

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cholinesterase (Homo sapiens (Human)) | BDBM50468733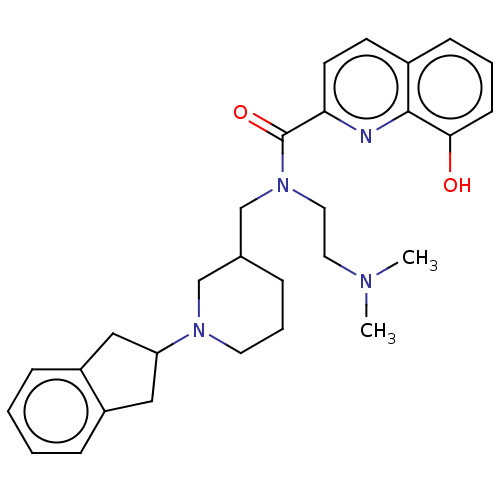 (CHEMBL4294570) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Ljubljana Curated by ChEMBL | Assay Description Competitive inhibition of human BChE using butyrylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition and measured... | Eur J Med Chem 156: 598-617 (2018) Article DOI: 10.1016/j.ejmech.2018.07.033 BindingDB Entry DOI: 10.7270/Q27W6FW2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50468736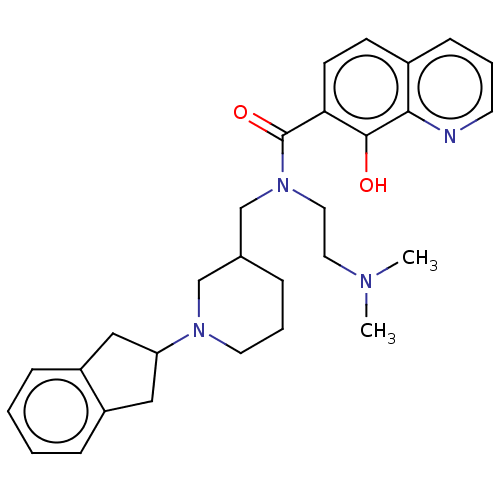 (CHEMBL4283585) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Ljubljana Curated by ChEMBL | Assay Description Competitive inhibition of human BChE using butyrylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition and measured... | Eur J Med Chem 156: 598-617 (2018) Article DOI: 10.1016/j.ejmech.2018.07.033 BindingDB Entry DOI: 10.7270/Q27W6FW2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-IIb/beta-3 (Homo sapiens (Human)) | BDBM50165956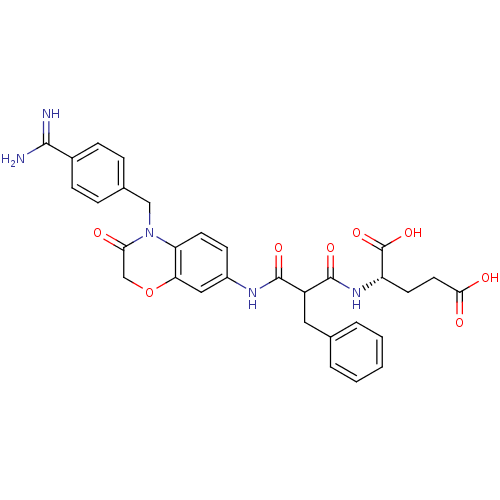 ((S)-2-{2-[4-(4-Carbamimidoyl-benzyl)-3-oxo-3,4-dih...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 206 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Ljubljana Curated by ChEMBL | Assay Description Inhibitory activity against binding of Fibrinogen to thrombocyte alpha IIb beta-3 integrin | J Med Chem 48: 3110-3 (2005) Article DOI: 10.1021/jm048984g BindingDB Entry DOI: 10.7270/Q2K0752H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-IIb/beta-3 (Homo sapiens (Human)) | BDBM50165952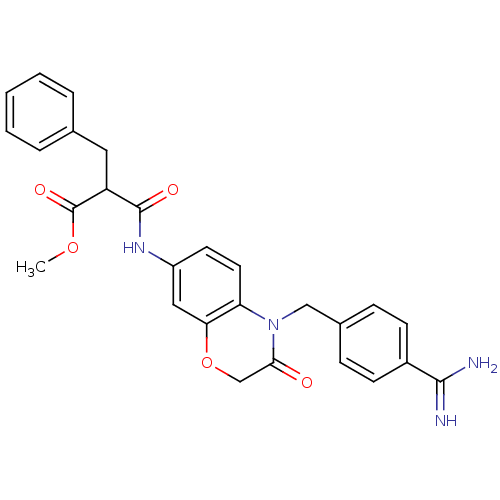 (2-Benzyl-N-[4-(4-carbamimidoyl-benzyl)-3-oxo-3,4-d...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 973 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Ljubljana Curated by ChEMBL | Assay Description Inhibitory activity against binding of Fibrinogen to thrombocyte alpha IIb beta-3 integrin | J Med Chem 48: 3110-3 (2005) Article DOI: 10.1021/jm048984g BindingDB Entry DOI: 10.7270/Q2K0752H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-IIb/beta-3 (Homo sapiens (Human)) | BDBM50165953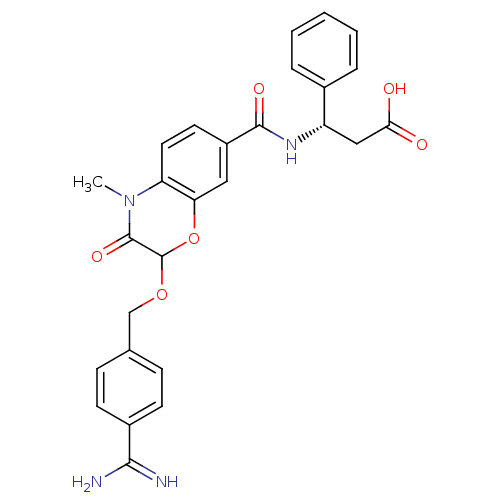 ((S)-3-{[2-(4-Carbamimidoyl-benzyloxy)-4-methyl-3-o...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.64E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Ljubljana Curated by ChEMBL | Assay Description Inhibitory activity against binding of Fibrinogen to thrombocyte alpha IIb beta-3 integrin | J Med Chem 48: 3110-3 (2005) Article DOI: 10.1021/jm048984g BindingDB Entry DOI: 10.7270/Q2K0752H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-IIb/beta-3 (Homo sapiens (Human)) | BDBM50165955 ((S)-2-{2-[4-(4-Carbamimidoyl-benzyl)-3-oxo-3,4-dih...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.66E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Ljubljana Curated by ChEMBL | Assay Description Inhibitory activity against binding of Fibrinogen to thrombocyte alpha IIb beta-3 integrin | J Med Chem 48: 3110-3 (2005) Article DOI: 10.1021/jm048984g BindingDB Entry DOI: 10.7270/Q2K0752H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-IIb/beta-3 (Homo sapiens (Human)) | BDBM50165957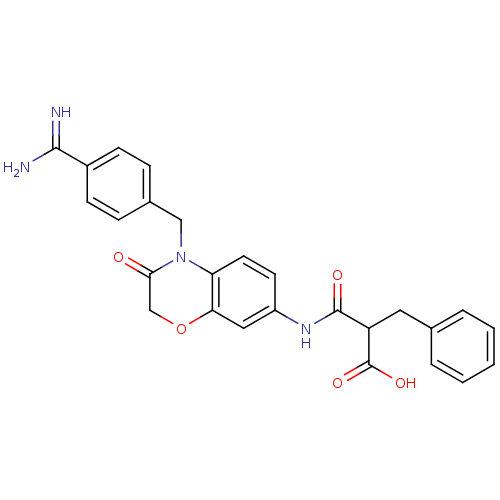 (2-Benzyl-N-[4-(4-carbamimidoyl-benzyl)-3-oxo-3,4-d...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.94E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Ljubljana Curated by ChEMBL | Assay Description Inhibitory activity against binding of Fibrinogen to thrombocyte alpha IIb beta-3 integrin | J Med Chem 48: 3110-3 (2005) Article DOI: 10.1021/jm048984g BindingDB Entry DOI: 10.7270/Q2K0752H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-IIb/beta-3 (Homo sapiens (Human)) | BDBM50165954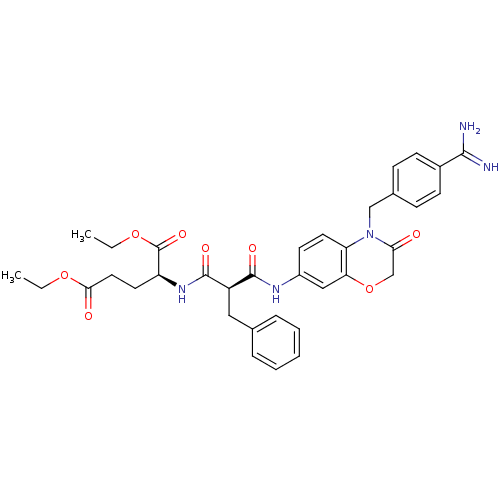 ((S)-2-{(S)-2-[4-(4-Carbamimidoyl-benzyl)-3-oxo-3,4...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.94E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Ljubljana Curated by ChEMBL | Assay Description Inhibitory activity against binding of Fibrinogen to thrombocyte alpha IIb beta-3 integrin | J Med Chem 48: 3110-3 (2005) Article DOI: 10.1021/jm048984g BindingDB Entry DOI: 10.7270/Q2K0752H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-IIb/beta-3 (Homo sapiens (Human)) | BDBM50165959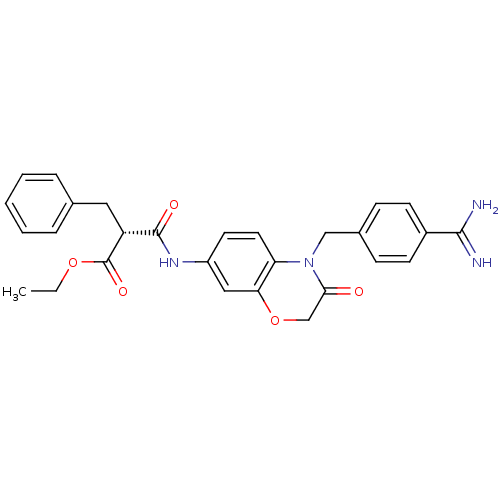 ((R)-2-Benzyl-N-[4-(4-carbamimidoyl-benzyl)-3-oxo-3...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5.19E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Ljubljana Curated by ChEMBL | Assay Description Inhibitory activity against binding of Fibrinogen to thrombocyte alpha IIb beta-3 integrin | J Med Chem 48: 3110-3 (2005) Article DOI: 10.1021/jm048984g BindingDB Entry DOI: 10.7270/Q2K0752H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-IIb/beta-3 (Homo sapiens (Human)) | BDBM50165960 (CHEMBL264985 | {2-[4-(4-Carbamimidoyl-benzyl)-3-ox...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6.84E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Ljubljana Curated by ChEMBL | Assay Description Inhibitory activity against binding of Fibrinogen to thrombocyte alpha IIb beta-3 integrin | J Med Chem 48: 3110-3 (2005) Article DOI: 10.1021/jm048984g BindingDB Entry DOI: 10.7270/Q2K0752H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-IIb/beta-3 (Homo sapiens (Human)) | BDBM50165962 ((S)-2-{2-[4-(4-Carbamimidoyl-benzyl)-3-oxo-3,4-dih...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Ljubljana Curated by ChEMBL | Assay Description Inhibitory activity against binding of Fibrinogen to thrombocyte alpha IIb beta-3 integrin | J Med Chem 48: 3110-3 (2005) Article DOI: 10.1021/jm048984g BindingDB Entry DOI: 10.7270/Q2K0752H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-IIb/beta-3 (Homo sapiens (Human)) | BDBM50165961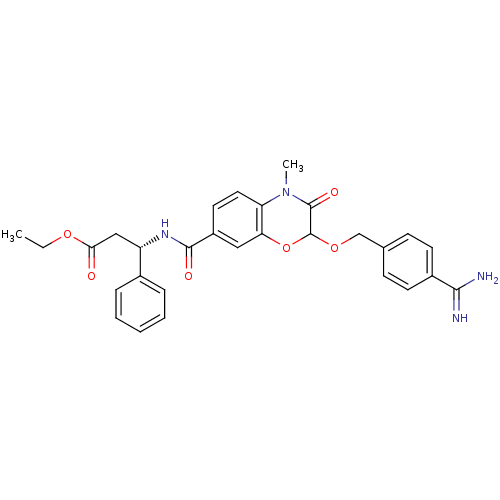 ((S)-3-{[2-(4-Carbamimidoyl-benzyloxy)-4-methyl-3-o...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.46E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Ljubljana Curated by ChEMBL | Assay Description Inhibitory activity against binding of Fibrinogen to thrombocyte alpha IIb beta-3 integrin | J Med Chem 48: 3110-3 (2005) Article DOI: 10.1021/jm048984g BindingDB Entry DOI: 10.7270/Q2K0752H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-IIb/beta-3 (Homo sapiens (Human)) | BDBM50165958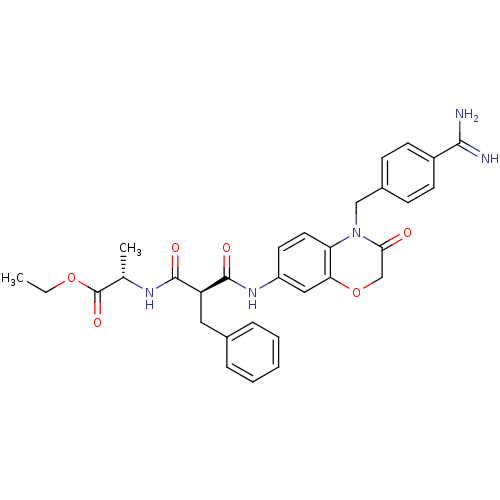 ((S)-2-{(S)-2-[4-(4-Carbamimidoyl-benzyl)-3-oxo-3,4...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5.91E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Ljubljana Curated by ChEMBL | Assay Description Inhibitory activity against binding of Fibrinogen to thrombocyte alpha IIb beta-3 integrin | J Med Chem 48: 3110-3 (2005) Article DOI: 10.1021/jm048984g BindingDB Entry DOI: 10.7270/Q2K0752H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50431886 (CHEMBL1413473) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.90 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Ljubljana Curated by ChEMBL | Assay Description Inhibition of human recombinant BChE using butyrylthiocholine as substrate preincubated for 300 secs followed by substrate addition and measured for ... | Eur J Med Chem 156: 598-617 (2018) Article DOI: 10.1016/j.ejmech.2018.07.033 BindingDB Entry DOI: 10.7270/Q27W6FW2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50468744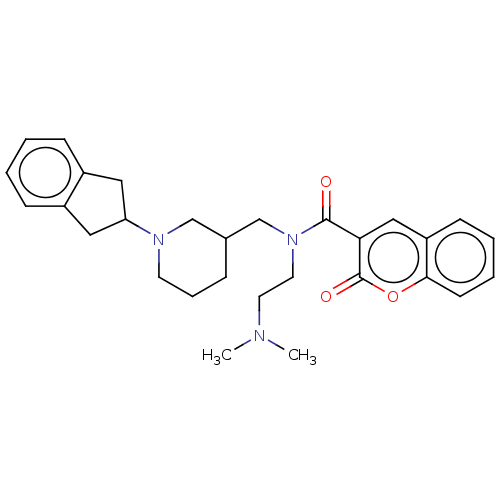 (CHEMBL4280947) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 9.5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Ljubljana Curated by ChEMBL | Assay Description Inhibition of human BChE using butyrylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition and measured for 1 min b... | Eur J Med Chem 156: 598-617 (2018) Article DOI: 10.1016/j.ejmech.2018.07.033 BindingDB Entry DOI: 10.7270/Q27W6FW2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50468733 (CHEMBL4294570) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Ljubljana Curated by ChEMBL | Assay Description Inhibition of human BChE using butyrylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition and measured for 1 min b... | Eur J Med Chem 156: 598-617 (2018) Article DOI: 10.1016/j.ejmech.2018.07.033 BindingDB Entry DOI: 10.7270/Q27W6FW2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM8961 (1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Ljubljana Curated by ChEMBL | Assay Description Inhibition of human recombinant BChE using butyrylthiocholine iodide as substrate after 5 mins by Ellman's method | Bioorg Med Chem 23: 4442-52 (2015) Article DOI: 10.1016/j.bmc.2015.06.010 BindingDB Entry DOI: 10.7270/Q2W097PZ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50468736 (CHEMBL4283585) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Ljubljana Curated by ChEMBL | Assay Description Inhibition of human BChE using butyrylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition and measured for 1 min b... | Eur J Med Chem 156: 598-617 (2018) Article DOI: 10.1016/j.ejmech.2018.07.033 BindingDB Entry DOI: 10.7270/Q27W6FW2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50468741 (CHEMBL4291165) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Ljubljana Curated by ChEMBL | Assay Description Inhibition of human BChE using butyrylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition and measured for 1 min b... | Eur J Med Chem 156: 598-617 (2018) Article DOI: 10.1016/j.ejmech.2018.07.033 BindingDB Entry DOI: 10.7270/Q27W6FW2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50027376 (CHEMBL3338391) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Ljubljana Curated by ChEMBL | Assay Description Inhibition of human recombinant BChE using butyrylthiocholine iodide as substrate after 5 mins by Ellman's method | Bioorg Med Chem 23: 4442-52 (2015) Article DOI: 10.1016/j.bmc.2015.06.010 BindingDB Entry DOI: 10.7270/Q2W097PZ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM8960 ((+/-)-2-[(1-benzylpiperidin-4-yl)methyl]-5,6-dimet...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Ljubljana Curated by ChEMBL | Assay Description Inhibition of AChE (unknown origin) | Eur J Med Chem 156: 598-617 (2018) Article DOI: 10.1016/j.ejmech.2018.07.033 BindingDB Entry DOI: 10.7270/Q27W6FW2 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM8961 (1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Ljubljana Curated by ChEMBL | Assay Description Inhibition of human BChE using butyrylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition and measured for 1 min b... | Eur J Med Chem 156: 598-617 (2018) Article DOI: 10.1016/j.ejmech.2018.07.033 BindingDB Entry DOI: 10.7270/Q27W6FW2 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50468739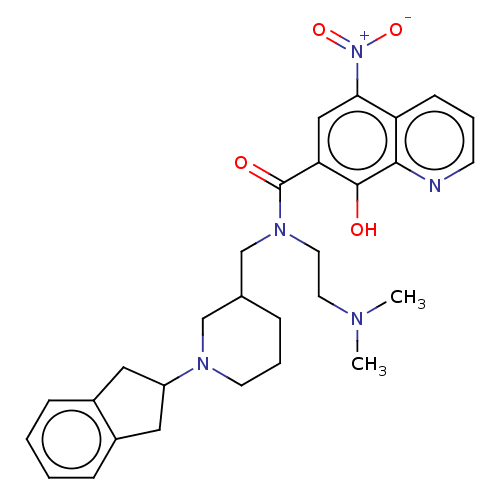 (CHEMBL4288890) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Ljubljana Curated by ChEMBL | Assay Description Inhibition of human BChE using butyrylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition and measured for 1 min b... | Eur J Med Chem 156: 598-617 (2018) Article DOI: 10.1016/j.ejmech.2018.07.033 BindingDB Entry DOI: 10.7270/Q27W6FW2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM11682 (2,3-dihydroxybutanedioic acid; 3-[(1S)-1-(dimethyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid UniChem Similars | DrugBank Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Ljubljana Curated by ChEMBL | Assay Description Inhibition of BChE (unknown origin) | Eur J Med Chem 156: 598-617 (2018) Article DOI: 10.1016/j.ejmech.2018.07.033 BindingDB Entry DOI: 10.7270/Q27W6FW2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50468731 (CHEMBL4287944) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 48 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Ljubljana Curated by ChEMBL | Assay Description Inhibition of human BChE using butyrylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition and measured for 1 min b... | Eur J Med Chem 156: 598-617 (2018) Article DOI: 10.1016/j.ejmech.2018.07.033 BindingDB Entry DOI: 10.7270/Q27W6FW2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50468735 (CHEMBL4288849) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 71 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Ljubljana Curated by ChEMBL | Assay Description Inhibition of human BChE using butyrylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition and measured for 1 min b... | Eur J Med Chem 156: 598-617 (2018) Article DOI: 10.1016/j.ejmech.2018.07.033 BindingDB Entry DOI: 10.7270/Q27W6FW2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50468748 (CHEMBL4277303) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 103 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Ljubljana Curated by ChEMBL | Assay Description Inhibition of human BChE using butyrylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition and measured for 1 min b... | Eur J Med Chem 156: 598-617 (2018) Article DOI: 10.1016/j.ejmech.2018.07.033 BindingDB Entry DOI: 10.7270/Q27W6FW2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Mus musculus (mouse)) | BDBM8961 (1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 106 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Ljubljana Curated by ChEMBL | Assay Description Inhibition of mouse recombinant AChE using butyrylthiocholine iodide as substrate after 5 mins by Ellman's method | Bioorg Med Chem 23: 4442-52 (2015) Article DOI: 10.1016/j.bmc.2015.06.010 BindingDB Entry DOI: 10.7270/Q2W097PZ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM8961 (1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 115 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Ljubljana Curated by ChEMBL | Assay Description Inhibition of human AChE using acetylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition and measured for 1 min by... | Eur J Med Chem 156: 598-617 (2018) Article DOI: 10.1016/j.ejmech.2018.07.033 BindingDB Entry DOI: 10.7270/Q27W6FW2 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50468743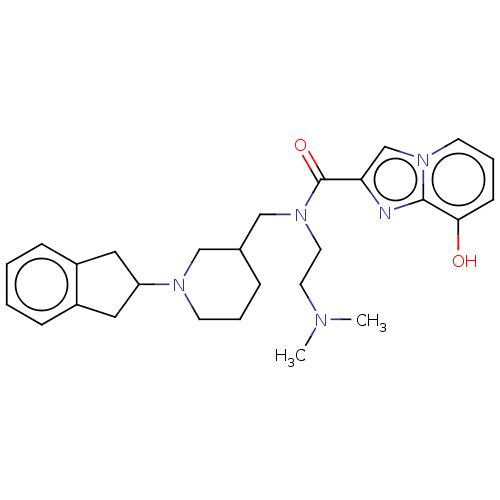 (CHEMBL4284357) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 135 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Ljubljana Curated by ChEMBL | Assay Description Inhibition of human BChE using butyrylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition and measured for 1 min b... | Eur J Med Chem 156: 598-617 (2018) Article DOI: 10.1016/j.ejmech.2018.07.033 BindingDB Entry DOI: 10.7270/Q27W6FW2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50096250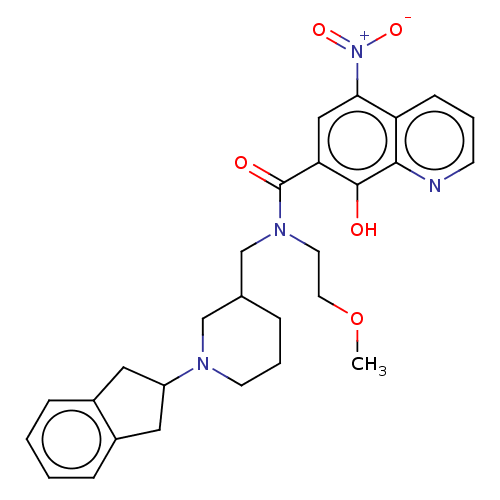 (CHEMBL3593658) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 215 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Ljubljana Curated by ChEMBL | Assay Description Inhibition of human recombinant BChE using butyrylthiocholine iodide as substrate after 5 mins by Ellman's method | Bioorg Med Chem 23: 4442-52 (2015) Article DOI: 10.1016/j.bmc.2015.06.010 BindingDB Entry DOI: 10.7270/Q2W097PZ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50468742 (CHEMBL4287764) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 229 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Ljubljana Curated by ChEMBL | Assay Description Inhibition of human BChE using butyrylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition and measured for 1 min b... | Eur J Med Chem 156: 598-617 (2018) Article DOI: 10.1016/j.ejmech.2018.07.033 BindingDB Entry DOI: 10.7270/Q27W6FW2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50468732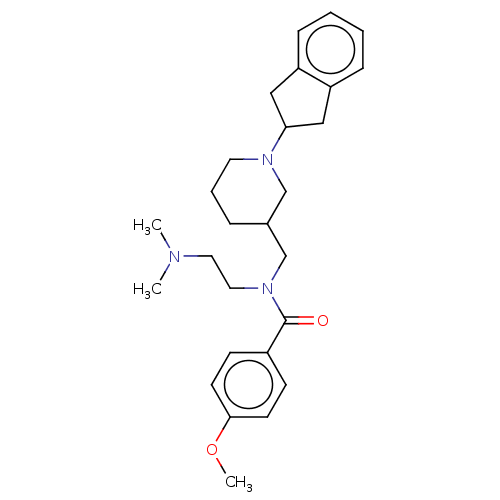 (CHEMBL4279606) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 237 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Ljubljana Curated by ChEMBL | Assay Description Inhibition of human BChE using butyrylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition and measured for 1 min b... | Eur J Med Chem 156: 598-617 (2018) Article DOI: 10.1016/j.ejmech.2018.07.033 BindingDB Entry DOI: 10.7270/Q27W6FW2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50468737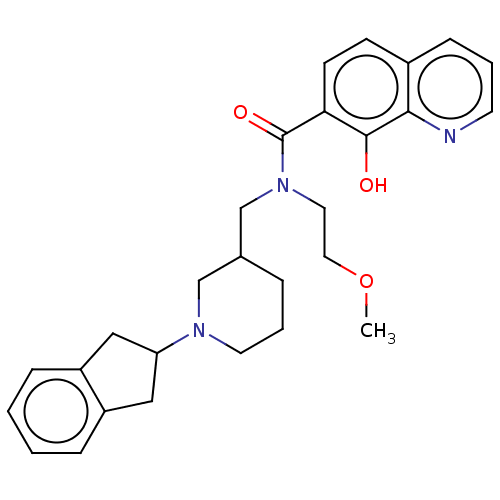 (CHEMBL4278201) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 266 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Ljubljana Curated by ChEMBL | Assay Description Inhibition of human BChE using butyrylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition and measured for 1 min b... | Eur J Med Chem 156: 598-617 (2018) Article DOI: 10.1016/j.ejmech.2018.07.033 BindingDB Entry DOI: 10.7270/Q27W6FW2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM10404 ((1S,12S,14R)-9-methoxy-4-methyl-11-oxa-4-azatetrac...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Ljubljana Curated by ChEMBL | Assay Description Inhibition of AChE (unknown origin) | Eur J Med Chem 156: 598-617 (2018) Article DOI: 10.1016/j.ejmech.2018.07.033 BindingDB Entry DOI: 10.7270/Q27W6FW2 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50468747 (CHEMBL4287511) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 460 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Ljubljana Curated by ChEMBL | Assay Description Inhibition of human BChE using butyrylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition and measured for 1 min b... | Eur J Med Chem 156: 598-617 (2018) Article DOI: 10.1016/j.ejmech.2018.07.033 BindingDB Entry DOI: 10.7270/Q27W6FW2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50468734 (CHEMBL4277107) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 635 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Ljubljana Curated by ChEMBL | Assay Description Inhibition of human BChE using butyrylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition and measured for 1 min b... | Eur J Med Chem 156: 598-617 (2018) Article DOI: 10.1016/j.ejmech.2018.07.033 BindingDB Entry DOI: 10.7270/Q27W6FW2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Diacylglycerol acyltransferase/mycolyltransferase Ag85C (Mycobacterium tuberculosis) | BDBM50148588 (CHEMBL325304 | Heptyl-phosphonic acid monoethyl es...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.06E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Ljubljana Curated by ChEMBL | Assay Description In vitro concentration required to inhibit antigen 85C mycolyltransferase activity in Mycobaterium tuberculosis | Bioorg Med Chem Lett 14: 3559-62 (2004) Article DOI: 10.1016/j.bmcl.2004.04.052 BindingDB Entry DOI: 10.7270/Q2C828RQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Diacylglycerol acyltransferase/mycolyltransferase Ag85C (Mycobacterium tuberculosis) | BDBM50148588 (CHEMBL325304 | Heptyl-phosphonic acid monoethyl es...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Ljubljana Curated by ChEMBL | Assay Description Inhibition of Mycobacterium tuberculosis recombinant antigen 85C mycolyltransferase | Eur J Med Chem 42: 54-63 (2007) Article DOI: 10.1016/j.ejmech.2006.08.007 BindingDB Entry DOI: 10.7270/Q2F18ZB3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Diacylglycerol acyltransferase/mycolyltransferase Ag85C (Mycobacterium tuberculosis) | BDBM50148590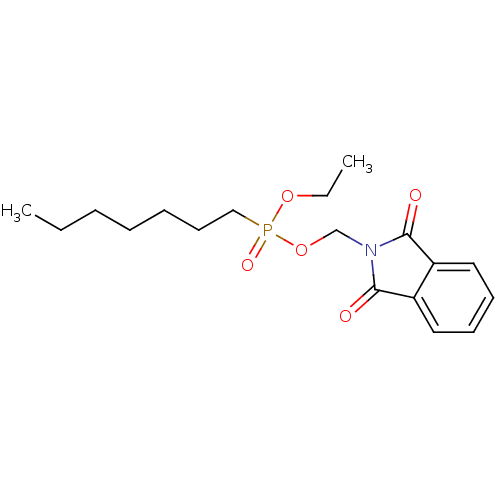 ((1,3-dioxo-1,3-dihydro-2H-isoindol-2-yl)-methyl et...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Ljubljana Curated by ChEMBL | Assay Description Inhibition of Mycobacterium tuberculosis recombinant antigen 85C mycolyltransferase | Eur J Med Chem 42: 54-63 (2007) Article DOI: 10.1016/j.ejmech.2006.08.007 BindingDB Entry DOI: 10.7270/Q2F18ZB3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Diacylglycerol acyltransferase/mycolyltransferase Ag85C (Mycobacterium tuberculosis) | BDBM50148590 ((1,3-dioxo-1,3-dihydro-2H-isoindol-2-yl)-methyl et...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.31E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Ljubljana Curated by ChEMBL | Assay Description In vitro concentration required to inhibit antigen 85C mycolyltransferase activity in Mycobaterium tuberculosis | Bioorg Med Chem Lett 14: 3559-62 (2004) Article DOI: 10.1016/j.bmcl.2004.04.052 BindingDB Entry DOI: 10.7270/Q2C828RQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Diacylglycerol acyltransferase/mycolyltransferase Ag85C (Mycobacterium tuberculosis) | BDBM50148587 (CHEMBL326268 | Heptyl-phosphonic acid mono-[2-(1,3...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.47E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Ljubljana Curated by ChEMBL | Assay Description In vitro concentration required to inhibit antigen 85C mycolyltransferase activity in Mycobaterium tuberculosis | Bioorg Med Chem Lett 14: 3559-62 (2004) Article DOI: 10.1016/j.bmcl.2004.04.052 BindingDB Entry DOI: 10.7270/Q2C828RQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50468740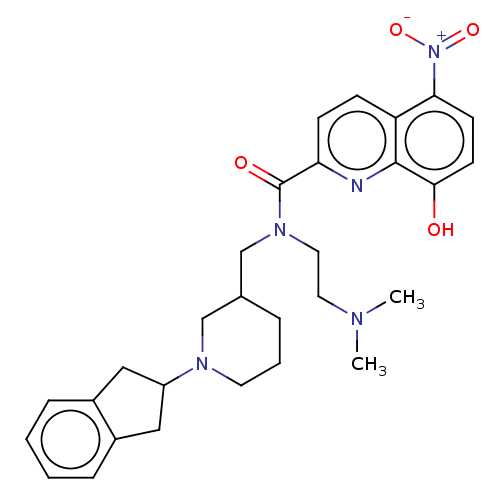 (CHEMBL4283974) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.61E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Ljubljana Curated by ChEMBL | Assay Description Inhibition of human BChE using butyrylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition and measured for 1 min b... | Eur J Med Chem 156: 598-617 (2018) Article DOI: 10.1016/j.ejmech.2018.07.033 BindingDB Entry DOI: 10.7270/Q27W6FW2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50468746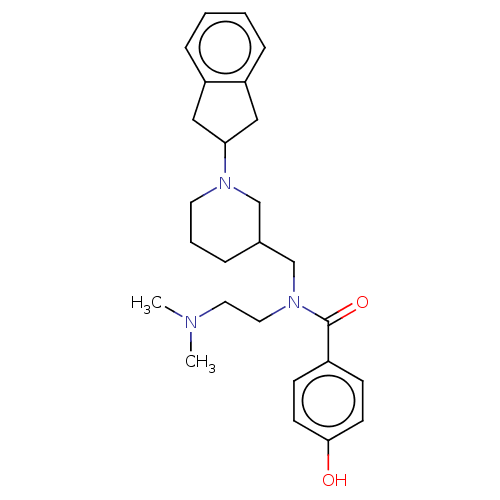 (CHEMBL4294752) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.79E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Ljubljana Curated by ChEMBL | Assay Description Inhibition of human BChE using butyrylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition and measured for 1 min b... | Eur J Med Chem 156: 598-617 (2018) Article DOI: 10.1016/j.ejmech.2018.07.033 BindingDB Entry DOI: 10.7270/Q27W6FW2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Diacylglycerol acyltransferase/mycolyltransferase Ag85C (Mycobacterium tuberculosis) | BDBM50195746 (CHEMBL227546 | ethyl 3-phenoxybenzyl butylphosphon...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Ljubljana Curated by ChEMBL | Assay Description Inhibition of Mycobacterium tuberculosis recombinant antigen 85C mycolyltransferase | Eur J Med Chem 42: 54-63 (2007) Article DOI: 10.1016/j.ejmech.2006.08.007 BindingDB Entry DOI: 10.7270/Q2F18ZB3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Diacylglycerol acyltransferase/mycolyltransferase Ag85C (Mycobacterium tuberculosis) | BDBM50148586 (Butyl-phosphonic acid ethyl ester 3-phenoxy-benzyl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.01E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Ljubljana Curated by ChEMBL | Assay Description In vitro concentration required to inhibit antigen 85C mycolyltransferase activity in Mycobaterium tuberculosis | Bioorg Med Chem Lett 14: 3559-62 (2004) Article DOI: 10.1016/j.bmcl.2004.04.052 BindingDB Entry DOI: 10.7270/Q2C828RQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50096251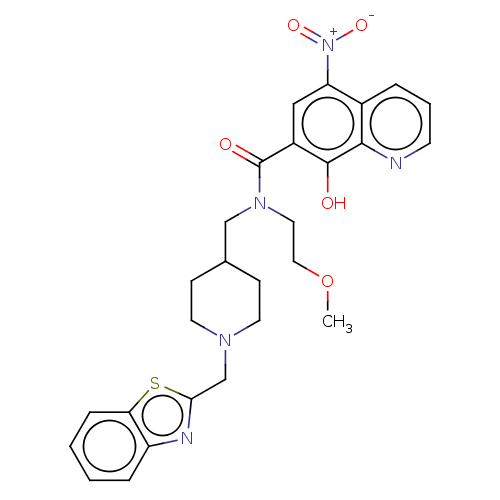 (CHEMBL3593652) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.14E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Ljubljana Curated by ChEMBL | Assay Description Inhibition of human recombinant BChE using butyrylthiocholine iodide as substrate after 5 mins by Ellman's method | Bioorg Med Chem 23: 4442-52 (2015) Article DOI: 10.1016/j.bmc.2015.06.010 BindingDB Entry DOI: 10.7270/Q2W097PZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50468745 (CHEMBL4284141) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.49E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Ljubljana Curated by ChEMBL | Assay Description Inhibition of human BChE using butyrylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition and measured for 1 min b... | Eur J Med Chem 156: 598-617 (2018) Article DOI: 10.1016/j.ejmech.2018.07.033 BindingDB Entry DOI: 10.7270/Q27W6FW2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM11682 (2,3-dihydroxybutanedioic acid; 3-[(1S)-1-(dimethyl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid UniChem Similars | DrugBank Article PubMed | n/a | n/a | 3.03E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Ljubljana Curated by ChEMBL | Assay Description Inhibition of AChE (unknown origin) | Eur J Med Chem 156: 598-617 (2018) Article DOI: 10.1016/j.ejmech.2018.07.033 BindingDB Entry DOI: 10.7270/Q27W6FW2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Diacylglycerol acyltransferase/mycolyltransferase Ag85C (Mycobacterium tuberculosis) | BDBM50148593 (CHEMBL119521 | Hexyl-phosphonic acid monoethyl est...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 3.56E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Ljubljana Curated by ChEMBL | Assay Description In vitro concentration required to inhibit antigen 85C mycolyltransferase activity in Mycobaterium tuberculosis | Bioorg Med Chem Lett 14: 3559-62 (2004) Article DOI: 10.1016/j.bmcl.2004.04.052 BindingDB Entry DOI: 10.7270/Q2C828RQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 94 total ) | Next | Last >> |