEMBL5179430)Show SMILES COc1cc(ccc1NC(=O)[C@@H]1N[C@@H](CC(C)(C)C)[C@@](CNCc2ccncc2)([C@H]1c1cccc(Cl)c1Cl)c1ccc(Cl)cc1F)C(O)=O |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00095
BindingDB Entry DOI: 10.7270/Q2V69PP3 |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM50398008
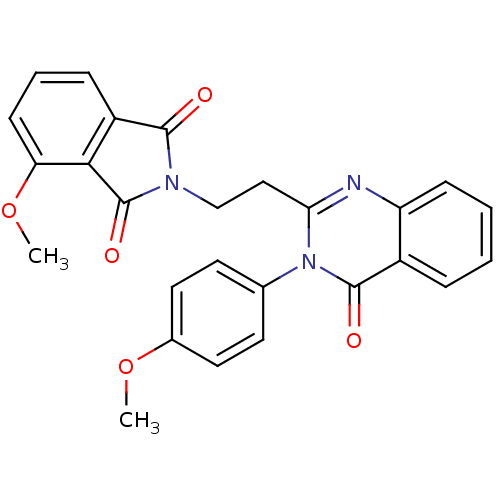
(CHEMBL2180426)Show SMILES COc1ccc(cc1)-n1c(CCN2C(=O)c3cccc(OC)c3C2=O)nc2ccccc2c1=O Show InChI InChI=1S/C26H21N3O5/c1-33-17-12-10-16(11-13-17)29-22(27-20-8-4-3-6-18(20)25(29)31)14-15-28-24(30)19-7-5-9-21(34-2)23(19)26(28)32/h3-13H,14-15H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of PDE10A (unknown origin) |
Bioorg Med Chem Lett 25: 4893-8 (2015)
Article DOI: 10.1016/j.bmcl.2015.05.080
BindingDB Entry DOI: 10.7270/Q2RJ4M9Z |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(MOUSE) | BDBM50408813
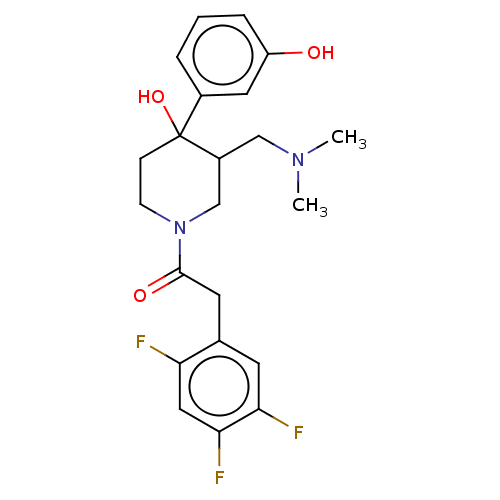
(CHEMBL5273356)Show SMILES N[C@H](CCCNC(N)=N)C(=O)N[C@@H](CCCNC(N)=N)C(=O)NCCCCCCCCCCCC(=O)N[C@@H](CO)C(=O)N1Cc2ccccc2C[C@@H]1C(=O)N1[C@H]2CCCC[C@H]2C[C@H]1C(O)=O Show InChI InChI=1S/C46H76N12O8/c47-33(19-14-24-53-45(48)49)40(61)56-34(20-15-25-54-46(50)51)41(62)52-23-13-7-5-3-1-2-4-6-8-22-39(60)55-35(29-59)42(63)57-28-32-18-10-9-16-30(32)26-37(57)43(64)58-36-21-12-11-17-31(36)27-38(58)44(65)66/h9-10,16,18,31,33-38,59H,1-8,11-15,17,19-29,47H2,(H,52,62)(H,55,60)(H,56,61)(H,65,66)(H4,48,49,53)(H4,50,51,54)/t31-,33+,34-,35-,36-,37+,38-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity at MTL receptor in rabbit duodenum homogenate |
Citation and Details
|
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM50605111
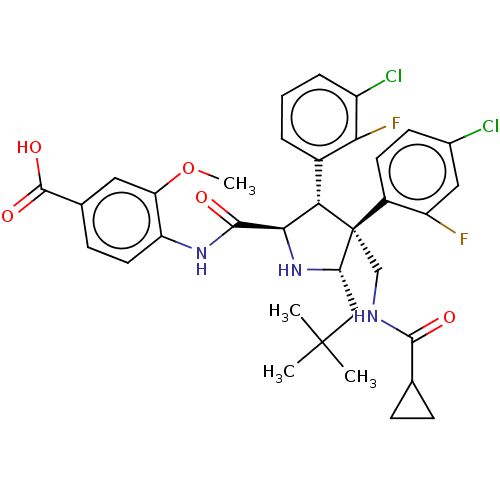
(CHEMBL5173985)Show SMILES COc1cc(ccc1NC(=O)[C@@H]1N[C@@H](CC(C)(C)C)[C@@](CNC(=O)C2CC2)([C@H]1c1cccc(Cl)c1F)c1ccc(Cl)cc1F)C(O)=O |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00095
BindingDB Entry DOI: 10.7270/Q2V69PP3 |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM50605120
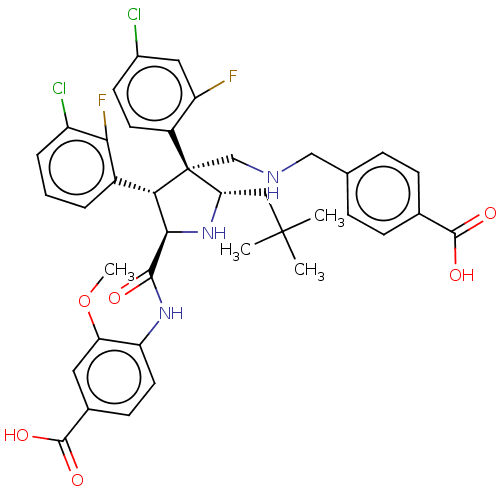
(CHEMBL5185334)Show SMILES COc1cc(ccc1NC(=O)[C@@H]1N[C@@H](CC(C)(C)C)[C@@](CNCc2ccc(cc2)C(O)=O)([C@H]1c1cccc(Cl)c1F)c1ccc(Cl)cc1F)C(O)=O |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00095
BindingDB Entry DOI: 10.7270/Q2V69PP3 |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM50605127
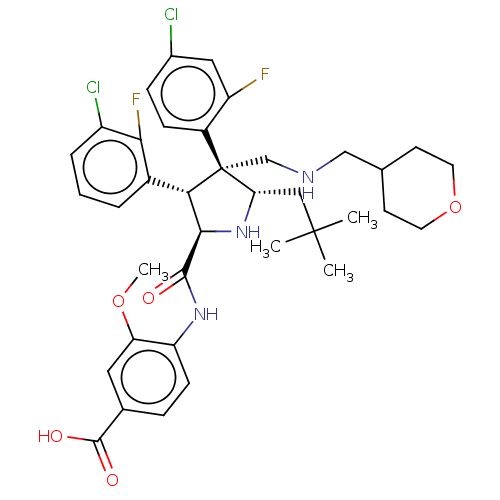
(CHEMBL5185988)Show SMILES COc1cc(ccc1NC(=O)[C@@H]1N[C@@H](CC(C)(C)C)[C@@](CNCC2CCOCC2)([C@H]1c1cccc(Cl)c1F)c1ccc(Cl)cc1F)C(O)=O |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00095
BindingDB Entry DOI: 10.7270/Q2V69PP3 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(MOUSE) | BDBM50408514
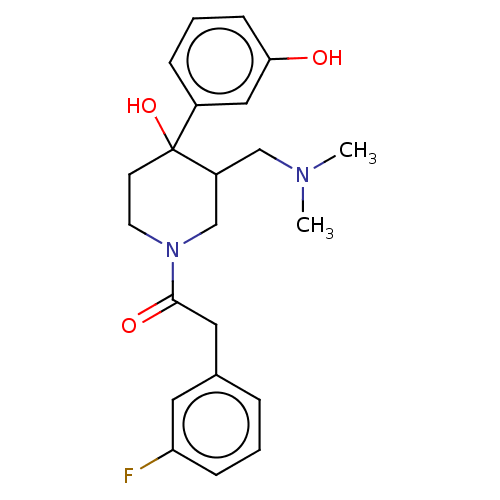
(CHEMBL5281561)Show InChI InChI=1S/C18H22N2OS/c1-19(2)10-5-11-20-15-6-3-4-7-17(15)22-18-9-8-14(13-21)12-16(18)20/h3-4,6-9,12,21H,5,10-11,13H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity at cloned muscarinic M3 receptor expressed in CHO cells assessed as acetylcholine-induced change in cytosolic calcium concentrati... |
Citation and Details
|
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM50605121
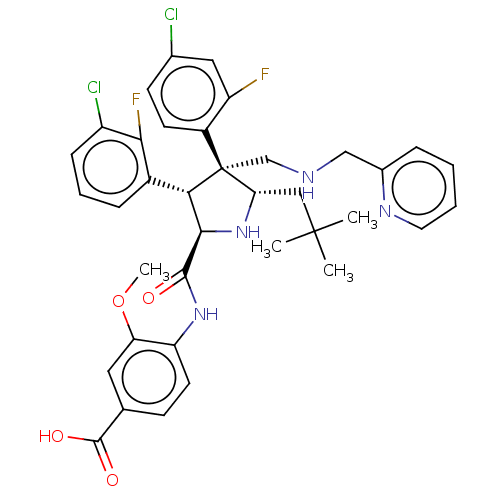
(CHEMBL5176086)Show SMILES COc1cc(ccc1NC(=O)[C@@H]1N[C@@H](CC(C)(C)C)[C@@](CNCc2ccccn2)([C@H]1c1cccc(Cl)c1F)c1ccc(Cl)cc1F)C(O)=O |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00095
BindingDB Entry DOI: 10.7270/Q2V69PP3 |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM50605106

(CHEMBL5199459)Show SMILES CCNC[C@@]1([C@H](CC(C)(C)C)N[C@H]([C@@H]1c1cccc(Cl)c1F)C(=O)Nc1ccc(cc1OC)C(O)=O)c1ccc(Cl)cc1F |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00095
BindingDB Entry DOI: 10.7270/Q2V69PP3 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(MOUSE) | BDBM21015

((2S)-2-{2-[(2R)-2-[(2S)-2-amino-3-(4-hydroxyphenyl...)Show SMILES C[C@@H](NC(=O)[C@@H](N)Cc1ccc(O)cc1)C(=O)NCC(=O)N(C)[C@@H](Cc1ccccc1)C(=O)NCCO Show InChI InChI=1S/C26H35N5O6/c1-17(30-25(36)21(27)14-19-8-10-20(33)11-9-19)24(35)29-16-23(34)31(2)22(26(37)28-12-13-32)15-18-6-4-3-5-7-18/h3-11,17,21-22,32-33H,12-16,27H2,1-2H3,(H,28,37)(H,29,35)(H,30,36)/t17-,21+,22+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity at cloned muscarinic M2 receptor-Gqi5 chimeric protein expressed in CHO cells assessed as acetylcholine-induced change in cytosol... |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM21008
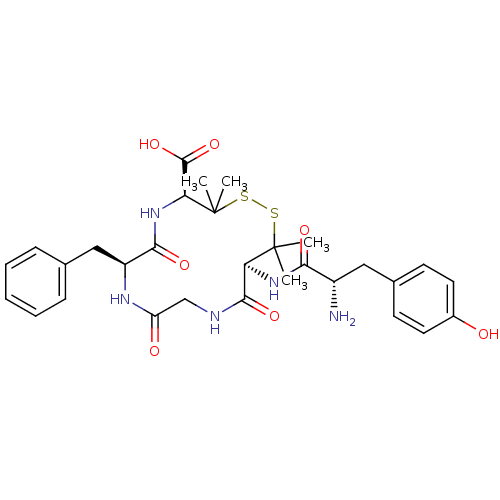
((4S,7S,13S)-13-[(2S)-2-amino-3-(4-hydroxyphenyl)pr...)Show SMILES CC1(C)SSC(C)(C)[C@@H](NC(=O)[C@@H](N)Cc2ccc(O)cc2)C(=O)NCC(=O)N[C@@H](Cc2ccccc2)C(=O)N[C@H]1C(O)=O Show InChI InChI=1S/C30H39N5O7S2/c1-29(2)23(34-25(38)20(31)14-18-10-12-19(36)13-11-18)27(40)32-16-22(37)33-21(15-17-8-6-5-7-9-17)26(39)35-24(28(41)42)30(3,4)44-43-29/h5-13,20-21,23-24,36H,14-16,31H2,1-4H3,(H,32,40)(H,33,37)(H,34,38)(H,35,39)(H,41,42)/t20-,21-,23-,24-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity at human recombinant EP1 receptor expressed in CHO cells assessed as luciferase activity by Schild assay |
Citation and Details
|
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM50605130
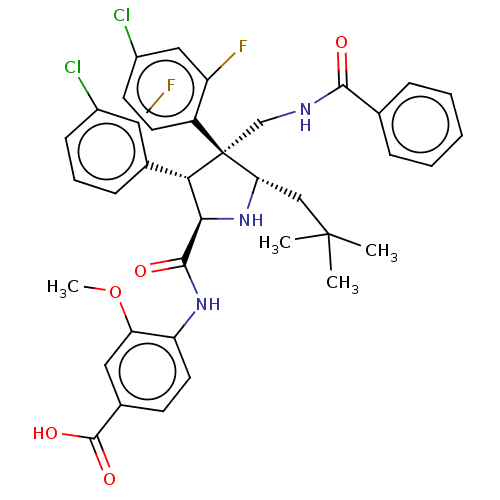
(CHEMBL5195734)Show SMILES COc1cc(ccc1NC(=O)[C@@H]1N[C@@H](CC(C)(C)C)[C@@](CNC(=O)c2ccccc2)([C@H]1c1cccc(Cl)c1F)c1ccc(Cl)cc1F)C(O)=O |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00095
BindingDB Entry DOI: 10.7270/Q2V69PP3 |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM50605113
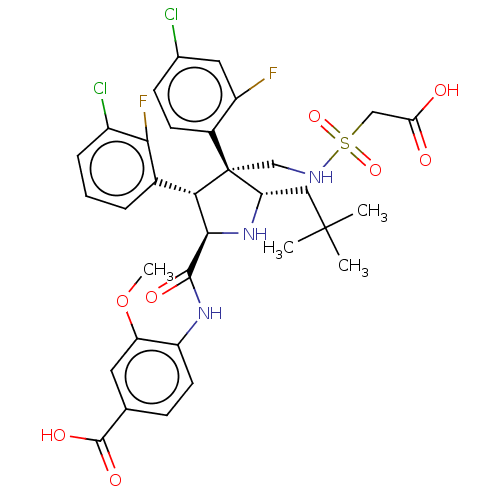
(CHEMBL5183808)Show SMILES COc1cc(ccc1NC(=O)[C@@H]1N[C@@H](CC(C)(C)C)[C@@](CNS(=O)(=O)CC(O)=O)([C@H]1c1cccc(Cl)c1F)c1ccc(Cl)cc1F)C(O)=O |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00095
BindingDB Entry DOI: 10.7270/Q2V69PP3 |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM50605119
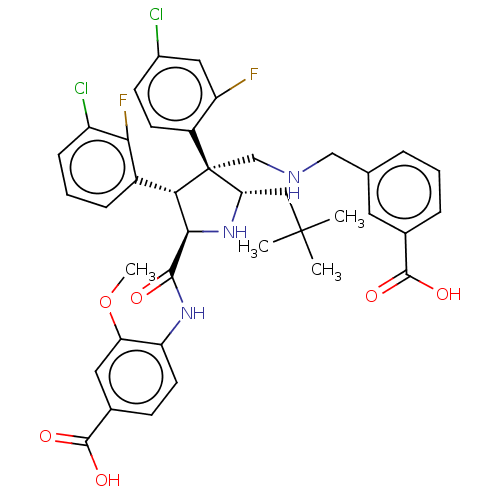
(CHEMBL5190459)Show SMILES COc1cc(ccc1NC(=O)[C@@H]1N[C@@H](CC(C)(C)C)[C@@](CNCc2cccc(c2)C(O)=O)([C@H]1c1cccc(Cl)c1F)c1ccc(Cl)cc1F)C(O)=O |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00095
BindingDB Entry DOI: 10.7270/Q2V69PP3 |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM50605118
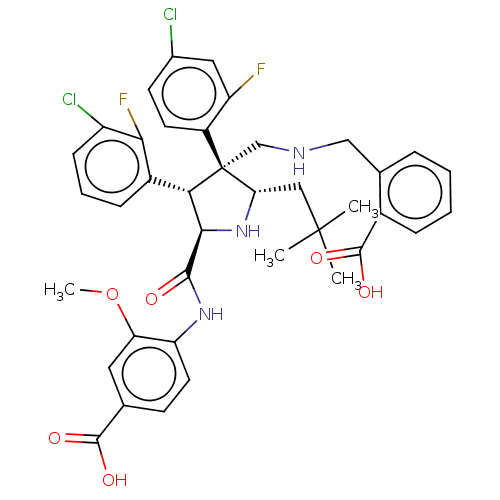
(CHEMBL5200925)Show SMILES COc1cc(ccc1NC(=O)[C@@H]1N[C@@H](CC(C)(C)C)[C@@](CNCc2ccccc2C(O)=O)([C@H]1c1cccc(Cl)c1F)c1ccc(Cl)cc1F)C(O)=O |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00095
BindingDB Entry DOI: 10.7270/Q2V69PP3 |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM50605110
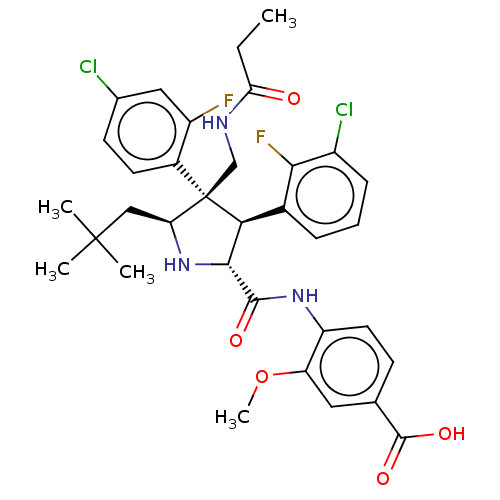
(CHEMBL5186698)Show SMILES CCC(=O)NC[C@@]1([C@H](CC(C)(C)C)N[C@H]([C@@H]1c1cccc(Cl)c1F)C(=O)Nc1ccc(cc1OC)C(O)=O)c1ccc(Cl)cc1F |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00095
BindingDB Entry DOI: 10.7270/Q2V69PP3 |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM50605122
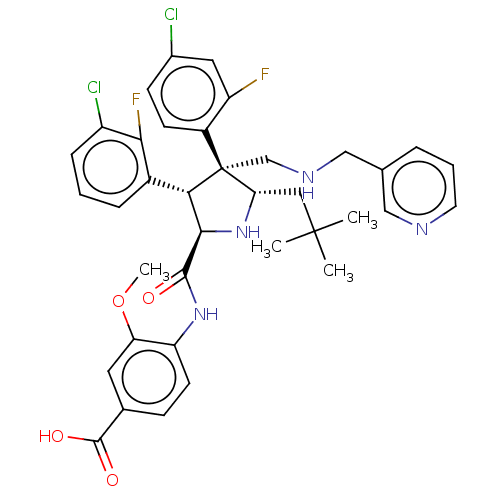
(CHEMBL5188708)Show SMILES COc1cc(ccc1NC(=O)[C@@H]1N[C@@H](CC(C)(C)C)[C@@](CNCc2cccnc2)([C@H]1c1cccc(Cl)c1F)c1ccc(Cl)cc1F)C(O)=O |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00095
BindingDB Entry DOI: 10.7270/Q2V69PP3 |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM50605109
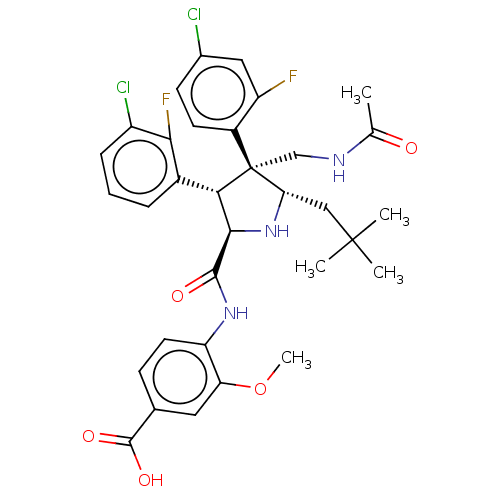
(CHEMBL5194615)Show SMILES COc1cc(ccc1NC(=O)[C@@H]1N[C@@H](CC(C)(C)C)[C@@](CNC(C)=O)([C@H]1c1cccc(Cl)c1F)c1ccc(Cl)cc1F)C(O)=O |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00095
BindingDB Entry DOI: 10.7270/Q2V69PP3 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(MOUSE) | BDBM50408669
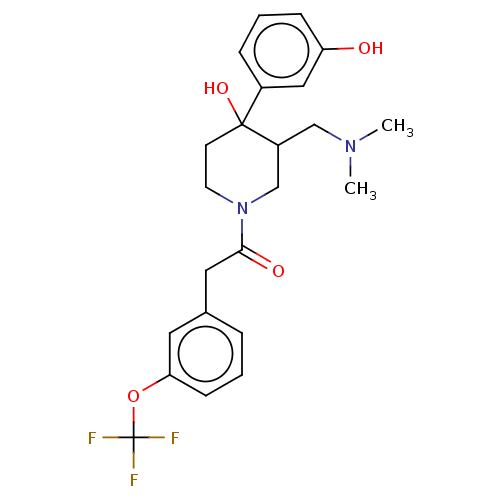
(CHEMBL5272171)Show SMILES COc1cc2nc(nc(N)c2cc1OC)N(C)CCCCCCN(C)C(=O)c1ccccc1CN(C)C Show InChI InChI=1S/C28H40N6O3/c1-32(2)19-20-13-9-10-14-21(20)27(35)33(3)15-11-7-8-12-16-34(4)28-30-23-18-25(37-6)24(36-5)17-22(23)26(29)31-28/h9-10,13-14,17-18H,7-8,11-12,15-16,19H2,1-6H3,(H2,29,30,31) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity at cloned muscarinic M3 receptor expressed in CHO cells assessed as acetylcholine-induced change in cytosolic calcium concentrati... |
Citation and Details
|
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM50605115
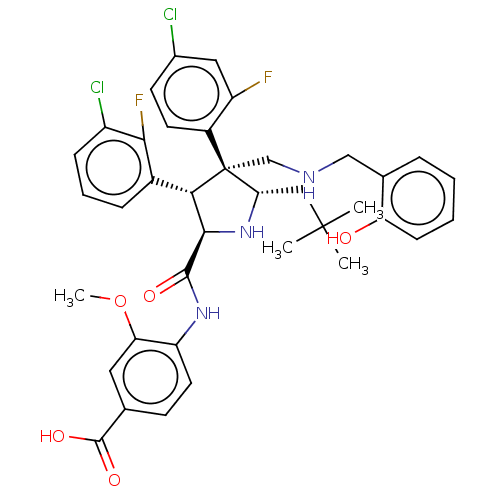
(CHEMBL5191798)Show SMILES COc1cc(ccc1NC(=O)[C@@H]1N[C@@H](CC(C)(C)C)[C@@](CNCc2ccccc2O)([C@H]1c1cccc(Cl)c1F)c1ccc(Cl)cc1F)C(O)=O |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00095
BindingDB Entry DOI: 10.7270/Q2V69PP3 |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM50605129
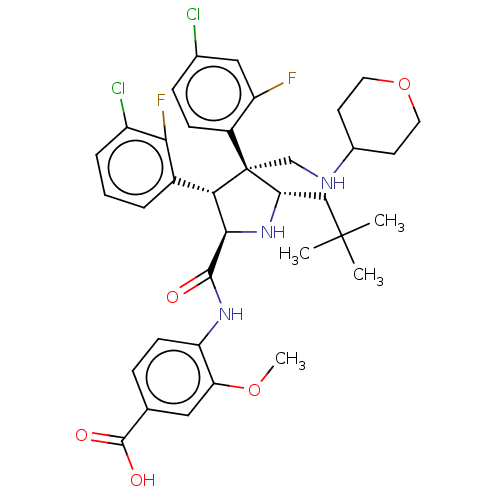
(CHEMBL5196353)Show SMILES COc1cc(ccc1NC(=O)[C@@H]1N[C@@H](CC(C)(C)C)[C@@](CNC2CCOCC2)([C@H]1c1cccc(Cl)c1F)c1ccc(Cl)cc1F)C(O)=O |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 3.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00095
BindingDB Entry DOI: 10.7270/Q2V69PP3 |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM50605133
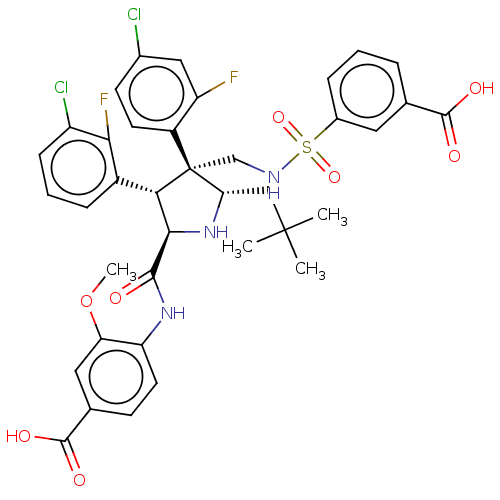
(CHEMBL5186741)Show SMILES COc1cc(ccc1NC(=O)[C@@H]1N[C@@H](CC(C)(C)C)[C@@](CNS(=O)(=O)c2cccc(c2)C(O)=O)([C@H]1c1cccc(Cl)c1F)c1ccc(Cl)cc1F)C(O)=O |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 3.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00095
BindingDB Entry DOI: 10.7270/Q2V69PP3 |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM50605139
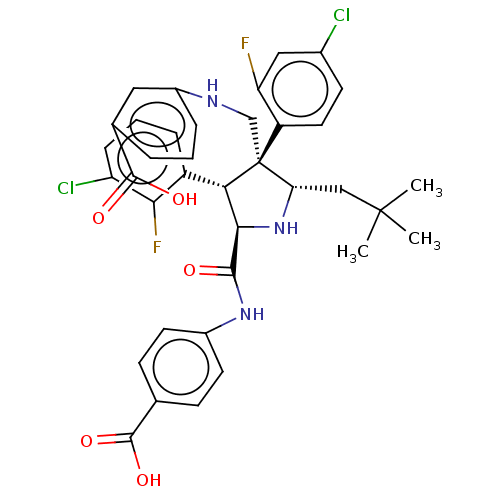
(CHEMBL5184577)Show SMILES CC(C)(C)C[C@@H]1N[C@H]([C@H](c2cccc(Cl)c2F)[C@@]1(CNc1cccc(c1)C(O)=O)c1ccc(Cl)cc1F)C(=O)Nc1ccc(cc1)C(O)=O |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 3.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00095
BindingDB Entry DOI: 10.7270/Q2V69PP3 |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM50605108
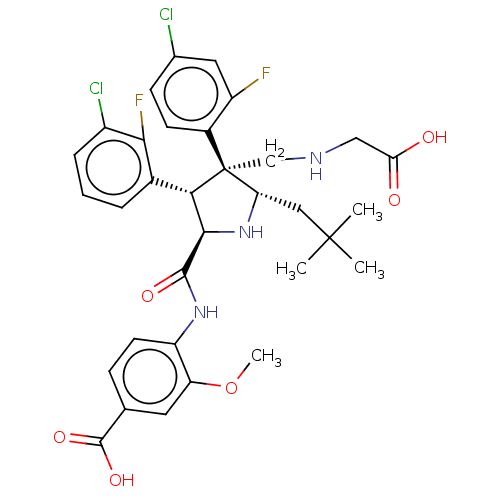
(CHEMBL5204732)Show SMILES COc1cc(ccc1NC(=O)[C@@H]1N[C@@H](CC(C)(C)C)[C@@](CNCC(O)=O)([C@H]1c1cccc(Cl)c1F)c1ccc(Cl)cc1F)C(O)=O |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 3.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00095
BindingDB Entry DOI: 10.7270/Q2V69PP3 |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM50605137
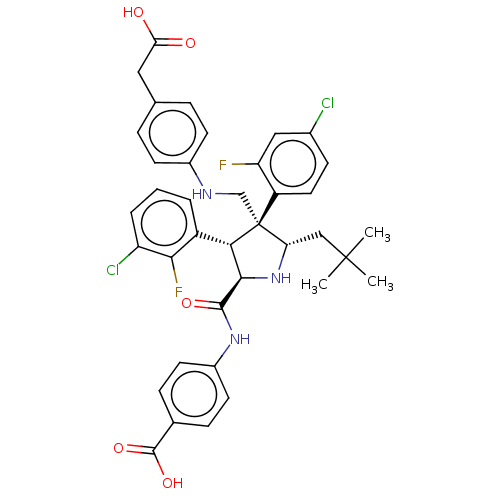
(CHEMBL5183859)Show SMILES CC(C)(C)C[C@@H]1N[C@H]([C@H](c2cccc(Cl)c2F)[C@@]1(CNc1ccc(CC(O)=O)cc1)c1ccc(Cl)cc1F)C(=O)Nc1ccc(cc1)C(O)=O |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 3.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00095
BindingDB Entry DOI: 10.7270/Q2V69PP3 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data