

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50342649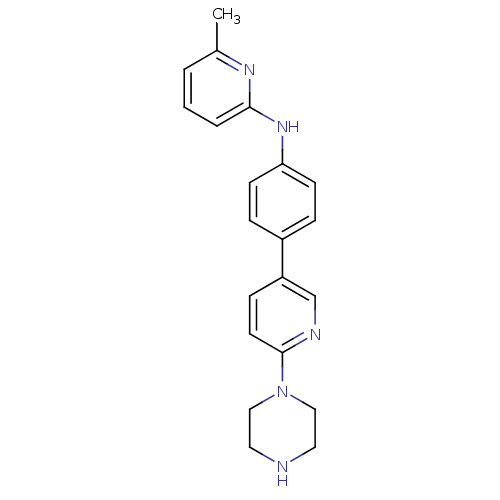 (6-methyl-N-(4-(6-(piperazin-1-yl)pyridin-3-yl)phen...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity to human ERG | Bioorg Med Chem Lett 21: 2646-9 (2011) Article DOI: 10.1016/j.bmcl.2010.12.115 BindingDB Entry DOI: 10.7270/Q2QV3MVJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TSC22 domain family protein 3 (Human) | BDBM476583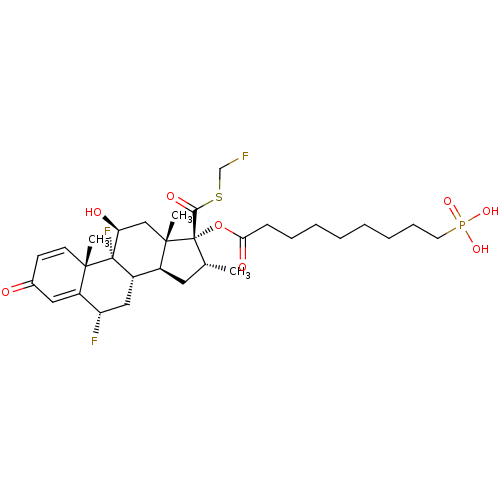 (US10869929, Compound 18 | US11554172, Compound 18) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
MERCK SHARP & DOHME CORP. US Patent | Assay Description HUT78 cells were cultured in IMEM plus 20% heat inactivated FBS and cell density was maintained between 0.1 to 1.2 million/mL. 786-O cells were cultu... | US Patent US10869929 (2020) BindingDB Entry DOI: 10.7270/Q2N58QG7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM476583 (US10869929, Compound 18 | US11554172, Compound 18) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The potency of binding to gucocorticoid receptor by small molecule compounds was measured with the PolarScreen™ Glucocorticoid Receptor Competitor As... | Citation and Details BindingDB Entry DOI: 10.7270/Q2639TPT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TSC22 domain family protein 3 (Human) | BDBM476584 (US10869929, Compound 19) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a |
MERCK SHARP & DOHME CORP. US Patent | Assay Description HUT78 cells were cultured in IMEM plus 20% heat inactivated FBS and cell density was maintained between 0.1 to 1.2 million/mL. 786-O cells were cultu... | US Patent US10869929 (2020) BindingDB Entry DOI: 10.7270/Q2N58QG7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM589245 (US11554172, Compound 19) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The potency of binding to gucocorticoid receptor by small molecule compounds was measured with the PolarScreen™ Glucocorticoid Receptor Competitor As... | Citation and Details BindingDB Entry DOI: 10.7270/Q2639TPT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TSC22 domain family protein 3 (Human) | BDBM50354849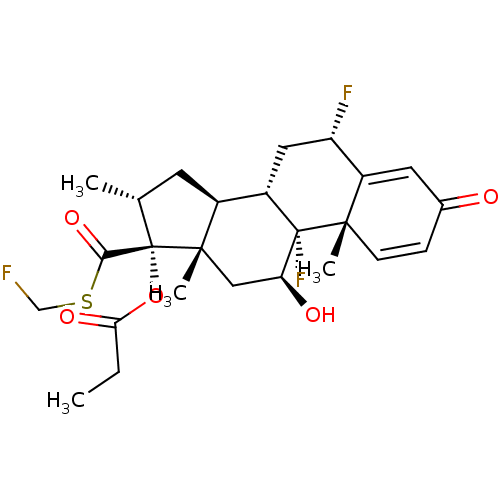 (CCI-18781 | Cutivate | FLUTICASONE PROPIONATE | Fl...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase DrugBank MCE PC cid PC sid UniChem Patents Similars | US Patent | n/a | n/a | 0.150 | n/a | n/a | n/a | n/a | n/a | n/a |
MERCK SHARP & DOHME CORP. US Patent | Assay Description HUT78 cells were cultured in IMEM plus 20% heat inactivated FBS and cell density was maintained between 0.1 to 1.2 million/mL. 786-O cells were cultu... | US Patent US10869929 (2020) BindingDB Entry DOI: 10.7270/Q2N58QG7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM589230 (US11554172, Compound Fluticasone-Propionate) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | US Patent | n/a | n/a | 0.150 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The potency of binding to gucocorticoid receptor by small molecule compounds was measured with the PolarScreen™ Glucocorticoid Receptor Competitor As... | Citation and Details BindingDB Entry DOI: 10.7270/Q2639TPT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM476575 (US10869929, Compound 8 | US11554172, Compound 8) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The potency of binding to gucocorticoid receptor by small molecule compounds was measured with the PolarScreen™ Glucocorticoid Receptor Competitor As... | Citation and Details BindingDB Entry DOI: 10.7270/Q2639TPT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TSC22 domain family protein 3 (Human) | BDBM476575 (US10869929, Compound 8 | US11554172, Compound 8) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a |
MERCK SHARP & DOHME CORP. US Patent | Assay Description HUT78 cells were cultured in IMEM plus 20% heat inactivated FBS and cell density was maintained between 0.1 to 1.2 million/mL. 786-O cells were cultu... | US Patent US10869929 (2020) BindingDB Entry DOI: 10.7270/Q2N58QG7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM476572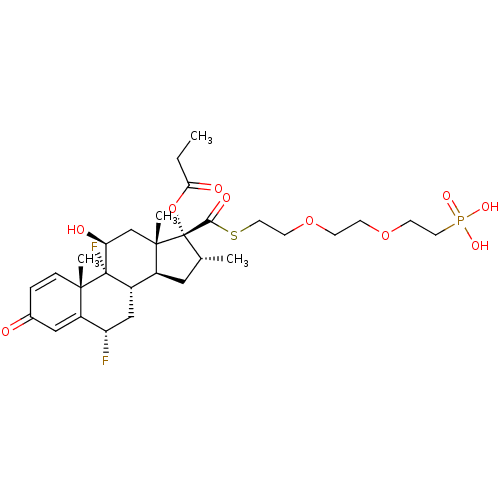 (US10869929, Compound 6 | US11554172, Compound 6) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.320 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The potency of binding to gucocorticoid receptor by small molecule compounds was measured with the PolarScreen™ Glucocorticoid Receptor Competitor As... | Citation and Details BindingDB Entry DOI: 10.7270/Q2639TPT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TSC22 domain family protein 3 (Human) | BDBM476572 (US10869929, Compound 6 | US11554172, Compound 6) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.320 | n/a | n/a | n/a | n/a | n/a | n/a |
MERCK SHARP & DOHME CORP. US Patent | Assay Description HUT78 cells were cultured in IMEM plus 20% heat inactivated FBS and cell density was maintained between 0.1 to 1.2 million/mL. 786-O cells were cultu... | US Patent US10869929 (2020) BindingDB Entry DOI: 10.7270/Q2N58QG7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM476567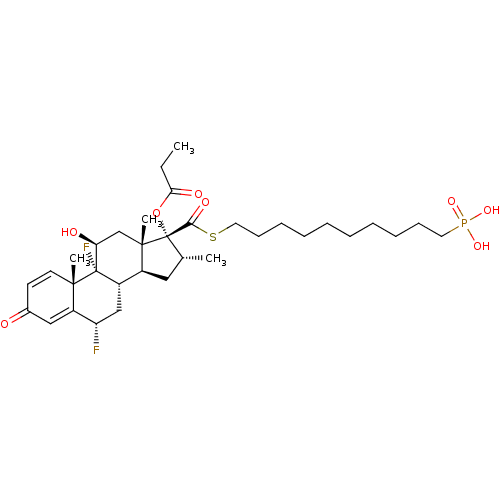 (US10869929, Compound 3 | US11554172, Compound 3) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.440 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The potency of binding to gucocorticoid receptor by small molecule compounds was measured with the PolarScreen™ Glucocorticoid Receptor Competitor As... | Citation and Details BindingDB Entry DOI: 10.7270/Q2639TPT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TSC22 domain family protein 3 (Human) | BDBM476567 (US10869929, Compound 3 | US11554172, Compound 3) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.440 | n/a | n/a | n/a | n/a | n/a | n/a |
MERCK SHARP & DOHME CORP. US Patent | Assay Description HUT78 cells were cultured in IMEM plus 20% heat inactivated FBS and cell density was maintained between 0.1 to 1.2 million/mL. 786-O cells were cultu... | US Patent US10869929 (2020) BindingDB Entry DOI: 10.7270/Q2N58QG7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TSC22 domain family protein 3 (Human) | BDBM476573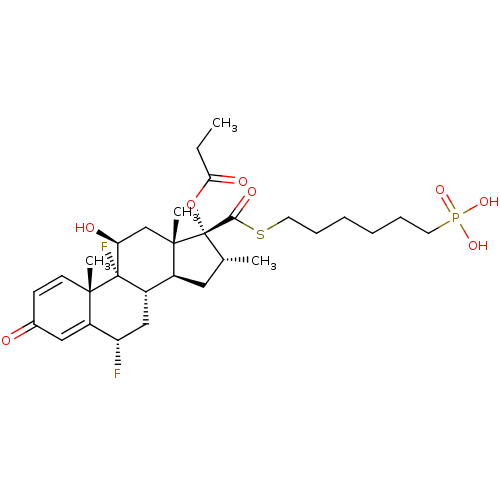 (US10869929, Compound 7 | US11554172, Compound 7) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.450 | n/a | n/a | n/a | n/a | n/a | n/a |
MERCK SHARP & DOHME CORP. US Patent | Assay Description HUT78 cells were cultured in IMEM plus 20% heat inactivated FBS and cell density was maintained between 0.1 to 1.2 million/mL. 786-O cells were cultu... | US Patent US10869929 (2020) BindingDB Entry DOI: 10.7270/Q2N58QG7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM476573 (US10869929, Compound 7 | US11554172, Compound 7) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.450 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The potency of binding to gucocorticoid receptor by small molecule compounds was measured with the PolarScreen™ Glucocorticoid Receptor Competitor As... | Citation and Details BindingDB Entry DOI: 10.7270/Q2639TPT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TSC22 domain family protein 3 (Human) | BDBM476568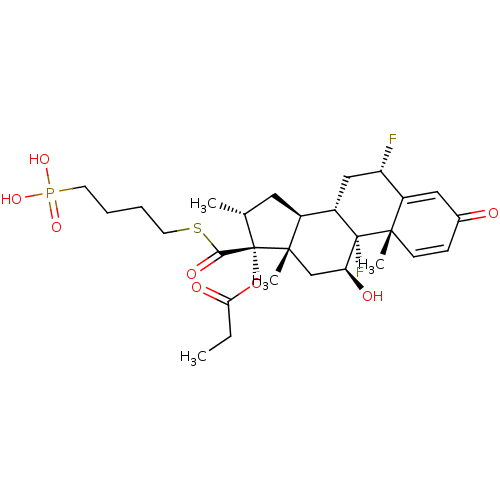 (US10869929, Compound 4 | US11554172, Compound 4) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.710 | n/a | n/a | n/a | n/a | n/a | n/a |
MERCK SHARP & DOHME CORP. US Patent | Assay Description HUT78 cells were cultured in IMEM plus 20% heat inactivated FBS and cell density was maintained between 0.1 to 1.2 million/mL. 786-O cells were cultu... | US Patent US10869929 (2020) BindingDB Entry DOI: 10.7270/Q2N58QG7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM476568 (US10869929, Compound 4 | US11554172, Compound 4) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.710 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The potency of binding to gucocorticoid receptor by small molecule compounds was measured with the PolarScreen™ Glucocorticoid Receptor Competitor As... | Citation and Details BindingDB Entry DOI: 10.7270/Q2639TPT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TSC22 domain family protein 3 (Human) | BDBM50354850 (BUDESONIDE | US10869929, Compound Budesonide | US1...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase DrugBank MCE PC cid PC sid UniChem Patents Similars | US Patent | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
MERCK SHARP & DOHME CORP. US Patent | Assay Description HUT78 cells were cultured in IMEM plus 20% heat inactivated FBS and cell density was maintained between 0.1 to 1.2 million/mL. 786-O cells were cultu... | US Patent US10869929 (2020) BindingDB Entry DOI: 10.7270/Q2N58QG7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50354850 (BUDESONIDE | US10869929, Compound Budesonide | US1...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE PC cid PC sid UniChem Patents Similars | DrugBank US Patent | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The potency of binding to gucocorticoid receptor by small molecule compounds was measured with the PolarScreen™ Glucocorticoid Receptor Competitor As... | Citation and Details BindingDB Entry DOI: 10.7270/Q2639TPT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM476576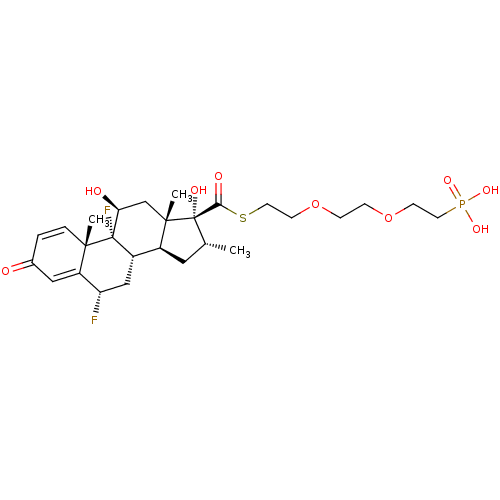 (US10869929, Compound 9 | US11554172, Compound 9) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.67 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The potency of binding to gucocorticoid receptor by small molecule compounds was measured with the PolarScreen™ Glucocorticoid Receptor Competitor As... | Citation and Details BindingDB Entry DOI: 10.7270/Q2639TPT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TSC22 domain family protein 3 (Human) | BDBM476576 (US10869929, Compound 9 | US11554172, Compound 9) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.67 | n/a | n/a | n/a | n/a | n/a | n/a |
MERCK SHARP & DOHME CORP. US Patent | Assay Description HUT78 cells were cultured in IMEM plus 20% heat inactivated FBS and cell density was maintained between 0.1 to 1.2 million/mL. 786-O cells were cultu... | US Patent US10869929 (2020) BindingDB Entry DOI: 10.7270/Q2N58QG7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM476577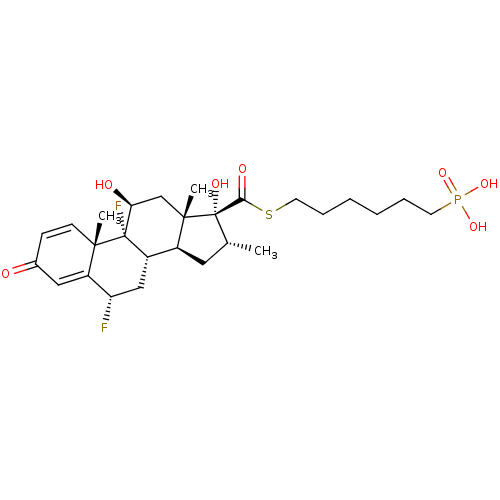 (US10869929, Compound 10 | US11554172, Compound 10) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.99 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The potency of binding to gucocorticoid receptor by small molecule compounds was measured with the PolarScreen™ Glucocorticoid Receptor Competitor As... | Citation and Details BindingDB Entry DOI: 10.7270/Q2639TPT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TSC22 domain family protein 3 (Human) | BDBM476577 (US10869929, Compound 10 | US11554172, Compound 10) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.99 | n/a | n/a | n/a | n/a | n/a | n/a |
MERCK SHARP & DOHME CORP. US Patent | Assay Description HUT78 cells were cultured in IMEM plus 20% heat inactivated FBS and cell density was maintained between 0.1 to 1.2 million/mL. 786-O cells were cultu... | US Patent US10869929 (2020) BindingDB Entry DOI: 10.7270/Q2N58QG7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TSC22 domain family protein 3 (Human) | BDBM476585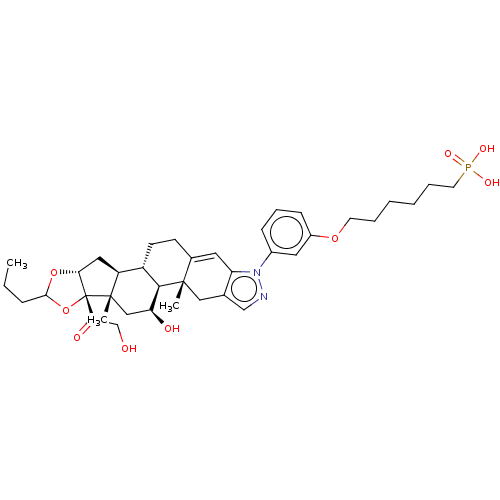 (US10869929, Compound 27 | US11554172, Compound 27) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
MERCK SHARP & DOHME CORP. US Patent | Assay Description HUT78 cells were cultured in IMEM plus 20% heat inactivated FBS and cell density was maintained between 0.1 to 1.2 million/mL. 786-O cells were cultu... | US Patent US10869929 (2020) BindingDB Entry DOI: 10.7270/Q2N58QG7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 5 (Homo sapiens (Human)) | BDBM504572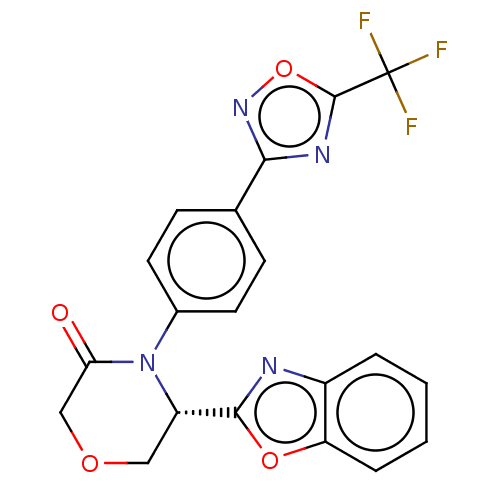 ((S)-5-(Benzo[d]oxazol-2-yl)-4-(4-(5-(trifluorometh...) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description HDAC5 reagents: N-terminal GST tagged HDAC5 was purchased from BPS Bioscience [catalog #50045]. Assays were performed with buffer containing 20 mM HE... | Citation and Details BindingDB Entry DOI: 10.7270/Q2DN485Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM476585 (US10869929, Compound 27 | US11554172, Compound 27) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The potency of binding to gucocorticoid receptor by small molecule compounds was measured with the PolarScreen™ Glucocorticoid Receptor Competitor As... | Citation and Details BindingDB Entry DOI: 10.7270/Q2639TPT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM504755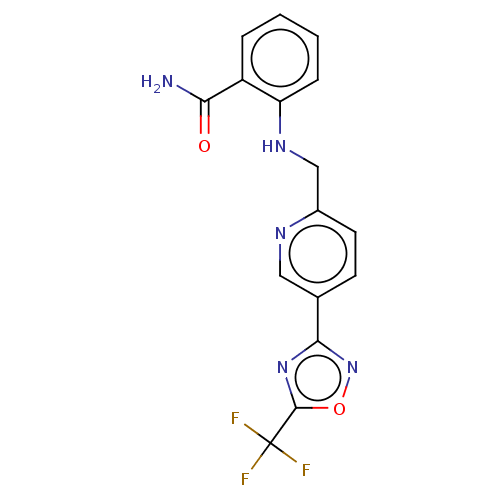 (2-(((5-(5-(Trifluoromethyl)-1,2,4-oxadiazol-3-yl)p...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description HDAC1 and 6 reagents: FLAG-tagged HDACs 1 and 6 were prepared in-house by protein expression in HEK293F cells followed by anti-FLAG affinity purifica... | Citation and Details BindingDB Entry DOI: 10.7270/Q2DN485Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TSC22 domain family protein 3 (Human) | BDBM18207 ((1R,2S,10S,11S,13R,14R,15S,17S)-1-fluoro-14,17-dih...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | US Patent | n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
MERCK SHARP & DOHME CORP. US Patent | Assay Description HUT78 cells were cultured in IMEM plus 20% heat inactivated FBS and cell density was maintained between 0.1 to 1.2 million/mL. 786-O cells were cultu... | US Patent US10869929 (2020) BindingDB Entry DOI: 10.7270/Q2N58QG7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM18207 ((1R,2S,10S,11S,13R,14R,15S,17S)-1-fluoro-14,17-dih...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB US Patent | n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The potency of binding to gucocorticoid receptor by small molecule compounds was measured with the PolarScreen™ Glucocorticoid Receptor Competitor As... | Citation and Details BindingDB Entry DOI: 10.7270/Q2639TPT | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM476591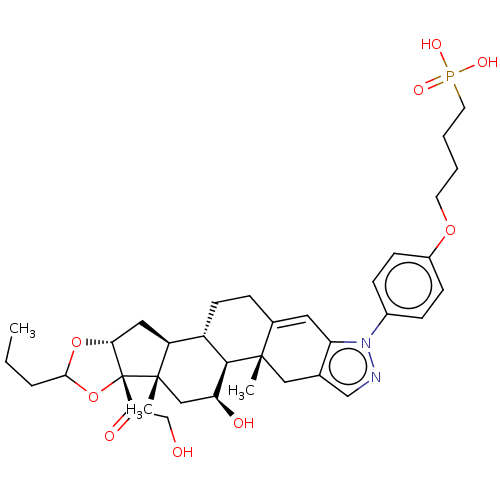 (US10869929, Compound 30 | US11554172, Compound 30) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The potency of binding to gucocorticoid receptor by small molecule compounds was measured with the PolarScreen™ Glucocorticoid Receptor Competitor As... | Citation and Details BindingDB Entry DOI: 10.7270/Q2639TPT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TSC22 domain family protein 3 (Human) | BDBM476591 (US10869929, Compound 30 | US11554172, Compound 30) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
MERCK SHARP & DOHME CORP. US Patent | Assay Description HUT78 cells were cultured in IMEM plus 20% heat inactivated FBS and cell density was maintained between 0.1 to 1.2 million/mL. 786-O cells were cultu... | US Patent US10869929 (2020) BindingDB Entry DOI: 10.7270/Q2N58QG7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM504971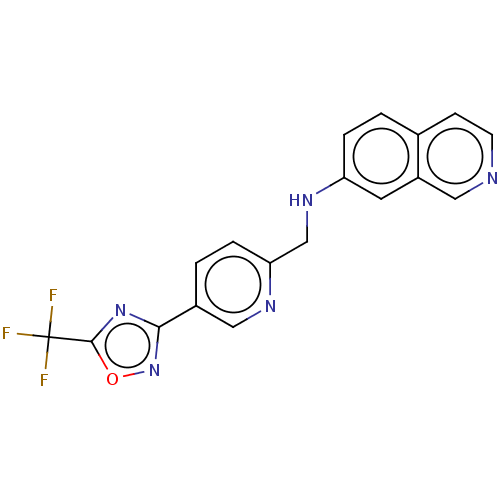 (N-({5-[5- (trifluoro- methyl)- 1,2,4-oxa- diazol-3...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description HDAC1 and 6 reagents: FLAG-tagged HDACs 1 and 6 were prepared in-house by protein expression in HEK293F cells followed by anti-FLAG affinity purifica... | Citation and Details BindingDB Entry DOI: 10.7270/Q2DN485Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 5 (Homo sapiens (Human)) | BDBM504735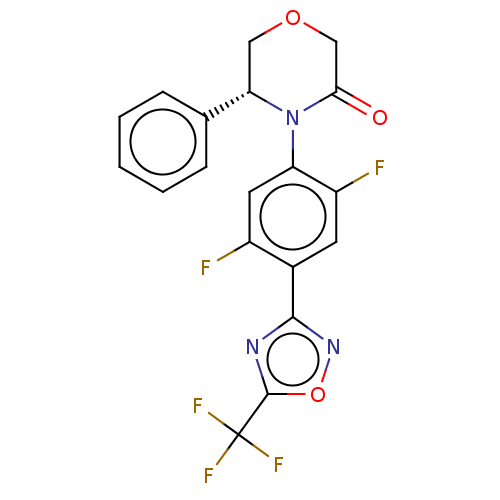 ((R)-4-(2,5- difluoro-4-(5- (trifluoromethyl)- 1,2,...) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description HDAC5 reagents: N-terminal GST tagged HDAC5 was purchased from BPS Bioscience [catalog #50045]. Assays were performed with buffer containing 20 mM HE... | Citation and Details BindingDB Entry DOI: 10.7270/Q2DN485Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM505072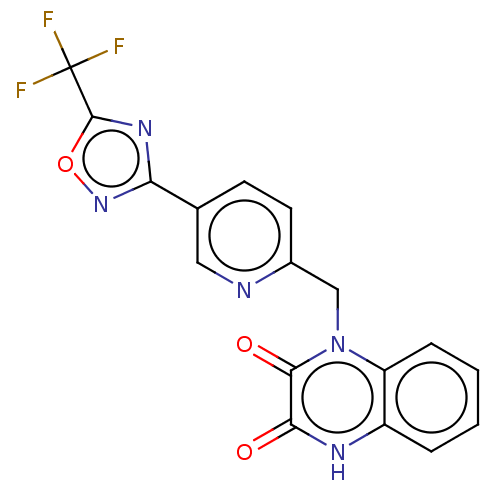 (1-({5-[5- (trifluoro- methyl)- 1,2,4-oxa- diazol-3...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description HDAC1 and 6 reagents: FLAG-tagged HDACs 1 and 6 were prepared in-house by protein expression in HEK293F cells followed by anti-FLAG affinity purifica... | Citation and Details BindingDB Entry DOI: 10.7270/Q2DN485Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM505023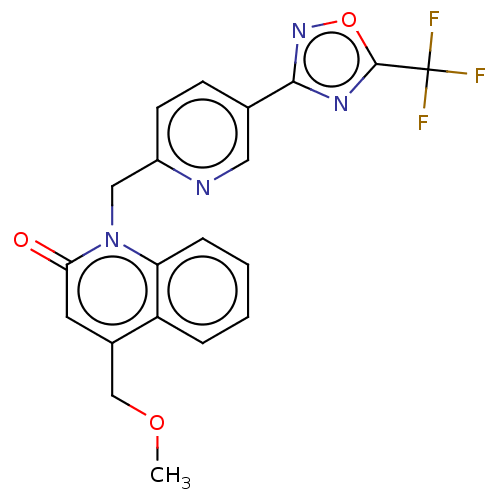 (4-(methoxy- methyl)- 1-({5-[5- (trifluoro- methyl)...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description HDAC1 and 6 reagents: FLAG-tagged HDACs 1 and 6 were prepared in-house by protein expression in HEK293F cells followed by anti-FLAG affinity purifica... | Citation and Details BindingDB Entry DOI: 10.7270/Q2DN485Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TSC22 domain family protein 3 (Human) | BDBM476593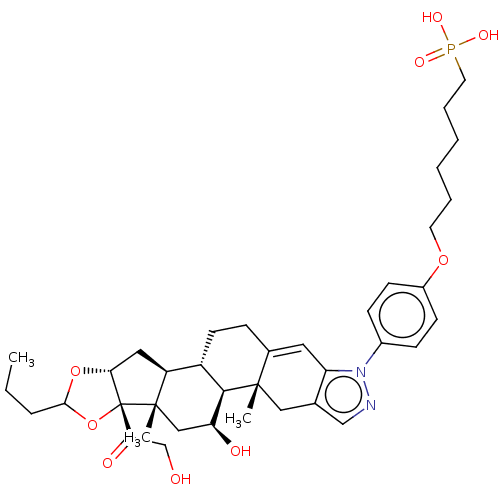 (US10869929, Compound 31 | US11554172, Compound 31) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a |
MERCK SHARP & DOHME CORP. US Patent | Assay Description HUT78 cells were cultured in IMEM plus 20% heat inactivated FBS and cell density was maintained between 0.1 to 1.2 million/mL. 786-O cells were cultu... | US Patent US10869929 (2020) BindingDB Entry DOI: 10.7270/Q2N58QG7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM504529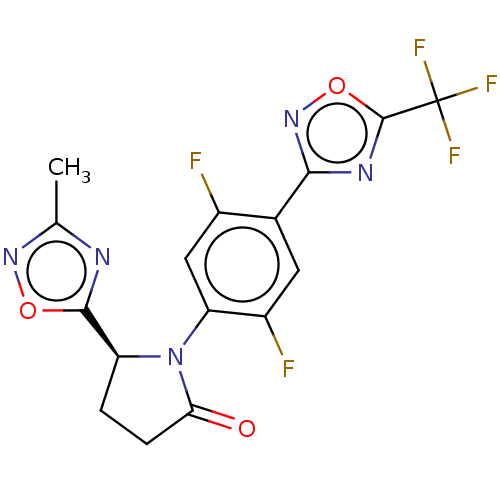 ((S)-1-(2,5-Difluoro-4-(5-(trifluoromethyl)-1,2,4-o...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description HDAC1 and 6 reagents: FLAG-tagged HDACs 1 and 6 were prepared in-house by protein expression in HEK293F cells followed by anti-FLAG affinity purifica... | Citation and Details BindingDB Entry DOI: 10.7270/Q2DN485Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM504913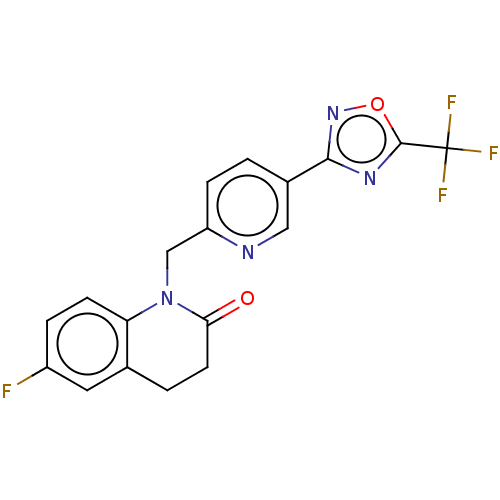 (6-fluoro- 1-({5-[5- (trifluoro- methyl)- 1,2,4-oxa...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description HDAC1 and 6 reagents: FLAG-tagged HDACs 1 and 6 were prepared in-house by protein expression in HEK293F cells followed by anti-FLAG affinity purifica... | Citation and Details BindingDB Entry DOI: 10.7270/Q2DN485Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM476593 (US10869929, Compound 31 | US11554172, Compound 31) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The potency of binding to gucocorticoid receptor by small molecule compounds was measured with the PolarScreen™ Glucocorticoid Receptor Competitor As... | Citation and Details BindingDB Entry DOI: 10.7270/Q2639TPT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TSC22 domain family protein 3 (Human) | BDBM476589 (US10869929, Compound 28 | US11554172, Compound 28) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4.10 | n/a | n/a | n/a | n/a | n/a | n/a |
MERCK SHARP & DOHME CORP. US Patent | Assay Description HUT78 cells were cultured in IMEM plus 20% heat inactivated FBS and cell density was maintained between 0.1 to 1.2 million/mL. 786-O cells were cultu... | US Patent US10869929 (2020) BindingDB Entry DOI: 10.7270/Q2N58QG7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM476589 (US10869929, Compound 28 | US11554172, Compound 28) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The potency of binding to gucocorticoid receptor by small molecule compounds was measured with the PolarScreen™ Glucocorticoid Receptor Competitor As... | Citation and Details BindingDB Entry DOI: 10.7270/Q2639TPT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM504678 ((S)-5-(1H- benzo[d]imidazol- 2-yl)-1-(2-fluoro- 4-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description HDAC1 and 6 reagents: FLAG-tagged HDACs 1 and 6 were prepared in-house by protein expression in HEK293F cells followed by anti-FLAG affinity purifica... | Citation and Details BindingDB Entry DOI: 10.7270/Q2DN485Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM505052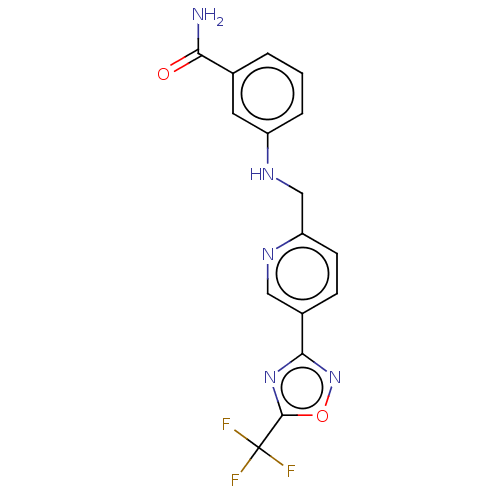 (3-[({5-[5- (trifluoro- methyl)- 1,2,4-oxa- diazol-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description HDAC1 and 6 reagents: FLAG-tagged HDACs 1 and 6 were prepared in-house by protein expression in HEK293F cells followed by anti-FLAG affinity purifica... | Citation and Details BindingDB Entry DOI: 10.7270/Q2DN485Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM504919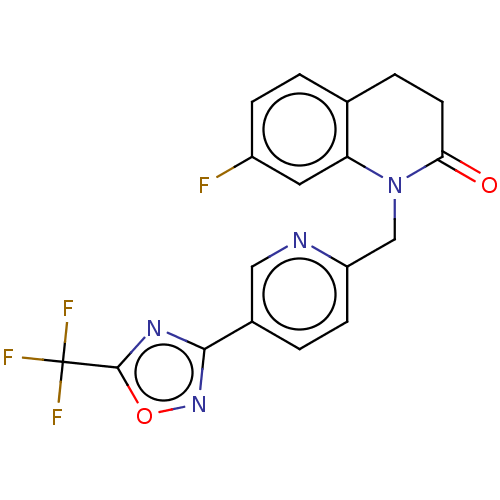 (7-fluoro- 1-({5-[5- (trifluoro- methyl)- 1,2,4-oxa...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description HDAC1 and 6 reagents: FLAG-tagged HDACs 1 and 6 were prepared in-house by protein expression in HEK293F cells followed by anti-FLAG affinity purifica... | Citation and Details BindingDB Entry DOI: 10.7270/Q2DN485Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM505048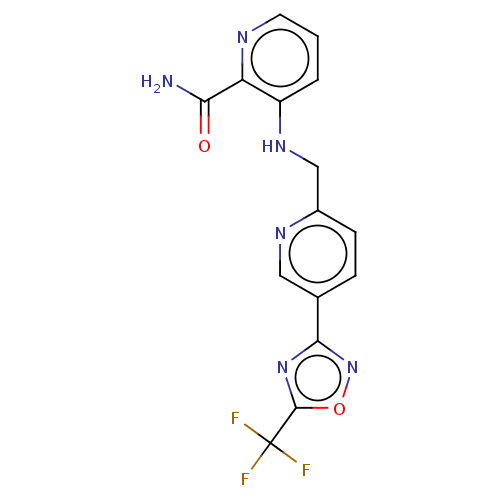 (3-[({5-[5- (trifluoro- methyl)- 1,2,4-oxa- diazol-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description HDAC1 and 6 reagents: FLAG-tagged HDACs 1 and 6 were prepared in-house by protein expression in HEK293F cells followed by anti-FLAG affinity purifica... | Citation and Details BindingDB Entry DOI: 10.7270/Q2DN485Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM505063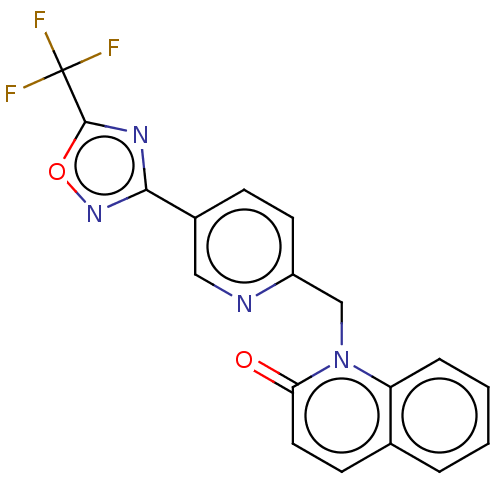 (1-({5-[5- (trifluoro- methyl)- 1,2,4-oxa- diazol-3...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description HDAC1 and 6 reagents: FLAG-tagged HDACs 1 and 6 were prepared in-house by protein expression in HEK293F cells followed by anti-FLAG affinity purifica... | Citation and Details BindingDB Entry DOI: 10.7270/Q2DN485Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM504550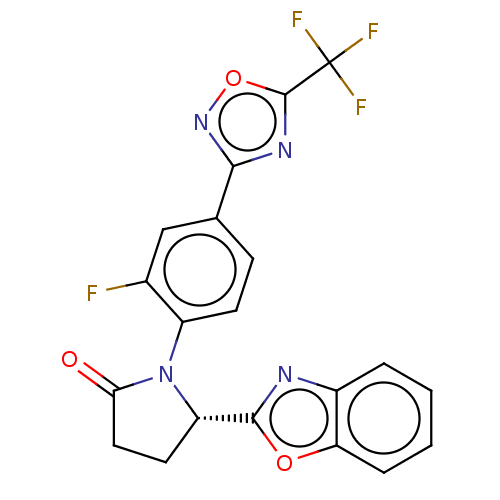 ((S)-5-(Benzo[d]oxazol-2-yl)-1-(2-fluoro-4-(5-(trif...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description HDAC1 and 6 reagents: FLAG-tagged HDACs 1 and 6 were prepared in-house by protein expression in HEK293F cells followed by anti-FLAG affinity purifica... | Citation and Details BindingDB Entry DOI: 10.7270/Q2DN485Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM505049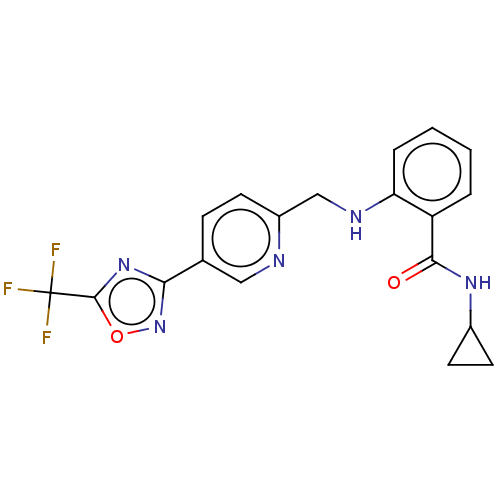 (N-cyclo- propyl-2- [({5-[5- (trifluoro- methyl)- 1...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description HDAC1 and 6 reagents: FLAG-tagged HDACs 1 and 6 were prepared in-house by protein expression in HEK293F cells followed by anti-FLAG affinity purifica... | Citation and Details BindingDB Entry DOI: 10.7270/Q2DN485Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM504958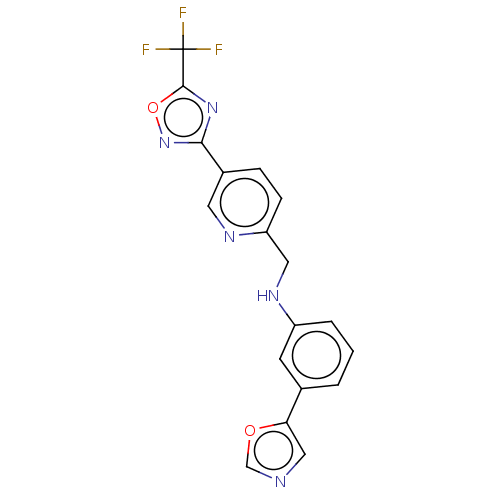 (3-(1,3- oxazol- 5-yl)- N-({5-[5- (trifluoro- methy...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description HDAC1 and 6 reagents: FLAG-tagged HDACs 1 and 6 were prepared in-house by protein expression in HEK293F cells followed by anti-FLAG affinity purifica... | Citation and Details BindingDB Entry DOI: 10.7270/Q2DN485Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM504543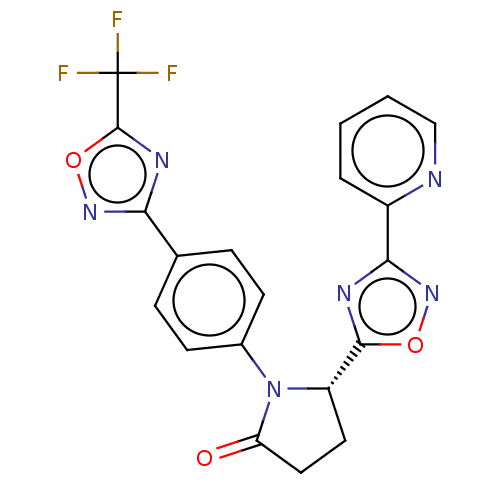 ((S)-5-(3-(Pyridin-2-yl)-1,2,4-oxadiazol-5-yl)-1-(4...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description HDAC1 and 6 reagents: FLAG-tagged HDACs 1 and 6 were prepared in-house by protein expression in HEK293F cells followed by anti-FLAG affinity purifica... | Citation and Details BindingDB Entry DOI: 10.7270/Q2DN485Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 1692 total ) | Next | Last >> |