 Found 1417 hits with Last Name = 'myers' and Initial = 'rw'
Found 1417 hits with Last Name = 'myers' and Initial = 'rw' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Histone deacetylase
(Cryptosporidium parvum) | BDBM50238632
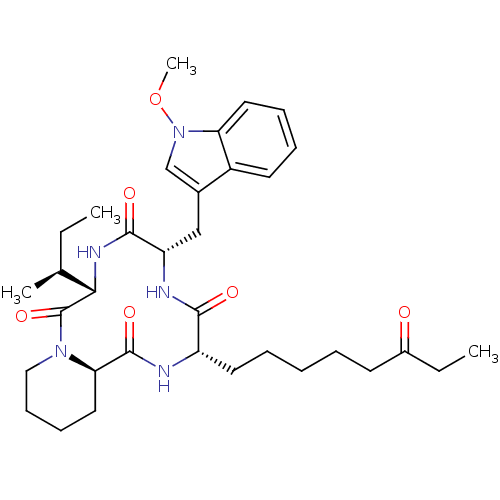
((6S,9S,12S,14aR)-6-((S)-sec-Butyl)-9-(1-methoxy-1H...)Show SMILES CC[C@H](C)[C@@H]1NC(=O)[C@H](Cc2cn(OC)c3ccccc23)NC(=O)[C@H](CCCCCC(=O)CC)NC(=O)[C@H]2CCCCN2C1=O |r| Show InChI InChI=1S/C34H49N5O6/c1-5-22(3)30-34(44)38-19-13-12-18-29(38)33(43)35-26(16-9-7-8-14-24(40)6-2)31(41)36-27(32(42)37-30)20-23-21-39(45-4)28-17-11-10-15-25(23)28/h10-11,15,17,21-22,26-27,29-30H,5-9,12-14,16,18-20H2,1-4H3,(H,35,43)(H,36,41)(H,37,42)/t22-,26-,27-,29+,30-/m0/s1 | KEGG
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| PubMed
| 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity towards HDAC enzyme derived from Eimeria tenella protozoa |
Bioorg Med Chem Lett 11: 113-7 (2001)
BindingDB Entry DOI: 10.7270/Q2M045ZB |
More data for this
Ligand-Target Pair | |
Histone deacetylase
(Cryptosporidium parvum) | BDBM50366737
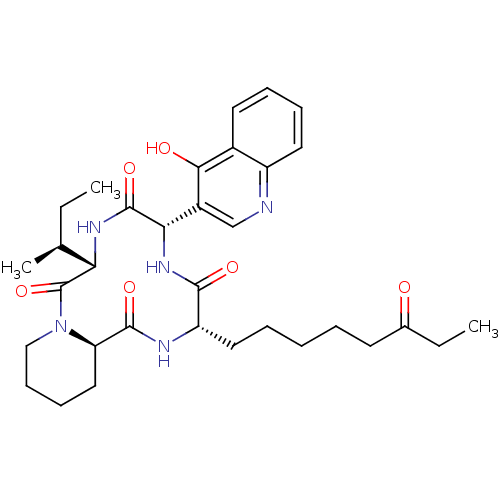
(CHEMBL1793971)Show SMILES CC[C@H](C)[C@@H]1NC(=O)[C@@H](NC(=O)[C@H](CCCCCC(=O)CC)NC(=O)[C@H]2CCCCN2C1=O)c1cnc2ccccc2c1O Show InChI InChI=1S/C33H45N5O6/c1-4-20(3)27-33(44)38-18-12-11-17-26(38)31(42)35-25(16-8-6-7-13-21(39)5-2)30(41)37-28(32(43)36-27)23-19-34-24-15-10-9-14-22(24)29(23)40/h9-10,14-15,19-20,25-28H,4-8,11-13,16-18H2,1-3H3,(H,34,40)(H,35,42)(H,36,43)(H,37,41)/t20-,25-,26+,27-,28-/m0/s1 | KEGG
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity towards HDAC enzyme derived from Eimeria tenella protozoa |
Bioorg Med Chem Lett 11: 113-7 (2001)
BindingDB Entry DOI: 10.7270/Q2M045ZB |
More data for this
Ligand-Target Pair | |
Histone deacetylase
(Cryptosporidium parvum) | BDBM50366726

(CHEMBL1793982)Show SMILES CC[C@H](C)[C@@H]1NC(=O)[C@H](NC(=O)[C@H](CCCCCC(=O)CC)NC(=O)[C@H]2CCCCN2C1=O)c1cnc2ccccc2c1N1CCCCC1 Show InChI InChI=1S/C38H54N6O5/c1-4-25(3)32-38(49)44-23-15-12-20-31(44)36(47)40-30(19-9-6-8-16-26(45)5-2)35(46)42-33(37(48)41-32)28-24-39-29-18-11-10-17-27(29)34(28)43-21-13-7-14-22-43/h10-11,17-18,24-25,30-33H,4-9,12-16,19-23H2,1-3H3,(H,40,47)(H,41,48)(H,42,46)/t25-,30-,31+,32-,33+/m0/s1 | KEGG
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity towards HDAC enzyme derived from Eimeria tenella protozoa |
Bioorg Med Chem Lett 11: 113-7 (2001)
BindingDB Entry DOI: 10.7270/Q2M045ZB |
More data for this
Ligand-Target Pair | |
Histone deacetylase
(Cryptosporidium parvum) | BDBM50366733
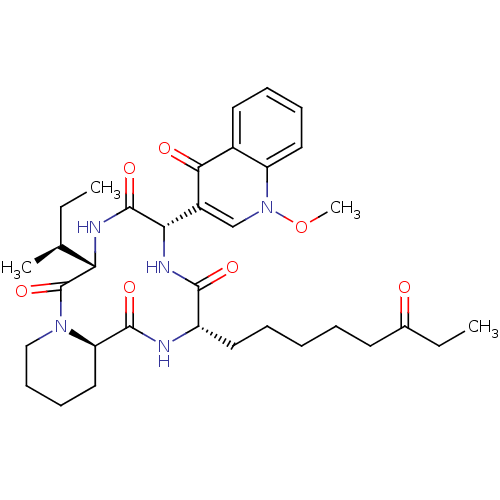
(CHEMBL1793973)Show SMILES CC[C@H](C)[C@@H]1NC(=O)[C@@H](NC(=O)[C@H](CCCCCC(=O)CC)NC(=O)[C@H]2CCCCN2C1=O)c1cn(OC)c2ccccc2c1=O Show InChI InChI=1S/C34H47N5O7/c1-5-21(3)28-34(45)38-19-13-12-18-27(38)32(43)35-25(16-9-7-8-14-22(40)6-2)31(42)37-29(33(44)36-28)24-20-39(46-4)26-17-11-10-15-23(26)30(24)41/h10-11,15,17,20-21,25,27-29H,5-9,12-14,16,18-19H2,1-4H3,(H,35,43)(H,36,44)(H,37,42)/t21-,25-,27+,28-,29-/m0/s1 | KEGG
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity towards HDAC enzyme derived from Eimeria tenella protozoa |
Bioorg Med Chem Lett 11: 113-7 (2001)
BindingDB Entry DOI: 10.7270/Q2M045ZB |
More data for this
Ligand-Target Pair | |
Histone deacetylase
(Cryptosporidium parvum) | BDBM50366749
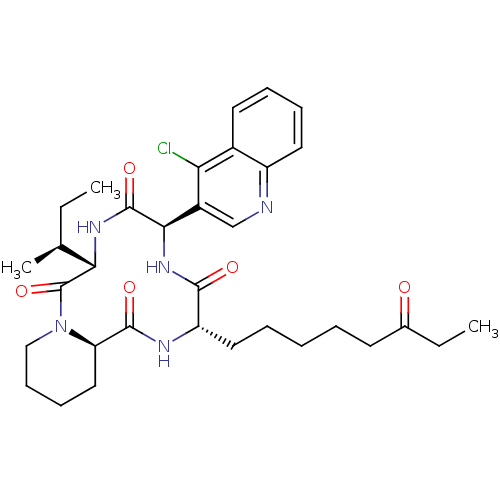
(CHEMBL1793983)Show SMILES CC[C@H](C)[C@@H]1NC(=O)[C@H](NC(=O)[C@H](CCCCCC(=O)CC)NC(=O)[C@H]2CCCCN2C1=O)c1cnc2ccccc2c1Cl Show InChI InChI=1S/C33H44ClN5O5/c1-4-20(3)28-33(44)39-18-12-11-17-26(39)31(42)36-25(16-8-6-7-13-21(40)5-2)30(41)38-29(32(43)37-28)23-19-35-24-15-10-9-14-22(24)27(23)34/h9-10,14-15,19-20,25-26,28-29H,4-8,11-13,16-18H2,1-3H3,(H,36,42)(H,37,43)(H,38,41)/t20-,25-,26+,28-,29+/m0/s1 | KEGG
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity towards HDAC enzyme derived from Eimeria tenella protozoa |
Bioorg Med Chem Lett 11: 113-7 (2001)
BindingDB Entry DOI: 10.7270/Q2M045ZB |
More data for this
Ligand-Target Pair | |
Histone deacetylase
(Cryptosporidium parvum) | BDBM50366736

(CHEMBL1793972)Show SMILES CC[C@H](C)[C@@H]1NC(=O)[C@@H](NC(=O)[C@H](CCCCCC(=O)CC)NC(=O)[C@H]2CCCCN2C1=O)c1cn(CC)c2ccccc2c1=O Show InChI InChI=1S/C35H49N5O6/c1-5-22(4)29-35(46)40-20-14-13-19-28(40)33(44)36-26(17-10-8-9-15-23(41)6-2)32(43)38-30(34(45)37-29)25-21-39(7-3)27-18-12-11-16-24(27)31(25)42/h11-12,16,18,21-22,26,28-30H,5-10,13-15,17,19-20H2,1-4H3,(H,36,44)(H,37,45)(H,38,43)/t22-,26-,28+,29-,30-/m0/s1 | KEGG
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity towards HDAC enzyme derived from Eimeria tenella protozoa |
Bioorg Med Chem Lett 11: 113-7 (2001)
BindingDB Entry DOI: 10.7270/Q2M045ZB |
More data for this
Ligand-Target Pair | |
Histone deacetylase
(Cryptosporidium parvum) | BDBM50366727
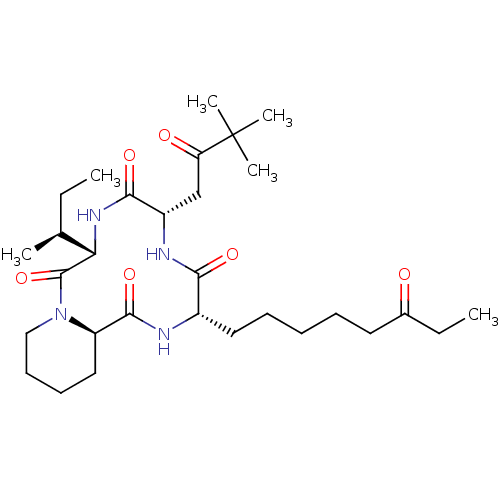
(CHEMBL1793981)Show SMILES CC[C@H](C)[C@@H]1NC(=O)[C@H](CC(=O)C(C)(C)C)NC(=O)[C@H](CCCCCC(=O)CC)NC(=O)[C@H]2CCCCN2C1=O Show InChI InChI=1S/C30H50N4O6/c1-7-19(3)25-29(40)34-17-13-12-16-23(34)28(39)31-21(15-11-9-10-14-20(35)8-2)26(37)32-22(27(38)33-25)18-24(36)30(4,5)6/h19,21-23,25H,7-18H2,1-6H3,(H,31,39)(H,32,37)(H,33,38)/t19-,21-,22-,23+,25-/m0/s1 | KEGG
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity towards HDAC enzyme derived from Eimeria tenella protozoa |
Bioorg Med Chem Lett 11: 113-7 (2001)
BindingDB Entry DOI: 10.7270/Q2M045ZB |
More data for this
Ligand-Target Pair | |
Histone deacetylase
(Cryptosporidium parvum) | BDBM50366729
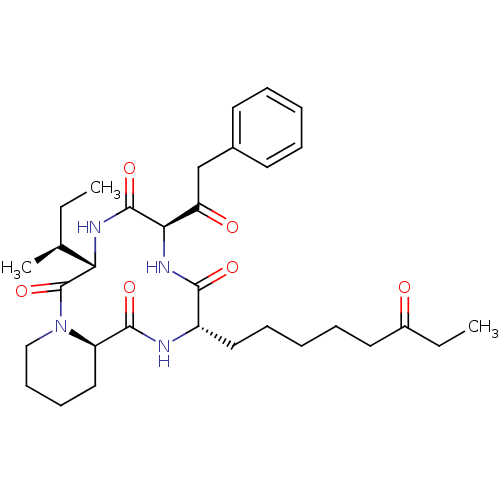
(CHEMBL1793969)Show SMILES CC[C@H](C)[C@@H]1NC(=O)[C@H](NC(=O)[C@H](CCCCCC(=O)CC)NC(=O)[C@H]2CCCCN2C1=O)C(=O)Cc1ccccc1 Show InChI InChI=1S/C32H46N4O6/c1-4-21(3)27-32(42)36-19-13-12-18-25(36)30(40)33-24(17-11-7-10-16-23(37)5-2)29(39)35-28(31(41)34-27)26(38)20-22-14-8-6-9-15-22/h6,8-9,14-15,21,24-25,27-28H,4-5,7,10-13,16-20H2,1-3H3,(H,33,40)(H,34,41)(H,35,39)/t21-,24-,25+,27-,28+/m0/s1 | KEGG
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity towards HDAC enzyme derived from Eimeria tenella protozoa |
Bioorg Med Chem Lett 11: 113-7 (2001)
BindingDB Entry DOI: 10.7270/Q2M045ZB |
More data for this
Ligand-Target Pair | |
Histone deacetylase
(Cryptosporidium parvum) | BDBM50366752
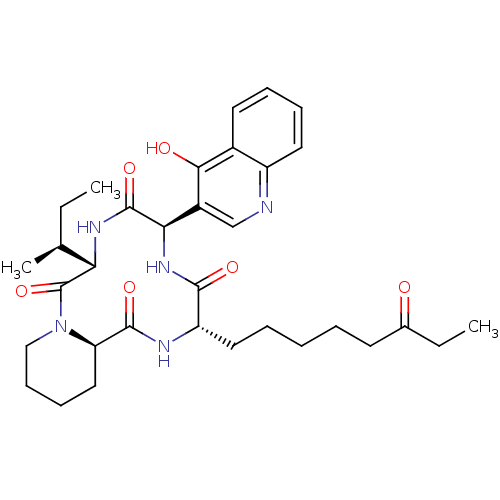
(CHEMBL1793970)Show SMILES CC[C@H](C)[C@@H]1NC(=O)[C@H](NC(=O)[C@H](CCCCCC(=O)CC)NC(=O)[C@H]2CCCCN2C1=O)c1cnc2ccccc2c1O Show InChI InChI=1S/C33H45N5O6/c1-4-20(3)27-33(44)38-18-12-11-17-26(38)31(42)35-25(16-8-6-7-13-21(39)5-2)30(41)37-28(32(43)36-27)23-19-34-24-15-10-9-14-22(24)29(23)40/h9-10,14-15,19-20,25-28H,4-8,11-13,16-18H2,1-3H3,(H,34,40)(H,35,42)(H,36,43)(H,37,41)/t20-,25-,26+,27-,28+/m0/s1 | KEGG
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 250 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity towards HDAC enzyme derived from Eimeria tenella protozoa |
Bioorg Med Chem Lett 11: 113-7 (2001)
BindingDB Entry DOI: 10.7270/Q2M045ZB |
More data for this
Ligand-Target Pair | |
Histone deacetylase
(Cryptosporidium parvum) | BDBM50366728

(CHEMBL1793978)Show SMILES CC[C@H](C)[C@@H]1NC(=O)[C@H](CC(=O)Cc2ccccc2)NC(=O)[C@H](CCCCCC(=O)CC)NC(=O)[C@H]2CCCCN2C1=O Show InChI InChI=1S/C33H48N4O6/c1-4-22(3)29-33(43)37-19-13-12-18-28(37)32(42)34-26(17-11-7-10-16-24(38)5-2)30(40)35-27(31(41)36-29)21-25(39)20-23-14-8-6-9-15-23/h6,8-9,14-15,22,26-29H,4-5,7,10-13,16-21H2,1-3H3,(H,34,42)(H,35,40)(H,36,41)/t22-,26-,27-,28+,29-/m0/s1 | KEGG
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity towards HDAC enzyme derived from Eimeria tenella protozoa |
Bioorg Med Chem Lett 11: 113-7 (2001)
BindingDB Entry DOI: 10.7270/Q2M045ZB |
More data for this
Ligand-Target Pair | |
Histone deacetylase
(Cryptosporidium parvum) | BDBM50366750
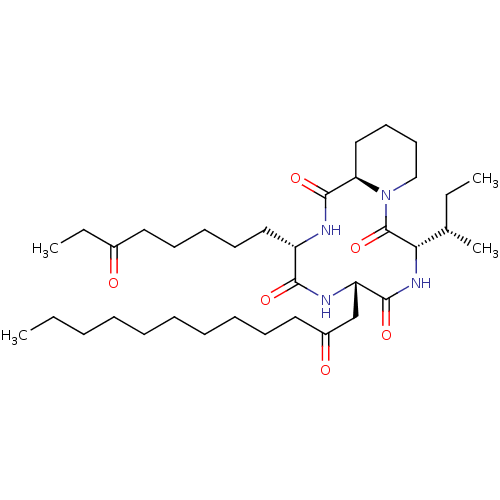
(CHEMBL1793987)Show SMILES CCCCCCCCCCC(=O)C[C@@H]1NC(=O)[C@H](CCCCCC(=O)CC)NC(=O)[C@H]2CCCCN2C(=O)[C@@H](NC1=O)[C@@H](C)CC Show InChI InChI=1S/C36H62N4O6/c1-5-8-9-10-11-12-13-15-21-28(42)25-30-34(44)39-32(26(4)6-2)36(46)40-24-19-18-23-31(40)35(45)37-29(33(43)38-30)22-17-14-16-20-27(41)7-3/h26,29-32H,5-25H2,1-4H3,(H,37,45)(H,38,43)(H,39,44)/t26-,29-,30-,31+,32-/m0/s1 | KEGG
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity towards HDAC enzyme derived from Eimeria tenella protozoa |
Bioorg Med Chem Lett 11: 113-7 (2001)
BindingDB Entry DOI: 10.7270/Q2M045ZB |
More data for this
Ligand-Target Pair | |
Histone deacetylase 3
(Homo sapiens (Human)) | BDBM50568214

(CHEMBL4860000)Show SMILES CCC(=O)CCCCC[C@@H]1NC(=O)[C@H]2CC22CCN(CC2)CCCCCCCCn2ccc3cc(ccc3c2=O)-c2cnc1[nH]2 |r,wU:13.12,wD:9.8,(54.03,-3.3,;52.63,-2.65,;51.39,-3.55,;51.52,-5.08,;49.99,-2.91,;48.73,-3.8,;47.34,-3.16,;46.08,-4.04,;44.69,-3.4,;43.44,-4.3,;43.59,-5.82,;44.98,-6.47,;46.23,-5.57,;45.14,-7.99,;46.02,-9.24,;44.49,-9.38,;43.11,-8.59,;41.76,-9.38,;41.75,-10.96,;43.12,-11.75,;44.51,-10.96,;40.36,-11.76,;38.97,-10.95,;37.58,-11.75,;36.09,-10.67,;34.26,-10.81,;32.5,-6.91,;30.88,-5.99,;31.12,-4.14,;32.53,-3.43,;33.01,-1.95,;34.54,-1.65,;35.57,-2.81,;37.06,-2.53,;38.07,-3.68,;37.59,-5.14,;36.08,-5.44,;35.07,-4.29,;33.55,-4.59,;33.04,-6.11,;39.58,-3.38,;40.22,-1.98,;41.75,-2.16,;42.05,-3.66,;40.71,-4.41,)| | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of HDAC3 (unknown origin) |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128168
BindingDB Entry DOI: 10.7270/Q2QR51V7 |
More data for this
Ligand-Target Pair | |
Nuclear receptor corepressor 1
(Homo sapiens (Human)) | BDBM50544215
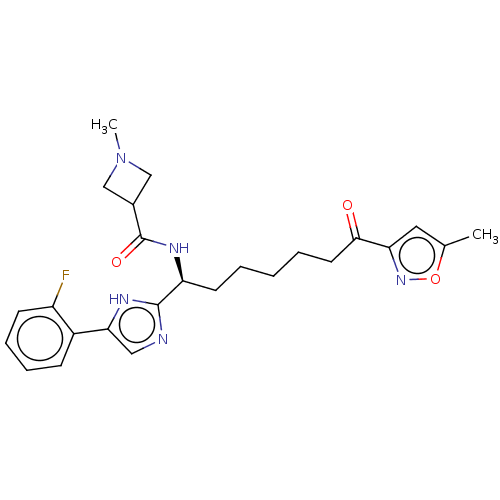
(CHEMBL4637689)Show SMILES CN1CC(C1)C(=O)N[C@@H](CCCCCC(=O)c1cc(C)on1)c1ncc([nH]1)-c1ccccc1F |r| Show InChI InChI=1S/C25H30FN5O3/c1-16-12-21(30-34-16)23(32)11-5-3-4-10-20(29-25(33)17-14-31(2)15-17)24-27-13-22(28-24)18-8-6-7-9-19(18)26/h6-9,12-13,17,20H,3-5,10-11,14-15H2,1-2H3,(H,27,28)(H,29,33)/t20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Inhibition of full length human recombinant FLAG-tagged HDAC3/His6-tagged SMRT (1 to 899 residues) expressed in HEK293F cells using FLUOR DE LYS as s... |
ACS Med Chem Lett 11: 1476-1483 (2020)
Article DOI: 10.1021/acsmedchemlett.0c00302
BindingDB Entry DOI: 10.7270/Q2RV0S85 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50544211

(CHEMBL4641682)Show SMILES CN1CC(C1)C(=O)N[C@@H](CCCCCC(=O)c1ccon1)c1ncc([nH]1)-c1ccccc1F |r| Show InChI InChI=1S/C24H28FN5O3/c1-30-14-16(15-30)24(32)28-20(9-3-2-4-10-22(31)19-11-12-33-29-19)23-26-13-21(27-23)17-7-5-6-8-18(17)25/h5-8,11-13,16,20H,2-4,9-10,14-15H2,1H3,(H,26,27)(H,28,32)/t20-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Inhibition of full length human recombinant FLAG-tagged HDAC1 expressed in HEK293F cells using FLUOR DE LYS as substrate preincubated for 3 hrs follo... |
ACS Med Chem Lett 11: 1476-1483 (2020)
Article DOI: 10.1021/acsmedchemlett.0c00302
BindingDB Entry DOI: 10.7270/Q2RV0S85 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50543650
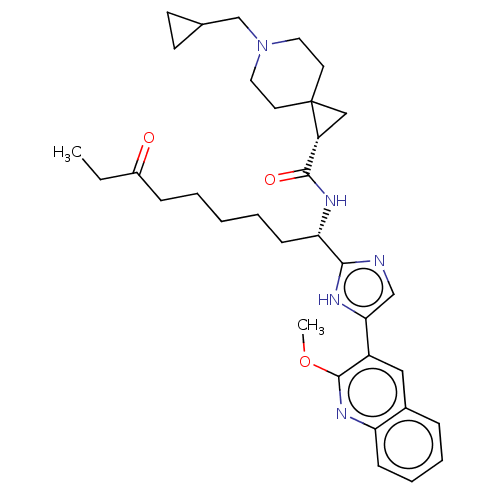
(CHEMBL4649205)Show SMILES CCC(=O)CCCCC[C@H](NC(=O)[C@H]1CC11CCN(CC2CC2)CC1)c1ncc([nH]1)-c1cc2ccccc2nc1OC |r| Show InChI InChI=1S/C34H45N5O3/c1-3-25(40)10-5-4-6-12-29(37-32(41)27-20-34(27)15-17-39(18-16-34)22-23-13-14-23)31-35-21-30(36-31)26-19-24-9-7-8-11-28(24)38-33(26)42-2/h7-9,11,19,21,23,27,29H,3-6,10,12-18,20,22H2,1-2H3,(H,35,36)(H,37,41)/t27-,29+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.0850 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Inhibition of HDAC1 (unknown origin) |
Bioorg Med Chem Lett 30: (2020)
Article DOI: 10.1016/j.bmcl.2020.127197
BindingDB Entry DOI: 10.7270/Q2CZ3BQC |
More data for this
Ligand-Target Pair | |
Nuclear receptor corepressor 1
(Homo sapiens (Human)) | BDBM50544211

(CHEMBL4641682)Show SMILES CN1CC(C1)C(=O)N[C@@H](CCCCCC(=O)c1ccon1)c1ncc([nH]1)-c1ccccc1F |r| Show InChI InChI=1S/C24H28FN5O3/c1-30-14-16(15-30)24(32)28-20(9-3-2-4-10-22(31)19-11-12-33-29-19)23-26-13-21(27-23)17-7-5-6-8-18(17)25/h5-8,11-13,16,20H,2-4,9-10,14-15H2,1H3,(H,26,27)(H,28,32)/t20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.0870 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Inhibition of full length human recombinant FLAG-tagged HDAC3/His6-tagged SMRT (1 to 899 residues) expressed in HEK293F cells using FLUOR DE LYS as s... |
ACS Med Chem Lett 11: 1476-1483 (2020)
Article DOI: 10.1021/acsmedchemlett.0c00302
BindingDB Entry DOI: 10.7270/Q2RV0S85 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 3
(Homo sapiens (Human)) | BDBM50543650

(CHEMBL4649205)Show SMILES CCC(=O)CCCCC[C@H](NC(=O)[C@H]1CC11CCN(CC2CC2)CC1)c1ncc([nH]1)-c1cc2ccccc2nc1OC |r| Show InChI InChI=1S/C34H45N5O3/c1-3-25(40)10-5-4-6-12-29(37-32(41)27-20-34(27)15-17-39(18-16-34)22-23-13-14-23)31-35-21-30(36-31)26-19-24-9-7-8-11-28(24)38-33(26)42-2/h7-9,11,19,21,23,27,29H,3-6,10,12-18,20,22H2,1-2H3,(H,35,36)(H,37,41)/t27-,29+/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.0930 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Inhibition of HDAC3 (unknown origin) |
Bioorg Med Chem Lett 30: (2020)
Article DOI: 10.1016/j.bmcl.2020.127197
BindingDB Entry DOI: 10.7270/Q2CZ3BQC |
More data for this
Ligand-Target Pair | |
Histone deacetylase
(Homo sapiens (Human)) | BDBM50366693
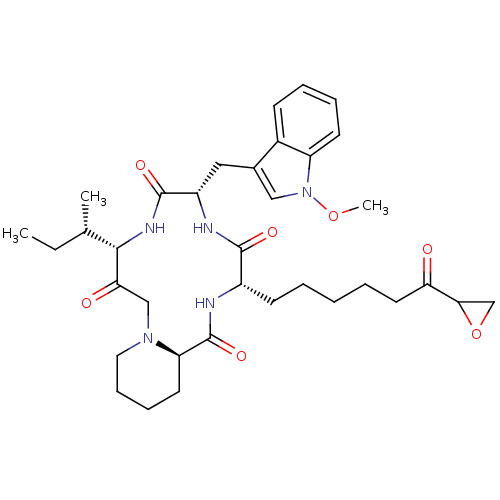
(CHEMBL1793812)Show SMILES CC[C@H](C)[C@@H]1NC(=O)[C@H](Cc2cn(OC)c3ccccc23)NC(=O)[C@H](CCCCCC(=O)C2CO2)NC(=O)[C@H]2CCCCN2CC1=O Show InChI InChI=1S/C35H49N5O7/c1-4-22(2)32-30(42)20-39-17-11-10-15-28(39)35(45)36-25(13-6-5-7-16-29(41)31-21-47-31)33(43)37-26(34(44)38-32)18-23-19-40(46-3)27-14-9-8-12-24(23)27/h8-9,12,14,19,22,25-26,28,31-32H,4-7,10-11,13,15-18,20-21H2,1-3H3,(H,36,45)(H,37,43)(H,38,44)/t22-,25-,26-,28+,31?,32-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | <0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against histone deacetylase (HDAC) derived from partially purified extracts of human HeLa cells |
Bioorg Med Chem Lett 11: 107-11 (2001)
BindingDB Entry DOI: 10.7270/Q2QR4XNX |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50543649
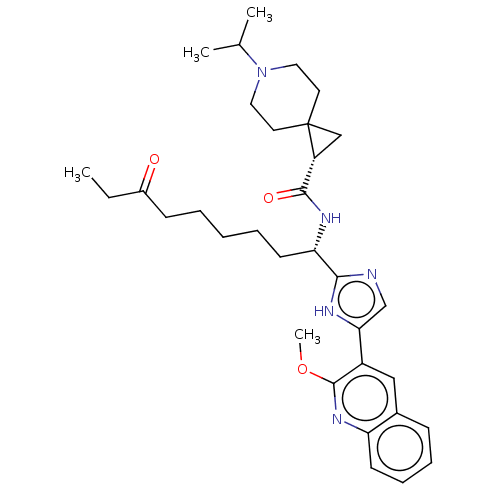
(CHEMBL4644038)Show SMILES CCC(=O)CCCCC[C@H](NC(=O)[C@H]1CC11CCN(CC1)C(C)C)c1ncc([nH]1)-c1cc2ccccc2nc1OC |r| Show InChI InChI=1S/C33H45N5O3/c1-5-24(39)12-7-6-8-14-28(36-31(40)26-20-33(26)15-17-38(18-16-33)22(2)3)30-34-21-29(35-30)25-19-23-11-9-10-13-27(23)37-32(25)41-4/h9-11,13,19,21-22,26,28H,5-8,12,14-18,20H2,1-4H3,(H,34,35)(H,36,40)/t26-,28+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Inhibition of HDAC1 (unknown origin) |
Bioorg Med Chem Lett 30: (2020)
Article DOI: 10.1016/j.bmcl.2020.127197
BindingDB Entry DOI: 10.7270/Q2CZ3BQC |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50568215
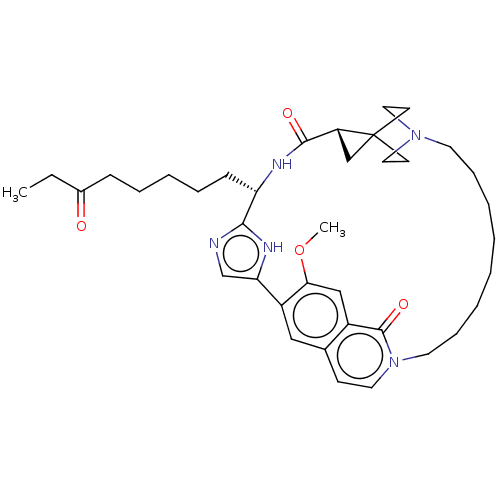
(CHEMBL4878197)Show SMILES CCC(=O)CCCCC[C@@H]1NC(=O)[C@H]2CC22CCN(CC2)CCCCCCCCn2ccc3cc(-c4cnc1[nH]4)c(OC)cc3c2=O |r,wU:13.12,wD:9.8,(81.04,-4.01,;79.64,-3.37,;78.4,-4.27,;78.53,-5.8,;77,-3.63,;75.75,-4.52,;74.35,-3.88,;73.09,-4.76,;71.71,-4.12,;70.45,-5.02,;70.6,-6.54,;71.99,-7.19,;73.24,-6.29,;72.15,-8.71,;73.03,-9.95,;71.5,-10.1,;70.13,-9.31,;68.77,-10.1,;68.77,-11.67,;70.13,-12.47,;71.52,-11.68,;67.37,-12.47,;65.99,-11.67,;64.6,-12.47,;63.21,-11.22,;61.32,-11.48,;59.29,-7.36,;57.73,-6.26,;58.02,-4.74,;59.54,-4.15,;60.03,-2.67,;61.55,-2.37,;62.58,-3.53,;64.07,-3.25,;65.09,-4.4,;66.6,-4.1,;67.23,-2.7,;68.76,-2.88,;69.06,-4.38,;67.72,-5.13,;64.6,-5.85,;65.62,-7,;65.13,-8.45,;63.1,-6.16,;62.08,-5.01,;60.56,-5.31,;60.05,-6.83,)| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of HDAC1 (unknown origin) |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128168
BindingDB Entry DOI: 10.7270/Q2QR51V7 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50568211
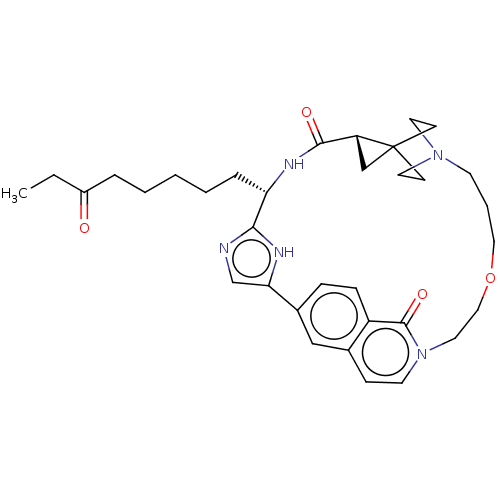
(CHEMBL4876610)Show SMILES CCC(=O)CCCCC[C@@H]1NC(=O)[C@H]2CC22CCN(CC2)CCCOCCn2ccc3cc(ccc3c2=O)-c2cnc1[nH]2 |r,wU:13.12,wD:9.8,(53.94,-55.95,;52.55,-55.3,;51.3,-56.19,;51.44,-57.72,;49.9,-55.56,;48.65,-56.45,;47.25,-55.81,;45.99,-56.69,;44.61,-56.06,;43.35,-56.95,;43.49,-58.47,;44.88,-59.12,;46.14,-58.23,;45.04,-60.64,;45.93,-61.89,;44.4,-62.03,;43.02,-61.24,;41.66,-62.03,;41.66,-63.61,;43.03,-64.41,;44.41,-63.62,;40.26,-64.41,;38.55,-63.17,;36.55,-63.64,;33.33,-59.89,;30.34,-58.69,;30.85,-56.4,;32.42,-56.08,;32.92,-54.6,;34.44,-54.29,;35.47,-55.46,;36.96,-55.18,;37.98,-56.33,;37.49,-57.79,;35.99,-58.09,;34.97,-56.93,;33.45,-57.24,;32.94,-58.76,;39.48,-56.03,;40.12,-54.63,;41.65,-54.8,;41.95,-56.31,;40.62,-57.06,)| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of HDAC1 (unknown origin) |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128168
BindingDB Entry DOI: 10.7270/Q2QR51V7 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 3
(Homo sapiens (Human)) | BDBM50568215

(CHEMBL4878197)Show SMILES CCC(=O)CCCCC[C@@H]1NC(=O)[C@H]2CC22CCN(CC2)CCCCCCCCn2ccc3cc(-c4cnc1[nH]4)c(OC)cc3c2=O |r,wU:13.12,wD:9.8,(81.04,-4.01,;79.64,-3.37,;78.4,-4.27,;78.53,-5.8,;77,-3.63,;75.75,-4.52,;74.35,-3.88,;73.09,-4.76,;71.71,-4.12,;70.45,-5.02,;70.6,-6.54,;71.99,-7.19,;73.24,-6.29,;72.15,-8.71,;73.03,-9.95,;71.5,-10.1,;70.13,-9.31,;68.77,-10.1,;68.77,-11.67,;70.13,-12.47,;71.52,-11.68,;67.37,-12.47,;65.99,-11.67,;64.6,-12.47,;63.21,-11.22,;61.32,-11.48,;59.29,-7.36,;57.73,-6.26,;58.02,-4.74,;59.54,-4.15,;60.03,-2.67,;61.55,-2.37,;62.58,-3.53,;64.07,-3.25,;65.09,-4.4,;66.6,-4.1,;67.23,-2.7,;68.76,-2.88,;69.06,-4.38,;67.72,-5.13,;64.6,-5.85,;65.62,-7,;65.13,-8.45,;63.1,-6.16,;62.08,-5.01,;60.56,-5.31,;60.05,-6.83,)| | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.130 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of HDAC3 (unknown origin) |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128168
BindingDB Entry DOI: 10.7270/Q2QR51V7 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50544215

(CHEMBL4637689)Show SMILES CN1CC(C1)C(=O)N[C@@H](CCCCCC(=O)c1cc(C)on1)c1ncc([nH]1)-c1ccccc1F |r| Show InChI InChI=1S/C25H30FN5O3/c1-16-12-21(30-34-16)23(32)11-5-3-4-10-20(29-25(33)17-14-31(2)15-17)24-27-13-22(28-24)18-8-6-7-9-19(18)26/h6-9,12-13,17,20H,3-5,10-11,14-15H2,1-2H3,(H,27,28)(H,29,33)/t20-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Inhibition of full length human recombinant FLAG-tagged HDAC1 expressed in HEK293F cells using FLUOR DE LYS as substrate preincubated for 3 hrs follo... |
ACS Med Chem Lett 11: 1476-1483 (2020)
Article DOI: 10.1021/acsmedchemlett.0c00302
BindingDB Entry DOI: 10.7270/Q2RV0S85 |
More data for this
Ligand-Target Pair | |
Nuclear receptor corepressor 1
(Homo sapiens (Human)) | BDBM50544227
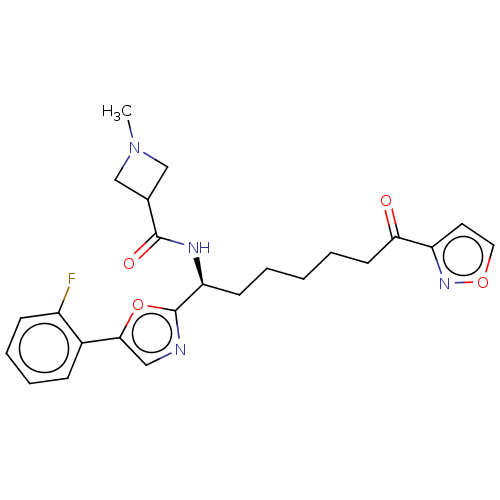
(CHEMBL4634501)Show SMILES CN1CC(C1)C(=O)N[C@@H](CCCCCC(=O)c1ccon1)c1ncc(o1)-c1ccccc1F |r| Show InChI InChI=1S/C24H27FN4O4/c1-29-14-16(15-29)23(31)27-20(9-3-2-4-10-21(30)19-11-12-32-28-19)24-26-13-22(33-24)17-7-5-6-8-18(17)25/h5-8,11-13,16,20H,2-4,9-10,14-15H2,1H3,(H,27,31)/t20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.170 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Inhibition of full length human recombinant FLAG-tagged HDAC3/His6-tagged SMRT (1 to 899 residues) expressed in HEK293F cells using FLUOR DE LYS as s... |
ACS Med Chem Lett 11: 1476-1483 (2020)
Article DOI: 10.1021/acsmedchemlett.0c00302
BindingDB Entry DOI: 10.7270/Q2RV0S85 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 3
(Homo sapiens (Human)) | BDBM50543649

(CHEMBL4644038)Show SMILES CCC(=O)CCCCC[C@H](NC(=O)[C@H]1CC11CCN(CC1)C(C)C)c1ncc([nH]1)-c1cc2ccccc2nc1OC |r| Show InChI InChI=1S/C33H45N5O3/c1-5-24(39)12-7-6-8-14-28(36-31(40)26-20-33(26)15-17-38(18-16-33)22(2)3)30-34-21-29(35-30)25-19-23-11-9-10-13-27(23)37-32(25)41-4/h9-11,13,19,21-22,26,28H,5-8,12,14-18,20H2,1-4H3,(H,34,35)(H,36,40)/t26-,28+/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.180 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Inhibition of HDAC3 (unknown origin) |
Bioorg Med Chem Lett 30: (2020)
Article DOI: 10.1016/j.bmcl.2020.127197
BindingDB Entry DOI: 10.7270/Q2CZ3BQC |
More data for this
Ligand-Target Pair | |
Histone deacetylase 3
(Homo sapiens (Human)) | BDBM50543637
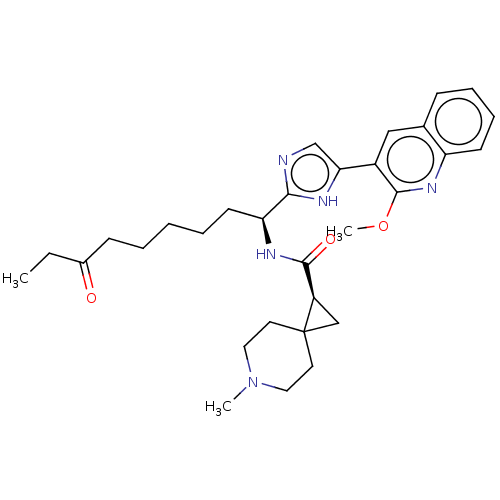
(CHEMBL4642518)Show SMILES CCC(=O)CCCCC[C@H](NC(=O)[C@H]1CC11CCN(C)CC1)c1ncc([nH]1)-c1cc2ccccc2nc1OC |r| Show InChI InChI=1S/C31H41N5O3/c1-4-22(37)11-6-5-7-13-26(34-29(38)24-19-31(24)14-16-36(2)17-15-31)28-32-20-27(33-28)23-18-21-10-8-9-12-25(21)35-30(23)39-3/h8-10,12,18,20,24,26H,4-7,11,13-17,19H2,1-3H3,(H,32,33)(H,34,38)/t24-,26+/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.190 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Inhibition of HDAC3 (unknown origin) |
Bioorg Med Chem Lett 30: (2020)
Article DOI: 10.1016/j.bmcl.2020.127197
BindingDB Entry DOI: 10.7270/Q2CZ3BQC |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50543637

(CHEMBL4642518)Show SMILES CCC(=O)CCCCC[C@H](NC(=O)[C@H]1CC11CCN(C)CC1)c1ncc([nH]1)-c1cc2ccccc2nc1OC |r| Show InChI InChI=1S/C31H41N5O3/c1-4-22(37)11-6-5-7-13-26(34-29(38)24-19-31(24)14-16-36(2)17-15-31)28-32-20-27(33-28)23-18-21-10-8-9-12-25(21)35-30(23)39-3/h8-10,12,18,20,24,26H,4-7,11,13-17,19H2,1-3H3,(H,32,33)(H,34,38)/t24-,26+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.190 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Inhibition of HDAC1 (unknown origin) |
Bioorg Med Chem Lett 30: (2020)
Article DOI: 10.1016/j.bmcl.2020.127197
BindingDB Entry DOI: 10.7270/Q2CZ3BQC |
More data for this
Ligand-Target Pair | |
Histone deacetylase 3
(Homo sapiens (Human)) | BDBM50552770

(CHEMBL4792257)Show SMILES CCN1CCC2(C[C@@H]2C(=O)N[C@@H](CCCCCC(=O)CC)c2ncc([nH]2)-c2cc(C)ccc2OC)CC1 |r| | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | <0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of HDAC3 (unknown origin) using compound purified by microgram-scale HPLC work-flow |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00596
BindingDB Entry DOI: 10.7270/Q2HD809G |
More data for this
Ligand-Target Pair | |
Histone deacetylase 3
(Homo sapiens (Human)) | BDBM50552772

(CHEMBL4753460)Show SMILES CCN1CCC2(C[C@@H]2C(=O)N[C@@H](CCCCCC(=O)CC)c2ncc([nH]2)-c2cc(ccc2OC)C(C)C)CC1 |r| | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of HDAC3 (unknown origin) using compound purified by microgram-scale HPLC work-flow |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00596
BindingDB Entry DOI: 10.7270/Q2HD809G |
More data for this
Ligand-Target Pair | |
Histone deacetylase 3
(Homo sapiens (Human)) | BDBM50552785
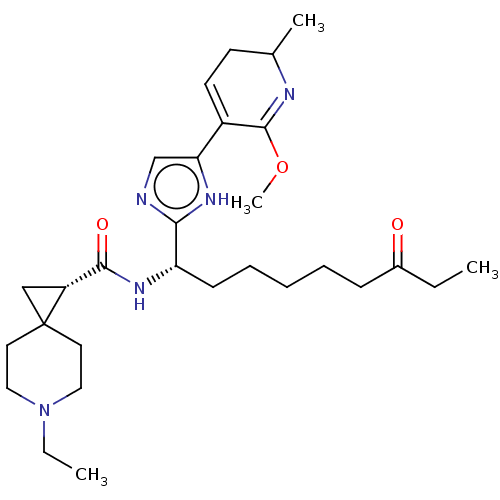
(CHEMBL4780974)Show SMILES CCN1CCC2(C[C@@H]2C(=O)N[C@@H](CCCCCC(=O)CC)c2ncc([nH]2)C2=CCC(C)N=C2OC)CC1 |r,c:33,t:28| | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | <0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of HDAC3 (unknown origin) using compound purified by microgram-scale HPLC work-flow |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00596
BindingDB Entry DOI: 10.7270/Q2HD809G |
More data for this
Ligand-Target Pair | |
Histone deacetylase
(Homo sapiens (Human)) | BDBM50366696

(CHEMBL1793811)Show SMILES CC[C@H](C)[C@@H]1NC(=O)[C@H](Cc2cn(OC)c3ccccc23)NC(=O)[C@H](CCCCCC(=O)NO)NC(=O)[C@H]2CCCCN2CC1=O Show InChI InChI=1S/C33H48N6O7/c1-4-21(2)30-28(40)20-38-17-11-10-15-27(38)33(44)34-24(13-6-5-7-16-29(41)37-45)31(42)35-25(32(43)36-30)18-22-19-39(46-3)26-14-9-8-12-23(22)26/h8-9,12,14,19,21,24-25,27,30,45H,4-7,10-11,13,15-18,20H2,1-3H3,(H,34,44)(H,35,42)(H,36,43)(H,37,41)/t21-,24-,25-,27+,30-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against histone deacetylase (HDAC) derived from partially purified extracts of human HeLa cells |
Bioorg Med Chem Lett 11: 107-11 (2001)
BindingDB Entry DOI: 10.7270/Q2QR4XNX |
More data for this
Ligand-Target Pair | |
Histone deacetylase 3
(Homo sapiens (Human)) | BDBM50552754

(CHEMBL4742026)Show SMILES CCC(=O)CCCCC[C@H](NC(=O)[C@H]1CC11CCN(C)CC1)c1ncc([nH]1)-c1cn(C)c2ccccc12 |r| | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of HDAC3 (unknown origin) using compound purified by traditional preparative HPLC work-flow |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00596
BindingDB Entry DOI: 10.7270/Q2HD809G |
More data for this
Ligand-Target Pair | |
Histone deacetylase 3
(Homo sapiens (Human)) | BDBM50552755
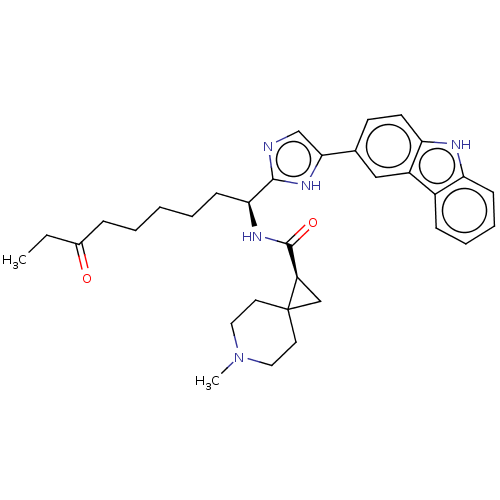
(CHEMBL4780309)Show SMILES CCC(=O)CCCCC[C@H](NC(=O)[C@H]1CC11CCN(C)CC1)c1ncc([nH]1)-c1ccc2[nH]c3ccccc3c2c1 |r| | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of HDAC3 (unknown origin) using compound purified by traditional preparative HPLC work-flow |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00596
BindingDB Entry DOI: 10.7270/Q2HD809G |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50552755

(CHEMBL4780309)Show SMILES CCC(=O)CCCCC[C@H](NC(=O)[C@H]1CC11CCN(C)CC1)c1ncc([nH]1)-c1ccc2[nH]c3ccccc3c2c1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of HDAC1 (unknown origin) using compound purified by traditional preparative HPLC work-flow |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00596
BindingDB Entry DOI: 10.7270/Q2HD809G |
More data for this
Ligand-Target Pair | |
Histone deacetylase 3
(Homo sapiens (Human)) | BDBM50552762
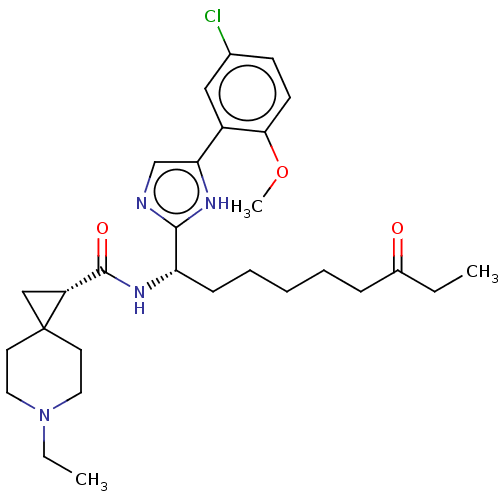
(CHEMBL4753452)Show SMILES CCN1CCC2(C[C@@H]2C(=O)N[C@@H](CCCCCC(=O)CC)c2ncc([nH]2)-c2cc(Cl)ccc2OC)CC1 |r| | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | <0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of HDAC3 (unknown origin) using compound purified by microgram-scale HPLC work-flow |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00596
BindingDB Entry DOI: 10.7270/Q2HD809G |
More data for this
Ligand-Target Pair | |
Histone deacetylase
(Cryptosporidium parvum) | BDBM50366738
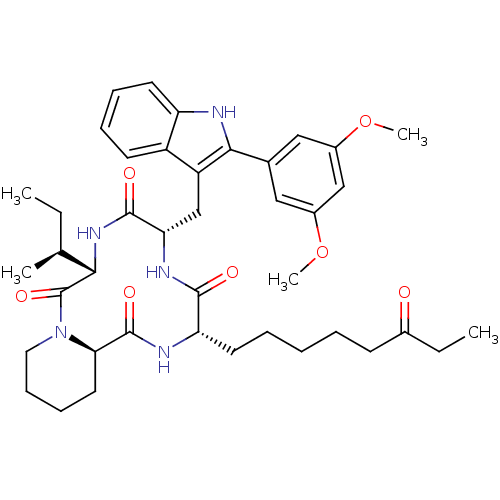
(CHEMBL1793985)Show SMILES CC[C@H](C)[C@@H]1NC(=O)[C@H](Cc2c([nH]c3ccccc23)-c2cc(OC)cc(OC)c2)NC(=O)[C@H](CCCCCC(=O)CC)NC(=O)[C@H]2CCCCN2C1=O Show InChI InChI=1S/C41H55N5O7/c1-6-25(3)36-41(51)46-20-14-13-19-35(46)40(50)43-33(18-10-8-9-15-27(47)7-2)38(48)44-34(39(49)45-36)24-31-30-16-11-12-17-32(30)42-37(31)26-21-28(52-4)23-29(22-26)53-5/h11-12,16-17,21-23,25,33-36,42H,6-10,13-15,18-20,24H2,1-5H3,(H,43,50)(H,44,48)(H,45,49)/t25-,33-,34-,35+,36-/m0/s1 | KEGG
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against histone deacetylase enzyme derived from partially purified extracts of Eimeria tenella protozoa using [3H]11 as radioliga... |
Bioorg Med Chem Lett 11: 113-7 (2001)
BindingDB Entry DOI: 10.7270/Q2M045ZB |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50568214

(CHEMBL4860000)Show SMILES CCC(=O)CCCCC[C@@H]1NC(=O)[C@H]2CC22CCN(CC2)CCCCCCCCn2ccc3cc(ccc3c2=O)-c2cnc1[nH]2 |r,wU:13.12,wD:9.8,(54.03,-3.3,;52.63,-2.65,;51.39,-3.55,;51.52,-5.08,;49.99,-2.91,;48.73,-3.8,;47.34,-3.16,;46.08,-4.04,;44.69,-3.4,;43.44,-4.3,;43.59,-5.82,;44.98,-6.47,;46.23,-5.57,;45.14,-7.99,;46.02,-9.24,;44.49,-9.38,;43.11,-8.59,;41.76,-9.38,;41.75,-10.96,;43.12,-11.75,;44.51,-10.96,;40.36,-11.76,;38.97,-10.95,;37.58,-11.75,;36.09,-10.67,;34.26,-10.81,;32.5,-6.91,;30.88,-5.99,;31.12,-4.14,;32.53,-3.43,;33.01,-1.95,;34.54,-1.65,;35.57,-2.81,;37.06,-2.53,;38.07,-3.68,;37.59,-5.14,;36.08,-5.44,;35.07,-4.29,;33.55,-4.59,;33.04,-6.11,;39.58,-3.38,;40.22,-1.98,;41.75,-2.16,;42.05,-3.66,;40.71,-4.41,)| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of HDAC1 (unknown origin) |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128168
BindingDB Entry DOI: 10.7270/Q2QR51V7 |
More data for this
Ligand-Target Pair | |
Histone deacetylase
(Homo sapiens (Human)) | BDBM50218940

(CHEMBL1793991)Show SMILES [H][C@](C)(CC)[C@]1([H])NC(=O)[C@]([H])(Cc2cn(CN3CCCC3)c3ccccc23)NC(=O)[C@]([H])(CCCCCC(=O)CC)NC(=O)[C@@]2([H])CCCCN2C1=O Show InChI InChI=1S/C38H56N6O5/c1-4-26(3)34-38(49)44-22-12-11-19-33(44)37(48)39-30(17-8-6-7-15-28(45)5-2)35(46)40-31(36(47)41-34)23-27-24-43(25-42-20-13-14-21-42)32-18-10-9-16-29(27)32/h9-10,16,18,24,26,30-31,33-34H,4-8,11-15,17,19-23,25H2,1-3H3,(H,39,48)(H,40,46)(H,41,47)/t26-,30-,31-,33+,34-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against histone deacetylase (HDAC) enzyme derived from partially purified extracts of human HeLa cells using [3H]11 as radioligan... |
Bioorg Med Chem Lett 11: 113-7 (2001)
BindingDB Entry DOI: 10.7270/Q2M045ZB |
More data for this
Ligand-Target Pair | |
Histone deacetylase
(Homo sapiens (Human)) | BDBM50366738

(CHEMBL1793985)Show SMILES CC[C@H](C)[C@@H]1NC(=O)[C@H](Cc2c([nH]c3ccccc23)-c2cc(OC)cc(OC)c2)NC(=O)[C@H](CCCCCC(=O)CC)NC(=O)[C@H]2CCCCN2C1=O Show InChI InChI=1S/C41H55N5O7/c1-6-25(3)36-41(51)46-20-14-13-19-35(46)40(50)43-33(18-10-8-9-15-27(47)7-2)38(48)44-34(39(49)45-36)24-31-30-16-11-12-17-32(30)42-37(31)26-21-28(52-4)23-29(22-26)53-5/h11-12,16-17,21-23,25,33-36,42H,6-10,13-15,18-20,24H2,1-5H3,(H,43,50)(H,44,48)(H,45,49)/t25-,33-,34-,35+,36-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against histone deacetylase (HDAC) enzyme derived from partially purified extracts of human HeLa cells using [3H]11 as radioligan... |
Bioorg Med Chem Lett 11: 113-7 (2001)
BindingDB Entry DOI: 10.7270/Q2M045ZB |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50544227

(CHEMBL4634501)Show SMILES CN1CC(C1)C(=O)N[C@@H](CCCCCC(=O)c1ccon1)c1ncc(o1)-c1ccccc1F |r| Show InChI InChI=1S/C24H27FN4O4/c1-29-14-16(15-29)23(31)27-20(9-3-2-4-10-21(30)19-11-12-32-28-19)24-26-13-22(33-24)17-7-5-6-8-18(17)25/h5-8,11-13,16,20H,2-4,9-10,14-15H2,1H3,(H,27,31)/t20-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Inhibition of full length human recombinant FLAG-tagged HDAC1 expressed in HEK293F cells using FLUOR DE LYS as substrate preincubated for 3 hrs follo... |
ACS Med Chem Lett 11: 1476-1483 (2020)
Article DOI: 10.1021/acsmedchemlett.0c00302
BindingDB Entry DOI: 10.7270/Q2RV0S85 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50543647

(CHEMBL4637357)Show SMILES CCN1CCC2(C[C@@H]2C(=O)N[C@@H](CCCCCC(=O)CC)c2ncc([nH]2)-c2cc3ccccc3nc2OC)CC1 |r| Show InChI InChI=1S/C32H43N5O3/c1-4-23(38)12-7-6-8-14-27(35-30(39)25-20-32(25)15-17-37(5-2)18-16-32)29-33-21-28(34-29)24-19-22-11-9-10-13-26(22)36-31(24)40-3/h9-11,13,19,21,25,27H,4-8,12,14-18,20H2,1-3H3,(H,33,34)(H,35,39)/t25-,27+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.220 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Inhibition of HDAC1 (unknown origin) |
Bioorg Med Chem Lett 30: (2020)
Article DOI: 10.1016/j.bmcl.2020.127197
BindingDB Entry DOI: 10.7270/Q2CZ3BQC |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50543643
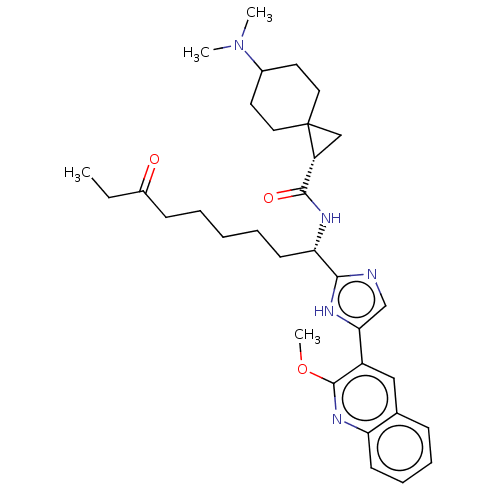
(CHEMBL4642023)Show SMILES CCC(=O)CCCCC[C@H](NC(=O)[C@H]1CC11CCC(CC1)N(C)C)c1ncc([nH]1)-c1cc2ccccc2nc1OC |r,wD:9.9,13.12,(21.19,-2.07,;19.64,-2.07,;18.88,-3.41,;19.65,-4.74,;17.34,-3.41,;16.57,-4.75,;15.03,-4.75,;14.26,-6.09,;12.72,-6.09,;11.95,-7.43,;12.72,-8.76,;14.27,-8.76,;15.03,-7.42,;15.03,-10.08,;15.04,-11.61,;16.36,-10.84,;17.12,-12.17,;18.64,-12.17,;19.41,-10.85,;18.65,-9.53,;17.12,-9.52,;20.94,-10.86,;21.7,-12.19,;21.72,-9.54,;10.41,-7.43,;9.64,-6.1,;8.13,-6.43,;7.98,-7.96,;9.39,-8.57,;6.66,-8.74,;5.32,-7.97,;3.99,-8.75,;2.65,-7.98,;1.32,-8.76,;1.32,-10.3,;2.66,-11.07,;3.99,-10.3,;5.33,-11.06,;6.66,-10.29,;8,-11.05,;8.01,-12.59,)| Show InChI InChI=1S/C33H45N5O3/c1-5-24(39)12-7-6-8-14-28(36-31(40)26-20-33(26)17-15-23(16-18-33)38(2)3)30-34-21-29(35-30)25-19-22-11-9-10-13-27(22)37-32(25)41-4/h9-11,13,19,21,23,26,28H,5-8,12,14-18,20H2,1-4H3,(H,34,35)(H,36,40)/t23?,26-,28+,33?/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.240 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Inhibition of HDAC1 (unknown origin) |
Bioorg Med Chem Lett 30: (2020)
Article DOI: 10.1016/j.bmcl.2020.127197
BindingDB Entry DOI: 10.7270/Q2CZ3BQC |
More data for this
Ligand-Target Pair | |
Histone deacetylase 3
(Homo sapiens (Human)) | BDBM50543640
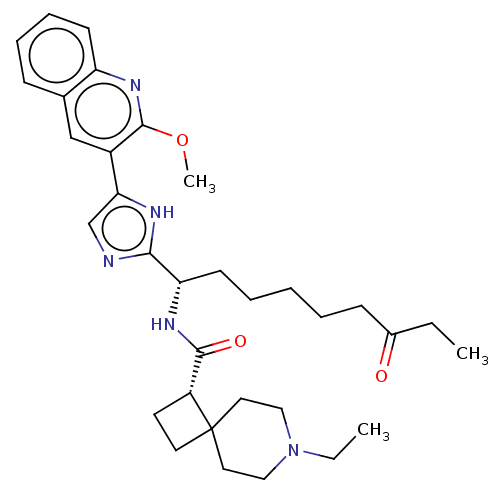
(CHEMBL4638264)Show SMILES CCN1CCC2(CC[C@@H]2C(=O)N[C@@H](CCCCCC(=O)CC)c2ncc([nH]2)-c2cc3ccccc3nc2OC)CC1 |r| Show InChI InChI=1S/C33H45N5O3/c1-4-24(39)12-7-6-8-14-28(36-31(40)26-15-16-33(26)17-19-38(5-2)20-18-33)30-34-22-29(35-30)25-21-23-11-9-10-13-27(23)37-32(25)41-3/h9-11,13,21-22,26,28H,4-8,12,14-20H2,1-3H3,(H,34,35)(H,36,40)/t26-,28+/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.280 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Inhibition of HDAC3 (unknown origin) |
Bioorg Med Chem Lett 30: (2020)
Article DOI: 10.1016/j.bmcl.2020.127197
BindingDB Entry DOI: 10.7270/Q2CZ3BQC |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50606543
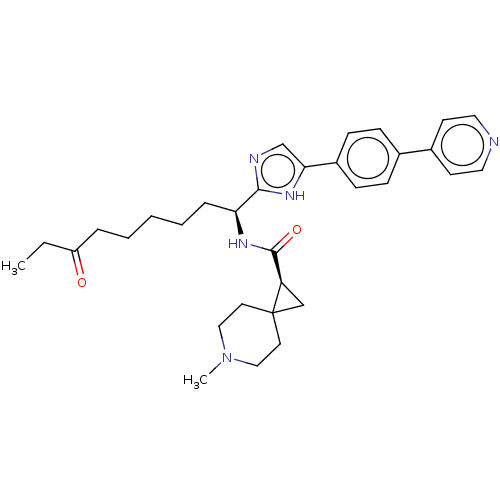
(CHEMBL5218926)Show SMILES CCC(=O)CCCCC[C@H](NC(=O)[C@H]1CC11CCN(C)CC1)c1ncc([nH]1)-c1ccc(cc1)-c1ccncc1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.280 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c02150
BindingDB Entry DOI: 10.7270/Q2N58RGN |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50543640

(CHEMBL4638264)Show SMILES CCN1CCC2(CC[C@@H]2C(=O)N[C@@H](CCCCCC(=O)CC)c2ncc([nH]2)-c2cc3ccccc3nc2OC)CC1 |r| Show InChI InChI=1S/C33H45N5O3/c1-4-24(39)12-7-6-8-14-28(36-31(40)26-15-16-33(26)17-19-38(5-2)20-18-33)30-34-22-29(35-30)25-21-23-11-9-10-13-27(23)37-32(25)41-3/h9-11,13,21-22,26,28H,4-8,12,14-20H2,1-3H3,(H,34,35)(H,36,40)/t26-,28+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.290 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Inhibition of HDAC1 (unknown origin) |
Bioorg Med Chem Lett 30: (2020)
Article DOI: 10.1016/j.bmcl.2020.127197
BindingDB Entry DOI: 10.7270/Q2CZ3BQC |
More data for this
Ligand-Target Pair | |
Histone deacetylase
(Homo sapiens (Human)) | BDBM50366712
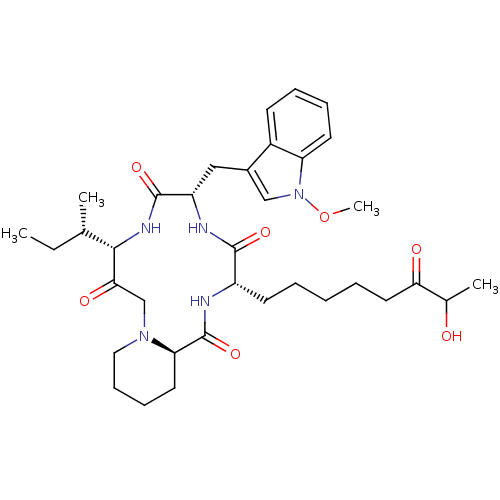
(CHEMBL1793822)Show SMILES CC[C@H](C)[C@@H]1NC(=O)[C@H](Cc2cn(OC)c3ccccc23)NC(=O)[C@H](CCCCCC(=O)C(C)O)NC(=O)[C@H]2CCCCN2CC1=O Show InChI InChI=1S/C35H51N5O7/c1-5-22(2)32-31(43)21-39-18-12-11-16-29(39)35(46)36-26(14-7-6-8-17-30(42)23(3)41)33(44)37-27(34(45)38-32)19-24-20-40(47-4)28-15-10-9-13-25(24)28/h9-10,13,15,20,22-23,26-27,29,32,41H,5-8,11-12,14,16-19,21H2,1-4H3,(H,36,46)(H,37,44)(H,38,45)/t22-,23?,26-,27-,29+,32-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against histone deacetylase (HDAC) derived from partially purified extracts of human HeLa cells |
Bioorg Med Chem Lett 11: 107-11 (2001)
BindingDB Entry DOI: 10.7270/Q2QR4XNX |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50606566
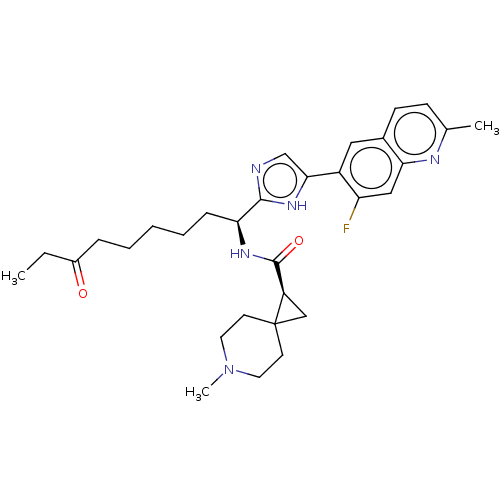
(CHEMBL5220233)Show SMILES CCC(=O)CCCCC[C@H](NC(=O)[C@H]1CC11CCN(C)CC1)c1ncc([nH]1)-c1cc2ccc(C)nc2cc1F |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | <0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c02150
BindingDB Entry DOI: 10.7270/Q2N58RGN |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50606568
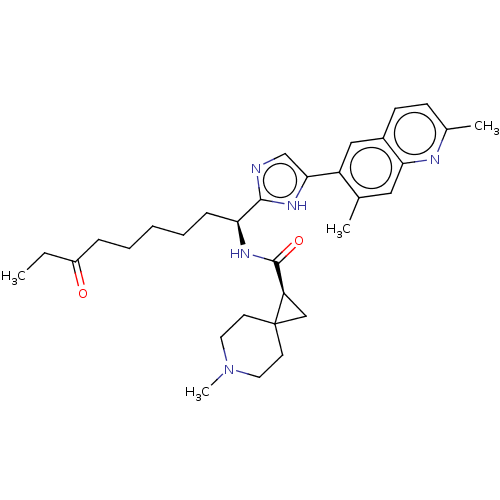
(CHEMBL5219115)Show SMILES CCC(=O)CCCCC[C@H](NC(=O)[C@H]1CC11CCN(C)CC1)c1ncc([nH]1)-c1cc2ccc(C)nc2cc1C |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | <0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c02150
BindingDB Entry DOI: 10.7270/Q2N58RGN |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50606569

(CHEMBL5220254)Show SMILES CCC(=O)CCCCC[C@H](NC(=O)[C@H]1CC11CCN(C)CC1)c1ncc([nH]1)-c1cc2ccc(C)nc2cc1CC |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | <0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c02150
BindingDB Entry DOI: 10.7270/Q2N58RGN |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50606572
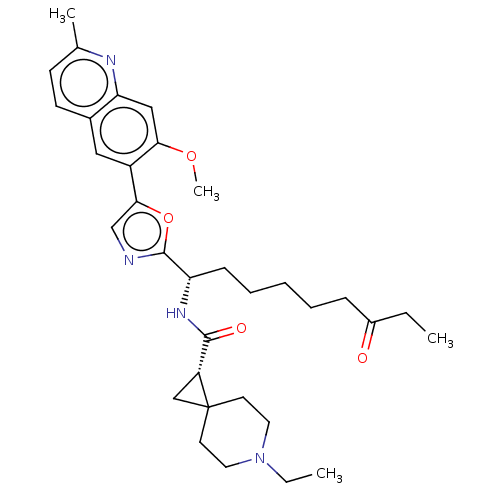
(CHEMBL5218693)Show SMILES CCN1CCC2(C[C@@H]2C(=O)N[C@@H](CCCCCC(=O)CC)c2ncc(o2)-c2cc3ccc(C)nc3cc2OC)CC1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | <0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c02150
BindingDB Entry DOI: 10.7270/Q2N58RGN |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data