 Found 57 hits with Last Name = 'mirand' and Initial = 'c'
Found 57 hits with Last Name = 'mirand' and Initial = 'c' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Low molecular weight phosphotyrosine protein phosphatase
(Mus musculus) | BDBM50096252
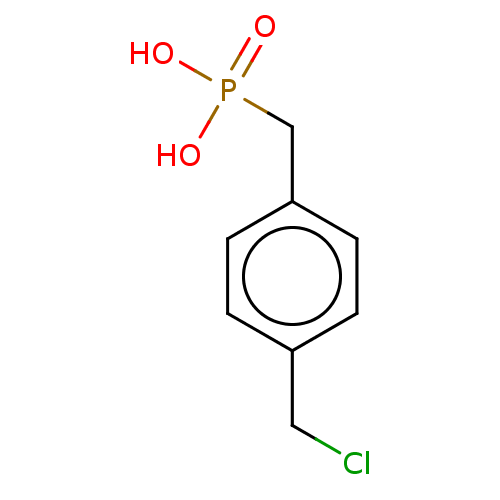
(CHEMBL3594198)Show InChI InChI=1S/C36H62N4O6/c1-5-8-9-10-11-12-13-15-21-28(42)25-30-34(44)39-32(26(4)6-2)36(46)40-24-19-18-23-31(40)35(45)37-29(33(43)38-30)22-17-14-16-20-27(41)7-3/h26,29-32H,5-25H2,1-4H3,(H,37,45)(H,38,43)(H,39,44)/t26?,29-,30-,31+,32-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 4.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Campinas
Curated by ChEMBL
| Assay Description
Competitive inhibition of mouse LMW-PTP expressed in Escherichia coli using pNPP substrate by Dixon plot |
Bioorg Med Chem 23: 4462-71 (2015)
Article DOI: 10.1016/j.bmc.2015.06.017
BindingDB Entry DOI: 10.7270/Q2R78GZ7 |
More data for this
Ligand-Target Pair | |
Low molecular weight phosphotyrosine protein phosphatase
(Mus musculus) | BDBM50096253
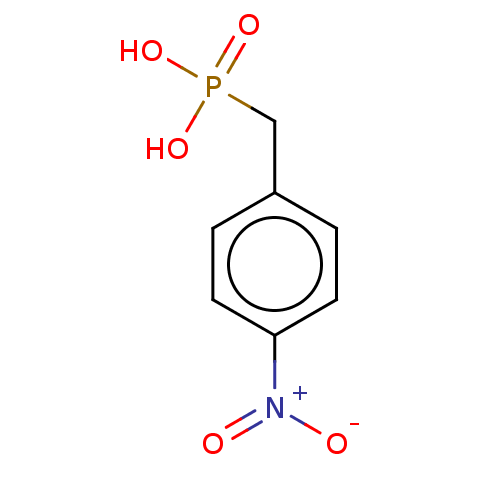
(CHEMBL3593255)Show InChI InChI=1S/C35H49N5O7/c1-4-22(3)31-35(47)40-18-12-11-17-29(40)34(46)36-26(15-8-6-7-13-24(41)5-2)32(44)37-27(33(45)38-31)19-23-20-39(21-30(42)43)28-16-10-9-14-25(23)28/h9-10,14,16,20,22,26-27,29,31H,4-8,11-13,15,17-19,21H2,1-3H3,(H,36,46)(H,37,44)(H,38,45)(H,42,43)/t22?,26-,27-,29+,31-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.24E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Campinas
Curated by ChEMBL
| Assay Description
Competitive inhibition of mouse LMW-PTP expressed in Escherichia coli using pNPP substrate by Dixon plot |
Bioorg Med Chem 23: 4462-71 (2015)
Article DOI: 10.1016/j.bmc.2015.06.017
BindingDB Entry DOI: 10.7270/Q2R78GZ7 |
More data for this
Ligand-Target Pair | |
Alkaline phosphatase, placental type
(Homo sapiens (Human)) | BDBM50080274
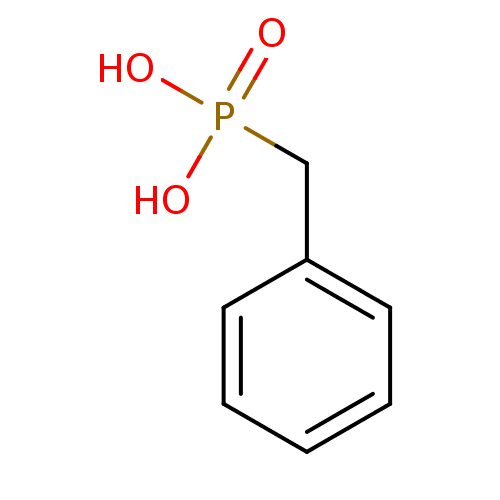
(Benzyl-phosphonic acid | CHEMBL299737 | Phenyl-met...)Show InChI InChI=1S/C7H9O3P/c8-11(9,10)6-7-4-2-1-3-5-7/h1-5H,6H2,(H2,8,9,10) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 5.80E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Campinas
Curated by ChEMBL
| Assay Description
Inhibition of human placental alkaline phosphatase using p-nitrophenyl phosphate substrate |
Bioorg Med Chem 23: 4462-71 (2015)
Article DOI: 10.1016/j.bmc.2015.06.017
BindingDB Entry DOI: 10.7270/Q2R78GZ7 |
More data for this
Ligand-Target Pair | |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 3
(Bos taurus) | BDBM50080274

(Benzyl-phosphonic acid | CHEMBL299737 | Phenyl-met...)Show InChI InChI=1S/C7H9O3P/c8-11(9,10)6-7-4-2-1-3-5-7/h1-5H,6H2,(H2,8,9,10) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 1.40E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Campinas
Curated by ChEMBL
| Assay Description
Inhibition of bovine intestinal 5'-nucleotide phosphodiesterase using 4-nitrophenyl phenylphosphonate substrate by spectrophotometry |
Bioorg Med Chem 23: 4462-71 (2015)
Article DOI: 10.1016/j.bmc.2015.06.017
BindingDB Entry DOI: 10.7270/Q2R78GZ7 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50080274

(Benzyl-phosphonic acid | CHEMBL299737 | Phenyl-met...)Show InChI InChI=1S/C7H9O3P/c8-11(9,10)6-7-4-2-1-3-5-7/h1-5H,6H2,(H2,8,9,10) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 3.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Campinas
Curated by ChEMBL
| Assay Description
Inhibition of PTP1B (unknown origin) using nPP substrate |
Bioorg Med Chem 23: 4462-71 (2015)
Article DOI: 10.1016/j.bmc.2015.06.017
BindingDB Entry DOI: 10.7270/Q2R78GZ7 |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50062351

((R)-N*4*-Hydroxy-N*1*-[(S)-2-(1H-indol-3-yl)-1-met...)Show SMILES CNC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](CC(C)C)CC(=O)NO |r| Show InChI InChI=1S/C20H28N4O4/c1-12(2)8-13(10-18(25)24-28)19(26)23-17(20(27)21-3)9-14-11-22-16-7-5-4-6-15(14)16/h4-7,11-13,17,22,28H,8-10H2,1-3H3,(H,21,27)(H,23,26)(H,24,25)/t13-,17+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 0.104 | n/a | n/a | n/a | n/a | n/a | n/a |
UMR/CNRS 6013
Curated by ChEMBL
| Assay Description
Inhibition of human matrix metalloproteinase-2 |
Bioorg Med Chem Lett 13: 2843-6 (2003)
BindingDB Entry DOI: 10.7270/Q2PN952R |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50062351

((R)-N*4*-Hydroxy-N*1*-[(S)-2-(1H-indol-3-yl)-1-met...)Show SMILES CNC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](CC(C)C)CC(=O)NO |r| Show InChI InChI=1S/C20H28N4O4/c1-12(2)8-13(10-18(25)24-28)19(26)23-17(20(27)21-3)9-14-11-22-16-7-5-4-6-15(14)16/h4-7,11-13,17,22,28H,8-10H2,1-3H3,(H,21,27)(H,23,26)(H,24,25)/t13-,17+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 0.191 | n/a | n/a | n/a | n/a | n/a | n/a |
UMR/CNRS 6013
Curated by ChEMBL
| Assay Description
Inhibition of human matrix metalloproteinase-3 |
Bioorg Med Chem Lett 13: 2843-6 (2003)
BindingDB Entry DOI: 10.7270/Q2PN952R |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50062351

((R)-N*4*-Hydroxy-N*1*-[(S)-2-(1H-indol-3-yl)-1-met...)Show SMILES CNC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](CC(C)C)CC(=O)NO |r| Show InChI InChI=1S/C20H28N4O4/c1-12(2)8-13(10-18(25)24-28)19(26)23-17(20(27)21-3)9-14-11-22-16-7-5-4-6-15(14)16/h4-7,11-13,17,22,28H,8-10H2,1-3H3,(H,21,27)(H,23,26)(H,24,25)/t13-,17+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Université de Reims-Champagne-Ardenne
Curated by ChEMBL
| Assay Description
Inhibition of MMP9 |
Bioorg Med Chem 15: 4753-66 (2007)
Article DOI: 10.1016/j.bmc.2007.05.001
BindingDB Entry DOI: 10.7270/Q2Q23ZXN |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50062351

((R)-N*4*-Hydroxy-N*1*-[(S)-2-(1H-indol-3-yl)-1-met...)Show SMILES CNC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](CC(C)C)CC(=O)NO |r| Show InChI InChI=1S/C20H28N4O4/c1-12(2)8-13(10-18(25)24-28)19(26)23-17(20(27)21-3)9-14-11-22-16-7-5-4-6-15(14)16/h4-7,11-13,17,22,28H,8-10H2,1-3H3,(H,21,27)(H,23,26)(H,24,25)/t13-,17+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Université de Reims-Champagne-Ardenne
Curated by ChEMBL
| Assay Description
Inhibition of MMP2 |
Bioorg Med Chem 15: 4753-66 (2007)
Article DOI: 10.1016/j.bmc.2007.05.001
BindingDB Entry DOI: 10.7270/Q2Q23ZXN |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50135601

((S,E)-N1-(3-(1H-indol-3-yl)-1-(methylamino)-1-oxop...)Show SMILES CNC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)C(\CC(=O)NO)=C\C(C)C Show InChI InChI=1S/C20H26N4O4/c1-12(2)8-13(10-18(25)24-28)19(26)23-17(20(27)21-3)9-14-11-22-16-7-5-4-6-15(14)16/h4-8,11-12,17,22,28H,9-10H2,1-3H3,(H,21,27)(H,23,26)(H,24,25)/b13-8+/t17-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
UMR/CNRS 6013
Curated by ChEMBL
| Assay Description
Inhibition of human matrix metalloprotease-2 |
Bioorg Med Chem Lett 13: 2843-6 (2003)
BindingDB Entry DOI: 10.7270/Q2PN952R |
More data for this
Ligand-Target Pair | |
Interstitial collagenase
(Homo sapiens (Human)) | BDBM50062351

((R)-N*4*-Hydroxy-N*1*-[(S)-2-(1H-indol-3-yl)-1-met...)Show SMILES CNC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](CC(C)C)CC(=O)NO |r| Show InChI InChI=1S/C20H28N4O4/c1-12(2)8-13(10-18(25)24-28)19(26)23-17(20(27)21-3)9-14-11-22-16-7-5-4-6-15(14)16/h4-7,11-13,17,22,28H,8-10H2,1-3H3,(H,21,27)(H,23,26)(H,24,25)/t13-,17+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Université de Reims-Champagne-Ardenne
Curated by ChEMBL
| Assay Description
Inhibition of MMP1 |
Bioorg Med Chem 15: 4753-66 (2007)
Article DOI: 10.1016/j.bmc.2007.05.001
BindingDB Entry DOI: 10.7270/Q2Q23ZXN |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50062351

((R)-N*4*-Hydroxy-N*1*-[(S)-2-(1H-indol-3-yl)-1-met...)Show SMILES CNC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](CC(C)C)CC(=O)NO |r| Show InChI InChI=1S/C20H28N4O4/c1-12(2)8-13(10-18(25)24-28)19(26)23-17(20(27)21-3)9-14-11-22-16-7-5-4-6-15(14)16/h4-7,11-13,17,22,28H,8-10H2,1-3H3,(H,21,27)(H,23,26)(H,24,25)/t13-,17+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Université de Reims-Champagne-Ardenne
Curated by ChEMBL
| Assay Description
Inhibition of MMP3 |
Bioorg Med Chem 15: 4753-66 (2007)
Article DOI: 10.1016/j.bmc.2007.05.001
BindingDB Entry DOI: 10.7270/Q2Q23ZXN |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50135602
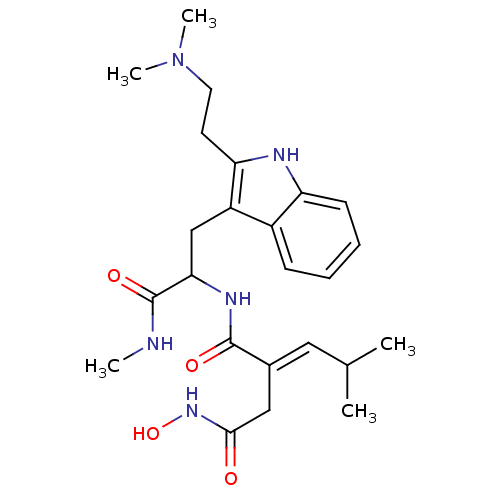
(CHEMBL420259 | N*1*-{2-[2-(2-Dimethylamino-ethyl)-...)Show SMILES CNC(=O)C(Cc1c(CCN(C)C)[nH]c2ccccc12)NC(=O)C(\CC(=O)NO)=C\C(C)C Show InChI InChI=1S/C24H35N5O4/c1-15(2)12-16(13-22(30)28-33)23(31)27-21(24(32)25-3)14-18-17-8-6-7-9-19(17)26-20(18)10-11-29(4)5/h6-9,12,15,21,26,33H,10-11,13-14H2,1-5H3,(H,25,32)(H,27,31)(H,28,30)/b16-12+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 5.70 | n/a | n/a | n/a | n/a | n/a | n/a |
UMR/CNRS 6013
Curated by ChEMBL
| Assay Description
Inhibition of human matrix metalloproteinase-2 |
Bioorg Med Chem Lett 13: 2843-6 (2003)
BindingDB Entry DOI: 10.7270/Q2PN952R |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50135600

((S)-2-((E)-2-Hydroxycarbamoylmethyl-4-methyl-pent-...)Show SMILES CCOC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)C(\CC(=O)NO)=C\C(C)C Show InChI InChI=1S/C21H27N3O5/c1-4-29-21(27)18(10-15-12-22-17-8-6-5-7-16(15)17)23-20(26)14(9-13(2)3)11-19(25)24-28/h5-9,12-13,18,22,28H,4,10-11H2,1-3H3,(H,23,26)(H,24,25)/b14-9+/t18-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
UMR/CNRS 6013
Curated by ChEMBL
| Assay Description
Inhibition of human matrix metalloproteinase-2 |
Bioorg Med Chem Lett 13: 2843-6 (2003)
BindingDB Entry DOI: 10.7270/Q2PN952R |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50135601

((S,E)-N1-(3-(1H-indol-3-yl)-1-(methylamino)-1-oxop...)Show SMILES CNC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)C(\CC(=O)NO)=C\C(C)C Show InChI InChI=1S/C20H26N4O4/c1-12(2)8-13(10-18(25)24-28)19(26)23-17(20(27)21-3)9-14-11-22-16-7-5-4-6-15(14)16/h4-8,11-12,17,22,28H,9-10H2,1-3H3,(H,21,27)(H,23,26)(H,24,25)/b13-8+/t17-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Université de Reims-Champagne-Ardenne
Curated by ChEMBL
| Assay Description
Inhibition of MMP2 |
Bioorg Med Chem 15: 4753-66 (2007)
Article DOI: 10.1016/j.bmc.2007.05.001
BindingDB Entry DOI: 10.7270/Q2Q23ZXN |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-14
(Homo sapiens (Human)) | BDBM50135601

((S,E)-N1-(3-(1H-indol-3-yl)-1-(methylamino)-1-oxop...)Show SMILES CNC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)C(\CC(=O)NO)=C\C(C)C Show InChI InChI=1S/C20H26N4O4/c1-12(2)8-13(10-18(25)24-28)19(26)23-17(20(27)21-3)9-14-11-22-16-7-5-4-6-15(14)16/h4-8,11-12,17,22,28H,9-10H2,1-3H3,(H,21,27)(H,23,26)(H,24,25)/b13-8+/t17-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Université de Reims-Champagne-Ardenne
Curated by ChEMBL
| Assay Description
Inhibition of MMP14 |
Bioorg Med Chem 15: 4753-66 (2007)
Article DOI: 10.1016/j.bmc.2007.05.001
BindingDB Entry DOI: 10.7270/Q2Q23ZXN |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-14
(Homo sapiens (Human)) | BDBM50062351

((R)-N*4*-Hydroxy-N*1*-[(S)-2-(1H-indol-3-yl)-1-met...)Show SMILES CNC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](CC(C)C)CC(=O)NO |r| Show InChI InChI=1S/C20H28N4O4/c1-12(2)8-13(10-18(25)24-28)19(26)23-17(20(27)21-3)9-14-11-22-16-7-5-4-6-15(14)16/h4-7,11-13,17,22,28H,8-10H2,1-3H3,(H,21,27)(H,23,26)(H,24,25)/t13-,17+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Université de Reims-Champagne-Ardenne
Curated by ChEMBL
| Assay Description
Inhibition of MMP14 |
Bioorg Med Chem 15: 4753-66 (2007)
Article DOI: 10.1016/j.bmc.2007.05.001
BindingDB Entry DOI: 10.7270/Q2Q23ZXN |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50135601

((S,E)-N1-(3-(1H-indol-3-yl)-1-(methylamino)-1-oxop...)Show SMILES CNC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)C(\CC(=O)NO)=C\C(C)C Show InChI InChI=1S/C20H26N4O4/c1-12(2)8-13(10-18(25)24-28)19(26)23-17(20(27)21-3)9-14-11-22-16-7-5-4-6-15(14)16/h4-8,11-12,17,22,28H,9-10H2,1-3H3,(H,21,27)(H,23,26)(H,24,25)/b13-8+/t17-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Université de Reims-Champagne-Ardenne
Curated by ChEMBL
| Assay Description
Inhibition of MMP9 |
Bioorg Med Chem 15: 4753-66 (2007)
Article DOI: 10.1016/j.bmc.2007.05.001
BindingDB Entry DOI: 10.7270/Q2Q23ZXN |
More data for this
Ligand-Target Pair | |
Interstitial collagenase
(Homo sapiens (Human)) | BDBM50135601

((S,E)-N1-(3-(1H-indol-3-yl)-1-(methylamino)-1-oxop...)Show SMILES CNC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)C(\CC(=O)NO)=C\C(C)C Show InChI InChI=1S/C20H26N4O4/c1-12(2)8-13(10-18(25)24-28)19(26)23-17(20(27)21-3)9-14-11-22-16-7-5-4-6-15(14)16/h4-8,11-12,17,22,28H,9-10H2,1-3H3,(H,21,27)(H,23,26)(H,24,25)/b13-8+/t17-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Université de Reims-Champagne-Ardenne
Curated by ChEMBL
| Assay Description
Inhibition of MMP1 |
Bioorg Med Chem 15: 4753-66 (2007)
Article DOI: 10.1016/j.bmc.2007.05.001
BindingDB Entry DOI: 10.7270/Q2Q23ZXN |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50451552
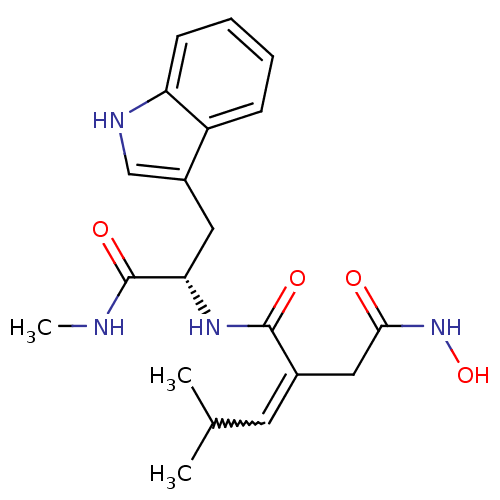
(CHEMBL2111573)Show SMILES CNC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)C(CC(=O)NO)=CC(C)C Show InChI InChI=1S/C20H26N4O4/c1-12(2)8-13(10-18(25)24-28)19(26)23-17(20(27)21-3)9-14-11-22-16-7-5-4-6-15(14)16/h4-8,11-12,17,22,28H,9-10H2,1-3H3,(H,21,27)(H,23,26)(H,24,25)/t17-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
UMR/CNRS 6013
Curated by ChEMBL
| Assay Description
Inhibition of human matrix metalloproteinase-2 |
Bioorg Med Chem Lett 13: 2843-6 (2003)
BindingDB Entry DOI: 10.7270/Q2PN952R |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50213416
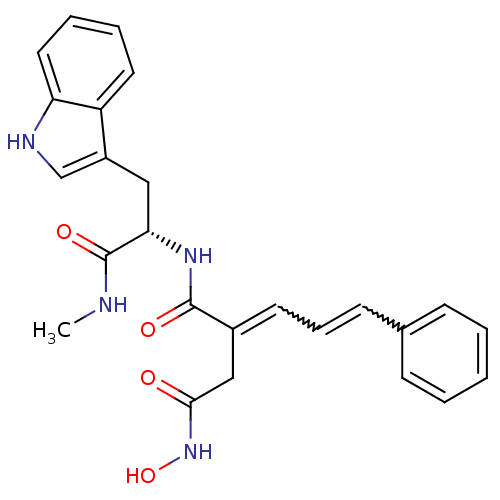
((2E)-3-(N-hydroxycarbamoyl)-2-[(2E)-3-phenylprop-2...)Show SMILES CNC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)C(CC(=O)NO)=CC=Cc1ccccc1 |w:24.26,26.28| Show InChI InChI=1S/C25H26N4O4/c1-26-25(32)22(14-19-16-27-21-13-6-5-12-20(19)21)28-24(31)18(15-23(30)29-33)11-7-10-17-8-3-2-4-9-17/h2-13,16,22,27,33H,14-15H2,1H3,(H,26,32)(H,28,31)(H,29,30)/t22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 38 | n/a | n/a | n/a | n/a | n/a | n/a |
Université de Reims-Champagne-Ardenne
Curated by ChEMBL
| Assay Description
Inhibition of MMP9 |
Bioorg Med Chem 15: 4753-66 (2007)
Article DOI: 10.1016/j.bmc.2007.05.001
BindingDB Entry DOI: 10.7270/Q2Q23ZXN |
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM19149

(CHEMBL98 | N-hydroxy-N'-phenyloctanediamide | SAHA...)Show InChI InChI=1S/C14H20N2O3/c17-13(15-12-8-4-3-5-9-12)10-6-1-2-7-11-14(18)16-19/h3-5,8-9,19H,1-2,6-7,10-11H2,(H,15,17)(H,16,18) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| n/a | n/a | 42 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bras£lia
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC6 using ZMAL (Z-(Ac)Lys-AMC fluorogenic substrate incubated for 90 mins by fluorescence method |
ACS Med Chem Lett 10: 671-676 (2019)
Article DOI: 10.1021/acsmedchemlett.9b00071
BindingDB Entry DOI: 10.7270/Q2BP068P |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50213419

((2E)-3-(N-hydroxycarbamoyl)-2-(3-phenylpropylidene...)Show SMILES CNC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)C(CC(=O)NO)=CCCc1ccccc1 |w:24.26| Show InChI InChI=1S/C25H28N4O4/c1-26-25(32)22(14-19-16-27-21-13-6-5-12-20(19)21)28-24(31)18(15-23(30)29-33)11-7-10-17-8-3-2-4-9-17/h2-6,8-9,11-13,16,22,27,33H,7,10,14-15H2,1H3,(H,26,32)(H,28,31)(H,29,30)/t22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 45 | n/a | n/a | n/a | n/a | n/a | n/a |
Université de Reims-Champagne-Ardenne
Curated by ChEMBL
| Assay Description
Inhibition of MMP9 |
Bioorg Med Chem 15: 4753-66 (2007)
Article DOI: 10.1016/j.bmc.2007.05.001
BindingDB Entry DOI: 10.7270/Q2Q23ZXN |
More data for this
Ligand-Target Pair | |
Neutrophil collagenase
(Homo sapiens (Human)) | BDBM50213418
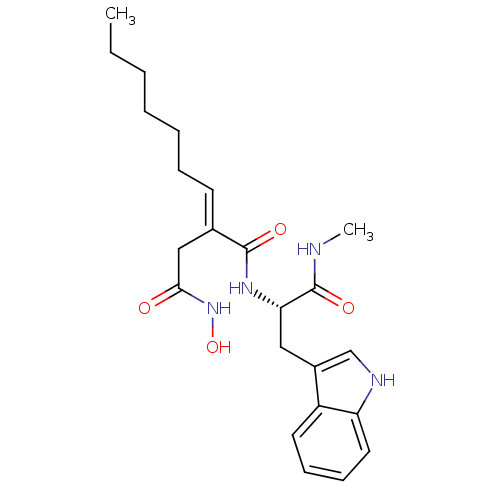
((2E)-3-(N-hydroxycarbamoyl)-2-heptylidenepropionyl...)Show SMILES CCCCCC\C=C(/CC(=O)NO)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)NC Show InChI InChI=1S/C23H32N4O4/c1-3-4-5-6-7-10-16(14-21(28)27-31)22(29)26-20(23(30)24-2)13-17-15-25-19-12-9-8-11-18(17)19/h8-12,15,20,25,31H,3-7,13-14H2,1-2H3,(H,24,30)(H,26,29)(H,27,28)/b16-10+/t20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 101 | n/a | n/a | n/a | n/a | n/a | n/a |
Université de Reims-Champagne-Ardenne
Curated by ChEMBL
| Assay Description
Inhibition of MMP8 |
Bioorg Med Chem 15: 4753-66 (2007)
Article DOI: 10.1016/j.bmc.2007.05.001
BindingDB Entry DOI: 10.7270/Q2Q23ZXN |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50213416

((2E)-3-(N-hydroxycarbamoyl)-2-[(2E)-3-phenylprop-2...)Show SMILES CNC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)C(CC(=O)NO)=CC=Cc1ccccc1 |w:24.26,26.28| Show InChI InChI=1S/C25H26N4O4/c1-26-25(32)22(14-19-16-27-21-13-6-5-12-20(19)21)28-24(31)18(15-23(30)29-33)11-7-10-17-8-3-2-4-9-17/h2-13,16,22,27,33H,14-15H2,1H3,(H,26,32)(H,28,31)(H,29,30)/t22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
Université de Reims-Champagne-Ardenne
Curated by ChEMBL
| Assay Description
Inhibition of MMP2 |
Bioorg Med Chem 15: 4753-66 (2007)
Article DOI: 10.1016/j.bmc.2007.05.001
BindingDB Entry DOI: 10.7270/Q2Q23ZXN |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM19149

(CHEMBL98 | N-hydroxy-N'-phenyloctanediamide | SAHA...)Show InChI InChI=1S/C14H20N2O3/c17-13(15-12-8-4-3-5-9-12)10-6-1-2-7-11-14(18)16-19/h3-5,8-9,19H,1-2,6-7,10-11H2,(H,15,17)(H,16,18) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bras£lia
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC1 using ZMAL (Z-(Ac)Lys-AMC fluorogenic substrate incubated for 90 mins by fluorescence method |
ACS Med Chem Lett 10: 671-676 (2019)
Article DOI: 10.1021/acsmedchemlett.9b00071
BindingDB Entry DOI: 10.7270/Q2BP068P |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Collagenase 3
(Homo sapiens (Human)) | BDBM50213418

((2E)-3-(N-hydroxycarbamoyl)-2-heptylidenepropionyl...)Show SMILES CCCCCC\C=C(/CC(=O)NO)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)NC Show InChI InChI=1S/C23H32N4O4/c1-3-4-5-6-7-10-16(14-21(28)27-31)22(29)26-20(23(30)24-2)13-17-15-25-19-12-9-8-11-18(17)19/h8-12,15,20,25,31H,3-7,13-14H2,1-2H3,(H,24,30)(H,26,29)(H,27,28)/b16-10+/t20-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 122 | n/a | n/a | n/a | n/a | n/a | n/a |
Université de Reims-Champagne-Ardenne
Curated by ChEMBL
| Assay Description
Inhibition of MMP13 |
Bioorg Med Chem 15: 4753-66 (2007)
Article DOI: 10.1016/j.bmc.2007.05.001
BindingDB Entry DOI: 10.7270/Q2Q23ZXN |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50213418

((2E)-3-(N-hydroxycarbamoyl)-2-heptylidenepropionyl...)Show SMILES CCCCCC\C=C(/CC(=O)NO)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)NC Show InChI InChI=1S/C23H32N4O4/c1-3-4-5-6-7-10-16(14-21(28)27-31)22(29)26-20(23(30)24-2)13-17-15-25-19-12-9-8-11-18(17)19/h8-12,15,20,25,31H,3-7,13-14H2,1-2H3,(H,24,30)(H,26,29)(H,27,28)/b16-10+/t20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 123 | n/a | n/a | n/a | n/a | n/a | n/a |
Université de Reims-Champagne-Ardenne
Curated by ChEMBL
| Assay Description
Inhibition of MMP2 |
Bioorg Med Chem 15: 4753-66 (2007)
Article DOI: 10.1016/j.bmc.2007.05.001
BindingDB Entry DOI: 10.7270/Q2Q23ZXN |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50135601

((S,E)-N1-(3-(1H-indol-3-yl)-1-(methylamino)-1-oxop...)Show SMILES CNC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)C(\CC(=O)NO)=C\C(C)C Show InChI InChI=1S/C20H26N4O4/c1-12(2)8-13(10-18(25)24-28)19(26)23-17(20(27)21-3)9-14-11-22-16-7-5-4-6-15(14)16/h4-8,11-12,17,22,28H,9-10H2,1-3H3,(H,21,27)(H,23,26)(H,24,25)/b13-8+/t17-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 179 | n/a | n/a | n/a | n/a | n/a | n/a |
UMR/CNRS 6013
Curated by ChEMBL
| Assay Description
Inhibition of PKD2 ( assessed as residual activity at 1 uM ) by TR-FRET assay |
Bioorg Med Chem Lett 13: 2843-6 (2003)
BindingDB Entry DOI: 10.7270/Q2PN952R |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50135601

((S,E)-N1-(3-(1H-indol-3-yl)-1-(methylamino)-1-oxop...)Show SMILES CNC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)C(\CC(=O)NO)=C\C(C)C Show InChI InChI=1S/C20H26N4O4/c1-12(2)8-13(10-18(25)24-28)19(26)23-17(20(27)21-3)9-14-11-22-16-7-5-4-6-15(14)16/h4-8,11-12,17,22,28H,9-10H2,1-3H3,(H,21,27)(H,23,26)(H,24,25)/b13-8+/t17-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 179 | n/a | n/a | n/a | n/a | n/a | n/a |
Université de Reims-Champagne-Ardenne
Curated by ChEMBL
| Assay Description
Inhibition of MMP3 |
Bioorg Med Chem 15: 4753-66 (2007)
Article DOI: 10.1016/j.bmc.2007.05.001
BindingDB Entry DOI: 10.7270/Q2Q23ZXN |
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50535444

(CHEMBL4463058)Show InChI InChI=1S/C15H23NO3/c1-19-14-10-7-9-13(12-14)8-5-3-2-4-6-11-15(17)16-18/h7,9-10,12,18H,2-6,8,11H2,1H3,(H,16,17) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 190 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bras£lia
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC6 using ZMAL (Z-(Ac)Lys-AMC fluorogenic substrate incubated for 90 mins by fluorescence method |
ACS Med Chem Lett 10: 671-676 (2019)
Article DOI: 10.1021/acsmedchemlett.9b00071
BindingDB Entry DOI: 10.7270/Q2BP068P |
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50535445

(CHEMBL4455730)Show InChI InChI=1S/C17H25NO5/c1-22-14-11-8-10-13(16(14)17(20)23-2)9-6-4-3-5-7-12-15(19)18-21/h8,10-11,21H,3-7,9,12H2,1-2H3,(H,18,19) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 215 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bras£lia
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC6 using ZMAL (Z-(Ac)Lys-AMC fluorogenic substrate incubated for 90 mins by fluorescence method |
ACS Med Chem Lett 10: 671-676 (2019)
Article DOI: 10.1021/acsmedchemlett.9b00071
BindingDB Entry DOI: 10.7270/Q2BP068P |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50213419

((2E)-3-(N-hydroxycarbamoyl)-2-(3-phenylpropylidene...)Show SMILES CNC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)C(CC(=O)NO)=CCCc1ccccc1 |w:24.26| Show InChI InChI=1S/C25H28N4O4/c1-26-25(32)22(14-19-16-27-21-13-6-5-12-20(19)21)28-24(31)18(15-23(30)29-33)11-7-10-17-8-3-2-4-9-17/h2-6,8-9,11-13,16,22,27,33H,7,10,14-15H2,1H3,(H,26,32)(H,28,31)(H,29,30)/t22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 280 | n/a | n/a | n/a | n/a | n/a | n/a |
Université de Reims-Champagne-Ardenne
Curated by ChEMBL
| Assay Description
Inhibition of MMP2 |
Bioorg Med Chem 15: 4753-66 (2007)
Article DOI: 10.1016/j.bmc.2007.05.001
BindingDB Entry DOI: 10.7270/Q2Q23ZXN |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50535444

(CHEMBL4463058)Show InChI InChI=1S/C15H23NO3/c1-19-14-10-7-9-13(12-14)8-5-3-2-4-6-11-15(17)16-18/h7,9-10,12,18H,2-6,8,11H2,1H3,(H,16,17) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 316 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bras£lia
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC1 using ZMAL (Z-(Ac)Lys-AMC fluorogenic substrate incubated for 90 mins by fluorescence method |
ACS Med Chem Lett 10: 671-676 (2019)
Article DOI: 10.1021/acsmedchemlett.9b00071
BindingDB Entry DOI: 10.7270/Q2BP068P |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50135602

(CHEMBL420259 | N*1*-{2-[2-(2-Dimethylamino-ethyl)-...)Show SMILES CNC(=O)C(Cc1c(CCN(C)C)[nH]c2ccccc12)NC(=O)C(\CC(=O)NO)=C\C(C)C Show InChI InChI=1S/C24H35N5O4/c1-15(2)12-16(13-22(30)28-33)23(31)27-21(24(32)25-3)14-18-17-8-6-7-9-19(17)26-20(18)10-11-29(4)5/h6-9,12,15,21,26,33H,10-11,13-14H2,1-5H3,(H,25,32)(H,27,31)(H,28,30)/b16-12+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 665 | n/a | n/a | n/a | n/a | n/a | n/a |
UMR/CNRS 6013
Curated by ChEMBL
| Assay Description
Inhibition of human matrix metalloproteinase-3 |
Bioorg Med Chem Lett 13: 2843-6 (2003)
BindingDB Entry DOI: 10.7270/Q2PN952R |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50535445

(CHEMBL4455730)Show InChI InChI=1S/C17H25NO5/c1-22-14-11-8-10-13(16(14)17(20)23-2)9-6-4-3-5-7-12-15(19)18-21/h8,10-11,21H,3-7,9,12H2,1-2H3,(H,18,19) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 775 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bras£lia
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC1 using ZMAL (Z-(Ac)Lys-AMC fluorogenic substrate incubated for 90 mins by fluorescence method |
ACS Med Chem Lett 10: 671-676 (2019)
Article DOI: 10.1021/acsmedchemlett.9b00071
BindingDB Entry DOI: 10.7270/Q2BP068P |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50451552

(CHEMBL2111573)Show SMILES CNC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)C(CC(=O)NO)=CC(C)C Show InChI InChI=1S/C20H26N4O4/c1-12(2)8-13(10-18(25)24-28)19(26)23-17(20(27)21-3)9-14-11-22-16-7-5-4-6-15(14)16/h4-8,11-12,17,22,28H,9-10H2,1-3H3,(H,21,27)(H,23,26)(H,24,25)/t17-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 910 | n/a | n/a | n/a | n/a | n/a | n/a |
UMR/CNRS 6013
Curated by ChEMBL
| Assay Description
Inhibition of human matrix metalloproteinase-3 |
Bioorg Med Chem Lett 13: 2843-6 (2003)
BindingDB Entry DOI: 10.7270/Q2PN952R |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-14
(Homo sapiens (Human)) | BDBM50213417

((2E)-3-(N-hydroxycarbamoyl)-2-[(2E)-but-2-en-1-yli...)Show SMILES CNC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)C(CC=CC)=CC(=O)NO |w:23.25,21.23| Show InChI InChI=1S/C20H24N4O4/c1-3-4-7-13(11-18(25)24-28)19(26)23-17(20(27)21-2)10-14-12-22-16-9-6-5-8-15(14)16/h3-6,8-9,11-12,17,22,28H,7,10H2,1-2H3,(H,21,27)(H,23,26)(H,24,25)/t17-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 913 | n/a | n/a | n/a | n/a | n/a | n/a |
Université de Reims-Champagne-Ardenne
Curated by ChEMBL
| Assay Description
Inhibition of MMP14 |
Bioorg Med Chem 15: 4753-66 (2007)
Article DOI: 10.1016/j.bmc.2007.05.001
BindingDB Entry DOI: 10.7270/Q2Q23ZXN |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50213417

((2E)-3-(N-hydroxycarbamoyl)-2-[(2E)-but-2-en-1-yli...)Show SMILES CNC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)C(CC=CC)=CC(=O)NO |w:23.25,21.23| Show InChI InChI=1S/C20H24N4O4/c1-3-4-7-13(11-18(25)24-28)19(26)23-17(20(27)21-2)10-14-12-22-16-9-6-5-8-15(14)16/h3-6,8-9,11-12,17,22,28H,7,10H2,1-2H3,(H,21,27)(H,23,26)(H,24,25)/t17-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 974 | n/a | n/a | n/a | n/a | n/a | n/a |
Université de Reims-Champagne-Ardenne
Curated by ChEMBL
| Assay Description
Inhibition of MMP9 |
Bioorg Med Chem 15: 4753-66 (2007)
Article DOI: 10.1016/j.bmc.2007.05.001
BindingDB Entry DOI: 10.7270/Q2Q23ZXN |
More data for this
Ligand-Target Pair | |
Interstitial collagenase
(Homo sapiens (Human)) | BDBM50213417

((2E)-3-(N-hydroxycarbamoyl)-2-[(2E)-but-2-en-1-yli...)Show SMILES CNC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)C(CC=CC)=CC(=O)NO |w:23.25,21.23| Show InChI InChI=1S/C20H24N4O4/c1-3-4-7-13(11-18(25)24-28)19(26)23-17(20(27)21-2)10-14-12-22-16-9-6-5-8-15(14)16/h3-6,8-9,11-12,17,22,28H,7,10H2,1-2H3,(H,21,27)(H,23,26)(H,24,25)/t17-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 984 | n/a | n/a | n/a | n/a | n/a | n/a |
Université de Reims-Champagne-Ardenne
Curated by ChEMBL
| Assay Description
Inhibition of MMP1 |
Bioorg Med Chem 15: 4753-66 (2007)
Article DOI: 10.1016/j.bmc.2007.05.001
BindingDB Entry DOI: 10.7270/Q2Q23ZXN |
More data for this
Ligand-Target Pair | |
Interstitial collagenase
(Homo sapiens (Human)) | BDBM50213416

((2E)-3-(N-hydroxycarbamoyl)-2-[(2E)-3-phenylprop-2...)Show SMILES CNC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)C(CC(=O)NO)=CC=Cc1ccccc1 |w:24.26,26.28| Show InChI InChI=1S/C25H26N4O4/c1-26-25(32)22(14-19-16-27-21-13-6-5-12-20(19)21)28-24(31)18(15-23(30)29-33)11-7-10-17-8-3-2-4-9-17/h2-13,16,22,27,33H,14-15H2,1H3,(H,26,32)(H,28,31)(H,29,30)/t22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.24E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Université de Reims-Champagne-Ardenne
Curated by ChEMBL
| Assay Description
Inhibition of MMP1 |
Bioorg Med Chem 15: 4753-66 (2007)
Article DOI: 10.1016/j.bmc.2007.05.001
BindingDB Entry DOI: 10.7270/Q2Q23ZXN |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50213419

((2E)-3-(N-hydroxycarbamoyl)-2-(3-phenylpropylidene...)Show SMILES CNC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)C(CC(=O)NO)=CCCc1ccccc1 |w:24.26| Show InChI InChI=1S/C25H28N4O4/c1-26-25(32)22(14-19-16-27-21-13-6-5-12-20(19)21)28-24(31)18(15-23(30)29-33)11-7-10-17-8-3-2-4-9-17/h2-6,8-9,11-13,16,22,27,33H,7,10,14-15H2,1H3,(H,26,32)(H,28,31)(H,29,30)/t22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.92E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Université de Reims-Champagne-Ardenne
Curated by ChEMBL
| Assay Description
Inhibition of MMP3 |
Bioorg Med Chem 15: 4753-66 (2007)
Article DOI: 10.1016/j.bmc.2007.05.001
BindingDB Entry DOI: 10.7270/Q2Q23ZXN |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50135600

((S)-2-((E)-2-Hydroxycarbamoylmethyl-4-methyl-pent-...)Show SMILES CCOC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)C(\CC(=O)NO)=C\C(C)C Show InChI InChI=1S/C21H27N3O5/c1-4-29-21(27)18(10-15-12-22-17-8-6-5-7-16(15)17)23-20(26)14(9-13(2)3)11-19(25)24-28/h5-9,12-13,18,22,28H,4,10-11H2,1-3H3,(H,23,26)(H,24,25)/b14-9+/t18-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 2.34E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
UMR/CNRS 6013
Curated by ChEMBL
| Assay Description
Inhibition of human matrix metalloproteinase-3 |
Bioorg Med Chem Lett 13: 2843-6 (2003)
BindingDB Entry DOI: 10.7270/Q2PN952R |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-14
(Homo sapiens (Human)) | BDBM50213416

((2E)-3-(N-hydroxycarbamoyl)-2-[(2E)-3-phenylprop-2...)Show SMILES CNC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)C(CC(=O)NO)=CC=Cc1ccccc1 |w:24.26,26.28| Show InChI InChI=1S/C25H26N4O4/c1-26-25(32)22(14-19-16-27-21-13-6-5-12-20(19)21)28-24(31)18(15-23(30)29-33)11-7-10-17-8-3-2-4-9-17/h2-13,16,22,27,33H,14-15H2,1H3,(H,26,32)(H,28,31)(H,29,30)/t22-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.49E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Université de Reims-Champagne-Ardenne
Curated by ChEMBL
| Assay Description
Inhibition of MMP14 |
Bioorg Med Chem 15: 4753-66 (2007)
Article DOI: 10.1016/j.bmc.2007.05.001
BindingDB Entry DOI: 10.7270/Q2Q23ZXN |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-14
(Homo sapiens (Human)) | BDBM50213418

((2E)-3-(N-hydroxycarbamoyl)-2-heptylidenepropionyl...)Show SMILES CCCCCC\C=C(/CC(=O)NO)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)NC Show InChI InChI=1S/C23H32N4O4/c1-3-4-5-6-7-10-16(14-21(28)27-31)22(29)26-20(23(30)24-2)13-17-15-25-19-12-9-8-11-18(17)19/h8-12,15,20,25,31H,3-7,13-14H2,1-2H3,(H,24,30)(H,26,29)(H,27,28)/b16-10+/t20-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.66E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Université de Reims-Champagne-Ardenne
Curated by ChEMBL
| Assay Description
Inhibition of MMP14 |
Bioorg Med Chem 15: 4753-66 (2007)
Article DOI: 10.1016/j.bmc.2007.05.001
BindingDB Entry DOI: 10.7270/Q2Q23ZXN |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50213417

((2E)-3-(N-hydroxycarbamoyl)-2-[(2E)-but-2-en-1-yli...)Show SMILES CNC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)C(CC=CC)=CC(=O)NO |w:23.25,21.23| Show InChI InChI=1S/C20H24N4O4/c1-3-4-7-13(11-18(25)24-28)19(26)23-17(20(27)21-2)10-14-12-22-16-9-6-5-8-15(14)16/h3-6,8-9,11-12,17,22,28H,7,10H2,1-2H3,(H,21,27)(H,23,26)(H,24,25)/t17-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.61E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Université de Reims-Champagne-Ardenne
Curated by ChEMBL
| Assay Description
Inhibition of MMP3 |
Bioorg Med Chem 15: 4753-66 (2007)
Article DOI: 10.1016/j.bmc.2007.05.001
BindingDB Entry DOI: 10.7270/Q2Q23ZXN |
More data for this
Ligand-Target Pair | |
Interstitial collagenase
(Homo sapiens (Human)) | BDBM50213419

((2E)-3-(N-hydroxycarbamoyl)-2-(3-phenylpropylidene...)Show SMILES CNC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)C(CC(=O)NO)=CCCc1ccccc1 |w:24.26| Show InChI InChI=1S/C25H28N4O4/c1-26-25(32)22(14-19-16-27-21-13-6-5-12-20(19)21)28-24(31)18(15-23(30)29-33)11-7-10-17-8-3-2-4-9-17/h2-6,8-9,11-13,16,22,27,33H,7,10,14-15H2,1H3,(H,26,32)(H,28,31)(H,29,30)/t22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9.13E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Université de Reims-Champagne-Ardenne
Curated by ChEMBL
| Assay Description
Inhibition of MMP1 |
Bioorg Med Chem 15: 4753-66 (2007)
Article DOI: 10.1016/j.bmc.2007.05.001
BindingDB Entry DOI: 10.7270/Q2Q23ZXN |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50213416

((2E)-3-(N-hydroxycarbamoyl)-2-[(2E)-3-phenylprop-2...)Show SMILES CNC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)C(CC(=O)NO)=CC=Cc1ccccc1 |w:24.26,26.28| Show InChI InChI=1S/C25H26N4O4/c1-26-25(32)22(14-19-16-27-21-13-6-5-12-20(19)21)28-24(31)18(15-23(30)29-33)11-7-10-17-8-3-2-4-9-17/h2-13,16,22,27,33H,14-15H2,1H3,(H,26,32)(H,28,31)(H,29,30)/t22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Université de Reims-Champagne-Ardenne
Curated by ChEMBL
| Assay Description
Inhibition of MMP3 |
Bioorg Med Chem 15: 4753-66 (2007)
Article DOI: 10.1016/j.bmc.2007.05.001
BindingDB Entry DOI: 10.7270/Q2Q23ZXN |
More data for this
Ligand-Target Pair | |
Interstitial collagenase
(Homo sapiens (Human)) | BDBM50213418

((2E)-3-(N-hydroxycarbamoyl)-2-heptylidenepropionyl...)Show SMILES CCCCCC\C=C(/CC(=O)NO)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)NC Show InChI InChI=1S/C23H32N4O4/c1-3-4-5-6-7-10-16(14-21(28)27-31)22(29)26-20(23(30)24-2)13-17-15-25-19-12-9-8-11-18(17)19/h8-12,15,20,25,31H,3-7,13-14H2,1-2H3,(H,24,30)(H,26,29)(H,27,28)/b16-10+/t20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Université de Reims-Champagne-Ardenne
Curated by ChEMBL
| Assay Description
Inhibition of MMP1 |
Bioorg Med Chem 15: 4753-66 (2007)
Article DOI: 10.1016/j.bmc.2007.05.001
BindingDB Entry DOI: 10.7270/Q2Q23ZXN |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-14
(Homo sapiens (Human)) | BDBM50213419

((2E)-3-(N-hydroxycarbamoyl)-2-(3-phenylpropylidene...)Show SMILES CNC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)C(CC(=O)NO)=CCCc1ccccc1 |w:24.26| Show InChI InChI=1S/C25H28N4O4/c1-26-25(32)22(14-19-16-27-21-13-6-5-12-20(19)21)28-24(31)18(15-23(30)29-33)11-7-10-17-8-3-2-4-9-17/h2-6,8-9,11-13,16,22,27,33H,7,10,14-15H2,1H3,(H,26,32)(H,28,31)(H,29,30)/t22-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.31E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Université de Reims-Champagne-Ardenne
Curated by ChEMBL
| Assay Description
Inhibition of MMP14 |
Bioorg Med Chem 15: 4753-66 (2007)
Article DOI: 10.1016/j.bmc.2007.05.001
BindingDB Entry DOI: 10.7270/Q2Q23ZXN |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data